Significance
WNT/β-catenin signaling is critical for lung development, and homeostasis and it has also been implicated in inflammatory lung diseases. However, the underlying molecular mechanisms, especially those at play during inflammatory conditions, are unclear. Here, we show that loss of the WNT coreceptor Related to receptor tyrosine kinase (RYK) specifically in mesenchymal cells results in lung inflammation. Our data indicate that RYK signaling through β-catenin and Nuclear Factor kappa B (NF-κB) is part of a safeguard mechanism against mesenchymal cell death, excessive inflammatory cytokine production, and inflammatory cell recruitment and accumulation.
Keywords: WNT/RYK signaling, lung mesenchyme, inflammatory lung disease
Abstract
A number of inflammatory lung diseases, including chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, and pneumonia, are modulated by WNT/β-catenin signaling. However, the underlying molecular mechanisms remain unclear. Here, starting with a forward genetic screen in mouse, we identify the WNT coreceptor Related to receptor tyrosine kinase (RYK) acting in mesenchymal tissues as a cell survival and antiinflammatory modulator. Ryk mutant mice exhibit lung hypoplasia and inflammation as well as alveolar simplification due to defective secondary septation, and deletion of Ryk specifically in mesenchymal cells also leads to these phenotypes. By analyzing the transcriptome of wild-type and mutant lungs, we observed the up-regulation of proapoptotic and inflammatory genes whose expression can be repressed by WNT/RYK signaling in vitro. Moreover, mesenchymal Ryk deletion at postnatal and adult stages can also lead to lung inflammation, thus indicating a continued role for WNT/RYK signaling in homeostasis. Our results indicate that RYK signaling through β-catenin and Nuclear Factor kappa B (NF-κB) is part of a safeguard mechanism against mesenchymal cell death, excessive inflammatory cytokine production, and inflammatory cell recruitment and accumulation. Notably, RYK expression is down-regulated in the stromal cells of pneumonitis patient lungs. Altogether, our data reveal that RYK signaling plays critical roles as an antiinflammatory modulator during lung development and homeostasis and provide an animal model to further investigate the etiology of, and therapeutic approaches to, inflammatory lung diseases.
During inflammatory lung diseases, lung mesenchymal cells play a crucial role in the inflammatory process and also contribute to tissue remodeling. Lung mesenchymal cells, including airway smooth muscle cells and fibroblasts, can produce and secrete a variety of inflammatory cytokines and chemokines, cell adhesion molecules, as well as extracellular matrix proteins, which lead to inflammatory cell recruitment and activation (1, 2). Occasionally, the inflammatory response in the lung is associated with structural cells undergoing apoptotic cell death (3, 4). In addition, apoptotic cells also secrete inflammatory cytokines and can recruit immune cells (5–7). A delicate balance of immune cell recruitment and apoptosis is required for lung homeostasis; however, the molecular mechanisms underlying mesenchymal–immune cell cross-talk are not fully understood.
Many components of the WNT signaling cascade are expressed in lung mesenchymal tissues during development and homeostasis. For instance, WNT-2, FZD-1, -4, and -7 as well as LEF1 are expressed selectively in mesenchymal cells (8–10). Moreover, dysregulated WNT/β-catenin signaling contributes to the pathogenesis of inflammatory lung diseases, including chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and asthma (10, 11). However, the precise role of WNT/β-catenin signaling during lung inflammation remains unclear.
Related to receptor tyrosine kinase (RYK) functions as a WNT coreceptor and belongs to the atypical receptor tyrosine kinase family (12, 13). In a previous study, we reported that RYK is strongly expressed in airway epithelial cells and moderately expressed in lung alveolar epithelial and mesenchymal cells. Ryk knockout (KO) and SL (stop loss) mice exhibit a combination of goblet cell hyperplasia and inflammation, and this latter phenotype is not observed in mice lacking Ryk function in epithelial cells (14). A few studies have focused on tissue- or cell type–specific functions of RYK. For example, WNT/RYK participates in motor cortex remapping after spinal cord injury and in neuropil formation in the outer retina (15, 16). An in vitro study showed that WNT-5A/RYK signaling in endothelial cells controls vascular permeability through cytoskeleton remodeling (17). However, the role of RYK in mesenchymal tissues has not yet been studied.
Here we reveal a role for RYK in mesenchymal cell survival and the inflammatory response during lung development and homeostasis. Our results from in vivo and in vitro manipulations suggest that alterations of WNT/RYK signaling through NF-κB contribute to the pathogenesis of inflammatory lung diseases. Two different mesenchymal Ryk deletion models display extensive inflammation at developmental and adult stages, thereby providing a useful platform to further investigate the etiology and treatment of inflammatory lung diseases.
Results
Ryk Mutant Mice Exhibit Lung Inflammation and Alveolar Simplification.
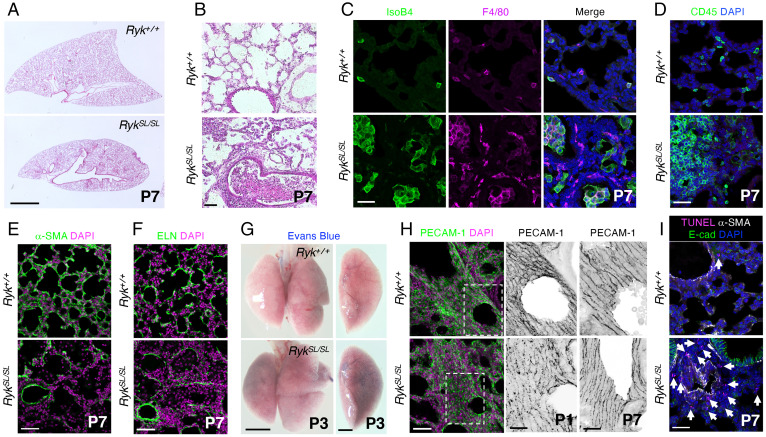
In a previous study, we reported the identification and analysis of a SL mutant allele of Ryk, which leads to lung hypoplasia and inflammation (Fig. 1 A and B) (14). Epithelial-specific deletion of Ryk leads to goblet cell hyperplasia and mucus hypersecretion without lung inflammation (14). Here, we focus on the inflammation phenotype. To determine which immune cell types accumulate in RykSL/SL lungs, we performed histological analyses and immunostaining. At the histological level, RykSL/SL lungs display infiltration of macrophages and monocytes, neutrophils, and also lymphocytes (SI Appendix, Fig. S1A). The number of both alveolar and interstitial macrophages was markedly increased in RykSL/SL lungs at postnatal day 7 (P7) (Fig. 1C). In addition, immunostaining analyses revealed increased numbers in P7 RykSL/SL lungs of hematopoietic cells (CD45+) (Fig. 1D), including lymphocytes (CD3+ and CD19+) and macrophages (MAC2+) (SI Appendix, Fig. S1 B–D). We next investigated the stage at which an accumulation of inflammatory cells is first observed in RykSL/SL lungs. While RykSL/SL lungs were partially collapsed at P0, inflammatory cells were first recruited in mutant lungs starting around P1 (SI Appendix, Fig. S1 E and F), indicating that the loss of Ryk function causes the recruitment of inflammatory cells into the lung starting at postnatal stages. Moreover, many immune cells were proliferating in RykSL/SL lungs (SI Appendix, Fig. S2 A and B), indicating that the marked increase in the number of inflammatory cells is due to both recruitment from other tissues and local proliferation. We next performed α-smooth muscle actin (α-SMA) and elastin (ELN) immunostaining to visualize secondary septa in P7 lungs. RykSL/SL mice exhibited severe defects in secondary septa formation and a significant reduction in α-SMA and ELN expression at P7 (Fig. 1 E and F), suggesting that RYK participates in myofibroblast development or maintenance and, consequently, secondary septa formation during lung maturation.
Fig. 1.
Ryk deficiency leads to lung hypoplasia and inflammation. (A) Hematoxylin and eosin (H&E) staining of P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (B) High magnification of H&E-stained P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (C) IsoB4 staining (immune cells) and F4/80 immunostaining (alveolar and interstitial macrophages) in P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (D) Immunostaining for CD45 (hematopoietic cells) in P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (E) Immunostaining for α-SMA (to mark myofibroblasts) in P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (F) Immunostaining for ELN (to mark secondary septa) in P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. (G) Representative images of P3 Ryk+/+ (n = 6) and RykSL/SL (n = 6) lungs injected with Evans blue dye. (H) Immunostaining for PECAM-1 (endothelial cells) in P1 and P7 Ryk+/+ (n = 5) and RykSL/SL (n = 5) lung sections. High-magnification image of the areas in the dashed boxes is shown in the Middle panels. (I) TUNEL staining and immunostaining for α-SMA and E-cadherin in P7 Ryk+/+ (n = 10) and RykSL/SL (n = 12) lung sections. Arrows point to TUNEL-positive cells. (Scale bars: 2 mm for A and G, Left, 1 mm for G, Right; 50 μm for B, E, F, and H, Left; and 30 μm for C, D, H, Right, and I.)
Loss of vascular integrity in inflammatory conditions can trigger immune cell infiltration into the lung parenchyma and alveolar spaces (18). To evaluate vessel integrity in RykSL/SL lungs, we injected Evans blue dye intracardially at P3. While Evans blue dye was retained in the blood vessels of wild-type (WT) lungs, it diffused into the RykSL/SL lung parenchyma (Fig. 1G). In addition, the postnatal expression of platelet endothelial cell adhesion molecule-1 (PECAM-1), an adhesion molecule that contributes to the maintenance of vascular integrity (19), appeared to be reduced in RykSL/SL lungs compared with Ryk+/+ siblings, and its localization at endothelial cell junction also appeared more punctate (Fig. 1H and SI Appendix, Fig. S2C). These data indicate that Ryk deficiency leads to defects in lung vessel wall integrity as well as increased permeability at postnatal stages.
Since cell death can induce endothelial barrier dysfunction and inflammatory cell recruitment (3, 5), we examined cell death in RykSL/SL lungs using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. The number of dead cells was significantly increased in RykSL/SL lungs at postnatal stages but not at embryonic day 18.5 (E18.5) (Fig. 1I and SI Appendix, Fig. S2D). Moreover, most dead cells were observed in the mesenchymal compartment surrounding blood vessels (Fig. 1I and SI Appendix, Fig. S2D), indicating that RYK is required for the survival of mesenchymal cells in the postnatal lung. Notably, RykSL/SL mice exhibited no obvious defects in other organs, including the heart, kidney, and liver (SI Appendix, Fig. S2E). Overall, these data indicate that in the postnatal lung, loss of Ryk function leads to cell death in the mesenchyme and triggers inflammatory cell recruitment and local proliferation.
Mesenchymal Ryk Deletion Leads to Lung Inflammation and Alveolar Simplification.
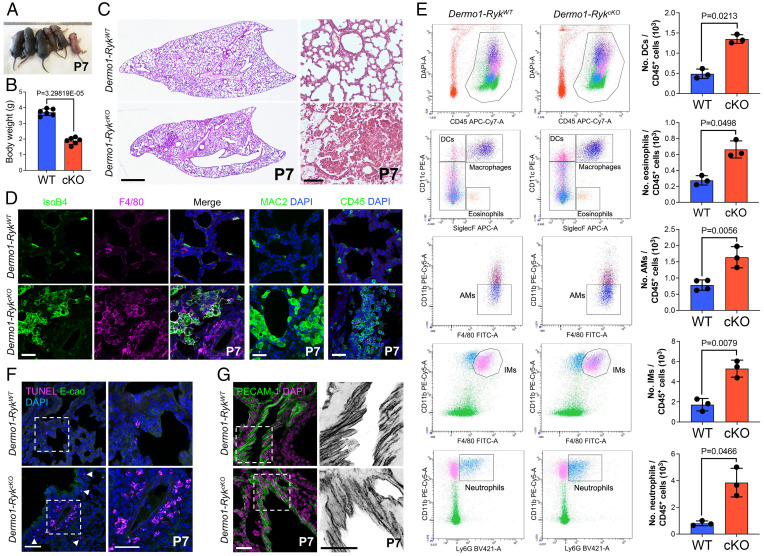
To investigate the cell type–specific functions of Ryk during mouse lung development, we deleted the gene in mesenchymal cells using a Dermo1/Twist2-Cre line (hereafter Dermo1-Cre−-Rykfl/+ or Dermo1-RykWT and Dermo1-Cre+-Rykfl/fl or Dermo1-RykcKO) and obtained specific loss of Ryk expression in lung mesenchymal cells (SI Appendix, Fig. S3A). Similar to Ryk KO and SL mice (14, 20), Dermo1-RykcKO mice exhibited growth retardation and hypoplastic lungs at P7 (Fig. 2 A–C) and subsequently died around P14. In addition, Dermo1-RykcKO lungs displayed inflammatory cell accumulation and alveolar simplification at P7 (SI Appendix, Fig. S3 B–D). The inflammatory cells observed in Dermo1-RykcKO lungs included macrophages, monocytes, neutrophils, and lymphocytes, as judged by cell morphology and marker analysis (Fig. 2D and SI Appendix, Fig. S3E). To quantitatively characterize the various inflammatory cell populations, we performed immunophenotyping using flow cytometry (SI Appendix, Fig. S3F). The number of immune cells (CD45+) was markedly increased in Dermo1-RykcKO lungs compared with Dermo1-RykWT lungs (Fig. 2 D and E). Dermo1-RykcKO lungs exhibited increased numbers of eosinophils (CD11c− SiglecF+), dendritic cells (CD11C+ SiglecF−), and neutrophils (CD11bhi Ly6Ghi) (Fig. 2E). In addition, the number of macrophages (CD11chi SiglecFhi), including alveolar macrophages (CD11b− F4/80hi) and interstitial macrophages (CD11bhi F4/80hi), was significantly increased in Dermo1-RykcKO lungs (Fig. 2E). These data indicate that Ryk deficiency in the mesenchyme leads to an increase of both resident and circulating immune cells in the lung. We next examined cell death in Dermo1-RykcKO lungs using TUNEL staining. Similar to our observations in Ryk SL mice, the number of dead cells was greatly increased in the perivascular mesenchyme of P7 Dermo1-RykcKO lungs (Fig. 2F). This increase in cell death in Dermo1-RykcKO lungs was apparent starting at around P1 (SI Appendix, Fig. S4A). In addition, we observed defects in vessel integrity in Dermo1-RykcKO lungs at P3 and P7 but not at P0 (Fig. 2G and SI Appendix, Fig. S4 B and C), indicating that mesenchymal cell death causes vessel integrity defects in Dermo1-RykcKO lungs. However, using Shh-Cre, Tek-CreERT2, and Lyz2-Cre lines, epithelial-, endothelial-, or myeloid lineage–specific Ryk deletion caused no obvious inflammatory cell recruitment and accumulation into the lungs (14) (SI Appendix, Fig. S5 A–E). Overall, these data indicate that in the lung mesenchyme, RYK functions as a survival factor and/or immunomodulator starting at early postnatal stages.
Fig. 2.
Mesenchymal Ryk deletion leads to lung inflammation. (A) General appearance of P7 Dermo1-RykWT (n = 3) and Dermo1-RykcKO (n = 3) mice. (B) Body weight of P7 Dermo1-RykWT (n = 6) and Dermo1-RykcKO (n = 5) mice. (C) H&E staining of P7 Dermo1-RykWT (n = 10) and Dermo1-RykcKO (n = 9) lung sections. (D) IsoB4 staining (immune cells) and immunostaining for F4/80 (alveolar and interstitial macrophages), MAC2 (alveolar and interstitial macrophages), and CD45 (hematopoietic cells) of P7 Dermo1-RykWT (n = 10) and Dermo1-RykcKO (n = 9) lung sections. (E) Representative flow cytometry analysis of CD45+ hematopoietic cells from the lungs of P3 Dermo1-RykWT (n = 3) and Dermo1-RykcKO (n = 4) mice. Bar graphs representing the number of alveolar macrophages (AMs), dendritic cells (DCs), eosinophils, interstitial macrophages (IMs), and neutrophils as measured by flow cytometry in the lungs of P3 Dermo1-RykWT (n = 3) and Dermo1-RykcKO (n = 4) mice. (F) TUNEL staining and E-cadherin immunostaining of P7 Dermo1-RykWT (n = 10) and Dermo1-RykcKO (n = 9) lung sections. High-magnification image of the areas in the dashed boxes is shown on the Right. Arrowheads point to TUNEL-positive cells. (G) Immunostaining for PECAM-1 of P7 Dermo1-RykWT (n = 10) and Dermo1-RykcKO (n = 9) lung sections. High-magnification image of the areas in the dashed boxes is shown on the Right. Error bars are means ± SEM, two-tailed Student’s t test. (Scale bars: 1 mm for C, Left; 100 μm for C, Right; 50 μm for F, Left; and 30 μm for D, F, Right, and G.)
RYK Signaling Inhibits Apoptosis-Associated Gene Expression and Restricts the Immune Response.
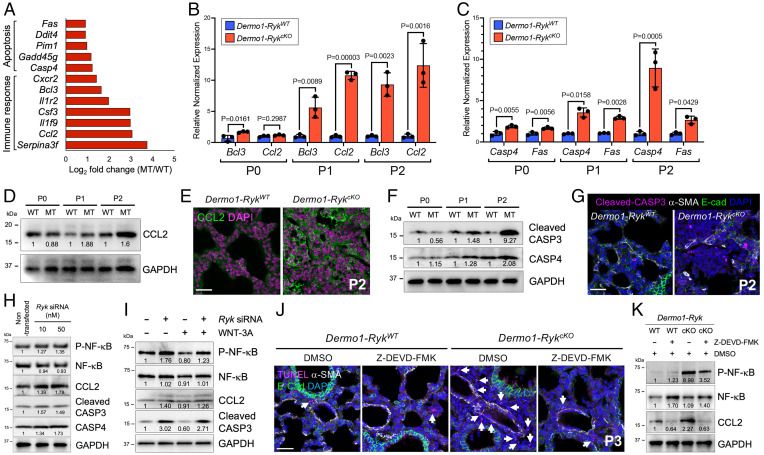
To identify mesenchymal genes regulated by Ryk function that are involved in postnatal lung development, we performed transcriptomic analysis of P0 Dermo1-RykWT and Dermo1-RykcKO lungs. From gene ontology (GO) analysis, the up-regulated genes were mostly associated with immune response and apoptosis, and the down-regulated genes with WNT signaling (Fig. 3A and SI Appendix, Fig. S6A and Dataset S1). We focused on the up-regulated genes and first validated their expression by qPCR. The expression of immune response genes, including Bcl3, Ccl2, Il1f9, Il1r2, and Serpina3f, was significantly up-regulated in Dermo1-RykcKO lungs starting at P1 (Fig. 3B and SI Appendix, Fig. S6B). In addition, the expression of apoptosis genes, including Casp4, Fas, Ddit4, Pim1, and Gadd45g, was significantly up-regulated already at P0 in Dermo1-RykcKO lungs compared with wild type (Fig. 3C and SI Appendix, Fig. S6C).
Fig. 3.
RYK inhibits cell death in the lung mesenchyme. (A) RNA-sequencing fold change (log2) of differentially expressed genes in P0 Dermo1-RykWT (n = 3) compared with Dermo1-RykcKO (n = 3) lungs. (B) qPCR analysis of Bcl3 and Ccl2 mRNA levels in P0, P1, and P2 Dermo1-RykWT and Dermo1-RykcKO lungs. (C) qPCR analysis of Casp4 and Fas mRNA levels in P0, P1, and P2 Dermo1-RykWT and Dermo1-RykcKO lungs. (D) Representative Western blot (from three individual sets of lung lysates) for CCL2 in P0, P1, and P2 Dermo1-RykWT and Dermo1-RykcKO lungs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Values represent the densitometric ratio after normalization to GAPDH. (E) Immunostaining for CCL2 in P2 Dermo1-RykWT (n = 6) and Dermo1-RykcKO (n = 6) lung sections. (F) Representative Western blot (from three individual sets of lung lysates) for cleaved CASP3 and CASP4 in P0, P1, and P2 Dermo1-RykWT and Dermo1-RykcKO lungs. Values represent the densitometric ratio after normalization to GAPDH. (G) Immunostaining for cleaved CASP3, α-SMA, and E-cadherin in P2 Dermo1-RykWT (n = 6) and Dermo1-RykcKO (n = 6) lung sections. (H) Representative Western blot (from three individual sets of cell lysates) for NF-κB, phospho–NF-κB, CCL2, cleaved CASP3, and CASP4 in nontransfected and Ryk siRNA (10 nM or 50 nM)-transfected NIH 3T3 fibroblasts. Values represent densitometric ratios after normalization to GAPDH, except for phospho–NF-κB, which was normalized to GAPDH and total NF-κB. (I) Representative Western blot (from three individual sets of cell lysates) for NF-κB, phospho–NF-κB, CCL2, and cleaved CASP3 upon stimulation with WNT-3A and/or cotransfection of Ryk siRNA in NIH 3T3 fibroblasts. Values represent densitometric ratios after normalization to GAPDH, except for phospho–NF-κB, which was normalized to GAPDH and total NF-κB. (J) TUNEL staining and immunostaining for α-SMA (to mark smooth muscle) and E-cadherin in DMSO- or Z-DEVD-FMK–treated P3 Dermo1-RykWT (n = 6) and Dermo1-RykcKO (n = 6) lung sections. Arrows point to TUNEL-positive cells. (K) Representative Western blot (from three individual sets of lung lysates) for NF-κB, phospho–NF-κB, and CCL2 in DMSO- or Z-DEVD-FMK–treated P3 Dermo1-RykWT and Dermo1-RykcKO lungs. Values represent densitometric ratios after normalization to GAPDH, except for phospho–NF-κB, which was normalized to GAPDH and total NF-κB. Error bars are means ± SEM, two-tailed Student’s t test. Ct values are listed in SI Appendix, Table S2. (Scale bars: 50 μm for G and 30 μm for E and J.)
We next examined the expression of Ccl2/CCL2 and Bcl3/BCL3 by qPCR, in situ hybridization, and Western blotting or immunostaining. The expression of Ccl2 was strongly up-regulated and that of Bcl3 moderately up-regulated in P2 Dermo1-RykcKO lungs (SI Appendix, Fig. S6 D and E). CCL2 expression was also increased in mesenchymal cells and immune cells in P2 Dermo1-RykcKO lungs (Fig. 3 D and E and SI Appendix, Fig. S7 A–C), indicating that RYK can repress Ccl2 expression in the lung mesenchyme at postnatal stages. We also examined the expression of the apoptosis genes Casp4 and Fas in developing lungs. Casp4 and Fas expression was significantly increased in mesenchymal cells of P2 Dermo1-RykcKO (SI Appendix, Fig. S6 D and F). Protein levels of CASP4 and cleaved CASP3, the latter a hallmark of active apoptosis (21), were significantly increased in Dermo1-RykcKO lungs starting at P1, and even more strongly at P2 (Fig. 3 F and G). These data indicate that loss of Ryk function in lung mesenchymal cells can cause their death, and that subsequently the dying cells induce the expression of cytokines.
To test whether Ryk deficiency also leads to the up-regulation of downstream genes in vitro, we performed Ryk knockdown in NIH3T3 mouse embryonic fibroblasts (SI Appendix, Fig. S7D). Since NF-κB signaling is a master regulator of the inflammatory response (22, 23), we first examined the activation status of NF-κB in Ryk knockdown cells. Ryk knockdown led to increased phosphorylation of NF-κB and IκKαβ (a positive regulator of NF-κB signaling that is activated by phosphorylation), as well as IκBα (a negative regulator of NF-κB signaling that is inactivated by phosphorylation) (Fig. 3H and SI Appendix, Fig. S7 E and F). In addition, expression of CCL2, cleaved CASP3, and CASP4 was also increased when Ryk was knocked down in NIH 3T3 cells (Fig. 3H). As RYK is known to modulate both β-catenin–dependent and –independent signaling (12, 13), we examined whether RYK participates in WNT/β-catenin signaling by testing GSK-3β–induced phosphorylation of CTNNB1/β-catenin. Ryk knockdown led to increased phosphorylation of CTNNB1 (SI Appendix, Fig. S7G) and could prevent the activation of WNT/β-catenin by WNT-3A, but it had no effect on the blockade of this pathway by WNT-5A (SI Appendix, Fig. S7H). These data indicate that RYK participates in β-catenin–dependent signaling in fibroblasts. WNT-3A treatment alone led to a reduction in NF-κB phosphorylation and lower levels of cleaved CASP3 in fibroblasts (Fig. 3I). Conversely, Ryk knockdown in combination with WNT-3A treatment led to an increase in NF-κB phosphorylation and CCL2 expression as well as cleaved CASP3 levels, reverting the effect of WNT/β-catenin activation (Fig. 3I). In summary, RYK participates in the WNT/β-catenin signaling pathway, and it can repress the expression of proinflammatory and proapoptotic mediators/factors in fibroblasts.
To examine whether Caspase inactivation could ameliorate the lung phenotype in Dermo1-RykcKO mice, we used a broad-spectrum Caspase inhibitor (Benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone, Z-VAD-FMK) as well as a Caspase-3 inhibitor (Benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone, Z-DEVD-FMK) in vivo. These compounds were injected intraperitoneally into lactating mothers at P0, P1, and P2, and the lungs were collected at P3. Dimethyl sulfoxide (DMSO)-treated Dermo1-RykcKO mice exhibited severe lung inflammation at P3 (SI Appendix, Fig. S8 A–C). Conversely, the lung phenotypes of Dermo1-RykcKO mice were partially rescued by treatment with the Caspase inhibitors (SI Appendix, Fig. S8 A–C). Whereas a high number of dying cells was observed in Dermo1-RykcKO lungs, this number was significantly decreased in Caspase inhibitor–treated Dermo1-RykcKO lungs (Fig. 3J and SI Appendix, Fig. S8D). In addition, the levels of NF-κB phosphorylation and CCL2 expression were also reduced in Caspase inhibitor–treated Dermo1-RykcKO lungs compared with control (Fig. 3K and SI Appendix, Fig. S8E). These data indicate that in Ryk-deficient conditions, cell death leads to lung inflammation. Altogether, these data suggest that WNT/RYK signaling has an antiapoptotic and/or antiinflammatory role in fibroblasts and developing lung mesenchymal cells.
RYK Participates in Lung Maturation and Homeostasis.
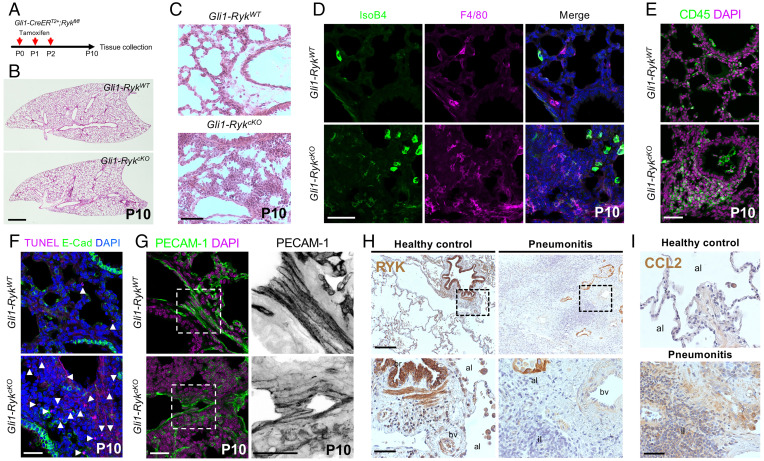
Since lung inflammation and cell death in Ryk-deficient mice were observed specifically at postnatal stages, we also investigated Ryk function in the mesenchyme during lung maturation and homeostasis. When we deleted Ryk in mesenchymal cells by injecting tamoxifen between P0 and P2 in Gli1-CreERT2;Ryk-floxed mice (hereafter Gli1-CreERT2--Rykfl/fl or Gli1-RykWT and Gli1-CreERT2+-Rykfl/fl or Gli1-RykcKO), we found no obvious growth retardation or lung hypoplasia at P10 (Fig. 4 A and B), unlike what we observed in Ryk SL and Dermo1-RykcKO mice. Instead, these Gli1-RykcKO mice exhibited inflammatory cell accumulation in the lungs (Fig. 4C and SI Appendix, Fig. S9A). At P10, most inflammatory cells in Gli1-RykcKO mice were macrophages (IsoB4, F4/80, and MAC2+) and lymphocytes (CD45+) (Fig. 4 D and E and SI Appendix, Fig. S9 A and B). In addition, an increased number of dead cells was observed in the mesenchymal cells surrounding the blood vessels of P10 Gli1-RykcKO lungs (Fig. 4F). Gli1-RykcKO mice also exhibited vessel integrity defects, as revealed by the lower expression of PECAM-1 in the lungs and diffusion of Evans blue dye into the lung parenchyma (Fig. 4G and SI Appendix, Fig. S9C). These data suggest that RYK also has a crucial function in preventing mesenchymal cell death and excessive inflammatory cell recruitment during lung maturation. To determine whether RYK participates in lung homeostasis, we deleted Ryk at adult stages by employing a Rosa26-CreERT2 driver line (hereafter Rosa26-CreERT2--Rykfl/fl or Rosa26-RykWT and Rosa26-CreERT2+-Rykfl/fl or Rosa26-RykcKO) (SI Appendix, Fig. S9D). Inflammatory cells accumulated in the lungs of Rosa26-RykcKO mice at adult stages (SI Appendix, Fig. S9 E and F), suggesting that RYK functions as an antiinflammatory modulator in the lung during homeostasis as well. Next, we selectively deleted Ryk in mesenchymal cells at adult stages using the Gli1-CreERT2 line (SI Appendix, Fig. S9G). Gli1-RykcKO lungs exhibited inflammatory cell accumulation and disrupted alveolar structure, as well as an increased number of dead cells (SI Appendix, Fig. S9 H–J), indicating that Ryk deficiency in mesenchymal cells is sufficient for inflammatory cell recruitment during lung homeostasis. Overall, RYK functions in the mesenchyme to promote cell survival and restrict the immune response during lung maturation and homeostasis.
Fig. 4.
RYK acts as a survival factor and antiinflammatory modulator during lung maturation and homeostasis. (A) Diagram indicating the time points of tamoxifen injections and tissue collection for the early postnatal Gli1-RykcKO mice. (B) H&E staining of P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. (C) High magnification of H&E-stained P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. (D) IsoB4 staining (immune cells) and F4/80 immunostaining (alveolar and interstitial macrophages) in P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. (E) Immunostaining for CD45 (hematopoietic cells) in P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. (F) TUNEL staining and E-cadherin immunostaining in P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. Arrowheads point to TUNEL-positive cells. (G) Immunostaining for PECAM-1 in P10 Gli1-RykWT (n = 6) and Gli1-RykcKO (n = 6) lung sections. High-magnification image of the areas in the dashed boxes is shown on the Right. (H) Immunostaining for RYK in the lungs of healthy controls (n = 5) and pneumonitis patients (n = 10). High-magnification image of the areas in the dashed boxes is shown on the Bottom. (I) Immunostaining for CCL2 in the lungs of healthy controls and pneumonitis patients. Abbreviations: al, alveoli; br, bronchiole; bv, blood vessel; and il, inflammatory lesion. (Scale bars: 1 mm for B; 200 μm for H, Top; 50 μm for H, Bottom, and I; and 30 μm for D, F, and G.)
RYK Is Down-Regulated in Patients with Pneumonitis.
Since WNT/β-catenin signaling plays antiinflammatory roles during inflammatory lung diseases in mice and humans (24, 25), we tested RYK expression in the lungs of five healthy controls and 10 pneumonitis patients. Similar to our previous findings (14), in the lungs of healthy controls, high levels of RYK were detected in the airway epithelium and moderate levels in immune cells and stromal cells (Fig. 4H and SI Appendix, Fig. S10 A and B). However, in the lungs of pneumonitis patients, RYK expression was markedly reduced in stromal cells, even though it was still high in epithelial cells of the airway and alveoli (SI Appendix, Fig. S10 A and B). We also examined CCL2 expression in those lungs. While CCL2 expression was specifically detected in immune cells in the lungs of healthy controls, it was significantly increased in stromal cells of pneumonitis patients (Fig. 4I and SI Appendix, Fig. S10C). These results are consistent with our mouse data, further indicating that in the lung, RYK restricts the immune response, including inflammatory cell recruitment.
Discussion
Here, we identify a role for RYK in the lung mesenchyme during lung development and maturation, as well as during homeostasis. In mesenchyme-specific Ryk-deficient lungs, inflammatory cells accumulate in airway lumens, alveolar spaces, and lung parenchyma, a phenotype accompanied by increased mesenchymal cell death and defective vessel integrity. We show that RYK inhibits proapoptotic and inflammatory genes in part through NF-κB. At later stages, encompassing lung maturation and homeostasis, RYK continues to promote mesenchymal cell survival and to dampen the immune response.
Abnormal inflammatory responses accompanied by infiltration of activated immune cells and release of proinflammatory mediators are observed in patients with inflammatory lung diseases such as COPD, IPF, asthma, as well as pneumonia (3, 4). WNT signaling plays a key role in both acute and chronic inflammation, and dysregulation of WNT/β-catenin signaling has been linked with the pathogenesis of these diseases (10, 11, 26). RYK, a coreceptor for WNT ligands, has been shown to participate in β-catenin–dependent and –independent signaling in a cell- and tissue-specific manner (12, 13, 27). In the present study, we found that, through dysregulation of β-catenin–dependent signaling, loss of Ryk function in the mouse lung mesenchyme leads to severe inflammation accompanied by overproduction of inflammatory cytokines, a process that is regulated by NF-κB signaling. As WNT/β-catenin activation inhibits NF-κB signaling (22), our data support a model where WNT/RYK signaling constitutively inhibits the activation of NF-κB signaling, thereby repressing the expression and release of inflammatory cytokines from the lung mesenchyme.
Recently, a role for RYK in endothelial cells has been reported. RYK knockdown in human coronary artery endothelial cells in culture prevents WNT-5A–induced hyperpermeability, which occurs through cytoskeleton remodeling (17). In contrast, our in vivo data suggest that Ryk deficiency in lung mesenchymal cells, but not lung endothelial cells, is sufficient to cause defects in endothelial cell junctions and vessel permeability/integrity. It has previously been reported that Ryk is expressed in the bone marrow and that its expression is regulated during hematopoietic development and maturation (28). In addition, Ryk deficiency in hematopoietic stem cells (HSCs) from the fetal liver can reduce HSC self-renewal and lead to proliferation-induced apoptosis (29). We found that mice with myeloid lineage-specific Ryk deletion (Lyz2-RykcKO) display no obvious inflammatory phenotypes in the lung, suggesting that RYK does not participate in myeloid cell proliferation and maturation in a cell-autonomous manner.
In the present study, the Ryk N-ethyl-N-nitrosourea (ENU) mutant mice (RykSL/SL) and the Dermo1-RykcKO mice, which express Cre recombinase in lung mesenchymal cells, all exhibit severe inflammation as well as developmental defects, including lung hypoplasia and leaky blood vessels. In contrast, when we activated CreERT2 in Gli1-RykcKO mice between P0 and P2 to conditionally delete Ryk in lung mesenchymal cells after birth, we observed lung inflammation and leaky blood vessels without obvious lung hypoplasia. The weaker phenotype in Gli-RykcKO mice compared with both the RykSL/SL and the Dermo1-RykcKO mice may be due to the fairly restricted vs. broader domain of Cre expression in Gli1-CreERT2 and Dermo1-Cre mice, respectively. These observations, together with our results from the knockdown of Ryk in NIH 3T3 fibroblasts, indicate that the inflammation phenotype derives directly, or indirectly, from a cell-autonomous defect in lung mesenchymal cells. Nevertheless, we cannot exclude the possibility that the increased immune response is in part due to developmental defects, such as growth retardation.
The stimulation of WNT/β-catenin signaling leads to the up-regulation of antiapoptotic factors in hepatocytes (30). Activated WNT/β-catenin signaling inhibits apoptosis during development and tissue repair, as well as during tumorigenesis (31, 32). Moreover, WNT/β-catenin signaling has been shown to suppress the apoptosis of IPF myofibroblasts (7, 33). We found that Ryk deficiency leads to the increased expression of proapototic genes in mesenchymal cells, increased interstitial cell death, vessel permeability defects, and excessive immune cell recruitment and local proliferation. Inhibition of Caspase activity can attenuate the inflammation phenotype in Ryk mutant mice, possibly in part by reducing NF-κB phosphorylation. Overall, these results suggest that the increased expression of inflammatory cytokines observed in Ryk mutants is due to both direct and indirect effects, including signals released by dying cells.
Recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease 19 (COVID-19) has been declared a pandemic. SARS-CoV-2 can, not only activate antiviral immune responses, but also cause uncontrolled inflammatory responses characterized by a cytokine storm (34, 35). Relevant to these processes, the mouse models in the present study recapitulate features of inflammatory lung diseases characterized by combined disruption of endothelial cell barrier function and excess immune responses.
In conclusion, we propose that during lung development and homeostasis, WNT signaling, through RYK, β-catenin, and NF-κB, plays an important role in safeguarding the lung mesenchyme against cell death and an exacerbated immune response, including increased inflammatory cytokine production and inflammatory cell recruitment and accumulation. This signaling axis could represent an important therapeutic target against inflammatory lung diseases, such as pneumonia and COPD.
Materials and Methods
All animal care and experimental procedures in this study were approved by the local animal ethics committee at the Regierungspräsidium Darmstadt, Hessen, Germany. Human pneumonitis lung samples and healthy control lungs were provided by the Universities of Giessen and Marburg Lung Center Biobank, which is a member of the Deutsches Zentrum für Lungenforschung (DZL) Platform Biobanking. The study protocol was approved by the ethics committee of the Justus Liebig University School of Medicine (no. 58/2015), and informed consent was obtained in written form from each patient. All human studies were performed in adherence to the relevant ethical guidelines.
For the transcriptome analysis, total RNA was isolated from P0 lungs of three Dermo1-RykWT and three Dermo1-RykcKO mice. For immune cell phenotyping, cells were obtained from P4 Dermo1-RykWT and Dermo1-RykcKO lungs.
The materials and methods used in this study are described in detail in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Young-June Jin for providing critical comments and sharing materials, Saverio Bellusci for the Gli1-CreERT2 mouse line, Nina Wettschureck and Stefan Offermanns for the Tek-CreERT2 mouse line, Johnny Kim and Thomas Braun for the ROSA26-CreERT2 mouse line, and Christian Stockmann for the Lyz2-Cre mouse line. We also thank Beate Grohmann, Carmen Buettner, Simon Perathoner, and Haaglim Cho for support and Susanne Herold for critical advice. This work was supported in part by National Research Foundation of Korea grants funded by the Korea government (Ministry of Science and Information and communications Technology (MSIT)) (2019R1A5A8083404 and 2020R1A2C1100479) (to H.-T.K.) and funds from the Max Planck Society (to D.Y.R.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201707119/-/DCSupplemental.
Data Availability
The RNA-Sequencing dataset produced in this study (36) is available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141974.
References
- 1.Alkhouri H., Poppinga W. J., Tania N. P., Ammit A., Schuliga M., Regulation of pulmonary inflammation by mesenchymal cells. Pulm. Pharmacol. Ther. 29, 156–165 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Broekman W., et al. , Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 73, 565–574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt E. P., Tuder R. M., Role of apoptosis in amplifying inflammatory responses in lung diseases. J. Cell Death 3, 41–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy B. D., Serhan C. N., Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 76, 467–492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L., Kepp O., Kroemer G., Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Wallach D., Kang T. B., Kovalenko A., Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat. Rev. Immunol. 14, 51–59 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Sauler M., Bazan I. S., Lee P. J., Cell death in the lung: The apoptosis-necroptosis axis. Annu. Rev. Physiol. 81, 375–402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M., Shi J., Huang Y., Lai L., Expression of canonical WNT/β-CATENIN signaling components in the developing human lung. BMC Dev. Biol. 12, 21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ota C., Baarsma H. A., Wagner D. E., Hilgendorff A., Königshoff M., Linking bronchopulmonary dysplasia to adult chronic lung diseases: Role of WNT signaling. Mol. Cell Pediatr. 3, 34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain M., et al. , Wnt/β-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 3226–3242 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Königshoff M., Eickelberg O., WNT signaling in lung disease: A failure or a regeneration signal? Am. J. Respir. Cell Mol. Biol. 42, 21–31 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Green J., Nusse R., van Amerongen R., The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb. Perspect. Biol. 6, 6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy J. P., Halford M. M., Stacker S. A., The biochemistry, signalling and disease relevance of RYK and other WNT-binding receptor tyrosine kinases. Growth Factors 36, 15–40 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Kim H. T., et al. , WNT/RYK signaling restricts goblet cell differentiation during lung development and repair. Proc. Natl. Acad. Sci. U.S.A. 116, 25697–25706 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollis E. R. II, et al. , Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat. Neurosci. 19, 697–705 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarin S., et al. , Role for Wnt signaling in retinal neuropil development: Analysis via RNA-Seq and in vivo somatic CRISPR mutagenesis. Neuron 98, 109–126.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skaria T., Bachli E., Schoedon G., Wnt5A/Ryk signaling critically affects barrier function in human vascular endothelial cells. Cell Adhes. Migr. 11, 24–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green C. E., Turner A. M., The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir. Res. 18, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lertkiatmongkol P., Liao D., Mei H., Hu Y., Newman P. J., Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 23, 253–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halford M. M., et al. , Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat. Genet. 25, 414–418 (2000). [DOI] [PubMed] [Google Scholar]
- 21.McArthur K., Kile B. T., Apoptotic caspases: Multiple or mistaken identities? Trends Cell Biol. 28, 475–493 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Ma B., Hottiger M. O., Crosstalk between Wnt/β-Catenin and NF-κB signaling pathway during inflammation. Front. Immunol. 7, 378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q., Lenardo M. J., Baltimore D., 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell 168, 37–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogendijk A. J., Diks S. H., van der Poll T., Peppelenbosch M. P., Wieland C. W., Kinase activity profiling of pneumococcal pneumonia. PLoS One 6, e18519 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai W., et al. , Blockade of Wnt/β-Catenin pathway aggravated silica-induced lung inflammation through Tregs regulation on Th immune responses. Mediators Inflamm. 2016, 6235614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae W. J., Bothwell A. L. M., Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 39, 830–847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halford M. M., Macheda M. L., Stacker S. A., “The RYK receptor family” in Receptor Tyrosine Kinases: Family and Subfamilies, Wheeler D. L., Yarden Y., Eds. (Humana, 2015), pp. 685–741. [Google Scholar]
- 28.Simoneaux D. K., et al. , The receptor tyrosine kinase-related gene (Ryk) demonstrates lineage and stage-specific expression in hematopoietic cells. J. Immunol. 154, 1157–1166 (1995). [PubMed] [Google Scholar]
- 29.Famili F., et al. , The non-canonical Wnt receptor Ryk regulates hematopoietic stem cell repopulation in part by controlling proliferation and apoptosis. Cell Death Dis. 7, e2479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Götschel F., et al. , Inhibition of GSK3 differentially modulates NF-kappaB, CREB, AP-1 and beta-catenin signaling in hepatocytes, but fails to promote TNF-alpha-induced apoptosis. Exp. Cell Res. 314, 1351–1366 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Chen S., et al. , Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 152, 87–96 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pećina-Slaus N., Wnt signal transduction pathway and apoptosis: A review. Cancer Cell Int. 10, 22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang W., et al. , SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J. Biol. Chem. 285, 8196–8206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teuwen L. A., Geldhof V., Pasut A., Carmeliet P., COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 20, 389–391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matacic C., Blood vessel injury may spur disease’s fatal second phase. Science 368, 1039–1040 (2020). [DOI] [PubMed] [Google Scholar]
- 36.H. T. Kim, S. Guenther, D. Y. R. Stainier. Transcriptome of P0 Dermo1-RykWT and Dermo1-RykcKO lungs. Gene Expression Omnibus (GEO) database. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141974. Deposited 13 December 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-Sequencing dataset produced in this study (36) is available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141974.