Abstract
Gastrointestinal disease is a frequently encountered problem among captive common marmoset (Callithrix jacchus) colonies. Management can be challenging due to the number of etiologies responsible for gastrointestinal disease in this species, limitations on diagnostic capabilities, and lack of effective treatments. Understanding commonly described GI diseases in the captive marmoset can provide insight on the impact these diseases have on research studies and aid in the development of appropriate management strategies. A review of commonly encountered GI disease processes as well as routinely implicated causes of GI disease in the common marmoset are provided. Current strategies in clinical management of GI disease in the common marmoset, including approaches to colony health, diagnostic testing, and commonly employed treatments are discussed.
Keywords: diagnostics, diarrhea, gastrointestinal, management, marmoset, pathogens, treatment
INTRODUCTION
The popularity of the common marmoset as a model for biomedical research continues to grow and so do concerns regarding gastrointestinal (GI) disease in this species. GI diseases are the most frequently reported clinical problem throughout captive colonies, with diarrhea being the most commonly reported symptom.1,2 GI diseases in this species result in a significant amount of lost resources, including time and money spent on diagnostics and treatment, delays in completing research studies, disqualification of unhealthy animals from study resulting in the need for replacement, and loss of animal life. Enrollment of healthy animals in research studies is of the utmost importance to ensure reliable scientific findings and to minimize the introduction of unwanted variables.
The use of well-defined clinical terminology (Table 1) is needed to promote collaboration across institutions with marmoset colonies. Standardization is imperative for the comparison of disease processes and determination of their prevalence throughout these colonies. Etiologic and morphologic descriptions of pathologic lesions and disease processes are essential to advancing understanding of GI disease in this species. Collaborative communication will facilitate investigation of causation as well as refine diagnostics and therapies of GI diseases in the marmoset.
Table 1.
Definitions of Commonly Used Terminology for Describing Gastrointestinal Diseases Affecting the Common Marmoset
| Term | Definition |
|---|---|
| Acute | Sudden or abrupt onset with short duration or course (0–14 d); may be more severe, self-limiting, or responsive to short-term medical treatment |
| Chronic | Consistent (≥50%) or continually recurs over long duration (>14 d), requiring multiple treatments or continual medical treatment |
| Intermittent | Occurs at intervals or alternating periods of normal and abnormal, not continuous, self-limiting or responsive to short-term medical treatment but returns after period of time |
| GI malabsorption | Poor or decreased uptake or incorporation of nutrients by GI tract due to intestinal inflammation or other disease process |
| IBD | Group of disorders characterized by chronic inflammation of GI tract |
| CLE | Type of IBD in the marmoset requiring histologic diagnosis, defined by villous blunting and fusion, crypt hyperplasia, and expansion of lamina propria due to many lymphocytes |
| Emesis/vomiting | Expulsion of material from stomach and/or intestinal tract through mouth |
| Hematochezia | Expulsion of fresh or frank blood from anus or presence of frank blood associated with feces |
| Melena | Dark, sticky feces containing digested blood |
| Mucoid | Contains viscous, slippery substance consisting of mucus (mucin, water, salts) |
| Metabolic bone disease | Group of disorders resulting in remodeling of bone tissue (eg, osteopenia, fibrous osteodystrophy, rickets) |
| Hepatomegaly | Enlargement of liver or liver tissue, clinically diagnosed by palpation of liver tissue extending beyond margins of costal arch |
| Thin | Body condition score ≤2.0 and/or weight ≤325 g |
| Obese | Body condition score ≥4.0 and/or weight ≥450 g |
CLE = chronic lymphocytic enteritis; GI = gastrointestinal; IBD = inflammatory bowel disease.
MANAGEMENT OF COLONY HEALTH
Establishment of routine colony surveillance measures is a key to a good preventative medicine program and overall colony health. Daily health monitoring, routine weighing and weight tracking, and standardized reporting of health abnormalities are tools that can aid in the early detection of GI and other diseases in marmosets. Early detection of disease facilitates prompt intervention and may help prevent minor clinical problems from progressing to more severe or chronic disturbances. Adherence to strict quarantine procedures can prevent the introduction and spread of infectious agents throughout a colony from animals that may be harboring disease.
Daily Health Observations
Each morning should begin with a systematic approach to evaluating the health of each animal in the colony. Daily health observations consist of a prompt and thorough visual inspection of the animal within its home enclosure. Marmosets are adept at hiding illness and can appear healthy despite significant ongoing disease. Health observations should include assessment of attitude and activity level with attention to ambulation, posture, hair coat, and vocalizations. Healthy animals are alert, interactive, with upright posture, and typically found in the upper portion of the enclosure as opposed to animals in poor health that may be reluctant to move, are lethargic, or have decreased responsiveness.3
Following evaluation of the animals, a thorough inspection of the enclosure should be performed. The enclosure, including shelves, perches, and nest box, should be checked for evidence of abnormal feces, vomitus, blood, or urine. A system allowing the detection of abnormal appetite should be utilized as a decrease in food consumption can be the first indication of a problem. A small scale is employed at the authors’ institution to weigh leftover food within the enclosure each morning during daily health observations making an abrupt increase in leftover food easily noticeable. Inclusion of food dropped to the floor and food moved around the enclosure by the animals will result in a more accurate measurement. Considerations should be made for overnight desiccation of the food (loss of water weight) because many marmoset diets have a high moisture content.
A simple and routine system for recording health abnormalities is an essential part of a marmoset colony health program. Electronic record systems are convenient and easy to use, but high expense prevents their widespread utilization. Easily accessible paper records can be used as an effective alternative. Abnormal findings detected during daily health observations by husbandry or other staff members should be readily available to both veterinary and research staff to facilitate the prompt evaluation of health concerns. It is essential for clinicians to also have access to past findings, dating back weeks, months, or even years, to assess trends. Urgent concerns require a verbal reporting system for veterinary staff to address emergencies in a timely manner.
Health observations performed by staff that are experienced in marmoset husbandry will improve detection of abnormalities and potential ongoing illness. Additionally, consistency among staff members results in increased familiarity with individual animals and their behaviors, resulting in improved ability to identify subtle changes. Unfamiliar or frequently changing staff may contribute to changes in animal stress levels or animal health, potentially exacerbating underlying problems or introducing variables in research outcomes.4 Staff performing health observations should receive regular reminders that emphasize the importance of detection and reporting of health abnormalities. Allocation of an appropriate amount of time for staff to thoroughly perform this task is essential.
Weights, Weight Tracking, and Body Condition Scoring
Preventative weight monitoring is another essential component of the marmoset colony health program. Common marmosets are known for drastic weight changes over short periods of time, indicative of environmental stressors or ongoing disease. Changes in weight may easily go unrecognized during daily health observations and cage-side examination of animals. All animals at the Wisconsin National Primate Research Center (WNPRC) are weighed at least every 30 days and animals with a history of GI disease or other health problems are weighed every 2 weeks. Animals undergoing treatment or requiring supportive care are weighed with increased frequency (1–3 times per week). It is standard procedure to weigh animals that are being handled, moved, or undergoing procedures to increase the number of recorded weights.
Recording weight data over time establishes a baseline for each animal and allows for quick detection of weight loss. Availability of historic weight data and the ability to display the data in list or graphic form is an invaluable tool for a clinician to quickly identify significant weight changes. In addition to screening for health problems, weight data can provide insight on effectiveness of treatments, changes in diet, or other interventions.
Standard procedures to allow regular weighing of all the animals in the colony should be developed. Detachable nest boxes mounted on the wall of the enclosure serve the dual purpose of nesting and transport. Placement of the nest box with the animals inside on the scale allows for quick and easy weighing of large numbers of animals. The scale is set to zero and when a marmoset is removed from the next box, the negative weight displayed indicates the weight of that marmoset; the marmoset may be placed in another nest box or returned to the home enclosure. This process is repeated until all marmosets in the next box have been weighed. Placement of scales within the animal enclosure has been described and is conducted at the Southwest National Primate Research Center (SNPRC), reducing the need for handling while providing a positive human interaction experience for the animals.5 It is a way to engage staff in positive reinforcement training, potentially resulting in a more enjoyable experience for the both the animals and the staff. It is helpful to record the time a weight is obtained to account for daily fluctuations, as animals may gain a significant amount of weight after feeding.
Employment of a standardized body condition scoring (BCS) system is recommended during physical examination to compliment weight findings. Evaluation of the soft tissues of the animal using palpation allows for a subjective measurement of the amount of muscle and fat mass on an animal. In macaques, BCS has been shown to be a better predictor of average percentage of body fat than weight alone.6 A common marmoset BCS chart was previously described and is employed at the author’s institution.3 An animal is assigned a score, using the chart, ranging from 1 (emaciated) to 5 (grossly obese) that provides the clinician information not reflected in weight data alone. BCS will discern a tall and thin animal from a short and obese animal that may be similar in weight.
Feces Description
The use of a standard fecal assessment results in efficient recognition and treatment of GI disease and aids in evaluation of clinical treatment effects. A fecal scoring system was used to aid in evaluation of the treatment of Giardia in marmosets.7 The use of a similar system is employed at the WNPRC and SNPRC during daily animal health checks. Visual aids along with detailed descriptions of different types of feces (firm, normal, soft, diarrhea, and watery diarrhea) provide a good reference (Figure 1) for staff performing health observations and help maintain consistency in reporting. In addition to fecal consistency, qualifiers that provide timeframe (acute, chronic), quantity (increased or decreased volume or number of bowel movements), color (tan, black, light, dark), and contents (blood, mucus, undigested food items) are useful to the clinician. Use of well-defined standard language (Table 1) can provide insight on etiology and allows for the association of abnormal feces with different disease processes.
Figure 1 .

Fecal scoring chart for the common marmoset. Images and descriptions of varying consistencies of stool found in the common marmoset (Callithrix jacchus).
Quarantine
Quarantine procedures are indicated when infectious diseases are detected or are suspected to be present within a marmoset colony. Newly arrived animals pose a risk of introducing novel pathogens into an established colony with potentially dire consequences, necessitating strict quarantine procedure.3 Transportation of animals may result in stress and potential recrudescence of latent infections in subclinical animals. The animals should remain quarantined until it is certain that no infectious diseases are present. The Centers for Disease Control and Protection guidelines state that imported nonhuman primates should be administered 3 tuberculin skin tests at least 2 weeks apart for a minimum quarantine of 31 days. Cohorts that have positive tests or are suspicious should be quarantined for 5 additional tuberculin skin tests.8 Quarantine length may be shortened for reliable domestic sources at the discretion of the receiving facility. A thorough evaluation of each animal to screen for ongoing disease should include a physical exam, blood work, fecal analysis, tuberculin testing, and radiographs. In addition to following quarantine procedures during importation, such practices should be followed prior to initiation of infectious disease work as well as after outbreaks of spontaneous disease to reduce the spread of pathogens.
Separation of quarantined animals from unaffected animals with a physical barrier is the first step in preventing the spread of infectious disease through the colony. Dedicated space for newly arrived animals and infectious disease studies should be located as far away as possible from the established colony and preferably have a separate entrance and dedicated air handling units to eliminate the possibility of aerosolized transmission of pathogens. If spontaneous disease occurs in a colony housing room, quarantining the animals in place will likely be the best option. Diagnostic screening of all animals in the room may be necessary to identify those affected. Clear identifying markers should be placed on affected enclosures and an attempt should be made to physically distance the affected animals from the unaffected. Tasks such as feeding and husbandry should be performed on unaffected enclosures prior to affected enclosures to minimize cross-contamination. Changing personal protective equipment between enclosures or animals is a good practice. Proactive decision making and well-defined standard operating procedures detailing the prompt implementation of quarantine can prevent losses in time, resources, and animal life.
Once an area has been quarantined, access should be limited to trained personnel and clear signage should be posted. Appropriate use of personal protective equipment should be employed, including dedicated space for removal of soiled items and their disposal. Entrance into the quarantined area should be minimized, and staff should change clothing and shower prior to entrance into nonquarantined areas. Disposable items should replace non-disposable items, and multidose vials should be left out of the area or discarded following use. Equipment removed from the quarantine area will require appropriate disinfection.8
Predetermined protocols for the management of potentially devastating infectious disease outbreaks in marmoset colonies such as Klebsiella pneumoniae or measles virus can provide a template in the event of an outbreak.9,10 Affected animals may require intense supportive care and antibiotics or other treatments. If the disease is known to cause high mortality, culling of affected animals may be appropriate to protect the colony. Any exposed animals may also require diagnostic screening for the disease of concern, possibly necessitating large-scale, repeated testing. Following resolution of symptoms, testing should be performed to evaluate effectiveness of treatment and to ensure animals are no longer shedding pathogens. The authors’ institution requires 3 negative fecal tests for GI pathogens, such as Shigella or Salmonella species, prior to release from quarantine.
HUSBANDRY AND DIET
Husbandry and diet are commonly implicated as contributors to GI symptoms seen in the common marmoset. A survey of the members of the European Association of Zoos and Aquariums suggests limited concentrate feeds and increased total dietary fiber results in a protective effect against developing disease and also suggests habitat designs that are less naturalistic and those that provide insufficient privacy or hide areas may increase chronic stress for callitrichid species.11 Housing density, stress, nutrition, and dietary components should all be considered when evaluating marmoset colony health.
Housing Density and Stress
Visual, auditory, and scent access to unrelated families or groups within a housing room may result in increased tension or stress due to the territorial nature of the marmoset family group. Clinical signs including diarrhea have been attributed to stress from increased housing density within rooms and introduction of new animals.4 Increased number of animals in an enclosure as well as increased number of enclosures located within close proximity influences social stress and may cause increased infectious disease transmission,12 potentially leading to increased incidence of pathogenic diarrhea. Increased housing density is often anecdotally described as a contributing factor of stress, weight loss, and diarrhea in captive-housed marmosets, but a controlled study has not been completed. The Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act13,14 provides guidelines for the recommended minimum height and floor space for nonhuman primate primary enclosures, but guidelines for density within secondary enclosures, such as housing rooms, are not addressed. Investigation of the optimal housing density for captive common marmosets is a much-needed endeavor.
Nutrition and Diet Components
Dietary requirements of the common marmoset remain incompletely understood. There are numerous commercially available diets containing varying amounts of nutrients and ingredients. It is common for additional food items to be fed along with or closely after providing primary diet. This “cafeteria style” diet can lead to variation in experimental outcomes and may also be contributing to some of the more common clinical diseases seen in this species in captivity.15 Dietary recommendations have been provided by the National Research Council, American Zoo and Aquarium Association, and the European Association of Zoos and Aquaria.11,16,17 There are limited evidence-based studies regarding impact of diet on physiology,18–20 and most recommendations are anecdotal.
Historic reports of inadequate dietary protein in Callitrichids have been implicated in the development of disease with GI signs, including weight loss and diarrhea.21,22 Marmosets should receive a minimum of 15% dry matter protein in the diet.23 Many commercial diets contain higher levels of protein. Protein and other nutrient deficiencies may still be a concern if animals are preferentially choosing low-nutrient supplemental items, resulting in an inadequate amount of balanced commercial diet intake.15
Consumption of the wheat protein gliadin, commonly found in marmoset diets, has been shown to produce antibodies and an immune-mediated reaction leading to weight loss and GI disease consistent with gluten sensitivity. GI symptoms were alleviated following the withdrawal of gluten from the diet.24 An immune reaction to gliadin in the colon of the marmoset has been demonstrated as well as increased deposition of IgA in the glomerulus of animals with increased IgA-gliadin antibodies.24–26
Vitamins and Minerals
Vitamins and minerals are essential components of the marmoset diet and are needed for growth, reproduction, and health.15 Commercially available marmoset diets contain adequate amounts of vitamins and minerals, minimizing concerns of historically reported deficiencies. Marmosets with poor digestive efficiency and intestinal inflammation have been shown to be deficient in vitamin D as well as calcium, resulting in the need for supplementation. Bone mineral density was positively associated with apparent digestibility of energy, vitamin D, and serum calcium.18,19 In 1 colony, marmosets were over 7 times more likely to have concurrent bone and GI disease than lesions in only 1 of the organ systems.27 It is likely that chronic intestinal malabsorption will lead to deficiency of other essential vitamins and minerals.
Conversely, excess dietary intake of iron and iron supplementation in marmosets can be a concern. Marmosets fed a high-iron diet (350–500 ppm) were shown to develop hepatic hemosiderosis with increased mortality. Feeding a diet with 100–200 ppm of iron is common practice, but appropriate levels remain uncertain.28 Marmosets were shown to maintain very high total liver iron (>2 year) following experimenmtally induced iron-overload and chelation, suggesting the condition is long-lasting and reversal is difficult.29 The use of iron dextran injections to treat conditions such as microcytic anemia in the marmoset should be performed judiciously by the clinician while understanding that hemosiderosis may be a potential outcome.
PATHOGENIC DISEASE
Bacterial, viral, and parasitic pathogens cause GI disease in marmosets. Pathogens may be a primary cause of clinical signs or may contribute in accumulation with other variables (ie, stress, diet, inflammation). In additional to supportive care, treatment of pathogenic diseases of the GI tract should be targeted at the pathogen that is detected or suspected to be the causative agent. Empiric antibiotic or antiparasitic treatments can be employed based on colony history and presenting signs while diagnostics are pending.
Bacterial Disease
Campylobacter jejuni and Campylobacter coli, Clostridium difficile, Escherichia coli, K pneumoniae, and Salmonella species have all been implicated as contributors to GI disease in marmosets. A complete review of these pathogens is provided in this volume.
Microbiome and Dysbiosis
Dysbiosis is a lack of balance in the microorganisms normally residing within an environment. When the composition is disrupted, some organisms may be reduced in number while others may become more prevalent, and microbial diversity is often reduced. Factors influencing dysbiosis include diet, antibiotic use, stress, and GI motility.30 Bacterial dysbiosis has been implicated as a significant cause of GI disease in humans.30,31 Environment had the strongest effect on microbiome composition when taxon, hybridization, and environment were investigated in marmosets. Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria were the predominant gut microbiome phyla in wild marmosets, and captive marmosets showed reduced microbiome diversity with higher levels of Enterobacteriaceae. The wild marmoset microbiome reflects carbohydrate metabolism whereas the captive marmoset microbiome is consistent with utilization of nucleotides and amino acids, raising concerns of maladaptation and potential long-term GI health effects of captive animals.32
Analysis of captive, semi-captive, and wild populations of red shanked duoc and mantled howler monkeys’ microbiomes suggest that decreased dietary plant fiber is the primary cause of microbiome changes.30 Captive marmoset diets consist mostly of commercially available gel or pelleted formulations differing greatly from their wild, omnivorous counterpart’s diet, which includes fruits, flowers, nectar, gum, insects, and small vertebrates. These differences in diet influence microbiome composition, which will likely play a factor in GI disease. Bifidobacterium species were found to vary between 3 captive marmoset colonies maintained on 3 different diets.33 Bifidobacterium has been suggested as a critical component of a healthy marmoset microbiome, and dysbiosis has been linked to chronic diarrhea in marmosets.34 Stress impacts gut microbiome through the brain gut axis (hypothalamic–pituitary–adrenal axis, autonomic nervous system, enteric nervous system) and may predispose humans to inflammatory bowel disease (IBD) development.35–37 Captive marmoset stress may be influenced by room traffic, human activity level, and density of marmosets within an enclosure or room. Fecal transplants have been used to treat C difficile infections, but no other reports of gut microbiome manipulation are available.38
Viral Disease
Viral diseases associated with GI manifestations are not common in modern marmoset colonies. Reduced importation from wild colonies and strict species separation may contribute to decreased incidence. Reviews of morbilliviruses, Saimiriine herpesvirus 2, and additional experimental viral infections with GI clinical signs are available.1
Callitrichid herpesvirus 3 (CHV-3), a gammaherpesvirus, was first isolated from spontaneous B-cell lymphomas in marmosets in 2000 and was the first lymphocryptovirus to be isolated from New World primates.39,40 Investigation was prompted after 16 cases of lymphoproliferative disease in male and female marmosets from 16 months to 9 years of age were reported over a 4-year time span. Clinical signs were often GI in origin and included weight loss, inappetence, diarrhea, and, in some cases, a palpable mid-abdominal mass. Mesenteric lymph nodes and colon sections from all 16 cases were found to contain neoplastic round cells. Some cases also had neoplastic cells in the jejunum, duodenum, ileum, liver, kidneys, and lungs. Immunohistochemical analysis identified the neoplastic cells as B-lymphocytes; CHV-3 was isolated from tumor tissue. At the time of discovery, 52 of 84 clinically normal marmosets were found to have a titer to Epstein Barr Virus, a closely related lymphocryptovirus.40
Prevalence was reported to be similar at 2 primate centers (WNPRC and SNPRC), but incidence of B-cell lymphomas was greater at WNPRC. This suggests additional factors contribute to malignant presentations.39 A more recent survey of another colony (Barshop Institute, San Antonio, TX) reports prevalence of 60% in a group of 1- to 4-year-old animals.41 Persistent asymptomatic infections are characteristic of Epstein Barr Virus in humans, and lymphocryptoviruses have been found to be common in Old World primates.42 Factors that contribute to malignant presentations have yet to be determined. The role CHV-3 plays in the development of lymphoma in marmosets remains uncertain as association has been demonstrated but causation has not been established.
Parasitic Disease
A complete review of protozoan, trematode, cestode, nematode, acanthocephalan, and arthropod parasites has been compiled.1 Commonly isolated parasites in contemporary laboratory animal facilities will be discussed.
Giardia intestinalis is likely the most commonly isolated parasite based on multiple reports.7,41,43,44 Incidence in colonies has been reported between 42% and 55% and was found to be persistent over a 3-year evaluation period.7 Prevalence was 20% in a population of 25 marmosets under 1 year of age.43 Giardia is a zoonotic protozoan parasite that produces infectious cysts easily spread via the fecal oral route. Trophozoites elicit clinical signs through binding to small intestinal epithelial cells.1,7 Clinical presentation is described as acute disease, consisting of acute, intermittent, or progressing to chronic diarrhea (may be mucoid or malodorous) and/or weight loss, and a chronic asymptomatic carrier state.7 A systematic evaluation of 1 colony found no significant difference in fecal scores and weight between marmosets positive or negative for Giardia.7 Chronic or intermittent diarrhea may lead to a poorly groomed hair coat with a greasy appearance, potentially secondary to high fat content of stool due to GI malabsorption.44 Giardia has been suggested as a common co-pathogen contributing to infectious diarrhea and chronic malabsorption.44 Diagnosis is via commercially available antigen and polymerase chain reaction exams or a direct fecal smear or float (less sensitive exams).1 Shedding may be intermittent; the authors recommend the use of antigen exams to increase likelihood of detection. Treatment with metronidazole (25–50 mg/kg PO SID to BID for 10 days) and tinidazole (150 mg/kg PO SID once and 77 mg/kg PO SID once 4 days later) is efficacious.1,41,45 Tinidazole has the advantage of 2 treatments total, but administration must be via oral gavage due to extreme unpalatability.7 Elimination of Giardia, Cryptosporidium, Clostridium perfringens, CHV-3, and GBV-A in marmosets housed in a barrier colony resulted in a plateau effect on adult longevity that differs from typical linear decline seen in most primate colonies.41 A significant increase in body weight 1 year post treatment for Giardia was found in 1 colony.7
Cryptosporidium parvum is a coccidian parasite that has been associated with enterocolitis in marmosets. A case study describes clinical presentation of weight loss and diarrhea with mucous and blood. Treatment with paromomycin (15 mg/kg PO BID for 14 days) and nutritional support was successful.46 Prevalence was 16% in a population of 25 marmosets under 1 year of age.43
NONPATHOGENIC AND MALABSORPTIVE GI DISEASE
GI symptoms can persist despite multiple attempts at conventional therapy (antibiotics and supportive care) targeted at pathogens, warranting exploration of alternate disease processes. Unfortunately, chronic conditions affecting the GI tract in captive marmosets including GI amyloidosis, GI neoplasia, and IBD are common. Recent reports describing large numbers of marmosets affected by duodenal disease are also of concern.47,48 Premortem diagnosis of these diseases is challenging due to shared nonspecific presenting signs such as weight loss and diarrhea and clinical pathology abnormalities including hypoalbuminemia and anemia. Chronic GI malabsorption is a sequela of many GI diseases and results in poor body condition and weight loss despite adequate caloric intake.
Duodenal Diseases
Vomiting is typically associated with the upper GI tract (stomach and duodenum) and may help focus the list of potential diseases to those affecting these organs. The major and minor duodenal papilla are sites at which the duodenal lumen connects to the common bile duct and the accessory pancreatic duct, respectively,49 making the duodenum susceptible to complications from pancreatic or liver diseases. Recent reports from 2 large common marmoset colonies demonstrate duodenal disease as a significant cause of morbidity and mortality.
At the first facility (Central Institute for Experimental Animals), a series of 49 cases was reported to have a novel GI disease referred to as marmoset duodenal dilation syndrome.48 Clinical signs include vomiting, weight loss, bloating, and diarrhea. The disease is characterized by dilation and obstruction of the proximal duodenum due to adhesions of the duodenum to the colon along with peritonitis, ulceration, inflammatory infiltrate, and fibrosis.48 Clinical pathology findings consisted of hypoalbuminemia, hypochloremia, elevated creatinine, and elevated gamma-glutamyl transferase. Histology also revealed cholangitis and cholecystitis, chronic lymphocytic enteritis (CLE), and pancreatic ductitis in several of the animals. The authors describe ultrasonography and contrast radiography as imaging techniques for diagnosis.47 The cause remains unknown, but the authors suspect a multifactorial process that may include bacterial infection, ulceration, and fibrosis leading to stricture, and diet factors including overeating or consumption of highly fermentable food. No successful treatment was reported.48
The second facility (Massachusetts Institute of Technology) reports similar findings affecting 21 animals. 70% of these animals were reported to be under 3 years of age.47 Clinical signs consisted of vomiting, diarrhea, and weight loss. Examination revealed mid-cranial abdominal organomegaly, palpable thickening of GI tract, and palpable masses. Clinical pathology abnormalities consisted of hypoalbuminemia, hypocalcemia, elevated alkaline phosphatase, anemia, and leukocytosis.47 Ultrasonography revealed ulceration and perforation of the duodenum distal to the pylorus of the stomach. Pathologic examination revealed reddened areas of the duodenal serosa, and histology revealed duodenal mucosal ulcerations with associated chronic active granulocytic and lymphohistiocytic inflammation with variable fibrosis and reactive epithelial proliferation at stricture sites. Secondary lesions include cholecystitis, choldochitis, and chronic active pancreatitis.47 Duodenal dilation has also been observed in marmosets at the WNPRC (Figure 2).
Figure 2 .
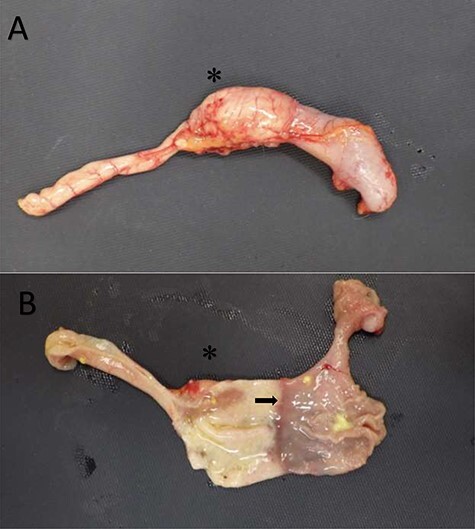
Duodenal dilation in the common marmoset. (A) The upper GI tract of a common marmoset with duodenal dilation. (B) The upper GI tract of the same marmoset with the tissue transected to expose the lumen. The duodenum (left) and stomach (right) are clearly demarcated (as denoted by the arrow) but are similar in diameter. The asterisk, in both images, is located at the dilated aspect of the duodenum. Images courtesy of Andres Mejia, WNPRC.
Additional reports of duodenal lesions in marmosets include cystic Brunner’s glands in 102 of 200 (51%) animals in a pathology survey of spontaneous lesions, suggesting the condition is a common finding.50 Cystic Brunner’s glands are typically considered a benign polypoid or nodular lesion in humans and reported to be exceedingly rare.51,52 There is also a single report of an inflammatory fibroid polyp in the duodenal wall of a common marmoset.53
Neoplasia: Small Intestinal Adenocarcinoma
Small intestinal adenocarcinoma is reported as the most common neoplasia found in the GI tract of the marmoset. An 8.1% prevalence was reported at the time of necropsy in the New England Primate Research Center (NEPRC) marmoset colony, with the average age of affected animals being 6.6 years old.1,2,54,55 Additionally, cases have been described in marmosets at The German Primate Center and WNPRC (Figure 3).56,57 Clinical signs are nonspecific, including weight loss, inappetence, diarrhea, and lethargy. This disease should be in the list of differentials for GI disease, particularly for older animals.
Figure 3 .
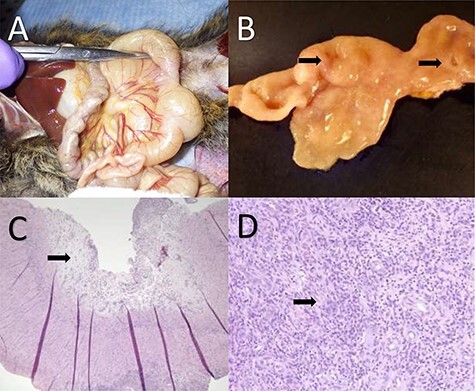
Gastrointestinal adenocarcinoma in the common marmoset. (A) Two strictures are present in the small intestine with associated discoloration and notable dilation proximal to each stricture. (B) The tissue has been transected and mucosal lesions (denoted by the two arrows) can be seen resulting in localized proliferation of the mucosa and strictures of the lumen. (C) Histology of gastrointestinal adenocarcinoma with mucosal erosion, edema and loss of normal mucosal architecture. The arrow denotes highly edematous tissue associated with the erosion. (D) Higher magnification photomicrograph of proliferative neoplastic cells effacing the intestinal mucosa. The arrow denotes a cluster of abnormal cells that are not forming normal glandular architecture within the tissue. Images courtesy of Heather Simmons and Andres Mejia, WNPRC.
Premortem diagnosis is difficult and may rely on ruling out several other disease processes. Palpation of focal thickening in the duodenum or proximal jejunum or distension of the GI tract proximal to the tumor may be detected by the astute clinician (Figure 3). Invasion to local lymph nodes is common,54 which may also be able to be detected by palpation. Clinical pathology changes associated with this disease include microcytic, hypochromic anemia, and hypoalbuminemia.2 Radiology or ultrasonographic imaging may help confirm GI lesions.
Ulceration or complete perforation of the affected tissue has been reported to lead to septicemia and peritonitis and will likely result in rapid decline.2 The prognosis for this disease process is poor, and successful treatment has not been described. Surgical excision should be explored because successful excision of intestinal adenocarcinomas have been reported in macaques56 and would likely be possible in the marmoset. Gross examination of the affected tissue typically reveals a focal thickening that may or may not result in stricture of the intestinal lumen (Figure 3). Causation remains unknown. Helicobacter-like bacteria and CHV-3 were reported as unlikely etiologies in 1 study.54
Neoplasia: GI Lymphoma
GI lymphoma (or lymphosarcoma) is seen regularly in the marmoset. Lymphoma was the most common neoplasm and the second-most common cause of death in mature marmosets reported in a pathology survey at SNPRC, with the GI tract commonly affected.59 GI lymphoma has been reported in other marmoset colonies, but prevalence is unknown and has been suggested to vary. CHV-3 has been associated with the formation of GI lymphoma at the WNPRC, as described above, but causation has not been established.39,40,42 Clinical signs are nonspecific and may include weight loss, change in appetite, diarrhea, and lethargy. Physical examination may reveal thickening of the small intestine, enlarged mesenteric lymph nodes or other lymphadenopathy, splenomegaly, and hepatomegaly indicative of multicentric lymphoma. Clinical pathology may include leukocytosis or possible detection of neoplastic cells on blood smear. A biopsy of affected tissue can be diagnostic as has been previously described, with CD20+ lymphocytes invading the lamina propria indicative of B-cell origin.2 GI lymphoma is yet another differential for progressive weight loss and GI malabsorption.
The marmoset makes an excellent aberrant host for herpes viruses, typically resulting in malignant lymphoma. Inoculation with Ateline herpesvirus 2 and 3, Saimiriine herpesvirus 2, and human herpes virus 4 (Epstein–Barr Virus) can result in lymphoma and potential GI proliferative lesions.1 Strict separation of marmosets from other New World species is required due to risk of transmission of these herpes viruses. Exposure to these species should result in quarantine and observation to prevent possible spread throughout the colony. Diligent use of personal protective equipment by human staff is the best preventative measure for the spread of human viruses to marmosets. Increased incidence of GI lymphoma within a colony should increase suspicion of viral cause.1
Amyloidosis
Amyloid A (AA) amyloidosis, also referred to as reactive or secondary amyloidosis, is a common finding in older marmosets.60 It has been diagnosed in 12% of non-experimental common marmosets at necropsy in a survey performed at the SNPRC58 and in 17% of animals at the NEPRC.2,60,61 AA amyloidosis is typically associated with inflammatory processes resulting in elevated serum amyloid A–related protein.61 The N-terminal fragment of this protein is cleaved and deposited systemically into organs as B-pleated sheets, resulting in disease. The most commonly affected organs in the marmoset are the small intestine, liver, adrenals, kidney, stomach, colon, and spleen.61
Clinical signs include weight loss and lethargy as the disease will likely be advanced prior to detection. Clinical pathology reveals anemia, hypoalbuminemia, and elevated alkaline phosphatase. Physical exam may detect enlarged or firm organs such as the liver and spleen.1,2 The use of ultrasound may help detection of the hepatic or splenic amyloid due to hypoechoic areas. Biopsy of affected organs can be diagnostic when evaluated histologically. Pale eosinophilic material will be found between cells and replaces normal cell architecture. Congo red staining will result in birefringence.2 Successful treatment has not been reported, but animals may live with this disease for some time prior to organ dysfunction.
Inflammatory Bowel Disease
Marmoset wasting syndrome (MWS) has been used interchangeably with IBD and CLE; however, this term should be avoided because it does not refer to a specific disease entity but rather a clinical presentation with which multiple diseases and clinical signs (including chronic colitis, tubulointerstitial nephritis, pancreatitis, hindlimb paresis/paralysis, and alopecia) have been associated.2,22,62–64 IBD is defined as a chronic intestinal inflammatory disorder.65 Reviewing reports of IBD in previous literature may lead to confusion due to the use of a variety of common names for Callithrix jacchus and Sanguinus oedipus, in combination with multiple disease processes being grouped under MWS.22,62–64 In callitrichids, 2 different disease entities have been recognized under IBD, which include CLE (a form of small bowel IBD) and colitis, or large bowel IBD. Although cases of colitis occur in common marmosets, cases of CLE are much more common in the authors’ experience, whereas S oedipus (although no longer used in research) had been a model for IBD in humans due to their spontaneous development of chronic colitis and colonic adenocarcinoma,59,66–68 which is less well documented in marmosets. CLE has been reported in 60% of non-experimental animals during necropsy at the NEPRC.2,69 CLE (T-lymphocyte rich) has been demonstrated in 58% of animals with GI lesions at time of necropsy at the WNPRC.57 It appears to affect both juveniles and adults as well as males and females equally.2
No etiology has been identified as the definitive cause of CLE in marmosets. Underlying parasitic, viral, and bacterial causes have been investigated in animals with chronic malabsorption and clinical signs consistent with CLE. Giardia intestinalis and Trichospurura leptostoma have historically been associated with symptoms described for MWS.1,70 A novel simian pegivirus Southwest bike trail virus (SOBV) was isolated from marmosets with lymphocytic enteritis at the WNPRC. A larger screening of these animals revealed that healthy individuals were also harboring the virus. An association between animals with the virus and the development of lymphocytic enteritis was not demonstrated, and the role the virus plays in the development of lymphocytic enteritis remains unknown.71 Although pathogens might contribute to an initial inflammatory reaction, CLE does not appear to be caused by the aforementioned pathogens.
Several recent studies have implicated dietary causes for CLE due to its similarity to Celiac disease in humans.2,19 Gore et al demonstrated an immune reaction to gliadin in callitrichids fed a wheat diet compared with animals were fed a rice diet.26 Increased IgA antibodies to glycoprotein 2, tissue transglutaminase, gliadin, and de-aminated gliadin occurs in people with Celiac disease. Marmosets with suspected CLE were found to have elevated antibodies to these markers, which subsequently decreased after being weaned off a gluten diet.24 Unfortunately, improvement in clinical signs is not always seen following dietary modification.72 As is the case with IBD in humans, CLE is likely a multifactorial disease. A potential pathogenesis described by Mansfield12 proposes an initial environmental trigger (such as an enteric pathogen) causing a break in local tolerance and a graft-vs-host-disease–like condition (based on preliminary evidence of increased chimeric twin cells in inflammatory lesions) resulting in a subsequent cycle of GI inflammation, breakdown of GI mucosal barrier/architecture, and changes in the microbiome that lead to chronic malabsorption, diarrhea, and weight loss.
CLE is characterized by an infiltrate of T-lymphocytes in the lamina propria of the small intestines. Distribution of the infiltrate can be segmental or diffuse throughout the small intestine, with lymphocytes causing expansion and disruption of normal lamina propria architecture (Figure 4). Although there can be varying degrees of infiltrate, severe cases will often have a majority of CD3+ cells and only a few CD20+ cells (Figure 5). Additional histological features of CLE include increased numbers of intraepithelial lymphocytes, blunting and fusion of villi, and hyperplasia of the crypt epithelium.1,2
Figure 4 .
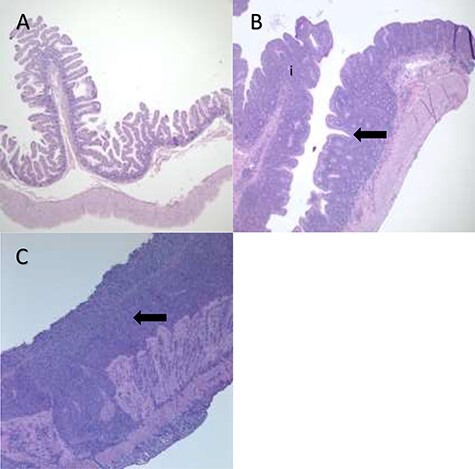
Degrees of Inflammation of the Small Intestine in the Common Marmoset. (A) Architecture of normal small intestines from a marmoset unaffected by CLE. Villi are thin and long, projecting into the lumen with few cells in the lamina propria. (B) Moderate small intestinal inflammation in a marmoset with CLE. The lamina propria is thicker with more inflammatory cells (i), and rounded, blunted villous projections (the arrow denotes an area in which there is little space between blunted villi). (C) Severe small intestinal inflammation in CLE with numerous inflammatory cells in the lamina propria and diffuse fusion of villi (denoted by the arrow). Images taken at 4X. Images courtesy of Heather Simmons and Andres Mejia, WNPRC.
Figure 5 .
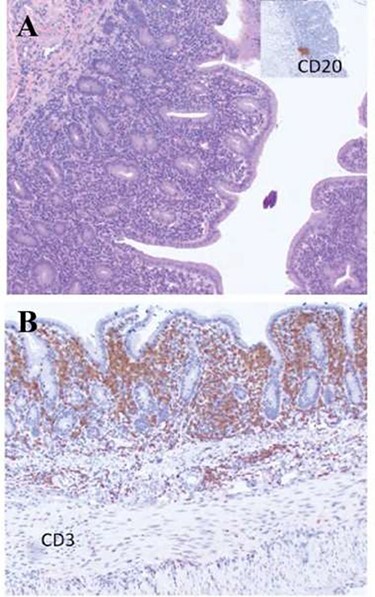
Immunohistochemistry of B- and T-Cell lymphocyte markers in the small intestines of a common marmoset with chronic lymphocytic enteritis. (A) Few CD20+ Cells (B-Lymphocytes) are present in the small intestine of a marmoset with CLE. The inset demonstrates a cluster of CD20+ cells in the lamina propria. (B) Numerous CD3+ cells (T-lymphocytes) are distributed through the mucosal epithelium and lamina propria, indicating a predominantly CD3+ (T-lymphocytic) infiltrate. Both images taken at 4×. Images courtesy of Heather Simmons and Andres Mejia, WNPRC.
Clinical manifestation of these lesions results in malabsorption, often accompanied by dehydration, chronic diarrhea, and weight loss. Given that clinical signs are rather non-specific, CLE is usually a diagnosis of exclusion. When a physical exam is performed, findings may include a reduced body condition score, thickened intestines, enlarged mesenteric lymph nodes on palpation, evidence of loose stool around perineum, and a prolonged skin tent. A serum albumin <3.5 g/dL in addition to a body weight of 325 g or less, and/or progressive body weight loss of 0.05% of the peak body weight per day has been used to accurately identify animals with end-stage disease.27 A complete blood count (CBC) may be considered, and anemia can sometimes be appreciated, specifically a microcytic, hypochromic anemia.12,63 As anticipated with dehydration, electrolyte abnormalities may be present on a chemistry panel as well as hypoproteinemia secondary to hypoalbuminemia and hypoglobulinemia. Several biomarkers for diagnosing CLE have also been explored and are covered in detail below. Diagnostic imaging is usually not very rewarding; however, gas dilation can often be seen on radiographs, and thickened loops of bowel may be appreciated by an experienced ultrasonographer.
Treatment of this common disease is frustrating because causation has not been established. The focus of treatment is centered on supportive care and anti-inflammatory therapeutics. In milder cases, initiating healthy supplemental food items to maintain the animal’s weight and administering isotonic saline subcutaneously for animals that are mildly dehydrated are basic supportive care measures that can be taken if reports of diarrhea become frequent. In the author’s experience, cases that are moderate to severe and/or chronic show symptomatic improvement when treated with doxycycline, as described in Table 2. During treatment, diarrhea and dehydration typically decrease in severity and may subside. Decreasing weight trends are typically slowed or stabilized, and weight gain is frequently demonstrated. Tetracyclines have been shown to enhance GI mucosal protection, suppress cytokine and chemokine production via mitogen-activated protein kinase and nuclear factor kappa B pathways, as well as inhibit matrix metalloproteinase expression and activity of tumor necrosis factor, interleuken-1, neutrophil elastase, nitric oxide, and reactive oxide intermediates.73–76 Corticosteroids have been a mainstay of treatment in humans and other species with IBD; however, their adverse side effects can be an encumbrance to their use.77–81 Alternatively, budesonide is often preferred due to its high hepatic clearance, high intestinal concentrations, and overall fewer side effects due to lower systemic concentrations.82,83 Animals may be managed on these drugs for a relatively long time, but overall prognosis for CLE remains poor because these treatments are focused on symptom relief rather than the cause of the disease. Rapid decompensation remains a common outcome. Other immunosuppressants such as azathioprine, cyclosporine, and chlorambucil are commonly used in other species with IBD but have yet to be explored in marmosets.77 Removal of animals with IBD from breeding programs should be considered because genetics are thought to play an important role in the development of IBD in humans.
Table 2.
Antibiotics and Antiparasitic Treatments for GI Disease in the Common Marmoset
| Drug | Dose | Comments |
|---|---|---|
| Azithromycin/erythromycin | Day 1: 40 mg/kg PO d 2–5: 20 mg/kg PO q24h | Treatment of Campylobacter; extended treatment duration may be necessary41 |
| Enrofloxacin | 5–10 mg/kg SC, PO, or IM q24h | To treat EPEC, Klebsiella, or Salmonella, severe colitis/enteritis2,41 |
| Doxycycline | d 1: 5 mg/kg PO q12h d 2–14: 5 mg/kg PO q24h | Tetracycline antibiotic with immunomodulatory effects; consider 2–4 wk administration; treatment of IBD/CLE/chronic malabsorption |
| Tinidazole | d 1: 150 mg/kg (62.5 mg) PO d 4: 77 mg/kg (31 mg) PO | Treatment of Giardia;114 contraindications include pregnancy and lactation; have utilized in young animals (3–4 mo); taste strongly aversive; recommend compounding to facilitate small volume administration (Wedgewood, 400 mg/mL as oil suspension with flavor); can be administered via oral gavage to non-sedated animals |
| Metronidazole | 25–50 mg/kg PO q12-24 h for 10 d | Treatment of Giardia, Clostridium difficile, or dysbiosis6 |
| Paromomycin | 15 mg/kg PO q12h for 28 d | Treatment for Cryptosporidium parvum42 |
CLE = chronic lymphocytic enteritis; EPEC = enteropathogenic Escherichia coli (E. coli); IBD = inflammatory bowel disease; IM = intramuscular; PO = per os; SC = subcutaneous.
DIAGNOSTICS
Signalment and Clinical Signs
A multitude of etiologies are responsible for GI diseases in the common marmoset, making diagnosis and treatment challenging. Signalment and clinical history are important considerations when beginning a clinical investigation into possible causes of disease and will help guide diagnostic testing. Signalment includes important information about the animal such as species, age, and sex. A clinical history review should include medical history as well as place of origin, environment (social or single housing, room density, and location within room), experimental use, and breeding history. The majority of disease processes involving the GI tract will present with nonspecific signs such as diarrhea and weight loss. Additional clinical signs may include vomiting, abnormal appetite, altered behavior, abdominal distension or flaccidity, unkempt hair coat, hunched posture, and lethargy. Maintaining a list of abnormal findings and differential diagnosis will guide diagnostic and therapeutic choices. Diagnostic flow charts for GI disease in the common marmoset are provided (Figures 6 and 7).
Figure 6 .
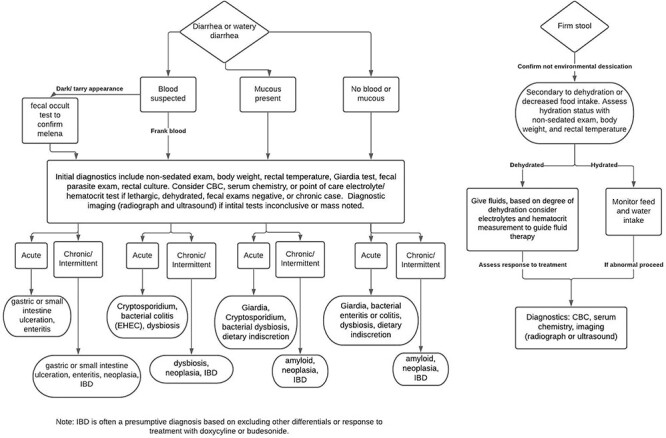
Diagnostic flow chart for abnormal stool in the common marmoset. A stepwise guide for the investigation of abnormal stool. This tool is designed to aid in the diagnosis of GI disease in the common marmoset. Starting at the top, work downward choosing the appropriate boxes and follow the arrows to reach the likely differentials.
Figure 7 .
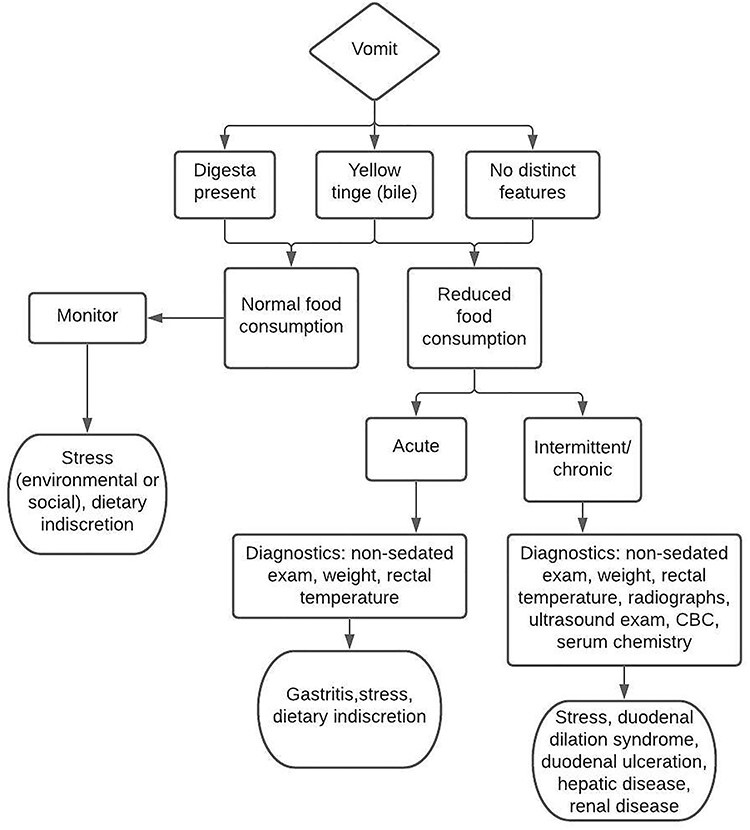
Diagnostic flow chart for vomiting in the common marmoset. A stepwise guide for the investigation of vomiting. This tool is designed to aid in the diagnosis of GI disease in the common marmoset. Starting at the top, work downward choosing the appropriate boxes and follow the arrows to reach the likely differentials.
Physical Examination
Physical examination performed by an experienced clinician is the first step in diagnostic testing. Guidelines for physical examination of the common marmoset have been outlined in detail.3 The procedure should include a thorough evaluation of each body system with a focus on those consistent with any presenting clinical signs. In the case of GI disease, a physical exam should begin with the oral cavity. Dentition abnormalities and periodontal disease should be noted and addressed. The abdominal cavity can be evaluated with palpation of the GI tract noting thickness of the mucosa, fluid or gas-filled areas, masses or strictures, and hepatomegaly. Localization of a lesion within the abdomen is possible by an experienced clinician with palpation alone. Inspection of the anus and perineum may reveal abnormal feces, skin irritation, or alopecia that may be attributable to diarrhea. Skin turgor can be evaluated by tenting the skin and observing skin elasticity; in adequate hydration, the skin should quickly return to normal appearance (<2 seconds). A delayed return or skin tent is indicative of dehydration. The skin over the abdomen and cranium is ideal for hydration assessment. Evaluation of mucous membranes should reveal pink, moist, and glistening tissue as opposed to dry, sticky, or tacky membranes, another indication of dehydration.
Fecal Analysis
The key to a thorough fecal analysis is the development of a good working relationship with a microbiology laboratory. Informing laboratory staff of colony trends and suspected pathogens will increase the value of results reported. Culture of fecal bacterial pathogens is a commonly employed tool and can be done on fresh whole feces or by using a swab placed in the rectum or colon. Submission of whole feces allows for multiple tests to be run on a single sample. Many fecal bacterial pathogens are easy to grow with standard culture techniques, but some are more fastidious and may require specialized media for their growth and identification in vitro. Recommendations for culture media for marmoset bacterial pathogens have been reviewed.44 If Salmonella spp. or enteropathogenic E coli (EPEC) is cultured, serotyping provides additional information on strains. Fecal flotation results in concentration of helminth eggs and certain protozoa on the surface of the floatation solution for easy detection by microscopy. Commercially available enzyme immunoassays allow for rapid detection of fecal protozoa such as Giardia spp., Cryptosporidium spp., and Entamoeba spp.7 Wet mount and direct smear techniques are simple and easy to perform and can aid in the detection of motile organisms such as protozoa by directly placing feces on a slide for microscopy, although sensitivity may be lower than other techniques. A summary of fecal diagnostic tests is provided in Table 3.
Table 3.
Fecal Diagnostic Tests Commonly Employed for GI Disease in the Common Marmoset
| Test | Indication | Comments |
|---|---|---|
| Fecal/rectal culture | Abnormal stool, suspect bacterial pathogen | Recommended as initial fecal diagnostic; determine which bacteria present within GI tract |
| Fecal EIA | Abnormal stool, suspect protozoal pathogen | Recommended as initial fecal diagnostic; EIA kits commercially available to detect protozoa: Giardia, Cryptosporidium, and Entamoeba |
| Fecal flotation test | Abnormal stool, suspect parasites | Recommended for animals with access to outdoor enclosures or new arrivals; kits commercially available |
| Direct fecal smear/wet mount prep | Abnormal stool, suspect protozoal pathogen or ova | Simple and easy to perform with light microscope; Giardia may be detected, but EIA is more sensitive; inexpensive |
| Antimicrobial susceptibility testing | Culture of pathogenic bacterial species | Performed on culture of bacteria; facilitates determination of appropriate antibiotic choice and monitoring trends in antibiotic resistance |
| E. coli virulence factor PCR | Culture of E. coli, suspect pathogenic strain of E. coli (EPEC) | Performed on fecal/rectal culture that grows E coli; virulence factors may distinguish pathogenic strains from normal commensal bacteria |
| Fecal occult blood | Suspect blood present in stool | Can be used to confirm blood present in stool when difficult to discern with naked eye; kits commercially available |
EIA = enzyme immunoassay; EPEC = enteropathogenic Escherichia coli (E. coli); GI = gastrointestinal; PCR = polymerase chain reaction.
Hematology and Serum Chemistry
Hematology and serum chemistry analysis reference ranges have been published for the marmoset.84–87 A CBC evaluates blood cells in circulation, providing insight to ongoing disease processes. Increased red blood cell parameters may be indicative of hemoconcentration suggestive of dehydration. Decreased red blood cell parameters (anemia) have been associated with many chronic GI disease processes in the marmoset but may also be evident secondary to acute blood loss through the GI tract (hematochezia or melena). Elevated white blood cell count may be indicative of infection or inflammatory disease. Common findings on serum chemistry in marmosets with GI disease include low blood albumin, due to malabsorption and/or decreased food intake, as well as electrolyte abnormalities secondary to loss via diarrhea or vomiting.27
Minimizing the amount of whole blood needed to perform blood analysis is imperative due to the small size of the common marmoset. Marmosets are estimated to have 60–70 mL/kg blood volume, making 10% of their blood volume approximately 2–4 mL.3 Blood volumes collected from dehydrated or anemic animals should be carefully tracked and replacement fluids given as needed. Point of care analyzers require less volume, such as the i-STAT 1 (Abaxis North America, Union City, CA), which can perform blood gas and chemistry analysis with as little as 100 μL.
Urinalysis
Urinalysis can be used to evaluate the urine concentration and abnormal components that may be found in the sample such as protein, glucose, bacteria, casts, or cells. Commercially available reagent strips are available. Urine specific gravity is a measure of the density of urine, with highly concentrated urine suggestive of dehydration. The mean urine specific gravity for captive marmosets was reported to be 1.02637 ± 0.006697 in 1 study and 1.024 ± 0.013 in another.88,89 Mild to moderate proteinuria as assessed via urine dipstick from free-catch samples is very common and has been suggested to be non-progressive and not suggestive of impaired renal function in marmosets.90 Renal protein loss occurs via the glomerulus and tubules. One study on spontaneous progressive glomerulonephropathy correlated degree of glomerular lesions (evaluated via histology and transmission electron microscopy) and urine obtained via cystocentesis; no correlation between lesion severity and proteinuria was found.91 Proteinuria, likely secondary to nephrosclerosis (glomerulosclerosis, tubulointerstitial fibrosis, and arteriosclerosis), is common in aged marmosets.91,92
Imaging
Radiography and ultrasonography are important imaging tools for the detection of abnormalities within the abdomen of the marmoset. The radiographic anatomy of the common marmoset has been described in detail.93 Ultrasound images of the kidneys, urinary bladder, spleen, adrenal glands, liver, and the GI tract are easily obtained, and reference images with organ measurements have been published for normal marmosets.94 Abdominal ultrasound is a useful technique for the investigation of signs of acute abdominal disease in non-human primates.95 The use of ultrasonography, radiography and contrast radiography was instrumental in the diagnosis of duodenal dilation.48 More advanced imaging technologies such as magnetic resonance imaging (MRI), dual energy X-ray absorptiometry (DEXA), positron emission tomography (PET), and computed tomography (CT) are often used in research studies but less so as diagnostic tools for clinical disease. A normal reference for CT of the abdomen is available.96
Metabolic bone disease occurs secondary to chronic GI malabsorption when vitamin D3 and calcium absorption is reduced.18,27,97 Lesions identified include rickets, fibrous osteodystrophy, and osteopenia.27,97 Assessment of bone radiodensity fraction of the distal femur has been used to identify marmosets with bone disease; values <0.5 differentiated marmosets with bone disease from marmosets without bone disease.27 Radiographs are less sensitive to mild bone density changes, and CT is a more sensitive modality to detect mild changes.98
Biopsy
Blind colonic biopsies have been obtained with 1.8-mm-outer diameter pediatric endoscopic biopsy forceps. Complications include hematochezia or peritonitis secondary to perforation of the colonic wall.3 Endoscopic visualization of the colon with biopsies is feasible with a pediatric bronchoscope 4.8-mm insertion tube and 2.0-mm biopsy channel.99 No reports of small intestine endoscopic equipment suitable for marmosets have been described. Full-thickness intestinal biopsies by surgical resection or introduction of endoscopic biopsy forceps via a 2- to 3-mm incision allows for examination of all layers of the intestine.2 Percutaneous and laparotomy liver biopsy techniques have been described.1,3 The large size of the biopsy needle relative to the liver predisposes marmosets to hemorrhage post procedure. A 2-cm paracostal laparotomy incision to isolate the right lateral liver lobe may be safer and facilitates visual assurance that hemorrhage has been controlled post biopsy.3
Biomarkers
The use of biomarkers for the detection of intestinal malabsorption in the marmoset has become a recent area of interest and shows great promise. Serum cobalamin and folate have been proposed as a marker for CLE and were reported to be moderately sensitive and specific for the disease process, warranting further investigation.100 N-methylhistamine has also been suggested as a possible marker for the detection of CLE. Investigation revealed 7 of 8 marmosets with CLE had increased fecal N-methylhistamine concentrations.101 Another study demostrated that serum albumin <3.5 g/dL or a weight <325 g had a 92% sensitivity, 100% specificity, and 100% positive predictive value for postmortem lymphoplasmacytic inflammation in the intestines of marmosets in the colony. Another group descibed increased serum matrix metalloproteinase 9 as a potential new marker for the diagnosis of IBD in marmosets characterized by inflammatory cell infiltrations in the intestine. Additional changes noted were decreases in hematocrit, hemoglobin, serum albumin, and calcium.102 Calprotectin is a marker for intestinal inflammation and plays a role in the induction of apoptosis. One group demonstrated that marmosets with chronic diarrhea had higher levels of fecal calprotectin.103 Fecal concentrations of alpha-1 proteinase inhibitor were significantly higher in marmosets with hypoalbuminemia than in healthy individuals. This trend was reversed on treatment, demonstrating that the cause of hypoalbuminemia is intestinal protein loss.104 A noninvasive promising biomarker for GI pathology impacting digestion is fecal fat. It has been suggested that normal marmosets have a fecal fat <5%, and marmosets with a fecal fat >10% may have GI pathology resulting in lipid malabsorption.19
Preventative Care
When pathogenic or nonpathogenic causes of GI disease are identified within a colony, it may be prudent to develop a screening program consisting of diagnostics that serve as biomarkers for disease processes of concern. Examples include periodic screening for Giardia and establishing minimum body weights or serum albumin values associated with GI disease in your colony.27 These diagnostics can be integrated into your marmoset colony health program and evolve as more sensitive and specific tests are developed.
TREATMENT STRATEGIES
Dosage recommendations are provided in Tables 2 and 4. Use of compounding pharmacies for appropriate oral concentrations suitable for small dose administration is recommended.
Table 4.
Supportive Care and Supplemental Treatments for Managing GI Disease in the Common Marmoset
| Drug | Dose | Comments |
|---|---|---|
| Bismuth subsalicylate | 5.25–10.5 mg PO q12-24 h | Gastroprotectant; decreases gut secretions and stool quantity |
| Budesonide | 0.25–0.75 mg PO q24h | Glucocorticoid for treatment of CLE/IBD/chronic malabsorption79 |
| Calcium citrate (powder) | 75 mg PO q24h | Recommended for animals diagnosed with moderate to severe GI malabsorption or animals with low calcium; calcium citrate has high bioavailability and can be mixed with food |
| Famotidine | 0.5–1 mg/kg PO, IM, IV q24h | GI protectant (H2 blocker); recommended peri-operatively and/or when appetite reduced |
| Maropitant | 1 mg/kg SC q24h | Antiemetic; recommended peri-operatively and/or when appetite reduced |
| Omeprazole | 0.5–1 mg/kg PO q24h | GI protectant (proton pump inhibitor); slower onset, but more efficacious than H2 blockers |
| Sucralfate | 100–200 mg/kg PO q12-24 h | Gastroprotectant; indicated for suspected ulcers |
| Vitamin D3 | 100–200 IU q24h or 500 IU 3×/wk or 400 IU EOD PO | Recommended for animals with chronic GI malabsorption (may note low calcium, phosphorous, and/or albumin on serum chemistry) |
| Yogurt/probiotic | 1 oz yogurt or probiotics as labeled PO q24h | Recommended for animals with chronic diarrhea or weight loss or suspected GI malabsorption |
CLE = chronic lymphocytic enteritis; EOD = every other day; GI = gastrointestinal; IBD = inflammatory bowel disease; IM = intramuscular; IU = international unit; IV = intravenous; PO = per os; SC = subcutaneous.
Supportive Care
Treatment should be directed at specific causes, but often diagnostics are inconclusive. General supportive care is critical to ensure return to normal function while a marmoset is undergoing diagnostics or therapy aimed at the primary GI disease cause. Food and water consumption should be monitored along with fecal and urine output. If inappetence is noted, provision of calorie-dense, palatable food items should be provided. If possible, modifying the primary diet by mixing with palatable liquids or food items is preferred to prevent additional GI upset with novel food items. The author recommends the use of high-quality yogurt as a dietary supplement for GI support. No controlled studies have been completed with probiotics in marmosets, but administration is unlikely to cause harm and may benefit the microbiome. Primate-specific formulations are available and easily administered to marmosets. If GI disease is chronic and malabsorption is suspected, micronutrient supplementation (eg, vitamin D3 as listed in Table 4) may be indicated.18,27,105
Fluid Therapy
GI disease is often associated with fluid loss through diarrhea or vomiting. Inappetence often exacerbates dehydration. Fluid therapy focuses on rehydration and restoration of any electrolyte imbalances. Dehydration can be assessed through weight history, skin tent test, mucus membrane evaluation, feces appearance and volume, CBC, serum chemistry, and urinalysis. Fluids can be provided orally, subcutaneously, or intravenously. Oral rehydration is appropriate only in marmosets consuming liquids and food. Intake should be carefully monitored. Mild to moderate dehydration can be corrected via subcutaneous fluids; 15–30 mL/kg, on average 8–10 mL, is feasible to administer in the loose skin overlying the abdomen or thorax. Although administration is feasible in non-sedated marmosets, the cost benefit of stress associated with restraint and therapeutic benefit must be considered. Moderate to severe dehydration may require intravenous fluid therapy. The saphenous and cephalic veins are commonly employed, and catheter placement has been described in detail.3 A catheter placed aseptically in the saphenous vein may be bandaged to allow repeated administration of fluids for approximately 24–36 hours.
Gastroprotectant and Anti-Nausea Therapies
Increased acid secretion by the stomach is common during stressful events and may lead to gastric erosions, ulcers, or hemorrhage.106,107 Gastric mucosal barriers are also compromised secondary to decreased microcirculation and mucus or bicarbonate secretion.107 This disease process has been termed stress-related mucosal disease in other species and likely affects marmosets.107 Famotidine is a H2-receptor antagonist and reduces acid secretion; it is available as injectable and oral formulations. Omeprazole, a proton-pump inhibitor, raises gastric pH higher and longer than H2-receptor antagonists; it is also available as injectable and oral formulations.104
Bismuth subsalicylate and sucralfate are mucosal protectants that coat the GI tract as well as potentially bind bacteria and toxins, and have been used with some success in the treatment of diarrhea in marmosets.106 Sucralfate binds to subepithelial proteins and forms a protective layer over the mucosa to facilitate regeneration.107 Sucralfate has been associated with lower risks of secondary bacterial overgrowth post administration in critically ill humans and may be an ideal choice for marmosets being treated for GI disease.109
Ondansetron (serotonin receptor antagonist) and maropitant (neurokinin receptor antagonist) are antiemetic agents used to prevent or treat vomiting and nausea. Maropitant results in reduced binding of neurotransmitter substance P within brainstem nuclei. This action at a common emesis control point may result in a more efficacious response to a broad variety of emetogenic stimuli.106 A combination of oral ondansetron and famotidine has been used by the author for treatment of chronic vomiting.
Anti-Inflammatories
Budesonide, a glucocorticoid, has been used to treat chronic malabsorption/CLE/IBD when no pathogen has been identified. A dose of 0.5 to 0.75 mg PO once a day was used to treat marmosets with a body weight <325 g and serum albumin <3.5 g/dL. Long-term treatment may be required; marmosets with severe disease or an acute presentation may not be responsive to treatment. Reoccurrence was reported and multiple or intermittent treatment may be required.82 In a similar fashion, the authors frequently use oral doxycycline for treatment of inflammatory GI disease and chronic malabsorption anecdotally resulting in improvement in fecal consistency, weight, and hydration. A combination of doxycycline and budesonide has also been used when either alone has been unsuccessful.
Antibiotics
The use of antibiotics in the common marmoset should be employed judiciously following the detection of bacterial or protozoan pathogens. Antimicrobial susceptibility testing will guide the choice of antibiotic, and review of historic reports will aid in monitoring antibacterial resistance trends. Fluoroquinolones are commonly used in the marmoset and have been described in the successful treatment of enteropathogenic Escherichia coli2,110,111 Campylobacter spp.,112 K pneumoniae,1,9,111 Salmonella spp.,44 and Francisella tularensis.113 Reported side effects specific to marmosets include reduced oocyte quality.114 Azithromycin and erythromycin have been used to treat Campylobacter enteritis in marmosets.44 Metronidazole has been reported for the treatment of the protozoan Chilomastix mesnili,115 C difficile,38 and Giardia.7 The use of metronidazole and tinidazole have been described in the treatment of Giardia spp. in marmosets.2,7 Fecal microbiota transfer was found to be a more successful treatment that metronidazole for marmosets infected with C difficile.38 Marmosets were treated with paromomycin (15 mg/kg PO) twice daily for 28 days, resulting in the resolution of clinical signs from C parvum infection.46 Commonly used antibiotic agents and dosages are provided (Table 2).
Analgesia
GI disease may present with behavior consistent with abdominal pain such as hunched posture and reluctance to ambulate. A complete review of analgesics is provided in this edition.
CONCLUSIONS
GI disease processes in the common marmoset have historically been lumped together due to shared, nonspecific clinical signs and lack of thorough clinical and pathological investigation. The term MWS has been applied to a multitude of disease process in the marmoset that result in progressive weight loss in combination with numerous other symptoms. The use of this term has resulted in confusion among clinicians and researchers due to the inability to determine what disease is being addressed and how to interpret the results of these studies. Most, if not all, diseases described above would fit the definition of MWS, and thus the use of this term should be avoided. The use of well-defined clinical terminology, such as those listed in Table 1, results in standardization and promotes collaboration across institutions with marmoset colonies. It is imperative to use well-defined terminology to facilitate comparison of disease processes and their prevalence across captive marmoset colonies. Morphologic descriptions of pathologic lesions and disease processes is essential to advancing the understanding of GI diseases in this species. To determine causation and refine diagnostics and therapies of GI diseases in the marmoset, consistent communication must be adopted.
Acknowledgments
This work was supported by the Wisconsin National Primate Research Center grant P51 OD011106 from the Office of Research Infrastructure Programs, National Institute of Health (to C.F, L.W., A.M., and H.S.); and by the Southwest National Primate Research Center grant P51 OD011133 from the Office of Research Infrastructure Programs, National Institute of Health (to A.G.).
Potential conflicts of interest. All authors: No reported conflicts.
Contributor Information
Casey Fitz, Wisconsin National Primate Research Center at the University of Wisconsin-Madison, Madison, Wisconsin, USA.
Anna Goodroe, Texas Biomedical Research Institute and Southwest National Primate Research Center in San Antonio, Texas, USA.
Lauren Wierenga, Wisconsin National Primate Research Center at the University of Wisconsin-Madison, Madison, Wisconsin, USA; Research Animal Resources and Compliance at the University of Wisconsin-Madison, Madison, Wisconsin, USA.
Andres Mejia, Wisconsin National Primate Research Center at the University of Wisconsin-Madison, Madison, Wisconsin, USA.
Heather Simmons, Wisconsin National Primate Research Center at the University of Wisconsin-Madison, Madison, Wisconsin, USA.
References
- 1. Kramer JA. Diseases of the gastrointestinal system. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 213–230. [Google Scholar]
- 2. Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med 2003; 53(4):369–382. [PubMed] [Google Scholar]
- 3. Burns M, Wachtman L. Physical examination, diagnosis, and common clinical procedures. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 145–175. [Google Scholar]
- 4. Layne-Colon D, Goodroe A, Burns M. Husbandry and housing of common marmosets. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 77–91. [Google Scholar]
- 5. Layne DG, Power RA. Husbandry, handling, and nutrition for marmosets. Comp Med 2003; 53(4):351–359. [PubMed] [Google Scholar]
- 6. Summers L, Clingerman KJ, Yang X. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition by using dual-energy X-ray absorptiometry. J Am Assoc Lab Anim Sci 2012; 51(1):88–93. [PMC free article] [PubMed] [Google Scholar]
- 7. Kramer JA, Hachey AM, Wachtman LM et al. Treatment of giardiasis in common marmosets (Callithrix jacchus) with tinidazole. Comp Med 2009; 59(2):174–179. [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts JA, Andrews K. Nonhuman primate quarantine: its evolution and practice. ILAR J 2008; 49(2):145–156. doi: 10.1093/ilar.49.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pisharath HR, Cooper TK, Brice AK et al. Septicemia and peritonitis in a colony of common marmosets (Callithrix jacchus) secondary to Klebsiella pneumoniae infection. Contemp Top Lab Anim Sci 2005; 44(1):35–37. [PubMed] [Google Scholar]
- 10. Albrecht P, Lorenz D, Klutch MJ et al. Fatal measles infection in marmosets pathogenesis and prophylaxis. Infect Immun 1980; 27(3):969–978. doi: 10.1128/IAI.27.3.969-978.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cabana F, Maguire R, Hsu CD et al. Identification of possible nutritional and stress risk factors in the development of marmoset wasting syndrome. Zoo Biol 2018; 37(2):98–106. doi: 10.1002/zoo.21398. [DOI] [PubMed] [Google Scholar]
- 12. The National Academies Collection . National Academies of Sciences Eg, and Medicine, Studies DoEaL, Research IfLA, Use RoSaWiLA. In: Anestidou L, Johnson AF, eds. Care, Use, and Welfare of Marmosets as Animal Models for Gene Editing-Based Biomedical Research: Proceedings of a Workshop. Washington, DC: National Academies Press, 2019. [PubMed] [Google Scholar]
- 13. National Research Council (U.S.) . Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the Care and Use of Laboratory Animals. 8th ed. Washington DC: National Academies Press; 2011:xxv. http://www.ncbi.nlm.nih.gov/books/NBK54050ebrary http://site.ebrary.com/id/10443276NationalAcademies Press http://www.nap.edu/catalog.php?record_id=12910NationalAcademies Press http://www.nap.edu/catalog.php?record_id=12910#tochttp://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=nap12910http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf. Accessed April 1, 2020. [Google Scholar]
- 14. United States . Animal and Plant Health Inspection Service, United States Animal Care (Program). In: Animal Welfare Act and Animal Welfare Regulations. Aphis 41. Washington, D.C.: United States Department of Agriculture. January 2017. [Google Scholar]
- 15. Power ML, Koutsos L. Marmoset nutrition and dietary husbandry. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 63–76. [Google Scholar]
- 16. Committee on Animal Nutrition, National Research Council (U.S.). Panel on nonhuman primate nutrition. Nutrient Requirements of Nonhuman Primates. 2nd rev. ed. National Washington, DC: Academy Press; 2003:xviii. [Google Scholar]
- 17. Crissey S, Gore M, Lintzenich B et al. Callitrichids: nutrition and dietary husbandry. Nutr Advis Hadb Fact Sheet 2003; 013:1–19. [Google Scholar]
- 18. Jarcho MR, Power ML, Layne-Colon DG et al. Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus). Am J Primatol 2013; 75(2):153–160. doi: 10.1002/ajp.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Power ML, Adams J, Solonika K et al. Diet, digestion and energy intake in captive common marmosets (Callithrix jacchus): research and management implications. Sci Rep 2019; 9(1):12134. doi: 10.1038/s41598-019-48643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Power ML, Ross CN, Schulkin J et al. Metabolic consequences of the early onset of obesity in common marmoset monkeys. Obesity (Silver Spring) 2013; 21(12):E592–E598. doi: 10.1002/oby.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnard D, Knapka J, Renquist D. The apparent reversal of a wasting syndrome by nutritional intervention in Saguinus mystax. Lab Anim Sci 1988; 38(3):282–288. [PubMed] [Google Scholar]
- 22. Brack M, Rothe H. Chronic tubulointerstitial nephritis and wasting disease in marmosets (Callithrix jacchus). Vet Pathol 1981; 18(Suppl 6):45–54. doi: 10.1177/0300985881018s0605. [DOI] [PubMed] [Google Scholar]
- 23. Tardif S, Jaquish C, Layne D et al. Growth variation in common marmoset monkeys (Callithrix jacchus) fed a purified diet: relation to care-giving and weaning behaviors. Lab Anim Sci 1998; 48(3):264–269. [PubMed] [Google Scholar]
- 24. Kuehnel F, Mietsch M, Buettner T et al. The influence of gluten on clinical and immunological status of common marmosets (Callithrix jacchus). J Med Primatol 2013; 42(6):300–309. doi: 10.1111/jmp.12055. [DOI] [PubMed] [Google Scholar]
- 25. Schroeder C, Osman AA, Roggenbuck D et al. IgA-gliadin antibodies, IgA-containing circulating immune complexes, and IgA glomerular deposits in wasting marmoset syndrome. Nephrol Dial Transplant 1999; 14(8):1875–1880. doi: 10.1093/ndt/14.8.1875. [DOI] [PubMed] [Google Scholar]
- 26. Gore MA, Brandes F, Kaup FJ et al. Callitrichid nutrition and food sensitivity. J Med Primatol 2001; 30(3):179–184. [DOI] [PubMed] [Google Scholar]
- 27. Baxter VK, Shaw GC, Sotuyo NP et al. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One 2013; 8(12):e82747. doi: 10.1371/journal.pone.0082747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller GF, Barnard DE, Woodward RA et al. Hepatic hemosiderosis in common marmosets, Callithrix jacchus: effect of diet on incidence and severity. Lab Anim Sci 1997; 47(2):138–142. [PubMed] [Google Scholar]
- 29. Sergejew T, Forgiarini P, Schnebli HP. Chelator-induced iron excretion in iron-overloaded marmosets. Br J Haematol 2000; 110(4):985–992. doi: 10.1046/j.1365-2141.2000.02260.x. [DOI] [PubMed] [Google Scholar]
- 30. Clayton JB, Vangay P, Huang H et al. Captivity humanizes the primate microbiome. Proc Natl Acad Sci U S A 2016; 113(37):10376–10381. doi: 10.1073/pnas.1521835113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Enck P, Mazurak N. Dysbiosis in functional bowel disorders. Ann Nutr Metab 2018; 72(4):296–306. doi: 10.1159/000488773. [DOI] [PubMed] [Google Scholar]
- 32. Malukiewicz J, Cartwright RA, Dergam JA et al. The effects of host taxon, hybridization, and environment on the gut microbiome of Callithrix marmosets. Am J Phys Anthropol 2020; 171:172–173. [Google Scholar]
- 33. Brown CJ, Mtui D, Oswald BP et al. Comparative genomics of Bifidobacterium species isolated from marmosets and humans. Am J Primatol 2019; 81(10–11):e983. doi: 10.1002/ajp.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shigeno Y, Toyama M, Nakamura M et al. Comparison of gut microbiota composition between laboratory-bred marmosets (Callithrix jacchus) with chronic diarrhea and healthy animals using terminal restriction fragment length polymorphism analysis. Microbiol Immunol 2018; 62(11):702–710. doi: 10.1111/1348-0421.12655. [DOI] [PubMed] [Google Scholar]
- 35. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013; 144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36. Sun YH, Li J, Shu HJ et al. Serum immunoinflammation-related protein complexes discriminate between inflammatory bowel disease and colorectal cancer. Clin Transl Oncol 2019; 21(12):1680–1686. doi: 10.1007/s12094-019-02100-3. [DOI] [PubMed] [Google Scholar]
- 37. Vedamurthy A, Ananthakrishnan AN. Influence of environmental factors in the development and outcomes of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019; 15(2):72–82. [PMC free article] [PubMed] [Google Scholar]
- 38. Yamazaki Y, Kawarai S, Morita H et al. Faecal transplantation for the treatment of Clostridium difficile infection in a marmoset. BMC Vet Res 2017; 13(1):150. doi: 10.1186/s12917-017-1070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho Y, Ramer J, Rivailler P et al. An Epstein-Barr-related herpesvirus from marmoset lymphomas. Proc Natl Acad Sci U S A Jan 30 2001; 98(3):1224–1229. doi: 10.1073/pnas.98.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramer JC, Garber RL, Steele KE et al. Fatal lymphoproliferative disease associated with a novel gammaherpesvirus in a captive population of common marmosets. Comp Med 2000; 50(1):59–68. [PubMed] [Google Scholar]
- 41. Ross CN, Austad S, Brasky K et al. The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging (Albany NY). 2017; 9(12):2544–2558. doi: 10.18632/aging.101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivailler P, Cho YG, Wang F. Complete genomic sequence of an Epstein-Barr virus-related herpesvirus naturally infecting a new world primate: a defining point in the evolution of oncogenic lymphocryptoviruses. J Virol 2002; 76(23):12055–12068. doi: 10.1128/jvi.76.23.12055-12068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalishman J, Paul-Murphy J, Scheffler J et al. Survey of cryptosporidium and giardia spp. in a captive population of common marmosets. Lab Anim Sci 1996; 46(1):116–119. [PubMed] [Google Scholar]
- 44. Izzi J. Select Topics in Marmoset Veterinary Care. [Oral presentation] ILAR Roundtable on Care, Use and Welfare of Marmosets: October 22-23, Washington, DC: Academies Press. [Google Scholar]
- 45. Kramer JA, Hachey AM, Wachtman LM et al. Treatment of giardiasis in common marmosets (Callithrix jacchus) with tinidazole. Comp Med 2009; 59(2):174–179. [PMC free article] [PubMed] [Google Scholar]
- 46. Hahn NE, Capuano SV 3rd. Successful treatment of cryptosporidiosis in 2 common marmosets (Callithrix jacchus) by using paromomycin. J Am Assoc Lab Anim Sci 2010; 49(6):873–875. [PMC free article] [PubMed] [Google Scholar]
- 47. Burns M. Gastrointestinal Disease: Common Presentations in Large Colonies; [Virtual oral presentation], National Primate Research Center - Marmoset Working Group 2020.
- 48. Mineshige T, Inoue T, Yasuda M et al. Novel gastrointestinal disease in common marmosets characterised by duodenal dilation: a clinical and pathological study. Sci Rep 2020; 10(1):3793. doi: 10.1038/s41598-020-60398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Casteleyn C, Bakker J. The anatomy of the common marmoset. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 17–41. [Google Scholar]
- 50. Kaspareit J, Friderichs-Gromoll S, Buse E et al. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp Toxicol Pathol 2006; 57(5–6):405–410. doi: 10.1016/j.etp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 51. Powers M, Sayuk GS, Wang HL. Brunner gland cyst: report of three cases. Int J Clin Exp Pathol 2008; 1(6):536–538. [PMC free article] [PubMed] [Google Scholar]
- 52. Park BJ, Kim MJ, Lee JH et al. Cystic Brunner's gland hamartoma in the duodenum: a case report. World J Gastroenterol 2009; 15(39):4980–4983. doi: 10.3748/wjg.15.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yokouchi Y, Imaoka M, Sayama A et al. Inflammatory fibroid polyp in the duodenum of a common marmoset (Callithrix jacchus). Toxicol Pathol 2013; 41(1):80–85. doi: 10.1177/0192623312452491. [DOI] [PubMed] [Google Scholar]
- 54. Miller AD, Kramer JA, Lin KC et al. Small intestinal adenocarcinoma in common marmosets (Callithrix jacchus). Vet Pathol 2010; 47(5):969–976. doi: 10.1177/0300985810369905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chalifoux LV. Adenocarcinoma of the small intestine in a common marmoset (Callithrix jacchus). Lab Anim Sci 1990; 40(2):210–212. [PubMed] [Google Scholar]
- 56. Brack M. Gastrointestinal tumors observed in nonhuman primates at the German primate center. J Med Primatol 1998; 27(6):319–324. doi: 10.1111/j.1600-0684.1998.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 57. Fitz C. Gastrointestinal Disease: Common Presentations in Large Colonies; [Virtual oral presentation], National Primate Research Center - Marmoset Working Group 2020.
- 58. Valverde CR, Tarara RP, Griffey SM et al. Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.). Comp Med 2000; 50(5):540–544. [PubMed] [Google Scholar]
- 59. David JM, Dick EJ, Hubbard GB. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). J Med Primatol 2009; 38(5):347–359. doi: 10.1111/j.1600-0684.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tardif SD, Mansfield KG, Ratnam R et al. The marmoset as a model of aging and age-related diseases. ILAR J 2011; 52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ludlage E, Murphy CL, Davern SM et al. Systemic AA amyloidosis in the common marmoset. Vet Pathol 2005; 42(2):117–124. doi: 10.1354/vp.42-2-117. [DOI] [PubMed] [Google Scholar]
- 62. Chalifoux LV, Bronson RT, Escajadillo A et al. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet Pathol Suppl 1982; 19(Suppl 7):141–162. [PubMed] [Google Scholar]
- 63. Logan AC, Khan KN. Clinical pathologic changes in two marmosets with wasting syndrome. Toxicol Pathol 1996; 24(6):707–709. doi: 10.1177/019262339602400605. [DOI] [PubMed] [Google Scholar]
- 64. Chalmers DT, Murgatroyd LB, Wadsworth PF. A survey of the pathology of marmosets (Callithrix jacchus) derived from a marmoset breeding unit. Lab Anim 1983; 17(4):270–279. doi: 10.1258/002367783781062217. [DOI] [PubMed] [Google Scholar]
- 65. Guan, Q. Comprehensive Review GQA. Update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019; 2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chalifoux LV, Bronson RT. Colonic adenocarcinoma associated with chronic colitis in cotton top marmosets, Saguinus oedipus. Gastroenterology 1981; 80(5 pt 1):942–946. [PubMed] [Google Scholar]
- 67. Kirkwood JK, Pearson GR, Epstein MA. Adenocarcinoma of the large bowel and colitis in captive cotton-top tamarins Saguinus o. oedipus. J Comp Pathol 1986; 96(5):507–515. doi: 10.1016/0021-9975(86)90071-x. [DOI] [PubMed] [Google Scholar]
- 68. Stadnicki A, Colman RW. Experimental models of inflammatory bowel disease. Arch Immunol Ther Exp 2003; 51(3):149–155. [PubMed] [Google Scholar]
- 69. Ross CN, Davis K, Dobek G et al. Aging phenotypes of common marmosets (Callithrix jacchus). J Aging Res 2012; 2012:567143. doi: 10.1155/2012/567143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beglinger R, Illgen B, Pfister R et al. The parasite Trichospirura leptostoma associated with wasting disease in a colony of common marmosets, Callithrix jacchus. Folia Primatol (Basel) 1988; 51(1):45–51. doi: 10.1159/000156355. [DOI] [PubMed] [Google Scholar]
- 71. Heffron A, Lauck M, Somsen E et al. Discovery of a novel simian pegivirus in common marmosets (Callithrix jacchus) with lymphocytic enteritis. Microorganisms, 8(10):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mansfield K. Pathology of Marmoset Diseases. [Oral presentation] ILAR Roundtable on Care, Use and Welfare of Marmosets: October 22-23, Washington, DC: Academies Press; 2018. [Google Scholar]
- 73. Iwasaki H, Inoue H, Mitsuke Y et al. Doxycycline induces apoptosis by way of caspase-3 activation with inhibition of matrix metalloproteinase in human T-lymphoblastic leukemia CCRF-CEM cells. J Lab Clin Med 2002; 140(6):382–386. doi: 10.1067/mlc.2002.129308. [DOI] [PubMed] [Google Scholar]
- 74. Pasquale TR, Tan JS. Nonantimicrobial effects of antibacterial agents. Clin Infect Dis 2005; 40(1):127–135. doi: 10.1086/426545. [DOI] [PubMed] [Google Scholar]
- 75. Sun J, Shigemi H, Tanaka Y et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep 2015; 4:397–404. doi: 10.1016/j.bbrep.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garrido-Mesa J, Rodríguez-Nogales A, Algieri F et al. Immunomodulatory tetracyclines shape the intestinal inflammatory response inducing mucosal healing and resolution. Br J Pharmacol 2018; 175(23):4353–4370. doi: 10.1111/bph.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Malewska K, Rychlik A, Nieradka R et al. Treatment of inflammatory bowel disease (IBD) in dogs and cats. Pol J Vet Sci 2011; 14(1):165–171. doi: 10.2478/v10181-011-0026-7. [DOI] [PubMed] [Google Scholar]
- 78. Sturgess K, K S . Diagnosis and management of idiopathic inflammatory bowel disease in dogs and cats. In Pract 2005; 27:293–301. [Google Scholar]
- 79. Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis 2013; 31(3–4):368–373. doi: 10.1159/000354699. [DOI] [PubMed] [Google Scholar]
- 80. Prantera C, Marconi S. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Ther Adv Gastroenterol 2013; 6(2):137–156. doi: 10.1177/1756283X12473675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sturgess K. Diagnosis and management of idiopathic inflammatory bowel disease in dogs and cats. In Pract 2005; 27:293–301. [Google Scholar]
- 82. Otovic P, Smith S, Hutchinson E. The use of glucocorticoids in marmoset wasting syndrome. J Med Primatol 2015; 44(2):53–59. doi: 10.1111/jmp.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pietra M, Fracassi F, Diana A et al. Plasma concentrations and therapeutic effects of budesonide in dogs with inflammatory bowel disease. Am J Vet Res 2013; 74(1):78–83. doi: 10.2460/ajvr.74.1.78. [DOI] [PubMed] [Google Scholar]
- 84. Kramer R, Burns M. Normal clinical and biological parameters of the common marmoset (Callithrix jacchus). In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 93–107. [Google Scholar]
- 85. Yarbrough LW, Tollett JL, Montrey RD et al. Serum biochemical, hematological and body measurement data for common marmosets (Callithrix jacchus jacchus). Lab Anim Sci 1984; 34(3):276–280. [PubMed] [Google Scholar]
- 86. Davy CW, Jackson MR, Walker JM. The effect of haemolysis on some clinical chemistry parameters in the marmoset (Callithrix jacchus). Lab Anim 1984; 18(2):161–168. doi: 10.1258/002367784780891343. [DOI] [PubMed] [Google Scholar]
- 87. Kuehnel F, Grohmann J, Buchwald U et al. Parameters of haematology, clinical chemistry and lipid metabolism in the common marmoset and alterations under stress conditions. J Med Primatol 2012; 41(4):241–250. doi: 10.1111/j.1600-0684.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- 88. Winn CB, Issa EB, Curcillo CP et al. Daily water intake by common marmosets. J Am Assoc Lab Anim Sci 2019; 58(1):16–20. doi: 10.30802/AALAS-JAALAS-18-000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Drake P. Species Differences in Urinary Specific Gravity of Various Nonhuman Primates; Honors Projects: Grand Valley State University. Allendale, MI. 2015.
- 90. Collins MG, Rogers NM, Jesudason S et al. Spontaneous glomerular mesangial lesions in common marmoset monkeys (Callithrix jacchus): a benign non-progressive glomerulopathy. J Med Primatol 2014; 43(6):477–487. doi: 10.1111/jmp.12134. [DOI] [PubMed] [Google Scholar]
- 91. Yamada N, Sato J, Kanno T et al. Morphological study of progressive glomerulonephropathy in common marmosets (Callithrix jacchus). Toxicol Pathol 2013; 41(8):1106–1115. doi: 10.1177/0192623313478206. [DOI] [PubMed] [Google Scholar]
- 92. Lee HJ, Gonzalez O, Dick EJ et al. Marmoset as a model to study kidney changes associated with aging. J Gerontol A Biol Sci Med Sci 2019; 74(3):315–324. doi: 10.1093/gerona/gly237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wagner WM, Kirberger RM. Radiographic anatomy of the thorax and abdomen of the common marmoset (Callithrix jacchus). Vet Radiol Ultrasound 2005; 46(3):217–224. doi: 10.1111/j.1740-8261.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 94. Wagner WM, Kirberger RM. Transcutaneous ultrasonography of the abdomen in the normal common marmoset (Callithrix jacchus). Vet Radiol Ultrasound 2005; 46(3):251–258. doi: 10.1111/j.1740-8261.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 95. de Souza AC, Lange RR, Guerios SD et al. Ultrasonographic examination in non-human primates with acute abdomen signs. J Med Primatol 2013; 42(6):336–342. doi: 10.1111/jmp.12065. [DOI] [PubMed] [Google Scholar]
- 96. du Plessis WM, Groenewald HB, Elliott D. Computed tomography of the abdomen in eight clinically normal common marmosets (Callithrix jacchus). Anat Histol Embryol 2017; 46(4):365–372. doi: 10.1111/ahe.12278. [DOI] [PubMed] [Google Scholar]
- 97. Olson EJ, Shaw GC, Hutchinson EK et al. Bone disease in the common marmoset: radiographic and histological findings. Vet Pathol 2015; 52(5):883–893. doi: 10.1177/0300985815589354. [DOI] [PubMed] [Google Scholar]
- 98. Grohmann J, Kuehnel F, Buchwald U et al. Analysis of the bone metabolism by quantitative computer tomography and clinical chemistry in a primate model (Callithrix jacchus). J Med Primatol 2012; 41(1):1–10. doi: 10.1111/j.1600-0684.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 99. Clapp NK, McArthur AH, Carson RL et al. Visualization and biopsy of the colon in tamarins and marmosets by endoscopy. Lab Anim Sci 1987; 37(2):217–219. [PubMed] [Google Scholar]
- 100. Parambeth JC, Ross CN, Miller AD et al. Serum cobalamin and folate concentrations in common marmosets (Callithrix jacchus) with chronic lymphocytic enteritis. Comp Med 2019; 69(2):135–143. doi: 10.30802/aalas-cm-18-000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Parambeth JC, López FR, Lopez R et al. Fecal concentrations of N-methylhistamine in common marmosets. Comp Med 2019; 69(2):130–134. doi: 10.30802/AALAS-CM-18-000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yoshimoto T, Niimi K, Takahashi E. Serum matrix metalloproteinase 9 (MMP9) as a biochemical marker for wasting marmoset syndrome. J Vet Med Sci 2016; 78(5):837–843. doi: 10.1292/jvms.15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nakashima E, Okano Y, Niimi K et al. Detection of calprotectin and apoptotic activity in the colon of marmosets with chronic diarrhea. J Vet Med Sci 2013; 75(12):1633–1636. doi: 10.1292/jvms.13-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Niimi K, Morishita H, Usui M et al. Measurement of the α1-proteinase inhibitor (α1-antitrypsin) of common marmoset and intestinal protein loss in wasting syndrome. Biosci Rep 2019; 39(7):BSR20190562. doi: 10.1042/BSR20190562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goodroe AE, Fitz C, Power ML et al. Evaluation of vitamin D. Am J Primatol 2020; 82(6):e23131. doi: 10.1002/ajp.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Grimm KA. Veterinary Anesthesia and Analgesia. 5th ed. Hoboken, NJ: Wiley Blackwell; 2015:1 online resource, p. 1074. doi: 10.1002/9781119421375. [DOI] [Google Scholar]
- 107. Hill TL, Lascelles BDX, Blikslager AT. Effect of sucralfate on gastric permeability in an ex vivo model of stress-related mucosal disease in dogs. J Vet Intern Med 2018; 32(2):670–678. doi: 10.1111/jvim.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wissman MA. Husbandry and medical care of callitrichids. J Exot Pet Med 2014; 23(4):347–362. doi: 10.1053/j.jepm.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Huang J, Cao Y, Liao C et al. Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: a meta-analysis of 10 randomized controlled trials. Crit Care 2010; 14(5):R194. doi: 10.1186/cc9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hayashimoto N, Inoue T, Morita H et al. Survey and experimental infection of enteropathogenic Escherichia coli in common marmosets (Callithrix jacchus). PLoS One 2016; 11(8):e0160116. doi: 10.1371/journal.pone.0160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mansfield KG, Fox JG. Bacterial diseases. In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Cambridge, MA: Academic Press; 2019. p. 265–287. [Google Scholar]
- 112. Goodman LJ, Kaplan RL, Petrak RM et al. Effects of erythromycin and ciprofloxacin on chronic fecal excretion of campylobacter species in marmosets. Antimicrob Agents Chemother 1986; 29(2):185–187. doi: 10.1128/aac.29.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nelson M, Lever MS, Dean RE et al. Bioavailability and efficacy of levofloxacin against Francisella tularensis in the common marmoset (Callithrix jacchus). Antimicrob Agents Chemother 2010; 54(9):3922–3926. doi: 10.1128/AAC.00390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tkachenko OY, Scheerer-Bernhard JU, Delimitreva S et al. A retrospective analysis of adverse effects of an in vivo fluoroquinolone antibiotic enrofloxacin treatment on oocyte quality in the common marmoset. Reprod Toxicol 2018; 75:86–95. doi: 10.1016/j.reprotox.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 115. Jeong ES, Park JH, Ryu SH et al. Detection of Chilomastix mesnili in common marmoset (Callithrix jacchus) and treatment with metronidazole. Iran J Parasitol 2019; 14(2):334–339. [PMC free article] [PubMed] [Google Scholar]


