Significance
This randomized, controlled trial demonstrates that by relieving a constraint on household nutritional assets, here through reducing chicken mortality through vaccination, households make dietary choices for young children that increase consumption of protein- and micronutrient-rich foods and decrease relative consumption of high-carbohydrate, low-protein grains. The study provides causal evidence that this shift in diet results in improved height for age, a key measure of childhood stunting. Given the high prevalence of childhood growth failure in rural Africa, these results highlight the potential to increase the utility of a common household animal asset to reduce the burden of childhood stunting in these communities.
Keywords: child growth, nutrition, household decisions, animal source foods
Abstract
Childhood growth faltering remains unacceptably high in sub-Saharan Africa. Rural communities dependent on household food production with limited off-farm income or liquid assets to bridge seasonal food availability are especially vulnerable. A cross-sectional survey in Siaya County, Kenya identified 23.5 and 4.8% of children under 5 y of age as stunted and wasted, respectively, using height-for-age Z (HAZ) scores to detect stunting and weight-for-height Z (WHZ) scores for wasting. Although these households are classified as living in poverty or extreme poverty with very limited off-farm income, households commonly have on-farm resources that could be developed to improve nutrition. While 95% of these households have chickens and consumption of eggs was shown to increase childhood growth by an average of 5%, the average flock size is small and constrained by high mortality due to infectious disease. We hypothesized that interventions to relieve this constraint would translate into household decisions influencing the diets and growth of children. Here, we show that vaccination of chickens against Newcastle disease has a causal impact on children’s consumption of animal source foods rich in protein and micronutrients relative to a high-carbohydrate, grain-based diet. Children in treatment households (chicken vaccination) showed overall increases in scores for both HAZ and WHZ relative to control households, benefiting both girls and boys. The findings demonstrate the impact of directing interventions at common on-farm assets managed by women in rural communities and support programs to enhance productivity at the household level.
Child growth failure (stunting, wasting, and underweight in children under the age of 5) remains a major burden on the development of individuals, families, communities, and nations (1). Dramatic reductions in child growth failure are required to meet the World Health Organization’s Global Nutrition Targets of a reduction in stunting by 40% and wasting to less than 5% by 2025 (2, 3). Similarly, meeting the United Nations’ Sustainable Development Goals is dependent on these reductions as nutrition underlies 12 of the individual goals (4).
Growth failure, especially stunting and wasting, remains a major health and development challenge in rural western Kenya (5–7). A 2014 cross-sectional survey of 597 households in Siaya County identified that 23.5 and 4.8% of children under 5 y of age were stunted and wasted, respectively, using analyses of height-for-age Z (HAZ) scores to detect stunting and weight-for-height Z (WHZ) scores for wasting (6). Although the majority of these households are classified as living in poverty or extreme poverty with very limited off-farm income, households commonly have on-farm resources that could be developed to improve nutrition and growth outcomes (8, 9). While 95% of these households have chickens and consumption of eggs was shown to increase childhood growth by a mean of 5%, the average flock size is small (∼10, only half of which are potential sources of eggs or meat; the rest are young chicks), and only 16% of children over the age of 6 mo were reported to have consumed eggs in the 3 d prior to the study survey (7, 9). In a follow-up longitudinal census of chickens and decision-making in 1,908 households within the same community, mortality was identified as the primary constraint on flock size, representing 60% of all chicken losses, as opposed to a voluntary household decision to sell, consume, or gift chickens to meet household needs or to maintain a desired flock size based on optimizing the input cost of household labor or other management resources (10). The high mortality of chicks constrained growth of the flock both in total numbers and in composition, as fewer chicks survived to maturity and productivity. We hypothesized that interventions to relieve this constraint of involuntary loss due to infectious disease would translate into increased consumption of protein-rich diets by children and ideally, into improved growth outcomes.
In a two-arm randomized, controlled trial, we first determined whether vaccination against Newcastle disease virus (NDV), widely considered to be the predominant infectious cause of mortality in free range scavenging chickens globally (11, 12), would result in increased flock size. Quarterly vaccination over an 18-mo period resulted in an increase in average flock size from 11.63 ± 0.70 chickens at enrollment to 13.06 ± 0.29 chickens, a significantly greater increase (P = 0.0026) as compared with the unvaccinated arm of the trial (13). Based on this intervention, we analyzed whether NDV vaccination translated to increased children’s consumption of animal source foods (ASFs) rich in protein and micronutrients relative to a high-carbohydrate, low-protein, grain-based diet. Furthermore, we determined whether a shift in consumption affected childhood growth. Herein, we present the results of this analysis and discuss the results in the context of maximizing the utility of a commonly held household resource in addressing childhood growth failure in rural Africa.
Results
Household Participation.
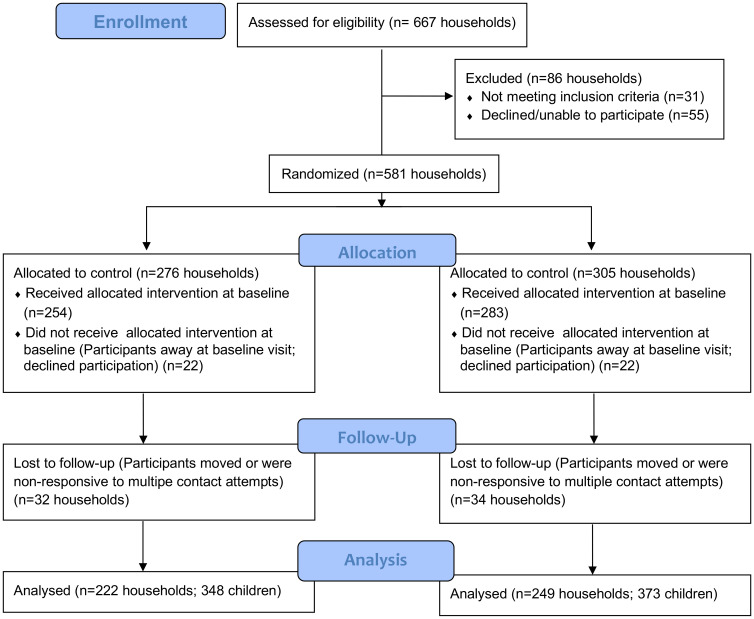
Fig. 1 provides detail on household selection and allocation to the treatment or control arms of the study, participation at baseline, and follow-up during the course of the trial. Not all households provided food consumption data and anthropometric measurements at every quarter, and some are not represented in the final quarter of the 18-mo trial (SI Appendix, Fig. S1, Supplementary Text S1, and Table S1) due to respondent or child absence, illness, or conflicting household activities following three attempts within the scheduled week. Of six planned data collection visits per child, the average number of completed visits per child is 4.24 in the treatment arm (1,307 records total) and 4.32 in the control arm (1,380 records total). Statistical analyses indicated no systematic sample selection affecting the results; nonetheless, inverse probability weighting was applied to minimize potential selectivity bias in estimation results (Materials and Methods and SI Appendix, Supplementary Text S1 and Tables S2 and S3). Final datal cleaning defines the number of records used for analysis (Fig. 1); each record is linked to an individual child within a household as some households had more than one enrolled child.
Fig. 1.
Consort diagram of study participation. Of the 667 households assessed for eligibility, 31 were excluded as not meeting the criteria of having a child <3 y of age and keeping chickens, and 55 declined participation. Households were then randomly allocated by computer to either the treatment arm of the study (vaccination of chickens against NDV plus parasite control) or the control arm (parasite control only). Households not present at baseline or that declined at baseline were excluded from the study, resulting in 254 treatment households and 283 control households entering the study. Households that did not participate in any subsequent quarters after three contact attempts were excluded from analysis, resulting in 222 treatment households with 348 children and 249 control households with 373 children for analysis. All households received the intervention (NDV vaccination and parasite control or parasite control alone) every quarter. Households that were assessed for child diet and growth on all quarterly visits and on multiple but not all quarterly visits were retained in the study (SI Appendix, Fig. S1, Supplementary Text S1, and Table S1). The procedures used to detect and control for any bias in intermittent participation are detailed in Materials and Methods and in SI Appendix, Supplementary Text S1 and Tables S2 and S3.
Demographics and Nutritional Status of Children at Baseline.
Household demographics and nutritional status at baseline are provided in Table 1. Based on HAZ and WHZ determination, stunting and wasting were present in 18.25 and 2.72% of the children, respectively. There were no significant differences between the treatment and control households at baseline (stunting: treatment households, 17.8%; control households, 18.6%; P = 0.793; wasting: treatment households, 3.5%; control households, 2.0%; P = 0.24). Using middle upper arm circumference (MUAC) measurements collected at enrollment, 8.6% of children suffered acute malnutrition: 2.8% with severe acute malnutrition (SAM) and 5.8% with moderate acute malnutrition (MAM).
Table 1.
Demographic characteristics and baseline child growth measurements
| Parameter | Treatment households* | Control households† |
|---|---|---|
| Household size (mean no. of occupants) | 6.3 ± 2.4‡ | 6.1 ± 2.2‡ |
| Daily household income (US dollars) | 2.7 ± 5.6‡ | 2.3 ± 4.7‡ |
| Maternal (caregiver§) age (mean y) | 36.7 ± 15.5‡ | 37.5 ± 17.9‡ |
| Maternal (caregiver§) education level | ||
| No formal education | 7 (2.0%) | 2 (0.54%) |
| Primary education | 234 (66.9%) | 277 (74.8%) |
| Secondary education | 100 (28.6%) | 83 (22.4%) |
| Postsecondary | 9 (2.6%) | 8 (2.2%) |
| Age of children (mean mo) | 21.2 ± 15.2‡ | 21.5 ± 14.8‡ |
| Gender of children, no. female (% female) | 170 (48.7%) | 187 (50.4%) |
| Stunted children¶ | 56 (17.8%) | 65 (18.6%) |
| Wasted children¶ | 11 (3.5%) | 7 (2.0%) |
| Diagnosis of MAM# | 25 (7.2%) | 17 (4.6%) |
| Diagnosis of SAM‖ | 8 (2.3%) | 12 (3.2%) |
*In total, 349 children were assessed at baseline from 221 households in the treatment arm.
†In total, 371 children were assessed at baseline from 246 households in the control arm.
‡Mean ±1 SD.
§Principal respondent and responsible for childcare in households where the mother was not present.
¶Some HAZ and WHZ scores were missing or flagged as suspect and were dropped from all analyses (in Fig. 1 under analysis), providing a total of 663 useable HAZ scores and 661 useable WHZ scores upon which these statistics are based.
#MAM is determined by measuring MUAC. Measurements between 115 and 124 mm indicate MAM.
‖SAM is determined by measuring MUAC. Measurements <115 mm indicate SAM.
Impact of Breastfeeding and Child Age on Food Consumption.
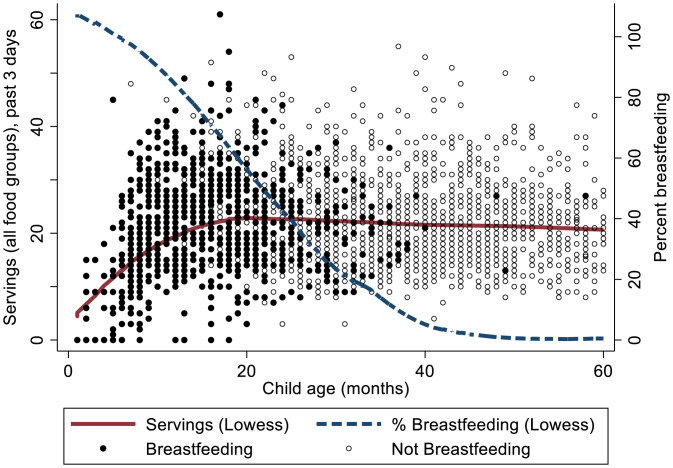
At baseline, 50.1% of children were breastfeeding (treatment households, 49.6%; control households, 50.7%; P = 0.76). In Fig. 2, food intake is represented by the sum of servings in the four food groups other than breast milk (ASFs, fruit, vegetables, grains) and plotted relative to breastfeeding and child age. The number of nonbreast milk food servings increases at a decreasing rate until ∼18 mo of age and thereafter, remains relatively constant with a slight decline, while the percentage of children being breastfed declines from ∼100% and approaches 0 around 40 mo (Fig. 2).
Fig. 2.
Transition from breastfeeding to solid foods by child age. The total number of solid food servings increases through about age 1.5 y (18 mo) and then, remains relatively constant with slight decline, while the fraction of children being breastfed declines to zero.
These dynamics of breastfeeding and food intake are captured in the four food intake regressions (Table 2) by the interactions between an age category indicator (≤18 or >18 mo), a breastfeeding indicator, child age, and child age squared. Overall, the regression results associated with these control variables are consistent with the intuitive progression of increasing solid food intake through 18 mo and that children not being breastfed tend to eat more solid foods than those breastfeeding. For example, the negative value on >18 mo × Breastfeeding of −0.179 in the Table 2 ASF regression indicates that children >18 mo of age being breastfed eat ∼18% less ASF than those in this age category not being breastfed (the base case, P = 0.077), and for children 18 mo or younger, children breastfeeding are fed less of each food category than their nonbreastfed counterparts (joint P value = 0.055). The parameters on ≤18 mo × Child Age and ≤18 mo × Child Age Sq. in the ln(A) regression of 0.234 and −0.007, respectively, show that for those ≤18 mo of age, ASF consumption increases at a decreasing rate until about 16.7 mo, consistent with the shape of the locally weighted scatterplot smoothing (LOWESS) regression line for total servings (Fig. 2). Similar results hold for the other food groups, with some statistically insignificant exceptions (Table 2). For children over the age of 18 mo, the age effects are not statistically significant except for a slight decline in grains consumption, also consistent with the nearly flat LOWESS curve after 18 mo.
Table 2.
Consumption of ASF, fruits, vegetables, and grains
| Independent variable† | Food category consumption (dependent variable)‡ | |||
|---|---|---|---|---|
| ln(A) | ln(F) | ln(V) | ln(G) | |
| Treatment × Months in Trial | 0.013* | 0.013 | 0.000 | −0.006 |
| Treatment | −0.038 | −0.140 | −0.144* | 0.003 |
| Time in Trial (mo) | 0.015*** | 0.034*** | 0.000 | 0.002 |
| MAM @ one or more visits | −0.119 | −0.404** | −0.186 | 0.067 |
| SAM @ one or more visits | −0.596**** | −0.598* | −0.291 | −1.186** |
| Time since MAM | 0.000 | 0.008 | 0.009 | 0.004 |
| Time since SAM | 0.053**** | 0.040 | 0.011 | 0.107** |
| >18 mo × Breastfeeding | −0.179* | −0.253* | −0.113**** | −0.092* |
| ≤18 mo × Not breastfeeding | −1.664*** | −3.541*** | −4.586*** | −6.240*** |
| ≤18 mo × Breastfeeding | −2.461*** | −4.037*** | −5.095*** | −6.542*** |
| >18 mo × Child Age | 0.007 | −0.027 | 0.002 | −0.003 |
| ≤18 mo × Child Age | 0.234*** | 0.445*** | 0.556*** | 1.054*** |
| >18 mo × Child Age sq. | 0.000 | 0.000 | 0.000 | 0.000 |
| ≤18 mo × Child Age sq. | −0.007** | −0.016*** | −0.016*** | −0.040*** |
| ln(Per capita income) | 0.016**** | 0.002 | 0.008 | −0.004 |
| Female Child | 0.016 | −0.001 | 0.024 | −0.039 |
| Mother’s (Caregiver§) Age | 0.001 | −0.006** | 0.004** | 0.002**** |
| Mother’s (Caregiver§) Education Level | 0.185*** | 0.126**** | 0.031 | 0.062** |
| cosmonth | 0.019 | 0.314*** | 0.073** | 0.049** |
| sinmonth | 0.035 | −0.238*** | −0.093*** | 0.017 |
| Intercept | 0.001 | 0.810 | 1.063*** | 2.321*** |
The number of observations for all equations is 2,549. The estimation method is the structural equation model with random effects at the child level. Food intake regressions are estimated simultaneously with the HAZ and WHZ regressions presented in Table 3 using the Stata 17 gsem routine with random effects at the individual child level. *Statistically significant at P < 0.1; **statistically significant at P < 0.05; ***statistically significant at P < 0.01; ****statistically significant at P < 0.15.
†Independent variables include the NDV vaccination treatment group (the control group is the base case), time since first diagnosis of MAM (MUAC of 115 to 124 mm) or SAM (MUAC of <115 mm), time in the trial in months, child age and breastfeeding status (18 mo+ × not breastfeeding is the base case), logarithm of per capita income, gender of the child, mother’s age, mother’s education level, and month of the year (to reflect seasonality). The use of 18 mo as an age reference is based on our data that the transition from breastfeeding as the primary source of child nutrition through a period of increased intake of other food sources occurs up to month 18 (Fig. 2). × represents the interaction between two variables.
‡Dependent variables are the natural logarithm of the number of servings for each food consumption category (ASF [A], fruits [F], vegetables [V], grains [G]).
§Principal respondent and responsible for child care.
Not unexpectedly, children ≤18 mo of age, regardless of breastfeeding status, consume less ASF than children >18 mo as implied by the negative associated parameter estimates (Table 2). This may explain, in part, the observation that servings slightly decrease for older children (Fig. 2) if they are consuming higher-calorie foods. However, a fractional multinomial logit model (SI Appendix, Supplementary Text S2) shows that the ASF share of total servings is larger for younger children, ≤18 mo, than for older children and is largest for younger children not breastfeeding (SI Appendix, Table S4). For the latter (≤18 mo × Not breastfeeding in SI Appendix, Table S4), the parameter estimate is 2.450 (P < 0.001). This represents an over 11-fold larger ratio of ASF to grains in children’s diet relative to the base case of children >18 mo and not breastfeeding [calculated as Exp(2.450) = 11.59]. Comparing the two parameter estimates for young children, the difference is 2.450 − 2.203 = 0.247 (SI Appendix, Table S4). Exponentiating provides Exp(0.247) = 1.28, implying that children ≤18 mo not breastfeeding consume 28% more ASF than children ≤18 mo breastfeeding. Taken together, the results comparing the combination of older vs. younger children and children breastfeeding or not show that while children ≤18 mo consume less ASF than children >18 mo (Table 2), their share of ASF servings is substantially larger relative to grains than in older children, and breastfeeding children consume relatively less ASF than nonbreastfeeding children of the same age group (SI Appendix, Table S4).
Impact of Household Income on Food Group Consumption.
Because the dependent variables in the food intake regression (Table 2) and per capita income (household income divided by number of household members) are both in logarithmic form, the parameters associated with per capita income represent elasticities: the percentage change in the number of total food servings in response to a 1% increase in per capita income. Elasticity is positive for ASF, fruit, and vegetables, while the elasticity is negative for grains (Table 2).
Additional perspective on how income affects substitution between food groups is provided from a fractional multinomial logit model presented in SI Appendix, Supplementary Text S2 and Table S4. Consumption of ASF and vegetables is significantly higher relative to grains (P < 0.068 and P < 0.031, respectively) for households with higher incomes, with the increase in ASF consumption higher than that of vegetables (SI Appendix, Table S4).
Impact of Season on Food Group Consumption.
Using the sine and cosine of the scaled month of year to capture seasonality, consumption of fruits, grains, and vegetables shows significant seasonal intake cycles (P < 0.01) (Table 2), whereas there is little evidence of seasonality for ASF. This is consistent with seasonal production and the limited ability to store fruit, vegetables, and to a lesser extent, grains at the household level as compared with more consistent availability of ASF. This seasonal relationship for fruits and vegetables but not ASF is also observed in the fractional multinomial logit model, where consumption is referenced relative to grains (SI Appendix, Table S4).
Impact of Household NDV Vaccination of Chickens on Food Group Consumption.
The effect of being in the treatment group (a household with quarterly NDV vaccination of all chickens) is captured by the parameter associated with the Treatment Group × Time in trial . A positive parameter estimate means that consumption for that food group increases over the course of the trial faster (and more) in the treatment group relative to intake of that food group by children in the control arm of the study (base case). ASF consumption increased faster in the treatment group by 1.3% per month (based on a parameter estimate of 0.013; P = 0.089) (Table 2). This implies a 24% increase in ASF consumption relative to the control group by the end of the trial. In contrast, estimated consumption of grains decreased in the treatment group relative to the control group over the course of the trial. These results suggest that the vaccination of chickens against NDV may have made ASF more available to the point that households substituted children’s food toward ASF and away from the other food groups, especially grains.
We examined this substitution effect associated with NDV treatment using the fractional multinomial logit model as well (SI Appendix, Supplementary Text S2 and Table S4). The results indicate that the share of ASF relative to grains increases at a significantly faster rate in the treatment group (P = 0.081), supporting the conclusion that NDV vaccination is inducing or allowing households to substitute toward ASF and away from grains as the trial proceeds. In contrast, there are no significant changes in the shares of consumed fruits and vegetables between the treatment and control groups (P = 0.450 and P = 0.645, respectively) (SI Appendix, Table S4).
Impact of Food Consumption on Child Growth.
For HAZ, higher average ASF consumption over the course of the trial has a relatively large, statistically strong, positive impact (0.165; P = 0.014) for children over 18 mo of age (Table 3). This result is consistent with the cumulative importance of protein consumption on HAZ as a growth measure (14). In contrast, average grain consumption over previous visits had a negative effect on HAZ for older children (−0.234; P = 0.095). For WHZ, the food consumption effects were mixed. Most statistically significant effects were negative, although the effect of vegetable consumption for older children was positive and significant (0.037; P = 0.027). The rest of the effects of food intake on older children are negative and/or are not significant at conventional test sizes. The coefficients on Time since MAM and Time since SAM are positive, suggesting that the intervention in response to MAM and SAM positively affected WHZ but not HAZ. Consistent with the seasonality of fruit and vegetable consumption (Table 2 and SI Appendix, Table S4), there appear to be seasonal effects on WHZ (Table 3). Note that sin(month) and cos(month) were omitted for HAZ because of the longer time frame of HAZ development.
Table 3.
Impacts on child growth: WHZ and HAZ
| Independent variable† | Child Z score (dependent variable) | |
|---|---|---|
| HAZ | WHZ | |
| Treatment × Month in Trial | 0.007 | 0.006 |
| Treatment | −0.205* | 0.108 |
| Time in Trial (mo) | 0.023*** | 0.006**** |
| MAM @ one or more visits | −1.217*** | −0.777*** |
| SAM @ one or more visits | −0.569* | −0.900*** |
| Time since MAM | −0.009 | 0.020**** |
| Time since SAM | −0.013 | 0.049** |
| >18 mo × ln([Avg]A)‡ | 0.165** | −0.019 |
| ≤18 mo × ln([Avg]A) | −0.029 | −0.017 |
| >18 mo × ln([Avg]F) | 0.070 | −0.022** |
| ≤18 mo × ln([Avg]F) | 0.000 | −0.029 |
| >18 mo × ln([Avg]V) | 0.081 | 0.037** |
| ≤18 mo × ln([Avg]V) | −0.036 | −0.092*** |
| >18 mo × ln([Avg]G) | −0.234* | −0.003 |
| ≤18 mo × ln([Avg]G) | −0.061 | −0.111*** |
| >18 mo × Breast(fed) [feeding]§ | −0.338** | −0.102* |
| ≤18 mo × Not breast(fed) [feeding] | 0.644 | 0.349** |
| ≤18 mo × Breast(fed) [feeding] | −0.685**** | 0.434*** |
| Child Age | −0.064*** | −0.022** |
| Child Age sq. | 0.001** | 0.000* |
| ln(Per capita income) | 0.028** | −0.007 |
| Female Child | 0.154**** | 0.076 |
| Mother’s (Caregiver’s) Age | 0.004 | 0.000 |
| Mother’s (Caregiver’s) Education Level | 0.096 | 0.050* |
| cosmonth | 0.052*** | |
| sinmonth | −0.045*** | |
| Intercept | 0.095 | −0.179 |
WHZ and HAZ scores are the dependent variables. The number of observations for all equations is 2,549. The estimation method is the structural equation model with random effects at the child level. Regressions are estimated (simultaneously with food category regressions in Table 2) using the gsem routine in Stata 17 with random effects at the individual child level. *Statistically significant at P < 0.1; **statistically significant at P < 0.05; ***statistically significant at P < 0.01; ****statistically significant at P < 0.15.
†Independent variables include the NDV vaccination treatment group (the control group is the base case), time since first diagnosis of MAM (MUAC of 115 to 124 mm) or SAM (MUAC of <115 mm), time in the trial in months, child age and breastfeeding status (18 mo+ × not breastfeeding is the base case), logarithm of per capita income, gender of the child, mother’s (or caregiver’s) age, mother’s (or caregiver’s) education level, and month of the year (to reflect seasonality). The use of 18 mo as an age reference is based on our data that the transition from breastfeeding as the primary source of child nutrition through a period of increased intake of other food sources occurs up to month 18 (Fig. 2). × represents the interaction between two variables.
‡Average servings reported over past visits were used for the HAZ regression, and current reported servings (last 3 d) were used for the WHZ regression. SI Appendix, Supplementary Text S3 and Table S5 have a robustness analysis of this specification. For the WHZ regression, ln(Food Group) is the logarithm of servings for the current visit. For the HAZ regression, ln(Food) is the logarithm of average servings for that food category reported in all household visits to date.
§For the WHZ regression, Breastfed and Not Breastfed indicate whether a child is currently being breastfed. For the HAZ regression, they indicate whether a child has ever been breastfed during the trial period to date.
Breastfeeding patterns have qualitatively similar effects on WHZ and HAZ (Table 3). The base case for comparison is children >18 mo of age not ever breastfed during the study period for HAZ and not currently breastfed for WHZ. Conditional on food consumption, older children being breastfed have lower HAZ and WHZ than older children not being breastfed. Younger children being breastfed have higher estimated WHZ than younger children not being breastfed, conditional on food intake. In contrast, older children being breastfed have lower WHZ than older children not being breastfed (−0.102; P = 0.085). These differences may reflect other unobserved differences in the diets of older breastfed children that affect these outcomes. Surprisingly, younger children never breastfed during the study have higher HAZ than younger children who have been breastfed [0.644 – (−0.685) = 1.329; P = 0.001]. This unintuitive result could be due to the coarseness of the variable “Has breastfed” (i.e., it may not capture either breastfeeding in the “Never breastfed” children that occurred just prior to study enrollment or the duration of breastfeeding in the “breastfed” children during the study). These effects are qualitatively the same across different specifications, including breastfeeding status (SI Appendix, Supplementary Text S3 and Table S5).
Direct, Indirect, and Total Impacts of Household NDV Vaccination of Chickens on Child Growth.
The increased child consumption of ASF in treatment households, both absolute and relative to grains (Table 2 and SI Appendix, Table S4), represents the primary driver of the effects of NDV vaccination on growth outcomes mediated through changes in food consumption, denoted as indirect effects. In addition, the WHZ and HAZ regressions show that there are also methodologically defined direct effects of treatment that are captured by our data but are not accrued to the nutritional data we collected. These may be treatment group impacts on nutrition not included or accurately measured in our assessments as well as unidentified behavioral or household management changes linked to being in the treatment arm of the study. Eq. 3 and associated discussion (Materials and Methods) describe how the parameter estimates (Tables 2 and 3) are used to calculate the indirect food consumption effects and the total effects of being in a treatment household on child growth.
The estimated direct, calculated indirect, and calculated total treatment effects measured at 18 mo after initiation of treatment are provided in Table 4 under the headings “Average treatment effect: HAZ” and “Average treatment effect: WHZ.” For HAZ, the estimated direct monthly NDV vaccine treatment effect is 0.0071 (P = 0.380), the indirect effect is 0.0045 (P = 0.084), and the total monthly effect is 0.0116 (P = 0.170). While the estimated direct effect is larger in magnitude than the indirect effect, the indirect effect leading to an increase in HAZ through food intake is statistically more compelling. Over the trial period of 18 mo, these estimates translate to a direct effect of 0.1269, an indirect effect of 0.0817, and a total effect of 0.2087 (P values are the same as corresponding monthly effects). For WHZ, the monthly estimated increase in WHZ through the direct effect is 0.0060 (P = 0.253; 0.1074 over 18 mo), the indirect effect is −0.0005 (−0.0095 over 18 mo; P = 0.286), and the total estimated effect is 0.0054 (0.0979 over 18 mo; P = 0.302).
Table 4.
Estimated direct, indirect, and total effects of treatment (NDV vaccination), time in trial, and MAM and SAM diagnoses and interventions
| Estimate | P > |z| | 90% CI | |||
|---|---|---|---|---|---|
| Average treatment effect: HAZ† | |||||
| Direct | Average monthly | 0.0071 | 0.380 | −0.0062 | 0.0203 |
| Indirect | Average monthly | 0.0045* | 0.084 | 0.0002 | 0.0089 |
| Total | Average monthly | 0.0116 | 0.170 | −0.0023 | 0.0255 |
| Direct | Full trial | 0.1269 | 0.380 | −0.1111 | 0.365 |
| Indirect | Full trial | 0.0817* | 0.084 | 0.0039 | 0.1596 |
| Total | Full trial | 0.2087 | 0.170 | −0.0416 | 0.459 |
| Average treatment effect: WHZ† | |||||
| Direct | Average monthly | 0.0060 | 0.253 | −0.0026 | 0.0145 |
| Indirect | Average monthly | −0.0005 | 0.286 | −0.0013 | 0.0003 |
| Total | Average monthly | 0.0054 | 0.302 | −0.0032 | 0.0141 |
| Direct | Full trial | 0.1074 | 0.253 | −0.0470 | 0.2619 |
| Indirect | Full trial | −0.0095 | 0.286 | −0.0243 | 0.0052 |
| Total | Full trial | 0.0979 | 0.302 | −0.0582 | 0.254 |
| Time in trial: HAZ‡ | |||||
| Direct | Average monthly | 0.0227*** | 0.008 | 0.0086 | 0.0368 |
| Indirect | Average monthly | 0.0045** | 0.043 | 0.0008 | 0.0082 |
| Total | Average monthly | 0.0272*** | 0.001 | 0.0135 | 0.0409 |
| Direct | Full trial | 0.4088** | 0.008 | 0.1546 | 0.6629 |
| Indirect | Full trial | 0.0810** | 0.043 | 0.0151 | 0.1470 |
| Total | Full trial | 0.4898*** | 0.001 | 0.2432 | 0.7364 |
| Time in trial: WHZ‡ | |||||
| Direct | Average monthly | 0.0063 | 0.138 | −0.0007 | 0.0134 |
| Indirect | Average monthly | −0.0011** | 0.045 | −0.0019 | −0.0002 |
| Total | Average monthly | 0.0053 | 0.219 | −0.0018 | 0.0124 |
| Direct | Full trial | 0.1143 | 0.138 | −0.0126 | 0.2411 |
| Indirect | Full trial | −0.0191** | 0.045 | −0.0348 | −0.0034 |
| Total | Full trial | 0.0951 | 0.219 | −0.0322 | 0.2225 |
| Time since MAM and SAM diagnosis intervention effect: HAZ‡ | |||||
| Direct | MAM | −0.0095 | 0.607 | −0.0398 | 0.0208 |
| Indirect | MAM | 0.0003 | 0.954 | −0.0076 | 0.0081 |
| Total | MAM | −0.0092 | 0.617 | −0.0395 | 0.0211 |
| Direct | SAM | −0.0133 | 0.562 | −0.0510 | 0.0244 |
| Indirect | SAM | −0.0124 | 0.429 | −0.0382 | 0.0134 |
| Total | SAM | −0.0257 | 0.312 | −0.0675 | 0.0161 |
| Time since MAM and SAM diagnosis intervention effect: WHZ‡ | |||||
| Direct | MAM | 0.0202 | 0.130 | −0.0018 | 0.0421 |
| Indirect | MAM | 0.0001 | 0.870 | −0.0011 | 0.0013 |
| Total | MAM | 0.0203 | 0.129 | −0.0017 | 0.0423 |
| Direct | SAM | 0.0494* | 0.042 | 0.0094 | 0.0893 |
| Indirect | SAM | −0.0017 | 0.708 | −0.0094 | 0.0059 |
| Total | SAM | 0.0476* | 0.056 | 0.0067 | 0.0886 |
Average treatment effect estimates for HAZ and WHZ are based on Eq. 3 (Materials and Methods) and the applicable parameter estimates from regressions presented in Tables 2 and 3. Time in trial effects were calculated based on Eq. 5 and parameter estimates from Tables 2 and 3. MAM and SAM diagnosis and intervention effects (applied to both the primary treatment group and the control group) are calculated based on Eq. 4 (Materials and Methods) and parameter estimates from Tables 2 and 3. All estimates in this table and associated P values and CIs were generated using Stata 17 nlcom routine. *Statistically significant at P < 0.10; **statistically significant at P < 0.05; ***statistically significant at P < 0.01.
†Treatment households only.
‡All households.
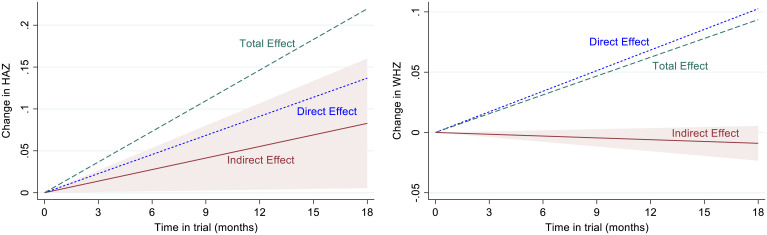
These results suggest a statistically clear positive effect of the NDV vaccination of household chickens on HAZ through changes in food consumption and especially, ASF consumption. The total effect of the NDV treatment on WHZ is positive but statistically weaker. Figs. 3 and 4 provide additional perspective on these outcomes. Fig. 3 illustrates total treatment effects on HAZ (Fig. 3, Upper) and WHZ (Fig. 3, Lower) in the context of the distribution of children in the control group at the end of the trial (trial participation through a minimum of 15 mo). Average total treatment effect for HAZ is about 17% of one SD of HAZ [100 × (0.2087/1.223)] of the control group at the end of the trial. The average total treatment effect for WHZ is about 10.6% of one SD of WHZ [100 × (0.0979/0.9221)]. Fig. 4 illustrates the estimated time path of average treatment effects over the course of the trial. The indirect component of the treatment effect for HAZ is the positive maroon line (Fig. 4, Left), with slope 0.0045 (P = 0.084) representing the cumulative indirect effect of observed food consumption differences between treatment and control groups over the course of the trial. The maroon shaded triangle is the associated 90% CI for the cumulative indirect effect. Estimated direct effects are larger but statistically weaker, suggesting there are other impacts of the NDV treatment process that are not captured by our data on food consumption and these regressions. In contrast, the indirect effect of NDV treatment on WHZ through food consumption (Fig. 4, Right) is negative, although small and not statistically different from zero (monthly effect = slope = −0.0005; P = 0.286). This weak indirect effect is more than compensated for by the positive direct effects. The opposing indirect treatment effects, strongly positive for HAZ and weakly negative for WHZ, suggest an apparent trade-off in growth characteristics due to the substitution away from grains toward other food groups, especially ASF, as shown in SI Appendix, Table S4. The estimated direct and total treatment effects are represented by the blue dashed lines and the green dashed lines, respectively, in each panel (Fig. 4).
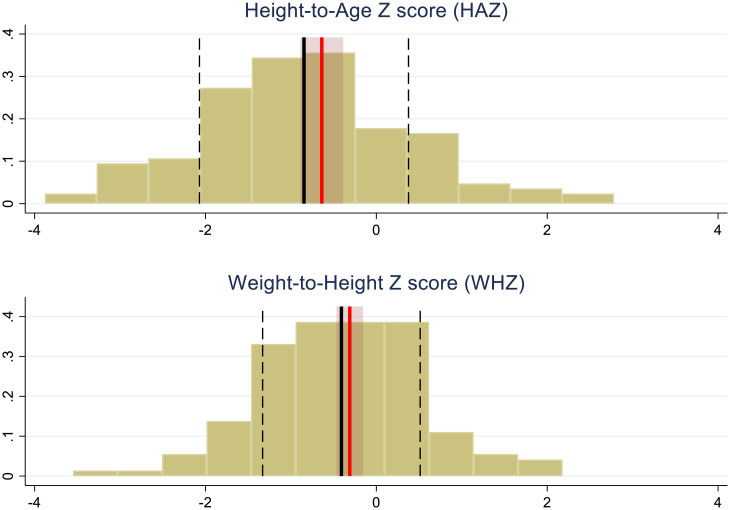
Fig. 3.
Overall effect of being in a treatment group household on child growth relative to the control group. The histograms represent the distributions of HAZ and WHZ scores for control group age over 18 mo of age for those who have been in the trial at least 15 mo. Thick solid black vertical lines are control group means, and the dashed black lines are one SD from the distribution mean (SDs are 1.22 for HAZ and 0.922 for WHZ). The red lines represent the mean Z score plus the total effect of treatment (direct plus indirect effects; 0.209 for HAZ and 0.098 for WHZ). The lightly shaded maroon areas are 90% CIs for the total treatment effect; P values for the total effects are P = 0.170 for HAZ and P = 0.302 for WHZ. Estimates for average total effects and CIs are taken from Table 4.
Fig. 4.
Estimated direct, indirect, and total effects of treatment on child growth over the course of the trial. The estimated indirect effect of vaccinating household chickens on child growth, through the effect on food intake, is the maroon solid line in each panel. The marginal monthly indirect effect is 0.0045 (P = 0.084) for HAZ and −0.0005 (P = 0.286) for WHZ (Table 4). These estimates are the slopes of the indirect effect lines in each panel. The maroon shaded triangles are 90% CIs for the indirect effects. Direct effects (blue dashed lines) and total effects (green dashed lines) are shown without CIs to limit visual complexity. The monthly total treatment effects are 0.0116 (P = 0.17) for HAZ and 0.0054 (P = 0.30) for WHZ, which are the slopes of the green dashed lines for HAZ and WHZ, respectively (Table 4). Fig. 3 provides a complementary perspective on the total treatment effect. Table 4 estimates are based on regression results presented in Tables 2 and 3.
Effects of Trial Participation.
Time in trial (months) captures any general effect on food consumption that might result from participating in the trial that is not otherwise captured as a treatment effect, such as the effect of chicken parasite control in both arms of the trial or the effects of other time-varying factors not otherwise controlled for in the regressions, whether they are induced by the trial itself or not (e.g., changes in weather or community economic conditions). The direct and indirect effects of Time in trial are calculated using parameter estimates from Tables 2 and 3 (based on Eq. 5 in Materials and Methods) and are summarized in Table 4. The monthly direct effect on HAZ of being in the trial is 0.0227 (P = 0.008), the indirect monthly effect through food consumption changes during the trial is 0.0045 (P = 0.043), and the total monthly effect is 0.0272 (P = 0.001). The total effect on HAZ over the full trial period for children 18 mo or older is 0.498 (P = 0.001). Time in trial effects for WHZ are mixed (Table 4). Monthly direct and total effects are positive but not statistically significant. Indirect monthly effects of time in trial on WHZ are −0.0011 (P = 0.045). The total effect over the trial period on WHZ for children 18 mo and older is 0.0951 (P = 0.219).
Impact of Nutritional Interventions for Acute Malnutrition.
If nutrition counseling after a finding of MAM or counseling and food supplementation after a finding of SAM had positive effects over time on the intake of a food category, then the associated parameters [Time since first MAM and Time since first SAM represented in Eqs. 1 and 2 as ] (Materials and Methods) should be positive (after controlling for baseline MAM and SAM incidence). Table 2 shows that the signs of the parameters on Time since MAM and Time since SAM are all positive in the food intake equations, although significance is weak. The two strongest effects are due to Time since SAM; ASF increases by about 5.3% per month (0.053; P = 0.129), and grains increase by about 10% per month (P = 0.014). These results suggest that counseling effectively promotes ASF consumption within the household as the supplemental feeding provided upon an SAM diagnosis during the trial did not have a specified ASF component. As with the NDV vaccination treatment effect, the nutritional interventions have both indirect and direct effects on HAZ and WHZ, shown in Table 4 (bottom two blocks of results; direct effects are also shown in the HAZ and WHZ regression results in Table 3). The estimated effects of MAM and SAM treatments on HAZ are all negative. This is somewhat unintuitive, especially given the effect of SAM on ASF consumption in the food regressions. Including MAM @ one or more visits is intended to control for the fact that HAZ will tend to be lower in children diagnosed with MAM. It could be that this summary statistic may not be fully controlling for this baseline characteristic sufficiently. In contrast, however, the effects of time since MAM and SAM have consistently positive effects on WHZ, except for the indirect effect of SAM. Despite this, the total estimated monthly increase in WHZ in response to an intervention for SAM is about 4.8% (P = 0.056). MAM interventions were information based, and SAM interventions were implemented on a quarterly timescale (between visits).
Effect of household income.
Households with higher per capita income show greater ASF consumption (Table 2), and conditional on food intake, HAZ is higher in higher-income households (0.028; P = 0.045) (Table 3). The total direct and indirect effect of household income on HAZ is 0.032 (P = 0.018). The direct and total effects of higher income on WHZ were not statistically significant (P = 0.373 and P = 0.270, respectively).
Effect of child gender.
Girls had weakly higher HAZ and WHZ as compared with boys (Table 3). The higher HAZ scores as measured directly with the Female Child indicator variable are in addition to the implied HAZ benefits from weakly higher ASF consumption (Table 2) and in ASF consumption relative to grains (SI Appendix, Table S4) shown for girls. The total direct and indirect estimated difference in HAZ of girls relative to boys is 0.15, indicating that growth benefits accrued at least equally to girls as well as boys.
Discussion
Despite measurable progress over the past two decades, childhood growth faltering, especially stunting, remains unacceptably high in many countries and communities in Africa (1, 6). Rural communities highly dependent on household food production and with limited off-farm income or liquid assets to bridge seasonal food availability are especially vulnerable (15, 16). The study community in Siaya County, Kenya reflects this vulnerability. We have focused on chickens as a widely held autochthonous resource, managed at the household level by women, that can provide foods high in protein and rich in micronutrients that are critically important in preventing stunting (17–19). We initiated the vaccine intervention recognizing that the pathway from veterinary vaccination to increased chicken productivity through household decision-making to improved nutrition and child growth is complex and impacted by multiple known and unknown factors. Nonetheless, our data show statistically significant impacts on the intermediate measure of increased ASF consumption, estimated at a 24% increase over the course of the trial, that translated into improved child growth parameters. This supports concerted NDV vaccination of household chickens, for which willingness to pay studies indicate strong household interest (20), as part of a multipronged approach to enhance childhood nutrition and reduce stunting in rural communities where chickens are a common household resource.
The gains measured in treatment households relative to control households very likely underestimate the impact of NDV vaccination on ASF consumption and childhood growth due to several inherencies of the trial design. First is that, for ethical reasons, the control households also received an intervention: medication for parasites in their chickens (as did the treatment households in addition to NDV vaccination). This medication plus any unmeasured veterinary advice provided at the time of treatment is reflected in the increase in flock size in the control households over baseline (13) and may be a driver of the significant effect of “time in trial” effects on ASF intake reported here. The second is that vaccine was only delivered at quarterly intervals—sufficient to maintain immunity in previously vaccinated chickens but misses all chicks hatched in the interim, the age group most susceptible to dying from NDV (11, 21). This challenge is addressed in the final discussion paragraph. Third, all households received data on their children’s growth and ad hoc dietary and poultry management guidance that may have influenced their decisions on both flock management and children’s diets. Notably, girls appear to be at least equal beneficiaries of the substitution toward ASF consumption. Although not linked to a treatment effect, overall the coincident increase in both HAZ and WHZ in girls relative to boys is discrepant from prior studies in other regions of Kenya, where girls had significantly lower HAZ and WHZ (22). We speculate that this reflects the primary role of women in management of household poultry linked to dietary consumption choices (23–26).
Translating the increase in flock size gained through NDV vaccination to a change in a young child’s diet is mediated through a household decision by, in rural Kenya, usually maternal or a female relative (6, 7, 17, 24, 27). The most significant increase in flock size in treatment households as compared with control households was due to an increase in laying hens (13). Based on prior studies of indigenous chicken productivity in this region, an increase in one hen per flock would result in average production of six to seven eggs per month, with a potential increase of 6 g of protein per egg (28, 29). The impact of increased egg production on child growth is supported by a study demonstrating that each instance of child consumption of an egg during a prior 3 d period was significantly linked to an increase in child height (7). In the present study, the positive and negative signs of the treatment coefficient in the ASF and grains regressions on HAZ and WHZ, respectively, are consistent with the substitution away from high-carbohydrate, low-protein grains toward high-protein ASF, leading a higher-protein diet but fewer total calories. This implies that although the treatment provided households with more in-home, accessible protein, there is still a trade-off in resource-constrained households: a small drop in WHZ for a larger increase in HAZ. This type of apparent substitution is not inevitable but is a common behavioral response in resource-constrained decision-making. In contrast to vegetables and fruits, ASF are much less influenced by season (30) and thus, may enhance HAZ to a greater degree due to HAZ reflecting growth over time.
Unidentified effects on child growth of being in a NDV treatment household, here denominated as direct treatment effects in the WHZ and HAZ regressions, are captured by the Treatment Group × Time in trial interaction term. As child growth measures, collection of data on the type and quantity of foods consumed by the child, and antiparasite medications given to the flocks were common to both treatment and control groups and we rigorously controlled for possible bias in household participation over time (SI Appendix, Fig. S1, Supplementary Text S1, and Tables S1–S3), we posit that the direct effects appear to derive from either households observing vaccination or the increased time that the animal health technicians were on the premises due to the additional time requirement for vaccination. The latter provided more time for household members to interact with the animal health technicians and potentially receive additional advice regarding poultry and livestock husbandry, crop management, or other issues affecting food production and availability. The direct observation of NDV vaccination may also have had an effect. Campbell et al. (31) identified that knowing a neighbor who vaccinated his or her chickens had the most significant impact on the decision of a given household to vaccinate. Whether households in the treatment group that routinely observed the vaccination process invested more of their own resources into poultry management was not captured in our study.
In addition to the effect of being in a treatment household on child growth mediated through food consumption, household income has consistent positive impacts on ASF consumption, ASF and vegetable consumption relative to grains, and HAZ. This result is consistent with prior findings that ASF and vegetables tend to be more income responsive than other food groups, while grains are less responsive to income or decline as a share of food expenditures (32, 33). Importantly, even modest income increases can help bridge the seasonal fluctuations in on-farm food availability and avoid periods of undernutrition in young children at the time they are most vulnerable to childhood stunting (15, 16, 34).
Prior studies have established a link between the health of poultry and livestock in rural smallholder farms and both decreased human disease and enhanced childhood growth (6, 9). While previous studies have documented the impacts of livestock ownership on child nutrition (6, 35, 36), we provide evidence on the impact of livestock health interventions. The positive impact of NDV vaccination on flock size and its translation into increased ASF consumption and improved child growth provide a compelling rationale for the minimal investments required for widespread vaccination in rural households. The high percentage of chicken ownership in our study site in Siaya County in western Kenya is representative of rural households across Africa and other rural, low-income regions within Asia and South and Central America. A willingness to pay study indicated that households in Tanzania with similar characteristics as in western Kenya were willing to pay roughly twice the market price for NDV vaccines, which are readily available in east Africa (20). However, the small number of chickens per household disincentivizes market-based delivery mechanisms, and the opportunity cost to individual household members, usually women, to travel and purchase vaccines is a disincentive (20, 21). This additional burden on women is consistent with studies examining the impact of early childhood interventions on mothers (37). Subsidizing animal health technicians to deliver vaccines to households is proposed to overcome these disincentives and provide a pathway to enhance household poultry productivity with impacts on household well-being and reduced childhood growth faltering.
Materials and Methods
Ethical Approval.
The research was approved by the Ethical and Animal Care and Use Committees (Scientific Steering Committee protocol no. 3159) of the Kenya Medical Research Institute and by the University of Nairobi Faculty of Veterinary Medicine Animal Use and Bioethics Committee to conduct research on enhancing childhood nutrition and growth. Informed consent covered the study overview, objectives of the study, potential benefits to the household participants, potential risks to the participants and mitigation steps, contact information for both medical and veterinary personnel involved in the study, and contact information for the ethical review committees. Participation was voluntary, and the decision was at the household level; there was no community-wide presentation or interdependency among households that may pressure a given household to participate. Both oral and written consents (in Luo, the language of the community) were obtained from the household head, and written consent has been retained.
Trial Design and Participant Selection.
The longitudinal, randomized, controlled trial was conducted in the Rarieda subcounty of Siaya County in western Kenya within a health and demographic surveillance system (HDSS) site run by the Kenya Medical Research Institute and the US Centers for Disease Control and Prevention (38). HDSS data from December 2016 (6 mo prior to the study initiation) indicated 667 potentially eligible households meeting the criteria of 1) chicken ownership, 2) a child 3 y or younger, and 3) location within a 5.5-km radius of St. Elizabeth Lwak Mission Hospital, which could provide nutritional support for children identified as suffering from acute malnutrition.
Vaccination.
Animal health technicians delivered the intervention (vaccination of chickens against NDV) quarterly, independent of the food consumption and anthropometric data collection. All chickens in treatment households were vaccinated with two drops of NDV AVIVAX I-2 thermostable vaccine (109.7 egg infectious doses per milliliter) intranasally or intraocularly (depending on the chicken’s age at recruitment) and then, every 3 mo thereafter (13). AVIVAX I-2 is a freeze-dried live attenuated Newcastle disease vaccine prepared from the La Sota strain manufactured by the Kenya Veterinary Vaccines Production Institute. In all households, treatment and control, all chickens were also treated with endo- and ectoparasiticides, piperazine citrate, and carbaryl every 3 mo by the animal health technicians.
Data Collection.
Assessments were conducted at the time of enrollment as a baseline and then, approximately quarterly for up to 18 mo to collect anthropometric data on children, the type and quantity of foods consumed, and household socioeconomic data. In the event the mother (or principal caregiver/respondent) or child was not present at the time of the scheduled visit, the interviewers returned twice more within the week. If the third attempt was unsuccessful, the household visit was scheduled for the next quarter. Child growth was measured using a Shorrboard for length (<2 y) and height (≥2 y) and caregiver/child standing scale for weight. MUAC was assessed using three standardized colored tapes: red (<115 mm) indicating SAM, which triggered referral to a program that provided vitamin A and fortified maize flour and nutritional counseling; yellow (115 to 124 mm) indicating MAM, which triggered nutritional counseling; and green (>125 mm), which was considered healthy growth and the caregiver was encouraged to continue with nutritious feeding. For dietary assessment, caregivers were requested to recall the type of food, quantity, and the number of times the child was fed each type of the food in the 3 d prior to the interview. The interviewers asked about each specific food and provided a standardized set of containers to assist in estimating the quantity of each food item. Socioeconomic data included the mother’s (or principal caregiver’s) age and level of education; the number of family members; and household income consisting of both on-farm and off-farm earnings, where Kenya shillings were converted to US dollars using the year 2016 average as an exchange rate reference (1 US dollar = 101.50 shillings) and discounted at an annual rate of 3.5%. All data were collected by community health interviewers in the local language (Luo), entered onto an electronic data capture tool, downloaded, and stored in a Microsoft Access database. All datasets underwent validation and consistency checks to identify and resolve errors before they were merged using unique household identifiers on each of the participating households.
Blinding.
Households were not notified of which group they belonged to but were informed (oral and written) that their chickens would receive treatment to prevent disease. The enumerators who conducted the household interviews and collected both food consumption data and the anthropometric measurements were blind to the control and treatment group allocation. The animal health technicians and the enumerators were never on the premises at the same time to prevent enumerators from identifying the group. Household allocation data were unmasked only at the end of the study by the investigators.
Data Analysis.
Child height, age, and sex were referenced to the World Health Organization standards to create continuous measures for HAZ score and WHZ score using Epi Info (39) upon importation of anthropometric variables. Stunting and wasting were defined as greater than two SDs below the World Health Organization reference mean for HAZ and WHZ, respectively. Analyses on all the relational factors over the study period were done using STATA, version 17 (40).
Statistical regression analysis was performed to 1) model the determinants of food consumption by children over the course of the study, including treatment effects, and 2) model the determinants of the biometric outcomes and WHZ and HAZ scores, including food consumption and treatment effects. The response variables in the food consumption regressions are the natural logarithms of the number of food servings for each of four food groups: ASF, fruits, vegetables, and grains. The food intake regression equations can be written as
| [1] |
where is the logarithmic transformation of the four food group servings, with representing ASF, vegetables, fruit, and grains, respectively. Greek letters are parameters to be estimated, is time since first household visit [Time in Trial (months)], and is an indicator variable equaling one if a household is in the Treatment Group and zero otherwise. represents two malnutrition indicators: MAM and SAM; the subscript identifies MAM and SAM indicator variables, the superscript indicates whether a child was ever diagnosed with MAM or SAM during the full trial, the superscript measures time since first record of MAM or SAM, respectively. represents other control variables (including interaction terms) in each equation, and is a random error term.
We hypothesize that a treatment effect of NDV vaccination of household chickens on a child’s diet would accumulate over the course of the trial. To capture this effect, we created an interaction variable in Eq. 1 by multiplying and . Because equals zero for control households and one for treatment households, equals the time in trial for treatment households and is zero for control households. The control group is, therefore, the base case, and represents the direction and rate of change in consumption of food group over the course of the trial in the control group. The parameter associated with represents the difference in the rate of change in food consumption in the treatment group relative to consumption in the control group. If , consumption of food category is increasing relative to consumption in the control group. The treatment indicator is included directly in each food and Z score equation to control for conditional baseline differences in treatment and control groups in relation to each dependent variable. The time in trial variable captures changes in the dependent variable over the course of the trial that are not attributable to the treatment itself.
The category of variables in Eq. 1 relates to two forms of malnutrition: MAM and SAM. The related category indicates whether a child was ever diagnosed with MAM or SAM during the trial and is used to control for unobserved baseline conditions that contribute to MAM and SAM risk. The base case is no acute malnutrition. The interaction term takes the value of zero for each individual until a first finding of either MAM or SAM, after which it counts months (in increments of days) since this first finding until the end of the trial to assess whether these interventions had measurable effects on outcomes over time.
Food consumption history in our data reflects the transition from breastfeeding as the primary source of child nutrition through a period of increased intake of other food sources up to month 18 (Fig. 2). This age-dependent transition was captured in the food consumption regressions (represented generally by in Eq. 1) by utilizing an indicator variable >18 mo taking a value of one if a child was over 18 mo old at the time of a household visit and zero otherwise and interacting this variable with child age, age squared (to capture the diminishing increase in servings through 18 mo), and a variable indicating whether a child was currently being breastfed (Breastfed = 1, Not Breastfed = 0). Further regressors in Eq. 1 include the natural logarithm of per capita household income, an indicator for female child (vs. male), the age and education level of the mother or principal caregiver, and a sine/cosine pair (trigonometric functions of month) to capture seasonality in food intake.
HAZ and WHZ regressions were estimated simultaneously with food intake equations represented by Eq. 1. These regressions can be represented as
| [2] |
where are weight-to-height and height-to-age measures, respectively, and the rest of the content shown in Eq. 2 is as described for Eq. 1. However, there are differences between regressions [1] and [2]. First, Eq. 2 includes the four categories of food intake, , as explanatory variables to capture food intake effect on biometric outcomes. Second, because height to age tends to reflect the cumulative effects of nutrition during the entire growth path of a child, while weight to height tends to more reflect recent nutrition, the explanatory variables in these regressions differ between the two regressions. Third, we include current breastfeeding status in the WHZ regression, but in the HAZ regression, we use an indicator of whether a child was ever breastfed (Never Breastfed vs. Breastfed Ever) during the trial prior to a given visit. We provide robustness analysis for specifying these differences in HAZ and WHZ regressors in SI Appendix, Supplementary Text S3 and Table S5.
Our data contain up to six records per child, one for each household visit. We apply a random effects model to account for unobserved similarities in children/households between visits. The error component of the regressions can be represented as , where and the and indices identify child and visit number, respectively (subscripts are omitted from Eqs. 1 and 2 to minimize notational clutter). Robust SEs are clustered by individual child. The food intake and Z-score regressions (six equations) were estimated simultaneously in Stata 17 using the gsem routine with random effects for each individual child (40). To account for systematic sample selectivity bias that might accompany this attrition, we use inverse probability weights (41–43) based on predicted probabilities generated from a Probit regression shown and discussed in SI Appendix, Supplementary Text S1 and Table S3.
Of particular interest are the effects of treatment on food intake, the effects of food intake on biometric scores, and the effects of treatment on Z scores conditional on food intake. Eqs. 1 and 2 allow for estimation of what we refer to as direct effects of treatment on food intake and biometric scores as well as indirect effects of treatment on Z scores through measured food consumption effects. The indirect effects are defined as the effects of treatment on Z scores through food consumption as measured in these regressions. The direct effect is defined as the measured effect of the treatment variables and included directly in the Z regressions and represents effects of treatment on nutritional outcomes not otherwise represented by our food consumption data and therefore, not captured in the food intake–related parameter estimates in regressions [1] and [2]. These effects are methodologically “direct” in the sense that they are captured directly in Eq. 2 parameters, but they may reflect complex and varied pathways from treatment to nutritional outcomes for the children in the study. The full effect of treatment on can be described as
The sum of direct and indirect treatment effects is calculated mathematically based on Eqs. 1 and 2 as
| [3] |
where is a treatment indicator variable equaling one if a child is in a treatment household and zero otherwise. The direct effects of treatment on Z are , and the indirect effects of treatment on Z through measured impacts of treatment on food consumption are the sum of the effects of treatment on food intake () and the effects of food intake on Z (). These effects are conditional on the duration of , up to a maximum of .
Household and child participation may affect both the control and treatment groups over the course of the trial. If a child was found to be moderately or acutely malnourished, they were referred to a therapeutic feeding program regardless of whether they were in the treatment or control group. A mathematically analogous calculation for estimating the direct effects of MAM and SAM intervention after diagnosis is calculated as
| [4] |
where in Eqs. 1 and 2 is abbreviated as ; ; and and are estimated by one parameter each in each equation in Tables 2 and 4, respectively, associated with Time since [first] MAM and Time since [first] SAM.
There are other known and unknown reasons that food intake and Z scores might be affected by trial participation independent of being in a treatment or control household. The full change in biometric scores Z that occurs over time during the course of study participation is
For the control group, the treatment indicator T = 0, so the “time in control group,” which nets out the treatment effect, simplifies to
| [5] |
Eq. 5 is the baseline for potential effects of trial participation on biometric outcomes. Because the control group status is the base case in the regression, it reflects effects of known and unknown factors affecting both groups. We designate this as the time in trial effect. Total effects of other regressors in both food and Z-score regressions are calculated from regression parameters in an analogous fashion. These indirect and total effects are calculated using the Stata nlcom routine (40).
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122389119/-/DCSupplemental.
Data Availability
All study data are included in the article, the SI Appendix, and on the Open Science Framework (OSF) at https://osf.io/m4vs2/?view_only=1428f4bb179b4d7bbde8a00c81d95b5d (or see ref. 44).
References
- 1.Local Burden of Disease Child Growth Failure Collaborators, Mapping child growth failure across low- and middle-income countries. Nature 577, 231–234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Onis M., et al. , The World Health Organization’s global target for reducing childhood stunting by 2025: Rationale and proposed actions. Matern. Child Nutr. 9 (suppl. 2), 6–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Global nutrition targets 2025 (2014). https://www.who.int/publications/i/item/WHO-NMH-NHD-14.2. Accessed 8 August 2021.
- 4.Grosso G., Mateo A., Rangelov N., Buzeti T., Birt C., Nutrition in the context of the Sustainable Development Goals. Eur. J. Public Health 30 (suppl. 1), i19–i23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloss E., Wainaina F., Bailey R. C., Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in western Kenya. J. Trop. Pediatr. 50, 260–270 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Mosites E., et al. , Relations between household livestock ownership, livestock disease, and young child growth. J. Nutr. 146, 1118–1124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosites E., et al. , Child height gain is associated with consumption of animal-source foods in livestock-owning households in Western Kenya. Public Health Nutr. 20, 336–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KIPPRA, Siaya County Integrated Development Plan 2018-2022 (2018). https://repository.kippra.or.ke/handle/123456789/1218. Accessed 8 August 2021.
- 9.Thumbi S. M., et al. , Linking human health and livestock health: A “one-health” platform for integrated analysis of human health, livestock health, and economic welfare in livestock dependent communities. PLoS One 10, e0120761 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otiang E., et al. , Mortality as the primary constraint to enhancing nutritional and financial gains from poultry: A multi-year longitudinal study of smallholder farmers in western Kenya. PLoS One 15, e0233691 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alders R., et al. , “Controlling Newcastle Disease in Village Chickens: A Training Manual” in ACIAR Monograph No. 86 (Australian Centre for International Agricultural Research, Canberra, ACT, Australia, 2002).
- 12.Njagi L. W., et al. , Prevalence of Newcastle disease virus in village indigenous chickens in varied agro-ecological zones in Kenya. Livest. Res. Rural Dev. 22, 95 (2010). [Google Scholar]
- 13.Otiang E., et al. , Impact of routine Newcastle disease vaccination on chicken flock size in smallholder farms in western Kenya. PLoS One 16, e0248596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulett J. L., et al. , Animal source foods have a positive impact on the primary school test scores of Kenyan schoolchildren in a cluster-randomised, controlled feeding intervention trial. Br. J. Nutr. 111, 875–886 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Livestock for Health, “Seasonality of malnutrition: Community knowledge on patterns and causes of undernutrition in children and women in Laisamis, Marsabit County, Kenya” (Rep., FOA, Food and Agricultural Organization of the United Nations, UNICEF, United Nations Children's Fund, Washington State University, 2021).
- 16.Catley A., Lotira R., Hopkins C., Hidden peaks: Women’s knowledge on seasonality and route causes of child malnutrition in Karamoja, Uganda and their programming preferences (2018). https://karamojaresilience.org/wp-content/uploads/2021/05/201811_krsu_hidden_peaks_nutrition_online.pdf. Accessed 1 March 2022.
- 17.Akinola L. A. F., Essien A., Relevance of rural poultry production in developing countries with special reference to Africa. Worlds Poult. Sci. J. 67, 697–705 (2011). [Google Scholar]
- 18.Allen L. H., Dror D. K., Effects of animal source foods, with emphasis on milk, in the diet of children in low-income countries. Nestle Nutr Works SE 67, 113–130 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Asare H., Rosi A., Faber M., Smuts C. M., Ricci C., Animal-source foods as a suitable complementary food for improved physical growth in 6 to 24-month-old children in low- and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 3, 1–11 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Campbell Z. A., Otieno L., Shirima G. M., Marsh T. L., Palmer G. H., Drivers of vaccination preferences to protect a low-value livestock resource: Willingness to pay for Newcastle disease vaccines by smallholder households. Vaccine 37, 11–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Absalón A. E., Cortés-Espinosa D. V., Lucio E., Miller P. J., Afonso C. L., Epidemiology, control, and prevention of Newcastle disease in endemic regions: Latin America. Trop. Anim. Health Prod. 51, 1033–1048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndiku M., Jaceldo-Siegl K., Singh P., Sabaté J., Gender inequality in food intake and nutritional status of children under 5 years old in rural Eastern Kenya. Eur. J. Clin. Nutr. 65, 26–31 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Gueye E. H., Women and family poultry production in rural Africa. Dev. Pract. 10, 98–102 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Guèye E. F., Gender aspects in family poultry management systems in developing countries. Worlds Poult. Sci. J. 61, 39–46 (2005). [Google Scholar]
- 25.Nordhagen S., Klemm R., Implementing small-scale poultry-for-nutrition projects: Successes and lessons learned. Matern. Child Nutr. 14 (suppl. 3), e2676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson K. R., Who reaps the benefits? Exploring access and social exclusion among village chicken producers in Kenya. Gend. Place Cult. 28, 1196–1200 (2021). [Google Scholar]
- 27.Ruel M. T., Alderman H.; Maternal and Child Nutrition Study Group, Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? Lancet 382, 536–551 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Chepkemoi M., et al. , “Nutritional diversity of meat and eggs of five poultry species in Kenya” in 2015 JKUAT Scientific Conference (Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 2016), pp. 124–131.
- 29.Olwande P. O., et al. , Assessing the productivity of indigenous chickens in an extensive management system in southern Nyanza, Kenya. Trop. Anim. Health Prod. 42, 283–288 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Kristjanson P., et al. , R., Are food insecure smallholder households making changes in their farming practices? Evidence from East Africa. Food Secur. 4, 381–397 (2012). [Google Scholar]
- 31.Campbell Z. A., Marsh T. L., Mpolya E. A., Thumbi S. M., Palmer G. H., Newcastle disease vaccine adoption by smallholder households in Tanzania: Identifying determinants and barriers. PLoS One 13, e0206058 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colen L., et al. , Income elasticities for food, calories and nutrients across Africa: A meta-analysis. Food Policy 77, 116–132 (2018). [Google Scholar]
- 33.Desiere S., Hung Y., Verbeke W., D’Haese M., Assessing current and future meat and fish consumption in sub-Sahara Africa: Learnings from FAO Food Balance Sheets and LSMS household survey data. Glob. Food Sec. 16, 116–126 (2018). [Google Scholar]
- 34.Leroy J. L., Ruel M., Habicht J. P., Frongillo E. A., Linear growth deficit continues to accumulate beyond the first 1000 days in low- and middle-income countries: Global evidence from 51 national surveys. J. Nutr. 144, 1460–1466 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Rawlins R., Pimkina S., Barrett C. B., Pedersen S., Wydick B., Got milk? The impact of Heifer International’s livestock donation programs in Rwanda on nutritional outcomes. Food Policy 44, 202–213 (2014). [Google Scholar]
- 36.Headey D., Hirvonen K., Hoddinott J., Animal sourced foods and child stunting. Am. J. Agric. Econ. 100, 1302–1319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans D. K., Jakiela P., Knauer H. A., The impact of early childhood interventions on mothers. Science 372, 794–796 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Feikin D. R., et al. , Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int. J. Epidemiol. 39, 450–458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, Epi Info (2019). https://www.cdc.gov/epiinfo/index.html. Accessed 5 December 2021.
- 40.StataCorp, Stata Statistical Software: Release 17 (StataCorp LLC, 2021). [Google Scholar]
- 41.Wooldridge J. M., Inverse probability weighted M-estimators for sample selection, attrition, and stratification. Port. Econ. J. 1, 117–139 (2002). [Google Scholar]
- 42.Huber M., Identification of average treatment effects in social experiments under alternative forms of attrition. J. Educ. Behav. Stat. 37, 443–474 (2012). [Google Scholar]
- 43.Weuve J., et al. , Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology 23, 119–128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.E. Otiang, Vaccination of household chickens results in a shift in young children’s diet and improves child growth. Open Science Framework. https://osf.io/m4vs2/?view_only=1428f4bb179b4d7bbde8a00c81d95b5d. Deposited 8 August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article, the SI Appendix, and on the Open Science Framework (OSF) at https://osf.io/m4vs2/?view_only=1428f4bb179b4d7bbde8a00c81d95b5d (or see ref. 44).