Abstract
Marmosets and closely related tamarins have become popular models for understanding aspects of human brain organization and function because they are small, reproduce and mature rapidly, and have few cortical fissures so that more cortex is visible and accessible on the surface. They are well suited for studies of development and aging. Because marmosets are highly social primates with extensive vocal communication, marmoset studies can inform theories of the evolution of language in humans. Most importantly, marmosets share basic features of major sensory and motor systems with other primates, including those of macaque monkeys and humans with larger and more complex brains. The early stages of sensory processing, including subcortical nuclei and several cortical levels for the visual, auditory, somatosensory, and motor systems, are highly similar across primates, and thus results from marmosets are relevant for making inferences about how these systems are organized and function in humans. Nevertheless, the structures in these systems are not identical across primate species, and homologous structures are much bigger and therefore function somewhat differently in human brains. In particular, the large human brain has more cortical areas that add to the complexity of information processing and storage, as well as decision-making, while making new abilities possible, such as language. Thus, inferences about human brains based on studies on marmoset brains alone should be made with a bit of caution.
Keywords: visual cortex, auditory cortex, somatosensory cortex, sensory system, motor cortex, posterior parietal cortex, frontal cortex
INTRODUCTION
Marmosets have recently become a very popular research model, largely due to their potential for study with optogenetic and related methods that are now applied to the brains of mice.1 Marmosets are also of special interest because they are highly vocal and social.2 Marmosets provide the research advantages of being a small, rapidly maturing and reproducing primate with a relatively smooth cortex of few fissures.3 Marmosets and the closely related tamarins comprise a total of 20 or more species in South and Central America that vary in appearances and size. Overall, they are the smallest of monkeys, with the pygmy marmoset weighing just over 100 g as an adult. As a small, New World monkey, their small brains still share many features with those of more studied primates such as New World squirrel monkeys and Old World macaque monkeys. Marmosets are thought to have evolved from larger ancestors.4,5 As they became smaller, marmosets were more able to feed in the thin outer branches of bushes and trees and cling to vertical trunks, giving them access to food less available to larger monkeys.6 As larger ancestors evolved into marmosets, their smaller brains likely became somewhat simplified, as small brains in small mammals face the dilemma of reducing the sizes and functions of cortical areas and subcortical nuclei overall, or reducing the numbers of distinct areas and nuclei to save space for the more critical cortical structures.7 There is some evidence that mammals, and especially primates, with larger brains have more cortical areas,8 and it seems rather certain that the large human brains have more functional subdivisions than most or all other primates, especially marmosets. Thus, marmoset brains can be studied for basic primate features, but the brains of other studied primates, such as macaques, may more closely resemble human brains.9,10 Other notable specializations of marmosets include having clawed fingers and toes for clinging to trees and generally giving birth to 2 offspring. The common marmoset, Callithrix jacchus, is the marmoset most used in research. The focus here is on the features of the marmoset forebrain, especially those in neocortex, compared with other primates. Fukushima et al11 provided an excellent recent review of marmoset neuroanatomy.
DEFINING ARCHITECTONIC AREAS AND NUCLEI
Defining the nuclei of the brainstem and thalamus and areas of neocortex is at the center of understanding how structures and networks in the nervous system process information, store memories, and plan and create motor responses. Defining these subdivisions of brains is basic to further advances that include understandings of their functions and roles in behavior. In this regard, researchers are fortunate in that the recently published stereotaxic atlas of the marmoset brain12 is one the best of modern efforts, and one that certainly exceeds the classical descriptions of Brodmann.13 While the atlas defines subcortical structures in ways that do not differ very much from earlier descriptions in monkeys and other primates,14 the Paxinos et al12 atlas recognizes a total of 116 histochemically defined cortical areas per hemisphere in marmosets, one that approaches the currently defined estimates for the much larger brains of macaque (~140)15 and humans (~180).16 In contrast, the classical description of cortical areas by Brodmann13 (1909) only included about 30 areas for marmosets and just 47 for humans. There are reasons for the larger numbers of areas now proposed for taxa of primates. First, there are now many more histological procedures for revealing structural differences that are used to histologically define areas and nuclei. Brodmann13 depended on the Nissl stain for revealing structural differences between proposed areas based on cell bodies, whereas the recent Paxinos et al12 atlas was based on coronal brain sections stained for Nissl substance, calbindin, acetylcholinesterase, and non-phosphorylated neurofilaments (SMI-32). In addition, there are many other histochemical and immunostaining procedures available,17 with each revealing somewhat different aspects of regional differences in brain structure. Second, and more importantly, modern atlases are guided by information from studies on the connections and functional maps of cortical and subcortical structures. For example, the early architectonic study of the cortical areas of a mouse lemur brain by Le Gros Clark in 1931 did not define an architectonically distinct area now found in all primates,18 the middle temporal visual area (MT),19 but the MT was included in later architectonic studies of mouse lemur cortex.20,21 Modern depictions of cortical areas in primates include an MT (Fig. 1), which is easily identified architectonically by multiple procedures.
Figure 1 .
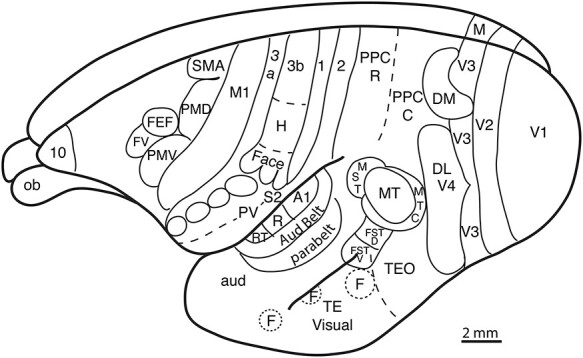
A dorsolateral view of a marmoset brain with proposed functional subdivisions. Visual areas have been named as follows. The primary or first visual area is V1 or area 17. Other commonly named visual areas include the second (V2), third (V3), and both (V4) visual areas. Alternatively, V4 is called the dorsolateral visual area (DL). DL appears to have rostral (DLr) and caudal (DLc) divisions. The nature of V3 in marmosets and other primates is uncertain. Here, V3 is disrupted by part of the dorsomedial visual area (DM) and the medial visual area (M) on the medial wall of the cerebral hemisphere. The middle temporal visual area (MT) is surrounded by functionally related visual areas: the MT crescent (MTc), the middle superior temporal area (MST), and the dorsal (FSTd) and ventral (FSTv) fundal superior temporal areas. The caudal temporal lobe is mainly visual, and 2 traditional divisions are illustrated, the temporal region (TE), and temporal-occipital region (TEO). Additionally, 3 of the regions activated by seeing faces (F) are outlined. Auditory cortex includes 3 primary areas, A1, the rostral area (R) and the rostral temporal area (RT). The second and third level of cortical processing include subdivisions of the auditory belt and the auditory parabelt (see Fig. 2). Other parts of the auditory temporal lobe are included in further processing of auditory information (Aud). Somatosensory cortex includes 4 somatotopically organized areas named after the numbering system of Brodmann,13 area 3b or the primary area, S1, and areas 3a, 1 and 2. The existence of area 2 in marmosets is not established. Other areas include the second somatosensory area (S2), the parietal ventral area (PV), and areas in the cortex of the lateral fissure (see Fig. 3). Motor areas include primary motor cortex (M1), the ventral (PMV) and dorsal (PMD) premotor areas, the supplementary motor area (SMA), the frontal eye field (FEF), and the related frontal visual area (FV). The frontal pole of the cerebral hemisphere is occupied by area 10 of Brodmann (09), just over the olfactory bulb. Posterior parietal cortex (PPC) includes rostral (R) PPC that is subdivided into a number of action specific domains with somatosensory and visual inputs and caudal (C) PPC with visual inputs. In area 3b (S1), the hand region is indicated (H) and 4 modules, representing teeth, tongue, teeth, and tongue, are outlined (compare with Fig. 4).
The point here is that more accurate depictions of areas and nuclei will always be guided by additional information and criteria, as areas and nuclei should be identified not only by their histological appearance but by connections with other structures, functional organization such as having a systematic representation of a sensory surface, and neuron response properties.22 The goal is to define the same structures (eg, homologous structures) in different taxa by shared characteristics while recognizing that shared structures have continued to evolve independently in different lines of descent so that they come to differ in many ways while still coming from a common ancestor. Overall, identifying homologous structures despite their acquired differences is not an easy task. By definition, homologous structures are those that were also present in a common ancestor of the species under consideration. But we do not have fossil brain structures from potential ancestors, so the inferences of homology are indirect. Thus, present conclusions should often be regarded as works in progress and subject to modification and change. This will become more apparent as the organizations of anatomical systems in the marmoset are outlined here. Fortunately, there have been many helpful studies of anatomical connections and sensory and motor maps in marmosets.
One of the most distinctive architectonic features of neocortex is that neurons vary in size across cortical layers and cortical areas. In general, cortical areas toward the front of the brain tend to have larger and less densely packed neurons on average than areas toward the back of the brain.23 In addition, primate brains have more densely packed neurons and therefore smaller neurons on average than rodents, carnivores, marsupials, and other mammals.24 Furthermore, the change in average neuron size from frontal to occipital cortex is much more pronounced in primates than other studied mammals. Marmosets fit nicely into this pattern.25 One possible explanation for the frontal-caudal gradients in cortex is that developmental programs in mammals of “late makes great”23,26 result in later maturing posterior regions of cortex having more neurons. An alternative explanation considers the functions of cortical areas and proposes that homologous areas across taxa may differ as they specialize in different ways. Considering only frontal-caudal gradient in average neuron size misses something that an area-by-area analysis reveals. Sensory areas tend to preserve the information coming in from the nuclei in the thalamus, and they therefore have small neurons with small dendritic arbors that are responsive to only a few thalamic inputs. This emphasis is most pronounced in layer 4 where neurons are activated by thalamic inputs, but pyramidal neurons with outputs to other cortical areas also tend to be smaller in sensory areas.27 Thus, neurons in primary sensory areas such as primary auditory, somatosensory, and especially visual cortex have the smallest and most densely packed neurons overall, especially in primates,28 including marmosets.25 Motor areas have little or no layer 4 and tend to have large pyramidal neurons that receive many inputs, with those in layer 5 projecting to distant subcortical targets. Thus, they have the largest neurons overall. Considerations of frontal-caudal gradients of neuron sizes and packing densities across cortex thereby overlook important areal differences in neuron packing densities that are more pronounced in some species than others.
The most common other model of the primate brain is that of the macaque monkey. Clear differences in many features, including brain size and numbers of neurons, suggest that different models best address different questions about human brain organization and functions. Marmosets are roughly 10 times smaller than macaques and have less than one-tenth the brain mass and one-tenth the number of neurons.24 The most comprehensive study of neuronal packing densities across cortical areas and regions is that of Atapour et al.25 As in other primates, the primary visual cortex (V1) of marmosets has the most densely packed neurons and thus the smallest neurons on average. Of course, the actual numbers depend on where in V1 the sample for counting is taken, as more neurons across the depth of the cortex occur in the part of V1 representing central vision compared with peripheral vision.
The Visual System: Subcortical Structures
A good place to start describing the marmoset brain is with the visual system as this system has been most extensively studied in marmosets and other primates. The projections of the retina have long been used to identify homologous visual structures in primates and other mammals. In particular, the major targets include the accessory optic nuclei, a part of the pulvinar complex (the lateral posterior “nucleus” of non-primate mammals), the pretectal nuclei, the ventral lateral geniculate nucleus, the dorsal lateral geniculate nucleus, and the superior colliculus (for identification, see the Paxinos et al atlas12). Here, the focus is on the dorsal lateral geniculate nucleus (LGN), the visual pulvinar, and the superior colliculus (SC). The retinal projections to the LGN have been frequently studied in part because the laminar pattern of neuron types and the distributions of inputs from the contralateral and ipsilateral eyes are highly variable across taxa.29 Early studies of the projections of the retina to the LGN of primates revealed major differences between the lamination patterns of strepsirrhine and platyrrhine (anthropoid) primates.30 Strepsirrhine primates were formerly known as prosimians, but this classification included tarsiers, which are now known to be an early branch of the anthropoid radiation.
The basic laminar pattern of the anthropoid LGN is of 2 ventral layers of large neurons (the magnocellular, or M layers) with inputs from the contralateral or ipsilateral eye, and 2 dorsal layers of smaller neurons (the parvocellular, or P layers) with inputs from the contralateral or ipsilateral eye, as shown for marmosets (Fig. 2),31,32 and a scattering of very small koniocellular, or K, neurons between layers (see Huo et al33 for marmosets). In large primates with proportionately more parvocellular neurons, the parvocellular layers partially subdivide and interdigitate to provide a more complex laminar pattern of parvocellular sublayers. The LGNs of strepsirrhine primates differ by having 2 distinct layers of koniocellular neurons between the 2 dorsal parvocellular layers. The 3 morphological and functional classes of LGN neurons get inputs from 3 matching classes of retinal ganglion cells,34 with roughly 80% of the retinal ganglion cells of primates projecting only to the parvocellular layers, while those projecting to the M or K layers also project to the SC.35,36 All LGN layers project to the primary visual cortex, V1, or area 17, with the P cell inputs being most important for object vision and the M cell for detecting motion and change.37 The K cells appear to have varied roles, but they were apparently important in early primates that were basically nocturnal. K cells also project to several visual areas in addition to V1. All of the LGN layers are retinotopically organized from central or foveal vision to the contralateral peripheral temporal vision. The layers with inputs from the contralateral eye include the representation of the extreme temporal vision, the monocular visual field.
Figure 2 .
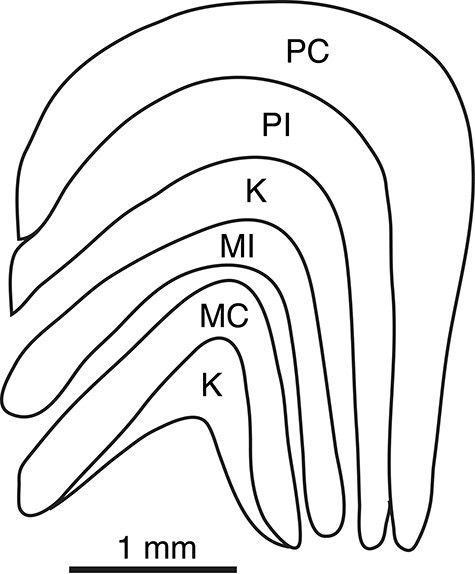
The lateral geniculate of a marmoset. The drawing is based on a coronal brain section with medial to the left. There are 4 obvious geniculate layers, including a pair of more dorsal parvocellular (M) layers and a more ventral pair of magnocellular (M) layers. The outer MC and PC layers receive retinal inputs from the contralateral retina, while the inner MI and PI layers receive inputs from the ipsilateral retina. Small koniocellular (K) neurons exist in the cell poor septal regions between layers, with a larger number between the inner M and P layers and below the MC layer. These septal regions of K neurons are not recognized as distinct layers.
In early vertebrates, the optic tectum (the SC) was the major target of the retinal projections, and the SC remains an important target in present-day mammals. In primates, the SC receives inputs from the M and K pathways that also project to the LGN, but not from the P pathway. The SC of primates represents the complete contralateral hemifields of both eyes but not the complete contralateral retina, as in other mammals. The retinal projections from the contralateral eye terminate most superficially in the superficial grey of the SC in marmosets, as in other primates, with the inputs from the ipsilateral eye terminating just below those of the contralateral eye.35 Other visual inputs from visual areas of cortex terminate deeper, as do somatosensory, auditory, and multisensory inputs from various sources.38 As in other primates, many cortical areas project to the SC in marmosets, with the densest projections coming from 3 visual areas: V1, V2, and MT.39 Outputs of the SC include those to the brainstem for evoking eye movements and those to the LGN and pulvinar nuclei.
The pulvinar of primates is a complex of several nuclei that are mostly visual in function.40 The traditional inferior pulvinar (PI) has 4 nuclei: the posterior (PIp), medial (PIm), central medial (PIcm), and central lateral. The traditional lateral pulvinar (PL) has 1 large nucleus (PL or PLvl) and 1 or 2 smaller nuclei. The traditional medial pulvinar (PM) is multisensory and may have functional subdivisions. These nuclei can be distinguished by their different histological characteristics and by their connections. While there has been some uncertainty about where the SC projections to the pulvinar terminate, results from marmosets were among the first to indicate that the SC projects densely to PIp and PIcm and not to PIm.41 PIm receives inputs from cortical area MT and projects back to MT.40 In marmosets, and probably in all other primates, the PIm gets inputs directly from the retina.31,42 PIp and PIcm get information about visual motion from the SC, and they project to visual areas around MT (areas MT crescent [MTc], medial superior temporal area [MST], FST, and PIcl (the central lateral nucleus of the inferior pulvinar) and PL have modulating interconnections with V1 and other visual areas.33,40,43 PM has connections with association areas of cortex and has roles in cognitive functions.44
Visual Cortex: Primary and Secondary Areas (V1 and V2) and Areas of the Third Tier
The organization of visual cortex in marmosets and other primates, including primary visual cortex and a number of adjoining or nearby visual areas (Fig. 1), is not completely settled. However, the primary area, V1 or area 17, is so obvious architectonically that there is no disagreement on its location and boundaries. Likewise, the MT is so distinct architectonically that there is general agreement about MT’s location and boundaries. The second area, V2 or area 18, has been well studied and is firmly established as a visual area. The main concerns are over the territory of visual area V3 and even its existence as well as the areal extent and organization of the “fourth” visual area, V4, also known as the dorsolateral visual area (DL).
The primary visual cortex is the largest area of the marmoset brain, occupying about 20% of the surface of neocortex.45 This is a larger proportion than in primates with larger brains, and it fits the premise that primary sensory areas occupy proportionately more of the cortex in small brains at the expense of higher order areas.46 Only the small and visually specialized tarsier has more cortex occupied by V1.47 The retinotopy of V1 has been mapped with microelectrode recordings in marmosets, and central vision has an expanded representation in V1,48,49 as in other diurnal anthropoid primates. The layers and sublayers of V1 (area 17) are well-differentiated in marmosets,50,51 as they are in other anthropoid primates, but the proposed homologs of these layers with other mammals, including strepsirrhine primates, has been reconsidered.37 V1 or area 17 is known across mammals for having a middle layer 4 of smaller, more densely packed neurons.
Brodmann13 described area 17 in a number of mammalian species and came to the conclusion that anthropoid primates had an expanded layer 4 with 2 deeper sublayers, 4Cα, and 4Cβ, of small, densely packed neurons separated from a narrow, more superficial thin layer of moderately small neurons, sublayer 4A, by a sublayer 4B of less densely packed larger neurons. In a more comprehensive review of the laminar pattern of area 17 in primates, Hassler52 revised Brodmann’s laminar pattern and concluded that only sublayers 4Cα and 4Cβ correspond to layer 4 of area 17 in other mammals and that sublayers 4A and 4B are actually sublayers of layer 3. This revision of the laminar pattern is strongly supported by more recent evidence that Brodmann’s layer 4B has neurons that project to area V2 and area MT of extrastriate cortex in marmosets53,54 and other primates.37 Sublayers of layer 4 are not expected to project to other cortical areas, whereas sublayers of layer 3 do project to other visual areas. Sublayer 4A of Brodmann in primates, however, is unusual in that this sublayer has smaller neurons and gets projections from the koniocellular LGN neurons.37 Layer 4 proper in marmosets51 and other primates receives inputs from the parvocellular and magnocellular LGN layers, with the magnocellular inputs superficial to the parvocellular inputs.37 Layer 6 projects back to the LGN and receives some LGN inputs; both layers 6 and 5 project to the pulvinar, while layer 5 projects to the SC.55
Area 17 is an area that has interhemispheric connections between the 2 hemispheres only along its outer border with V2. These connections extend further into V1 in marmosets than in macaques but not as far as in nocturnal primates.56 Area 17 also has other features that are shared with other primates, including the layer 3 (layer 4A of Brodmann) “blobs” of high cytochrome oxidase (CO) activity that receive inputs from the koniocellular layers of the LGN.37 The band-like ocular dominance columns that are related to the contralateral or ipsilateral eyes in macaques and humans are only weakly segregated from each other in marmosets.57
The second visual area, V2, is an area common to almost all mammals.58 An exception is the blind mole rat, which only has an architectonic remnant of area 17. Strangely, V2 is not always recognized in rats and mice by most current investigators. V2 borders V1 along adjoining representation of the zero-vertical meridian through the center of gaze. V2 contains a complete representation of the contralateral visual hemifield, with the outer border of V2 representing the zero-horizontal meridian.59 V2 has been mapped in detail by microelectrode recordings in marmosets.60 V2 has a prominent layer 4 and is densely packed with neurons but less so than in layer 4 of V1.25 In brain sections cut parallel to the brain surface and stained for CO, the width of V2 in marmosets is crossed by CO dark bands alternating with CO light bands,61,62 as in other anthropoid primates. These CO dark bands have been characterized as alternating between thick and thin types in macaques,63 leading to the conclusion, based on connections, that there are sets of 4 functionally distinct bands that repeat along the length of V2 in marmosets64 and other primates. Importantly, the thick CO bands in V2 relay magnocellular stream information from layer 3C neurons in V1 to MT.54,55 V2 also projects to V3 and DM.62 Subcortical projections of V1, V2, and V3 in marmosets and other monkeys include those to the SC39 and nuclei of the pulvinar.65
Area V3 is a cortical field that was proposed on the basis of demonstrated connections of dorsal V1 representing the lower visual hemifield to adjoining dorsal V2 and cortex just rostral to V2 in macaque monkeys.66 V3 was proposed as having a mirror image retinotopy of V2, reversing along a common representation of the zero-horizontal meridian at the V2-V3 border. Microelectrode mapping of the retinotopy of the V3 region in macaques subsequently provided compelling evidence for the proposed organization of V3.67 However, an early problem for this interpretation was that 2 locations along the rostral border of V2 did not represent the lower visual quadrant as expected for V3, but they represented instead locations in the upper visual quadrant, thus providing evidence for an upper visual quadrant representation of 2 proposed visual areas along the rostral border of dorsal V2, DM, and the medial area (M).68,69 Although ventral V3 represents the upper visual quadrant as expected for V3, researchers thought that the ventral V3 region, renamed the ventral posterior visual area (VP), did not receive inputs from V1, while clear evidence existed for such a projection to dorsal V3.70
These problems for the traditional interpretation of V3 were addressed in 2 different ways by researchers working with marmosets. As the problem of VP as a visual area is that it represented only the upper visual quadrant, researchers combined the lateral part of dorsal V3 with VP to create a new ventrolateral (VL) visual area with at least a partial representation of the lower visual quadrant. Another part of dorsal V3 remained as part of DM.71,72 This is the interpretation portrayed in the Paxinos et al12 atlas. Another interpretation followed a study of the projections of both dorsal and ventral V1 in marmosets62 and other primates.73,74 Injections of tracers were placed in different parts of ventral and dorsal V1, and dorsal or ventral parts of V3 were always labeled in expected locations just rostral to V2. This argues that dorsal and ventral parts of V3 combine to form a single retinotopic area just rostral to V2, as previously proposed for V3. However, this interpretation does not eliminate the possibility that dorsal V3 has 1 or 2 gaps that represent the upper visual field of other smaller areas. Such a gap has been mentioned as occurring in some cases of mapping V3 in macaque monkeys, and clear evidence for such a gap was obtained in optical image recordings from V3 in a strepsirrhine (prosimian) primate, the African galago.75 Thus, the interpretation of a V3 with 1 or more gaps, perhaps for the upper field representations of DM and M, seems compatible with more of the data.76 What is not explained is why dorsal V3, and not ventral V3, has gaps.
Visual Cortex: The Dorsal and Ventral Streams
In marmosets,62 and other primates,73,74 direct, topographically organized projections from V1 provide evidence for 4 retinotopically organized visual areas. One of those targets, V2, is broadly accepted as a valid visual area across primates and in other mammals.58 Another is the MT,19 which appears to exist only in primates. Others include visual area V3, which is open to other interpretations (see above), and the DM (Fig. 4). M gets little or no direct input from V1 and is known mainly from microelectrode mapping data.69,77 In marmosets, the part of area M (Fig. 4) that represents the upper visual quadrant along the border with V1 has also been called area 19 M.3 This region has been variously divided and named in macaques.78,79 Thus, there are uncertainties about the organization and name for the visual region of M. Area DM was first described in owl monkeys as a complete visual area representing both upper and lower visual quadrants, and having more myelination than adjoined cortex, in owl monkeys.68 The region of DM has been called DM in other primates, including macaques.80 Overall, the DM region has been somewhat differently described. For example, Rosa et al81 and Lyon and Kaas62 have different depictions in marmosets. However, it is clear that part of the rostral border of V2 in primates is joined by the cortex representing the upper visual cortex as originally portrayed,68 likely occurring in the “gap” in dorsal V3 that represents the upper visual quadrant,75 but whether cortex medial to this gap is part of dorsal V3 or the representation of the lower visual quadrant of DM is unsettled.
Figure 4 .
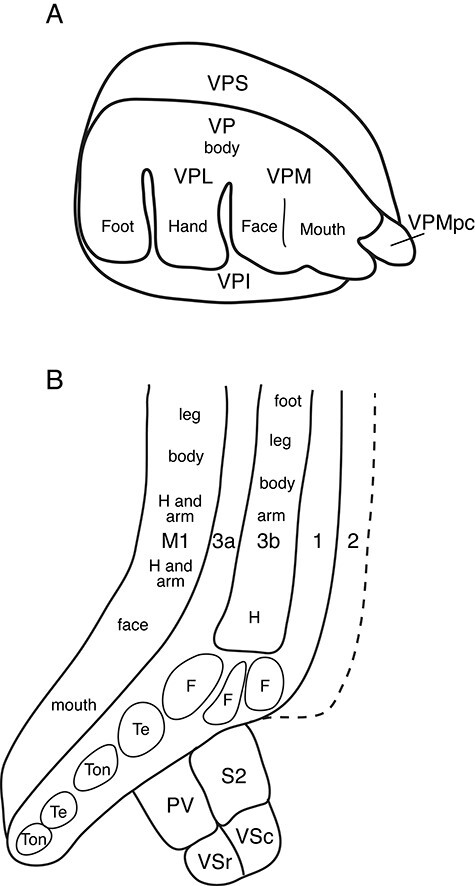
The somatosensory thalamus and cortex of marmosets. (A) The somatosensory thalamus includes 3 main nuclei, the ventroposterior nucleus (VP) which is divided into a lateral division (VPL) that represents the lower body, and a medial division (VPM) that represents the face and mouth. Cell-poor septa separate the hand representation in VPL from that of the face in VPM, as well as the hand and the foot representations in VPL. The more medial parts of VPM represent the teeth and tongue. Most medially, the ventroposterior medial parvocellular nucleus (VPMpc) represents taste. (B) Somatosensory areas and primary motor cortex (M1). Primary somatosensory cortex, S1 or area 3b, represents the contralateral body from tail and foot to hand (H), followed by 3 modules representing different parts of the face (F), and more ventrally by 4 modules representing the contralateral teeth (Te) and tongue (Ton) followed by the ipsilateral teeth and tongue. Area 3a, just rostral to 3b, represents proprioceptors, and parts of 3a may intrude into 3b. Area 1 contains a mirror image reversal of the somatotopy of 3b, and it appears to continue along representations of the face, teeth, and tongue in area 3b. In area 2, a representation of both touch and proprioception is expected, but there is only limited evidence for area 2 in marmosets. The second somatosensory area (S2) adjoins area 3b or an extension of area 1 and extends into the lateral sulcus. Another somatosensory representation, the parietal ventral area (PV) adjoins S2 ventrally. Deeper in the lateral sulcus, the ventral somatosensory region is divided into caudal (VSc) and rostral (VSr) areas. Just rostral to area 3a, the primary motor area (M1) contains crude somatotopic representation from leg to mouth, but M1 is more complexly organized into action-specific modules that may involve more than 1 body part (arm and hand, arm and face, arm and legs) when compared with Figure 1.
There is more agreement over the organization of the region of visual area MT in primates, including marmosets. MT is an easy area to define by its marked myelination and dense expression of CO. MT was first described in owl monkeys by clear histological characteristics, a representation of the visual hemifield, and neurons responsive to stimulus movement and stimulus onset.19 Major activating inputs come from both stellate and pyramidal neurons in V1.82 Optical imaging of evoked activity reveals that MT contains systematic maps of stimulus orientation and direction of motion responses relative to retinotopy.83 Studies on marmosets have the advantage of having MT completely located on the surface of cortex due to the short temporal sulcus, and MT and surrounding visual areas have been mapped with microelectrodes by Rosa and Elston in 1998.60 In addition, the cortical connections of MT with MST, FSTd, MTc (Fig. 1), and other regions have been described in marmosets.53,61,62 Subcortical inputs to MT include those from the medial nucleus (PIm) of inferior pulvinar, as well as from the koniocellular cells of the LGN.41 These connections are similar to those reported for other monkeys.61 Because it is easy to identify, MT has been found in all studied primates.
Studies of the postnatal development of MT in marmosets suggest that MT develops early, in part because of direct retinal inputs to the PIm of the pulvinar that relay to MT. Thereby, MT serves as an early organizer of surrounding visual areas and directs the subsequent development of visual-motor areas in the posterior parietal cortex.40,42,84 Much of MT’s influence on higher order visual processing comes from surrounding areas with short, direct inputs from MT. These areas include the MST, just rostral to MT; a ventral area, FST, which has been divided in dorsal (FSTd) and ventral (FSTv) areas with direct (FSTd) and indirect (FSTv) connections with MT; and a nearly surrounding band-like area, the MTc.85 MTc is especially distinct as a crescent of CO ovals in a CO light band around MT.62 All 3 areas have separate representations of the contralateral visual hemifield, and they have been studied in marmosets.53 These areas, together with MT and DM, are major contributors to visual and visuomotor areas of posterior parietal cortex that form the extensions of the dorsal stream of visual processing into parietal and frontal cortex.40,86
Visual areas of the ventral stream of visual processing in the temporal lobe are less well understood in marmosets. According to current views of the ventral stream of visual processing, which mediates object vision in primates,87 areas V1, V2, and V3 contribute to both dorsal and ventral streams via segregated pathways through these 3 areas.64 The cortical pathways with the information essential for the ventral stream terminate in the dorsolateral area DL (V4) located between central V3 and MT (Fig. 1). DL was originally described as a retinotopic visual area in owl monkeys located between V2 and MT.59,88 The original DL included the region of MTc and likely parts of V3. The main part of DL represented central vision, and the proposed extensions of DL along MT in what is now considered to be MTc were included because they added paracentral and peripheral vision. The DL region was called V4 in macaques, based on topographic connections with V1, V2, and V3,89 and V4 is the term most commonly used today. The V4-DL region in macaques appears to have 2 separate parallel representations within rostral and caudal bands, DLr and DLc, as the connections of these 2 regions differ, even after MTc is removed from V4/DL.90 Connection patterns with especially V2 have been used to delineate the dorsal border or DL/V4, but the location of the ventral border is still somewhat uncertain.90 The Paxinos et al12 2012 atlas of marmoset cortex illustrates a single area as V4 or the ventrolateral area (VL), but this V4 extends so far ventrally into the temporal lobe that much of this V4 or VLA area would represent the upper visual quadrant, making it much larger than the proposed more dorsal representation of the lower visual quadrant. While DLr appears to have connections more related to the dorsal stream of processing, DLc provides the major direct inputs to the upper temporal lobe (area TEO) as the next step in processing in the ventral stream. Further processing in the ventral stream involves areas and regions that have been defined functionally as critical in object vision, but they do not correspond to the proposed subdivisions of the temporal lobe based on classical cytoarchitecture.
In humans and macaques, parts of the lower temporal lobe are specialized for the processing of faces because faces are important in social signaling.91–93 More recently, such face-sensitive patches (Fig. 1) have been shown in the caudoventral temporal cortex of marmosets,94 suggesting that the face-processing network evolved in early monkeys at least 35 million years ago and that social visual perception relevant to humans can be studied in marmosets. See Kravitz et al95 for a review of the ventral visual pathway.
THE AUDITORY SYSTEM
Early mammals differed from their reptilian-like ancestors by modifying the mechanics of sound conduction to the inner ear so that hearing of higher frequencies was possible. Some species of bats are now known to have further adaptations that allow them to navigate and locate flying insects with very high frequency sounds. Primates do not have these extensive peripheral adaptations, but they do have specializations for vocal communications between members of the same species.96 The auditory system of marmosets has been studied, in part, because they are very social primates that communicate extensively with a variety of calls.97,98 As these studies have focused on higher levels of auditory processing, cortical processing is emphasized here.
Across mammals, the external ear and ear canal conduct sound pressure waves to the eardrum, where these vibrations are conducted by 3 bones to the oval window of the cochlea, a long-coiled tube of 3 compartments surrounded by bone. The middle compartment has inner sensory hair cells that respond to vibrations from sounds in the cochlear fluids, and these inner hair cells activate the afferents of ganglion cells that have axons that form the auditory nerve. The hair cells and axons of the auditory nerve encode information on the frequencies and intensities of sounds. Compared with the somatosensory and visual systems, more of the processing of sensory inputs are occurs subcortically in a complex of brainstem structures that are similar across mammalian taxa.99 The auditory nerve projects to divisions of the cochlear complex, the dorsal, posterior, and anterior cochlear nuclei that project contralaterally to brainstem structures and bilaterally to the superior olivary complex. The contralateral projections also go to the nuclei of the ascending pathway, the lateral lemniscus, and to the inferior colliculus. Other ascending projections include those from the superior olivary complex. The connections between the nuclei of the lateral lemniscus and the inferior colliculus of both sides further allow neurons to compare outputs from the 2 ears for information on sound location. The inferior colliculus receives inputs from cochlear and other subcortical nuclei as well as from descending inputs from the SC, thalamus, and cortex. The inferior colliculus contains at least 3 functionally distinct subdivisions.100,101 The large laminated central nucleus consists of sheets of neurons that systematically represent sound frequencies with low frequencies represented in sheets dorsolateral to those for high frequencies.102 The central nucleus of the inferior colliculus projects topographically to the tonotopically organized ventral nucleus of the medial geniculate complex of the auditory thalamus; while these projections are bilateral, the ipsilateral projections are much denser. The dorsal cortex and the external lateral nucleus of the inferior colliculus project to dorsal and medial nuclei of the medial geniculate complex of the auditory thalamus.103 As in other primates, the projections from auditory cortex to the inferior colliculus in marmosets are most dense in the dorsal cortex, but sparse in the central nucleus.104
The auditory thalamus includes 3 traditional architectonic divisions of the medial geniculate complex: the ventral (MGv), dorsal (MGd), and medial or magnocellular (MGm) nuclei.14 These nuclei have been identified in many species of mammals, including several primate species. In marmosets, these and other related nuclei have been fully described by de la Mothe et al105 and Bartlett and Wang.106 The large MGv has tightly packed neurons of medium size and most densely express CO and the glutamate transporter, VGLUT2. MGv receives tonotopically organized projections from the central nucleus of the ipsilateral inferior colliculus but also sparse projections from the contralateral central nucleus. The projections terminate in thin layers that successively represent low-to-high-tone frequencies in a ventrocaudal-to-dorsorostral progression.107 Relay neurons in MGv project densely to layer 4 of the primary areas of auditory cortex, where they are thought to be the major activating source of input. MGv has only sparse projections to secondary areas of the auditory belt.105,108 MGv has densely packed, smaller neurons.104 MGd receives inputs from the dorsal cortex of the inferior colliculus. Neurons in MGd are broadly responsive to tone frequencies and may respond best to complex sounds. Projections of MGd are to areas at the second and third levels of cortical processing outside the auditory core. MGm has neurons of several sizes, including the large magnocellular neurons.108 Neurons in MGm vary in responsiveness to tone and other stimuli, and some have very short response latencies.109 Both MGd and MGm project mainly to non-primary areas of auditory cortex, and where they terminate in superficial layers 2 and 3, with probably a modulatory role.104,105,107
Several other thalamic nuclei have roles in auditory processing, including the suprageniculate nucleus with multisensory inputs from the SC and broad connections to auditory and multisensory cortex.108 The PM projects broadly to multisensory areas of cortex, including the third cortical level of auditory processing, the auditory parabelt.
Auditory Cortex
Current theories for how auditory cortex are organized in primates came out of a series of experiments on macaque monkeys,108,110–113 owl monkeys,107,114 and marmosets.105,115–117 The cortical model that emerged (Figs. 1 and 3) appears to apply broadly to anthropoid primates, including apes and humans.118 This model of auditory cortex includes a core of 3 primary (or primary-like) areas, the traditional primary area (A1), the more recently defined rostral auditory area (R), and the rostrotemporal area (RT). Surrounding the core, a belt of secondary areas, named by location relative to the core, includes the caudolateral area, middle lateral area, and the rostrotemporal lateral area of the lateral belt and the caudolateral area, rostromedial area, and the rostrotemporal area of the medial belt. The parabelt region includes 2 divisions: the rostral parabelt and the caudal parabelt. The core is distinguished from the belt architectonically, and areas within the core are distinguished by patterns of tonotopic organization. The belt areas receive inputs directly from the core areas, and the areas of the belt are identified by their different patterns of connections. The parabelt region has dense inputs from the belt areas, and the 2 divisions of the parabelt are distinguished by their different patterns of input from the belt areas. Further study might reveal more divisions of the parabelt and other modifications of the cortical model, but it now is well supported by a large body of evidence.
Figure 3 .
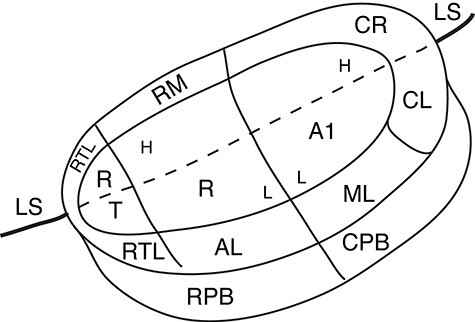
A surface view of flattened auditory cortex of marmosets. The region is divided into a ventral part that is visible on the exposed surface of the temporal lobe and a part unfolded from the caudal bank of the lateral sulcus (LS). Here both parts are shown on a flattened surface view of auditory cortex. The part of the lateral sulcus that divides auditory cortex is marked by a dashed line. The 3 primary areas are the traditional primary area (A1), the rostral area (R), and the rostral temporal area (RT). These areas represent high (H) to low (L) tone frequencies in progressions that reverse at areal borders. The auditory belt includes the rostral temporal lateral (RTL), the anterior lateral (AL), the medial lateral, and the caudal lateral (CL) areas of the lateral belt, and the rostral temporal medial (RTM), rostral medial (RM), and caudal rostral (CR) areas of the medial belt. The third level of auditory processing of the parabelt includes rostral (RPB) and caudal (CPB) parabelt divisions (compare with Fig. 1).
The primary area (A1) was the first area explored in microelectrode recording studies in primates.113 The general assumption was that mammals had only 1 primary area, as proposed for the visual and somatosensory systems.119 Through microelectrode mapping studies of tonotopic patterns, it became clear that rodents, cats, primates, and likely other mammals had more than 1 area with primary-like characteristics. The problem was determining which one of the primary-like areas was the primary area A1. As the primary-like areas in cats were described, A1 became the tonotopic area that occupied much of the territory of the originally described A1 with less precise methods. Yet it became clear that the adjoining anterior auditory field and the posterior auditory field in cats were also clearly primary-like in function and anatomy. Rodents also were found to have 3 primary-like fields with an AAF, A1, P progression of tonotopic maps, but some investigators called the AAF map A1. In primates, the core areas of A1, R, and RT do not have obvious homologues with the AAF, A1, P sequences in rodents and cats because the sequences of frequencies in the representations differ. In primates, the A1 name is well established, but it is possible that it is not the same area that is called A1 in other mammals. The existence of 2 or 3 primary-like areas in various mammals suggests that it is advantageous to provide 3 parallel streams at the first cortical stage of auditory processing instead of starting from a single primary area, as for vision and touch. The somewhat different response characteristics of neurons in R, RT, and A1 as well as their cortical connections suggest that R and RT are more associated with a ventral stream at auditory processing for the identification of sounds and communication calls, while A1 is more associated with the dorsal stream of processing for localizing the sources of sounds.120–122 A1 and adjacent belt and parabelt regions contribute more to dorsal cortical regions that project to dorsal parts of prefrontal cortex, while R and RT and adjacent cortex contribute more to the ventral cortex in the temporal lobe that projects to the ventral prefrontal cortex. In marmosets, RT has projections that include area 10 of prefrontal cortex.123 Overall, the core areas are densely myelinated and distinguished from the belt by expressing more parvalbumin, CO, and acetylcholinesterase. Activating inputs are from MGv. Layer 4 is thicker and more densely packed with smaller neurons in core areas, and neurons respond better to pure tones. One of the belt areas, area CM, is more like a primary area than other belt areas.117 CM has a tonotopic organization, and neurons respond to tones. CM has more of the architectonic characteristics of the core areas than other belt areas, but they are less pronounced than in primary areas. In addition, CM has thalamic inputs from MGd and not MGv, and lesions of A1 abolish the auditory responses of CM neurons.124 However, CM may be a key area of the dorsal stream, as neurons in CM are more selective for the locations of sounds.
The outputs of parabelt cortex involve caudally adjacent multisensory cortex, projections to more ventral temporal cortex, inputs to parts of posterior parietal cortex, and projections to frontal cortex.125 These connections have been used to identify parts of cortex involved in the dorsal and ventral streams of auditory cortex.112,126 Both auditory and visual areas project to overlapping regions of the frontal lobe, with a dorsal frontal region more involved with spatial locations and a ventral region more involved in object recognition.121,122 Some projections from multisensory regions of the temporal lobe in marmosets terminate in the frontal pole region area 10 of neocortex known as in other primates.127
THE SOMATOSENSORY SYSTEM
The somatosensory systems of marmosets are overall highly similar to those of other anthropoid primates. Yet, there are likely some specializations related to their small size and to their unique trait of having claws on all fingers and toes except the great toe, unlike other primates. These claws are not a vestigial trait of early primate or non-primate ancestors but a re-evolved trait or specialized feature that allows them to hold on while climbing up and down the vertical trunks of trees, much like squirrels do.6 Fingers and toes in other monkeys have nails to protect and reinforce the underlying digit and toe pads, which have high concentrations of tactile receptors. The hands of marmoset are more like paws and thereby less suitable for manipulating objects. Thus, marmosets may obtain proportionally less somatosensory information from their glabrous surface of their hands and feet than other anthropoid primates.
The somatosensory systems of marmosets and other primates include inputs from specialized receptors in the hand and other parts of the body that terminate on neurons in the dorsal horn of the grey matter of the spinal cord or in brainstem nuclei. Tactile inputs from the skin have branches that travel in the dorsal columns of the spinal cord to nuclei that represent the lower body (the gracile nucleus) or the forelimb (cuneate nucleus), or the face and mouth for the principal nucleus of the trigeminal complex in the brainstem. The representations of the hand and arm have been mapped in the cuneate nucleus of marmosets128 as well as in other primates. The brainstem nuclei project to the ventroposterior nucleus of the contralateral thalamus, which projects to primary somatosensory cortex, where information is then distributed to higher order somatosensory areas.129
The glabrous skin of the hands and feet of primates contain 4 types of low-threshold mechanoreceptors that respond to touch and pressure on the skin. These include the rapidly adapting type 1 that originates in Meissner corpuscles, responds on stimulus onset and offset to taps and flutters, and signals the texture of surface. The rapidly adapting type 1 afferent inputs are complimented by the slowly adapting type 1 afferents that originate in Merkel disks, respond during continued light pressure, and signal edges on surfaces. The less frequent SA2 and RA2 receptors signal skin stretch or vibration. The hairy skin has these types of receptors with some modifications, including touch domes and other receptors sensitive to hair and whisker movements or grooming. The stroking of the fur coat in social species, such as marmosets, may evoke a pleasant sensation known as affiliative touch.130 The inputs for affiliative touch and the high-dynamic range neurons, which may contribute to pain, terminate in the dorsal horn of spinal cord or on brainstem neurons that send projections to the contralateral thalamus via the spinothalamic pathways or its equivalent in the brainstem. These afferent pathways and relays to the thalamus have not been much studied in marmosets compared with other primates.
The somatosensory thalamus of primates (Fig. 4A) includes a large ventroposterior nucleus (VP) for inputs from the dorsal column nuclei and the principal nucleus of the trigeminal complex. These inputs are largely those that reflect the rapid and slowly adapting type 1 tactile afferents. VP contains 2 major subdivisions, the ventroposterior lateral subnucleus (VPL), which represents the contralateral body surface from tail to hand, arm, and neck; and the ventroposterior medial subnucleus (VPM), which represents the face, teeth, and tongue. Dorsomedial to VPM, the parvocellular ventroposterior nucleus (VPMpc) represents taste inputs from the tongue. The ventroposterior inferior nucleus (VPI) with inputs from the spinothalamic tract and its equivalent from the trigeminal spinal nucleus of the brainstem is just inferior to VP. The ventroposterior superior nucleus (VPS) just above VP receives proprioceptive inputs from the spinal cord and brainstem.129 As in other primates, these nuclei can be defined in marmosets by their architectonic characteristics as well as by inputs from subcortical structures and projections to cortical areas.
In Nissl and CO preparations, VP of marmosets stands out as more darkly stained than surrounding nuclei.12,131–133 VP is divided into VPL and VPM by a narrow, cell-poor septum that extends dorsally from VPI. Other less apparent septa separate the hand representation from the more lateral foot representation and the 5 digits of the hand from each other. Such septal regions also separate darkly stained clusters of neurons in VPM, such as those for representing the face, tongue, and teeth.131 the parvocellular ventroposterior nucleus for taste is distinguished from VPM by having somewhat smaller neurons and by being darker in sections stained for calbindin. VPI stains lightly for CO and is sparsely packed with neurons. Krubitzer and Kaas132 suggest that the poorly stained septal regions of VP are extensions of VPI into VP. VPS has fewer darkly packed neurons than VPM and less CO. As in other monkeys, VP projects densely to layer 4 of S1 (area 3b) in marmosets.131,132 The connections are somatotopic with injections of tracer in different parts of the body representation in S1 receiving inputs from different parts of VP. The major thalamic projections to the second somatosensory area (S2) and parietal ventral area (PV) are from VPI and the septal regions of VP.132,133 The major thalamic inputs to area 3a are from VPS.134
The somatosensory cortex in marmosets (Figs. 1 and 4B) includes areas S1 (area 3b), area 1, area 3a, area S2, area PV, and possibly area 2 (Fig. 4B). All these areas have been defined and studied in other primates.129 The first limited study of the somatotopic organization of S1 cortex in marmosets or tamarins was reported only in a brief abstract.135 Subsequently, a nearly complete representation of the contralateral body surface in tamarins was described in an extensive microelectrode mapping study.136 The representation was confined to architectonic area, 3b, and it extended from the foot most medially in precentral parietal cortex (note the lack of a central sulcus in these primates) through the leg, trunk, arm, and wrist to a large representation of the hand with digits 5 to 1 in a mediolateral sequence. More laterally and extending rostrally, representations of the face, teeth, and tongue were recorded, much as in Figure 1. The more ventral second representation of ipsilateral teeth and tongue were not described until much later.131 Later studies confirmed aspects of this overall somatotopic organization and provided further details in marmosets.61,137 Additional studies compared the normal organization of the hand in S1 of marmosets with the altered representation after the loss of some afferents from the hand.138,139 The architectonic features of S1 in marmosets include having densely packed neurons and dense myelination.12,137 In brain sections cut parallel to the cortical surface, S1 (area 3b) has a pronounced myelin-sparse septum between the hand and face representations61,140 but not the obvious septal regions between digit representations that can be seen in macaque monkeys.141 As in other monkeys, few intrinsic connections in S1 cross the hand-face border in marmosets.140
Unlike other monkeys that have been mapped with microelectrodes, there is only limited evidence for a complete representation of the contralateral body surface along the caudal border of S1, the area 1 representation, and only a hint of an area 2 representation.138 Likely these areas are present but are not very responsive under anesthesia, as suggested by Carlson et al.136 Area 2 representations have been described in macaques and New World cebus monkeys, but an area 2 somatotopic representation has not been reported in any small New World monkey. Thus, the existence of an area 2 has been questioned for marmosets as well as for owl monkeys and titi monkeys.142 However, there is good evidence for area 3a in marmosets that corresponds to area 3a of other primates as an area with proprioceptive inputs from VPS of the thalamus.134,137 In addition, there is evidence for the 2 somatosensory representations, the second somatosensory representation (S2) and the PV, in marmosets.61,133,136 These 2 areas depend on inputs from S1 for activation as lesions of S1 in marmosets render S2 and PV unresponsive.143 Other cortical connections of marmosets include sparse connections of S1 with area 3a, connections of 3b with the area 1–2 region, and dense connections between area 3a and area 1–2 region.134,140
Sensorimotor Cortex
There is experimental evidence that the frontal motor areas of other primates exist in marmosets (Fig. 1). Parts of posterior parietal cortex with parietal–frontal connections also exist, although parietal motor systems have not been extensively studied in marmosets. Importantly, the primary motor area (M1) has been identified and mapped with stimulating microelectrodes, and the M1 of marmosets, as in other primates, represents movements of the contralateral body from hindlimb to face in mediolateral sequences.137,144,145 M1 is coextensive with an agranular region of cortex that has large pyramidal neurons. Thalamic connections of M1 of marmosets, as expected, are with the ventrolateral nucleus.134 Movements have been evoked from area 3a and, at higher levels of current, from parts of S1. Premotor cortex was not very responsive, but limited evidence was obtained for the dorsal and ventral promotor areas (PMD and PMV) as well as the supplementary motor area.146 The cortical connections of caudal and rostral subdivisions of dorsal premotor cortex have been described in marmosets147 as similar to those in other monkeys. There is limited evidence from microsimulation and connections from visual areas for a frontal eye field and an adjoining frontal ventral area with visual connections.61,148 Connections of architectonic fields of prefrontal cortex, including the region of the frontal eye field, have been described in marmosets.149 Less is known about the organization of sensorimotor areas of posterior parietal cortex that have been well studied in other primates.86,150 However, single-unit recordings from neurons in posterior parietal cortex during a saccadic eye movement task revealed a region likely to be the lateral intraparietal area of other primates.151 Saccades were evoked from the same region by electrical stimulation.152 The proposed locations of other sensorimotor areas of posterior parietal cortex have been illustrated by Majka et al.153
OTHER CORTICAL AREAS
A description of other proposed architectonic fields and connections of marmosets is beyond the scope of this review. Other regions of cortex are presented in the Paxinos et al12 atlas and in Majka et al.153 Connections of the frontal pole of prefrontal cortex are described by Burman et al.127 Prefrontal cortex is important in the recognition of visual and auditory components of vocal communication, and marmosets are highly vocal.1 Chaplin et al154 indicate how proportions of regions of cortex have expanded differently in primates with large brains compared with the brains of marmosets. Atapour et al25 provide a detailed description of how the packing densities of neurons in the neocortex vary across cortical areas in marmosets. In addition, proportionally fewer neurons in marmosets are in primary sensory areas of cortex compared with the thalamic relay nuclei in small-brained marmosets than in primates with larger brains.155,156 This suggests that even for primary sensory areas, primates with larger brains are more dependent on cortical areas, the so called “corticalization of function.” Finally, resting-state functional magnetic resonance imaging has been used in marmosets to reveal large-scale brain networks.157
Already, marmosets have become one of the most studied of primates in neuroscience, but there is still so much to do.
CONCLUSION
Marmosets are presently considered to be a highly useful model of the primate brain where studies are highly relevant to understandings of the organization and functions of the human brain. As anthropoid primates, marmoset brains share many features with human brains, but human brains are much larger and more complex. Human brains have many more neurons and more functionally distinct subdivisions. Thus, inferences about human brains made from studies on marmoset brains should be made with caution, and information from studies on other model primates, especially from macaque monkeys, should be considered. Nevertheless, there is much to learn from studies on marmoset brains that will be informative and highly relevant. Marmosets offer the advantages of having brains that have already been extensively studied and having a small brain that offers many technical advantages. In addition, they mature and age more rapidly than large primates and thus are useful in studies on development and aging.
ACKNOWLEDGMENTS
Research on marmosets and other primates in the Kaas laboratory has been funded by long-standing grants from The National Institute of Health (USA).
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
References
- 1. Marx V. Neurobiology: learning from marmosets. Nat Methods 2016; 13(11):911–916. [DOI] [PubMed] [Google Scholar]
- 2. Miller CT, Freiwald WA, Leopold DA et al. Marmosets: a neuroscientific model of human social behavior. Neuron 2016; 90(2):219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solomon SG, Rosa MG. A simpler primate brain: the visual system of the marmoset monkey. Front Neural Circuits 2014; 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford SM. Callitrichids as phyletic dwarfs, and the place of the Callitrichidae in Platyrrhini. Primates 1980; 21:31–43. [Google Scholar]
- 5. Leutenegger W. Monogamy in callitrichids: a consequence of phyletic dwarfism? Int J Primatol 1980; 1(1):95–98. [Google Scholar]
- 6. Garber PA. Vertical clinging, small body size, and the evolution of feeding adaptations in the Callitrichinae. Am J Phys Anthropol 1992; 88(4):469–482. [DOI] [PubMed] [Google Scholar]
- 7. Catania KC, Lyon DC, Mock OB et al. Cortical organization in shrews: evidence from five species. J Comp Neurol 1999; 410(1):55–72. [PubMed] [Google Scholar]
- 8. Finlay BL, Brodsky P. Cortical evolution as the expression of a program for disproportionate growth and the proliferation of areas. In: Kaas JH, Krubitzer LA, eds. Evolution of Nervous System in Mammals. Vol 3. Oxford, UK: Elsevier; 2007:73–96. [Google Scholar]
- 9. Kaas JH, Preuss TM. Human brain evolution. In: Squire LR, ed. Functional Neuroscience. 4th ed. Oxford, UK: Elsevier; 2014:901–918. [Google Scholar]
- 10. Preuss TM. Critique of pure marmoset. Brain Behav Evol 2019; 93(2–3):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukushima M, Ichinohe N, Okano H. Neuroanatomy of the marmoset. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Oxford, UK: Elsevier; 2019. p. 43–62. [Google Scholar]
- 12. Paxinos G, Watson C, Peptrides M et al. The Marmoset Brain In Stereotaxic Coorinates. Oxford: Elsevier; 2012. [Google Scholar]
- 13. Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Barth; 1909. [Google Scholar]
- 14. Jones EG. The Thalamus. 2nd ed. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 15. Van Essen DC, Glasser MF, Dierker DL et al. Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cereb Cortex 2012; 22(10):2227–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Essen DC, Donahue CJ, Coalson TS et al. Cerebral cortical folding, parcellation, and connectivity in humans, nonhuman primates, and mice. Proc Natl Acad Sci U S A 2019; 116(S2):26173–26180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zilles K, Schlaug G, Matelli M et al. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat 1995; 187(Pt 3):515–537. [PMC free article] [PubMed] [Google Scholar]
- 18. Le Gros Clark WE. The brain of microcebus murinus. J Zool 1931; 101:463–486. [Google Scholar]
- 19. Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle tempral gyrus of the owl monkey (Aotus trivirgatus). Brain Res 1971; 31(1):85–105. [DOI] [PubMed] [Google Scholar]
- 20. Saraf MP, Balaram P, Pifferi F et al. Architectonic features and relative locations of primary sensory and related areas of neocortex in mouse lemurs. J Comp Neurol 2019; 527(3):625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zilles K, Rehkamper G, Stephan H et al. A quantitative approach to cytoarchitectonics. IV. The areal pattern of the cortex of Galago demidovii (e. Geoffroy, 1796), (lorisidae, primates). Anat Embryol 1979; 157(1):81–103. [DOI] [PubMed] [Google Scholar]
- 22. Kaas JH. The organization of neocortex in mammals: implications for theories of brain function. Annu Rev Psychol 1987; 38:129–151. [DOI] [PubMed] [Google Scholar]
- 23. Cahalane DJ, Charvet CJ, Finlay BL. Modeling local and cross-species neuron number variations in the cerebral cortex as arising from a common mechanism. Proc Natl Acad Sci U S A 2014; 111(49):17642–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herculano-Houzel S, Collins CE, Wong P et al. Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A 2007; 104(9):3562–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atapour N, Majka P, Wolkowicz IH et al. Neuronal distribution across the cerebral cortex of the marmoset monkey (Callithrix jacchus). Cereb Cortex 2019; 29(9):3836–3863. [DOI] [PubMed] [Google Scholar]
- 26. Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science 1995; 268(5217):1578–1584. [DOI] [PubMed] [Google Scholar]
- 27. Elston GN, Elston A, Kaas JH et al. Regional specialization in pyramidal cell structure in the visual cortex of the galago: an intracellular injection study of striate and extrastriate areas with comparative notes on new world and old world monkeys. Brain Behav Evol 2005; 66(1):10–21. [DOI] [PubMed] [Google Scholar]
- 28. Collins CE, Airey DC, Young NA et al. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A 2010; 107(36):15927–15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol 1972; 6(1):253–299. [DOI] [PubMed] [Google Scholar]
- 30. Kaas JH, Huerta MF, Weber JT et al. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J Comp Neurol 1978; 182(3):517–553. [DOI] [PubMed] [Google Scholar]
- 31. Warner CE, Kwan WC, Wright D et al. Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Curr Biol 2015; 25(4):424–434. [DOI] [PubMed] [Google Scholar]
- 32. White AJ, Wilder HD, Goodchild AK et al. Segregation of receptive field properties in the lateral geniculate nucleus of a new-world monkey, the marmoset Callithrix jacchus. J Neurophysiol 1998; 80(4):2063–2076. [DOI] [PubMed] [Google Scholar]
- 33. Huo BX, Zeater N, Lin MK et al. Relation of koniocellular layers of dorsal lateral geniculate to inferior pulvinar nuclei in common marmosets. Eur J Neurosci 2019; 50(12):4004–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon SG, White AJ, Martin PR. Extraclassical receptive field properties of parvocellular, magnocellular, and koniocellular cells in the primate lateral geniculate nucleus. J Neurosci 2002; 22(1):338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaas JH, Huerta MS. The subcortical visual sysem of primates. In: Inisteklis HP, ed. Comparative Primate Biology. Vol 4. New York: Alan R. Liss, 1988:327–391.
- 36. Weller RE, Kaas JH. Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates. Vis Neurosci 1989; 3(4):327–349. [DOI] [PubMed] [Google Scholar]
- 37. Casagrande VA, Kaas JH. The afferent, intrinsic, and effect connections of primary visual cortex in marmosets. In: Peters A, Rockland K, eds. Cerebral Cortex. Vol 10. New York: Plenurm Press; 1994:201–259. [Google Scholar]
- 38. May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 2006; 151:321–378. [DOI] [PubMed] [Google Scholar]
- 39. Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat Rec A Discov Mol Cell Evol Biol 2005; 285(1):619–627. [DOI] [PubMed] [Google Scholar]
- 40. Kaas JH, Baldwin MKL. The evolution of the Pulvinar complex in primates and its role in the dorsal and ventral streams of cortical processing. Vision (Basel) 2020; 4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci 1999; 11(2):469–480. [DOI] [PubMed] [Google Scholar]
- 42. Warner CE, Kwan WC, Bourne JA. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J Neurosci 2012; 32(48):17073–17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baldwin MKL, Balaram P, Kaas JH. The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol 2017; 525(15):3207–3226. [DOI] [PubMed] [Google Scholar]
- 44. Homman-Ludiye J, Bourne JA. The medial pulvinar: function, origin and association with neurodevelopmental disorders. J Anat 2019; 235(3):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pessoa VF, Abrahao JC, Pacheco RA et al. Relative sizes of cortical visual areas in marmosets: functional and phylogenetic implications. Exp Brain Res 1992; 88(2):459–462. [DOI] [PubMed] [Google Scholar]
- 46. Kaas JH. Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain and Mind 2000; 1:7–23. [Google Scholar]
- 47. Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. Anat Rec A Discov Mol Cell Evol Biol 2005; 287(1):1013–1025. [DOI] [PubMed] [Google Scholar]
- 48. Chaplin TA, Yu HH, Rosa MG. Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J Comp Neurol 2013; 521(5):1001–1019. [DOI] [PubMed] [Google Scholar]
- 49. Fritsches KA, Rosa MG. Visuotopic organisation of striate cortex in the marmoset monkey (Callithrix jacchus). J Comp Neurol 1996; 372(2):264–282. [DOI] [PubMed] [Google Scholar]
- 50. Gebhard R, Zilles K, Schleicher A et al. Distribution of seven major neurotransmitter receptors in the striate cortex of the new world monkey Callithrix jacchus. Neuroscience 1993; 56(4):877–885. [DOI] [PubMed] [Google Scholar]
- 51. Solomon SG. Striate cortex in dichromatic and trichromatic marmosets: neurochemical compartmentalization and geniculate input. J Comp Neurol 2002; 450(4):366–381. [DOI] [PubMed] [Google Scholar]
- 52. Hassler R. Comparative anatomy of the central visual system in day- and night-active primates. In: Hassler R, Stephan H, eds. Evolution of the Forebrain. Stuttgart, West Germany: Thiem Varlay; 1966. p. 419–434. [Google Scholar]
- 53. Palmer SM, Rosa MG. Quantitative analysis of the corticocortical projections to the middle temporal area in the marmoset monkey: evolutionary and functional implications. Cereb Cortex 2006; 16(9):1361–1375. [DOI] [PubMed] [Google Scholar]
- 54. Spatz WB. Topographically organized reciprocal connections between areas 17 and MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Exp Brain Res 1977; 27(5):559–572. [DOI] [PubMed] [Google Scholar]
- 55. Spatz WB. An efferent connection of the solitary cells of Meynert. A study with horseradish peroxidase in the marmoset Callithrix. Brain Res 1975; 92(3):450–455. [DOI] [PubMed] [Google Scholar]
- 56. Cusick CG, Gould HJ 3rd, Kaas JH. Interhemispheric connections of visual cortex of owl monkeys (Aotus trivirgatus), marmosets (Callithrix jacchus), and galagos (Galago crassicaudatus). J Comp Neurol 1984; 230(3):311–336. [DOI] [PubMed] [Google Scholar]
- 57. Markstahler U, Bach M, Spatz WB. Transient molecular visualization of ocular dominance columns (ODCs) in normal adult marmosets despite the desegregated termination of the retino-geniculo-cortical pathways. J Comp Neurol 1998; 393(1):118–134. [PubMed] [Google Scholar]
- 58. Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends Neurosci 1999; 22(6):242–248. [DOI] [PubMed] [Google Scholar]
- 59. Allman JM, Kaas JH. The organization of the second visual area (V II) in the owl monkey: a second order transformation of the visual hemifield. Brain Res 1974; 76(2):247–265. [DOI] [PubMed] [Google Scholar]
- 60. Rosa MG, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J Comp Neurol 1998; 393(4):505–527. [PubMed] [Google Scholar]
- 61. Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci 1990; 10(3):952–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lyon DC, Kaas JH. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J Neurosci 2001; 21(1):249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci 1984; 4(1):309–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Federer F, Ichida JM, Jeffs J et al. Four projection streams from primate V1 to the cytochrome oxidase stripes of V2. J Neurosci 2009; 29(49):15455–15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev 2007; 55(2):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zeki SM. Representation of central visual fields in prestriate cortex of monkey. Brain Res 1969; 14(2):271–291. [DOI] [PubMed] [Google Scholar]
- 67. Gattass R, Sousa AP, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. J Neurosci 1988; 8(6):1831–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allman JM, Kaas JH. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Res 1975; 100(3):473–487. [DOI] [PubMed] [Google Scholar]
- 69. Allman JM, Kaas JH. Representation of the visual field on the medial wall of occipital-parietal cortex in the owl monkey. Science 1976; 191(4227):572–575. [DOI] [PubMed] [Google Scholar]
- 70. Burkhalter A, Felleman DJ, Newsome WT et al. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res 1986; 26(1):63–80. [DOI] [PubMed] [Google Scholar]
- 71. Angelucci A, Rosa MG. Resolving the organization of the third tier visual cortex in primates: a hypothesis-based approach. Vis Neurosci 2015; 32:E010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosa MG, Tweedale R. Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. J Comp Neurol 2000; 422(4):621–651. [DOI] [PubMed] [Google Scholar]
- 73. Lyon DC, Kaas JH. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of new world monkeys. J Comp Neurol 2002; 449(3):281–297. [DOI] [PubMed] [Google Scholar]
- 74. Lyon DC, Kaas JH. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron 2002; 33(3):453–461. [DOI] [PubMed] [Google Scholar]
- 75. Fan RH, Baldwin MK, Jermakowicz WJ et al. Intrinsic signal optical imaging evidence for dorsal V3 in the prosimian galago (Otolemur garnettii). J Comp Neurol 2012; 520(18):4254–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaas JH, Roe AW, Baldwin MK et al. Resolving the organization of the territory of the third visual area: a new proposal. Vis Neurosci 2015; 32:E016. [DOI] [PubMed] [Google Scholar]
- 77. Rosa MG, Schmid LM. Visual areas in the dorsal and medial extrastriate cortices of the marmoset. J Comp Neurol 1995; 359(2):272–299. [DOI] [PubMed] [Google Scholar]
- 78. Colby CL, Gattass R, Olson CR et al. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol 1988; 269(3):392–413. [DOI] [PubMed] [Google Scholar]
- 79. Galletti C, Gamberini M, Kutz DF et al. The relationship between V6 and PO in macaque extrastriate cortex. Eur J Neurosci 2005; 21(4):959–970. [DOI] [PubMed] [Google Scholar]
- 80. Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in old world macaque monkeys. J Comp Neurol 1999; 406(4):487–502. [PubMed] [Google Scholar]
- 81. Rosa MG, Palmer SM, Gamberini M et al. Resolving the organization of the new world monkey third visual complex: the dorsal extrastriate cortex of the marmoset (Callithrix jacchus). J Comp Neurol 2005; 483(2):164–191. [DOI] [PubMed] [Google Scholar]
- 82. Mundinano IC, Kwan WC, Bourne JA. Retinotopic specializations of cortical and thalamic inputs to area MT. Proc Natl Acad Sci U S A 2019; 116(46):23326–23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kaskan PM, Dillenburger BC, Lu HD et al. Orientation and direction-of-motion response in the middle temporal visual area (MT) of new world owl monkeys as revealed by intrinsic-signal optical imaging. Front Neuroanat 2010; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bourne JA, Rosa MG. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT). Cereb Cortex 2006; 16(3):405–414. [DOI] [PubMed] [Google Scholar]
- 85. Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J Neurosci 1993; 13(2):534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kaas JH, Qi HX, Stepniewska I. The evolution of parietal cortex in primates. Handb Clin Neurol 2018; 151:31–52. [DOI] [PubMed] [Google Scholar]
- 87. Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 1983; 6(10):414–417. [Google Scholar]
- 88. Allman JM, Kaas JH. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgatus). Brain Res 1974; 81(2):199–213. [DOI] [PubMed] [Google Scholar]
- 89. Zeki SM. The functional organization of projections from striate to prestriate visual cortex in the rhesus monkey. Cold Spring Harb Symp Quant Biol 1976; 40:591–600. [DOI] [PubMed] [Google Scholar]
- 90. Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex 2005; 15(6):809–822. [DOI] [PubMed] [Google Scholar]
- 91. Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17(11):4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ku SP, Tolias AS, Logothetis NK et al. fMRI of the face-processing network in the ventral temporal lobe of awake and anesthetized macaques. Neuron 2011; 70(2):352–362. [DOI] [PubMed] [Google Scholar]
- 93. Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature 2006; 442(7102):572–575. [DOI] [PubMed] [Google Scholar]
- 94. Hung CC, Yen CC, Ciuchta JL et al. Functional mapping of face-selective regions in the extrastriate visual cortex of the marmoset. J Neurosci 2015; 35(3):1160–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kravitz DJ, Saleem KS, Baker CI et al. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci 2013; 17(1):26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ghazanfar AA, Hauser MD. The neuroethology of primate vocal communication: substrates for the evolution of speech. Trends Cogn Sci 1999; 3(10):377–384. [DOI] [PubMed] [Google Scholar]
- 97. Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cereb Cortex 2005; 15(10):1510–1523. [DOI] [PubMed] [Google Scholar]
- 98. Ghazanfar AA, Hauser MD. The auditory behaviour of primates: a neuroethological perspective. Curr Opin Neurobiol 2001; 11(6):712–720. [DOI] [PubMed] [Google Scholar]
- 99. Kaas JH, O'Brien BMJ, Hackett TA. Auditory processing in primate brains. In: Gollapher M, Nelson RJ, eds. Handbook of Psychology. Vol 3. Hoboken, NJ: John Wiley and Sons; 2013: 254–285. [Google Scholar]
- 100. FitzPatrick KA. Cellular architecture and topographic organization of the inferior colliculus of the squirrel monkey. J Comp Neurol 1975; 164(2):185–207. [DOI] [PubMed] [Google Scholar]
- 101. Huffman RF, Henson OW Jr. The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Brain Res Rev 1990; 15(3):295–323. [DOI] [PubMed] [Google Scholar]
- 102. Bulkin DA, Groh JM. Systematic mapping of the monkey inferior colliculus reveals enhanced low frequency sound representation. J Neurophysiol 2011; 105(4):1785–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res 2003; 153(4):543–549. [DOI] [PubMed] [Google Scholar]
- 104. Luethke LE, Krubitzer LA, Kaas JH. Connections of primary auditory cortex in the new world monkey, Saguinus. J Comp Neurol 1989; 285(4):487–513. [DOI] [PubMed] [Google Scholar]
- 105. de la Mothe LA, Blumell S, Kajikawa Y et al. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol 2006; 496(1):72–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. J Neurophysiol 2011; 105(6):2647–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Morel A, Kaas JH. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol 1992; 318(1):27–63. [DOI] [PubMed] [Google Scholar]
- 108. Hackett TA, Stepniewska I, Kaas JH. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol 1998; 400(2):271–286. [DOI] [PubMed] [Google Scholar]
- 109. Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci 1983; 3(11):2365–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hackett TA, De La Mothe LA, Ulbert I et al. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol 2007; 502(6):924–952. [DOI] [PubMed] [Google Scholar]
- 111. Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol 1998; 394(4):475–495. [DOI] [PubMed] [Google Scholar]
- 112. Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A 2000; 97(22):11793–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res 1973; 50(2):275–296. [DOI] [PubMed] [Google Scholar]
- 114. Imig TJ, Ruggero MA, Kitzes LM et al. Organization of auditory cortex in the owl monkey (Aotus trivirgatus). J Comp Neurol 1977; 171(1):111–128. [DOI] [PubMed] [Google Scholar]
- 115. Aitkin LM, Merzenich MM, Irvine DR et al. Frequency representation in auditory cortex of the common marmoset (Callithrix jacchus jacchus). J Comp Neurol 1986; 252(2):175–185. [DOI] [PubMed] [Google Scholar]
- 116. Philibert B, Beitel RE, Nagarajan SS et al. Functional organization and hemispheric comparison of primary auditory cortex in the common marmoset (Callithrix jacchus). J Comp Neurol 2005; 487(4):391–406. [DOI] [PubMed] [Google Scholar]
- 117. de la Mothe LA, Blumell S, Kajikawa Y et al. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol 2006; 496(1):27–71. [DOI] [PubMed] [Google Scholar]
- 118. Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol 2001; 441(3):197–222. [DOI] [PubMed] [Google Scholar]
- 119. Kaas JH. The evolution of auditory cortex: the core areas. In: Winer JA, Schreiner CE, eds. The Auditory Cortex. New York: Springer; 2011:407–427. [Google Scholar]
- 120. Kaas JH, Hackett TA. 'What' and 'where' processing in auditory cortex. Nat Neurosci 1999; 2(12):1045–1047. [DOI] [PubMed] [Google Scholar]
- 121. Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol 2005; 93(2):734–747. [DOI] [PubMed] [Google Scholar]
- 122. Romanski LM, Tian B, Fritz J et al. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci 1999; 2(12):1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rosa MG, Palmer SM, Gamberini M et al. Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J Neurosci 2009; 29(14):4548–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rauschecker JP, Tian B, Pons T et al. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol 1997; 382(1):89–103. [PubMed] [Google Scholar]
- 125. Hackett TA. Information flow in the auditory cortical network. Hear Res 2011; 271(1–2):133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc Natl Acad Sci U S A 2000; 97(22):11800–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Burman KJ, Reser DH, Yu HH et al. Cortical input to the frontal pole of the marmoset monkey. Cereb Cortex 2011; 21(8):1712–1737. [DOI] [PubMed] [Google Scholar]
- 128. Xu J, Wall JT. Cutaneous representations of the hand and other body parts in the cuneate nucleus of a primate, and some relationships to previously described cortical representations. Somatosens Mot Res 1996; 13(3–4):187–197. [DOI] [PubMed] [Google Scholar]
- 129. Kaas JH. Somatosensory system. In: Paxinos G, Mai JK, eds. The Human Nervous System. 2nd ed. Oxford: Elsevier; 2004:1059–1092. [Google Scholar]
- 130. Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci 2010; 11(6):417–428. [DOI] [PubMed] [Google Scholar]
- 131. Iyengar S, Qi HX, Jain N et al. Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of new world monkeys. J Comp Neurol 2007; 501(1):95–120. [DOI] [PubMed] [Google Scholar]
- 132. Krubitzer LA, Kaas JH. The somatosensory thalamus of monkeys: cortical connections and a redefinition of nuclei in marmosets. J Comp Neurol 1992; 319(1):123–140. [DOI] [PubMed] [Google Scholar]
- 133. Qi HX, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus). J Comp Neurol 2002; 443(2):168–182. [DOI] [PubMed] [Google Scholar]
- 134. Huffman KJ, Krubitzer L. Thalamo-cortical connections of areas 3a and M1 in marmoset monkeys. J Comp Neurol 2001; 435(3):291–310. [DOI] [PubMed] [Google Scholar]
- 135. Woolsey CN. Localization patterns in a lissencephalic primate (Hapale). Am J Physiol 1954; 179:686. [Google Scholar]
- 136. Carlson M, Huerta MF, Cusick CG et al. Studies on the evolution of multiple somatosensory representations in primates: the organization of anterior parietal cortex in the new world Callitrichid, Saguinus. J Comp Neurol 1986; 246(3):409–426. [DOI] [PubMed] [Google Scholar]
- 137. Burish MJ, Stepniewska I, Kaas JH. Microstimulation and architectonics of frontoparietal cortex in common marmosets (Callithrix jacchus). J Comp Neurol 2008; 507(2):1151–1168. [DOI] [PubMed] [Google Scholar]
- 138. Bowes C, Burish M, Cerkevich C et al. Patterns of cortical reorganization in the adult marmoset after a cervical spinal cord injury. J Comp Neurol 2013; 521(15):3451–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wall JT, Huerta MF, Kaas JH. Changes in the cortical map of the hand following postnatal ulnar and radial nerve injury in monkeys: organization and modification of nerve dominance aggregates. J Neurosci 1992; 12(9):3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fang PC, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of new world monkeys. J Comp Neurol 2002; 454(3):310–319. [DOI] [PubMed] [Google Scholar]
- 141. Qi HX, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp Neurol 2004; 477(2):172–187. [DOI] [PubMed] [Google Scholar]
- 142. Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do new world monkeys have an area 2? Cereb Cortex 2005; 15(12):1938–1963. [DOI] [PubMed] [Google Scholar]
- 143. Garraghty PE, Pons TP, Kaas JH. Ablations of areas 3b (SI proper) and 3a of somatosensory cortex in marmosets deactivate the second and parietal ventral somatosensory areas. Somatosens Mot Res 1990; 7(2):125–135. [DOI] [PubMed] [Google Scholar]
- 144. Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex 2001; 11(9):849–867. [DOI] [PubMed] [Google Scholar]
- 145. Burman KJ, Palmer SM, Gamberini M et al. Anatomical and physiological definition of the motor cortex of the marmoset monkey. J Comp Neurol 2008; 506(5):860–876. [DOI] [PubMed] [Google Scholar]
- 146. Bakola S, Burman KJ, Rosa MG. The cortical motor system of the marmoset monkey (Callithrix jacchus). Neurosci Res 2015; 93:72–81. [DOI] [PubMed] [Google Scholar]
- 147. Burman KJ, Bakola S, Richardson KE et al. Patterns of afferent input to the caudal and rostral areas of the dorsal premotor cortex (6DC and 6DR) in the marmoset monkey. J Comp Neurol 2014; 522(16):3683–3716. [DOI] [PubMed] [Google Scholar]
- 148. Kaas JH, Krubitzer LA. Subdivisions of visuomotor and visual cortex in the frontal lobe of primates: the frontal eye field and the target of the middle temporal visual area. Soc Neurosci Abstracts 1988; 14:820. [Google Scholar]
- 149. Reser DH, Burman KJ, Yu HH et al. Contrasting patterns of cortical input to architectural subdivisions of the area 8 complex: a retrograde tracing study in marmoset monkeys. Cereb Cortex 2013; 23(8):1901–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stepniewska I, Gharbawie OA, Burish MJ et al. Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation. J Neurophysiol 2014; 111(5):1100–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ma L, Selvanayagam J, Ghahremani M et al. Single-unit activity in marmoset posterior parietal cortex in a gap saccade task. J Neurophysiol 2020; 123(3):896–911. [DOI] [PubMed] [Google Scholar]
- 152. Ghahremani M, Johnston KD, Ma L et al. Electrical microstimulation evokes saccades in posterior parietal cortex of common marmosets. J Neurophysiol 2019; 122(4):1765–1776. [DOI] [PubMed] [Google Scholar]
- 153. Majka P, Chaplin TA, Yu HH et al. Towards a comprehensive atlas of cortical connections in a primate brain: mapping tracer injection studies of the common marmoset into a reference digital template. J Comp Neurol 2016; 524(11):2161–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chaplin TA, Yu HH, Soares JG et al. A conserved pattern of differential expansion of cortical areas in simian primates. J Neurosci 2013; 33(38):15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Collins CE, Leitch DB, Wong P et al. Faster scaling of visual neurons in cortical areas relative to subcortical structures in non-human primate brains. Brain Struct Funct 2013; 218(3):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wong P, Peebles JK, Asplund CL et al. Faster scaling of auditory neurons in cortical areas relative to subcortical structures in primate brains. Brain Behav Evol 2013; 81(4):209–218. [DOI] [PubMed] [Google Scholar]
- 157. Belcher AM, Yen CC, Stepp H et al. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci 2013; 33(42):16796–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]


