Significance
The Galápagos Islands are an iconic evolutionary and ecological setting, recognized to be both species-poor and ecologically sensitive. Here, we show an indirect ecological cascade initiated by whalers harvesting tortoises near the coast in the 1790s, which had lasting impacts on the highland interior of San Cristóbal Island. Our data also reveal how the replacement of endemic herbivores with exotic herbivores, namely, cattle, impacted the local vegetation. We advocate for the restoration of preimpact shrub species and tortoises to promote habitat rewilding, restoration, and especially the socioeconomic value of these highland ecosystems in providing tourist experiences.
Keywords: giant tortoise, whaling, overharvesting, migration, endemic
Abstract
Oceanic islands support unique biotas but often lack ecological redundancy, so that the removal of a species can have a large effect on the ecosystem. The larger islands of the Galápagos Archipelago once had one or two species of giant tortoise that were the dominant herbivore. Using paleoecological techniques, we investigate the ecological cascade on highland ecosystems that resulted from whalers removing many thousands of tortoises from the lowlands. We hypothesize that the seasonal migration of a now-extinct tortoise species to the highlands was curtailed by decreased intraspecific competition. We find the trajectory of plant community dynamics changed within a decade of the first whaling vessels visiting the islands. Novel communities established, with a previously uncommon shrub, Miconia, replacing other shrubs of the genera Alternanthera and Acalypha. It was, however, the introduction of cattle and horses that caused the local extirpation of plant species, with the most extreme impacts being evident after c. 1930. This modified ecology is considered the natural state of the islands and has shaped subsequent conservation policy and practice. Restoration of El Junco Crater should emphasize exclusion of livestock, rewilding with tortoises, and expanding the ongoing plantings of Miconia to also include Acalypha and Alternanthera.
Owing to their isolation, island systems often have simplified food webs, with less ecological redundancy than mainland systems (1). Islands also account for a disproportionately high number of critically endangered and recently extinct species (2), with the majority of those extinctions occurring soon after the initial colonization by humans (3). Here, we describe the cascading ecological effects of a whaling-precipitated vegetation change in the highlands of the Galápagos Islands, mediated through the depletion of tortoises and the introduction of livestock. Tortoises have repeatedly proven to be successful colonists of remote oceanic islands (e.g., Aldabra, the Mascarene Islands, the Seychelles, and the Galápagos Islands), where they evolve to become the dominant, and usually the only, megaherbivore. On the semidesert islands of the Galápagos, a classic coevolutionary adaptive arms race of leg and neck elongation between saddle-backed Chelonoidis spp. giant tortoises and trunk height in Opuntia spp. cactus highlights the struggle between predator and prey (4). But in the limited food webs on the islands, the Opuntia cactus are reliant on the tortoises for the dispersal of their seeds (5, 6). The other main morphotype of Chelonoidis tortoises, the dome-shelled tortoise, does not show such tight coevolution as they have a broader diet and are not confined to the lowlands. During the cool months of October to February, tagged tortoise telemetry shows that dome-shelled tortoises migrate up to about 420-m elevation (7). From evolutionary data, it is evident that this elevation has not been a physiological boundary as modern tortoise populations within the archipelago are resident on high calderas at elevations >1,000 m when cut off from the lowlands by lava fields (8). Thus, the height of the observed migration may reflect energetic tradeoffs that could be influenced by forage quality and competition (9). Prior paleoecological analyses from the island of Santa Cruz used the occurrence of the coprophilous fungus Sporormiella spp. to suggest that tortoises visited bogs at c. 700-m elevation until c. 700 to 500 y ago (10).
The Galápagos Islands were only visited by pirates and wayward sailors prior to the 1780s (Fig. 1) (12). However, rising demand for whale oil and overexploitation of Atlantic whale stocks forced whalers to explore the Pacific Ocean (13). Whalers began operating around the Galápagos in the 1790s, and by the early 1800s they were regularly visiting the islands (13). Each whaling voyage lasted about a year, and tortoises were an essential source of fresh meat, with each whaler commonly taking aboard 200 to 300 tortoises, each weighing 150 to 200 kg (14). The tortoises could be stored in the hold of the vessel, living without food or water for up to a year (14). It is estimated that more than 200,000 tortoises were removed by an estimated 700 whaling ships that visited the islands between 1800 and 1870 (13). Although comprehensive documentation of tortoise harvesting does not exist, excerpts of 79 logbooks of whaling ships that captured ∼13,000 tortoises provide insights into the harvest between 1831 and 1870 (8). These logs record the overharvesting of tortoises, manifested in the time taken to capture 200 animals more than doubling between 1830 and 1860 (13). The weight of the tortoises and the harsh terrain meant that sailors generally hunted close to the coast. Hence, depletions were strongest close to natural harbors and water sources and on “tortoise roads”—well-traveled tracks created by the animals. The replacement of whale oil with kerosene in the 1860s led to the collapse of whaling around the Galápagos.
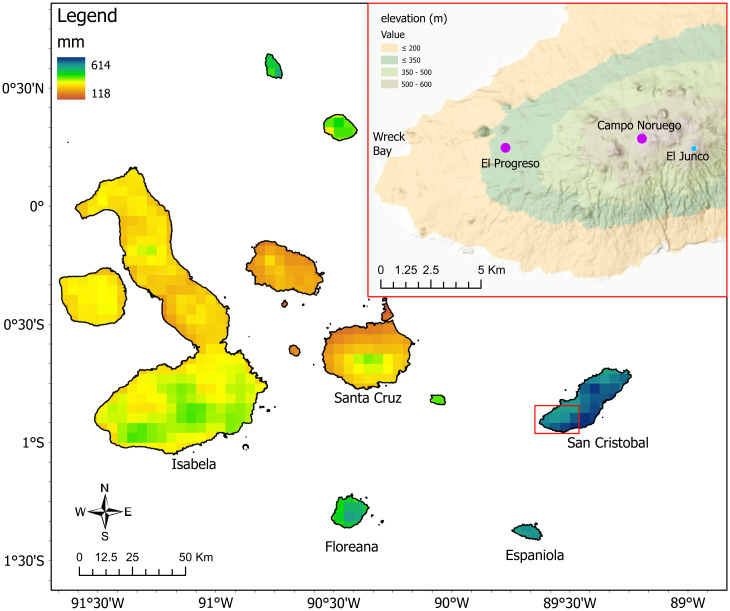
Fig. 1.
Map of the Galápagos Islands showing annual precipitation (11) and elevation (Inset). The area in the Inset of western San Cristóbal is shown by the red rectangle.
The slow reproduction and long lifespans of tortoises condemned some tortoise species to extinction (15). By the 1880s, scientists mounted a series of expeditions (1888, 1891, 1897, 1899, and 1905 to 1996) to capture tortoises but only found them on the islands of Isabela and Pinzón (Fig. 1) (16). Thus, by the late 1800s, giant tortoises were close to extinct on all but two islands in the archipelago. Some recovery of giant tortoises has taken place, but modern populations are thought to exist at about 10% of their former density (17).
El Junco Crater Lake (hereafter El Junco) lies at 660-m elevation on the island of San Cristóbal (Fig. 2), and is the only permanent freshwater lake in the Galápagos Archipelago. A sedimentary core was recovered from the center of El Junco in 2004 and analyzed for its fossil pollen and spore content (18, 19). We use spores of the dung fungus Sporormiella (expressed as a percentage of the terrestrial pollen sum) as a proxy for megaherbivore presence (20, 21), thereby documenting megaherbivore history around the lake. The lake has also provided a detailed record of climate change (18, 22), but significant changes in temperature do not align closely either with Sporormiella abundance or vegetation change (SI Appendix). While we do not discount the possibility that recent warming may influence restoration efforts, it probably does not account for the species declines documented here.
Fig. 2.
Data on tortoise capture from 79 whaling ship logbooks for the period from 1831 to 1870 CE (13). Tortoise harvesting effort is based on our interpretation of the ships’ logbooks to tabulate the number of tortoises captured per day of hunting. The logbook entries are not detailed enough to quantify this as tortoises per person per day, as the number of sailors in the hunting party was seldom defined. Also shown is the number of ships represented by the logbooks each year.
The El Junco paleoecological record shows a reduction in Sporormiella abundance in the 1790s that is coincident with the onset of whaling activity (Fig. 3). For >1,000 y prior to the 1790s, when tortoise populations are unaffected by humans, the fossil pollen spectra of El Junco are rich in two shrubby genera, Alternanthera and Acalypha (SI Appendix), both of which are eaten by tortoises (19, 23). Between 1790 and 1927, however, Alternanthera gradually declines (Fig. 3), while Miconia increases by a factor of four between 1790 and 1900. Detrended correspondence analysis (DCA) (Fig. 3), which depicts the overall trajectory of the vegetation and had been largely stable for 1,500 y (SI Appendix), produces an inflection point at c. 1790 and a new trajectory that continued into modern times.
Fig. 3.
Percentage occurrence of selected pollen and spores from the El Junco paleoecological record. Sporormiella percentages of the pollen sum and concentrations (spores per cubic centimeter) are shown. The axis 1 scores of the DCA of the fossil pollen flora for the period from c. 1560 to 2000 CE are plotted against time. The periods of whaling and the settlement of the San Cristóbal highlands are indicated. The background is an inferred grazing pressure around El Junco based on our data and the literature, with green representing tortoise grazing and gray that of introduced livestock. The dashed lines mark inflection in vegetation history.
By the 1840s, tortoises were rare on San Cristóbal (16) and were unlikely to account for increasing amounts of Sporormiella found between 1840 and 1890. Rather, these spores probably represent the spread of introduced livestock. Donkeys introduced by whalers to help transport tortoises became feral on some islands, though no specifics are known of their introduction to San Cristóbal. The introduction of pigs and goats to San Cristóbal is thought to have been in the 1830s (24). Cattle were probably first introduced to San Cristóbal in 1842 (25), when the first settlement was made at Wreck Bay (now Puerto Baquerizo Moreno), though by 1854 there were enough feral cattle on Isabel and San Cristóbal for a small company to be formed to exploit them for tallow, but that enterprise had failed by 1860 (26). In 1869, the village of El Progreso (Fig. 1) was founded at c. 200-m elevation, about 8 km from El Junco. A zooarchaeological survey of the associated midden did not find giant tortoise (27), consistent with observations that the animals were already rare in this part of the island. A governmental visitor to the island in 1890 estimated there to be about 10,000 wild cattle on San Cristóbal. We therefore infer that the peaks of Sporormiella seen in the late 1800s come from these exotic livestock. An example of an endemic plant species that appears to have thrived under this regime of disturbance is the tree fern Cyathea weatherbyana, the spores of which reach four times the terrestrial pollen sum.
A sharp increase in Sporormiella, evident at c. 1927, may reflect the arrival in 1926 and settlement of Norwegian colonists at Campo Noruega, just 2 km from El Junco (Fig. 1) (28). Only one family of Norwegians continued to inhabit the area after 1930, but the vegetation around El Junco clearly transitioned from mildly to extremely impacted at this time (Fig. 3). From the 1930s until the 1960s, San Cristóbal was the most populous island in the archipelago, but very little information on land use or herd densities exists for this period. After 1933, both Alternanthera and Acalypha gradually decline to background (<5%) levels. Cyathea and Poaceae show marked increases in abundance that peak in 1953 and 1973, respectively (Fig. 3). Eckhardt (29) cites a resident stating that hunting reduced cattle densities in the 1940s, and that open grassy areas were recolonized by shrub. In the last 30 y of the record, Psidium begins to increase from <2% in the 1970s to >12% in 1986 (Fig. 3). The Psidium pollen type includes both the endemic Psidium galapageium, a small, fairly uncommon tree of midelevations, and the exotic Psidium guajava, whose fruit (guava) is spread by cattle, horses, and tortoises (30). By outcompeting all other shrubs, P. guajava forms near-monodominant stands (31).
Discussion
We attribute the presence of Sporormiella prior to c. 1800 to the population of endemic giant tortoises, while that of the period post 1840 is suggested to reflect the introduction and maintenance of (exotic to the region) grazing livestock. The ecological cascades caused by the extinction of ectothermic, energetically efficient tortoises and their replacement with larger, less energetically efficient endotherms, namely cattle and horses, resulted in increased herbivory and the extirpation of some plant species.
Prior to 1800 almost all samples contain >2% Sporormiella (though with some variability), indicating the regular presence of the animals around the lake (21). The modern ecology of giant tortoises suggests that they were not permanent residents at the lake but rather formed a migratory population (7). Currently, tortoises migrate out of the midelevations into the lowlands to take advantage of young plant growth and to nest. Where tortoise migration has been documented, however, they do not migrate as high as the 660-m elevation of El Junco, but this may reflect their low modern population density. We suggest that when much larger tortoise populations existed on the islands, as encountered by the first visitors, competition would have caused population ranges to extend further upslope. These higher-than-modern distributions were almost by definition suboptimal, otherwise modern tortoises would likely migrate that high. Current climate change has not been seen to cause substantial changes in midelevation vegetation but, in the agricultural zones where migration studies have been made, habitat alteration could be influencing these migratory patterns. Prior to human alteration of landscapes (i.e., prior to 1800), variance is evident in the Sporormiella abundance record from El Junco. Because tortoises live up to 170 y (32), decadal-scale variability in their abundance at El Junco is far more likely to reflect the need for animals to visit the lake as a function of food availability and competition lower on the hillslopes, rather than island population size.
After 1800, we find evidence of oscillations in Sporormiella abundance in the paleoecological record from El Junco that coincide with known changes in human activity on the Galápagos Islands. A sharp reduction in Sporormiella abundance occurred at c. 1800 (Fig. 3), suggesting the sudden loss of the tortoise population from this setting. As whaling reduced tortoise population density by removing animals from the lowlands, the competition pressure was relaxed, meaning that tortoises no longer had to migrate as far upslope.
When tortoise migration stopped short of the crater (Fig. 4), there would have been a rapid reduction in dung production around the lake and hence Sporormiella representation would have fallen. That this response was triggered by reduced midelevation tortoise competition meant that the highland Sporormiella record was highly sensitive to the onset of whalers collecting tortoises in the lowlands. Thus, the observed vegetation changes in the highlands were the indirect effects of increased predation in the coastal area. The abandonment of the site by the largest herbivore had an immediate effect on the vegetation. Alternanthera was the genus most obviously negatively impacted by the loss of grazers. It is not immediately clear why Alternanthera should have become rarer, as it is eaten by tortoises (23), but it probably needed some disturbance to prevent it from being outcompeted by other species. While it is evident that the balance of species was altered by the loss of tortoises, there is no suggestion that local extirpations of plant populations occurred. Miconia pollen increased in abundance as Alternanthera fell and, while these were not numerically similar changes, they were of great ecological significance. Alternanthera is a wind-pollinated taxon and is likely to be very strongly overrepresented, perhaps by a factor of >10, whereas Miconia is entomophilous (pollinated by insects) and is likely to be somewhat underrepresented (33). The values of 4% attained after c. 1900 were consistent with modern pollen representation in areas rich in Miconia, but not immediately beside a Miconia bush (34). While Alternanthera abundance fell as tortoises were lost, there was a temporal lag before the major increase in Miconia occurred about 1880, concurrent with the arrival of cattle in the highlands. Whether it was browsing, altered regimes of mechanical damage, or fertilization by cattle dung that favored Miconia cannot be determined.
Fig. 4.
Inferred ecological consequences of the transition from tortoises to exotic livestock for the plants of El Junco, Galápagos. Schematic diagram showing the potential influence of tortoise capture, its effect on tortoise migration, and the introduction of exotic livestock on native and exotic floral elements around El Junco. Purple-colored icons indicate rewilded conspecifics or the nearest ecological equivalent brought from other islands. Extant endemic plants, especially Alternanthera spp., have such reduced populations that they will need active cultivation and restoration.
The first floristic description of the Galápagos highlands was made in 1905 to 1906 (35) and provided support for a floral zonation developed by Wiggins and Porter (36) that identified a “Miconia zone.” Had a botanist visited El Junco at any time in the prior 2,000 y, they would not have found such an abundance of Miconia, and this would probably have been more generically described as a “mixed shrub zone.” Because the first scientific observations of highland vegetation on the Galápagos (1905 to 1906) occurred after a century of human influence, those initial descriptions did not document the natural state but a shifted baseline (sensu ref. 37). We note that the only two islands where a Miconia zone is described, on Santa Cruz and San Cristóbal, are both islands that have probably lost domed tortoises from their upper migrational range where Miconia grows today and that gained cattle.
The abrupt increase in Sporormiella in the late 1920s aligns with the arrival of 14 Norwegian families who tried to settle the highlands in 1926 (38). The settlers established homesteads and attempted to cultivate crops brought from Norway. Most met with failure and quickly abandoned the land, but over the next decades a cattle export industry developed, with El Junco being an important water source. Throughout the period from 1800 to 1960 the pollen of Alternanthera declined in abundance, matched by a decline in Acalypha after 1925 (Fig. 3). This was the first time in the last 1,500 y (SI Appendix) that both Alternanthera and Acalypha experienced synchronous declines (19). Indeed, between 1925 and 1950 the highland flora was so modified that it had no counterpart in the last 1,500 y. The loss of native shrubs allowed introduced Poaceae (grasses) to increase in abundance (39).
It was the direct grazing impact of cattle and horses that had the largest impacts on individual taxon abundances, and it was probably within this period that Acalypha was extirpated. Seberg (40) suggested that the Acalypha pollen from El Junco was attributable to Acalypha baurii, which was collected from the island in 1891 but has not been seen since. A. baurii was probably widespread in the interior highlands, but was lost from all the islands that were extensively modified by humans; it appears to be very vulnerable to human disturbance (40). The initial disturbance to El Junco did not eliminate Acalypha. Indeed, Acalypha seems to have thrived in the 1930s despite grazing activity. But the change in land use that caused the expansion of C. weatherbyana between 1930 and 1950 appears correlated with the precipitous decline in Acalypha. These observations underscore that not all megaherbivores are equal. The endemic tortoise populations fell to extinction without inducing plant extinctions. In contrast, the high energetic demand of cattle and horses increased grazing impacts, while the mechanical damage by hooves of animals two to three times the size of a Galápagos tortoise was likely far more damaging than that of the broad flat feet of tortoises.
The decline in the local cattle industry during the 1970s and 1980s was marked by a rise in Psidium pollen. Endemic P. galapageium had a consistent <2% presence throughout the record (22), but in the 1980s the rise of Psidium pollen reflected the spread of exotic guava, P. guajava, which had been previously held in check by cattle grazing (29, 41). P. guajava was introduced to El Progreso in the 1890s, but was not documented in a detailed floristic description of the island conducted in 1905 to 1906 (35). In 1966, P. guajava was documented as spreading but scattered in the highlands (42), but by the 1990s as cattle density fell, P. guajava became the dominant vegetation of large highland areas (43). Although cattle probably caused the extirpation of Acalypha from the highlands, and its local extinction on San Cristóbal, it can still be found in the highlands of other islands. Hence, we term its loss an extirpation rather than an extinction. It was probably the rapid invasion of exotic trees that sealed the fate for Alternanthera recovery, contra the observations that exotic trees did not reduce endemic species diversity on Santa Cruz (44).
The dome-shelled tortoise of San Cristóbal appears to have been in decline since possibly the late 1700s, with a migrational range curtailed and local extirpation in the early 1800s. Although a skeleton of the dome-shelled species was found in a cave on San Cristóbal in 1906, live animals were not collected in any of the five expeditions between 1886 and 1906 (16). The dome-shelled species of San Cristóbal probably went extinct between the 1880s and 1930s (45, 46).
El Junco has become the emblematic highland scene for many tourists and its restoration is feasible when that of the broader highlands may be beyond reach (47). Continued efforts to remove exotic plant species, especially Rubus nivaeus and P. guajava, should be paired with reintroduction of A. baurii, and the two species of Alternanthera known to have existed in the San Cristóbal highlands, Alternanthera halimifolia and Alternanthera rugulosa. Certainly, A. baurii would have to be reintroduced from another island, whose individuals probably have a somewhat different genetic composition from the extirpated form. As such, this would constitute rewilding. Restoration of Alternanthera spp. may be possible through active cultivation of individuals remaining on San Cristóbal, or through reintroduction from populations on other islands. We suggest that it would also be beneficial to rewild (48) the landscape by reintroducing a few dome-shelled tortoises to El Junco, at least seasonally. Giant tortoises are known to be ecosystem engineers (49) and so their presence would be integral to restoring the full functioning of the ecosystem. Indeed, the rewilding of tortoises Chelonoidis hoodensis from Espaniola Island to replace the extinct tortoises of Santa Fe Island appears to have been highly successful (50). In addition to their ecological benefits, the presence of tortoises would increase the attractiveness of this setting as a tourist destination.
Materials and Methods
El Junco lies at 660-m elevation, about 6.5 km from the nearest potential anchorage and 14.5 km from the natural harbor of Puerto Baquerizo Moreno. El Junco is c. 8 m deep and is the only permanent freshwater lake on the islands. The vegetation around El Junco is being restored to a Miconia-zone shrubland, following the archipelago’s habitat zonation of Wiggins and Porter (36). Invasive exotic species such as P. guajava and R. nivaeus are being controlled as this is now one of the most-visited highland habitats and constitutes a showpiece for ecotourists.
In 2004, a sediment core was raised from the center of the lake using a Colinvaux–Vohnout piston sampler, and prior publications describe the fossil pollen record that was reconstructed (19, 51). Pollen samples were prepared using standard protocols, the addition of styrene microspheres (52) and 10% KOH, acetolysis (53), and mounting in glycerol. The fossil pollen extracts from analyses made by Restrepo et al. (19) were examined for Sporormiella, Podospora, and Cercophora, all of which have been used as proxies for megaherbivores (10, 54). Here, we report the Sporormiella data as these are the only one (of the three) that is an obligate dung fungus. Cercophora data are included in SI Appendix but are not interpreted. Samples were counted until the same number of exotic microspheres had been counted as in the original pollen counts, thereby providing an equivalent to the number of spores per 300 pollen grains. Where insufficient extract remained to achieve this count, fresh samples were prepared from the same core depths using standard protocols (20, 53) and spiked with the same concentration of microspheres as in the original sample.
The chronology for the El Junco record (18) was developed based on 210Pb, 137Cs, and 14C dating. A revised version of this chronology was developed using rbacon (55); an IntCal20 calibration is adopted (SI Appendix).
The fossil pollen data previously reported (19) were reanalyzed for the period from 1630 to modern times and plotted on the new chronology. A detrended correspondence analysis (56), using R package vegan (57), was applied to percentile values of all pollen taxa that are found on the islands.
Supplementary Material
Acknowledgments
We thank the Charles Darwin Station and the Parque Nacional Galápagos. This work was supported by NSF grant AGS-2002504.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203752119/-/DCSupplemental.
Data Availability
Fossil pollen and spore data reported in this article have been deposited in the Neotoma Paleoecology database (https://www.neotomadb.org) and at GitHub (https://github.com/markbbush/Galapagos-pollen-and-spores.git) (58).
All study data are included in the article and/or SI Appendix.
References
- 1.Whittaker R. J., Fernández-Palacios J. M., Island Biogeography: Ecology, Evolution, and Conservation (Oxford University Press, 2007). [Google Scholar]
- 2.Tershy B. R., Shen K.-W., Newton K. M., Holmes N. D., Croll D. A., The importance of islands for the protection of biological and linguistic diversity. Bioscience 65, 592–597 (2015). [Google Scholar]
- 3.Nogué S., et al. , The human dimension of biodiversity changes on islands. Science 372, 488–491 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Fritts T. H., “Morphometrics of Galápagos tortoises: Evolutionary implications” in Patterns of Evolution in Galápagos Organisms, R. I. Bowman, M. Berson, A. E. Leviton, Eds. (AAAS Pacific Division, San Francisco, CA, 1983), pp. 107–122. [Google Scholar]
- 5.Hamann O., On vegetation recovery, goats and giant tortoises on Pinta Island, Galápagos, Ecuador. Biodivers. Conserv. 2, 138–151 (1993). [Google Scholar]
- 6.Gibbs J. P., Marquez C., Sterling E. J., The role of endangered species reintroduction in ecosystem restoration: Tortoise–cactus interactions on Española Island, Galápagos. Restor. Ecol. 16, 88–93 (2008). [Google Scholar]
- 7.Blake S., et al. , “Movement ecology” in Galapagos Giant Tortoises, J. P. Gibbs, L. J. Cayot, W. Tapia, Eds. (Elsevier, 2021), pp. 261–279. [Google Scholar]
- 8.Beaman K. R., “Seasonal variation in population location of the Galapagos tortoise, Geochelone elephantopus vandenburghi, on Volcan Alcedo, Isabela Island, Galapagos Archipelago,” Master’s thesis, Loma Linda University, Loma Linda, CA (1985).
- 9.Mysterud A., et al. , Partial migration in expanding red deer populations at northern latitudes—A role for density dependence? Oikos 120, 1817–1825 (2011). [Google Scholar]
- 10.Froyd C. A., et al. , The ecological consequences of megafaunal loss: Giant tortoises and wetland biodiversity. Ecol. Lett. 17, 144–154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 12.Kricher J. C., Galápagos: A Natural History (Princeton University Press, 2006). [Google Scholar]
- 13.Townsend C. H., The Galápagos tortoises in their relation to the whaling industry: A study of old logbooks. Zoologica 4, 55–135 (1925). [Google Scholar]
- 14.Porter D., Journal of a Cruise Made to the Pacific Ocean in the United States Frigate Essex: In the Years 1812, 1813, and 1814 (Bradford and Inskeep, 1815), vol. 1. [Google Scholar]
- 15.Poulakakis N., et al. , Historical DNA analysis reveals living descendants of an extinct species of Galápagos tortoise. Proc. Natl. Acad. Sci. U.S.A. 105, 15464–15469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Denbergh J. V., The Gigantic Land Tortoises of the Galapagos Archipelago (Proceedings of the California Academy of Sciences ser. 4, San Francisco, CA, 1914), vol. 2, pp. 203–374. [Google Scholar]
- 17.Tapia W., et al. , Giant Tortoise Restoration Initiative: Beyond rescue to full recovery. Galapagos Report 2015–2016 (2017). https://www.galapagos.org/about_galapagos/about-galapagos/library/galapagos-reports/2015-2016/ (Accessed 19 May 2022).
- 18.Conroy J. L., Overpeck J. T., Cole J. E., Shanahan T. M., Steinitz-Kannan M., Holocene changes in eastern tropical Pacific climate inferred from a Galápagos lake sediment record. Quat. Sci. Rev. 27, 1166–1180 (2008). [Google Scholar]
- 19.Restrepo A., et al. , Impacts of climate variability and human colonization on the vegetation of the Galápagos Islands. Ecology 93, 1853–1866 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Raper D., Bush M. B., A test of Sporormiella representation as a predictor of megaherbivore presence and abundance. Quat. Res. 71, 490–496 (2009). [Google Scholar]
- 21.Davis O. K., Shafer D. S., Sporormiella fungal spores, a palynological means of detecting herbivore density. Palaeogeogr. Palaeoclimatol. Palaeoecol. 237, 40–50 (2006). [Google Scholar]
- 22.Conroy J. L., et al. , Unprecedented recent warming of surface temperatures in the eastern tropical Pacific Ocean. Nat. Geosci. 2, 46–50 (2009). [Google Scholar]
- 23.Blake S., Tapia P. I., Safi K., Ellis-Soto D., “Diet, behavior, and activity patterns” in Galapagos Giant Tortoises, J. P. Gibbs, L. J. Cayot, W. Tapia, Eds. Galapagos Giant Tortoises (Elsevier, 2021), pp. 207–239. [Google Scholar]
- 24.Cayot L. J., Campbell K., Carrión V., “Invasive species: Impacts, control, and eradication” in Galapagos Giant Tortoises, J. P. Gibbs, L. J. Cayot, W. Tapia, Eds. (Elsevier, 2021), pp. 381–399. [Google Scholar]
- 25.Lundh J. P., “The Galapagos: A brief history” (2001). http://www.lundh.no/jacob/galapagos/pg05.htm. Accessed 19 May 2022.
- 26.Latorre O., Netherly P., de Salomón J. D., Cummins K., The Curse of the Giant Tortoise: Tragedies, Mysteries and Crimes in the Galápagos Islands (Libri Mundi, 1990). [Google Scholar]
- 27.Stahl P. W., Zooarchaeology of Hacienda El Progreso (San Cristóbal, Ecuador, 2017). [Google Scholar]
- 28.Stahl P. W., Astudillo F. J., Jamieson R. W., Quiroga D., Delgado F., Historical Ecology and Archaeology in the Galápagos Islands: A Legacy of Human Occupation (University Press of Florida, 2020). [Google Scholar]
- 29.Eckhardt R. C., Introduced plants and animals in the Galapagos Islands. Bioscience 22, 585–590 (1972). [Google Scholar]
- 30.Phillips R. B., Wiedenfeld D. A., Snell H. L., Current status of alien vertebrates in the Galápagos Islands: Invasion history, distribution, and potential impacts. Biol. Invasions 14, 461–480 (2012). [Google Scholar]
- 31.Mauchamp A., Threats from alien plant species in the Galápagos Islands. Conserv. Biol. 11, 260–263 (1997). [Google Scholar]
- 32.Spector W. S., Ed., Handbook of Biological Data (Saunders, Philadelphia, PA, 1956). [Google Scholar]
- 33.Bush M. B., Neotropical plant reproductive strategies and fossil pollen representation. Am. Nat. 145, 594–609 (1995). [Google Scholar]
- 34.Collins A., Bush M. B., An analysis of modern pollen representation and climatic conditions on the Galápagos Islands. Holocene 21, 237–250 (2011). [Google Scholar]
- 35.Stewart A., Some Observations Concerning the Botanical Conditions on the Galápagos Islands (Transactions of the Wisconsin Academy of Sciences, Arts and Letters, 1915), vol. 18, pp. 272–340. [Google Scholar]
- 36.Wiggins I. L., Porter D. M., Flora of the Galapagos Islands (Stanford University Press, Stanford, CA, 1971). [Google Scholar]
- 37.Pauly D., Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Hoff S., Drømmen om Galapagos (Grodahl & Son, Oslo, Norway, 2014). [Google Scholar]
- 39.Laegaard S., Pozo García P., Invasive grasses in the Galapagos Islands. Lyonia 6, 171–175 (2004). [Google Scholar]
- 40.Seberg O., Taxonomy and phytogeny of the genus Acalypha (Euphorbiaceae) in the Galápagos Archipelago. Nord. J. Bot. 4, 159–190 (1984). [Google Scholar]
- 41.Schofield E. K., Effects of introduced plants and animals on island vegetation: Examples from Galápagos Archipelago. Conserv. Biol. 3, 227–239 (1989). [Google Scholar]
- 42.Colinvaux P. A., Schofield E. K., Historical ecology in the Galapagos Islands. I. A Holocene pollen record from El Junco Lake, Isla San Cristobal. J. Ecol. 64, 986–1012 (1976). [Google Scholar]
- 43.Urquía D., et al. , Psidium guajava in the Galapagos Islands: Population genetics and history of an invasive species. PLoS One 14, e0203737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jäger H., Kowarik I., Tye A., Destruction without extinction: Long-term impacts of an invasive tree species on Galápagos highland vegetation. J. Ecol. 97, 1252–1263 (2009). [Google Scholar]
- 45.Russello M. A., et al. , A cryptic taxon of Galápagos tortoise in conservation peril. Biol. Lett. 1, 287–290 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciofi C., et al. , “Genetics and conservation on islands: The Galapagos giant tortoise as a case study” in Population Genetics for Animal Conservation G. Bertorelle, M. W. Bruford, H. C. Hauffe, A. Rizzoli, C. Vernesi, Eds. (Cambridge University Press, Cambridge, 2009), pp. 269–293. [Google Scholar]
- 47.Vince G., Conservation ecology. Embracing invasives. Science 331, 1383–1384 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Donlan J., Re-wilding North America. Nature 436, 913–914 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Gibbs J. P., Sterling E. J., Zabala F. J., Giant tortoises as ecological engineers: A long‐term quasi‐experiment in the Galápagos Islands. Biotropica 42, 208–214 (2010). [Google Scholar]
- 50.Tapia W., Goldspiel H. B., Gibbs J. P., Introduction of giant tortoises as a replacement “ecosystem engineer” to facilitate restoration of Santa Fe Island, Galapagos. Restor. Ecol. 30, e13476 (2022). [Google Scholar]
- 51.Bush M. B., Restrepo A., Collins A. F., Galápagos history, restoration, and a shifted baseline. Restor. Ecol. 22, 296–298 (2014). [Google Scholar]
- 52.Battarbee R. W., Kneen M. J., The use of electronically counted microspheres in absolute diatom analysis. Limnol. Oceanogr. 27, 184–188 (1982). [Google Scholar]
- 53.Faegri K., Iversen J., Textbook of Pollen Analysis (Wiley, Chichester, UK, ed. 4, 1989). [Google Scholar]
- 54.Rozas-Davila A., Correa-Metrio A., McMichael C. N., Bush M. B., When the grass wasn’t greener: Megafaunal ecology and paleodroughts. Quat. Sci. Rev. 266, 107073 (2021). [Google Scholar]
- 55.Blaauw M., Christen J. A., rbacon: Age-Depth Modelling Using Bayesian Statistics (R Package Version 2.4, 2018).
- 56.Hill M. O., DECORANA. A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging (Ecological and Systematics Department, Cornell University, Ithaca, NY, 1979). [Google Scholar]
- 57.Oksanen J., et al. , vegan: Community Ecology Package (Version 2.5-6, The Comprehensive R Archive Network, 2019).
- 58.Busha M. B., et al., Galapagos pollen and spores. GitHub. https://github.com/markbbush/Galapagos-pollen-and-spores.git. Deposited 19 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fossil pollen and spore data reported in this article have been deposited in the Neotoma Paleoecology database (https://www.neotomadb.org) and at GitHub (https://github.com/markbbush/Galapagos-pollen-and-spores.git) (58).
All study data are included in the article and/or SI Appendix.