Abstract
We herein present an overview of the upcoming 5th edition of the World Health Organization Classification of Haematolymphoid Tumours focussing on lymphoid neoplasms. Myeloid and histiocytic neoplasms will be presented in a separate accompanying article. Besides listing the entities of the classification, we highlight and explain changes from the revised 4th edition. These include reorganization of entities by a hierarchical system as is adopted throughout the 5th edition of the WHO classification of tumours of all organ systems, modification of nomenclature for some entities, revision of diagnostic criteria or subtypes, deletion of certain entities, and introduction of new entities, as well as inclusion of tumour-like lesions, mesenchymal lesions specific to lymph node and spleen, and germline predisposition syndromes associated with the lymphoid neoplasms.
Subject terms: Diagnosis, Lymphoma
Introduction
Evidence-based classification of disease is fundamental for the treatment of individual patients, monitoring of global disease incidence, and investigating all aspects of disease causation, prevention and therapy. The World Health Organization (WHO) classification of lymphoid tumours has provided a global reference for the diagnosis of lymphoid neoplasms since its 3rd edition in 2001 [1] which was based on the R.E.A.L Classification developed by the International Lymphoma Study Group (ILSG) in the early 1990s [2]. The definitions laid down in the successive WHO classifications [3, 4] have not only been adopted for use by pathologists, clinicians, and basic and translational research scientists, but they have also been incorporated into the International Classification of Diseases (ICD) codes, and thereby serve as a global reference for epidemiological monitoring across national and international health policy organizations. In this article, we provide the conceptual framework and major developments in lymphoid neoplasms in the upcoming 5th edition of the WHO Classification of Haematolymphoid Tumours (WHO-HAEM5) scheduled to be published in 2022. An overview of myeloid neoplasms will be published separately.
The International Agency for Research on Cancer (IARC) initiated the process culminating in WHO-HAEM5 in 2018 by laying out the governance rules and classification principles for the entire 5th Edition series of the WHO classification of tumours, comprising 14 volumes, each dedicated to neoplasia of specific organ systems and/or clinical contexts (Paediatric Tumours and Genetic Tumour Syndromes). In 2021, expert members of the editorial board and authors were invited to contribute to WHO-HAEM5 based on their records of diagnostic and/or scientific expertise, regional representation, equity and lack of potential conflicts-of-interest. For most chapters, a multidisciplinary author team was formed including haematopathologists, haematologists, oncologists, geneticists, epidemiologists and molecular biologists. Experts from other disciplines, such as radiation oncologists and immunologists, were also involved where appropriate. Author teams worked “virtually”, in close collaboration, further supported by regular online meetings with the editorial team despite (and possibly in part thanks to) the challenges encountered during the COVID-19 pandemic. In addition, major issues arising during the development of the classification were discussed, resolved and harmonized across entities, both within WHO-HAEM5 and across other WHO volumes that cover some of the same entities in different clinical and/or organ-specific contexts. This was accomplished via regular meetings among expert groups and further dedicated conferences, including a clinical forum with all clinicians involved in the WHO-HAEM5. Public consultation was sought on an initial classification draft. Final decisions were taken based on principles of evidence-based medicine.
The resulting WHO-HAEM5 is a systematic evolution of the prior classifications. To allow for continuity in daily practice and ongoing clinical trials, a relatively conservative approach was taken in making changes to nomenclature. The WHO-HAEM5, like all 5th Edition WHO tumour volumes, applies a hierarchical system for classification. That is, it organises diseases in order of increasing levels of specification: category (e.g., mature B-cell), family/class (e.g., large B-cell lymphomas), entity/type (e.g., diffuse large B-cell lymphoma, not otherwise specified) and subtype (e.g., diffuse large B cell lymphoma, not otherwise specified, germinal center B-cell-like). Entities and subtypes have been formulated such that the implementation of the WHO-HAEM5 classification system is possible globally, in all settings. The WHO-HAEM5 recognizes the increasing importance of genetic and other molecular data in the evaluation of lymphoid neoplasia; however, consideration has also been given to the fact that the required diagnostic resources are not universally available. Thus, to facilitate a pragmatic approach to diagnosis while also encouraging the adoption of molecular testing where required, “essential“ and “desirable“ diagnostic criteria for each entity are defined in a hierarchical way. “Essential criteria” are minimal criteria to allow the diagnosis of an entity as universally as possible, although molecular criteria are inevitably included for some entities. “Desirable criteria” are those that aid in confirmation and refinement of the diagnosis, and usually require the application of advanced techniques. In circumstances where resources are not available to reach a definitive diagnosis of an entity (or when suboptimal quality or quantity of material is limiting), a diagnostic label based on the family name of that entity can be applied.
Provisional entities were not created in WHO-HAEM5 as these, by definition, lack sufficient evidence. Novel potential subtypes have been restrictively proposed for some entities, such as in Burkitt lymphoma, where besides the three traditional epidemiologic variants, the distinction of EBV-positive and EBV-negative Burkitt lymphoma subtypes is recommended.
The order of classification follows the traditional major subgrouping according to cell lineage, with precursor cell neoplasms followed by mature malignancies. Within a family, the entities are generally arranged in an order commencing with more indolent and progressing to increasingly aggressive ones. For the first time, in an effort to prevent the over-diagnosis of lymphoma and to improve the recognition of clinicopathologically distinct entities, non-neoplastic conditions mimicking lymphoma or representing an important differential diagnosis, have been included in WHO-HAEM5. Similarly, in light of the increasing clinical importance of germline tumour predisposition syndromes, which are frequently associated with lymphoid neoplasms, such as ataxia telangiectasia, dedicated chapters have been introduced. In addition, the rapid development in the understanding of lymphoid proliferations associated with inborn errors of immunity (primary immunodeficiencies) and acquired immune disorders justified significant updates, and these have been included in WHO-HAEM5.
The following sections represent an overview of the most significant changes made in WHO-HAEM5 compared with WHO-HAEM4R (Tables 1–3).
Table 1.
WHO Classification of Haematolymphoid Tumours, 5th edition: B-cell lymphoid proliferations and lymphomas.
| WHO Classification, 5th edition | WHO Classification, revised 4th edition |
|---|---|
| Tumour-like lesions with B-cell predominance | |
| Reactive B-cell-rich lymphoid proliferations that can mimic lymphoma | Not previously included |
| IgG4-related disease | Not previously included |
| Unicentric Castleman disease | Not previously included |
| Idiopathic multicentric Castleman disease | Not previously included |
| KSHV/HHV8-associated multicentric Castleman disease | Multicentric Castleman disease |
| Precursor B-cell neoplasms | |
| B-cell lymphoblastic leukaemias/lymphomas | |
| B-lymphoblastic leukaemia/lymphoma, NOS | (Same) |
| B-lymphoblastic leukaemia/lymphoma with high hyperdiploidy | B-lymphoblastic leukaemia/lymphoma with hyperdiploidy |
| B-lymphoblastic leukaemia/lymphoma with hypodiploidy | (Same) |
| B-lymphoblastic leukaemia/lymphoma with iAMP21 | (Same) |
| B-lymphoblastic leukaemia/lymphoma with BCR::ABL1 fusion | B-lymphoblastic leukaemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1 |
| B-lymphoblastic leukaemia/lymphoma with BCR::ABL1-like features | B-lymphoblastic leukaemia/lymphoma, BCR-ABL1-like |
| B-lymphoblastic leukaemia/lymphoma with KMT2A rearrangement | B-lymphoblastic leukaemia/lymphoma with t(v;11q23.3); KMT2A-rearranged |
| B-lymphoblastic leukaemia/lymphoma with ETV6::RUNX1 fusion | B-lymphoblastic leukaemia/lymphoma with t(12;21)(p13.2;q22.1); ETV6-RUNX1 |
| B-lymphoblastic leukaemia/lymphoma with ETV6::RUNX1-like features | Not previously included |
| B-lymphoblastic leukaemia/lymphoma with TCF3::PBX1 fusion | B-lymphoblastic leukaemia/lymphoma with t(1;19)(q23;p13.3); TCF3-PBX1 |
| B-lymphoblastic leukaemia/lymphoma with IGH::IL3 fusion | B-lymphoblastic leukaemia/lymphoma with t(5;14)(q31.1;q32.1); IGH/IL3 |
| B-lymphoblastic leukaemia/lymphoma with TCF3::HLF fusion | Not previously included |
| B-lymphoblastic leukaemia/lymphoma with other defined genetic abnormalities | (Same) |
| Mature B-cell neoplasms | |
| Pre-neoplastic and neoplastic small lymphocytic proliferations | |
| Monoclonal B-cell lymphocytosis | (Same) |
| Chronic lymphocytic leukaemia/small lymphocytic lymphoma | (Same) |
| (Entity deleted) | B-cell prolymphocytic leukaemia |
| Splenic B-cell lymphomas and leukaemias | |
| Hairy cell leukaemia | (Same) |
| Splenic marginal zone lymphoma | (Same) |
| Splenic diffuse red pulp small B-cell lymphoma | (Same) |
| Splenic B-cell lymphoma/leukaemia with prominent nucleoli | Not previously included (encompassing hairy cell leukaemia variant and some cases of B-cell prolymphocytic leukaemia) |
| Lymphoplasmacytic lymphoma | |
| Lymphoplasmacytic lymphoma | (Same) |
| Marginal zone lymphoma | |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue | (Same) |
| Primary cutaneous marginal zone lymphoma | Not previously included (originally included under “extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue”) |
| Nodal marginal zone lymphoma | (Same) |
| Paediatric marginal zone lymphoma | (Same) |
| Follicular lymphoma | |
| In situ follicular B-cell neoplasm | In situ follicular neoplasia |
| Follicular lymphoma | (Same) |
| Paediatric-type follicular lymphoma | (Same) |
| Duodenal-type follicular lymphoma | (Same) |
| Cutaneous follicle centre lymphoma | |
| Primary cutaneous follicle centre lymphoma | (Same) |
| Mantle cell lymphoma | |
| In situ mantle cell neoplasm | In situ mantle cell neoplasia |
| Mantle cell lymphoma | (Same) |
| Leukaemic non-nodal mantle cell lymphoma | (Same) |
| Transformations of indolent B-cell lymphomas | |
| Transformations of indolent B-cell lymphomas | Not previously included |
| Large B-cell lymphomas | |
| Diffuse large B-cell lymphoma, NOS | (Same) |
| T-cell/histiocyte-rich large B-cell lymphoma | (Same) |
| Diffuse large B-cell lymphoma/ high grade B-cell lymphoma with MYC and BCL2 rearrangements | High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements |
| ALK-positive large B-cell lymphoma | (Same) |
| Large B-cell lymphoma with IRF4 rearrangement | (Same) |
| High-grade B-cell lymphoma with 11q aberrations | Burkitt-like lymphoma with 11q aberration |
| Lymphomatoid granulomatosis | (Same) |
| EBV-positive diffuse large B-cell lymphoma | EBV-positive diffuse large B-cell lymphoma, NOS |
| Diffuse large B-cell lymphoma associated with chronic inflammation | (Same) |
| Fibrin-associated large B-cell lymphoma | Not previously included (Previously considered a subtype of diffuse large B-cell lymphoma associated with chronic inflammation) |
| Fluid overload-associated large B-cell lymphoma | Not previously included |
| Plasmablastic lymphoma | (Same) |
| Primary large B-cell lymphoma of immune-privileged sites | Not previously included, encompassing primary diffuse large B-cell lymphoma of the CNS in revised 4th edition (plus primary large B-cell lymphoma of the vitreoretina and primary large B-cell lymphoma of the testis) |
| Primary cutaneous diffuse large B-cell lymphoma, leg type | (Same) |
| Intravascular large B-cell lymphoma | (Same) |
| Primary mediastinal large B-cell lymphoma | (Same) |
| Mediastinal grey zone lymphoma | B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classic Hodgkin lymphoma |
| High-grade B-cell lymphoma, NOS | (Same) |
| Burkitt lymphoma | |
| Burkitt lymphoma | (Same) |
| KSHV/HHV8-associated B-cell lymphoid proliferations and lymphomas | |
| Primary effusion lymphoma | (Same) |
| KSHV/HHV8-positive diffuse large B-cell lymphoma | HHV8-positive diffuse large B-cell lymphoma, NOS |
| KSHV/HHV8-positive germinotropic lymphoproliferative disorder | HHV8-positive germinotropic lymphoproliferative disorder |
| Lymphoid proliferations and lymphomas associated with immune deficiency and dysregulation | |
| Hyperplasias arising in immune deficiency/dysregulation | Not previously included, encompassing non-destructive post-transplant lymphoproliferative disorders, among others |
| Polymorphic lymphoproliferative disorders arising in immune deficiency/dysregulation | Not previously included, encompassing polymorphic posttransplant lymphoproliferative disorders, other iatrogenic immunodeficiency-associated lymphoproliferative disorders, among others |
| EBV-positive mucocutaneous ulcer | (Same) |
| Lymphomas arising in immune deficiency / dysregulation | Not previously included, encompassing monomorphic posttransplant lymphoproliferative disorders, classic Hodgkin lymphoma posttransplant lymphoproliferative disorders, lymphomas associated with HIV infection, among others |
| Inborn error of immunity-associated lymphoid proliferations and lymphomas | Lymphoproliferative diseases associated with primary immune disorders |
| Hodgkin lymphoma | |
| Classic Hodgkin lymphoma | (Same) |
| Nodular lymphocyte predominant Hodgkin lymphoma | (Same) |
| Plasma cell neoplasms and other diseases with paraproteins | |
| Monoclonal gammopathies | |
| Cold agglutinin disease | Not previously included |
| IgM monoclonal gammopathy of undetermined significance | (Same) |
| Non-IgM monoclonal gammopathy of undetermined significance | (Same) |
| Monoclonal gammopathy of renal significance | Not previously included |
| Diseases with monoclonal immunoglobulin deposition | |
| Immunoglobulin-related (AL) amyloidosis | Primary amyloidosis |
| Monoclonal immunoglobulin deposition disease | Light chain and heavy chain deposition disease |
| Heavy chain diseases | |
| Mu heavy chain disease | (Same) |
| Gamma heavy chain disease | (Same) |
| Alpha heavy chain disease | (Same) |
| Plasma cell neoplasms | |
| Plasmacytoma | (Same) |
| Plasma cell myeloma | (Same) |
|
Plasma cell neoplasms with associated paraneoplastic syndrome -POEMS syndrome -TEMPI syndrome -AESOP syndrome |
(Same) Except AESOP syndrome not previously included |
Table 3.
WHO Classification of Haematolymphoid Tumours, 5th edition: Stroma-derived neoplasms of lymphoid tissues.
| WHO Classification, 5th edition | WHO Classification, revised 4th edition |
|---|---|
| Mesenchymal dendritic cell neoplasms | |
| Follicular dendritic cell sarcoma | (Same) |
| EBV-positive inflammatory follicular dendritic cell sarcoma | Inflammatory pseudotumour-like follicular/fibroblastic dendritic cell sarcoma |
| Fibroblastic reticular cell tumour | (Same) |
| Myofibroblastic tumour | |
| Intranodal palisaded myofibroblastoma | Not previously included |
| Spleen-specific vascular-stromal tumours | |
| Splenic vascular-stromal tumours | |
| Littoral cell angioma | Not previously included |
| Splenic hamartoma | Not previously included |
| Sclerosing angiomatoid nodular transformation of spleen | Not previously included |
B-cell lymphoid proliferations and lymphomas
New addition to WHO-HAEM5: Tumour-like lesions with B-cell predominance
For the first time, the WHO ‘Blue Book’ on haematolymphoid tumours introduces tumour-like lesions, including five entities in a distinct class of tumour-like lesions with B-cell predominance. Castleman disease is not a single disease but rather three clinicopathologically distinct entities: unicentric Castleman disease, idiopathic multicentric Castleman disease, and KSHV/HHV8-associated multicentric Castleman disease. The diagnostic algorithm for the classification of Castleman disease requires an integrated approach, including histological, haematological, immunological, and clinical parameters [5–9]. Also included in this section is IgG4-related disease; IgG4-related lymphadenopathy has features that can overlap with Castleman disease. The fifth chapter covers other non-neoplastic B-cell predominant lymphoid proliferations involving lymph nodes and/or extranodal sites that can mimic lymphomas, including progressive transformation of germinal centers, infectious mononucleosis, florid reactive lymphoid hyperplasia/lymphoma-like lesion of the female genital tract, and systemic lupus erythematosus.
B-lymphoblastic leukaemias/lymphomas (B-ALL): New genetically defined entities and subtypes
Following the principles of ‘essential’ and ‘desirable’ diagnostic criteria outlined above, B-lymphoblastic leukaemia/lymphoma (B-ALL) can be diagnosed at the family/class level on morphology and immunophenotype alone as B-ALL, not further classified (NFC). Most entities can be classified based on broadly-available cytogenetic testing, although molecular genetic subtyping is required for some entities based on the current state-of-the-art. B-ALL NOS, is to be reserved for cases that cannot be classified even after comprehensive testing. The majority of precursor B-cell neoplasms are classified in WHO-HAEM5 according to ploidy changes, such as hyperdiploidy and hypodiploidy, as well as chromosomal rearrangements or the presence of other genetic drivers [10]. In most cases, well-known drivers underlie B-ALL pathogenesis: iAMP21, BCR::ABL1 fusion, KMT2A rearrangements, ETV6::RUNX1 fusion, TCF3::PBX1 fusion or IGH::IL3 fusion. The classification based on these groups remains largely unchanged from WHO-HAEM4R; however, the nomenclature focuses on the molecular events rather than cytogenetic alterations, to allow for the application of differing techniques for their detection (Table 1). Other minor updates reflect the incorporation of additional genetic findings and refinements in the definitions of entities based on shared gene expression features. The rare B-ALL with TCF3::HLF fusion has been added to WHO-HAEM5; it is distinct from B-ALL with TCF3::PBX1 fusion and is characterized by a particularly aggressive behaviour [11, 12]. B-ALL with BCR::ABL1-like features is now an entity (previously a provisional entity), by definition sharing gene expression and phenotypic features of B-ALL with BCR::ABL1 fusion; it is prevalent across all age groups [13, 14] and shows significant benefit from targeted therapies [15–17]. Similarly, advances in diagnostic methodologies have allowed identification of a new entity, B-ALL with ETV6::RUNX1-like features, the description of which follows the section on B-ALL with ETV6::RUNX1 fusion [18].
Recent gene expression and sequencing studies have identified a number of novel genetic drivers that appear to confer distinct clinical, phenotypic and/or prognostic features. Considering emerging, yet limited, evidence for separating them in the future as potential novel entities, these new subtypes are subsumed under “B-ALL with other defined genetic abnormalities”. These include B-ALL with DUX4 [18, 19], MEF2D [20], ZNF384 [21] or NUTM1 [22] rearrangements, with IG::MYC fusion [23, 24], and with PAX5alt [25] or PAX5 p.P80R (NP_057953.1) [26] abnormalities. Intriguingly, B-ALL with ZNF384 rearrangement, DUX4 rearrangement or PAX5 p.P80R may show monocytic differentiation following therapy and even at diagnosis [27, 28], broadening concepts of the plasticity of leukemic lineages. This plasticity has important implications for disease management, including minimal residual disease (MRD) assessment [27].
Mature B-cell neoplasms
The category of mature B-cell neoplasms comprises 12 families. The hierarchical structure is outlined in Table 1.
Pre-neoplastic and neoplastic small lymphocytic proliferations: MBL and CLL/SLL remain; B-PLL is no longer recognized as an entity
This family comprises two entities: Monoclonal B-cell Lymphocytosis (MBL) and Chronic Lymphocytic Leukaemia/Small Lymphocytic Lymphoma (CLL/SLL). WHO-HAEM5 recognizes three subtypes of monoclonal B-cell lymphocytosis (MBL):
Low-count MBL or clonal B-cell expansion: clonal CLL/SLL-phenotype B-cell count below 0.5 x 109/L with no other features diagnostic of B-lymphoproliferative disorder. The arbitrary threshold is based on the distribution of clonal B-cell counts in population studies compared to clinical cohorts [29].
CLL/SLL-type MBL: monoclonal CLL/SLL-phenotype B-cell count ≥0.5 x 109/L and total B-cell count less than 5 x 109/L with no other features diagnostic of CLL/SLL [30]. The threshold of less than 5 x 109/L is arbitrary but identifies a group with a very low likelihood of requiring treatment compared to individuals with B-cell counts between 5–10 x 109/L [31].
non-CLL/SLL-type MBL: ANY monoclonal non-CLL/SLL phenotype B-cell expansion with no symptoms or features diagnostic of another mature B-cell neoplasm. The majority of cases have features consistent with a marginal zone (MZ) origin [32].
All subtypes of MBL are clinically characterized by immune impairment with sub-optimal response to vaccinations and increased risk of infection [33–37]. In the diagnosis of CLL, CD5, CD19, CD20, CD23, and surface or cytoplasmic kappa and lambda light chains are regarded as essential markers, and CD10, CD43, CD79b, CD81, CD200 and ROR1 as additional targets useful in the differential diagnosis from other small B-cell lymphomas/leukaemias [38]. In addition to del(11q), del(13q), del(17p), and trisomy 12 assessment, TP53 mutational analysis, immunoglobulin gene heavy chain variable (IGHV) region somatic hypermutation (SHM) analysis and B-cell receptor stereotype subset analysis (subset #2 configuration) are all essential for full prognostic evaluation of CLL/SLL [39–41]. Detection of karyotypic complexity and BTK, PLCG2, and BCL2 mutation status all remain desirable additional investigations in the context of targeted therapy. IGHV mutation and TP53 aberration status are both included in the CLL-international prognostic index (CLL-IPI) [42], along with age, clinical stage and beta 2-microglobulin level. The International Prognostic Score for early-stage CLL/SLL (IPS-E) includes IGHV mutation status, absolute lymphocyte count >15 × 109/L, and presence of palpable lymph nodes [43]. In the setting of transformation, use of the term “Richter transformation” is recommended over “Richter Syndrome”.
B-prolymphocytic leukaemia (B-PLL) of WHO-HAEM4R is no longer recognized in WHO-HAEM5 in view of its heterogeneous nature. Cases that have been labeled as B-PLL include: (1) a variant of mantle cell lymphoma, characterized by presence of IGH::CCND1; (2) prolymphocytic progression of CLL/SLL, defined by CD5-positive non-mantle B-cell neoplasm with >15% prolymphocytes in the peripheral blood and/or bone marrow [44–47], and (3) other cases, now classified as “splenic B-cell lymphoma/leukaemia with prominent nucleoli”.
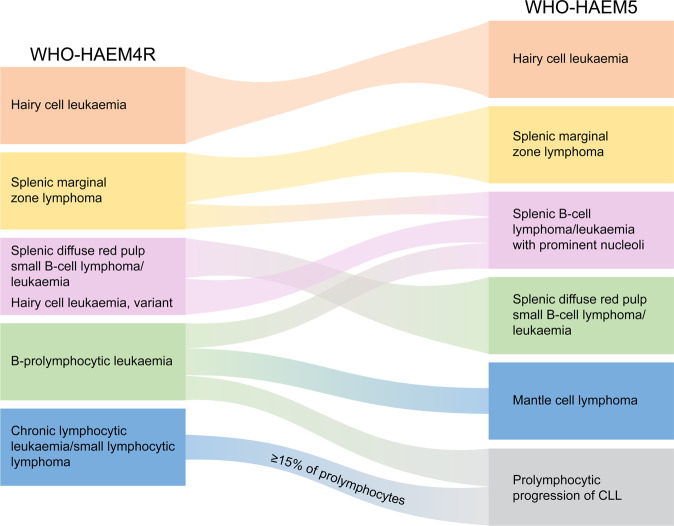
Splenic B-cell lymphomas and leukaemias: The term “splenic B-cell lymphoma/ leukaemia with prominent nucleoli” replaces “hairy cell leukaemia variant” and “CD5-negative B-cell prolymphocytic leukaemia”
The splenic B-cell lymphoma and leukaemia family in WHO-HAEM5 includes hairy cell leukaemia (HCL), splenic B-cell lymphoma/leukaemia with prominent nucleoli (SBLPN), splenic diffuse red pulp small B-cell lymphoma (SDRPL) and splenic marginal zone lymphoma (SMZL) (Fig. 1). In contrast to WHO-HAEM4R, SBLPN and SDRPL are now separately classified, with a nomenclature change in the former. Hairy cell leukaemia is a mature B-cell neoplasm with distinctive clinicopathologic features and BRAF p.V600E (NP_004324.2) somatic mutation in ≥95% of cases [48]. Other splenic small B-cell lymphomas usually lack BRAF mutations.
Fig. 1. Summary of the relationship between splenic B-cell lymphoma entities as named and defined in the revised 4th edition of the WHO classification (WHO-HAEM4R) and in the present 5th edition (WHO-HAEM5).
Some cases previously classified as B-prolymphocytic leukaemia do represent (blastoid) mantle cell lymphoma (as was already indicated in WHO-HAEM4R) or prolymphocytic progression of CLL. Cases classified in WHO-HAEM4R as CLL/SLL with ≥ 15% of prolymphocytes are now classified as prolymphocytic progression of CLL, cases with <15% of prolymphocytes remain CLL/SLL in WHO-HAEM5. Remaining cases are now renamed as “splenic B-cell lymphoma/leukaemia with prominent nucleoli” (SBLPN). This latter entity has absorbed cases formerly classified as hairy cell leukaemia variant (HCLv) and very rare cases of splenic marginal zone lymphoma with similar morphological features. It should be noted that the distinction between the various entities cannot always be made in the absence of a splenectomy specimen.
The new entity splenic B-cell lymphoma/leukaemia with prominent nucleoli (SBLPN) replaces the previous term “hairy-cell leukaemia variant“, in recognition that this proliferation is biologically distinct from HCL, although the leukaemic cells may partly resemble the “hairy cells” of HCL. Moreover, this entity also absorbs all cases previously termed CD5-negative B-prolymphocytic leukaemia (B-PLL) per WHO-HAEM4R. Although data from the literature cannot be directly extrapolated to the new class, it can be stated that SBLPN is rare, comprising approximately 0.4% of chronic lymphoid malignancies [49–53], and affects mainly elderly patients. The neoplastic cells have prominent nucleoli and are negative for HCL markers CD25, annexin A1, TRAP, and CD123. SBLPN is clinically more aggressive than HCL and resistant to cladribine as single-agent treatment. More recently improved sensitivity to cladribine in combinations with rituximab or bendamustine has been shown [49, 54–56].
Splenic diffuse red pulp small B-cell lymphoma (SDRPL) has some features overlapping with HCL and SBLPN but can be distinguished on careful evaluation of morphologic and immunophenotypic characteristics. A CD200 mean fuorescence intensity (MFI)/CD180 MFI ratio <0.5 on flow cytometry favours a diagnosis of SDRPL over HCL, SMZL, and SBLPN [57]. These entities can be best discriminated by pathologic examination of the spleen; in the absence of a splenectomy specimen, bone marrow examination shows characteristic features in SDRPL with a predominant intrasinusoidal pattern, while SMZL and SBLPN have a more diverse growth pattern in the bone marrow and HCL shows a typical diffuse pattern with reticulin fibrosis [58, 59]. In absence of a splenectomy specimen, however, the distinction is often not possible.
Lymphoplasmacytic lymphoma: IgM matters
WHO-HAEM5 recognizes two subtypes of lymphoplasmacytic lymphoma (LPL), the most common being the IgM-LPL/Waldenström Macroglobulinaemia (WM) type. Non-WM type LPL represents around 5% of LPL and includes: (1) cases with IgG or IgA monoclonal proteins, (2) non-secretory LPL, and (3) IgM LPL without bone marrow involvement [60–65].
There are two molecular subsets of IgM-LPL/WM type based on the presence or absence of the MYD88 p.L265P (NP_002459.2) mutation, which is regarded as the hallmark driver mutation in the vast majority of LPL (>90%) [66–69]. Demonstration of the MYD88 p.L265P mutation may aid in the difficult differential diagnosis with nodal and extranodal marginal zone lymphomas (MZL) with plasmacytoid differentiation and plasma cell (multiple) myeloma. The two latter entities generally lack the MYD88 p.L265P mutation with the exception of rare cases of MZL. CXCR4 mutations occur in up to 40% of all LPLs, usually concurrent with MYD88 mutations. It is desirable to perform CXCR4 mutational analysis for patients considered for treatment with a BTK inhibitor, since this genetic context is not only associated with shorter time to treatment, but especially with resistance to ibrutinib therapy [70].
Marginal zone lymphomas: cytogenetic and mutational profiles differ by anatomic site, and cutaneous MZL achieves independence
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL) and nodal marginal zone lymphoma (NMZL), featured as distinct entities in WHO-HAEM4R, are retained in WHO-HAEM5. Paediatric nodal marginal zone lymphoma (pNMZL) is upgraded from a subtype under nodal marginal zone lymphoma to a separate entity. Although it shows overlapping features with paediatric-type follicular lymphoma, current published evidence is considered insufficient to group these two indolent paediatric diseases into one family at this time. Primary cutaneous marginal zone lymphoma (PCMZL) has also been designated as a separate entity in WHO-HAEM5, owing to its distinctive clinicopathologic features.
EMZL, NMZL, and PCMZL have overlapping histologic and immunophenotypic features: the neoplastic cells are mature small B cells typically negative for CD5 and CD10. Plasmacytic differentiation is common, and associated reactive lymphoid follicles are often present. However, despite some shared features, they have different etiologies and pathogenesis, with further differences among EMZLs arising in different anatomic sites. Trisomy of chromosomes 3 and 18 are common in all. Gains of chromosomes 2p and 6p, and loss of 1p and 6q are frequent in NMZL [71–77]; however, gain of 6p and loss of 6q are recurrently seen only in EMZL of the ocular adnexa [78]. Translocations involving MALT1 such as t(11;18)(q21;q21), resulting in BIRC3::MALT1 fusion, are recurrent in gastric and pulmonary EMZL but rare at other sites [79–83]. In contrast, no recurrent gene fusions or rearrangements are described in PCMZL or NMZL.
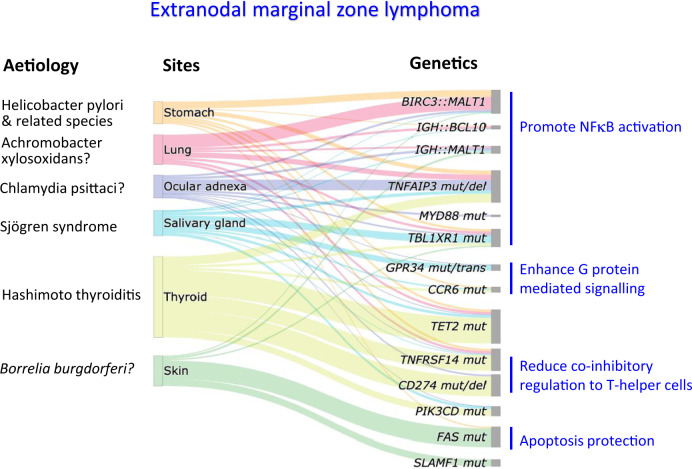
The mutational profiles of EMZL and NMZL differ [76, 84–86]. In addition, there are significant genetic differences among EMZLs arising in different anatomic sites (Fig. 2): e.g., ocular adnexal EMZL commonly shows TNFAIP3 mutation/deletion [87, 88]; salivary gland EMZL shows recurrently mutated GPR34 [89, 90]; most thyroid EMZL carry deleterious mutations of CD274, TNFRSF14 and/or TET2 [91]; and PCMZL often shows FAS mutations [92]. Somatic variants of KMT2D, PTPRD, NOTCH2, KLF2, and others are frequent in NMZL [76, 84, 85, 93] but not in EMZL. Better definition of the underlying molecular genetic changes of these lymphomas may potentially open the door to improved treatment options.
Fig. 2. Aetiology and recurrent genetic abnormalities in extranodal marginal zone lymphoma (EMZL) of various sites.
An important clinical application is that BIRC3::MALT1 identifies those cases of the gastric EMZL not responding to H. pylori eradication. As many of the genes involved in EMZL have not been uniformly investigated across different sites, only the recurrent genetic changes fundamental to the understanding of EMZL pathogenesis are presented. The height of the boxes under sites does not reflect the frequencies of these lymphomas. trans translocation, mut mutation, del: deletion.
Follicular lymphoma (FL): from classic grading to biological grouping
The family of follicular lymphoma encompasses follicular lymphoma, in situ follicular B-cell neoplasm (ISFN), paediatric-type FL and duodenal-type FL. There are no significant updates on the latter three entities in WHO-HAEM5. In contrast, the entity of follicular lymphoma has undergone significant revision. The vast majority of FL (85%) have at least in part a follicular growth pattern, are composed of centrocytes and centroblasts and harbour the t(14;18)(q32;q21) translocation associated with IGH::BCL2 fusion; these are now termed classic FL (cFL) and set apart from two related subtypes/groups, follicular large B-cell lymphoma (FLBL) and FL with uncommon features (uFL).
In WHO-HAEM5, grading of FL, which is only pertinent to cFL, is no longer mandatory. This decision was made after extensive discussions and evaluation of the literature centering on the reproducibility of grading and on its questionable clinical significance for individual patients in the era of modern therapy. Poor reproducibility may result from various causes, including sampling (complete lymph node excision versus core needle biopsy), definition and recognition of centroblasts, and methods of enumeration. Since grading of FL is based on the enumeration of centroblasts per high-power field (HPF), one of the challenges is the lack of a consistent definition of a HPF using a 40x microscope objective (400x magnification), where the size of the microscopic field has changed over the years even at the same magnification [94]. Lack of consensus regarding the morphological spectrum of centroblasts and using conventional methods of counting further negatively impacts reproducibility [95]. Clinical outcomes among patients with FL of grades 1, 2, and 3A seem not to be significantly different. Currently, patients are treated with similar protocols both in and outside clinical trials in many parts of the world [96–99]. While attempts have been made to improve reproducibility through digital applications or by using immunohistochemical supportive data, such methods have not been compared to patient outcome. Hence, it was deemed premature to include them in WHO-HAEM5 [100–102]. Taken together, for histopathologic as well as clinical reasons, it was felt timely to make grading of FL to be optional in the subtyping of cFL.
Rare cases of cFL grade 3A may show a focal or extensive diffuse growth pattern. In WHO-HAEM4R, the recommended diagnosis in such cases was “DLBCL with follicular lymphoma”, even though sheets of large cells are not often present. Currently, it is uncertain whether such cases should better be classified as cFL or DLBCL [103] and therefore, treatment decisions in individual patients should not be based on pathology information alone but rather be made in multidisciplinary conference settings and await research to define more objective criteria to predict clinical course. The subtype of FLBL largely equals WHO-HAEM4R FL grade 3B, and renaming was done for reasons of consistency throughout the classification.
The newly introduced subtype of uFL includes two subsets that significantly diverge from cFL: one with “blastoid” or “large centrocyte” variant cytological features, and the other with a predominantly diffuse growth pattern [104, 105]. FL with “blastoid” or “large centrocyte” cytological features more frequently display variant immunophenotypic and genotypic characteristics and may show inferior survival [106]. They need to be distinguished from large B-cell lymphoma with IRF4 rearrangement [107]. FL with a predominantly diffuse growth pattern frequently occurs as a large tumour in the inguinal region and is associated with CD23 expression, an absence of IGH::BCL2 fusion [108], and frequent STAT6 mutations along with 1p36 deletion or TNFRSF14 mutation [104, 109]. Separating such cases from cFL will support research to clarify disease biology, allowing a better definition in future classifications.
Mantle cell lymphoma: Improved risk stratification
WHO-HAEM5 groups mantle cell neoplasia into three individual chapters. In situ mantle cell neoplasm (ISMCN) is rare and typically an incidental finding. It represents colonization of mantle zones of lymphoid follicles by B cells carrying an IG::CCND1 fusion leading to cyclin D1 overexpression [110].
The IGH::CCND1 fusion associated with t(11;14)(q13;q32) is the genetic hallmark of mantle cell lymphoma (MCL), present in ≥95% of cases (i.e., cyclin D1-positive MCL subtype) [111, 112]. Occasionally, IGK or IGL serve as the CCND1 translocation partner [113]. In the occasional cases of MCL that strongly express cyclin D1 protein but show no CCND1 rearrangement by FISH, genomic studies have revealed cryptic rearrangements of IGK or IGL enhancers with CCND1 [114–116]. In the small subset of MCL negative for cyclin D1 expression and CCND1 rearrangement (i.e., cyclin D1-negative MCL subtype), CCND2, CCND3, or CCNE rearrangements have been identified as alternative mechanisms of cell cycle dysregulation [117]. In recent years, the median overall survival of patients with MCL has dramatically increased due to improved therapies. Hence, the identification of prognostic subgroups has become highly relevant. Widely available and best-established biomarkers of high-risk MCL include cytomorphology (pleomorphic or blastoid appearance), high Ki67 proliferative index, p53 expression and TP53 mutation [118, 119].
Non-nodal MCL (nnMCL) is characterized by involvement of blood, bone marrow and spleen, little or no lymphadenopathy, a mostly asymptomatic presentation, and a better clinical outcome compared to MCL. Biologically, nnMCL differs from MCL by: (i) lack of SOX11 expression [120, 121], low Ki67 index and frequent lack of CD5 expression [122]; (ii) differences in the usage of IGHV gene segments with biased usage of the IGHV1-8 gene [122] together with a higher somatic hypermutation load [121, 123, 124]; and (iii) fewer genetic alterations and infrequent genomic complexity [120, 125].
High-grade transformation steps forth
For the first time, WHO-HAEM5 now includes a section with the description of High-grade transformation of indolent B- cell lymphomas including a summary of the incidence of known and driver genes.
Large B-cell lymphomas: new names and new umbrellas
The family of large B-cell lymphomas comprises a wide spectrum of tumours. Although these are generally composed of medium-sized to large cells with round to ovoid nuclei and vesicular chromatin, cases with intermediate-sized and blastoid cells may also meet criteria for this family. These require delineation from morphologically similar entities, such as the blastoid variant of mantle cell lymphoma and lymphoblastic leukaemia/lymphoma.
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) represents the most common entity, and is defined by large-cell morphology as above, mature B-cell phenotype, and lack of criteria defining specific large B-cell lymphoma entities. The lymphomas encompassed within DLBCL, NOS are morphologically and molecularly heterogeneous. Since most DLBCL, NOS broadly recapitulate the differentiation and maturation mechanisms active in germinal centers (GC), two main subtypes previously defined in WHO-HAEM4R continue to be recognized. The germinal centre B-cell-like (GCB) subtype has a gene expression profile (GEP) related to a GC cell of origin (COO), and is enriched for IGH::BCL2 fusion due to t(14;18)(q32;q21) and mutations of genes instrumental for GC development, GC dark zone and light zone transitions and microenvironmental interactions, such as EZH2, GNA13, MEF2B, KMT2D, TNFRSF14, B2M and CREBBP [126]. The activated B-cell-like (ABC) subtype derives from cells of GC exit or post GC origin, with either germinal center-exit or early plasmablastic phenotype. It is characterized by dependence on BCR signaling and NFκB activities, is negative for most GC markers, and expresses IRF4/MUM1 [127]. It is enriched for BCR pathway mutations such as in MYD88 (mostly p.L265P), CD79B and PIM1, as well as genetic changes that block the B-cell differentiation program such as BCL6 rearrangements and PRDM1/BLIMP1 mutation/deletion [126]. It is recommended to continue rendering the GCB/ABC (GCB/nonGCB) distinction although it has become apparent that the clinical impact of COO stratification is relatively limited outside clinical trials. Although IHC algorithms obviously do not recognize the “unclassified” GEP category and have concordance issues with GEP, they are widely used in routine practice. Recent data from next generation sequencing studies have illustrated a heterogeneous molecular landscape of DLBCL, NOS with around 150 genetic drivers that are recurrently mutated in DLBCL, with a mean of approximately 8% of these genes mutated per patient [128]. Interestingly, in spite of the use of various sequencing approaches and clustering algorithms, the genetic landscape of DLBCL, NOS can be used for sub-classification with broad concordance suggesting that the underlying disease biology can be captured by mutational analysis. Some of the genetic groups harbour a mutational profile that in part overlaps with those of FL or MZL, suggesting either transformation from these low-grade lymphomas or a common path in their early pathogenesis. However, no unifying concept for proposed clusters and the significance of their genetic drivers has been established so far, precluding the definition of a unified genetic framework of DLBCL, NOS at the present time. Moreover, the impact of these genetic clusters on outcome and as a basis for targeted treatment approaches is currently unclear and awaits evidence from clinical trials. Therefore, it was considered premature to introduce such molecular classifications in WHO-HAEM5.
WHO-HAEM5 recognizes 17 specific entities as “large B-cell lymphomas” other than DLBCL, NOS (Table 1 and Fig. 3). For most of these entities, biological concepts and diagnostic strategies have remained largely unchanged compared with WHO-HAEM4R. However, the names of some entities have been modified for reasons of consistency, from “diffuse large B-cell lymphoma“ to “large B-cell lymphoma“, acknowledging the fact that a diffuse growth pattern is either not apparent/present or cannot be assessed in some entities (e.g., fibrin-associated large B-cell lymphoma or fluid-overload associated large B-cell lymphoma).
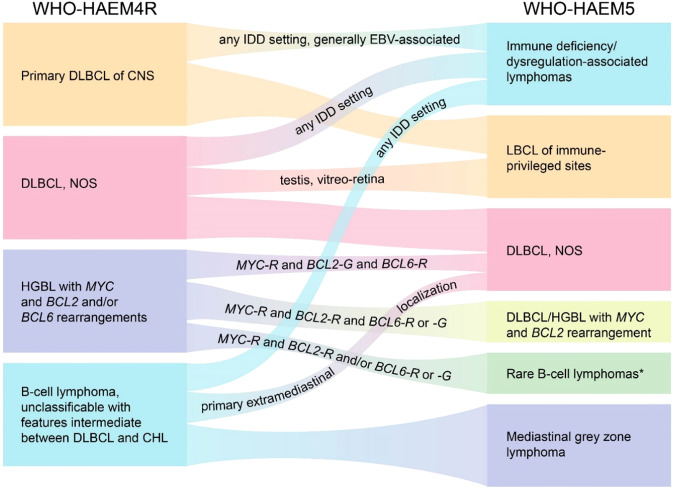
Fig. 3. Summary of the relationship between large B-cell lymphoma (LBCL) entities as named and defined in the revised 4th edition of the WHO classification (WHO-HAEM4R) and in the present 5th edition (WHO-HAEM5).
* “Rare B-cell lymphomas” refer to those fulfilling definitions of specific clinico-pathological entities while incidentally bearing concomitant MYC and BCL2 rearrangements. Examples are fluid-overload-associated large B-cell lymphomas and rare follicular lymphomas. R rearrangement, G germline configuration.
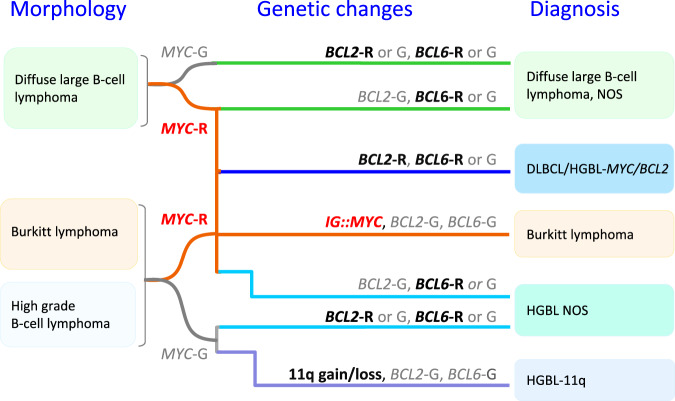
The WHO-HAEM4R entity of high-grade B-cell lymphoma with dual rearrangements of MYC and BCL2 and/or BCL6 has been conceptually reframed and reassigned. In recognition of their variable morphologies but homogeneous dark zone biologic features and gene expression characteristics, the WHO-HAEM5 renames the entity diffuse large B-cell lymphoma/high-grade B-cell lymphoma with MYC and BCL2 rearrangements (DLBCL/HGBL-MYC/BCL2) to encompass tumours defined by the presence of dual MYC and BCL2 rearrangements that may be composed of large or intermediate or blastoid cells (Fig. 4). Hence, the primary morphological categorization of the neoplasm can be maintained after determining the genetic constitution. This group of cases forms a homogeneous entity with an exclusive GC gene expression profile, a close pathogenetic relationship to FL and molecular GC-like DLBCL subsets [129–132]. In addition, gene expression signatures associated with DLBCL/HGBL-MYC/BCL2 (MHG, DHITsig) [130, 133] significantly overlap with those of Burkitt lymphoma (BL). In contrast, lymphoid neoplasms with dual MYC and BCL6 rearrangements represent a more diverse spectrum [129] with variable gene expression profiles and mutational spectra, markedly differing from DLBCL/HGBL-MYC/BCL2. Hence, these cases have been excluded from the DLBCL/HGBL-MYC/BCL2 entity and are now classified either as a subtype of DLBCL, NOS or HGBL, NOS according to their cytomorphological features (Fig. 4).
Fig. 4. Algorithm for classification of aggressive B-cell lymphomas in WHO-HAEM5 in the light of MYC, BCL2 and BCL6 rearrangement and complex 11q gain/loss patterns.
HGBL high grade B-cell lymphoma, R rearrangement, G germline configuration.
High-grade B-cell lymphoma with 11q aberration (HGBL-11q), formerly known as Burkitt-like lymphoma with 11q aberration in WHO-HAEM4R, is an aggressive MYC rearrangement-negative mature B-cell lymphoma with a morphology similar to Burkitt lymphoma (BL) or with an intermediate or blastoid appearance, an immunophenotype (CD10+, BCL6+, BCL2-), and/or gene expression profile (GEP) similar to BL, and a characteristic chromosome 11q-gain/loss pattern. The losses in 11q24qter are more specific to this entity than the centromeric gains but rarely might be substituted by copy-number neutral losses of heterozygosity. More recent studies have also confirmed that the mutational spectrum, besides the pattern of genomic imbalances, is different from that of BL and is more similar to that of DLBCL of GCB type. Of note, genomic alterations affecting the ID3-TCF3 complex, one of the molecular hallmarks of BL, are only rarely, if at all, seen in HGBL-11q [134, 135]. Cases of B-cell lymphoma with a Burkitt-like appearance that lack MYC rearrangement, therefore, should be tested for the 11q gain/loss pattern [136] (Fig. 4). It should be noted that the morphological spectrum of HGBL-11q as defined by the specific 11q-gain/loss pattern is more restricted than that of DLBCL/HGBL-MYC/BCL2.
Large B-cell lymphomas (LBCL) of immune-privileged sites is a new umbrella term introduced in WHO-HAEM5 to acknowledge common biological features of a group of aggressive B-cell lymphomas that arise as primary tumours in the central nervous system (CNS), the vitreoretinal compartment, and the testes of immunocompetent patients. This new entity now combines the previous entity of primary DLBCL of CNS with DLBCL of the vitreoretina and testis that were previously included among DLBCL, NOS. They arise in immune sanctuaries created by their respective anatomical structures (e.g., the blood-brain, blood-retinal, and blood-testicular barriers), and immune regulation systems within their respective primary sites, and share immunophenotypic and molecular features [137–139] (Table 4). Information on this group of tumours is rapidly accruing: it appears that some lymphomas arising at other distinct sites such as the breast and skin share some of these features, and thus, this group of ‘immune-privileged lymphomas’ might expand in future classifications.
Table 4.
Distinctive features of primary large B-cell lymphomas of immune privileged sites.
| Subtypes | Primary large B-cell lymphoma of the CNS |
| Primary large B-cell lymphoma of the vitreoretina | |
| Primary large B-cell lymphoma of the testis | |
| Clinical | Usually in adults over age of 60 years |
| Lymphoma tends to “home” to other immune privileged sites: vitreoretina tumour may occur concurrently with or follow CNS tumour; testicular tumour tends to relapse in CNS or contralateral testis | |
| Aggressive tumours with generally poor prognosis | |
| Morphology | Large B-cell lymphoma |
| Immunophenotype | Activated B-cell immunophenotype: Usually CD10-, MUM1+, BCL6+ |
| EBV negative | |
| Mutational profile | Concomitant MYD88 and CD79B mutations |
| Immune evasion: genetic inactivation of MHC class I and II and B2M (β 2-microglobulin) with subsequent loss of protein expression | |
| Showing DLBCL genomic signature C5/MCD/MYD88 |
Fluid overload-associated large B-cell lymphoma is a new addition to the list of large B-cell lymphomas in WHO-HAEM5, being distinct from primary effusion lymphoma (PEL). This entity has been briefly alluded to in the 5th Edition of the WHO Classification of Thoracic Tumours, under the names “PEL-like lymphoma” or “HHV8-unrelated PEL-like lymphoma” [140]. Patients usually are adults, predominantly elderly, without underlying immunodeficiency, who present with exclusive involvement of body cavities, most commonly the pleural cavity [141–143]. They frequently have an underlying condition causing fluid overload, such as chronic heart failure, renal failure, protein-losing enteropathy or liver failure/cirrhosis. The neoplastic large cells exhibit a mature B-cell rather than plasmablastic immunophenotype. KSHV/HHV8 is negative, while EBV is positive in 13–30% of cases and the genomic landscape differs essentially from primary effusion lymphoma (PEL) [141, 142]. The prognosis appears to be fairly favorable, yet another reason for distinction from PEL.
Mediastinal gray zone lymphoma (MGZL) is a B-cell lymphoma with overlapping features between primary mediastinal B-cell lymphoma (PMBL) and classic Hodgkin lymphoma (CHL), especially nodular sclerosis CHL (NSCHL). This entity replaces the term “B-cell-lymphoma, unclassifiable with features intermediate between DLBCL and classic Hodgkin lymphoma” of the WHO-HAEM4R, taking into account that lymphomas with these features are specific to the mediastinum and are part of a single biologic group with a morphologic and immunophenotypic spectrum from CHL to PMBL, with MGZL straddling the two. Current evidence indicates that cases with morphologic and immunophenotypic features similar to MGZL, but occurring outside and without involvement of the mediastinum, harbour different gene expression profiles and DNA alterations [143]. Hence, these cases are better classified as DLBCL, NOS.
High grade B-cell lymphoma, NOS (HGBL, NOS) represents aggressive mature B-cell lymphomas composed of medium-sized or blastoid cells that do not fit into other well-defined entities. NGS-based analyses of the mutational spectrum and gene expression signatures suggest that HGBL, NOS is a heterogeneous category, also including activated B-cell lymphomas with mutations of MYD88, CD79B, or TBL1XR1. Most frequent mutations are found in KMT2D (43%) and TP53 (30%). By GEP, most cases of HGBL, NOS have been reported to group into the “unclassified” cluster, and the remainder are variably classified in the other clusters [144]. Interestingly, gene expression profiling showed that 54% of HGBL, NOS harbour the “double hit” signature (DHITsig) characteristic of LBCL/HGBL with MYC/BCL2 despite lacking rearrangements of these genes [144].
Burkitt lymphoma: EBV matters
The definition of Burkitt lymphoma (BL) in WHO-HAEM5 remains largely unchanged, describing BL as an aggressive mature B-cell neoplasm composed of medium-sized cells with a germinal center B-cell phenotype CD10+, BCL6+, BCL2-/weak, high Ki67 index (>95%) and an IG::MYC juxtaposition (Fig. 4). Whereas three subtypes of BL have been historically recognized (“endemic”, “non-endemic or sporadic”, and “immunodeficiency-associated”) [145], more recent data suggest that EBV-positive BL and EBV-negative BL form discrete biologic groups based on their molecular features regardless of epidemiologic context and geographic location and therefore supersede the epidemiological subtyping [146–151]. EBV infection plays an essential role early in pathogenesis causing B cells to evade apoptosis [152, 153]. Emerging evidence suggests a dual mechanism of BL pathogenesis: virus-driven versus mutational, depending on EBV status [147]. EBV-positive and EBV-negative BL share evidence of coding mutations affecting pathways such as BCR and PI3K signaling, apoptosis, SWI/SNF complex and GPCR signaling [149, 154, 155]. In comparison with EBV-negative BL, EBV-positive BL shows significantly higher levels of somatic hypermutation particularly in noncoding sequences close to the transcription start site [149], harbours fewer driver mutations, particularly in the apoptosis pathway [149], and shows a lower frequency of mutations in the genes encoding the transcription factor TCF3 or its repressor ID3 [149]. To acknowledge these recent insights into BL biology, the distinction of the two subtypes, EBV-positive BL vs. EBV-negative BL, is recommended by WHO-HAEM5.
KSHV/HHV8-associated B-cell lymphoid proliferations and lymphomas
WHO-HAEM5 recognizes the full spectrum of lymphoid proliferations related to Kaposi sarcoma herpesvirus/human herpesvirus 8 (KSHV/HHV8) infection, which in parallel with the terminology for other herpesviruses is now indicated as KSHV/HHV8 to accommodate both the common practices of haematopathologists and of virologists. These lymphoid proliferations include KSHV/HHV8-associated multicentric Castleman disease (KSHV/HHV8-MCD) [covered under the category “Tumour-like lesions with B-cell predominance”], germinotropic lymphoproliferative disorder (KSHV/HHV8-GLPD), primary effusion lymphoma (PEL), extracavitary PEL (EC-PEL) and KSHV/HHV8-positive diffuse large B-cell lymphoma (KSHV/HHV8-DLBCL) [156, 157]. PEL/EC-PEL and KSHV/HHV8-DLBCL are characteristically seen in HIV patients, but can be seen in other immunodeficiency settings. KSHV/HHV8-GLPD, in contrast, is more prevalent in elderly patients without overt immunodeficiency, although it has also been reported in HIV-positive individuals. In addition, KSHV/HHV8-MCD is seen in both HIV-positive and HIV-negative patients, but the latter are overall older [158–160]. The diagnosis of prototypical examples of the various KSHV/HHV8-associated entities is often straightforward based on the definitions as formulated in WHO-HAEM5. It has become clear, however, that the morphological and clinical spectrum of KSHV/HHV8-associated entities is broader than previously appreciated [161]. Moreover, there are individual patients in whom clinical, histologic and viral features (KSHV/HHV8 with or without EBV) overlap among entities. Observations of synchronous and metachronous presentation of different KSHV/HHV8-associated lesions, and cases that possess morphological and/or clinical features of more than one entity support the notion that these equivocal cases may result from the special biology of KSHV/HHV8, which is not adequately captured by current disease-defining criteria [158, 161, 162]. For example, distinction between lymph node-based extracavitary PEL and KSHV/HHV8-positive DLBCL is difficult and may be arbitrary. WHO-HAEM5 acknowledges the limitations of its definitions. While more data to support biology-defined boundaries among the entities are awaited, it is recommended that decisions on classification and optimal therapy should be resolved in a multidisciplinary setting in challenging cases.
Lymphoid proliferations and lymphomas associated with immune deficiency and dysregulation: a new approach to order patterns
WHO-HAEM5 has introduced major changes to the classification of immunodeficiency-associated lymphoproliferative disorders (Fig. 5). In prior classifications, these disorders were grouped according to the disease background in which they arose and were discussed in separate chapters: primary immunodeficiencies, HIV infection, post-transplantation and other iatrogenic immunodeficiencies. This approach has been valuable for many years in supporting clinical decision-making and as a basis for translational and basic research. Knowledge that has resulted from this approach supports the notion that morphological features and, to a certain extent, the biology of many of these entities overlap and that the spectrum of immunodeficiency settings is broader than previously recognized. Therefore, it was considered timely to introduce an overarching framework and a standardized nomenclature to cover the different settings of immune dysfunction, according to the unifying nomenclature proposed at the Workshop on Immunodeficiency and Dysregulation organized by the Society of Hematopathology and European Association for Haematopathology in 2015 [163]. This framework aims to focus attention on shared histologic and pathogenetic features as well as to accommodate distinct causal associations of specific lesions and specific clinical and/or therapeutic consequences [163, 164]
Fig. 5. Summary of the relationship between immunodeficiency-associated lymphoid proliferations and lymphomas as named and defined in the revised 4th edition of the WHO Classification (WHO-HAEM4R) and in the present 5th edition (WHO-HAEM5).
The overarching concept applied in WHO-HAEM5 recognizes the pathological and biological similarities between proliferations presenting in various immune deficiency settings, while acknowledging their specific features. Outside the shared entities, unique proliferations are especially typical for various inborn errors of immunity (IEI). EBVMCU: EBV-positive mucocutaneous ulcer.
The new standardized nomenclature builds on an integrated approach to diagnosis that combines all relevant data into a reporting system as follows (Table 5):
Table 5.
Three-part nomenclature for lymphoid proliferations and lymphomas arising in the setting of immune deficiency/dysregulation.
| Histological diagnosis | Viral association | Immune deficiency/dysregulation setting |
|---|---|---|
|
◦ Hyperplasia (specify type) ◦ Polymorphic lymphoproliferative disorder ◦ Mucocutaneous ulcer ◦ Lymphoma (classify as for immunocompetent patients) |
◦ EBV +/− ◦ KSHV/HHV8 +/− |
◦ Inborn error of immunity (specify type) ◦ HIV infection ◦ Posttransplant (specify: solid organ/bone marrow) ◦ Autoimmune disease ◦ Iatrogenic/therapy-related (specify) ◦ Immune senescence |
1) Histological diagnosis according to accepted criteria and terminology;
2) Presence or absence of one or more oncogenic virus(es); and
3) The clinical setting/immunodeficiency background.
This nomenclature addresses existing inconsistencies in terminology and diagnostic criteria for similar lesions in different immunodeficiency settings, may improve communication among multidisciplinary teams in guiding appropriate clinical management as well as research studies, and facilitate incorporation of emerging knowledge in the field. As the same pathological entity, e.g., polymorphic lymphoproliferative disorder, does not necessarily have the same pathogenesis or clinical behaviour in different immune deficiency/dysregulation settings, this underscores the need to include the immune deficiency/dysregulation setting as a required part of the three-part nomenclature.
New types of immunodeficiency settings continue to be recognized [165–167]. Poly-chemotherapy for treatment of solid tumours and haematologic neoplasms has been largely accepted as an underlying cause for immunodeficiency. However, it is as yet unclear which polychemotherapy regimens confer this risk, and how long the risk persists [168]. In addition, with increasing use of novel immune modulatory agents, unanticipated types of immune dysfunction are emerging, e.g., in the aftermath of CAR-T cell and/or checkpoint inhibition therapies. Immune senescence is another setting that is poorly understood and as yet not possible to define or exclude; thereby use of an arbitrary age as cut-off does not have a scientific basis [169]. All these emerging concepts call into question the adequacy of the term “immunodeficiency”, which does not capture the extent, depth or phenotypic variation of immune suppression and the milieu of deregulated immune cell subsets. Therefore, WHO-HAEM5 has adopted “immune deficiency/dysregulation” (IDD) as the preferred term to encompass this expanding disease spectrum.
Primary immunodeficiencies, associated with germline mutations, have been renamed “inborn errors of immunity” (IEI) by the International Union of Immunological Societies, a terminology adopted by WHO-HAEM5 [170]. Patients with IEI may develop distinctive types of lymphoid proliferations unique to the particular IEI, as well as those described in the acquired IDD settings. The types and frequency of these proliferations are largely dependent on the immune dysregulation conferred by the germline aberration underyling a respective IEI. Given the overlap with other IDD settings, IEI-associated lymphoid proliferations and lymphomas have been incorporated into the overarching framework and nomenclature of “lymphoid proliferations and lymphomas associated with immune deficiency and dysregulation.”
The new approach to the classification of IDD-associated lymphoid proliferations and lymphomas impacts other lymphoid entities that are described in separate WHO chapters. This especially holds true for diagnoses in which EBV plays a defining or important role, including EBV-positive DLBCL, lymphomatoid granulomatosis, and CHL. In WHO-HAEM5, harmonization of diagnostic criteria among these categories has been undertaken as much as currently feasible, while at the same time acknowledging that some terminologies are arbitrary. For example, should an elderly patient with a DLBCL harbouring EBV be diagnosed as having EBV+ DLBCL or DLBCL, EBV+, in an IDD setting based upon presumed immune senescence? Clarification of these disease boundaries awaits further clinico-pathological data and further insights into disease pathogenesis, which will allow evidence-based refinements to the classification.
Hodgkin lymphoma: CHL clearly defined from its mimickers, NLPHL on the way to, but not yet NLPBCL
Classic Hodgkin lymphoma (CHL) comprises a group of B-cell neoplasms derived from germinal center B-cells, characterized by a low number of tumour cells embedded in a reactive microenvironment rich in immune cells. The large diagnostic Hodgkin and Reed-Sternberg (HRS) cells characteristically show a defective B-cell program. The defining immunophenotype of HRS cells remains unchanged from WHO-HAEM4R, as are criteria for nodular sclerosis (NSCHL), mixed cellularity (MCCHL), lymphocyte rich (LRCHL), and lymphocyte depleted (LDCHL) subtypes. With modern treatment protocols, these subtypes have lost most of their prognostic relevance. However, there is still merit in describing these subtypes to support epidemiological and translational studies, since specific subtypes are associated with different clinical features and underlying biologies [171]. While the basic description has not changed substantially since the last century, WHO-HAEM5 includes a comprehensive section on the etiology and pathogenesis of CHL, in particular incorporating new data on the crucial role of the microenvironment in modulating the disease [172, 173]. Recent biological insights have led to the recognition of an expanding spectrum of pitfalls, grey zones and mimickers, among them nodal T follicular helper cell lymphomas and lymphoproliferative disorders arising in immune deficiency/dysregulation settings that may contain EBV-positive HRS-like cells [163, 174, 175]. Caution should be exercised, therefore, when considering the diagnosis of CHL in the IDD setting; the same applies to purely extranodal CHL-like lymphoproliferations.
WHO-HAEM5 continues to list nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) under the family of Hodgkin lymphoma; the existing terminology of NLPHL (Hodgkin lymphoma) is maintained so as not to interfere with ongoing clinical trials. However, NLPHL may be more accurately called “nodular lymphocyte predominant B-cell lymphoma” since the neoplastic cells have a functional B-cell program, and therefore this term is now considered acceptable in preparation of future definitive adoption of the new nomenclature. An important issue in NLPHL is the recognition of the different growth patterns [176] overlapping with T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) at the extreme end (Table 6) [177]. These patterns occur across all age groups. Some variant patterns (patterns C, D and E) have been associated with more aggressive clinical behaviour in retrospective analyses [177–179] and may thus reflect the natural development and progression of the tumour [180, 181]. In some cases, a clear distinction between NLPHL Pattern E and THRLBCL may not be possible since both diseases present with advanced clinical stage. Distinction is especially difficult on small biopsies, which may not be representative.
Table 6.
Immuno-morphological growth patterns of NLPHL.
| Designation | Description |
|---|---|
| Pattern A | Classic B-cell nodular |
| Pattern B | Serpiginous/interconnected |
| Pattern C | Prominent extra-nodular LP cells |
| Pattern D | T-cell-rich nodular |
| Pattern E | Diffuse THRLBCL/DLBCL-like |
| Pattern F | Diffuse moth-eaten, B-cell-rich |
THRLBCL T-cell/histiocyte-rich large B-cell lymphoma.
Plasma cell neoplasms and other diseases with paraproteins: new conditions from AESOP to TEMPI
The section on plasma cell neoplasms in WHO-HAEM5 recognizes new entities and incorporates structural modifications as a step forward from WHO HAEM4R. New conditions included are monoclonal gammopathy of renal significance (MGRS), cold agglutinin disease (CAD), as well as TEMPI syndrome (a provisional entity in WHO-HAEM4R, characterized by telangiectasias, elevated erythropoietin and erythrocytosis, monoclonal gammopathy, perinephric fluid collection, and intrapulmonary shunting) and AESOP syndrome (adenopathy and extensive skin patch overlying a plasmacytoma). Sections based on types of paraproteins and disease burden have been reorganised. CAD, IgM and non-IgM MGUS and MGRS are grouped as monoclonal gammopathies, and diseases with abnormal monoclonal immunoglobulin deposits are grouped together. The heavy chain diseases (HCD) are now included in the plasma cell neoplasms section.
Cold agglutinin disease (CAD) is an autoimmune haemolytic anemia mediated by monoclonal cold agglutinins and driven by an underlying clonal B-cell lymphoid proliferation not fulfilling criteria for a B-cell lymphoma. The annual incidence of this rare disease is estimated at 1–1.8 per million; its prevalence is four-fold higher in colder countries [182–184]. Monoclonal gammopathy of renal significance (MGRS) represents a plasma cell or B-cell proliferation that does not meet accepted criteria for malignancy but secretes a monoclonal immunoglobulin or immunoglobulin fragment resulting in kidney injury [185, 186]. About 1.5% of patients whose disease would otherwise be classified as MGUS have MGRS [187].
The risk stratification model for IgM MGUS and non-IgM MGUS has been updated. Presence of all 3 risk factors consisting of: (1) an abnormal serum free light chain ratio, (2) IgA or IgM type MGUS, and (3) serum M-protein value >1.5 g/dL is considered high risk with approximately 50–60% risk of progression at 20 years, whereas the risk is only 5% when none of the risk factors are present [188]. A diagnosis of TEMPI syndrome is primarily made on clinical and imaging investigations. The bone marrow is unremarkable in the majority of cases; a few cases show erythroid hyperplasia and a low-volume of light chain-restricted plasma cells [189, 190]. Skin biopsies of patients with AESOP syndrome show diffuse hyperplasia of dermal vessels associated with surrounding dermal mucin, and lymph nodes can show features mimicking Castleman disease [191, 192].
New data have emerged concerning the progression from precursor states to plasma cell (multiple) myeloma (PCM), involving branching evolutionary patterns, novel mutations, biallelic hits in tumour suppressor genes, and segmental copy number changes [193]. While 1q21 gain is often an early event, translocations and additional amplifications of 1q21 emerge later during pathogenesis [194]. Staging of PCM according to the Revised International Staging System for Multiple Myeloma proposed by the International Myeloma Working Group has been adopted [195]. The important role of minimal/measurable residual disease (MRD) using next-generation flow cytometry or next-generation sequencing of immunoglobulin gene rearrangements as well as PET/CT in assessing prognosis and risk stratification in patients with PCM has been detailed [196, 197].
T-cell and NK-cell lymphoid proliferations and lymphomas
WHO-HAEM5 has reorganized entities that were listed as mature T- and NK-cell neoplasms in WHO HAEM4R to include a broader group of entities under the heading of “T-cell and NK-cell lymphoid proliferations and lymphomas” (Table 2). Notably, included is a family/class of tumour-like lesions with T cell predominance. Precursor T-lymphoblastic neoplasms are also included under this overarching category as a separate family. The mature T-cell and NK-cell neoplasms are grouped into 9 families based on diverse concepts: cell of origin/differentiation state, clinical scenario, disease localization, and cytomorphology. While most T- or NK-cell neoplasms can be assigned to the respective T- or NK-cell lineage, they are not separated as two categories in WHO-HAEM5 because some entities comprise a spectrum of tumours of NK, T, hybrid or indeterminate phenotype, such as in extranodal NK/T-cell lymphoma, EBV+ nodal T- and NK-cell lymphoma, chronic active EBV disease and severe mosquito bite allergy. In other instances, distinction between T- and NK-cell origin may be unclear or difficult to determine.
Table 2.
WHO Classification of Haematolymphoid Tumours, 5th edition: T-cell and NK-cell lymphoid proliferations and lymphomas.
| WHO Classification, 5th edition | WHO Classification, revised 4th edition |
| Tumour-like lesions with T-cell predominance | |
| Kikuchi-Fujimoto disease | Not previously included |
| Indolent T-lymphoblastic proliferation | Not previously included |
| Autoimmune lymphoproliferative syndrome | Not previously included |
| Precursor T-cell neoplasms | |
| T-lymphoblastic leukaemia/lymphoma | |
| T-lymphoblastic leukaemia / lymphoma, NOS | T-lymphoblastic leukaemia/lymphoma |
| Early T-precursor lymphoblastic leukaemia / lymphoma | Early T-cell precursor lymphoblastic leukaemia |
| (Entity deleted) | NK-lymphoblastic leukaemia/lymphoma |
| Mature T-cell and NK-cell neoplasms | |
| Mature T-cell and NK-cell leukaemias | |
| T-prolymphocytic leukaemia | (Same) |
| T-large granular lymphocytic leukaemia | T-cell large granular lymphocytic leukaemia |
| NK-large granular lymphocytic leukaemia | Chronic lymphoproliferative disorder of NK cells |
| Adult T-cell leukaemia/lymphoma | (Same) |
| Sezary syndrome | (Same) |
| Aggressive NK-cell leukaemia | (Same) |
| Primary cutaneous T-cell lymphomas | |
| Primary cutaneous CD4-positive small or medium T-cell lymphoproliferative disorder | (Same) |
| Primary cutaneous acral CD8-positive lymphoproliferative disorder | Primary cutaneous acral CD8-positive T-cell lymphoma |
| Mycosis fungoides | (Same) |
| Primary cutaneous CD30-positive T-cell lymphoproliferative disorder: Lymphomatoid papulosis | (Same) |
| Primary cutaneous CD30-positive T-cell lymphoproliferative disorder: Primary cutaneous anaplastic large cell lymphoma | (Same) |
| Subcutaneous panniculitis-like T-cell lymphoma | (Same) |
| Primary cutaneous gamma/delta T-cell lymphoma | (Same) |
| Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma | (Same) |
| Primary cutaneous peripheral T-cell lymphoma, NOS | Not previously included |
| Intestinal T-cell and NK-cell lymphoid proliferations and lymphomas | |
| Indolent T-cell lymphoma of the gastrointestinal tract | Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract |
| Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract | Not previously included |
| Enteropathy-associated T-cell lymphoma | (Same) |
| Monomorphic epitheliotropic intestinal T-cell lymphoma | (Same) |
| Intestinal T-cell lymphoma, NOS | (Same) |
| Hepatosplenic T-cell lymphoma | |
| Hepatosplenic T-cell lymphoma | (Same) |
| Anaplastic large cell lymphoma | |
| ALK-positive anaplastic large cell lymphoma | Anaplastic large cell lymphoma, ALK-positive |
| ALK-negative anaplastic large cell lymphoma | Anaplastic large cell lymphoma, ALK-negative |
| Breast implant-associated anaplastic large cell lymphoma | (Same) |
| Nodal T-follicular helper (TFH) cell lymphoma | |
| Nodal TFH cell lymphoma, angioimmunoblastic-type | Angioimmunoblastic T-cell lymphoma |
| Nodal TFH cell lymphoma, follicular-type | Follicular T-cell lymphoma |
| Nodal TFH cell lymphoma, NOS | Nodal peripheral T-cell lymphoma with TFH phenotype |
| Other peripheral T-cell lymphomas | |
| Peripheral T-cell lymphoma, not otherwise specified | (Same) |
| EBV-positive NK/T-cell lymphomas | |
| EBV-positive nodal T- and NK-cell lymphoma | Not previously included |
| Extranodal NK/T-cell lymphoma | Extranodal NK/T-cell lymphoma, nasal-type |
| EBV-positive T- and NK-cell lymphoid proliferations and lymphomas of childhood | |
| Severe mosquito bite allergy | (Same) |
| Hydroa vacciniforme lymphoproliferative disorder | Hydroa vacciniforme-like lymphoproliferative disorder |
| Systemic chronic active EBV disease | Chronic active EBV infection of T- and NK-cell type, systemic form |
| Systemic EBV-positive T-cell lymphoma of childhood | (Same) |
Tumour-like lesions with T-cell predominance: a new class of tumour-like lesions
The new family of tumour-like lesions with T-cell predominance in WHO-HAEM5 includes three distinct entities: indolent T-lymphoblastic proliferation (ITLP), Kikuchi-Fujimoto disease (KFD), and autoimmune lymphoproliferative syndrome (ALPS). These expansions of T cells can potentially be mistaken for lymphoma. Indolent T-lymphoblastic proliferation (ITLP) may occur by itself or in association with benign and neoplastic follicular dendritic cell proliferations and other malignancies. It shows clusters or confluent sheets of lymphoid cells which can range in appearance from small lymphocytes to slightly larger cells with more open chromatin (morphologically consistent with thymocytes as seen in the normal thymus), which may be mistaken for T-lymphoblastic leukaemia/lymphoma due to TdT expression [198–205]. However, ITLP may distort, but typically does not obliterate the architecture of the involved tissues, the TdT+ cells are not as atypical as those encountered in lymphoblastic leukaemia/lymphoma, and ITLP does not show monoclonal TCR gene rearrangement. Kikuchi-Fujimoto disease (KFD) commonly shows large aggregates and sheets of T immunoblasts and histiocytes, accompanied by prominent apoptosis in lymph nodes, mimicking peripheral T-cell lymphoma NOS. Clues to the correct diagnosis include the typical clinical scenario of cervical lymphadenopathy in a young woman, the circumscribed and non-expansile nature of the nodal infiltrate, presence of a significant component of plasmacytoid dendritic cells (CD123+) and presence of many histiocytes that express myeloperoxidase. Autoimmune lymphoproliferative syndrome (ALPS), which is associated with autoimmunity and germline or somatic pathogenetic changes in genes involved in FAS-mediated apoptosis [206], has nodal or extranodal infiltrates of CD4-, CD8- T cells, which may appear as atypical medium-sized cells with clear cytoplasm that may mimic lymphoma. The clinical setting (young patient age) and lack of destructive infiltrate may provide clues to its benign nature [207].
Precursor T-cell neoplasms: uncertainties about NK-lymphoblastic leukaemia/lymphoma
T-lymphoblastic leukaemia/lymphoma (T-ALL) are precursor T-cell neoplasms, comprising T-lymphoblastic leukaemia/lymphoma NOS and early T-precursor lymphoblastic leukaemia/lymphoma, as in WHO-HAEM4R. The latter shows a gene expression signature corresponding to that of earlier stages of normal precursor T cells as compared with the former entity, and shows a unique immunophenotype that includes expression of stem cell and/or myeloid markers. Despite significant advances in our understanding of the genetic background of T-ALL [208], there is as yet not sufficient evidence to establish genetically defined types of T-ALL with clinical relevance.
NK-lymphoblastic leukaemia/lymphoma, considered a provisional entity in WHO-HAEM4R, is not separately listed in WHO-HAEM5 because of lack of clear-cut and reliable diagnostic criteria, lack of published information on expression on NK-cell-associated antigens such as CD94 and CD161, and marked morphologic and immunophenotypic overlap with other entities, such as blastic plasmacytoid dendritic cell neoplasm, CD56+ T-ALL, CD56+ acute myeloid leukaemia and CD56+ acute undifferentiated leukaemia [209].
Mature T-cell and NK-cell leukaemias: a family is growing
The family of mature T-cell and NK-cell leukaemias encompasses neoplastic T- and NK-cell proliferations that primarily present as leukaemic disease, including T-prolymphocytic leukaemia (T-PLL), T-large granular lymphocytic leukaemia (T-LGLL), NK-large granular lymphocytic leukaemia (NK-LGLL), adult T-cell leukaemia/lymphoma (ATLL), Sezary syndrome (SS) and aggressive NK-cell leukaemia (ANKL). Enhanced molecular understanding is considered sufficiently mature to permit incorporation of such features in the diagnostic criteria or prognostic markers of these diseases, where relevant.
T-prolymphocytic leukaemia (T-PLL) is a rare form of mature T-cell leukaemia with a heterogeneous clinical course. Recent efforts to standardize diagnosis, staging and treatment response [210] have led to unified diagnostic criteria, which include T lymphocytosis (>5 x 109/L) with appropriate phenotype, T-cell monoclonality and the presence of genetic aberrations including structural variants with breakpoints affecting the TCL1A or MTCP1 locus or expression of TCL1. There is emerging evidence of clinical and phenotypic significance of specific mutations in T-large granular lymphocytic leukaemia (T-LGLL). STAT3 mutation, found preferentially in CD8+ T-LGLL and gamma/delta T-LGLL, is associated with neutropenia and poorer overall survival [211–214]. STAT5B mutation is over-represented in the rare CD4+ T-LGLLs (present in up to 30% of cases); it is associated with a poor prognosis in CD8+ T-LGLL, but has no prognostic impact in CD4+ T-LGLL and gamma/delta T-LGLL [214]. “Chronic lymphoproliferative disorder of NK cells” in WHO-HAEM4R has been renamed NK-large granular lymphocytic leukaemia (NK-LGLL), given recent evidence that this is a monoclonal or oligoclonal expansion of NK cells that has many similarities with T-LGLL. Genetic analyses of adult T-cell leukaemia/lymphoma (ATLL) have revealed novel events that highlight the importance of immune evasion including CTLA4::CD28 and ICOS::CD28 fusions, REL C-terminal truncations [215, 216], recurrent alterations in HLA-A and HLA-B and structural variations disrupting the 3′-untranslated region of CD274 (PD-L1) [217]. Furthermore, the frequency and pattern of somatic alterations appear to be correlated with clinical behavior. Specifically, aggressive subtypes show more genetic alterations, whereas STAT3 mutations are more common in indolent subtypes. Based on clinical and serological features, prognostic indices of ATLL have been better defined, and a prognostically meaningful genetic classification has recently been proposed [218]. Sezary syndrome (SS), while closely related to mycosis fungoides but a distinct entity, is included in this section to highlight its primary site of clinical presentation and consideration in the differential diagnosis of mature T-cell leukaemias. Comprehensive analyses of genomic signatures [219] highlight the contribution of cellular aging and UV exposure observed in SS. Genome-wide sequencing studies have provided novel insights into pathogenetic events in aggressive NK-cell leukaemia (ANKL). They implicate mutations in genes of the JAK/STAT and RAS/MAPK pathways, epigenetic modifiers (TET2, CREBBP, KMT2D), and immune checkpoint molecules CD274 (PD-L1)/PDCD1LG2 (PD-L2) [220–223] in disease pathogenesis.
Primary cutaneous T-cell lymphoid proliferations and lymphomas (CTCL): rare subtypes become entities
Primary cutaneous T-cell lymphoid proliferations and lymphomas (CTCL) comprise a dedicated family within the mature T/NK-cell neoplasms chapter in WHO-HAEM5, and include nine entities.
In WHO-HAEM4R, primary cutaneous gamma/delta T-cell lymphoma, CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma, acral CD8-positive T-cell lymphoproliferative disorder and CD4-positive small or medium T-cell lymphoproliferative disorder were grouped together under the term ‘cutaneous peripheral T-cell lymphoma, rare subtypes’, but are now each listed as separate entities in WHO-HAEM5 acknowledging their specific clinicopathological and genetic characteristics. The variants of mycosis fungoides from WHO-HAEM4R remain in place as subtypes; however, within the folliculotropic category, clinical early versus advanced stage patterns are described, and should be distinguished, to acknowledge differing clinical outcomes. There still remain rare cases that do not fit into the other known CTCL entities, and that are grouped into the newly coined entity “primary cutaneous peripheral T-cell lymphoma, NOS”, awaiting further studies to clarify their nature [224].
As there is morphologic and immunophenotypic overlap among the various forms of primary CTCL, correlation with clinical history, signs and symptoms is a key element of the diagnostic work-up. Thus, dermatological examination and clinical photographic documentation are indispensable in reaching the correct diagnosis [224, 225].
Intestinal T-cell and NK-cell lymphoid proliferations and lymphomas: indolent NK-cell lymphoproliferative disorder as the new kid in town
In WHO-HAEM5, the main changes in the classification of intestinal T-cell and NK-cell lymphomas include: new nomenclature for indolent T-cell lymphoproliferative disorder of the gastrointestinal tract, now designated “indolent T-cell lymphoma of the gastrointestinal (GI) tract”, and addition of a new entity, “indolent NK-cell lymphoproliferative disorder of the GI tract” (iNKLPD) (Table 7). For indolent T-cell lymphoma of the gastrointetinal (GI) tract, the change from the conservative designation of “lymphoproliferative disorder” to “lymphoma” is justified by the significant morbidity related to the tumour and the ability of the disease to disseminate, while the qualifier “indolent” remains to indicate its protracted clinical course [226–230]. There are interesting correlations between T-cell subsets and genetic changes in this neoplasm: alterations in JAK-STAT pathway genes and mutations in epigenetic modifier genes (e.g., TET2, KMT2D) preferentially occur in CD4+, CD4+/CD8+, and CD4-/CD8- subsets, with CD4+ cases sometimes displaying STAT3::JAK2 fusions. In contrast, some CD8+ cases have been shown to harbor structural alterations involving the IL2 gene [227, 230]. Indolent NK-cell lymphoproliferative disorder of the GI tract (iNKLPD), (Fig. 6) formerly known as lymphomatoid gastropathy or NK-cell enteropathy and previously thought to be a reactive process, is included as an entity because of recent findings supporting its neoplastic nature. Somatic mutations in various genes have been identified, including recurrent JAK3 mutations (K563_C565del; NP_000206). Moreover, immunophenotypic features support a role for JAK3-STAT5 pathway activation in pathogenesis [231]. Nonetheless, the disease has a benign clinical outcome: individual lesions usually spontaneously regress in a few months, although lesions may persist or new lesions may develop over a period of years. Progression to aggressive disease is not reported, justifying its designation as “lymphoproliferative disorder” [231–233]. An interesting observation is that this tumour may not be entirely confined to the GI tract, with rare cases reported to involve gallbladder, adjacent lymph nodes and the vagina [234–236]. It is most important not to misinterpret iNKLPD as extranodal NK/T-cell lymphoma, the immunophenotype of which can be largely identical with the exception of crucial differential EBV association. While the infiltrate of atypical medium-sized lymphoid cells is worrisome, the small size and superficial nature of the lesions, expansile rather than highly destructive growth and presence of paranuclear brightly eosinophilic granules may provide a clue to the diagnosis, which can be further confirmed by the lack of EBV.
Table 7.
Comparison of different types of T and NK cell lymphoproliferative disorders and lymphomas involving the gastrointestinal tract (GIT).
| Indolent T-cell lymphoma of the GIT | Indolent NK-cell LPD of the GIT | Enteropathy-associated T-cell lymphoma | Monomorphic epitheliotropic intestinal T-cell lymphoma | Extranodal NK/T-cell lymphoma | |
|---|---|---|---|---|---|
| Major clinical presentation | Abdominal symptoms | Asymptomatic or nonspecific GI symptoms | Abdominal symptoms; bowel perforation or obstruction common. | Abdominal symptoms; bowel perforation or obstruction common. | Abdominal symptoms; bowel perforation common. |
| Association with celiac disease | – | – | + | – | – |
| Clinical course | Chronic persistent or relapsing | Usually spontaneous regression, but may persist or develop new lesions | Aggressive | Aggressive | Aggressive |
| Commonest localization in GIT | Small bowel or colon | Stomach, small and large intestines | Small intestine | Small intestine | Small and large intestines |
| Depth of involvement | Superficial | Superficial | Deep | Deep | Deep |
| Cytomorphology | Small lymphoid cells with minimal nuclear atypia | Atypical medium-sized cells with pale cytoplasm and eosinophilic granules | Pleomorphic large or medium-sized cells, often with prominent inflammatory background | Monomorphic small to medium-sized cells | Variable cytomorphology, from small to medium-sized to large cells |
| Epitheliotropism | −/ focal | −/ minimal | + | + | – |
| Necrosis | – | – | +/− | Usually – | + |
| EBV association | – | – | – | – | + |
| Lineage | T cell, CD4+ >CD8+ | NK cell | T cell, most often CD4-, CD8- | T cell, most often CD8+ | NK cell (commoner) or T cell |
| Molecular genetics | JAK2::STAT3 fusion; mutations of JAK-STAT pathway genes and epigenetic modifier genes | JAK3 mutation | Gains of 9q34; loss of 16q12; mutations of JAK-STAT pathway genes (commonly JAK1, STAT3) | Gains of 9q34; loss of 16q12; mutations of SETD2 and JAK-STAT pathway genes (commonly JAK3, STAT5B) | 6q21-25 deletion; Mutations of JAK-STAT pathway genes, epigenetic regulators, tumor suppressor genes (TP53, MGA) and RNA helicase (DDX3X) |
Fig. 6. Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract involving the stomach.
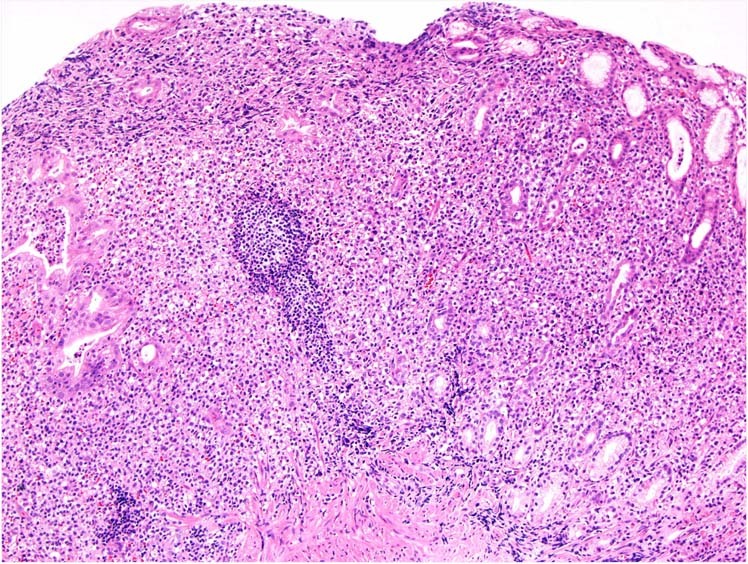
The gastric mucosa shows expansion of the lamina propria by an atypical lymphoid infiltrate. The tumour cells are medium-sized, often with pale-staining cytoplasm.
Hepatosplenic T-cell lymphoma: not confined to the young
Various new findings regarding hepatosplenic T-cell lymphoma (HSTCL) since WHO-HAEM4R have led to refinements in WHO-HAEM5. Recent studies have shown that HSTCL is not necessarily a disease of the young; only 49% of patients were younger than 60 years of age in a recent study [237]. Of note, dyspoiesis of bone marrow elements mimicking myelodysplastic syndrome is not uncommon in marrow smears of HSTCL patients, although this does not have any clinical impact [238]. While most cases express TCRγδ (~75%) followed by TCRαβ (~25%), a small subset of cases, about 5%, are TCR-silent [239].
Anaplastic large cell lymphoma: more genetic data in an otherwise well-defined entity
WHO-HAEM5 recognizes 3 entities within the family of anaplastic large cell lymphomas (ALCL), which are mature T-cell lymphomas characterized by pleomorphic tumour cells with uniform strong expression of CD30 and often defective expression of T-lineage markers. Primary cutaneous ALCL is grouped under primary cutaneous T-cell lymphoid proliferations and lymphomas acknowledging its clinico-pathological relation to these disorders and highly favorable outcome in contrast to systemic ALK- ALCL [240, 241]. ALK positive anaplastic large cell lymphoma (ALK+ ALCL) has been separated from ALK-negative ALCL (ALK- ALCL) since WHO-HAEM4 based on its distinct pathogenesis [242, 243] and clinical course. ALK- ALCL was acknowledged as a heterogeneous entity. Recent genomic analyses have led to recognition of several genetic contexts with prognostic implications, although there are currently insufficient data to determine if these are best regarded as prognostic markers or molecular subtypes. ALK-negative ALCL bearing TP63 rearrangements [244], loss of TP53 [244–246] and/or overexpression of IL-2Rα [247] are associated with poor outcomes. Although initial reports suggested DUSP22 rearrangement to be associated with a favorable 5-year overall survival comparable to ALK+ ALCL [248], more recent studies have not confirmed this association [249]. Some specific molecular alterations in ALK- ALCL have been shown to correlate with morphologic features. ALCLs with DUSP22 rearrangement are characterized by neoplastic cells with a “doughnut cell” appearance [250] and sheet-like growth pattern with less pleomorphic cells; LEF1 expression may serve as a surrogate marker for this molecular alteration [251]. A subset of cases with Hodgkin-like morphology shows aberrant ERBB4 protein expression [252], while more anaplastic cells are seen in cases with JAK2 rearrangement [253]. Breast implant-associated ALCL (BIA-ALCL) is an entity distinct from other ALK- ALCL; notably it is a usually non-invasive neoplasm arising in association with textured-surface breast implants and is associated with an excellent outcome [254]. Invasion of adjacent structures, however, worsens the prognosis. Recent studies highlight the importance of TH2 allergic inflammatory response, a role for immune-evasion via amplification of 9p24.1 and overexpression of PD-L1 in over 50% of the cases and constitutive JAK-STAT activation by somatic mutations of STAT3, STAT5B, JAK1 and JAK2 and loss-of function mutations of SOCS1 and SOCS3 [255–261].
Nodal T-follicular helper cell lymphomas: New nomenclature to unite family members
This family includes three entities of nodal T-cell lymphomas that bear the phenotype and gene expression signatures of T-follicular helper (TFH) cells [262, 263]. While the conceptual basis for the recognition of these entities is consistent with that proposed in WHO-HAEM4R, a common family terminology of nodal T-follicular helper cell lymphomas (nTFHLs) is introduced in WHO-HAEM5, with previously recognized diseases now regarded as entities within this family. Accordingly, diseases previously referred to as “angioimmunoblastic T-cell lymphoma”, “follicular T-cell lymphoma” and “peripheral T cell lymphoma with TFH phenotype” are renamed nTFHL angioimmunoblastic-type (nTFHL-AI), nTFHL follicular-type (nTFHL-F) and nTFHL not otherwise specified (nTFHL-NOS), respectively. This is to recognize their significant clinical and immunophenotypic overlap and plasticity [264, 265], as well as similar TFH gene expression signature and mutation profiles. Research in the coming years may provide data to further define the boundaries in biology between these entities or rather refute such differences. The current classification provides a platform for such studies.
Nodal T-follicular helper cell lymphoma, angioimmunoblastic-type (nTFHL-AI) is the prototype with well-defined clinical, morphologic (Fig. 7), immunophenotypic and characteristic mutational profiles. Genetically, nTFHL-AI is characterized by a stepwise acquisition of somatic changes with TET2 and DNMT3A mutations occurring early in haematopoietic stem cells, while RHOA and IDH2 mutations are also present in the neoplastic TFH cell population. In contrast, nTFHL-F and nTFHL-NOS (Fig. 7) represent less well-studied nodal lymphomas, which also express TFH markers such as PD1, ICOS, CXCL13, CD10, and BCL6 [266–277] and show mutation profiles similar to those of nTFHL-AI [265, 266, 278–280].
Fig. 7. Nodal TFH-cell lymphoma (nTFHL).
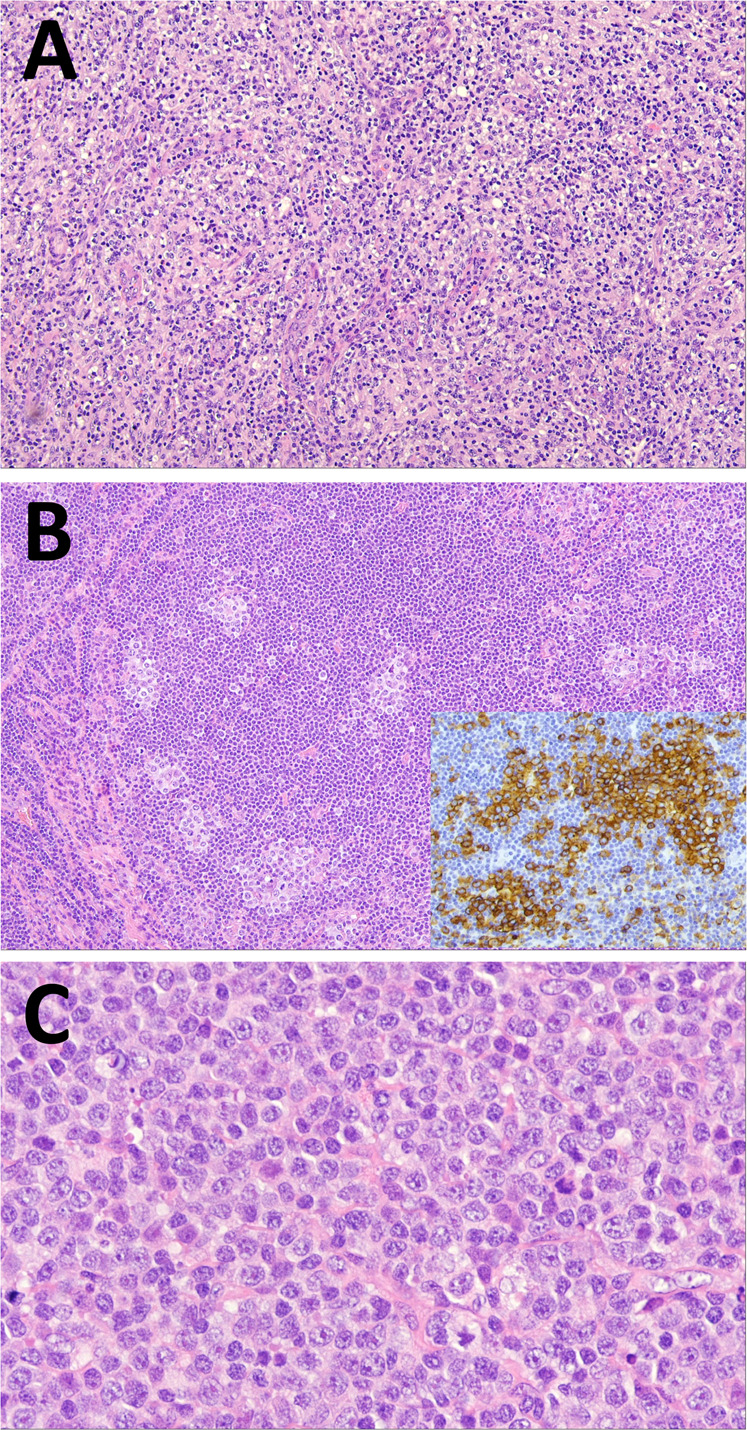
A Nodal TFH-cell lymphoma, angioimmunoblastic-type (nTFHL-AI). The normal architecture of the lymph node is effaced. There is a diffuse infiltrate of medium-sized, slightly atypical lymphocytes, sometimes with clear cytoplasm. One of the hallmarks of the disease is the proliferation of arborizing post-capillary vessels consistent with high endothelial venules. B Nodal TFH-cell lymphoma, follicular-type (nTFHL-F). In this example, progressive transformation of germinal centre-like nTFHL-F, clusters of atypical lymphoid cells with pale cytoplasm are embedded in a background of small lymphocytes of mantle zone type. The inset shows strong expression of PD1 in the tumour cells. C Nodal TFH-cell lymphoma, not otherwise specified. This tumour is composed of a sheet-like proliferation of medium-sized to large neoplastic cells.
Although the individual entities are defined predominantly by histopathological features, there is considerable morphologic overlap and inter-observer variability. nTFHL-NOS is the recommended term for CD4+ lymphomas with TFH phenotype but that do not meet criteria for nTFHL-AI or nTFHL-F. The generic term nTFHL rather than nTFHL-NOS is recommended when interpreting core biopsies to prevent misclassification due to inadequate sampling. The TFH phenotype is defined as the presence of at least two TFH markers in addition to CD4. Further studies are required to determine if this definition is sufficiently robust in differentiating nTFHL-NOS from PTCL, NOS, as most cases of the former often express the less specific TFH markers such as PD1 and ICOS. In essence, the diagnosis may be challenging with many pitfalls. An integrated approach is recommended, at the very minimum requiring correlation of clinical, morphologic and immunophenotypic features, with input from genomics for clonality and mutational profiles in difficult cases.
Other peripheral T-cell and NK-cell lymphomas: nodal EBV+ T- and NK-cell lymphoma counterpart of extranodal NK/T-cell lymphoma
In WHO-HAEM5, peripheral T-cell lymphoma NOS (PTCL-NOS) remains a heterogeneous category and a diagnosis of exclusion, with a differential diagnosis that in particular includes nodal T-follicular helper cell lymphomas, among others. Two possible biological variants of PTCL-NOS, PTCL-TBX21 and PTCL-GATA3, have been characterized by the transcriptional program of T-helper-1 and T-helper-2 cells, respectively [281]. While PTCL-GATA3 has a uniform molecular genetic profile, PTCL-TBX21 is heterogeneous and may include a subgroup with a cytotoxic gene expression program and aggressive behavior [281, 282]. The current status of knowledge on the genetic landscape, clinico-pathological context and prognostic implications of these possible biological variants of PTCL-NOS are deemed, however, insufficient to justify a formal status as “subtype” [283]. Extranodal NK/T-cell lymphoma (ENKTL) will have the qualifier “nasal-type” dropped from its name in WHO-HAEM5 in accordance with the recognized presentation of this disease at various extranodal sites. The introduction of L-asparaginase-based chemotherapy in combination with radiotherapy has led to markedly improved outcomes for this lymphoma [284]. Immune checkpoint inhibitor therapy has recently shown great promise for relapsed or refractory disease [285–287], in keeping with the finding that immune evasion is a critical pathway for ENKTL cell survival [288, 289]. Intravascular NK/T-cell lymphoma was considered a form of ENKTL in WHO-HAEM4R [290–295]. This highly aggressive lymphoma is often, but not invariably, EBV positive, does not present with mass lesions and shows a predilection for skin and central nervous system. Since its nosological nature is still unclear, it is now described under aggressive NK-cell leukaemia rather than extranodal NK/T-cell lymphomas, pending further studies to determine where it fits best.
Nodal EBV-positive T and NK-cell lymphoma, which occurs mostly in East Asians [296–300], is now recognized as a distinct entity in WHO-HAEM5; previously it was subsumed as a subtype under the entity of PTCL-NOS. Patients typically present with lymphadenopathy with or without extranodal involvement, advanced-stage disease and B symptoms; they have a dismal prognosis. Morphologically, this lymphoma often resembles diffuse large B-cell lymphoma, lacking the coagulative necrosis and angioinvasion characteristic of ENKTL (Fig. 8). It more often shows a cytotoxic T-cell than NK-cell immunophenotype; EBV is positive, by definition. The genetic landscape differs from that of ENKTL, with the most commonly mutated gene being TET2 [300].
Fig. 8. Nodal EBV-positive T- and NK-cell lymphoma.
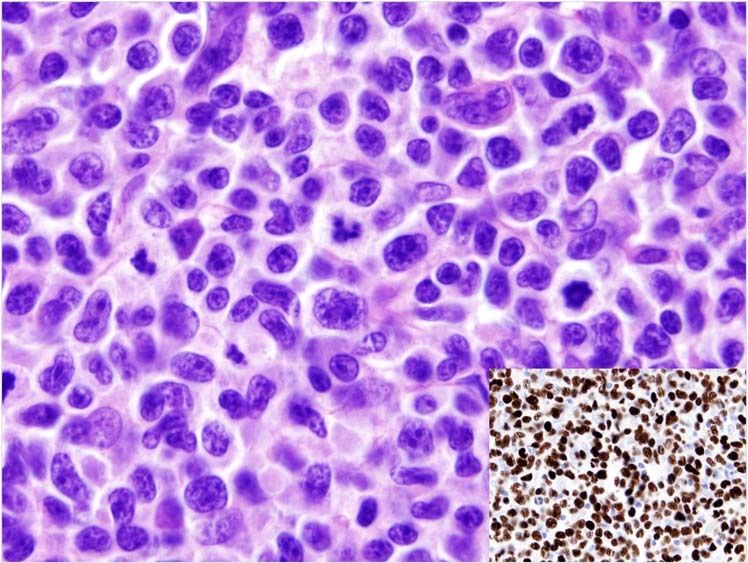
This lymphoma shows a diffuse infiltrate of relatively monotonous, medium-sized to large cells, sometimes reminiscent of centroblasts. Inset: in-situ hybridization for EBERs identifies EBV infection in virtually all tumour cells.
EBV-positive T- and NK-cell lymphoid proliferations and lymphomas of childhood: revised terminology
EBV-associated lymphoid proliferations and lymphomas of childhood are uncommon T- and NK-cell disorders with a predilection for Asian and native American ethnic groups [301–305]; occurrence in adults is also reported [306]. This family includes chronic active EBV disease (CAEBVD) and systemic EBV-positive T-cell lymphoma of childhood. CAEBVD is characterized by a broad clinical spectrum varying from localized and/or indolent forms (severe mosquito bite allergy and hydroa vacciniforme lymphoproliferative disorder [HVLPD] classic form), to systemic disease with fever, hepatosplenomegaly, and lymphadenopathy, with or without cutaneous manifestations (HVLPD systemic form and systemic CAEBVD).
This classification introduces several changes in terminology to reflect the morphologic overlap among different entities, such as HVLPD systemic form and systemic CAEBVD, and the need for clinicopathologic correlation in diagnosis. “Hydroa vacciniforme-like lymphoproliferative disorder” in WHO-HAEM4R has been renamed HVLPD, with identification of a classic and a systemic form. Systemic HVLPD shows persistent systemic symptoms of CAEBVD or extracutaneous disease, and should be distinguished from systemic CAEBVD without HVLPD, which is characteried by an even more aggressive clinical course [307–310]. Moreover, the usually fatal outcome in the absence of haematopoietic stem cell transplantation has led to replacement of the former terminology “chronic active EBV infection, systemic form” with “systemic chronic active EBV disease” [308, 309].
Stroma-derived neoplasms of lymphoid tissues: some tumour types are unique to lymph node or spleen
A new category of stroma-derived neoplasms of lymphoid tissues is introduced in WHO-HAEM5 (Table 3). Mesenchymal tumours specific to lymph node (including intranodal palisaded myofibroblastoma) and spleen (including littoral cell angioma, splenic hamartoma and sclerosing angiomatoid nodular transformation) are covered, while various soft tissue tumours not specific to lymph node or spleen (such as haemangioma, lymphangiomyoma, Kaposi sarcoma and angiosarcoma) are covered in the WHO Classification of Soft Tissue and Bone Tumours (5th edition, 2020). Furthermore, follicular dendritic cell and fibroblastic reticular cell neoplasms have been moved from the “histiocytic and dendritic cell neoplasms” category to this new category, because follicular dendritic cells are not derived from haematopoietic stem cells but rather are of mesenchymal origin [311–313]. Given its distinctive clinicopathologic features, EBV-positive inflammatory follicular dendritic cell sarcoma is delineated as an entity separate from follicular dendritic cell sarcoma [314], together with a nomenclature change from “inflammatory pseudotumour-like follicular/fibroblastic dendritic cell sarcoma”, a change first introduced in the WHO Classification of Digestive Tract Tumours (5th edition, 2019) [315].
Genetic predisposition syndromes: increasing importance of germline genetics
To acknowledge the growing number of known germline predispositions associated with haematologic neoplasms, lymphoid neoplasms occurring in the context of clinical syndromes should be separately recognized, similar to classification in other organ systems. To this end, WHO-HAEM5 introduces new chapters on genetic predisposition. With regard to lymphoid neoplasms, Ataxia telangiectasia (AT) and Nijmegen-Breakage syndrome are particularly relevant. These are linked to germline mutations in ATM and NBN, respectively. The detection of such underlying syndromes associated with germline predisposition is clinically important not only with regards to treatment planning (e.g., increased toxicities) but also surveillance of carriers and counselling of relatives. In this regard, leukaemias and lymphomas should be diagnosed using conventional criteria but should be designated as “AT-related” or “NBS-related”. Besides the separate chapter on genetic predisposition syndromes, aspects of germline predisposition including recommendations for germline testing have been systematically incorporated in individual chapters.
Epilogue
The dramatic increase in information regarding lymphoid tumours and their molecular complexity suggests that Fyodor Dostoyevsky’s famous words [316], “Reality is infinitely diverse (…and) Reality resists classification” hold true for lymphoma classifications.
We are aware that any classification is arbitrary and subject to further evolution as new evidence arises. Moreover, since the development and differentiation of lymphocytes represent a continuous spectrum rather than a sequence of distinct steps, we acknowledge that any classification system breaks up a disease continuum into groups using arbitrary (and yet evidence-based) borders. Furthermore, our daily work demands the naming, and hence, the diagnosis, of discrete entities to allow for treatment decisions and for patient management. Therefore, following the principles of Linnaeus, classification also provides the basis for preserving knowledge and providing a template for future work.
We are grateful to - and stand on the shoulders of - countless individuals and research teams, who have contributed significantly to establish the foundations of the current lymphoma classification. We thank the numerous authors and contributors whose input and thoughts have created the herein outlined ‘snapshot-in-time’ of the classification. We are confident that the present proposal, albeit by definition imperfect, will provide a robust framework for future generations of scientists to continue our efforts, to further disentangle the universe of lymphoma biology, for the benefit of patient care.
Disclaimer
The content of this article represents the personal views of the authors and does not represent the views of the authors’ employers and associated institutions. This work is intended to provide a preview and summary of content whose copyright belongs solely to the International Agency for Research on Cancer/World Health Organization. Any or all portions of the material in this work may appear in future International Agency for Research on Cancer/World Health Organization publications.
Acknowledgements
The authors thank the leadership and staff of the International Agency for Research on Cancer, especially Ms. Asiedua Asante, for her tireless efforts, and also Jasmine Singh and Kim Vu for their expert assistance in graphic presentation of the river plot figures.
Author contributions
JK and AJL are standing members of the WHO Classification of Tumours editorial board. RA, JKCC, WJC, SEC, SSD, DDJ, MQD, JF, SG, AH, MSL, KNN, GO, SS, AS, WS, RS, and BW are expert members of the Haematolymphoid Tumours 5th edition blue book editorial board. CA, IA, ADA, IBOA, EB, GB, AMB, DB, MC, AC, WC, JKC, SSC, MC, KSEJ, JG, DG, JG, MH, CJH, SH, PMJ, KK, WK, HK, WK, AEK, SK, SL, LL, NL, VL, XQL, WPL, ALj, AM, LJM, MM, RNM, CM, SMM, WM, VN, YN, SBN, IO, MP, MP, SVJ, ACR, KR, AR, JS, CS, CS, ARS, RT, ATG, FV, BV, ADW, LX, and WX contributed as responsible authors in the book. All authors and editors contributed to discussions on the content of the book chapters. All listed authors edited and approved the manuscript.
The following colleagues are acknowledged for their expert contributions as authors in the WHO Classification of Haematolymphoid Tumours blue book on lymphoid topics, mesenchymal lesions specific to lymph node and spleen, and germline predisposition syndromes associated with the lymphoid neoplasms. Their affiliations were provided as given in the International Agency for Research on Cancer (IARC) databases. Where authors are identified as personnel of IARC/World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Norah Olubunmi Akinola68, Yassmine Akkari69, Luis M. Allende70, Katsuyuki Aozasa71, Iguaracyra Araujo72, Luca Arcaini73, Kirit M. Ardeshna74, Naoko Asano75, Andishe Attarbaschi76, Chris M. Bacon27, Sharon Louise Barrans55, Tracy Batchelor77, Maxime Battistella78, Linda B. Baughn48, Amir Behdad79, Sigbjørn Berentsen80, Giada Bianchi81, Jacob Bledsoe26, Peter Borchmann82, Mark Bower83, Barbara Buldini84, Jan Andreas Burger85, Birgit Burkhardt86, Ryan D. Cassaday87, Giovanni Cazzaniga88, Nadine Cerf-Bensussan89, Ethel Cesarman11, Mammen Chandy90, Jennifer R. Chapman91, Björn Chapuy92, Xueyan Chen93, Chee Leong Cheng94, Carlos Chiattone95, Nicholas Chiorazzi96, Lucy B. Cook97, Wendy A. Cooper98, Gregory Philip Corboy99, Andrew John Cowan87, Immacolata Cozzolino100, Ian A Cree101, Emanuele S.G. d’Amore102, Andrew John Davies103, Martina Deckert104, Jan Delabie105, Elizabeth G. Demicco105, Vikram Deshpande22, Arianna Di Napoli106, Arjan Diepstra107, Daan Dierickx108, Kieron Dunleavy109, Barbara Eichhorst110, Daisuke Ennishi111, David C. Fajgenbaum112, Pedro Farinha113, Carlos Fernández de Larrea114, Kevin E. Fisher44, Jude Fitzgibbon115, Melina Flanagan116, Jonathan Fromm93, Juan F. Garcia117, William Robert Geddie105, Morie Gertz37, Ajay Gopal87, Satish Gopal118, Patricia Theresa Greipp48, Alejandro Gru119, Ritu Gupta120, Martin-Leo Hansmann121, Konnie M. Hebeda122, Klaus Herfarth123, Marco Herling124, Olivier Hermine125, Khe Hoang-Xuan126, Jennelle Hodge127, Shimin Hu33, Yuhua Huang128, Yin Pun Hung22, Stephen Hunger129, Hiroto Inaba130, Hiroshi Inagaki131, Javeed Iqbal132, Kenji Ishitsuka133, Noriko Iwaki134, Keiji Iwatsuki135, Nitin Jain85, Yoon Kyung Jeon136, Marshall Kadin137, Sachiko Kaji138, Aanchal Kakkar120, Anastasios Karadimitris139, Keisuke Kataoka140, Seiichi Kato141, Marie José Kersten142, Rhett P. Ketterling48, Ji Eun Kim143, Christian P. Kratz144, Robert Kridel145, Sigurdur Kristinsson146, Ralf Küppers147, Isinsu Kuzu148, Yok-Lam Kwong149, Ann Lacasce150, Laurence Lamant-Rochaix151, Thierry Lamy152, Ola Landgren153, Siddhartha Laskar154, William Bradlyn Laskin155, Georg Lenz156, Shaoying Li33, Gan Di Li43, Pei Lin33, Franco Locatelli157, Robert Brian Lorsbach158, Izidore Lossos153, Thomas P. Jr. Loughran159, William R. Macon48, Joseph J. Maleszewski48, Pankaj Malhotra160, Teresa Marafioti161, Dai Maruyama162, Alexander Marx163, Sam M. Mbulaiteye164, Veronique Meignin78, Ester Mejstrikova165, Pamela Michelow166, Markku Miettinen167, Rodney R. Miles168, Hiroaki Miyoshi169, Thierry Jo Molina170, Manuela Mollejo171, Shuji Momose172, Tetsuya Mori173, William G. Morice48, Bertrand Nadel174, Hirokazu Nagai175, Motoo Nagane176, Reena Nair90, Naoya Nakamura177, Atsuko Nakazawa178, Samih Nasr48, Andrew Gordon Nicholson179, Alina Nicolae180, Robert Shigeo Ohgami181, Naoki Oishi182, Timothy S. Olson129, Nicolas Ortonne183, Bruno Paiva184, Qiang Pan-Hammarström185, Mayur Parihar186, Marco Paulli187, Andrea Petersen188, Jennifer Picarsic189, Alessandro Pileri190, Nicola Pimpinelli191, Jose A Plaza192, Karen R. Rabin193, Markus Raderer194, Kanti Rai195, Ulla Randen196, Huilan Rao128, Alistair Robson197, Rosemary Rochford198, Richard Rosenquist199, Davide Rossi200, Esther D. Rossi201, Simon Rule202, Grzegorz Rymkiewicz203, Elena Sabattini204, Vaskar Saha205, Mamiko Sakata-Yanagimoto206, Christian A. Sander207, J. Martin Sangueza208, Omar P. Sangüeza209, Marco Santucci210, Yasuharu Sato211, Akira Satou141, Kristian Theo Schafernak212, Fernando Schmitt213, Gianpietro Semenzato214, Manju Sengar90, Tait Shanafelt215, Kazuyuki Shimada216, Graham W. Slack113, Susan Slager217, Riccardo Soffietti218, David A. Solomon181, Kostas Stamatopoulos219, Christian Steidl220, Stephan Stilgenbauer221, Narittee Sukswai222, Kengo Takeuchi223, Giovanni Tallini224, Junichi Tamaru225, Soo-Yong Tan50, Prashant Tembhare226, Enrico Tiacci227, Yoshiki Tokura228, Olivier Tournilhac229, Steven Treon230, Lorenz Truemper231, Kunihiro Tsukasaki232, Frits van Rhee233, Abraham Varghese234, Maarten H. Vermeer235, Philippe Vielh236, Brian Walker237, Michael Wang238, Huan-You Wang239, Zhe Wang240, Takashi Watanabe241, Oliver Weigert242, David Weinstock243, Sean J. Whittaker244, Rein Willemze235, Wilhelm Woessmann245, Catherine J. Wu243, Motoko Yamaguchi246, Hidetaka Yamamoto247, Daisuke Yamashita248, Shenmiao Yang249, David T Yang250, Takahiko Yasuda251, Wei-Hua Yin252, Yoh Zen253, Sha Zhao43, Wei-Li Zhao254 68Haematology and Immunology, Obafemi Awolowo University, Ile Ife, Nigeria; 69Cytogenetics and Molecular Pathology, Legacy Health, Portland, OR, USA; 70Immunology, Hopsital 12 de Octubre, Portland, OR, USA; 71Department of Pathology, Osaka University Graduate School of Medicine, Suita, Osaka, Japan; 72Pathology and Forensic Medicine, Federal University of Bahia, Salvador, Bahia, Brazil; 73Fondazione IRCCS Policlinico San Matteo and Departement of Molecular Medicine, University of Pavia, University of Pavia, Pavia, Italy; 74Cancer Division, University College London Hospitals, London, UK; 75Molecular Diagnostics, Nagano Prefectural Shinshu Medical Center, Suzaka, Japan; 76Pediatric Hematology and Oncology, St. Anna Children’s Hospital, Vienna, Austria; 77Department of Neurology, Brigham and Women’s Hospital, Boston MA, USA; 78Pathology Department, Hôpital Saint Louis, AP-HP Université Paris, Paris, France; 79Department of Pathology, Northwestern University, Chicago, IL, USA; 80Department of Research and Innovation, Helse Fonna Hospital Trust, Haugesund, Norway; 81Division of Hematology, Department of Medicine, Brigham and Women’s Hospital, Boston MA, USA; 821st Dept of Internal Medicine, University Hospital of Cologne, Cologne, Germany; 83National Centre for HIV Malignancy, Chelsea & Westminster Hospital, London, UK; 84Maternal and Child’s Health Department, University of Padova, Padova, Italy; 85Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 86Pediatric Hematology, Oncology and BMT, University Hospital Muenster, Muenster, Germany; 87Department of Medicine, University of Washington School of Medicine, Seattle, WA, USA; 88Pediatrics and Medical Genetics, Univ Milan Bicocca—Centro Ricerca Tettamanti, Barzano, Italy; 89Laboratory Intestinal Immunity, Institut Imagine-Inserm U1183, Université de Paris, Paris, France; 90Clinical Hematology & Medical Oncology, Tata Medical Center, Kolkata, India; 91Pathology and Laboratory Medicine, University of Miami, Miami, FL, USA; 92Hematology, Oncology and Tumor Immunology, Charité, University Medical Center Berlin, Berlin, Germany; 93Laboratory Medicine and Pathology, University of Washington, Seattle, WA, USA; 94Anatomical Pathology, Singapore General Hospital, Singapore; 95Hematology and Oncology, Santa Casa Midical School, Sao Paulo, Brazil; 96Karches Center for Oncology Research, The Feinstein Institutes for Medical Research, Manhasset, NY, USA; 97Department of Haematology, Imperial College Healthcare NHS Trust, London, UK; 98Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, NSW Health Pathology, Camperdown NSW, Australia; 99Department of Haematology, Pathology Queensland, Herston, Australia; 100Pathology Unit. Department of Mental and Physical Health and Preventive Medicine, University of Campania “L. Vanvitelli”, Naples, Italy 101Evidence synthesis and classification branch, International Agency for Research on Cancer, Lyon, France; 102UOC di Anatomia Patologica, Ospedale San Bortolo, Vicenza, Italy; 103Cancer Sciences Unit, University of Southampton, Southampton, UK; 104Department of Neuropathology, University Hospital of Cologne, Cologne, Germany; 105Laboratory Medicine Program, University Health Network and University of Toronto, Toronto, Canada; 106Department of Clinical and Molecular Medicine, Sapienza University of Rome, Sant’Andrea Hospital, Roma, Italy; 107Pathology and Laboratory Medicine, University Medical Center Groningen, Groningen, The Netherlands; 108Department of Hematology, University Hospitals Leuven, Leuven, Belgium; 109Hematology and Oncology, Lombardi Cancer Center, Georgetown University, Washington DC, USA; 110Department I for Internal Medicine and Center for Integrated Oncology Aachen, Bonn, Cologne, Duesseldorf, University of Cologne, Cologne, Germany; 111Department of Hematology and Oncology, Okayama University Hospital, Okayama, Japan; 112Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 113Department of Pathology, BC Cancer Agency, Vancouver, Canada; 114Amyloidosis and Myeloma Unit, Department of Hematology, Hospital Clínic of Barcelona, IDIBAPS, University of Barcelona, Barcelona, Spain; 115Genomics and Computational Biology, Queen Mary University of London, London, UK; 116Department of Pathology, West Virginia University School of Medicine, Morgantown, WV, USA; 117Department of Pathology, MD Anderson Cancer Center Madrid, Madrid, Spain; 118Center for Global Health, National Cancer Institute, Rockville, MD, USA; 119Pathology & Dermatology, University of Virginia, Charlottesville, VA, USA; 120Laboratory Oncology, All India Institute of Medical Sciences, Delhi, New Delhi, India; 121Consultation Center for Hematopathology, Institute of Pathology and Molecular Pathology, Wuppertal, Germany; 122Department of Pathology, The Radboud University Medical Center, Nijmegen, the Netherlands; 123Radiation Oncology, University Hospital Heidelberg, Heidelberg, Germany; 124Hematology, Cellular Therapy, Hemostaseology, University of Leipzig, Leipzig, Germany; 125Hematology and laboratory of physiopathology and treatment of hematological disorders, Necker Hospital, APHP, INSERM U1163, Imagine, Paris University, Paris, France; 126Neuro-oncology, Hôpital Universitaire Pitié Salpêtrière, Paris, France; 127Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN, USA; 128Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, PR China; 129Division of Oncology, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; 130Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, USA; 131Department of Pathology and Molecular Diagnostics, Nagoya City University, Nagoya, Japan; 132Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA; 133Department of Hematology and Rheumatology, Kagoshima University, Kagoshima, Japan; 134Department of Hematology, National Cancer Center Hospital, Tsukiji, Chuo-ku, Tokyo, Japan; 135Departments of Dermatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan; 136Department of Pathology, Seoul National University College of Medicine, Seoul, South Korea; 137Department of Pathology and Laboratory Medicine, Brown University Alpert School of Medicine, Providence, RI, USA; 138Department of Pathology, Japanese Red Cross Narita Hospital, Narita, Chiba, Japan; 139Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK; 140Division of Hematology, Department of Medicine, Keio University School of Medicine, Tokyo, Japan; 141Department of Pathology and Molecular Diagnostics, Aichi Cancer Center Hospital, Nagoya, Japan; 142Department of Hematology, Amsterdam University Medical Centers, Amsterdam, The Netherlands; 143Department of Pathology, Seoul National University SNU SMG Boramae Hospital, Seoul, South Korea; 144Department of Pediatric Hematology and Oncology, Hannover Medical School, Hannover, Germany; 145Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre - UHN, Toronto, Canada; 146Faculty of Medicine, University of Iceland, Reykjavik, Iceland; 147Institute of Cell Biology (Cancer Research), University of Duisburg-Essen, Essen, Germany; 148Department of Pathology, University of Ankara, School of Medicine, Ankara, Turkey; 149Department of Medicine, University of Hong Kong, Queen Mary Hospital, Hong Kong; 150Medical Oncology, Dana Farber Cancer Institute, Boston, MA, USA; 151Departement of Pathology, Institut Universitaire du cancer Toulouse Oncopole, Toulouse, France; 152Department of Hematology, Rennes University Hospital, Rennes, France; 153Department of Medicine, Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA; 154Radiation Oncology, Tata Memorial Centre, Mumbai, India; 155Department of Pathology, Yale School of Medicine, New Haven, CT, USA; 156Department of Hematology, Oncology and Pneumology, University Hospital Münster, Münster, Germany; 157Pediatric Hematology/Oncology, Cell and Gene Therapy, Bambino Gesù Children’s Hospital, Rome, Italy; 158Division of Pathology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA; 159Hematology/Oncology, University of Virginia, Charlottesville, VA, USA; 160Clinical Hematology and Medical Oncology, Postgraduate Institute of Medical Education and Research, Chandigarh, India; 161Department of Cellular Pathology, University College Hospital London, London, UK; 162Hematology Oncology, Cancer Institute Hospital of Japanese Foundation of Cancer Research, Tokyo, Japan; 163Department of Pathology, University Medical Centre Mannheim, Mannheim, Germany; 164Infections and Immunoepidemiology Branch, DCEG, National Cancer Institute, Rockville, MD, USA; 165Department of Pediatric Hematology and Oncology, 2nd Faculty of Medicine, University Hospital Motol, Prague, the Czech Republic; 166Department of Anatomical Pathology, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, South Africa; 167Laboratory of Pathology, National Cancer Institute/NIH, Bethesda, MD, USA; 168Department of Pathology, University of Utah, Salt Lake City, UT, USA; 169Department of Pathology, Kurume University, School of Medicine, Kurume, Japan; 170Department of Pathology, AP-HP, Hôpital Necker-Enfants Malades and Robert Debré, Université de Paris, Paris, France; 171Department of Pathology, Hospital Universitario de Toledo, Toledo, Spain; 172Department of Pathology, Saitama Medical University, Saitama Medical Center, Kawagoe, Japan; 173Department of Pediatrics, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan; 174Centre d’Immunologie de Marseille-Luminy, Inserm CNRS AMU, Marseille, France; 175Clinical Research Center, National Hospital Organization Nagoya Medical Center, Nagoya, Japan; 176Department of Neurosurgery, Kyorin University Faculty of Medicine, Tokyo, Japan; 177Department of Pathology, Tokai University School of Medicine, Isehara, Japan; 178Clinical Research, Saitama Children’s Medical Center, Saitama, Japan; 179Department of Histopathology, Royal Brompton & Harefield NHS Foundation Trust, London, UK; 180Department of Pathology, Hautepierre, University Hospital Strasbourg, Strasbourg, France; 181Department of Pathology, University of California San Francisco, San Francisco, CA, USA; 182Department of Pathology, University of Yamanashi, Chuo, Yamanashi, Japan; 183Department of Pathology, AP-HP, Hopitaux universitaires Henri-Mondor, Creteil, France; 184Hemato-Oncology, Clinica Universidad de Navarra, Pamplona, Spain; 185Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden; 186Lab Haematology / Cytogenetics & Molecular Genetics, Tata Medical center, Kolkata, India; 187Pathology Unit, Dept. of Molecular Medicine, University of Pavia, Pavia, Italy; 188Pediatric Development and Rehab, Genetics Division, Legacy Health/Randall Children’s Hospital and Washington State University Elson S. Floyd College of Medicine, Portland, OR, USA; 189Department of Pathology, Cincinnati Childrens Hospital Medical Center, Cincinnati, OH, USA; 190Experimental, Diagnostic and Specialty Medicine Department, Dermatology Unit, Bologna, Italy; 191Health Sciences, University of Florence Medical School, Florence, Italy; 192Pathology and Dermatology, The Ohio State University Medical Center, Columbus, OH, USA; 193Baylor College of Medicine, Texas Children’s Cancer Center, Houston, TX, USA; 194Internal Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria; 195Department of Medicine, Northwell Health Cancer Institute, New Hyde Park, NY, USA; 196Department of Pathology, Akershus University Hospital/University of Oslo, Lørenskog, Norway; 197Departamento de Diagnostico Laboratorial, IPOLFG—Servico de Anatomia Patologica, Lisboa, Portugal; 198Immunology and Microbiology, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA; 199Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; 200Department of Hematology, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland; 201Department of anatomic pathology and histology, Fondazione Policlinico universitario Agostino Gemelli-IRCCS, Rome, Italy; 202Department of Haematology, Plymouth University Medical School, Plymouth, UK; 203Department of Pathology and Laboratory Diagnostics, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland; 204Haematopathology, IRCCS Azienda Ospedaliero-Universitaria, Bologna, Italy; 205Paediatric Haematology and Oncology, Tata Translational Cancer Research Centre, Kolkata, India; 206Department of Hematology, University of Tsukuba, Ibaraki, Japan; 207Department of Dermatology, Asklepios Klinik St. Georg, Hamburg, Germany; 208Pathology and Dermatology, Hospital Obrero Nro 1 CNS, Hospital General, La Paz, Spain; 209Dept of Pathology and Dermatology, Wake Forest University, School of Medicine Medical Center Boulevard, Winston-Salem, NC, USA; 210Department of Health Sciences, Division of Histopathology and Molecular Diagnostics, University of Florence School of Human Health Sciences, Firenze, Italy; 211Department of Pathology, Okayama University Graduate School of Health Sciences, Okayama, Japan; 212Pathology and Laboratory Medicine, Phoenix Children’s Hospital, Phoenix, AZ, USA; 213Molecular Pathology Unit, Medical Faculty, Porto University, Porto, Portugal; 214Department of Hematology, Veneto Institute of Molecular Medicine, University of Padua, Padova, Italy; 215Department of Medicine, Division of Hematology, Stanford University, Palo Alto, CA, USA; 216Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 217Medicine and Quality Health Sciences, Mayo Clinic, Rochester, MN, USA; 218Department of Neuro-Oncology, University of Turin and City of Health and Science Hospital, Turin, Italy; 219Centre for Research and Technology Hellas, Institute of Applied Biosciences, Thessaloniki, Greece; 220Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada; 221Comprehensive Cancer Center Ulm (CCCU), Ulm University, Ulm, Germany; 222Department of Pathology, Chulalongkorn University, Bangkok, Thailand; 223Division of Pathology, Cancer Institute, Japanese Foundation for Cancer Research, Tokyo, Japan; 224Department of Pathology, Policlinico S.Orsola-Malpighi, Bologna, Italy; 225Pathology, Biomedical and Sciences, Saitama Medical Center, Saitama Medical University, Kawagoe, Japan; 226Hematopathology Laboratory, Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Navi Mumbai, India; 227Department of Medicine and Surgery, Institute of Hematology and Center for Hemato-Oncology Research, Perugia, Italy; 228Allergic Disease Research Center, Chutoen General Medical Center, Kakegawa, Japan; 229Hematology and Cell Therapy, CHU de Clermont-Ferrand, Clermont-Ferrand, France; 230Department of Medicine, Harvard Medical School, Boston, MA, USA; 231Hematology and Medical Oncology, Universitätsmedizin Göttingen, Göttingen, Germany; 232Department of Hematology, International Medical Center, Saitama Medical University, Hidaka, Japan; 233Myeloma Center, UAMS Winthrop P. Rockefeller Cancer Institute, Little Rock, AR, USA; 234Department of Haematology, Little Flower Hospital and Research Centre, Angamaly, India; 235Department of Dermatology, Leiden University Medical Center, Leiden, The Netherlands; 236Department of Pathology, Medipath and American Hospital of Paris, Paris, France; 237Division of Hematology Oncology, Indiana University, Indianapolis, IN, USA; 238Lymphoma and Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA; 239Department of Pathology, University of California San Diego, San Diego, CA, USA; 240Department of Pathology, Fourth Military Medical University, Xi’an, PR China; 241Personalized Cancer Immunotherapy, Mie University Graduate School of Medicine, Tsu, Japan; 242Medical Department III, Ludwig-Maximilians-University (LMU) Hospital, Munich, Germany; 243Medical Oncology, Dana-Farber Cancer Institute and Havard Medical School, Boston, MA, USA; 244School of Basic and Medical Biosciences KCL, St. John’s Institute of Dermatology, London, UK; 245Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 246Department of Hematological Malignancies, Mie University Graduate School of Medicine, Tsu, Japan; 247Anatomic Pathology, Kyushu University, Fukuoka, Japan; 248Department of Pathology, Kobe City Medical Center General Hospital, Kobe, Japan; 249Department of Hematology, Peking University Peoples’ Hospital, Peking Institute of Hematology, Beijing, PR China; 250Department of Pathology and Laboratory Medicine, University of Wisconsin, Madison, WI, USA; 251Advanced Diagnosis, Nagoya Medical Center, Nagoya, Japan; 252Department of Pathology, Peking University Shenzhen Hospital, Shenzhen, PR China; 253Institute of Liver Studies, King’s College Hospital, London, UK; 254National Center of Translational Medicine, Institute of Hematology, Shanghai, PR China.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/19/2023
A Correction to this paper has been published: 10.1038/s41375-023-01962-5
Contributor Information
Ming-Qing Du, Email: mqd20@cam.ac.uk.
Judith Ferry, Email: JFERRY@mgh.harvard.edu.
German Ott, Email: German.Ott@rbk.de.
Reiner Siebert, Email: reiner.siebert@uni-ulm.de.
References
- 1.Jaffe ES, Harris N, Stein H, Vardiman JW (Eds.): World Health Organization classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 3rd ed. Lyon: IARC; 2001.
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92. [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, et al. (Eds.): World Health Organization classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC 2008.
- 4.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (Eds.): World Health Organization classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC; 2017.
- 5.Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, Srkalovic G, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646–57. doi: 10.1182/blood-2016-10-746933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Medeiros LJ. Castleman Disease. Surg Pathol Clin. 2019;12:849–63. doi: 10.1016/j.path.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura MF, Nishimura Y, Nishikori A, Maekawa Y, Maehama K, Yoshino T, et al. Clinical and pathological characteristics of hyaline-vascular type unicentric castleman disease: a 20-year retrospective analysis. Diagnostics. 2021;11. [DOI] [PMC free article] [PubMed]
- 8.Uldrick TS, Polizzotto MN, Aleman K, O’Mahony D, Wyvill KM, Wang V, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117:6977–86. doi: 10.1182/blood-2010-11-317610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gérard L, Bérezné A, Galicier L, Meignin V, Obadia M, De Castro N, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25:3350–6. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 10.Thol F. ALL is not the same in the era of genetics. Blood. 2021;138:915–6. doi: 10.1182/blood.2021011934. [DOI] [PubMed] [Google Scholar]
- 11.Panagopoulos I, Micci F, Thorsen J, Haugom L, Tierens A, Ulvmoen A, et al. A novel TCF3-HLF fusion transcript in acute lymphoblastic leukemia with a t(17;19)(q22;p13) Cancer Genet. 2012;205:669–72. doi: 10.1016/j.cancergen.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Fischer U, Forster M, Rinaldi A, Risch T, Sungalee S, Warnatz HJ, et al. Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet. 2015;47:1020–9. doi: 10.1038/ng.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129:3352–61. doi: 10.1182/blood-2016-12-758979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen IM, et al. High frequency and poor outcome of philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35:394–401. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells J, Jain N, Konopleva M. Philadelphia chromosome-like acute lymphoblastic leukemia: progress in a new cancer subtype. Clin Adv Hematol Oncol. 2017;15:554–61. [PubMed] [Google Scholar]
- 16.Cario G, Leoni V, Conter V, Baruchel A, Schrappe M, Biondi A. BCR-ABL1-like acute lymphoblastic leukemia in childhood and targeted therapy. Haematologica. 2020;105:2200–4. doi: 10.3324/haematol.2018.207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanasi I, Ba I, Sirvent N, Braun T, Cuccuini W, Ballerini P, et al. Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134:1351–5. doi: 10.1182/blood.2019001244. [DOI] [PubMed] [Google Scholar]
- 18.Lilljebjörn H, Henningsson R, Hyrenius-Wittsten A, Olsson L, Orsmark-Pietras C, von Palffy S, et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun. 2016;7:11790. doi: 10.1038/ncomms11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda T, Tsuzuki S, Kawazu M, Hayakawa F, Kojima S, Ueno T, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat Genet. 2016;48:569–74. doi: 10.1038/ng.3535. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Churchman M, Roberts K, Li Y, Liu Y, Harvey RC, et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat Commun. 2016;7:13331. doi: 10.1038/ncomms13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirabayashi S, Ohki K, Nakabayashi K, Ichikawa H, Momozawa Y, Okamura K, et al. ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica. 2017;102:118–29. doi: 10.3324/haematol.2016.151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hormann FM, Hoogkamer AQ, Beverloo HB, Boeree A, Dingjan I, Wattel MM, et al. NUTM1 is a recurrent fusion gene partner in B-cell precursor acute lymphoblastic leukemia associated with increased expression of genes on chromosome band 10p12.31-12.2. Haematologica. 2019;104:e455–e9. doi: 10.3324/haematol.2018.206961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagener R, López C, Kleinheinz K, Bausinger J, Aukema SM, Nagel I, et al. IG-MYC (+) neoplasms with precursor B-cell phenotype are molecularly distinct from Burkitt lymphomas. Blood. 2018;132:2280–5. doi: 10.1182/blood-2018-03-842088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobucci I, Kimura S, Mullighan CG, Biologic and therapeutic implications of genomic alterations in acute lymphoblastic leukemia. J Clin Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 25.Gu Z, Churchman ML, Roberts KG, Moore I, Zhou X, Nakitandwe J, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet. 2019;51:296–307. doi: 10.1038/s41588-018-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passet M, Boissel N, Sigaux F, Saillard C, Bargetzi M, Ba I, et al. PAX5 P80R mutation identifies a novel subtype of B-cell precursor acute lymphoblastic leukemia with favorable outcome. Blood. 2019;133:280–4. doi: 10.1182/blood-2018-10-882142. [DOI] [PubMed] [Google Scholar]
- 27.Novakova M, Zaliova M, Fiser K, Vakrmanova B, Slamova L, Musilova A, et al. DUX4r, ZNF384r and PAX5-P80R mutated B-cell precursor acute lymphoblastic leukemia frequently undergo monocytic switch. Haematologica. 2021;106:2066–75. doi: 10.3324/haematol.2020.250423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schinnerl D, Mejstrikova E, Schumich A, Zaliova M, Fortschegger K, Nebral K, et al. CD371 cell surface expression: a unique feature of DUX4-rearranged acute lymphoblastic leukemia. Haematologica. 2019;104:e352–e5. doi: 10.3324/haematol.2018.214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawstron AC, Shanafelt T, Lanasa MC, Landgren O, Hanson C, Orfao A, et al. Different biology and clinical outcome according to the absolute numbers of clonal B-cells in monoclonal B-cell lymphocytosis (MBL) Cytom B Clin Cytom. 2010;78(Suppl 1):S19–23. doi: 10.1002/cyto.b.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130:325–32. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 31.Shanafelt TD, Kay NE, Rabe KG, Call TG, Zent CS, Maddocks K, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27:3959–63. doi: 10.1200/JCO.2008.21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xochelli A, Oscier D, Stamatopoulos K. Clonal B-cell lymphocytosis of marginal zone origin. Best Pr Res Clin Haematol. 2017;30:77–83. doi: 10.1016/j.beha.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Shanafelt TD, Kay NE, Parikh SA, Achenbach SJ, Lesnick CE, Hanson CA, et al. Risk of serious infection among individuals with and without low count monoclonal B-cell lymphocytosis (MBL) Leukemia. 2021;35:239–44. doi: 10.1038/s41375-020-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker JA, Parikh SA, Shanafelt TD, Kay NE, Kennedy RB, Grill DE, et al. The humoral immune response to high-dose influenza vaccine in persons with monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL) Vaccine. 2021;39:1122–30. doi: 10.1016/j.vaccine.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira J, Rabe KG, Cerhan JR, Kay NE, Wilson JW, Call TG, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27:136–41. doi: 10.1038/leu.2012.187. [DOI] [PubMed] [Google Scholar]
- 36.Muchtar E, Koehler AB, Johnson MJ, Rabe KG, Ding W, Call TG, et al. Humoral and cellular immune responses to recombinant herpes zoster vaccine in patients with chronic lymphocytic leukemia and monoclonal B cell lymphocytosis. Am J Hematol. 2022;97:90–8. doi: 10.1002/ajh.26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criado I, Rodríguez-Caballero A, Gutiérrez ML, Pedreira CE, Alcoceba M, Nieto W, et al. Low-count monoclonal B-cell lymphocytosis persists after seven years of follow up and is associated with a poorer outcome. Haematologica. 2018;103:1198–208. doi: 10.3324/haematol.2017.183954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawstron AC, Kreuzer KA, Soosapilla A, Spacek M, Stehlikova O, Gambell P, et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytom B Clin Cytom. 2018;94:121–8. doi: 10.1002/cyto.b.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol. 2019;16:684–701. doi: 10.1038/s41571-019-0239-8. [DOI] [PubMed] [Google Scholar]
- 40.Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679–705. doi: 10.1002/ajh.26367. [DOI] [PubMed] [Google Scholar]
- 41.Jaramillo S, Agathangelidis A, Schneider C, Bahlo J, Robrecht S, Tausch E, et al. Prognostic impact of prevalent chronic lymphocytic leukemia stereotyped subsets: analysis within prospective clinical trials of the German CLL Study Group (GCLLSG) Haematologica. 2020;105:2598–607. doi: 10.3324/haematol.2019.231027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–90. [DOI] [PubMed]
- 43.Condoluci A, Terzi di Bergamo L, Langerbeins P, Hoechstetter MA, Herling CD, De Paoli L, et al. International prognostic score for asymptomatic early-stage chronic lymphocytic leukemia. Blood. 2020;135:1859–69. doi: 10.1182/blood.2019003453. [DOI] [PubMed] [Google Scholar]
- 44.Enno A, Catovsky D, O’Brien M, Cherchi M, Kumaran TO, Galton DA. ‘Prolymphocytoid’ transformation of chronic lymphocytic leukaemia. Br J Haematol. 1979;41:9–18. doi: 10.1111/j.1365-2141.1979.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 45.Melo JV, Catovsky D, Galton DA. The relationship between chronic lymphocytic leukaemia and prolymphocytic leukaemia. II. Patterns of evolution of ‘prolymphocytoid’ transformation. Br J Haematol. 1986;64:77–86. doi: 10.1111/j.1365-2141.1986.tb07575.x. [DOI] [PubMed] [Google Scholar]
- 46.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of chronic (mature) B and T lymphoid leukaemias. French-American-British (FAB) Cooperative Group. J Clin Pathol. 1989;42:567–84. doi: 10.1136/jcp.42.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiacci E, Pettirossi V, Schiavoni G, Falini B. Genomics of Hairy Cell Leukemia. J Clin Oncol. 2017;35:1002–10. doi: 10.1200/JCO.2016.71.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matutes E, Wotherspoon A, Catovsky D. The variant form of hairy-cell leukaemia. Best Pr Res Clin Haematol. 2003;16:41–56. doi: 10.1016/s1521-6926(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 50.Robak T. Current treatment options in hairy cell leukemia and hairy cell leukemia variant. Cancer Treat Rev. 2006;32:365–76. doi: 10.1016/j.ctrv.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Robak T. Hairy-cell leukemia variant: recent view on diagnosis, biology and treatment. Cancer Treat Rev. 2011;37:3–10. doi: 10.1016/j.ctrv.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4:547–59. doi: 10.1016/0145-2126(80)90066-1. [DOI] [PubMed] [Google Scholar]
- 53.Cannon T, Mobarek D, Wegge J, Tabbara IA. Hairy cell leukemia: current concepts. Cancer Invest. 2008;26:860–5. doi: 10.1080/07357900801965034. [DOI] [PubMed] [Google Scholar]
- 54.Tran J, Gaulin C, Tallman MS. Advances in the treatment of hairy cell leukemia variant. Curr Treat Options Oncol. 2022;23:99–116. doi: 10.1007/s11864-021-00927-z. [DOI] [PubMed] [Google Scholar]
- 55.Matutes E, Wotherspoon A, Brito-Babapulle V, Catovsky D. The natural history and clinico-pathological features of the variant form of hairy cell leukemia. Leukemia. 2001;15:184–6. doi: 10.1038/sj.leu.2401999. [DOI] [PubMed] [Google Scholar]
- 56.Matutes E, Wotherspoon A, Catovsky D. Differential diagnosis in chronic lymphocytic leukaemia. Best Pr Res Clin Haematol. 2007;20:367–84. doi: 10.1016/j.beha.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Favre R, Manzoni D, Traverse-Glehen A, Verney A, Jallades L, Callet-Bauchu E, et al. Usefulness of CD200 in the differential diagnosis of SDRPL, SMZL, and HCL. Int J Lab Hematol. 2018;40:e59–e62. doi: 10.1111/ijlh.12824. [DOI] [PubMed] [Google Scholar]
- 58.Angelova EA, Medeiros LJ, Wang W, Muzzafar T, Lu X, Khoury JD, et al. Clinicopathologic and molecular features in hairy cell leukemia-variant: single institutional experience. Mod Pathol. 2018;31:1717–32. doi: 10.1038/s41379-018-0093-8. [DOI] [PubMed] [Google Scholar]
- 59.Matutes E, Martínez-Trillos A, Campo E. Hairy cell leukaemia-variant: Disease features and treatment. Best Pr Res Clin Haematol. 2015;28:253–63. doi: 10.1016/j.beha.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Varettoni M, Boveri E, Zibellini S, Tedeschi A, Candido C, Ferretti VV, et al. Clinical and molecular characteristics of lymphoplasmacytic lymphoma not associated with an IgM monoclonal protein: A multicentric study of the Rete Ematologica Lombarda (REL) network. Am J Hematol. 2019;94:1193–9. doi: 10.1002/ajh.25600. [DOI] [PubMed] [Google Scholar]
- 61.King RL, Gonsalves WI, Ansell SM, Greipp PT, Frederick LA, Viswanatha DS, et al. Lymphoplasmacytic Lymphoma With a Non-IgM Paraprotein Shows Clinical and Pathologic Heterogeneity and May Harbor MYD88 L265P Mutations. Am J Clin Pathol. 2016;145:843–51. doi: 10.1093/ajcp/aqw072. [DOI] [PubMed] [Google Scholar]
- 62.Cao X, Medeiros LJ, Xia Y, Wang X, Thomas SK, Loghavi S, et al. Clinicopathologic features and outcomes of lymphoplasmacytic lymphoma patients with monoclonal IgG or IgA paraprotein expression. Leuk Lymphoma. 2016;57:1104–13. doi: 10.3109/10428194.2015.1096357. [DOI] [PubMed] [Google Scholar]
- 63.Kang J, Hong JY, Suh C. Clinical features and survival outcomes of patients with lymphoplasmacytic lymphoma, including non-IgM type, in Korea: a single-center experience. Blood Res. 2018;53:189–97. doi: 10.5045/br.2018.53.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castillo JJ, Itchaki G, Gustine JN, Meid K, Flynn CA, Demos MG, et al. A matched case-control study comparing features, treatment and outcomes between patients with non-IgM lymphoplasmacytic lymphoma and Waldenström macroglobulinemia. Leuk Lymphoma. 2020;61:1388–94. doi: 10.1080/10428194.2020.1719100. [DOI] [PubMed] [Google Scholar]
- 65.Tursz T, Brouet JC, Flandrin G, Danon F, Clauvel JP, Seligmann M. Clinical and pathologic features of Waldenström’s macroglobulinemia in seven patients with serum monoclonal IgG or IgA. Am J Med. 1977;63:499–502. doi: 10.1016/0002-9343(77)90193-0. [DOI] [PubMed] [Google Scholar]
- 66.Hunter ZR, Xu L, Yang G, Tsakmaklis N, Vos JM, Liu X, et al. Transcriptome sequencing reveals a profile that corresponds to genomic variants in Waldenström macroglobulinemia. Blood. 2016;128:827–38. doi: 10.1182/blood-2016-03-708263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123:1637–46. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 68.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–6. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 69.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl J Med. 2012;367:826–33. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 70.Treon SP, Xu L, Guerrera ML, Jimenez C, Hunter ZR, Liu X, et al. Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J Clin Oncol. 2020;38:1198–208. doi: 10.1200/JCO.19.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brynes RK, Almaguer PD, Leathery KE, McCourty A, Arber DA, Medeiros LJ, et al. Numerical cytogenetic abnormalities of chromosomes 3, 7, and 12 in marginal zone B-cell lymphomas. Mod Pathol. 1996;9:995–1000. [PubMed] [Google Scholar]
- 72.Krijgsman O, Gonzalez P, Ponz OB, Roemer MG, Slot S, Broeks A, et al. Dissecting the gray zone between follicular lymphoma and marginal zone lymphoma using morphological and genetic features. Haematologica. 2013;98:1921–9. doi: 10.3324/haematol.2013.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aamot HV, Micci F, Holte H, Delabie J, Heim S. G-banding and molecular cytogenetic analyses of marginal zone lymphoma. Br J Haematol. 2005;130:890–901. doi: 10.1111/j.1365-2141.2005.05706.x. [DOI] [PubMed] [Google Scholar]
- 74.Rinaldi A, Mian M, Chigrinova E, Arcaini L, Bhagat G, Novak U, et al. Genome-wide DNA profiling of marginal zone lymphomas identifies subtype-specific lesions with an impact on the clinical outcome. Blood. 2011;117:1595–604. doi: 10.1182/blood-2010-01-264275. [DOI] [PubMed] [Google Scholar]
- 75.van den Brand M, van Krieken JH. Recognizing nodal marginal zone lymphoma: recent advances and pitfalls. A systematic review. Haematologica. 2013;98:1003–13. doi: 10.3324/haematol.2012.083386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pillonel V, Juskevicius D, Ng CKY, Bodmer A, Zettl A, Jucker D, et al. High-throughput sequencing of nodal marginal zone lymphomas identifies recurrent BRAF mutations. Leukemia. 2018;32:2412–26. doi: 10.1038/s41375-018-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Callet-Bauchu E, Baseggio L, Felman P, Traverse-Glehen A, Berger F, Morel D, et al. Cytogenetic analysis delineates a spectrum of chromosomal changes that can distinguish non-MALT marginal zone B-cell lymphomas among mature B-cell entities: a description of 103 cases. Leukemia. 2005;19:1818–23. doi: 10.1038/sj.leu.2403909. [DOI] [PubMed] [Google Scholar]
- 78.Chanudet E, Ye H, Ferry J, Bacon CM, Adam P, Müller-Hermelink HK, et al. A20 deletion is associated with copy number gain at the TNFA/B/C locus and occurs preferentially in translocation-negative MALT lymphoma of the ocular adnexa and salivary glands. J Pathol. 2009;217:420–30. doi: 10.1002/path.2466. [DOI] [PubMed] [Google Scholar]
- 79.Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–8. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- 80.Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–6. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- 81.Ye H, Dogan A, Karran L, Willis TG, Chen L, Wlodarska I, et al. BCL10 expression in normal and neoplastic lymphoid tissue. Nuclear localization in MALT lymphoma. Am J Pathol. 2000;157:1147–54. doi: 10.1016/S0002-9440(10)64630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye H, Gong L, Liu H, Hamoudi RA, Shirali S, Ho L, et al. MALT lymphoma with t(14;18)(q32;q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression. J Pathol. 2005;205:293–301. doi: 10.1002/path.1715. [DOI] [PubMed] [Google Scholar]
- 83.Goatly A, Bacon CM, Nakamura S, Ye H, Kim I, Brown PJ, et al. FOXP1 abnormalities in lymphoma: translocation breakpoint mapping reveals insights into deregulated transcriptional control. Mod Pathol. 2008;21:902–11. doi: 10.1038/modpathol.2008.74. [DOI] [PubMed] [Google Scholar]
- 84.van den Brand M, Rijntjes J, Hebeda KM, Menting L, Bregitha CV, Stevens WB, et al. Recurrent mutations in genes involved in nuclear factor-κB signalling in nodal marginal zone lymphoma-diagnostic and therapeutic implications. Histopathology. 2017;70:174–84. doi: 10.1111/his.13015. [DOI] [PubMed] [Google Scholar]
- 85.Spina V, Khiabanian H, Messina M, Monti S, Cascione L, Bruscaggin A, et al. The genetics of nodal marginal zone lymphoma. Blood. 2016;128:1362–73. doi: 10.1182/blood-2016-02-696757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vela V, Juskevicius D, Dirnhofer S, Menter T, Tzankov A. Mutational landscape of marginal zone B-cell lymphomas of various origin: organotypic alterations and diagnostic potential for assignment of organ origin. Virchows Arch. 2022;480:403–13. doi: 10.1007/s00428-021-03186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Honma K, Tsuzuki S, Nakagawa M, Tagawa H, Nakamura S, Morishima Y, et al. TNFAIP3/A20 functions as a novel tumour suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 2009;114:2467–75. doi: 10.1182/blood-2008-12-194852. [DOI] [PubMed] [Google Scholar]
- 88.Moody S, Escudero-Ibarz L, Wang M, Clipson A, Ochoa Ruiz E, Dunn-Walters D, et al. Significant association between TNFAIP3 inactivation and biased immunoglobulin heavy chain variable region 4-34 usage in mucosa-associated lymphoid tissue lymphoma. J Pathol. 2017;243:3–8. doi: 10.1002/path.4933. [DOI] [PubMed] [Google Scholar]
- 89.Moody S, Thompson JS, Chuang SS, Liu H, Raderer M, Vassiliou G, et al. Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites. Haematologica. 2018;103:1329–36. doi: 10.3324/haematol.2018.191601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korona B, Korona D, Zhao W, Wotherspoon AC, Du MQ. GPR34 activation potentially bridges lymphoepithelial lesions to genesis of salivary gland MALT lymphoma. Blood. 2022;139:2186–97. doi: 10.1182/blood.2020010495. [DOI] [PubMed] [Google Scholar]
- 91.Wu F, Watanabe N, Tzioni MM, Akarca A, Zhang C, Li Y, et al. Thyroid MALT lymphoma: self-harm to gain potential T-cell help. Leukemia. 2021;35:3497–508. doi: 10.1038/s41375-021-01289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maurus K, Appenzeller S, Roth S, Kuper J, Rost S, Meierjohann S, et al. Panel sequencing shows recurrent genetic FAS alterations in primary cutaneous marginal zone lymphoma. J Invest Dermatol. 2018;138:1573–81. doi: 10.1016/j.jid.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 93.Swerdlow SH, Kuzu I, Dogan A, Dirnhofer S, Chan JK, Sander B, et al. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. 2016;468:259–75. doi: 10.1007/s00428-015-1858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters! Mod Pathol. 2021;34:1651–7. doi: 10.1038/s41379-021-00825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Metter GE, Nathwani BN, Burke JS, Winberg CD, Mann RB, Barcos M, et al. Morphological subclassification of follicular lymphoma: variability of diagnoses among hematopathologists, a collaborative study between the Repository Center and Pathology Panel for Lymphoma Clinical Studies. J Clin Oncol. 1985;3:25–38. doi: 10.1200/JCO.1985.3.1.25. [DOI] [PubMed] [Google Scholar]
- 96.Chau I, Jones R, Cunningham D, Wotherspoon A, Maisey N, Norman AR, et al. Outcome of follicular lymphoma grade 3: is anthracycline necessary as front-line therapy? Br J Cancer. 2003;89:36–42. doi: 10.1038/sj.bjc.6601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pham RN, Gooley TA, Keeney GE, Press OW, Pagel JM, Greisman HA, et al. The impact of histologic grade on the outcome of high-dose therapy and autologous stem cell transplantation for follicular lymphoma. Bone Marrow Transpl. 2007;40:1039–44. doi: 10.1038/sj.bmt.1705864. [DOI] [PubMed] [Google Scholar]
- 98.Wahlin BE, Yri OE, Kimby E, Holte H, Delabie J, Smeland EB, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156:225–33. doi: 10.1111/j.1365-2141.2011.08942.x. [DOI] [PubMed] [Google Scholar]
- 99.Rimsza LM, Li H, Braziel RM, Spier CM, Persky DO, Dunlap J, et al. Impact of histological grading on survival in the SWOG S0016 follicular lymphoma cohort. Haematologica. 2018;103:e151–e3. doi: 10.3324/haematol.2017.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lozanski G, Pennell M, Shana’ah A, Zhao W, Gewirtz A, Racke F, et al. Inter-reader variability in follicular lymphoma grading: Conventional and digital reading. J Pathol Inf. 2013;4:30. doi: 10.4103/2153-3539.120747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khieu ML, Broadwater DR, Aden JK, Coviello JM, Lynch DT, Hall JM. The Utility of Phosphohistone H3 (PHH3) in Follicular Lymphoma Grading: A Comparative Study With Ki-67 and H&E Mitotic Count. Am J Clin Pathol. 2019;151:542–50. doi: 10.1093/ajcp/aqz003. [DOI] [PubMed] [Google Scholar]
- 102.Kroft SH. Stratification of follicular lymphoma: time for a paradigm shift? Am J Clin Pathol. 2019;151:539–41. doi: 10.1093/ajcp/aqz008. [DOI] [PubMed] [Google Scholar]
- 103.Koch K, Hoster E, Ziepert M, Unterhalt M, Ott G, Rosenwald A, et al. Clinical, pathological and genetic features of follicular lymphoma grade 3A: a joint analysis of the German low-grade and high-grade lymphoma study groups GLSG and DSHNHL. Ann Oncol. 2016;27:1323–9. doi: 10.1093/annonc/mdw185. [DOI] [PubMed] [Google Scholar]
- 104.Nann D, Ramis-Zaldivar JE, Müller I, Gonzalez-Farre B, Schmidt J, Egan C, et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020;4:5652–65. doi: 10.1182/bloodadvances.2020002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siddiqi IN, Friedman J, Barry-Holson KQ, Ma C, Thodima V, Kang I, et al. Characterization of a variant of t(14;18) negative nodal diffuse follicular lymphoma with CD23 expression, 1p36/TNFRSF14 abnormalities, and STAT6 mutations. Mod Pathol. 2016;29:570–81. doi: 10.1038/modpathol.2016.51. [DOI] [PubMed] [Google Scholar]
- 106.Laurent C, Adélaïde J, Guille A, Tesson B, Gat E, Evrard S, et al. High-grade follicular lymphomas exhibit clinicopathologic, cytogenetic, and molecular diversity extending beyond Grades 3A and 3B. Am J Surg Pathol. 2021;45:1324–36. doi: 10.1097/PAS.0000000000001726. [DOI] [PubMed] [Google Scholar]
- 107.Salaverria I, Philipp C, Oschlies I, Kohler CW, Kreuz M, Szczepanowski M, et al. Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood. 2011;118:139–47. doi: 10.1182/blood-2011-01-330795. [DOI] [PubMed] [Google Scholar]
- 108.Katzenberger T, Kalla J, Leich E, Stöcklein H, Hartmann E, Barnickel S, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113:1053–61. doi: 10.1182/blood-2008-07-168682. [DOI] [PubMed] [Google Scholar]
- 109.Zamò A, Pischimarov J, Horn H, Ott G, Rosenwald A, Leich E. The exomic landscape of t(14;18)-negative diffuse follicular lymphoma with 1p36 deletion. Br J Haematol. 2018;180:391–4. doi: 10.1111/bjh.15041. [DOI] [PubMed] [Google Scholar]
- 110.Oishi N, Montes-Moreno S, Feldman AL. In situ neoplasia in lymph node pathology. Semin Diagn Pathol. 2018;35:76–83. doi: 10.1053/j.semdp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Li JY, Gaillard F, Moreau A, Harousseau JL, Laboisse C, Milpied N, et al. Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol. 1999;154:1449–52. doi: 10.1016/S0002-9440(10)65399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vandenberghe E, De Wolf-Peeters C, van den Oord J, Wlodarska I, Delabie J, Stul M, et al. Translocation (11;14): a cytogenetic anomaly associated with B-cell lymphomas of non-follicle centre cell lineage. J Pathol. 1991;163:13–8. doi: 10.1002/path.1711630104. [DOI] [PubMed] [Google Scholar]
- 113.Royo C, Salaverria I, Hartmann EM, Rosenwald A, Campo E, Beà S. The complex landscape of genetic alterations in mantle cell lymphoma. Semin Cancer Biol. 2011;21:322–34. doi: 10.1016/j.semcancer.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 114.Fuster C, Martín-Garcia D, Balagué O, Navarro A, Nadeu F, Costa D, et al. Cryptic insertions of the immunoglobulin light chain enhancer region near CCND1 in t(11;14)-negative mantle cell lymphoma. Haematologica. 2020;105:e408–e11. doi: 10.3324/haematol.2019.237073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peterson JF, Baughn LB, Ketterling RP, Pitel BA, Smoley SA, Vasmatzis G, et al. Characterization of a cryptic IGH/CCND1 rearrangement in a case of mantle cell lymphoma with negative CCND1 FISH studies. Blood Adv. 2019;3:1298–302. doi: 10.1182/bloodadvances.2019031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Polonis K, Schultz MJ, Olteanu H, Smadbeck JB, Johnson SH, Vasmatzis G, et al. Detection of cryptic CCND1 rearrangements in mantle cell lymphoma by next generation sequencing. Ann Diagn Pathol. 2020;46:151533. doi: 10.1016/j.anndiagpath.2020.151533. [DOI] [PubMed] [Google Scholar]
- 117.Salaverria I, Royo C, Carvajal-Cuenca A, Clot G, Navarro A, Valera A, et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1(-) mantle cell lymphoma. Blood. 2013;121:1394–402. doi: 10.1182/blood-2012-08-452284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, et al. Prognostic value of Ki-67 Index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the european mantle cell lymphoma network. J Clin Oncol. 2016;34:1386–94. doi: 10.1200/JCO.2015.63.8387. [DOI] [PubMed] [Google Scholar]
- 119.Aukema SM, Hoster E, Rosenwald A, Canoni D, Delfau-Larue MH, Rymkiewicz G, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood. 2018;131:417–20. doi: 10.1182/blood-2017-07-797019. [DOI] [PubMed] [Google Scholar]
- 120.Royo C, Navarro A, Clot G, Salaverria I, Giné E, Jares P, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia. 2012;26:1895–8. doi: 10.1038/leu.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Navarro A, Clot G, Royo C, Jares P, Hadzidimitriou A, Agathangelidis A, et al. Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biologic and clinical features. Cancer Res. 2012;72:5307–16. doi: 10.1158/0008-5472.CAN-12-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pouliou E, Xochelli A, Kanellis G, Stalika E, Sutton LA, Navarro A, et al. Numerous ontogenetic roads to mantle cell lymphoma: immunogenetic and immunohistochemical evidence. Am J Pathol. 2017;187:1454–8. doi: 10.1016/j.ajpath.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Orchard J, Garand R, Davis Z, Babbage G, Sahota S, Matutes E, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101:4975–81. doi: 10.1182/blood-2002-06-1864. [DOI] [PubMed] [Google Scholar]
- 124.Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:3088–95. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 125.Nadeu F, Martin-Garcia D, Clot G, Díaz-Navarro A, Duran-Ferrer M, Navarro A, et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood. 2020;136:1419–32. doi: 10.1182/blood.2020005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol. 2015;52:67–76. doi: 10.1053/j.seminhematol.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 128.Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481–94. doi: 10.1016/j.cell.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cucco F, Barrans S, Sha C, Clipson A, Crouch S, Dobson R, et al. Distinct genetic changes reveal evolutionary history and heterogeneous molecular grade of DLBCL with MYC/BCL2 double-hit. Leukemia. 2020;34:1329–41. doi: 10.1038/s41375-019-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ennishi D, Jiang A, Boyle M, Collinge B, Grande BM, Ben-Neriah S, et al. Double-hit gene expression signature defines a distinct subgroup of germinal Center B-Cell-like diffuse large B-Cell Lymphoma. J Clin Oncol. 2019;37:190–201. doi: 10.1200/JCO.18.01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse Large B Cell lymphoma with therapeutic implications. Cancer Cell. 2020;37:551–68.e14. doi: 10.1016/j.ccell.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131:2060–4. doi: 10.1182/blood-2017-12-820605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sha C, Barrans S, Cucco F, Bentley MA, Care MA, Cummin T, et al. Molecular High-Grade B-Cell lymphoma: defining a poor-risk group that requires different approaches to therapy. J Clin Oncol. 2019;37:202–12. doi: 10.1200/JCO.18.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagener R, Seufert J, Raimondi F, Bens S, Kleinheinz K, Nagel I, et al. The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood. 2019;133:962–6. doi: 10.1182/blood-2018-07-864025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J, Balagué O, Celis V, Verdu-Amoros J, et al. Burkitt-like lymphoma with 11q aberration: a germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica. 2019;104:1822–9. doi: 10.3324/haematol.2018.207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horn H, Kalmbach S, Wagener R, Staiger AM, Hüttl K, Mottok A, et al. A diagnostic approach to the identification of Burkitt-like Lymphoma with 11q aberration in aggressive B-cell lymphomas. Am J Surg Pathol. 2021;45:356–64. doi: 10.1097/PAS.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 137.Riemersma SA, Jordanova ES, Schop RF, Philippo K, Looijenga LH, Schuuring E, et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood. 2000;96:3569–77. [PubMed] [Google Scholar]
- 138.King RL, Goodlad JR, Calaminici M, Dotlic S, Montes-Moreno S, Oschlies I, et al. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020;476:647–65. doi: 10.1007/s00428-019-02698-3. [DOI] [PubMed] [Google Scholar]
- 139.Alame M, Cornillot E, Cacheux V, Rigau V, Costes-Martineau V, Lacheretz-Szablewski V, et al. The immune contexture of primary central nervous system diffuse large B cell lymphoma associates with patient survival and specific cell signaling. Theranostics. 2021;11:3565–79. doi: 10.7150/thno.54343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.WHO-Classification-of-Tumours-Editorial-Board, editor. Thoracic Tumours, WHO classification of tumours series. 5th ed. Lyon: IRAC; 2021.
- 141.Alexanian S, Said J, Lones M, Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. 2013;37:241–9. doi: 10.1097/PAS.0b013e318267fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kubota T, Sasaki Y, Shiozawa E, Takimoto M, Hishima T, Chong JMAge. and CD20 expression are significant prognostic factors in human herpes virus-8-negative effusion-based lymphoma. Am J Surg Pathol. 2018;42:1607–16. doi: 10.1097/PAS.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 143.Sarkozy C, Hung SS, Chavez EA, Duns G, Takata K, Chong LC, et al. Mutational landscape of gray zone lymphoma. Blood. 2021;137:1765–76. doi: 10.1182/blood.2020007507. [DOI] [PubMed] [Google Scholar]
- 144.Collinge B; Hilton L, Wong J, Ben-Neriah S, Rushton CK, Slack GW, et al. Characterization of the genetic landscape of high-grade B-cell lymphoma, NOS – an LLMPP project. Hematol Oncol;. 2021. 157-9.
- 145.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 146.Bellan C, Lazzi S, Hummel M, Palummo N, de Santi M, Amato T, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–6. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 147.Abate F, Ambrosio MR, Mundo L, Laginestra MA, Fuligni F, Rossi M, et al. Distinct viral and mutational spectrum of Endemic Burkitt Lymphoma. PLoS Pathog. 2015;11:e1005158. doi: 10.1371/journal.ppat.1005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaymaz Y, Oduor CI, Yu H, Otieno JA, Ong’echa JM, Moormann AM, et al. Comprehensive transcriptome and mutational profiling of Endemic Burkitt Lymphoma Reveals EBV Type-Specific Differences. Mol Cancer Res. 2017;15:563–76. doi: 10.1158/1541-7786.MCR-16-0305-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB, et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019;133:1313–24. doi: 10.1182/blood-2018-09-871418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Richter J, John K, Staiger AM, Rosenwald A, Kurz K, Michgehl U, et al. Epstein-Barr virus status of sporadic Burkitt lymphoma is associated with patient age and mutational features. Br J Haematol. 2022;196:681–9. doi: 10.1111/bjh.17874. [DOI] [PubMed] [Google Scholar]
- 151.Leoncini L. Epstein-Barr virus positivity as a defining pathogenetic feature of Burkitt lymphoma subtypes. Br J Haematol. 2022;196:468–70. doi: 10.1111/bjh.17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Allday MJ. How does Epstein-Barr virus (EBV) complement the activation of Myc in the pathogenesis of Burkitt’s lymphoma? Semin Cancer Biol. 2009;19:366–76. doi: 10.1016/j.semcancer.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fitzsimmons L, Boyce AJ, Wei W, Chang C, Croom-Carter D, Tierney RJ, et al. Coordinated repression of BIM and PUMA by Epstein-Barr virus latent genes maintains the survival of Burkitt lymphoma cells. Cell Death Differ. 2018;25:241–54. doi: 10.1038/cdd.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Panea RI, Love CL, Shingleton JR, Reddy A, Bailey JA, Moormann AM, et al. The whole-genome landscape of Burkitt lymphoma subtypes. Blood. 2019;134:1598–607. doi: 10.1182/blood.2019001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Greenough A, Dave SS. New clues to the molecular pathogenesis of Burkitt lymphoma revealed through next-generation sequencing. Curr Opin Hematol. 2014;21:326–32. doi: 10.1097/MOH.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 156.Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004;28:1401–16. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- 157.Diaz S, Higa HH, Hayes BK, Varki A. O-acetylation and de-O-acetylation of sialic acids. 7- and 9-o-acetylation of alpha 2,6-linked sialic acids on endogenous N-linked glycans in rat liver Golgi vesicles. J Biol Chem. 1989;264:19416–26. [PubMed] [Google Scholar]
- 158.Chadburn A, Said J, Gratzinger D, Chan JK, de Jong D, Jaffe ES, et al. HHV8/KSHV-positive lymphoproliferative disorders and the spectrum of plasmablastic and plasma cell neoplasms: 2015 SH/EAHP Workshop Report-Part 3. Am J Clin Pathol. 2017;147:171–87. doi: 10.1093/ajcp/aqw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W, Kanagal-Shamanna R, Medeiros LJ. Lymphoproliferative disorders with concurrent HHV8 and EBV infection: beyond primary effusion lymphoma and germinotropic lymphoproliferative disorder. Histopathology. 2018;72:855–61. doi: 10.1111/his.13428. [DOI] [PubMed] [Google Scholar]
- 160.Sanchez S, Veloza L, Wang L, López M, López-Guillermo A, Marginet M, et al. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B cell lymphoma: expanding the spectrum of HHV8 and EBV-associated lymphoproliferative disorders. Int J Hematol. 2020;112:734–40. doi: 10.1007/s12185-020-02897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cesarman E, Chadburn A, Rubinstein PG. KSHV/HHV8-mediated hematologic diseases. Blood. 2022;139:1013–25. doi: 10.1182/blood.2020005470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramaswami R, Lurain K, Polizzotto MN, Ekwede I, Waldon K, Steinberg SM, et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Adv. 2021;5:1660–70. doi: 10.1182/bloodadvances.2020004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Natkunam Y, Gratzinger D, Chadburn A, Goodlad JR, Chan JKC, Said J, et al. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood. 2018;132:1871–8. doi: 10.1182/blood-2018-04-842559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Natkunam Y, Gratzinger D, de Jong D, Chadburn A, Goodlad JR, Chan JK, et al. Immunodeficiency and Dysregulation: Report of the 2015 Workshop of the Society for Hematopathology/European Association for Haematopathology. Am J Clin Pathol. 2017;147:124–8. doi: 10.1093/ajcp/aqw200. [DOI] [PubMed] [Google Scholar]
- 165.Kluin-Nelemans HC, Coenen JL, Boers JE, van Imhoff GW, Rosati S. EBV-positive immunodeficiency lymphoma after alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Blood. 2008;112:1039–41. doi: 10.1182/blood-2008-02-138800. [DOI] [PubMed] [Google Scholar]
- 166.García-Barchino MJ, Sarasquete ME, Panizo C, Morscio J, Martinez A, Alcoceba M, et al. Richter transformation driven by Epstein-Barr virus reactivation during therapy-related immunosuppression in chronic lymphocytic leukaemia. J Pathol. 2018;245:61–73. doi: 10.1002/path.5060. [DOI] [PubMed] [Google Scholar]
- 167.Morscio J, Bittoun E, Volders N, Lurquin E, Wlodarska I, Gheysens O, et al. Secondary B-cell lymphoma associated with the Epstein-Barr virus in chronic lymphocytic leukemia patients. J Hematop. 2016;9:113–20. doi: 10.1007/s12308-016-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pina-Oviedo S, Miranda RN, Medeiros LJ. Cancer therapy-associated lymphoproliferative disorders: an under-recognized type of immunodeficiency-associated lymphoproliferative disorder. Am J Surg Pathol. 2018;42:116–29. doi: 10.1097/PAS.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 169.Mancuso S, Carlisi M, Santoro M, Napolitano M, Raso S, Siragusa S. Immunosenescence and lymphomagenesis. Immun Ageing. 2018;15:22. doi: 10.1186/s12979-018-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ebied A, Thanh Huan V, Makram OM, Sang TK, Ghorab M, Ngo HT, et al. The role of primary lymph node sites in survival and mortality prediction in Hodgkin lymphoma: a SEER population-based retrospective study. Cancer Med. 2018;7:953–65. doi: 10.1002/cam4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–7. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Attygalle AD, Cabeçadas J, Gaulard P, Jaffe ES, de Jong D, Ko YH, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64:171–99. doi: 10.1111/his.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Naresh KN, Menasce LP, Shenjere P, Banerjee SS. ‘Precursors’ of classical Hodgkin lymphoma in samples of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2008;141:124–6. doi: 10.1111/j.1365-2141.2008.07004.x. [DOI] [PubMed] [Google Scholar]
- 176.Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27:1346–56. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 177.Hartmann S, Eichenauer DA, Plütschow A, Mottok A, Bob R, Koch K, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG) Blood. 2013;122:4246–52. doi: 10.1182/blood-2013-07-515825. [DOI] [PubMed] [Google Scholar]
- 178.Xia D, Sayed S, Moloo Z, Gakinya SM, Mutuiri A, Wawire J, et al. Geographic variability of nodular lymphocyte-predominant Hodgkin Lymphoma. Am J Clin Pathol. 2022;157:231–43. doi: 10.1093/ajcp/aqab113. [DOI] [PubMed] [Google Scholar]
- 179.Shankar AG, Kirkwood AA, Hall GW, Hayward J, O’Hare P, Ramsay AD. Childhood and Adolescent nodular lymphocyte predominant Hodgkin lymphoma - A review of clinical outcome based on the histological variants. Br J Haematol. 2015;171:254–62. doi: 10.1111/bjh.13540. [DOI] [PubMed] [Google Scholar]
- 180.Hartmann S, Döring C, Vucic E, Chan FC, Ennishi D, Tousseyn T, et al. Array comparative genomic hybridization reveals similarities between nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. Br J Haematol. 2015;169:415–22. doi: 10.1111/bjh.13310. [DOI] [PubMed] [Google Scholar]
- 181.Schuhmacher B, Bein J, Rausch T, Benes V, Tousseyn T, Vornanen M, et al. JUNB, DUSP2, SGK1, SOCS1 and CREBBP are frequently mutated in T-cell/histiocyte-rich large B-cell lymphoma. Haematologica. 2019;104:330–7. doi: 10.3324/haematol.2018.203224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Berentsen S, Ulvestad E, Langholm R, Beiske K, Hjorth-Hansen H, Ghanima W, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91:460–6. [PubMed] [Google Scholar]
- 183.Berentsen S, Barcellini W, D’Sa S, Randen U, Tvedt THA, Fattizzo B, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136:480–8. doi: 10.1182/blood.2020005674. [DOI] [PubMed] [Google Scholar]
- 184.Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122:1114–21. doi: 10.1182/blood-2013-02-474437. [DOI] [PubMed] [Google Scholar]
- 185.Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 187.Klomjit N, Leung N, Fervenza F, Sethi S, Zand L. Rate and predictors of finding Monoclonal Gammopathy of Renal Significance (MGRS) lesions on kidney biopsy in patients with monoclonal gammopathy. J Am Soc Nephrol. 2020;31:2400–11. doi: 10.1681/ASN.2020010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, 3rd, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sykes DB, O’Connell C, Schroyens W. The TEMPI syndrome. Blood. 2020;135:1199–203. doi: 10.1182/blood.2019004216. [DOI] [PubMed] [Google Scholar]
- 190.Sykes DB, Schroyens W, O’Connell C. The TEMPI syndrome-a novel multisystem disease. N. Engl J Med. 2011;365:475–7. doi: 10.1056/NEJMc1106670. [DOI] [PubMed] [Google Scholar]
- 191.Farooq U, Choudhary S, McLeod MP, Torchia D, Rongioletti F.Romanelli P, Adenopathy and extensive skin patch over lying a Plasmacytoma (AESOP) Syndrome. J Clin Aesthet Dermatol. 2012;5:25–7. [PMC free article] [PubMed]
- 192.Rongioletti F, Romanelli P, Rebora A. Cutaneous mucinous angiomatosis as a presenting sign of bone plasmacytoma: a new case of (A)ESOP syndrome. J Am Acad Dermatol. 2006;55:909–10. doi: 10.1016/j.jaad.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 193.Boyle EM, Deshpande S, Tytarenko R, Ashby C, Wang Y, Bauer MA, et al. The molecular make up of smoldering myeloma highlights the evolutionary pathways leading to multiple myeloma. Nat Commun. 2021;12:293. doi: 10.1038/s41467-020-20524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maura F, Bolli N, Angelopoulos N, Dawson KJ, Leongamornlert D, Martincorena I, et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat Commun. 2019;10:3835. doi: 10.1038/s41467-019-11680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of (18)F-FDG-PET/CT according to deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39:116–25. doi: 10.1200/JCO.20.00386. [DOI] [PubMed] [Google Scholar]
- 197.Cavo M, San-Miguel J, Usmani SZ, Weisel K, Dimopoulos MA, Avet-Loiseau H, et al. Prognostic value of minimal residual disease negativity in myeloma: combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood. 2022;139:835–44. doi: 10.1182/blood.2021011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Strauchen JA. Indolent T-lymphoblastic proliferation: report of a case with an 11-year history and association with myasthenia gravis. Am J Surg Pathol. 2001;25:411–5. doi: 10.1097/00000478-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 199.Kim WY, Kim H, Jeon YK, Kim CW. Follicular dendritic cell sarcoma with immature T-cell proliferation. Hum Pathol. 2010;41:129–33. doi: 10.1016/j.humpath.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 200.Qian YW, Weissmann D, Goodell L, August D, Strair R. Indolent T-lymphoblastic proliferation in Castleman lymphadenopathy. Leuk Lymphoma. 2009;50:306–8. doi: 10.1080/10428190802645079. [DOI] [PubMed] [Google Scholar]
- 201.Ohgami RS, Zhao S, Ohgami JK, Leavitt MO, Zehnder JL, West RB, et al. TdT+ T-lymphoblastic populations are increased in Castleman disease, in Castleman disease in association with follicular dendritic cell tumours, and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2012;36:1619–28. doi: 10.1097/PAS.0b013e318264e223. [DOI] [PubMed] [Google Scholar]
- 202.Woo CG, Huh J. TdT+ T-lymphoblastic proliferation in Castleman disease. J Pathol Transl Med. 2015;49:1–4. doi: 10.4132/jptm.2014.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fromm JR, Edlefsen KL, Cherian S, Wood BL, Soma L, Wu D. Flow cytometric features of incidental indolent T lymphoblastic proliferations. Cytom B Clin Cytom. 2020;98:282–7. doi: 10.1002/cyto.b.21845. [DOI] [PubMed] [Google Scholar]
- 204.Walters M, Pittelkow MR, Hasserjian RP, Harris NL, Macon WR, Kurtin PJ, et al. Follicular dendritic cell sarcoma with indolent T-lymphoblastic proliferation is associated with paraneoplastic autoimmune multiorgan syndrome. Am J Surg Pathol. 2018;42:1647–52. doi: 10.1097/PAS.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 205.Chen J, Feng J, Xiao H, Ma Q, Chen Z. Indolent T-lymphoblastic proliferation associated with Castleman disease and low grade follicular dendric cell sarcoma: report of a case and review of literature. Int J Clin Exp Pathol. 2019;12:1497–505. [PMC free article] [PubMed] [Google Scholar]
- 206.Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153:1541–50. doi: 10.1016/S0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dumas G, Prendki V, Haroche J, Amoura Z, Cacoub P, Galicier L, et al. Kikuchi-Fujimoto disease: retrospective study of 91 cases and review of the literature. Medicine. 2014;93:372–82. doi: 10.1097/MD.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bardelli V, Arniani S, Pierini V, Di Giacomo D, Pierini T, Gorello P, et al. T-cell acute lymphoblastic leukemia: biomarkers and their clinical usefulness. Genes. 2021;12. [DOI] [PMC free article] [PubMed]
- 209.Weinberg OK, Chisholm KM, Ok CY, Fedoriw Y, Grzywacz B, Kurzer JH, et al. Clinical, immunophenotypic and genomic findings of NK lymphoblastic leukemia: a study from the Bone Marrow Pathology Group. Mod Pathol. 2021;34:1358–66. doi: 10.1038/s41379-021-00739-4. [DOI] [PubMed] [Google Scholar]
- 210.Staber PB, Herling M, Bellido M, Jacobsen ED, Davids MS, Kadia TM, et al. Consensus criteria for diagnosis, staging, and treatment response assessment of T-cell prolymphocytic leukemia. Blood. 2019;134:1132–43. doi: 10.1182/blood.2019000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sanikommu SR, Clemente MJ, Chomczynski P, Afable MG, 2nd, Jerez A, Thota S, et al. Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL) Leuk Lymphoma. 2018;59:416–22. doi: 10.1080/10428194.2017.1339880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Barilà G, Teramo A, Calabretto G, Vicenzetto C, Gasparini VR, Pavan L, et al. Stat3 mutations impact on overall survival in large granular lymphocyte leukemia: a single-center experience of 205 patients. Leukemia. 2020;34:1116–24. doi: 10.1038/s41375-019-0644-0. [DOI] [PubMed] [Google Scholar]
- 213.Qiu ZY, Fan L, Wang R, Gale RP, Liang HJ, Wang M, et al. Methotrexate therapy of T-cell large granular lymphocytic leukemia impact of STAT3 mutation. Oncotarget. 2016;7:61419–25. doi: 10.18632/oncotarget.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Teramo A, Barilà G, Calabretto G, Vicenzetto C, Gasparini VR, Semenzato G, et al. Insights into genetic landscape of large granular lymphocyte leukemia. Front Oncol. 2020;10:152. doi: 10.3389/fonc.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–15. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 216.Kogure Y, Kameda T, Koya J, Yoshimitsu M, Nosaka K, Yasunaga JI, et al. Whole-genome landscape of adult T-cell leukemia/lymphoma. Blood. 2022;139:967–82. doi: 10.1182/blood.2021013568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, et al. Aberrant PD-L1 expression through 3’-UTR disruption in multiple cancers. Nature. 2016;534:402–6. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- 218.Kataoka K, Iwanaga M, Yasunaga JI, Nagata Y, Kitanaka A, Kameda T, et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood. 2018;131:215–25. doi: 10.1182/blood-2017-01-761874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jones CL, Degasperi A, Grandi V, Amarante TD, Mitchell TJ, Nik-Zainal S, et al. Spectrum of mutational signatures in T-cell lymphoma reveals a key role for UV radiation in cutaneous T-cell lymphoma. Sci Rep. 2021;11:3962. doi: 10.1038/s41598-021-83352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tang YT, Wang D, Luo H, Xiao M, Zhou HS, Liu D, et al. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J. 2017;7:660. doi: 10.1038/s41408-017-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dufva O, Kankainen M, Kelkka T, Sekiguchi N, Awad SA, Eldfors S, et al. Aggressive natural killer-cell leukemia mutational landscape and drug profiling highlight JAK-STAT signaling as therapeutic target. Nat Commun. 2018;9:1567. doi: 10.1038/s41467-018-03987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang L, Liu D, Wang N, Ling S, Tang Y, Wu J, et al. Integrated genomic analysis identifies deregulated JAK/STAT-MYC-biosynthesis axis in aggressive NK-cell leukemia. Cell Res. 2018;28:172–86. doi: 10.1038/cr.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.El Hussein S, Patel KP, Fang H, Thakral B, Loghavi S, Kanagal-Shamanna R, et al. Genomic and Immunophenotypic Landscape of Aggressive NK-Cell Leukemia. Am J Surg Pathol. 2020;44:1235–43. doi: 10.1097/PAS.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 224.Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–14. doi: 10.1182/blood-2018-11-881268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kempf W, Mitteldorf C. Cutaneous T-cell lymphomas-An update 2021. Hematol Oncol. 2021;39(Suppl 1):46–51. doi: 10.1002/hon.2850. [DOI] [PubMed] [Google Scholar]
- 226.Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One. 2013;8:e68343. doi: 10.1371/journal.pone.0068343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma A, Oishi N, Boddicker RL, Hu G, Benson HK, Ketterling RP, et al. Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood. 2018;131:2262–6. doi: 10.1182/blood-2018-01-830968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599–606. doi: 10.1182/blood-2013-07-512830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Perry AM, Bailey NG, Bonnett M, Jaffe ES, Chan WC. Disease progression in a patient with indolent T-Cell lymphoproliferative disease of the gastrointestinal tract. Int J Surg Pathol. 2019;27:102–7. doi: 10.1177/1066896918785985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Soderquist CR, Patel N, Murty VV, Betman S, Aggarwal N, Young KH, et al. Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica. 2020;105:1895–906. doi: 10.3324/haematol.2019.230961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xiao W, Gupta GK, Yao J, Jang YJ, Xi L, Baik J, et al. Recurrent somatic JAK3 mutations in NK-cell enteropathy. Blood. 2019;134:986–91. doi: 10.1182/blood.2019001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mansoor A, Pittaluga S, Beck PL, Wilson WH, Ferry JA, Jaffe ES. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447–52. doi: 10.1182/blood-2010-08-302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Takeuchi K, Yokoyama M, Ishizawa S, Terui Y, Nomura K, Marutsuka K, et al. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631–7. doi: 10.1182/blood-2010-06-290650. [DOI] [PubMed] [Google Scholar]
- 234.Xia D, Morgan EA, Berger D, Pinkus GS, Ferry JA, Zukerberg LR. NK-cell enteropathy and similar indolent lymphoproliferative disorders: a case series with literature review. Am J Clin Pathol. 2019;151:75–85. doi: 10.1093/ajcp/aqy108. [DOI] [PubMed] [Google Scholar]
- 235.Krishnan R, Ring K, Williams E, Portell C, Jaffe ES, Gru AA. An Enteropathy-like indolent NK-cell proliferation presenting in the female genital tract. Am J Surg Pathol. 2020;44:561–5. doi: 10.1097/PAS.0000000000001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dargent JL, Tinton N, Trimech M, de Leval L. Lymph node involvement by enteropathy-like indolent NK-cell proliferation. Virchows Arch. 2021;478:1197–202. doi: 10.1007/s00428-020-02892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Foss FM, Horwitz SM, Civallero M, Bellei M, Marcheselli L, Kim WS, et al. Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol. 2020;95:151–5. doi: 10.1002/ajh.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yabe M, Medeiros LJ, Tang G, Wang SA, K PP, Routbort M, et al. Dyspoietic changes associated with hepatosplenic T-cell lymphoma are not a manifestation of a myelodysplastic syndrome: analysis of 25 patients. Hum Pathol. 2016;50:109–17. doi: 10.1016/j.humpath.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 239.Yabe M, Medeiros LJ, Tang G, Wang SA, Ahmed S, Nieto Y, et al. Prognostic factors of Hepatosplenic T-cell lymphoma: clinicopathologic study of 28 cases. Am J Surg Pathol. 2016;40:676–88. doi: 10.1097/PAS.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 240.Irshaid L, Xu ML. ALCL by any other name: the many facets of anaplastic large cell lymphoma. Pathology. 2020;52:100–10. doi: 10.1016/j.pathol.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 241.Pina-Oviedo S, Ortiz-Hidalgo C, Carballo-Zarate AA, Zarate-Osorno A ALK-negative anaplastic large cell lymphoma: current concepts and molecular pathogenesis of a heterogeneous group of large T-cell lymphomas. Cancers. 2021;13. [DOI] [PMC free article] [PubMed]
- 242.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugières L, Terrier-Lacombe MJ, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91:2076–84. [PubMed] [Google Scholar]
- 243.Pittaluga S, Wlodarska I, Pulford K, Campo E, Morris SW, Van den Berghe H, et al. The monoclonal antibody ALK1 identifies a distinct morphological subtype of anaplastic large cell lymphoma associated with 2p23/ALK rearrangements. Am J Pathol. 1997;151:343–51. [PMC free article] [PubMed] [Google Scholar]
- 244.Boi M, Rinaldi A, Kwee I, Bonetti P, Todaro M, Tabbò F, et al. PRDM1/BLIMP1 is commonly inactivated in anaplastic large T-cell lymphoma. Blood. 2013;122:2683–93. doi: 10.1182/blood-2013-04-497933. [DOI] [PubMed] [Google Scholar]
- 245.Lobello C, Tichy B, Bystry V, Radova L, Filip D, Mraz M, et al. STAT3 and TP53 mutations associate with poor prognosis in anaplastic large cell lymphoma. Leukemia. 2021;35:1500–5. doi: 10.1038/s41375-020-01093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Richardson AI, Yin CC, Cui W, Li N, Medeiros LJ, Li L, et al. p53 and β-Catenin Expression Predict Poorer Prognosis In Patients With Anaplastic Large-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:e385–e92. doi: 10.1016/j.clml.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 247.Liang HC, Costanza M, Prutsch N, Zimmerman MW, Gurnhofer E, Montes-Mojarro IA, et al. Super-enhancer-based identification of a BATF3/IL-2R-module reveals vulnerabilities in anaplastic large cell lymphoma. Nat Commun. 2021;12:5577. doi: 10.1038/s41467-021-25379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pedersen MB, Hamilton-Dutoit SJ, Bendix K, Ketterling RP, Bedroske PP, Luoma IM, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130:554–7. doi: 10.1182/blood-2016-12-755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hapgood G, Ben-Neriah S, Mottok A, Lee DG, Robert K, Villa D, et al. Identification of high-risk DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. Br J Haematol. 2019;186:e28–e31. doi: 10.1111/bjh.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.King RL, Dao LN, McPhail ED, Jaffe ES, Said J, Swerdlow SH, et al. Morphologic Features of ALK-negative Anaplastic Large Cell Lymphomas With DUSP22 Rearrangements. Am J Surg Pathol. 2016;40:36–43. doi: 10.1097/PAS.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ravindran A, Feldman AL, Ketterling RP, Dasari S, Rech KL, McPhail ED, et al. Striking Association of Lymphoid Enhancing Factor (LEF1) Overexpression and DUSP22 Rearrangements in Anaplastic Large Cell Lymphoma. Am J Surg Pathol. 2021;45:550–7. doi: 10.1097/PAS.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 252.Scarfò I, Pellegrino E, Mereu E, Kwee I, Agnelli L, Bergaggio E, et al. Identification of a new subclass of ALK-negative ALCL expressing aberrant levels of ERBB4 transcripts. Blood. 2016;127:221–32. doi: 10.1182/blood-2014-12-614503. [DOI] [PubMed] [Google Scholar]
- 253.Fitzpatrick MJ, Massoth LR, Marcus C, Vergilio JA, Severson E, Duncan D, et al. JAK2 rearrangements are a recurrent alteration in CD30+ systemic T-cell lymphomas with anaplastic morphology. Am J Surg Pathol. 2021;45:895–904. doi: 10.1097/PAS.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 254.Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–20. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Oishi N, Brody GS, Ketterling RP, Viswanatha DS, He R, Dasari S, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132:544–7. doi: 10.1182/blood-2017-12-821868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Blombery P, Thompson ER, Jones K, Arnau GM, Lade S, Markham JF, et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101:e387–90. doi: 10.3324/haematol.2016.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Laurent C, Nicolae A, Laurent C, Le Bras F, Haioun C, Fataccioli V, et al. Gene alterations in epigenetic modifiers and JAK-STAT signaling are frequent in breast implant-associated ALCL. Blood. 2020;135:360–70. doi: 10.1182/blood.2019001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Letourneau A, Maerevoet M, Milowich D, Dewind R, Bisig B, Missiaglia E, et al. Dual JAK1 and STAT3 mutations in a breast implant-associated anaplastic large cell lymphoma. Virchows Arch. 2018;473:505–11. doi: 10.1007/s00428-018-2352-y. [DOI] [PubMed] [Google Scholar]
- 259.Di Napoli A, Jain P, Duranti E, Margolskee E, Arancio W, Facchetti F, et al. Targeted next generation sequencing of breast implant-associated anaplastic large cell lymphoma reveals mutations in JAK/STAT signalling pathway genes, TP53 and DNMT3A. Br J Haematol. 2018;180:741–4. doi: 10.1111/bjh.14431. [DOI] [PubMed] [Google Scholar]
- 260.Los-de Vries GT, de Boer M, van Dijk E, Stathi P, Hijmering NJ, Roemer MGM, et al. Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood. 2020;136:2927–32. doi: 10.1182/blood.2020005372. [DOI] [PubMed] [Google Scholar]
- 261.Quesada AE, Zhang Y, Ptashkin R, Ho C, Horwitz S, Benayed R, et al. Next generation sequencing of breast implant-associated anaplastic large cell lymphomas reveals a novel STAT3-JAK2 fusion among other activating genetic alterations within the JAK-STAT pathway. Breast J. 2021;27:314–21. doi: 10.1111/tbj.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–60. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 264.Huang Y, Moreau A, Dupuis J, Streubel B, Petit B, Le Gouill S, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33:682–90. doi: 10.1097/PAS.0b013e3181971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dobay MP, Lemonnier F, Missiaglia E, Bastard C, Vallois D, Jais JP, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica. 2017;102:e148–e51. doi: 10.3324/haematol.2016.158428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:171–5. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 267.Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–3. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293–6. doi: 10.1182/blood-2013-10-531509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–63. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 270.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–10. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Marafioti T, Paterson JC, Ballabio E, Chott A, Natkunam Y, Rodriguez-Justo M, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432–9. doi: 10.3324/haematol.2009.010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood. 2005;106:1501–2. doi: 10.1182/blood-2005-03-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–33. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- 274.Roncador G, García Verdes-Montenegro JF, Tedoldi S, Paterson JC, Klapper W, Ballabio E, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92:1059–66. doi: 10.3324/haematol.10864. [DOI] [PubMed] [Google Scholar]
- 275.Dorfman DM, Shahsafaei A. CD200 (OX-2 membrane glycoprotein) is expressed by follicular T helper cells and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2011;35:76–83. doi: 10.1097/PAS.0b013e31820065c9. [DOI] [PubMed] [Google Scholar]
- 276.Murakami YI, Yatabe Y, Sakaguchi T, Sasaki E, Yamashita Y, Morito N, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2007;31:1695–702. doi: 10.1097/PAS.0b013e318054dbcf. [DOI] [PubMed] [Google Scholar]
- 277.Ree HJ, Kadin ME, Kikuchi M, Ko YH, Suzumiya J, Go JH. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30:403–11. doi: 10.1016/s0046-8177(99)90115-6. [DOI] [PubMed] [Google Scholar]
- 278.Vallois D, Dobay MP, Morin RD, Lemonnier F, Missiaglia E, Juilland M, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood. 2016;128:1490–502. doi: 10.1182/blood-2016-02-698977. [DOI] [PubMed] [Google Scholar]
- 279.Watatani Y, Sato Y, Miyoshi H, Sakamoto K, Nishida K, Gion Y, et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia. 2019;33:2867–83. doi: 10.1038/s41375-019-0473-1. [DOI] [PubMed] [Google Scholar]
- 280.Miyoshi H, Sakata-Yanagimoto M, Shimono J, Yoshida N, Hattori K, Arakawa F, et al. RHOA mutation in follicular T-cell lymphoma: Clinicopathological analysis of 16 cases. Pathol Int. 2020;70:653–60. doi: 10.1111/pin.12981. [DOI] [PubMed] [Google Scholar]
- 281.Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123:2915–23. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Heavican TB, Bouska A, Yu J, Lone W, Amador C, Gong Q, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood. 2019;133:1664–76. doi: 10.1182/blood-2018-09-872549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Amador C, Greiner TC, Heavican TB, Smith LM, Galvis KT, Lone W, et al. Reproducing the molecular subclassification of peripheral T-cell lymphoma-NOS by immunohistochemistry. Blood. 2019;134:2159–70. doi: 10.1182/blood.2019000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tse E, Au-Yeung R, Kwong YL. Recent advances in the diagnosis and treatment of natural killer/T-cell lymphomas. Expert Rev Hematol. 2019;12:927–35. doi: 10.1080/17474086.2019.1660640. [DOI] [PubMed] [Google Scholar]
- 285.Jiao W, Ji JF, Xu W, Bu W, Zheng Y, Ma A, et al. Distinct downstream signaling and the roles of VEGF and PlGF in high glucose-mediated injuries of human retinal endothelial cells in culture. Sci Rep. 2019;9:15339. doi: 10.1038/s41598-019-51603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lim JQ, Huang D, Tang T, Tan D, Laurensia Y, Peng RJ, et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia. 2020;34:3413–9. doi: 10.1038/s41375-020-1000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136:2754–63. doi: 10.1182/blood.2020007247. [DOI] [PubMed] [Google Scholar]
- 288.Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132:1146–58. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kuo TT, Chen MJ, Kuo MC. Cutaneous intravascular NK-cell lymphoma: report of a rare variant associated with Epstein-Barr virus. Am J Surg Pathol. 2006;30:1197–201. doi: 10.1097/01.pas.0000213263.99973.09. [DOI] [PubMed] [Google Scholar]
- 291.Cerroni L, Massone C, Kutzner H, Mentzel T, Umbert P, Kerl H. Intravascular large T-cell or NK-cell lymphoma: a rare variant of intravascular large cell lymphoma with frequent cytotoxic phenotype and association with Epstein-Barr virus infection. Am J Surg Pathol. 2008;32:891–8. doi: 10.1097/PAS.0b013e31815d29c9. [DOI] [PubMed] [Google Scholar]
- 292.Liu Y, Zhang W, An J, Li H, Liu S. Cutaneous intravascular natural killer-cell lymphoma: a case report and review of the literature. Am J Clin Pathol. 2014;142:243–7. doi: 10.1309/AJCP1JLYXLGDNOCH. [DOI] [PubMed] [Google Scholar]
- 293.Alegría-Landa V, Manzarbeitia F, Salvatierra Calderón MG, Requena L, Rodríguez-Pinilla SM. Cutaneous intravascular natural killer/T cell lymphoma with peculiar immunophenotype. Histopathology. 2017;71:994–1002. doi: 10.1111/his.13332. [DOI] [PubMed] [Google Scholar]
- 294.Klairmont MM, Cheng J, Martin MG, Gradowski JF. Recurrent cytogenetic abnormalities in intravascular Large B-cell lymphoma. Am J Clin Pathol. 2018;150:18–26. doi: 10.1093/ajcp/aqy023. [DOI] [PubMed] [Google Scholar]
- 295.Fujikura K, Yamashita D, Yoshida M, Ishikawa T, Itoh T, Imai Y. Cytogenetic complexity and heterogeneity in intravascular lymphoma. J Clin Pathol. 2021;74:244–50. doi: 10.1136/jclinpath-2020-206573. [DOI] [PubMed] [Google Scholar]
- 296.Jeon YK, Kim JH, Sung JY, Han JH, Ko YH. Epstein-Barr virus-positive nodal T/NK-cell lymphoma: an analysis of 15 cases with distinct clinicopathological features. Hum Pathol. 2015;46:981–90. doi: 10.1016/j.humpath.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 297.Jung KS, Cho SH, Kim SJ, Ko YH, Kim WS. Clinical features and treatment outcome of Epstein-Barr virus-positive nodal T-cell lymphoma. Int J Hematol. 2016;104:591–5. doi: 10.1007/s12185-016-2068-1. [DOI] [PubMed] [Google Scholar]
- 298.Ng SB, Chung TH, Kato S, Nakamura S, Takahashi E, Ko YH, et al. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica. 2018;103:278–87. doi: 10.3324/haematol.2017.180430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yamashita D, Shimada K, Takata K, Miyata-Takata T, Kohno K, Satou A, et al. Reappraisal of nodal Epstein-Barr Virus-negative cytotoxic T-cell lymphoma: Identification of indolent CD5(+) diseases. Cancer Sci. 2018;109:2599–610. doi: 10.1111/cas.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wai CMM, Chen S, Phyu T, Fan S, Leong SM, Zheng W, et al. Immune pathway upregulation and lower genomic instability distinguish EBV-positive nodal T/NK-cell lymphoma from ENKTL and PTCL-NOS. Haematologica. 2022. [DOI] [PMC free article] [PubMed]
- 301.Hong M, Ko YH, Yoo KH, Koo HH, Kim SJ, Kim WS, et al. EBV-Positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137–47. doi: 10.4132/KoreanJPathol.2013.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98:280–6. doi: 10.1182/blood.v98.2.280. [DOI] [PubMed] [Google Scholar]
- 303.Miyake T, Yamamoto T, Hirai Y, Otsuka M, Hamada T, Tsuji K, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol. 2015;172:56–63. doi: 10.1111/bjd.13411. [DOI] [PubMed] [Google Scholar]
- 304.Liu Y, Ma C, Wang G, Wang L. Hydroa vacciniforme-like lymphoproliferative disorder: Clinicopathologic study of 41 cases. J Am Acad Dermatol. 2019;81:534–40. doi: 10.1016/j.jaad.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 305.Cohen JI, Iwatsuki K, Ko YH, Kimura H, Manoli I, Ohshima K, et al. Epstein-Barr virus NK and T cell lymphoproliferative disease: report of a 2018 international meeting. Leuk Lymphoma. 2020;61:808–19. doi: 10.1080/10428194.2019.1699080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Isobe Y, Aritaka N, Setoguchi Y, Ito Y, Kimura H, Hamano Y, et al. T/NK cell type chronic active Epstein-Barr virus disease in adults: an underlying condition for Epstein-Barr virus-associated T/NK-cell lymphoma. J Clin Pathol. 2012;65:278–82. doi: 10.1136/jclinpath-2011-200523. [DOI] [PubMed] [Google Scholar]
- 307.Cohen JI, Manoli I, Dowdell K, Krogmann TA, Tamura D, Radecki P, et al. Hydroa vacciniforme-like lymphoproliferative disorder: an EBV disease with a low risk of systemic illness in whites. Blood. 2019;133:2753–64. doi: 10.1182/blood.2018893750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–86. doi: 10.1182/blood-2011-10-381921. [DOI] [PubMed] [Google Scholar]
- 309.Yonese I, Sakashita C, Imadome KI, Kobayashi T, Yamamoto M, Sawada A, et al. Nationwide survey of systemic chronic active EBV infection in Japan in accordance with the new WHO classification. Blood Adv. 2020;4:2918–26. doi: 10.1182/bloodadvances.2020001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Montes-Mojarro IA, Kim WY, Fend F, Quintanilla-Martinez L, Epstein - Barr virus positive T and NK-cell lymphoproliferations: Morphological features and differential diagnosis. Semin Diagn Pathol. 2020;37:32–46. doi: 10.1053/j.semdp.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 311.Bofill M, Akbar AN, Amlot PL. Follicular dendritic cells share a membrane-bound protein with fibroblasts. J Pathol. 2000;191:217–26. doi: 10.1002/(SICI)1096-9896(200006)191:2<217::AID-PATH586>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 312.van Nierop K, de Groot C. Human follicular dendritic cells: function, origin and development. Semin Immunol. 2002;14:251–7. doi: 10.1016/s1044-5323(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 313.Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. 2014;211:1109–22. doi: 10.1084/jem.20132409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jiang XN, Zhang Y, Xue T, Chen JY, Chan ACL, Cheuk W, et al. New clinicopathologic scenarios of EBV+ inflammatory follicular dendritic cell sarcoma: Report of 9 extrahepatosplenic cases. Am J Surg Pathol. 2021;45:765–72. doi: 10.1097/PAS.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 315.WHO-Classification-of-Tumour-Editorial-Board, editor. Digestive system tumours, WHO classification of tumours series. 5th ed. Lyon: IARC; 2019.
- 316.Dostoyevsky F, The House of the Dead; 1860–62.