Abstract
Background
Venous thromboembolism (VTE) after an inferior vena cava (IVC) injury is a devastating complication. Current practice involves variable use of anticoagulation and antiplatelet (AC/AP) agents. We hypothesized that AC/AP can reduce the incidence of VTE and that delayed institution of AC/AP is associated with increased VTE events.
Methods
We retrospectively reviewed IVC injuries cared for at a large urban adult academic level 1 trauma center between January 1, 2008 and December 31, 2020, surviving 72 hours. Patient demographics, injury mechanism, surgical repair, type and timing of AC, and type and timing of VTE events were characterized. Postoperative AC status during hospital course before an acute VTE event was delineated by grouping patients into four categories: full, prophylactic, prophylactic with concomitant AP, and none. The primary outcome was the incidence of an acute VTE event. IVC ligation was excluded from analysis.
Results
Of the 76 patients sustaining an IVC injury, 26 were included. The incidence of a new deep vein thrombosis distal to the IVC injury and a new pulmonary embolism was 31% and 15%, respectively. The median onset of VTE was 5 days (IQR 1–11). Four received full AC, 10 received prophylactic AC with concomitant AP, 8 received prophylactic AC, and 4 received no AC/AP. New VTE events occurred in 0.0% of full, in 30.0% of prophylactic with concomitant AP, in 50.0% of prophylactic, and in 50.0% without AC/AP. There was no difference in baseline demographics, injury mechanisms, surgical interventions, and bleeding complications.
Discussion
This is the first study to suggest that delay and degree of antithrombotic initiation in an IVC-injured patient may be associated with an increase in VTE events. Consideration of therapy initiation should be performed on hemostatic stabilization. Future studies are necessary to characterize the optimal dosing and temporal timing of these therapies.
Level of evidence
Therapeutic, level 3.
Keywords: adult, venous thromboembolism, venous thrombosis, vascular system injuries
WHAT IS ALREADY KNOWN ON THIS TOPIC
Traumatic injury lends an individual susceptible to venous thromboembolism (VTE) events.
Within injured patients, several cohorts exist that have a higher incidence of VTE, including patients with an injured inferior vena cava.
WHAT THIS STUDY ADDS
This study provides stratification to thromboprophylaxis regimens and suggests that more aggressive prophylaxis can mitigate VTE events.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
On hemostatic stabilization, a clinician should consider initiating appropriately aggressive antithrombotic medications.
Background
Inferior vena cava (IVC) injuries, inclusive of various types, locations, and severities, account for up to 40% of major abdominal vascular injuries and carry a mortality rate of 20% to 66%.1–10 Following injury, surgical exploration and repair can be arduous and inclusive of high-volume bleeding and major risk of death. Patients surviving IVC injury are at high risk of catastrophic complications, including major life-threatening bleeding, IVC thrombosis, deep vein thrombosis (DVT), and pulmonary embolus (PE).4 8–11 The incidence of venous thromboembolism (VTE) has been reported to be 1.1% to 18.9% in DVT and 0.4% to 4.6% in PE.8 9 11
Best practices for antithrombotic prophylaxis in patients with traumatic vascular injury remain unclear, as demonstrated by the American Association for the Surgery of Trauma in the multicenter PROOVIT (PROspective Observational Vascular Injury Trial), which found no consistent approach to anticoagulation across 542 patients with various vascular injuries.12 Surgical repair of the IVC further disrupts the endothelium and may also introduce luminal stenosis, both of which further increase the risk of VTE. In addition, a focal hematoma can compress the vessel, disrupting laminar flow.
This risk, however, must be balanced by acute bleeding concerns after an extensive retroperitoneal dissection needed for surgical repair,13 which can lend a risk of excess bleeding in addition to other concomitant injuries that may continue to bleed (ie, liver laceration, splenic laceration). Additionally, the plausibility of incomplete hemostasis, missed injuries, or iatrogenic injuries must be considered before instituting these modalities. Lastly, if the patient had other injuries that contaminated the repair including enteric contents, fecal contents, or pancreatic enzymes, then there is always the possibility of dehiscing the IVC suture line. This then renders a high-flow, low-pressure catastrophic bleed.
Antithrombotic agents are widely used postoperatively and even beyond hospital discharge to mitigate VTE events, but for patients with IVC injuries specifically no studies have conclusively demonstrated an ideal course for treatment.12 Herein, this study sought to evaluate the relationship between various antithrombotic regimens with the incidence of acute VTE in patients with an IVC injury. We conducted a retrospective clinical study in which our hypothesis was that varied anticoagulant and antiplatelet dose regimens exist, and these regimens will result in a varied incidence of acute VTE events after IVC injury. Such study is necessary as potentially preventable deaths could then be mitigated.
Methods
Patients and data collection
We performed a retrospective observational study at our large, urban, academic, medical center and level 1 trauma center between January 1, 2008 and December 31, 2020. Data were curated from our institutional trauma registry and electronic medical record (EMR). The registry was queried for all patients who sustained a traumatic IVC injury requiring hospital admission. On EMR review, patients who were ultimately found not to have sustained an IVC injury or if they died within the index 72 hours were excluded from analysis. An investigation of cause of death was performed on patients who survived beyond their index operation; however, succumbed to death within the first 72 hours. Furthermore, due to the inherent differences in underline presenting physiology, a patient was excluded from the VTE analysis if they underwent an IVC ligation (online supplemental figure 1).
tsaco-2022-000923supp003.pdf (45.2KB, pdf)
We classified patients into four cohorts: full-dose anticoagulation, prophylactic dose anticoagulation, prophylactic dose anticoagulation with concomitant antiplatelet agent, and no anticoagulant or antiplatelet agent. During the study period, standard-of-care prophylaxis was based on empiric dosing and not titrated to any measure. Patients were assigned to their cohort depending on their current treatment before an acute VTE event or hospital discharge, whichever occurred first. Therefore, if a patient received full-dose anticoagulation, but they were transitioned to prophylactic anticoagulation before sustaining an acute VTE, then the patient was analyzed in the prophylactic dose regimen. Pre-existing home medications were characterized. Our analysis included any available EMR data up to 6 months postacute hospitalization discharge.
Our primary outcome was the incidence of an acute VTE event. An acute VTE included IVC thrombus (presumed at the site of injury), any DVT distal to the IVC injury (ie, ileac veins), or PE. Only a patient’s first acute VTE event was recorded in the analysis for time to VTE event. If a patient sustained both a DVT and a PE, this was also characterized.
Descriptive variables included patient demographics (age, gender, body mass index), injury mechanism and details (mechanism, Injury Severity Score, Abbreviated Injury Scale score of the abdomen, trauma exsanguination protocol activation), surgical methods (type of operation, IVC filter placement), type and timing of all antithrombotic agents (including discharge regimens), timing of VTE events, and outcomes (mortality, bleeding complications, new VTE events after hospital discharge).
Although this is an observational study, it should be noted that during the investigation period our DVT surveillance protocol underwent several modifications, which may have affected our recorded incidence of DVT detection. Briefly, in the first 4 years of the study period, screening was optional in the high-risk and very high-risk patients; however, it became mandatory for this cohort in 2012. Then, in 2017, screening again de-escalated. When performed, asymptomatic screening typically involved duplex ultrasound imaging of the lower extremities, usually performed within the first 5 days of admission and continued every 5 to 7 days up to either 3 weeks or until three negative duplexes (online supplemental table 1). In addition, imaging was also obtained when clinical suspicion arose, at the provider’s discretion. Article preparation was guided by STROBE (Strengthening The Reporting of OBservational Studies in Epidemiology; checklist provided in online supplemental materials).
tsaco-2022-000923supp001.pdf (30.6KB, pdf)
tsaco-2022-000923supp002.pdf (100.2KB, pdf)
Results
Of the 84 patients screened from the registry query, 76 sustained an IVC injury on EMR review. 26 met all the inclusion and exclusion criteria. On EMR review, 8 were excluded as they did not have an IVC injury, 23 died in the emergency department, 22 died in the operating room, and 4 died within the index 72 hours (online supplemental figure 1). In addition, one patient underwent an IVC ligation.
Four patients were partitioned into the therapeutic dose anticoagulation. Ten patients received concomitant prophylactic dose anticoagulation with an antiplatelet agent. Eight patients received prophylactic dose anticoagulation. Four patients did not receive any anticoagulation or antiplatelet agents. Of the fully anticoagulated patients, two received a concomitant antiplatelet agent during part of their hospital course.
Overall, 26 (100.0%) were male and the median age was 26 (IQR 16–51). Of the patients, 24 (92.3%) sustained a penetrating injury and 22 (84.6%) sustained a gunshot wound. The descriptors of their injury burden and operative details are listed in table 1, including 24 (92.3%) who went to the operating theater. These 26 patients were admitted by 18 different trauma attendings. Table 2 depicts patients’ injuries, including their cava injuries.
Table 1.
Baseline demographic comparison of the AC and AP cohorts
| Entire cohort (N=26) | Full (n=4) | Prophylactic+AP (n=10) | Prophylactic (n=8) | None (n=4) | |
| Demographics and clinical variables | |||||
| Age (years) | 26 (16–51) | 24 (22–26) | 25 (16–57) | 29 (20–51) | 24 (16–28) |
| Male (%) | 26 (100) | 4 (100) | 10 (100) | 8 (100) | 4 (100) |
| BMI (kg/m2) | 24.4 (19.7–45.6) | 24.4 (19.9–30.9) | 26.3 (19.7–45.6) | 22.3 (18.9–47.0) | 24.4 (23.6–28.1) |
| Penetrating (%) | 24 (92) | 4 (100) | 9 (90) | 9 (100) | 3 (75) |
| ISS | 22 (9–38) | 21 (10–34) | 22 (9–34) | 18 (9–38) | 27 (9–43) |
| AIS score of abdomen | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 3 (3–4) |
| Trauma exsanguination protocol (%) | 17 (71), n=24 | 3 (75) | 6 (67), n=9 | 5 (71), n=7 | 3 (75) |
| Operative management | |||||
| Surgical intervention (%) | 24 (92) | 4 (100) | 9 (90) | 9 (100)* | 3 (75) |
| Postoperative outcomes | |||||
| IVC filter (%) | 5 (19) | 0 (0) | 2 (20) | 2 (22) | 1 (25) |
| Mortality (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
All values are n (%) or median (IQR).
There was no significant difference between the treatment groups with regard to baseline demographics.
*One patient required surgical intervention, but the retroperitoneum was not explored.
AC, anticoagulation; AIS, Abbreviated Injury Scale; AP, antiplatelet; BMI, body mass index; ISS, Injury Severity Score; IVC, inferior vena cava.
Table 2.
Description of patient injuries, including a description of the IVC injury
| Patient | Injury burden | IVC injury description |
| 1 | Right colectomy, mesenteric injury, SBR, embolization of the proximal SMA and ileocolic artery. | Suture line on the right lateral wall of the infrarenal IVC performed at OSH. |
| 2 | Aorta-caval fistula (stent), Gr 5 renal (nephrectomy), Gr 3 D2 injury ×2, SBR. | Nickel-size anterior hole extending inferior toward the renal hilum; 3–0 Prolene running. |
| 3 | Gr 3 D3 ×2 (primary), Gr 2 gastric greater curve (primary), G1 colon (right hemicolectomy), frx: L4. | 1 cm anterior and 1 cm posterior infrarenal injury; 4–0 Prolene running. |
| 4 | SBR ×2, ileocolic mesenteric injury ×5, ilium frx. | 2 cm proximal to the iliac confluence; single 5–0 Prolene figure 8. |
| 5 | Gr 4 liver, Gr 2 right renal, Gr 3 left renal, HTX, frx: L2 TP, olecranon, alar. | 2 cm lateral longitudinal near renal vein; 4–0 Prolene running and 2 figure 8 reinforcement. |
| 6 | Renal laceration, lumbar artery coil, PSA a embolize, PPTX, and contusion, frx: 4/5 rib, clavicle, L2–L4 TP, Le Fort 3, bilateral upper extremities | IVC not repaired. |
| 7 | Small bowel injury ×4 (SBR ×2), frx: humerus. | Infrarenal anterior longitudinal 2.5 cm and lateral 1.5 cm; 4–0 running <10% diameter loss. |
| 8 | 4 cm transverse D1 and 2 cm anterior D2 (both primarily), pancreas (drain), liver, renal hematoma. | 2–3 cm longitudinal anterior infrarenal injury; 3–0 running Prolene slight narrowing. |
| 9 | Liver, superior/lateral pancreas, duodenal ×2 (primary), pyloric exclusion, cholecystectomy, mid-jejunum (SBR multiple), right colon (colostomy), frx: L1/L2 (incomplete paraplegia), ulnar/radial, iliac. | Infrarenal injuries (×3): posterior injury longitudinal and linear, lateral injury, anterior injury that was extended; 4–0 Prolene. |
| 10 | Pancreas (drained), posterior duodenum (primarily) right hemicolectomy (mesenteric hematoma). | 2 cm lateral; 4–0 Prolene interrupted, then second layer of 4–0 Prolene running. |
| 11 | Liver (segments 4 and 5), pancreas (uncinate), CBD (T-tube), colon (right hemicolectomy), frx: femur, L3. | 3 cm defect anteromedially; 5–0 Prolene with significant stenosis. |
| 12 | D3 ×2 (primary), SBR (multiple), mesenteric injury. | Infrarenal extends to the iliac; running Prolene, reinforced with interrupted. |
| 13 | Gr 1 spleen, Gr 1 liver, right pulmonary contusion. | IVC not repaired. |
| 14 | 3 liver lacerations, branch of right hepatic artery (requiring IR embolization). | 2 mm anterior injury at the renal hilum junction; 4–0 Prolene figure 8. |
| 15 | Aorta-caval fistula (repair, stent, repair), D2 ×2 (primary), PTX. | Infrahepatic requiring venopulmonary artery bypass; 2 4–0 Prolene with pledgets. |
| 16 | Liver, gastric (greater curve, posterior) (primary), pancreatic head (drained), cholecystectomy, L3 partial hemiplegia. | Anterior and inferior posterior injury; 5–0 Prolene longitudinally, lost two-thirds diameter. |
| 17 | Gastric ×2 (gastric wedge, ultimately gastrojejunostomy and pyloric exclusion), duodenum ×2 (initially primary repair), transverse colon resection, Gr 1 liver, L3 (unstable). | 3 cm medial injury few centimeters inferior to the renal vein involving 50% circumference; 3–0 Prolene running with narrowing that was unavoidable. |
| 18 | Cecal and TI injury resulting in completion right hemicolectomy with right ileocolonic anastomosis. | Infrarenal lateral and posterior injuries; 3–0 Prolene whip stitch. |
| 19 | Gr 3 liver, transected gallbladder (cholecystecotmy), 2 cm lateral D2 (primary), Gr 1 bilateral kidney. | Infrahepatic 1.5 cm anterior; 4–0 running Prolene with 5–0 interrupted Prolene. |
| 20 | Small bowel (SBR), mesenteric injury, transected ureter (PCN). | Infrarenal 3 cm anterior injury; double running with 40% loss in diameter. |
| 21 | Gr 3 D3 and D4 anterior and posterior duodenal (primary), renal pseudoaneurysm ×2 IR, mesenteric. | Infrarenal right side 7 o’clock 1 cm and left side 3 o’clock 2 cm, double venorrhaphy. |
| 22 | Gr 5 pancreas and distal pancreas (drains/stent), partial hepatectomy, cholecystectomy, severed GDA, transect renal artery and vein. | 4 cm medial suprarenal with possible renal artery; 4–0 Prolene running without narrowing. |
| 23 | Small bowel injury (SBR), renal hematoma, L4/L5 (ASIA-B), right 12th rib. | IVC not repaired. |
| 24 | Liver, portal vein, pancreatic neck (distal pancreatectomy), splenectomy, nephrectomy, lumbar artery embolize, frx: L2/L3 TP, tibia/fibula, iliac wing. | Juxtarenal; 3–0 Prolene running without narrowing. |
| 25 | Nephrectomy, pancreas head (drain), colon splenic flexure (ostomy), adrenal, lumbar artery, frx: femur, tibia. | Infrarenal lateral through and through; 4–0 Prolene with some narrowing. |
| 26 | 85% infrarenal aortic (shunt/repair), jejunum (SBR), right tibial/peroneal artery, iliac wing frx. | Lateral infrarenal hole; running Prolene. |
ASIA, American Spinal Injury Association; BLUE, bilateral upper extremities; CBD, common bile duct; chole, cholecystectomy; D, duodenum; frx, fracture; GDA, gastroduodenal artery; Gr, grade; HTX, hemothorax; IR, interventional radiology; IVC, inferior vena cava; OSH, outside hospital; PCN, percutaneous nephrostomy; PSA, posterior superior alveolar; PTX, pneumothorax; SBR, small bowel resection; SMA, superior mesenteric artery; TI, terminal ileum; TP, transverse process.
None of the patients were noted to be on an anticoagulant as a home medication; however, one patient in the prophylactic anticoagulation with a concomitant antiplatelet agent was noted to have aspirin and effient as home medications. Four (15.4%) of the patietns had data missing on their home medications.However, none of these four patients had a pre-existing condition warranting an antithrombotic agent.
Nine (34.6%) acute VTE events were reported. Four PEs and eight DVTs occurred in nine different patients and the anatomic locations of the DVTs are described in table 3. New VTE events occurred in zero (0.0%) patient in the full-dose cohort, in three (30.0%) patients in the prophylactic dose with concomitant antiplatelet agent cohort, in four (50.0%) patients in the prophylactic cohort, and in two (50.0%) patients without antithrombotic agents (figure 1). Of note, three IVCs were thrombosed, two prophylactic patients and one patient without any antithrombotic agents. Additionally, one prophylactic patient whose IVC was stenosed sustained a right femoral DVT.
Table 3.
Location of deep vein thrombosis
| Patient | HD-DVT | Location of DVT |
| 17 | 1-DVT | IVC thrombosis. |
| 19 | 5-DVT | Soleal DVT that propagated on serial ultrasounds into the peroneal vein and posterior tibial vein meeting full-dose AC criteria. |
| 20 | 5-DVT | IVC stenosis (50%) with right femoral DVT. |
| 22 | 11-DVT | Right popliteal DVT with partial mobile tip. |
| 23 | 7-DVT | IVC thrombosis with occlusive iliac veins. Right profunda DVT and left soleal DVT. IVC/iliac vein recanalization recommended. |
| 24 | 3-DVT | IVC thrombosis and left popliteal vein thrombosis. |
| 25 | 10-DVT | Left iliac vein thrombosis extending toward the IVC and the left femoral vein. |
| 26 | 19-DVT | PE diagnosed during admission (HD 11) on AC, probable right external iliac DVT by CT. Workup would not change management. |
AC, anticoagulation; DVT, deep vein thrombosis; HD, hospital day; IVC, inferior vena cava; PE, pulmonary embolism.
Figure 1.
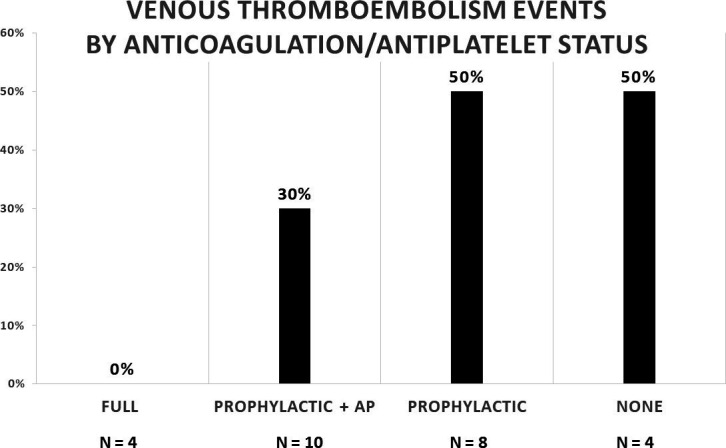
Bar graph with anticoagulation and antiplatelet (AP) as the independent variable and the incidence of acute venous thromboembolism events as the dependent variable.
Table 4 depicts the antithrombotic regimen throughout the hospital course, including changes to the daily antithrombotic medications along with hospital days of VTE events. Notably, one patient who was classified in the category without receiving antithrombotic agents had been on prophylactic dose anticoagulation from hospital days 3 to 8, then was transitioned to full-dose anticoagulation on day 8. On hospital day 10, the full-dose and prophylactic dose anticoagulation were discontinued due to a gastrointestinal bleed. The patient was then diagnosed with a pulmonary embolism on day 11 in addition to a DVT on day 19. They received an IVC filter on hospital day 11 and their full-dose anticoagulation resumed on hospital day 14. Of the four fully anticoagulated patients, none sustained an acute bleeding complication after full-dose anticoagulation initiation. Online supplemental table 2 provides a detailed account of the bleeding events.
Table 4.
Temporal timing of antithrombotics and venous thromboembolic events by patient
| Patient | VTE | Timing of antithrombotic and venous thromboembolic events |
| Full | ||
| 3 | N/A | HD 1 PPX enox, HD1–5 FD hep (HD 6–10 ASA 325), HD 5–10 PPX enox, 10–12 and 14–16 FD hep (HD 12–14 FD enox), HD 16-20 FD apix; LOS 20 days |
| 5 | N/A | HD 2–4 PPX hep (HD 2–5 ASA), HD 4–6 FD hep, 7–9 FD enox, 10–16 FD dabigatran; LOS 16 days |
| 14 | N/A | HD 4–8 PPX hep, HD 12–19 FD hep; LOS 19 days |
| 18 | N/A | HD 3–4 PPX hep, HD 4–5 FD warfarin, HD 6–11 FD enox; LOS 11 days |
| Prophylactic with concomitant antiplatelet | ||
| 1 | N/A | HD 9–44 PPX hep (HD 15–35 ASA), HD 44–46 FD hep (our HD 9), HD 52–71 PPX enox (HD 55–71 ASA), HD 1 IVC-F; LOS 71 days |
| 6 | N/A | HD 3–20 PPX enox with ASA; LOS 20 days |
| 7 | N/A | HD 1–7 PPX enox with ASA; LOS 7 days |
| 10 | N/A | HD 4–26 PPX dalteparin (HD 4–26 ASA 325); LOS 26 days |
| 11 | N/A | HD 4–12 FD hep, HD 24–54 PPX dalteparin (HD18-54 ASA 325); LOS 54 days |
| 12 | N/A | HD 3–8 PPX dalteparin (HD 4–6 ASA suppository, HD 7–8 ASA 325); LOS 8 days |
| 16 | N/A | HD 3–18 PPX hep (HD 17–18 ASA 325); LOS 18 days |
| 21 | 4 PE | HD 2–4 PPX enox (HD 2–4 ASA suppository), HD 4–20 FD enox (HD 4–23 ASA 325), HD 20–23 FD apix; LOS 23 days |
| 22 | 11 DVT | HD 2–19 PPX hep (HD 3–25 ASA 81), HD 19–33 FD hep, HD 34–44 PPX hep, HD 44–46 PPX enox, HD 12 IVC-F; LOS 59 |
| 25 | 10 DVT | HD 3–7 PPX hep (HD 4–51 ASA 81), HD 8–11 PPX dalteparin, HD 11–51 FD dalteparin, HD 31–51 warfarin (took a while for INR to become therapeutic); LOS 51 days |
| Prophylactic anticoagulation | ||
| 2 | N/A | HD 2–48 PPX hep (HD 6–10 ASA), HD 48–58 PPX enox; LOS 58 days |
| 4 | N/A | HD 0–6 PPX enox; LOS 6 days |
| 8 | N/A | HD 3–9 PPX enox; LOS 9 days |
| 9 | N/A | HD 1–125 PPX hep (HD 3–22 ASA suppository); LOS 125 days |
| 19 | 5 DVT, 23 PE | HD 3–5 PPX enox, HD 5–11 PPX hep (HD 7–9 ASA suppository, HD 10–28 ASA 325), HD 12–28 PPX enox, HD 29 FD hep, HD 30–77 FD enox; LOS 77 |
| 20 | 5 DVT | HD 2–5 PPX hep, HD 5–9 FD hep, HD 9–10 PPX hep, HD 10 IVC-F; LOS 17 |
| 23 | 7 DVT, 8 PE | HD 3–4 PPX hep, HD 4–8 PPX enox, HD 8–9 FD hep, HD 9–34 FD enox, HD 32–34 warfarin, HD 9 IVC-F; LOS 34 days |
| 24 | 3 DVT | HD 2–3 PPX hep, HD 3–36 FD hep, HD 34–44 warfarin; LOS 44 days |
| No antithrombotics | ||
| 13 | N/A | No antithrombotics; LOS 3 |
| 15 | N/A | No antithrombotics; LOS 41 days |
| 17 | 1 DVT | HD 3–4 PPX hep, HD 4–24 FD hep, HD 24–30 FD enox, HD 30–37 FD hep, HD 38–54 FD enox, HD 54 dalteparin; LOS 54 days |
| 26 | 11 PE, 19 DVT | HD 3–8 PPX hep, HD 8–10 FD hep, HD 14–29 FD hep, HD 11 IVC-F; LOS 34 days |
apix, apixaban; ASA, aspirin; DVT, deep vein thrombosis; enox, enoxaparin; FD, full dose; HD, hospital day; hep, heparin; INR, international normalized ratio; IVC-F, inferior vena cava filter; LOS, length of stay; N/A, not applicable; PE, pulmonary embolism; PPX, prophylactic dose; VTE, venous thromboembolism.
The median days to onset of an acute VTE event was 5 (IQR 1–11). The hospital days that the four cohorts sustained an acute VTE event with their respective median time to receiving anticoagulation and antiplatelet treatment modalities are included in table 5. Notably, the timing only depicts current antithrombotic initiation that the patient is on immediately before a VTE event. All patients who sustained an acute VTE event had undergone primary repair, except for one patient who did not undergo an operation. It should be noted that the incidence of DVT detection increased over time; conversely, the DVT screening process became less aggressive over time.
Table 5.
Hospital day of acute thrombotic event and of anticoagulant/antiplatelet initiation
| Full (n=4) | Prophylactic+antiplatelet (n=10) | Prophylactic (n=8) | None (n=4) | |
| First acute thrombosis hospital day | n=0 | 10 (4–11), n=3 | 5 (3–7), n=4 | 6 (1–11), n=2 |
| Full hospital day initiation | 3 (1–12), n=4 | N/A | N/A | N/A |
| Prophylactic hospital day initiation | 2 (1–4), n=4 | 3 (1–14), n=10 | 3 (0–3), n=8 | N/A |
| Antiplatelet hospital day initiation | 4 (2–6), n=2 | 3 (1–18), n=10 | N/A | N/A |
All values are median (IQR).
N/A, not applicable.
Online supplemental table 3 depicts patients’ discharge regimens after hospitalization. Most of the patients maintained their hospital regimen. Of note, one patient who was in the prophylactic anticoagulation category sustained a new VTE after discharge. Furthermore, on posthospitalization follow-up, there were expected VTE propagations of previously diagnosed DVTs. Two out of the three IVC that were thrombosed had further propagation distally on outpatient follow-up. Finally, one patient (3.8%) did not complete posthospitalization follow-up.
Of the four patients who died within the index 72 hours, one died immediately after their operation and the remainder had a re-exploratory laparotomy confirming coagulopathy as the cause of their death.
Discussion
Traumatically injured patients often have risk factors that lend them to be hypercoagulable, rendering them susceptible to VTE events.14 15 In this retrospective study of IVC-injured patients, we sought to determine the association between anticoagulation/antiplatelet status and the incidence of acute VTE events. Our results show this group to be very high risk, with a 50.0% acute VTE event rate in patients with prophylactic dose anticoagulation and in patients who were not receiving an antithrombotic agent. On the other hand, those receiving full-dose anticoagulation had a 0.0% acute VTE event rate. These novel and striking findings should prompt consideration of more aggressive anticoagulation/antiplatelet treatment therapies in this high-risk population.
The increased VTE event rate in patients with IVC injuries is thought to occur due to disturbance in the laminar flow through endothelial layer disruption and/or the disruption caused by an outflow obstruction when the repair becomes stenosed.16–19 To capture this pathology, we excluded all deaths within the first 72 hours. Of the 49 excluded patients, 48 died within the first 24 hours and the 49th patient died within the first 48 hours. Excluding these patients ensured that we were more likely to include patients who sustained an acute VTE event as the result of disruption of the laminar flow and less likely to include patients who had a non-survivable injury without enough time to contract an acute VTE.
Our 34.6% incidence of acute VTE events is compatible with the existing body of literature.14 20–28 In a large prospective study of 716 patients, it was found that traumatically injured patients have a high incidence of VTE events, with up to 57.6% incidence in those without prophylactic anticoagulation.14 Our cohort of four patients who did not have prophylactic anticoagulation had an incidence of DVT distal to the IVC injury of 50.0%. Prior literature has suggested that there may be a synergistic effect when antiplatelet agents are combined with pharmacological prophylactically dosed anticoagulants and our findings noted an improvement in the VTE event from 50% to 30% when an antiplatelet agent was added to the prophylactic regimen.29 These findings are strikingly different from those who received full-dose anticoagulation, as our incidence of VTE events in this cohort was 0.0%. The literature on the incidence of acute VTE events has widely variable data, with an incidence as high as 57.6% in the general trauma population and an incidence as low as 1.2% in the traumatic IVC injury population.8 9 14 30 Our fully anticoagulated patients had an event rate lower than what was previously described in the literature; however, there were only four patients partitioned into this cohort.
The strength of our study was the manual extraction of data allowing for detailed determination of the type, dosage, and timing of anticoagulation/antiplatelet initiation and the timing of any acute VTE events. In addition, patients were followed 6 months after hospital discharge for any further VTE events. Regional and national databases may list injuries and complications; however, they do not have the granularity of the treatment regimen provided. There are, however, some limitations to consider. This was a single-center study and the analysis was therefore limited. Operative interventions, VTE prophylaxis, and screening processes may not be the same at all other institutions.
In addition, with a final cohort of 26 patients, our study was underpowered to detect a statistical significance between the various cohorts. This further limited our ability to apply more rigorous excluding parameters, to perform a multivariable analysis or to stratify patients based on a number of confounding variables, as it is plausible that a selection bias exists in our fully anticoagulated patients. Furthermore, the classification scheme is not without inherent limitations. Given that group assignments were made based on the immediate last therapy, length of therapy and missed doses were not accounted for. Such a patient may have undergone a lengthy hospital course without receiving antithrombotic agents or sustaining an acute VTE event and then be placed on full-dose anticoagulation in the days preceding discharge. Consequently, this patient would be classified as receiving full-dose anticoagulation.
Our study also had a high prevalence of penetrating mechanisms with a patient population that was entirely male. Of note, in general female trauma patients31–33 and patients with a blunt mechanism34–36 are more likely to be of the hypercoagulable phenotype than of the hyperfibrinolytic phenotype. IVC filter placement can also mitigate some risk; however, placement is not without risk.37–43 As such, this is a controversial topic that needs further investigation in this population as treatment options vary widely in the literature.44–49 The granularity of our data presented a unique opportunity to demonstrate that patients receiving lower doses of anticoagulation/antiplatelet medications had an overall higher incidence of VTE events, all while not having any major bleeding complications in the fully anticoagulated cohort. This outcome is applicable with the understanding that the level of evidence is insufficient.
Although there is a growing body of literature regarding coagulopathy in traumatically injured patients, there remains a paucity of data regarding trauma subpopulations that may benefit from higher doses of VTE prophylaxis, including IVC-injured patients. We think that future prospective multicenter clinical trials should capture coagulation phenotypes and anticoagulation/antiplatelet initiation practices. These details would serve to inform clinical practice guidelines as future directives decide on how to best handle traumatic IVC-injured patients. Our findings can then facilitate a more robust power analysis for a future multicenter trial.
Conclusion
In this retrospective, single-center, observational study of a large, urban, academic, level 1 trauma center, we found that IVC-injured patients have a high incidence of acute VTE events. There appears to be a lower incidence of acute VTE events in patients who were prophylactically fully anticoagulated compared with patients who had other regimens of antithrombotic agents. Consideration of appropriate anticoagulation/antiplatelet initiation should ensue after hemostatic stabilization. A future prospective multicenter trial should capture the temporal characteristics of anticoagulation/antiplatelet treatment modalities to further inform clinical practice.
Footnotes
Contributors: AMH, DRS, JSP, PMR, and NM designed the study. AMH, BCB, and MJS searched the literature. AMH, SM, and BCB aquired the data. AMH analyzed the data. AMH, DRS, SM, JSP, PMR, MJS, and NM participated in data interpretation. AMH and BCB drafted the article, which all authors critically revised for important intellectual content and approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AMH accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was reviewed and approved by the University of Pennsylvania Institutional Review Board (protocol number: 843994). The investigators adhered to the policies regarding the protection of human subjects as prescribed by the Code of Federal Regulations Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
References
- 1.Mullins RJ, Lucas CE, Ledgerwood AM. The natural history following venous ligation for civilian injuries. J Trauma 1980;20:737–43. 10.1097/00005373-198009000-00005 [DOI] [PubMed] [Google Scholar]
- 2.Coimbra R, Hoyt D, Winchell R, Simons R, Fortlage D, Garcia J. The ongoing challenge of retroperitoneal vascular injuries. Am J Surg 1996;172:541–5. 10.1016/S0002-9610(96)00231-0 [DOI] [PubMed] [Google Scholar]
- 3.Tyburski JG, Wilson RF, Dente C, Steffes C, Carlin AM. Factors affecting mortality rates in patients with abdominal vascular injuries. J Trauma 2001;50:1020–6. 10.1097/00005373-200106000-00008 [DOI] [PubMed] [Google Scholar]
- 4.Navsaria PH, de Bruyn P, Nicol AJ. Penetrating abdominal vena cava injuries. Eur J Vasc Endovasc Surg 2005;30:499–503. 10.1016/j.ejvs.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 5.Huerta S, Bui TD, Nguyen TH, Banimahd FN, Porral D, Dolich MO. Predictors of mortality and management of patients with traumatic inferior vena cava injuries. Am Surg 2006;72:290–6. 10.1177/000313480607200402 [DOI] [PubMed] [Google Scholar]
- 6.Paul JS, Webb TP, Aprahamian C, Weigelt JA. Intraabdominal vascular injury: are we getting any better? J Trauma 2010;69:1393–7. 10.1097/TA.0b013e3181e49045 [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PS, Dente CJ, Patel S, Carmichael M, Srinivasan JK, Wyrzykowski AD, Nicholas JM, Salomone JP, Ingram WL, Vercruysse GA, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg 2010;199:500–6. 10.1016/j.amjsurg.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 8.Singer MB, Hadjibashi AA, Bukur M, Ley EJ, Mirocha J, Malinoski DJ, Margulies DR, Salim A. Incidence of venous thromboembolism after inferior vena cava injury. J Surg Res 2012;177:306–9. 10.1016/j.jss.2012.05.055 [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Jung K, Smith A, Coimbra R. Management of IVC injury: repair or ligation? A propensity score matching analysis using the National trauma data bank. J Am Coll Surg 2018;226:752–9. 10.1016/j.jamcollsurg.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 10.Klein SR, Baumgartner FJ, Bongard FS. Contemporary management strategy for major inferior vena caval injuries. J Trauma 1994;37:35–42. 10.1097/00005373-199407000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Burch JM, Feliciano DV, Mattox KL, Edelman M. Injuries of the inferior vena cava. Am J Surg 1988;156:548–52. 10.1016/S0002-9610(88)80550-6 [DOI] [PubMed] [Google Scholar]
- 12.DuBose JJ, Savage SA, Fabian TC, Menaker J, Scalea T, Holcomb JB, Skarupa D, Poulin N, Chourliaras K, Inaba K, et al. The American association for the surgery of trauma prospective observational vascular injury treatment (PROOVIT) registry: multicenter data on modern vascular injury diagnosis, management, and outcomes. J Trauma Acute Care Surg 2015;78:215–22. 10.1097/TA.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 13.Buckman RF, Pathak AS, Badellino MM, Bradley KM. Injuries of the inferior vena cava. Surg Clin North Am 2001;81:1431–47. 10.1016/S0039-6109(01)80016-5 [DOI] [PubMed] [Google Scholar]
- 14.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994;331:1601–6. 10.1056/NEJM199412153312401 [DOI] [PubMed] [Google Scholar]
- 15.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008;65:748–54. 10.1097/TA.0b013e3181877a9c [DOI] [PubMed] [Google Scholar]
- 16.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 1998;18:677–85. 10.1161/01.atv.18.5.677 [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci 2001;947:93–111. 10.1111/j.1749-6632.2001.tb03932.x [DOI] [PubMed] [Google Scholar]
- 18.Mammen EF. Pathogenesis of venous thrombosis. Chest 1992;102:640S–4. 10.1378/chest.102.6_Supplement.640S [DOI] [PubMed] [Google Scholar]
- 19.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327–87. 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano LMB, Perel P, Ker K, Cirocchi R, Farinella E, Morales CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev 2010;2010. 10.1002/14651858.CD008303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based report-part I: analysis of risk factors and evaluation of the role of vena caval filters. J Traumadiscussion 2000;49:132–8. 10.1097/00005373-200007000-00020 [DOI] [PubMed] [Google Scholar]
- 22.Frank B, Maher Z, Hazelton JP, Resnick S, Dauer E, Goldenberg A, Lubitz AL, Smith BP, Saillant NN, Reilly PM, et al. Venous thromboembolism after major venous injuries: competing priorities. J Trauma 2017;83:1095–101. 10.1097/TA.0000000000001655 [DOI] [PubMed] [Google Scholar]
- 23.Shackford SR, Davis JW, Hollingsworth-Fridlund P, Brewer NS, Hoyt DB, Mackersie RC. Venous thromboembolism in patients with major trauma. Am J Surg 1990;159:365–9. 10.1016/S0002-9610(05)81272-3 [DOI] [PubMed] [Google Scholar]
- 24.Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg 2017;83:413–9. 10.1097/TA.0000000000001618 [DOI] [PubMed] [Google Scholar]
- 25.Louis SG, Sato M, Geraci T, Anderson R, Cho SD, Van PY, Barton JS, Riha GM, Underwood S, Differding J, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg 2014;149:365–70. 10.1001/jamasurg.2013.3963 [DOI] [PubMed] [Google Scholar]
- 26.Knudson MM, Collins JA, Goodman SB, McCrory DW. Thromboembolism following multiple trauma. J Trauma 1992;32:2–11. 10.1097/00005373-199201000-00002 [DOI] [PubMed] [Google Scholar]
- 27.Knudson MM, Lewis FR, Clinton A, Atkinson K, Megerman J. Prevention of venous thromboembolism in trauma patients. J Trauma 1994;37:480–7. 10.1097/00005373-199409000-00025 [DOI] [PubMed] [Google Scholar]
- 28.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of surgeons national trauma data bank. Ann Surg 2004;240:490–6. 10.1097/01.sla.0000137138.40116.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collaborative overview of randomised trials of antiplatelet therapy--III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308:235–46. 10.1136/bmj.308.6923.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degiannis E, Velmahos GC, Levy RD, Souter I, Benn CA, Saadia R. Penetrating injuries of the abdominal inferior vena cava. Ann R Coll Surg Engl 1996;78:485–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Gorton HJ, Warren ER, Simpson NA, Lyons GR, Columb MO. Thromboelastography identifies sex-related differences in coagulation. Anesth Analg 2000;91:1279–81. 10.1097/00000539-200011000-00042 [DOI] [PubMed] [Google Scholar]
- 32.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma 2005;58:475–81. 10.1097/01.TA.0000153938.77777.26 [DOI] [PubMed] [Google Scholar]
- 33.Coleman JR, Moore EE, Samuels JM, Cohen MJ, Sauaia A, Sumislawski JJ, Ghasabyan A, Chandler JG, Banerjee A, Silliman CC, et al. Trauma resuscitation consideration: sex matters. J Am Coll Surg 2019;228:760–8. 10.1016/j.jamcollsurg.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg 2017;224:575–82. 10.1016/j.jamcollsurg.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 35.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 2016;222:347–55. 10.1016/j.jamcollsurg.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma 1997;42:716–22. discussion 720-2. 10.1097/00005373-199704000-00023 [DOI] [PubMed] [Google Scholar]
- 37.Vijay K, Hughes JA, Burdette AS, Scorza LB, Singh H, Waybill PN, Lynch FC, Recovery FB. Fractured bard recovery, G2, and G2 express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts. J Vasc Interv Radiol 2012;23:188–94. 10.1016/j.jvir.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Durack JC, Westphalen AC, Kekulawela S, Bhanu SB, Avrin DE, Gordon RL, Kerlan RK. Perforation of the IVC: rule rather than exception after longer indwelling times for the Günther tulip and Celect Retrievable filters. Cardiovasc Intervent Radiol 2012;35:299–308. 10.1007/s00270-011-0151-9 [DOI] [PubMed] [Google Scholar]
- 39.Sarosiek S, Rybin D, Weinberg J, Burke PA, Kasotakis G, Sloan JM. Association between inferior vena cava filter insertion in trauma patients and in-hospital and overall mortality. JAMA Surg 2017;152:75–81. 10.1001/jamasurg.2016.3091 [DOI] [PubMed] [Google Scholar]
- 40.Go MR, Keller-Biehl L, Starr JE. Penetration of the inferior vena cava and adjacent organs after filter placement is associated with retrievable filter type and length of time in place. J Vasc Surg Venous Lymphat Disord 2014;2:174–8. 10.1016/j.jvsv.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 41.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTe in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e227S. 10.1378/chest.11-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teo TKB, Angle JF, Shipp JI, Bluett MK, Gilliland CA, Turba UC, Matsumoto AH. Incidence and management of inferior vena cava filter thrombus detected at time of filter retrieval. J Vasc Interv Radiol 2011;22:1514–20. 10.1016/j.jvir.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 43.Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014;25:1181–5. 10.1016/j.jvir.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 44.Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, Sperry JL, Rizzo AG, Rosen NG, Brasel KJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: a Western trauma association critical decisions algorithm. J Trauma Acute Care Surg 2020;89:971–81. 10.1097/TA.0000000000002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haut ER, Garcia LJ, Shihab HM, Brotman DJ, Stevens KA, Sharma R, Chelladurai Y, Akande TO, Shermock KM, Kebede S, et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta-analysis. JAMA Surg 2014;149:194–202. 10.1001/jamasurg.2013.3970 [DOI] [PubMed] [Google Scholar]
- 46.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133:381S–453. 10.1378/chest.08-0656 [DOI] [PubMed] [Google Scholar]
- 47.Kelkar AH, Rajasekhar A. Inferior vena cava filters: a framework for evidence-based use. Hematology Am Soc Hematol Educ Program 2020;2020:619–28. 10.1182/hematology.2020000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care 2019;23:98. 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rappold JF, Sheppard FR, Carmichael II SP, Cuschieri J, Ley E, Rangel E, Seshadri AJ, Michetti CP. Venous thromboembolism prophylaxis in the trauma intensive care unit: an American association for the surgery of trauma critical care Committee clinical consensus document. Trauma Surg Acute Care Open 2021;6:e000643. 10.1136/tsaco-2020-000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tsaco-2022-000923supp003.pdf (45.2KB, pdf)
tsaco-2022-000923supp001.pdf (30.6KB, pdf)
tsaco-2022-000923supp002.pdf (100.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


