Dear Sirs,
Several cases of inflammatory disease of the central nervous system have been reported after vaccination against COVID-19. The most frequently reported form of demyelinating disorder in this situation is acute disseminated encephalomyelitis (ADEM) [1]. Cases of NMOSD, mostly AQP4+ , have been also reported after vaccination with inactivated vaccine [2], recombinant vaccination [3] and mRNA vaccines [4]. NMOSD is a chronic inflammatory autoimmune disease of the central nervous system in which cardinal symptoms are optic neuritis, extensive transverse myelitis and area postrema syndrome [5], but cerebral lesions are also described and involve mainly periependymal white matter regions [6]. We report here the case of an unusual strictly encephalic form of AQP4+ NMOSD occurring after the first dose of mRNA vaccination against COVID-19.
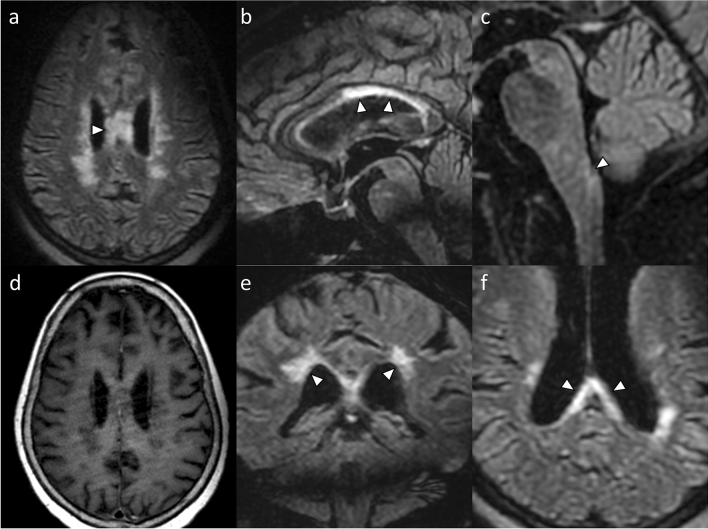
A 72-year-old woman originally from Guadeloupe, with no significant personal or familial medical history presented with paresthesia, hypoesthesia and weakness of the left arm and leg 1 week after receiving the first dose of COVID-19 Moderna (mRNA-1273) vaccination. She had no history of COVID-19 infection. Three weeks later, she developed fever, headaches, and alteration of consciousness. She was admitted to the intensive care unit and her physical examination revealed choreoathetosis of the left arm and leg. Brain magnetic resonance imaging (MRI) showed typical lesions of the corpus callosum, the area postrema and the periependymal regions of the lateral ventricles, without parenchymal or meningeal enhancement (Fig. 1). MRI of the optic nerves and the spinal cord showed no abnormality. The cerebrospinal fluid analysis (CSF) revealed lymphocytic pleocytosis (500 cells/mm3, 88% lymphocytes), with raised protein level (1.17 g/L) and normal glucose level. Infectious agents tested negative in the CSF. Immunological examination of the CSF showed negative oligoclonal bands. The blood tests were within normal ranges for hematological and biochemical findings, there was no immunodeficiency profile, and serology against human immunodeficiency virus was negative. The immunologic screening showed positive antinuclear antibodies (titer 1:160) and anti-SSA/Ro antibodies (titer > 8 UI/mL), with a non-contributive salivary gland biopsy. Serum AQP4 antibodies were positive in the indirect immunofluorescence assay and confirmed in a cell-based assay. Other immunological testing returned negative (including anti-DNA, anti-phospholipid antibodies, anti-neutrophil cytoplasmic antibodies, anti-myelin oligodendrocyte glycoprotein (MOG), anti-thyroid antibodies). Anti-SARS-CoV-2 IgG were positive. A contrast-enhanced computed tomography (CT) scan of the chest, abdomen and pelvis and a whole-body positron emission tomography (PET) scan were performed and showed no sign of a malignant lesion. Anti-paraneoplastic antibodies (tested in the CSF for anti-NMDA, anti-AMPA and anti-VGKC, and in the serum for anti-Yo, -Ri, -GAD, -Hu, -CV and -Tr antibodies) returned negative.
Fig. 1 .
Brain magnetic resonance imaging. Axial (a, f), sagittal (b, c), and coronal (e) fluid-attenuated inversion recovery images show hyperintense lesions of the corpus callosum (a, b, f), the area postrema (c), and the periependymal regions of the lateral ventricles (e). Lesions of the corpus callosum create typical “marbled” (a) and “arch bridge” (f) patterns. Axial post-contrast T1-weighted image (d) shows the absence of parenchymal or meningeal enhancement
Typical MRI findings associated with positive AQP4 antibodies and exclusion of differential diagnoses confirmed the diagnosis of AQP4+ neuromyelitis optica. The patient was treated with high dose of intravenous methylprednisolone pulse followed by cycles of plasma exchanges, with subsequent improvement of the vigilance status and resolution of abnormal movements. Rituximab was introduced afterward. Clinical examination was normal except for persistently altered cognitive functions. Lumbar puncture showed normalization of CSF analysis one month after disease onset (8 cells/mm3, protein level 0.8 g/dL).
We report an unusual strictly encephalic presentation of NMOSD occurring 1 month after the first dose of mRNA COVID-19 vaccination. This type of auto-immune serious adverse event has rarely been described after COVID-19 inactivated vaccine [2], recombinant vaccine [3], and mRNA vaccine [4]. NMOSD have also been described after live-attenuated vaccines such as yellow fever [7].
Knowledge of mRNA vaccine-related adverse events is scarce and their understanding has become of great importance since the dramatic increase of their use with the COVID-19 vaccination campaign. Our case shows that nucleic acid vaccines can act as auto-immune triggers as well as other types of vaccines.
In our case, NMOSD coexists with positive antinuclear and anti-SSA antibodies. NMOSD and Sjögren’s syndrome or antibodies are frequently associated [8]. It is still unclear if the auto-antibodies are directly triggered by the vaccination itself or if there is an underlying autoimmune background favoring the emergence of post-vaccination NMOSD.
Lesions in NMOSD are classically located in the spinal cord and optic nerve regions, but can also affect, as in our case, periventricular areas [6], linked with the high expression of AQP4 antigen in those regions. Our case thus presents an unusual encephalic presentation of NMOSD, with lesions limited to the encephalic white matter, sparing the optic nerves and spinal cord.
Our case of an unusual purely encephalic form of NMOSD occurring after COVID-19 mRNA vaccination contributes to the knowledge of vaccination-related serious adverse events but also to the understanding of the complex NMOSD pathophysiology and triggering factors. NMOSD is a diagnosis to consider in front of an acute febrile encephalopathy occurring in the weeks following a vaccination. Due to the large number of patients receiving COVID-19 vaccination, large prospective studies are needed to further explore the relationship between vaccines and NMOSD.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Declarations
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
ethical standard statement
This case report was performed according to the international ethical guidelines and followed our institutional ethical tenets. Permission was obtained from the patient for the publication of this report.
References
- 1.Dimitrios K, Panayiota P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Fan X-R, He S, et al. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci. 2021;42:3537–3539. doi: 10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badrawi N, Kumar N, Albastaki U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: case report & MRI findings. Radiol Case Rep. 2021 doi: 10.1016/j.radcr.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS infammation: a case series. J Neurol. 2022;269:1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, et al. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöberl F, Csanadi E, Eren O, et al. NMOSD triggered by yellow fever vaccination – an unusual clinical presentation with segmental painful erythema. Mult Scler Relat Disord. 2017 doi: 10.1016/j.msard.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, Lucchinetti CF, Zéphir H, Moder K, Weinshenker BG. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65(1):78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]