Key Points
Question
Among patients with persistent atrial fibrillation (AF), does the addition of magnetic resonance imaging (MRI)-guided fibrosis ablation to conventional catheter ablation affect atrial arrhythmia recurrence?
Findings
In this randomized clinical trial that included 843 patients with persistent AF, there was no significant difference in atrial arrhythmia recurrence in the MRI-guided fibrosis ablation group compared with the pulmonary vein isolation only group (hazard ratio, 0.95).
Meaning
Findings do not support the use of MRI-guided fibrosis ablation for the treatment of persistent atrial fibrillation.
Abstract
Importance
Ablation of persistent atrial fibrillation (AF) remains a challenge. Left atrial fibrosis plays an important role in the pathophysiology of AF and has been associated with poor procedural outcomes.
Objective
To investigate the efficacy and adverse events of targeting atrial fibrosis detected on magnetic resonance imaging (MRI) in reducing atrial arrhythmia recurrence in persistent AF.
Design, Setting, and Participants
The Efficacy of Delayed Enhancement-MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation of Atrial Fibrillation trial was an investigator-initiated, multicenter, randomized clinical trial involving 44 academic and nonacademic centers in 10 countries. A total of 843 patients with symptomatic or asymptomatic persistent AF and undergoing AF ablation were enrolled from July 2016 to January 2020, with follow-up through February 19, 2021.
Interventions
Patients with persistent AF were randomly assigned to pulmonary vein isolation (PVI) plus MRI-guided atrial fibrosis ablation (421 patients) or PVI alone (422 patients). Delayed-enhancement MRI was performed in both groups before the ablation procedure to assess baseline atrial fibrosis and at 3 months postablation to assess for ablation scar.
Main Outcomes and Measures
The primary end point was time to first atrial arrhythmia recurrence after a 90-day blanking period postablation. The primary safety composite outcome was defined by the occurrence of 1 or more of the following events within 30 days postablation: stroke, PV stenosis, bleeding, heart failure, or death.
Results
Among 843 patients who were randomized (mean age 62.7 years; 178 [21.1%] women), 815 (96.9%) completed the 90-day blanking period and contributed to the efficacy analyses. There was no significant difference in atrial arrhythmia recurrence between groups (fibrosis-guided ablation plus PVI patients, 175 [43.0%] vs PVI-only patients, 188 [46.1%]; hazard ratio [HR], 0.95 [95% CI, 0.77-1.17]; P = .63). Patients in the fibrosis-guided ablation plus PVI group experienced a higher rate of safety outcomes (9 [2.2%] vs 0 in PVI group; P = .001). Six patients (1.5%) in the fibrosis-guided ablation plus PVI group had an ischemic stroke compared with none in PVI-only group. Two deaths occurred in the fibrosis-guided ablation plus PVI group, and the first one was possibly related to the procedure.
Conclusions and Relevance
Among patients with persistent AF, MRI-guided fibrosis ablation plus PVI, compared with PVI catheter ablation only, resulted in no significant difference in atrial arrhythmia recurrence. Findings do not support the use of MRI-guided fibrosis ablation for the treatment of persistent AF.
Trial Registration
ClinicalTrials.gov Identifier: NCT02529319
This randomized clinical trial assesses the effects of pulmonary vein isolation (PVI) plus MRI-guided fibrosis ablation compared with PVI alone in treating symptomatic and asymptomatic patients with persistent atrial fibrillation.
Introduction
Ablation of persistent atrial fibrillation (AF) remains challenging as recurrence of atrial arrhythmia can be common despite multiple procedures.1,2,3 Different strategies, including posterior wall ablation, adding left-atrial roof line ablation, targeting atrial rotors (regions of re-entry), or targeting complex fractionated atrial electrograms (high-frequency electrical sources), have yet to show superiority over conventional pulmonary vein isolation (PVI) in multicenter clinical trials.4,5,6,7 Left-atrial fibrosis, a hallmark of atrial myopathy, plays an important role in the pathophysiology of AF.8 Higher baseline left atrial fibrosis, determined on delayed-enhancement magnetic resonance imaging (MRI), was independently associated with atrial arrhythmia recurrence after ablation.9 Moreover, higher residual fibrosis has been significantly associated with worse postprocedural outcomes,10 highlighting the role of fibrotic myopathy in maintaining an arrhythmogenic substrate. Nevertheless, the effectiveness of targeting atrial fibrotic tissue during ablation in improving rates of atrial arrhythmia recurrence in patients with persistent AF has not been tested in large randomized clinical trials.
The Efficacy of Delayed Enhancement-MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation of Atrial Fibrillation (The DECAAF II) trial was designed to investigate the hypothesis that targeting atrial fibrosis detected on delayed-enhancement MRI, in addition to performing PVI, would decrease atrial arrhythmia recurrence compared with performing PVI alone in patients with persistent AF.
Methods
Study Design and Oversight
The trial was approved by the ethics committee at each participating center. Written informed consent was obtained from all patients. The trial protocol and statistical analysis plan are available in Supplement 1. The trial rationale, design, and protocol have also been described previously.11 The principal investigator and the steering committee designed the trial. The trial included 44 academic and nonacademic sites across Europe, Australia, and the United States. A full list of participating sites and investigators is included in Supplement 2. Data management and the statistical analysis were provided by the data coordinating center at the University of Utah. Studies previously demonstrated racial differences in AF management and postablation outcomes, thus race and ethnicity were collected. Race and ethnicity were self-classified by the patient during the consent process based on fixed categories.
Patients
To be enrolled in the trial, patients had to have persistent AF (defined as 7 days or more of AF as evidenced by either rhythm strip or documentation on chart review) and must have been undergoing their first AF ablation. Major exclusion criteria were contraindication to gadolinium and/or MRI and previous AF ablation or valvular cardiac surgery (see a complete list of the exclusion criteria in eTable 1 in Supplement 3). From July 2016 through January 2020, patients were recruited and randomly assigned to undergo ablation targeting left atrial fibrosis, as detected on delayed-enhancement MRI, in addition to PVI or PVI only. Treatment assignment was masked from patients, and the duration of follow-up was 12 to 18 months after randomization.
Randomization
Before undergoing the baseline MRI, patients were randomly assigned in a 1:1 ratio to receive MRI-guided fibrosis ablation plus PVI or PVI alone. A computerized central randomization design using random permuted blocks (block size range, 2-6) was generated and stratified according to center and level of atrial fibrosis at baseline (fibrosis <20% and ≥20%).
Interventions
Fibrosis-Guided Ablation
For patients randomized to the fibrosis-guided ablation group, processed delayed-enhancement MRI images were merged with the 3D mapping system at each study site to be used during the procedure. All patients underwent PVI. After the PV entrance block had been confirmed, fibrosis-guided ablation was pursued. The operator either encircled or covered with ablation lesions all fibrotic areas observed on delayed-enhancement MRI. Details regarding the ablation protocol for both treatment groups are included in the protocol in section 4.7 of eAppendix 1 in Supplement 1.
PVI
All PVs were electrically isolated as described by the Heart Rhythm Society Consensus Statement.12 If normal sinus rhythm could not be restored, despite cardioversion at the end of the PVI portion of the procedure in patients randomized to this group, the operator had the choice to pursue further measures to eliminate recurrent arrhythmias if needed.
Imaging
Patients underwent a delayed-enhancement MRI within 30 days prior to the ablation procedure using the Merisight delayed-enhancement MRI protocol (MARREK Inc). The purpose of the baseline MRI was to quantify left atrial fibrosis in all patients. Patients’ randomized treatment group was masked from reviewers who assessed MRI quality. MARREK Inc assisted with image segmentation, processing, and quantification of left atrial fibrosis. Following ablation, delayed-enhancement MRIs were obtained at 90 to 180 days to quantify ablation-related scar formation.13
Follow-up
All patients received a handheld smartphone electrocardiogram (ECG) device (ECG Check, Cardiac Designs) and were required to record daily ECG strips, as well as to send a strip to the ECG core laboratory if they experienced symptoms during the study follow-up period. Ambulatory monitoring and 12-lead ECG data performed as part of clinical care were also included. ECG strips data were transmitted automatically to the ECG core laboratory for reading by trained experts masked from treatment assignment. Patients were scheduled for a follow-up visit at 3 months postablation. A postablation delayed-enhancement MRI was completed to document postablation fibrosis coverage and to quantify ablation-related scar formation. Scheduled follow-up telephone visits took place at 6- and 12-months postablation in which medication changes and smartphone ECG device compliance were assessed.
Outcomes
Primary Outcome
The primary end point of the study was the first confirmed recurrence of atrial arrhythmia (including AF, atrial flutter, or atrial tachycardia) lasting for at least 30 seconds after the 90-day blanking period, demonstrated by at least 2 consecutive 1-lead smartphone ECG device tracings, 1 positive reading on a clinical 12-lead ECG tracing, ambulatory monitor, or if the patient underwent repeat ablation. The daily smartphone ECGs were intended as the primary method for assessing atrial arrhythmia recurrence, but clinical and ambulatory ECGs served as back-up methods for detecting recurrence in patients who failed to reliably transmit smartphone ECG readings. A core laboratory at the University of Washington adjudicated the ECG findings.
Main Secondary, Prespecified, and Post Hoc Outcomes
Quality of life, as measured by the Toronto Atrial Fibrillation Symptom Severity Scale (AFSS),14 was the main secondary efficacy outcome. Patients filled the questionnaire at baseline, 3 months, and 12-month postablation periods, with 12 months serving as the primary assessment time for interpretation of results. The AFSS is a disease-specific instrument intended to measure the severity of arrhythmia-related symptoms. AFSS scores range from a minimum of 0 to 35 points, with higher scores indicating greater AF symptom severity. Other prespecified secondary outcomes included the individual components of the primary outcome (AF, atrial flutter, and atrial tachycardia), repeat ablation, a composite outcome of atrial arrhythmia recurrence, repeat ablation, prescription of an antiarrhythmic medication, the RAND physical function and mental health composite t-scores from version 1 the Short-Form 3615 (administered at baseline, month 3, and month 12), stroke, cardiovascular hospitalization, and symptomatic atrial arrhythmia recurrence. The final 2 secondary outcomes are not included in this article. The t-scores are normed to have a mean (SD) of 50 (10) in a healthy US population, with higher scores representing better health.
The composite of atrial arrhythmia recurrence, repeat ablation, new atrial arrhythmia medication, and cardioversion was analyzed as a post hoc outcome.
Safety Outcome
The primary safety composite outcome was defined by the occurrence of 1 or more of the following events during the 30-day period following the ablation procedure: stroke, PV stenosis, bleeding, heart failure, and death. Additional safety outcomes include each of the individual components of the primary safety composite as well as the occurrence of cardiac perforation or esophageal injury within 30 days of the ablation procedure. These safety end points were compiled from periprocedural complications reported by the operator and from adverse events occurring within 30 days after ablation. They were adjudicated by a 3-member outcomes committee based on the 2017 Heart Rhythm Society Consensus Statement.12 The outcome committee also adjudicated all strokes during the full follow-up period, which defined the stroke secondary outcome.
Evaluating Fibrosis Targeting and Scar Coverage
Left atrial ablation points were collected and superposed on 3D left atrial fibrosis images. Targeted fibrosis represented the baseline fibrosis that was covered by ablation points recorded during the procedure. Scar-covered fibrosis was determined based on ablation-induced scarring that covered or encircled baseline fibrosis when superposing the 3-month delayed-enhancement MRI image on the baseline delayed-enhancement MRI image. Five reviewers (masked from randomization group) were trained to identify the amount of fibrosis that was targeted and scar covered and to classify it based on a 5-level scale (level 1, none or little fibrosis covered or encircled; level 2, some fibrosis covered or encircled; level 3, half of fibrosis covered or encircled; level 4, majority of fibrosis covered or encircled; and level 5, nearly all or all fibrosis covered or encircled). Examples of the 5-level scale are shown in eFigure 1 in Supplement 3.
Sample Size Calculation
The trial was originally designed to be event driven, with the enrollment of approximately 888 patients expected to provide 517 events to provide 90% power with 2-sided α of .05 to detect a 25% hazard reduction between the MRI-guided ablation plus PVI group and the PVI-alone group. The targeted effect size of a 25% hazard reduction is similar to or smaller than effect sizes targeted in previous ablation clinical trials.4,16,17 Due to a lower than projected event rate, the protocol was modified on July 17, 2019, when 728 patients had been randomized to stipulate a target sample size of 900 patients irrespective of the number of primary outcome events. The trial ultimately enrolled 843 patients to provide 363 events.
Statistical Analyses
The primary atrial arrhythmia recurrence outcome and all secondary outcomes involving atrial arrhythmia recurrence or repeat ablation were performed in randomized patients who received an ablation procedure and remained in follow-up at the close of the 90-day blanking period. Analyses of quality-of-life outcomes and of stroke as a secondary outcome was performed in all randomized patients who received an ablation procedure; patients were analyzed in accordance with their randomized treatment groups. The analyses of safety outcomes were carried out in all randomized patients who received an ablation procedure, with patients assigned to the treatment received.
The primary efficacy analysis used a log-rank test stratified by fibrosis stage (<20% vs ≥20%) to compare the time to first atrial arrhythmia recurrence after the blanking period between the randomized treatment groups. An associated Cox proportional hazards regression with stratification of the baseline hazard by fibrosis stage estimated the hazard ratio (HR) between the fibrosis-guided ablation plus PVI group and the PVI-alone group. Similar stratified log-rank tests and Cox proportional hazards regressions were performed for secondary time-to-event outcomes. Post hoc sensitivity analyses repeated the Cox regression for the primary outcome, first with the 44 clinical centers incorporated as a stratification factor (leading to a total of 88 strata when combined with the fibrosis stage strata), and then using a γ frailty model in which center was treated as a random effect.18 A single interim analysis for efficacy was conducted using an O’Brien-Fleming stopping boundary19 after 179 events had been observed for the primary outcome. HRs for the primary and secondary time-to-event outcomes were computed separately for baseline fibrosis stages (<20% vs ≥20%), with the baseline hazard stratified by baseline fibrosis in the low baseline fibrosis stage (<10% vs ≥10%) and by baseline fibrosis in the higher baseline fibrosis stage (<30% vs ≥30%). The interaction between the treatment and baseline fibrosis stage, categorized as less than 20% vs 20% or greater, was tested based on the ratio of difference between treatment groups in the estimated log HRs to the standard error for this ratio. In prespecified analyses, the effects of randomized treatment assignment on quality-of-life outcomes at months 3 and 12 were estimated using constrained longitudinal mixed-effects models in which baseline mean quality-of-life scores were assumed equal between the randomized groups, with baseline fibrosis stratum included in the model as a covariate and with an unstructured covariance matrix to account for serial correlation in quality-of-life scores. A post hoc sensitivity analysis expanded the covariance model by considering center to be a random effect.
A Fisher exact test compared the primary safety composite outcome between the treatment groups. The weighted κ and the Gwet agreement coefficient20 were used to assess interrater agreement across the 5 raters of the level of scar coverage. All hypothesis tests were performed with a 2-sided significance level of .05, without adjustment for multiple comparisons.
Time-to-event analyses were right censored at the time of the final ECG transmission or the final study visit (whichever came last). The assumption of proportional hazards was evaluated using smooth Schoenfeld residual plots and tests of the interaction between randomized treatment and follow-up time. No violations of proportional hazards were detected for either the primary or secondary time-to-event outcomes. Analyses of quality-of-life outcomes incorporated all available data at each time point and under the mixed-effect model remain approximately unbiased under the assumption that data are missing at random after accounting for the observed data in the analysis.21
Due to the large number of secondary end points, analyses of secondary end points other than the main secondary outcome should be interpreted as exploratory. All analyses were performed in SAS Version 9.4 or R Version 3.4.1.
Results
Assessment and Evaluation
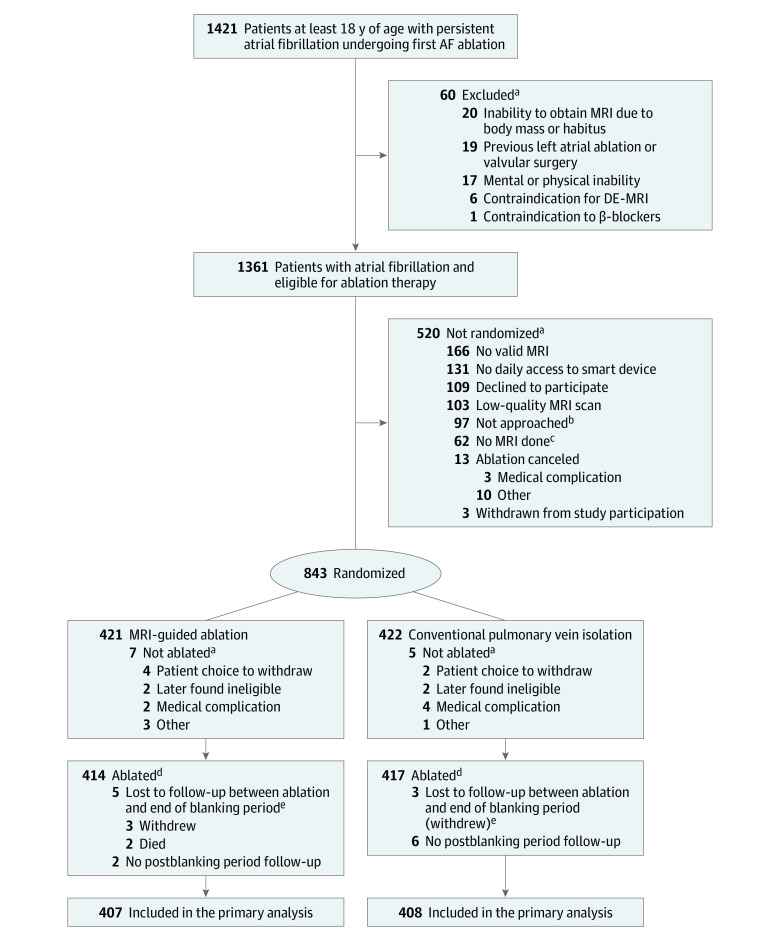
From July 2016 through January 2020, 843 patients were recruited. Four hundred and twenty-one patients were randomized to undergo fibrosis-guided ablation plus PVI, and 422 were assigned to receive PVI only. A flow diagram highlighting causes for exclusion is shown in Figure 1. Baseline characteristics were balanced between the randomized treatment groups (Table 1).
Figure 1. Patient Evaluation and Randomization for a Trial of MRI-Guided Fibrosis Ablation for Atrial Fibrillation.
aSubcategories are not mutually exclusive and may not sum because a single patient may have multiple reasons for being excluded, not randomized, or not followed-up after the blanking period.
bReasons not approached: attending physician preference (n = 11), site investigator and/or research coordinator resources were inadequate to recruit additional patients (n = 3), and other (n = 83).
cReasons magnetic resonance imaging (MRI) was not performed: patient noncompliance or refusal (n = 26), technical difficulties (n = 9), medical condition (n = 5), body habitus (n = 4), glomerular filtration rate too low (n = 3), insufficient time (n = 3), ablation cancelled (n = 1), and unknown (n = 11).
dThere were 12 patients randomized to MRI-guided ablation who were ablated using pulmonary vein isolation (PVI) alone, and there was 1 patient randomized to PVI alone who was ablated using MRI-guided ablation. Hence the total number of patients included in safety analyses were 414 – 12 + 1 = 403 for the MRI-guided ablation group and 417 + 12 – 1 = 428 for the PVI-alone group. More than 1 reason could be designated for not receiving ablation.
eThe blanking period was defined as 90 days postablation. Patients were not monitored for the primary outcome of atrial arrhythmia recurrence during this period.
AF indicates atrial fibrillation.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| MRI-guided (n = 421) | PVI only (n = 422) | |
| Age, median (IQR), y | 62.2 (57.0-68.2) | 63.2 (57.1-68.8) |
| >75 y | 24 (5.7) | 24 (5.7) |
| Women | 89 (21.1) | 89 (21.1) |
| Men | 332 (78.9) | 333 (78.9) |
| Ethnicity, No.a | 386 | 389 |
| Hispanic or Latino | 19 (4.9) | 11 (2.8) |
| Not Hispanic or Latino | 367 (95.1) | 378 (97.2) |
| Race, No.a | 396 | 398 |
| Alaska Native, Native Hawaiian or Other Pacific Islander | 0 | 3 (0.8) |
| Asian | 2 (0.5) | 0 |
| Black or African American | 4 (1.0) | 4 (1.0) |
| White | 390 (98.5) | 391 (98.2) |
| History of tobacco use | 147 (34.9) | 164 (38.9) |
| Medical history | ||
| Baseline fibrosis levels | ||
| <10% | 48 (11.4) | 50 (11.8) |
| 10%-<20% | 198 (47) | 196 (46.4) |
| 20%-<30% | 144 (34.2) | 137 (32.5) |
| ≥30% | 31 (7.4) | 39 (9.2) |
| Median (IQR) | 18.4 (12.7-23.4) | 18 (13.2-23.8) |
| Cardiovertedb | 353 (83.8) | 353 (83.6) |
| Hypertension (systolic >160 mm Hg) | 247 (58.7) | 247 (58.5) |
| Hyperlipidemia | 146 (34.7) | 142 (33.6) |
| Congestive heart failure or left ventricular dysfunctionc | 91 (21.6) | 70 (16.6) |
| Coronary artery disease | 56 (13.3) | 51 (12.1) |
| Vascular disease | 44 (10.5) | 40 (9.5) |
| Diabetes | 40 (9.5) | 45 (10.7) |
| Stroke, transient ischemic attack, or thromboembolism | 36 (8.6) | 34 (8.1) |
| Mitral valve disease | 23 (5.5) | 27 (6.4) |
| Rheumatic fever | 7 (1.7) | 4 (0.9) |
| Coronary artery bypass graft | 4 (1) | 9 (2.1) |
| Treatment details | ||
| Ever taken anti-arrhythmic medication that failed to control atrial arrhythmia | 240 (57.0) | 250 (59.2) |
| Anti-arrhythmic medications | 201 (47.7) | 195 (46.2) |
| Days from atrial fibrillation diagnosis to ablation, No. | 355 | 351 |
| Median (IQR) | 451 (159-1147) | 405 (188-1124) |
| At least 1 y from atrial fibrillation diagnosis | 355 | 351 |
| No. (%) | 192 (54.1) | 188 (53.6) |
| Cryotherapy catheter at ablation | 415 | 417 |
| No. (%) | 48 (11.6) | 64 (15.3) |
Baseline characteristics are summarized for the full randomized study population.
Race and ethnicity were classified based on self-report. Some patients did not report these data, as categorial number of patients does not randomized group number of patients.
Indicates cardioverted prior to randomization (no time limit).
Left ventricular dysfunction was not firmly defined.
Assessment of Fibrosis Targeting and Scar Coverage
The assessment of fibrosis targeting by ablation points in each treatment group showed that 80.9% of patients in the fibrosis-guided ablation plus PVI group and 16.7% of patients in the PVI-only group had a mean fibrosis targeted score of at least 3 (indicating half or more coverage or encirclement). The assessment of fibrosis coverage by ablation-induced scar on the 3-month MRI showed that 44.8% of patients in the fibrosis-guided ablation plus PVI group and 15.5% of patients in the PVI-only group had mean scores consistent with half or more of their fibrosis covered by scar. The distribution of fibrosis targeting and scar coverage in each trial group, according to the 5-level scale, is shown in eTable 2 in Supplement 3.
Primary Outcome
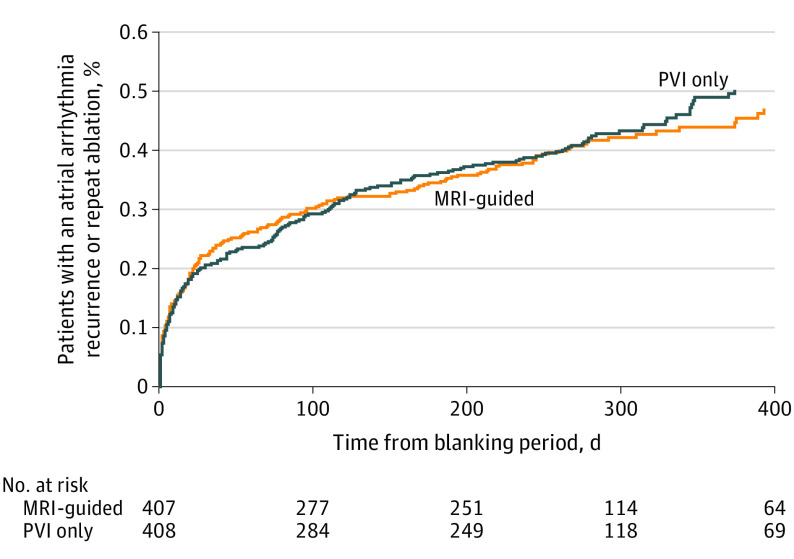
The event rate for the primary end point of atrial arrhythmia recurrence after ablation did not significantly differ between the fibrosis-guided group plus PVI and the PVI-only group (Figure 2). After a follow-up period of 12 to 18 months, the primary end point occurred in 175 (43.0%) patients in the fibrosis-guided ablation plus PVI group and in 188 (46.1%) in the PVI-only group (HR = 0.95 [95% CI, 0.77 to 1.17]; P = .63) (Table 2). Similar results were obtained under sensitivity analyses, which stratified by clinical center or treated center as a random effect (eTable 3 in Supplement 3).
Figure 2. Primary Composite of Atrial Arrhythmia Recurrence or Repeat Ablation.
The analysis was performed in randomized patients who remained in follow-up after the 90-day blanking period. Follow-up times are expressed in days following the end of the 90 day blanking period. No. at risk indicates the number of patients remaining at risk at the indicated follow-up times without a prior atrial arrhythmia–recurrence event. Cox model hazard ratio, 0.95 (95% CI, 0.77-1.17); log-rank P = .63; median observation time, 273 days (IQR, 51-321 days). MRI indicates magnetic resonance imaging; PVI, pulmonary vein isolation.
Table 2. Efficacy Outcomesa.
| No. (%) | Risk difference (95% CI)b | Hazard ratio (95% CI)c | P valued | ||
|---|---|---|---|---|---|
| MRI-guided (N = 407) | PVI only (N = 408) | ||||
| Primary outcome | |||||
| Atrial arrhythmia recurrence or repeat ablatione | 175 (43.0) | 188 (46.1) | −0.016 (−0.078 to 0.048) | 0.95 (0.77 to 1.17) | .63 |
| Components of the primary outcome (atrial arrhythmia types)f | |||||
| Atrial fibrillation | 129 (31.7) | 147 (36.0) | −0.029 (−0.089 to 0.036) | 0.90 (0.71 to 1.14) | .37 |
| Atrial flutter | 33 (8.1) | 26 (6.4) | 0.021 (−0.020 to 0.064) | 1.30 (0.78 to 2.17) | .32 |
| Atrial tachycardia | 7 (1.7) | 6 (1.5) | 0.003 (−0.018 to 0.024) | 1.18 (0.40 to 3.50) | .77 |
| Secondary outcomes | |||||
| Atrial arrhythmia recurrence, repeat ablation, or new atrial arrhythmia medicatione,g | 183 (45.0) | 196 (48.0) | −0.016 (−0.080 to 0.048) | 0.95 (0.78 to 1.16) | .62 |
| Repeat ablationh | 57 (14.0) | 72 (17.6) | −0.028 (−0.070 to 0.013) | 0.80 (0.56 to 1.12) | .20 |
| Post hoc outcome | |||||
| Atrial arrhythmia recurrence, repeat ablation, new atrial arrhythmia medication or cardioversione,g | 187 (45.9) | 198 (48.5) | −0.013 (−0.076 to 0.052) | 0.96 (0.79 to 1.17) | .69 |
Abbreviations: MRI, magnetic resonance imaging, PVI, pulmonary vein isolation.
Outcomes were evaluated in randomized patients who remained in follow-up after the 90-day blanking period.
Calculated as the difference in risk of the outcome in the MRI-guided group vs the PVI-guided group by day 275 after the start of the blanking period (95% CIs are percentile CIs from 2000 bootstrap samples).
Computed using Cox regression with baseline hazards stratified by baseline fibrosis (<20% vs ≥20%).
Computed from the log-rank test stratified by baseline fibrosis (<20% vs ≥20%).
The analysis evaluates the listed events as a composite outcome, with the first occurrence of any of the listed events counted as the composite event for the analysis.
Indicates atrial arrhythmia type for atrial arrhythmia recurrences designating the primary outcome.
Only new initiations of atrial arrhythmia medications are included in the atrial arrhythmia medication component of this composite outcome.
Repeat ablation is counted as an outcome even if there was an atrial arrhythmia recurrence, cardioversion, or start of atrial arrhythmia medications prior to the repeat ablation date.
The provision of ECG readings is summarized in eFigure 2 in Supplement 3. The percent of patients remaining at risk for the primary end point with at least 1 ECG reading over a 1-week period during the first week after the blanking period was 80.6% in the MRI-guided group and 83.2% in the PVI-only group. These percentages declined to 62.5% in the MRI-guided group and to 58.0% in the PVI-only group by 180 days after the blanking period, and they declined to 31.2% in the MRI-guided group and to 33.5% in the PVI-only group by 360 days after. The median total duration of follow-up for the atrial arrhythmia recurrence outcome was 9.0 months. Of 363 total atrial arrhythmia recurrence events, 230 (63.4%) were identified by smartphone readings, 79 (21.8%) by clinical ECGs, 39 (10.7%) by ambulatory ECG monitoring, and 15 (4.1%) by repeat ablations.
Main Secondary Outcome
In the prespecified main secondary analysis, the Toronto Atrial Fibrillation Symptom Severity Scale declined by a mean of 6.82 (95% CI, −7.52 to −6.08) points in the MRI-guided group and by a mean of 6.44 (95% CI, −7.13 to −5.71) points in the PVI-only group at 12-month follow-up, with a mean difference in change from baseline to 12 months of −0.38 (95% CI, −1.23 to 0.47) points (eTable 4A in Supplement 3). Similar results were obtained in the post hoc sensitivity analysis with site as a random effect (eTable 4B in Supplement 3).
Other Secondary Outcomes
There were no significant differences between treatment groups in individual components of the primary atrial arrhythmia composite end point or in other secondary end points related to atrial arrhythmia recurrence and repeat ablation (Table 2).
There were no significant differences between treatment groups in the mean changes in the Short Form-36 physical or mental health composite scores at 3-month or 12-month follow-up (eTable 4A in Supplement 3). Seven of the 414 patients in the MRI-guided group and 1 out of 417 in the PVI-only group had strokes during the 12- to 18-month follow-up period (P value = .04).
Prespecified Subgroups
In prespecified subgroup analysis comparing the primary atrial arrhythmia recurrence composite outcome between the fibrosis-guided ablation plus PVI and PVI-only groups, the HRs were 0.88 (95% CI 0.67-1.16) for patients with low-fibrosis stage (<20% baseline fibrosis) and 1.09 (0.80-1.50) for patients with high-fibrosis stage (≥20% baseline fibrosis) (eFigures 3A and 3B in Supplement 3). The HRs for the primary composite outcome did not differ significantly between the 2 baseline fibrosis groups (P value for interaction = .32). The individual components of the primary end point and related composite end points separated by fibrosis stage are summarized by treatment group in eTables 5A and 5B in Supplement 3.
Adverse Events
There was a statistically significant higher occurrence of the primary safety composite outcome in the fibrosis-guided ablation plus PVI group (9 of 403 patients [2.2%]) compared with the PVI-only group (0 of 428 patients; P = .001) (Table 3). Six patients (1.5%) in the fibrosis-guided ablation plus PVI group had an ischemic stroke within 30 days after the procedure compared with none in the PVI-only group. Two out of 6 of these patients (in the fibrosis-guided ablation plus PVI group) had a previous stroke episode. Characteristics of patients who experienced an ischemic stroke are shown in eTable 6 in Supplement 3. Anticoagulation was resumed for all patients after the procedure. None had an isolation of their left-atrial appendage. Five stroke events occurred between 0 to 3 days after ablation. One patient had an out-of-hospital ventricular fibrillation event 26 days after the ablation procedure. Only 1 of the 6 patients with stroke had no comorbidities. Two deaths occurred in the fibrosis-guided ablation plus PVI group, and the first one was possibly related to the procedure. The first death occurred 5 days postablation and was sudden with unknown cause. The second death occurred 34 days after ablation and was due to 2 strokes after ventricular fibrillation. eTable 7 in Supplement 3 summarizes the frequency of safety events by treatment group and fibrosis stage.
Table 3. Safety Outcomes in Total Populationa.
| No. (%) | ||
|---|---|---|
| MRI-guided (N = 403) | PVI alone (N = 428) | |
| Safety outcomes | ||
| Bleeding requiring transfusion | 1 (0.2) | 0 |
| Heart failure | 1 (0.2) | 0 |
| Pulmonary vein stenosis | 0 (0) | 0 |
| Stroke or transient ischemic attack | 6 (1.5) | 0 |
| Death | 2 (0.5) | 0 |
| Primary composite safety outcome, defined as ≥1 of the above eventsb | 9 (2.2) | 0 |
| Esophageal injuryc | 5 (1.2) | 1 (0.2) |
| Perforation or tamponadec | 5 (1.2) | 5 (1.2) |
Abbreviations: MRI, Magnetic resonance imaging; PVI, pulmonary vein isolation; TIA, transient ischemic attack.
Safety outcomes were evaluated according to the treatment received in the full safety population for the 30-day period following ablation. Therefore, referring to footnote c of Figure 1: for the safety analysis in the MRI group, N = 414 – 12 + 1 = 403, and for the PVI-only group, N = 417 + 12 – 1 = 428.
The P value for the comparison of the primary composite safety outcome between the MRI-guided and PVI-only groups computed using the Fisher-exact test was .001.
Esophageal injury and perforation or tamonade were initially identified by clinical sites and reviewed by the medical monitor for this trial. Final classifications were made by a safety outcome review committee.
Discussion
In this randomized clinical trial of patients with persistent AF, MRI-guided fibrosis-targeted ablation with PVI, compared with PVI alone did not significantly improve atrial arrhythmia recurrence at follow-up. Moreover, more strokes were observed when additional MRI defined fibrotic areas outside the PV ostia were targeted.
Targeting atrial fibrosis detected by electroanatomical mapping or using diagnostic imaging to treat patients with AF has shown promise in recent studies. Trials targeting low-voltage areas during ablation, either by homogenization or selective ablation, significantly increased the success rates of the procedure compared with PVI by reducing AF recurrence.22,23,24 Using regions of delayed enhancement to identify fibrotic remodeling, Akoum et al25 demonstrated that patients with persistent AF and more fibrosis targeted during ablation based on late gadolinium enhancement MRI had significantly less AF recurrence after the procedure. In the recently published ALICIA trial,17 investigators found no significant additional benefit of adding delayed-enhancement MRI–guided fibrosis ablation to PVI in 181 randomized patients with AF. Trial investigators excluded patients with large left atrium, and most randomized participants had paroxysmal AF with very low fibrotic burden. Similar outcomes were observed in this trial that included a larger cohort consisting exclusively of patients with persistent AF and a wider distribution of left-atrial fibrotic burdens.
Despite the different ablation strategies that have been explored to improve ablation outcomes in patients with persistent AF, none have shown significant superiority to PVI in randomized clinical trials.4,26,27 In this trial, 54% of patients with AF were free of AF recurrence at 12-month follow-up. This finding is similar to rates observed in other trials comparing PVI to other ablation strategies that also included paroxysmal AF.17,28,29 Reproducibility of PVI success rates across different studies can be explained by the fact that PVs are well-defined anatomical structures that can be targeted in a reproducible objective manner and by multiple existing ablation technologies.
The lack of benefit of fibrosis-guided ablation could be explained by several factors related to technical challenges and the pathophysiology of AF. While available data suggest a strong link between fibrosis and AF, the mechanism by which fibrosis leads to initiation or perpetuation of AF is not completely understood. Conversely, different types of fibrosis can co-exist in the atrial tissue, including interstitial and reparative fibrosis, unequally contributing to AF development. The arrhythmogenic propensity of fibrotic tissue can depend on the texture and spatial distribution of fibrosis. While not all fibrosis plays an active role in AF, current imaging techniques cannot make the distinction, limiting the benefit of extensively ablating fibrotic tissue. Second, applying a thermal injury to fibrotic tissue might not be an appropriate strategy to eliminate its arrhythmogenic potential. Atrial fibrosis can also progress to nonfibrotic areas with time, despite being ablated in previous procedures.30 Third, ablation parameters required to achieve lesion formation and transmurality are influenced by the type of underlying fibrosis and regional wall characteristics.31,32,33,34,35,36,37 Fourth, from a technical standpoint and unlike PVI, fibrosis ablation is not standardized and has no established end points among operators, leaving room for subjectivity in targeting strategies.
The higher rate of complications observed in the fibrosis-guided ablation plus PVI group was mainly driven by higher ischemic stroke events. The reported rate of cerebral injuries in the fibrosis-guided ablation plus PVI group in this trial were similar to the rates of stroke observed in other published ablation studies when additional AF mechanisms were targeted.38 In the STAR AF II trial,28 when lesions were extended outside the PVs, the incidence of strokes was approximately 1%. Fibrotic burden at baseline in the 6 patients who had a stroke was heterogenous, ranging from 8% to 30%, with 66% (4) patients having more than 20% fibrotic burden. Extensive atrial tissue injury during ablation can affect the function of the left atrium, as well as potentially increase the risk of clot formation at the ablation site, thus increasing the propensity for embolic stroke.39 Additionally, a higher rate of complications has been generally observed in ablation requiring longer procedural times, regardless of the strategy used. Based on the findings from DECAAFII and other trials, any additional ablation lesions targeting areas outside the PV ostia should be considered with caution.
Limitations
The trial has several limitations. First, the lack of investigator blinding with regard to randomization and treatment could have led to observation bias. Second, ECG smartphone compliance declined in similar fashion in both study groups over the duration of the trial, but other ECG monitoring methods were available, and the completeness of ECG tracings was similar between the 2 randomized groups. Third, the follow-up period was relatively short.
Conclusion
Among patients with persistent AF, MRI-guided fibrosis ablation plus PVI, compared with PVI catheter ablation only, resulted in no significant difference in atrial arrhythmia recurrence. Findings do not support the use of MRI-guided fibrosis ablation for the treatment of persistent AF.
Trial Protocol and Statistical Analysis Plan
Nonauthor Collaborators
eAppendix. Committees: Steering Committee, Data Safety and Monitoring Board, End Point Adverse Event Committee, Data Coordinating Center
eFigure 1. Examples of Ablation Points Targeting Fibrosis and Scar-Coverage/Encirclement of Fibrosis at 3 Months in Each Level on the 5-Level Scale as Assessed on 3-Month MRI
eFigure 2. Completeness of ECG Transmissions in Both Treatment Arms
eFigure 3. Primary End Point by Fibrosis Stages <20% and ≥20%
eTable 1. Eligibility Criteria
eTable 2. Descriptive Statistics of Mean Fibrosis Covered/Encircled and Mean Fibrosis Targeted in Each Treatment Arm
eTable 3. Sensitivity Analyses for Estimating the Hazard Ratio Comparing the Primary Atrial-Arrhythmia Recurrence Composite Outcome Between the MRI-Guided and PVI-Only Treatment Groups
eTable 4A. Effects of Randomized Interventions on Quality of Life Outcomes: Prespecified Analysis
eTable 4B. Effects of Randomized Interventions on Quality of Life Outcomes: Post Hoc Sensitivity Analysis Including Site as a Random Effect
eTable 5. Efficacy Outcomes by Fibrosis Stages: <20% and ≥20%
eTable 6. Details of Strokes in First 30 Days
eTable 7. Safety Outcomes by Fibrosis Stages
Data Sharing Statement
References
- 1.Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7(6):835-846. doi: 10.1016/j.hrthm.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 2.Sultan A, Lüker J, Andresen D, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep. 2017;7(1):16678. doi: 10.1038/s41598-017-16938-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. doi: 10.1161/JAHA.112.004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma A, Chen-yang J, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812-1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 5.Tamborero D, Mont L, Berruezo A, et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009;2(1):35-40. doi: 10.1161/CIRCEP.108.797944 [DOI] [PubMed] [Google Scholar]
- 6.Mun HS, Joung B, Shim J, et al. Does additional linear ablation after circumferential pulmonary vein isolation improve clinical outcome in patients with paroxysmal atrial fibrillation? prospective randomised study. Heart. 2012;98(6):480-484. doi: 10.1136/heartjnl-2011-301107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilz RR, Lenz C, Sommer P, et al. Focal impulse and rotor modulation ablation vs. pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: results from the FIRMAP AF study. Europace. 2021;23(5):722-730. doi: 10.1093/europace/euaa378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. 2017;3(5):425-435. doi: 10.1016/j.jacep.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311(5):498-506. doi: 10.1001/jama.2014.3 [DOI] [PubMed] [Google Scholar]
- 10.Akoum N, Wilber D, Hindricks G, et al. MRI assessment of ablation-induced scarring in atrial fibrillation: analysis from the DECAAF Study. J Cardiovasc Electrophysiol. 2015;26(5):473-480. doi: 10.1111/jce.12650 [DOI] [PubMed] [Google Scholar]
- 11.Marrouche NF, Greene T, Dean JM, et al. ; DECAAF II Investigators . Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: the DECAAF II trial: study design. J Cardiovasc Electrophysiol. 2021;32(4):916-924. doi: 10.1111/jce.14957 [DOI] [PubMed] [Google Scholar]
- 12.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chubb H, Karim R, Roujol S, et al. The reproducibility of late gadolinium enhancement cardiovascular magnetic resonance imaging of post-ablation atrial scar: a cross-over study. J Cardiovasc Magn Reson. 2018;20(1):21. doi: 10.1186/s12968-018-0438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermond RA, Crijns HJGM, Tijssen JGP, et al. ; RACE II investigators . Symptom severity is associated with cardiovascular outcome in patients with permanent atrial fibrillation in the RACE II study. Europace. 2014;16(10):1417-1425. doi: 10.1093/europace/euu151 [DOI] [PubMed] [Google Scholar]
- 15.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217-227. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 16.Fink T, Schlüter M, Heeger CH, et al. ; Georg Hospital for Long-Standing Persistent Atrial Fibrillation) . Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017;10(7):e005114. doi: 10.1161/CIRCEP.117.005114 [DOI] [PubMed] [Google Scholar]
- 17.Bisbal F, Benito E, Teis A, et al. Magnetic resonance imaging-guided fibrosis ablation for the treatment of atrial fibrillation: the ALICIA Trial. Circ Arrhythm Electrophysiol. 2020;13(11):e008707. doi: 10.1161/CIRCEP.120.008707 [DOI] [PubMed] [Google Scholar]
- 18.Munda M, Legrand C. Adjusting for centre heterogeneity in multicentre clinical trials with a time-to-event outcome. Pharm Stat. 2014;13(2):145-152. doi: 10.1002/pst.1612 [DOI] [PubMed] [Google Scholar]
- 19.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 20.Gwet K. Handbook of Inter-Rater Reliability: The Definitive Guide to Measuring the Extent of Agreement Among Raters. Advanced Analytics, LLC; 2012. [Google Scholar]
- 21.Little RJA, Rubin DB. (2019) Statistical Analysis with Missing Data (Vol. 793), Third ed. John Wiley & Sons; 2019. Accessed April 26, 2022. https://onlinelibrary.wiley.com/doi/book/10.1002/9781119482260
- 22.Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7(5):825-833. doi: 10.1161/CIRCEP.113.001251 [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Tsuchiya T, Nakahara S, et al. Efficacy of left atrial voltage-based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27(9):1055-1063. doi: 10.1111/jce.13019 [DOI] [PubMed] [Google Scholar]
- 24.Kircher S, Arya A, Altmann D, et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;20(11):1766-1775. doi: 10.1093/europace/eux310 [DOI] [PubMed] [Google Scholar]
- 25.Akoum N, Morris A, Perry D, et al. Substrate modification is a better predictor of catheter ablation success in atrial fibrillation than pulmonary vein isolation: an LGE-MRI study. Clin Med Insights Cardiol. 2015;9:25-31. doi: 10.4137/CMC.S22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estner HL, Hessling G, Biegler R, et al. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation—a prospective randomized study. Pacing Clin Electrophysiol. 2011;34(8):939-948. doi: 10.1111/j.1540-8159.2011.03100.x [DOI] [PubMed] [Google Scholar]
- 27.Mohanty S, Mohanty P, Trivedi C, et al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping: insights from a meta-analysis. Circ Arrhythm Electrophysiol. 2018;11(3):e005789. doi: 10.1161/CIRCEP.117.005789 [DOI] [PubMed] [Google Scholar]
- 28.Verma A, Jiang CY, Betts TR, et al. ; STAR AF II Investigators . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812-1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 29.Masuda M, Asai M, Iida O, et al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO Trial. J Am Heart Assoc. 2020;9(13):e015927. doi: 10.1161/JAHA.120.015927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kheirkhahan M, Baher A, Goldooz M, et al. Left atrial fibrosis progression detected by LGE-MRI after ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2020;43(4):402-411. doi: 10.1111/pace.13866 [DOI] [PubMed] [Google Scholar]
- 31.Motoike Y, Harada M, Ito T, et al. Wall thickness-based adjustment of ablation index improves efficacy of pulmonary vein isolation in atrial fibrillation: real-time assessment by intracardiac echocardiography. J Cardiovasc Electrophysiol. 2021;32(6):1620-1630. doi: 10.1111/jce.15000 [DOI] [PubMed] [Google Scholar]
- 32.Bishop M, Rajani R, Plank G, et al. Three-dimensional atrial wall thickness maps to inform catheter ablation procedures for atrial fibrillation. Europace. 2016;18(3):376-383. doi: 10.1093/europace/euv073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulder MJ, Kemme MJB, Hagen AMD, et al. Impact of local left atrial wall thickness on the incidence of acute pulmonary vein reconnection after ablation index-guided atrial fibrillation ablation. Int J Cardiol Heart Vasc. 2020;29:100574. doi: 10.1016/j.ijcha.2020.100574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segreti L, De Simone A, Schillaci V, et al. A novel local impedance algorithm to guide effective pulmonary vein isolation in atrial fibrillation patients: preliminary experience across different ablation sites from the CHARISMA pilot study. J Cardiovasc Electrophysiol. 2020;31(9):2319-2327. doi: 10.1111/jce.14647 [DOI] [PubMed] [Google Scholar]
- 35.Tao S, Guttman MA, Fink S, et al. Ablation lesion characterization in scarred substrate assessed using cardiac magnetic resonance. JACC Clin Electrophysiol. 2019;5(1):91-100. doi: 10.1016/j.jacep.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tofig BJ, Lukac P, Nielsen JM, et al. Radiofrequency ablation lesions in low-, intermediate-, and normal-voltage myocardium: an in vivo study in a porcine heart model. Europace. 2019;21(12):1919-1927. doi: 10.1093/europace/euz247 [DOI] [PubMed] [Google Scholar]
- 37.Glashan CA, Stevenson W, Zeppenfeld K. Lesion size and lesion maturation after radiofrequency catheter ablation for ventricular tachycardia in humans with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2021;14(8):e009808. doi: 10.1161/CIRCEP.121.009808 [DOI] [PubMed] [Google Scholar]
- 38.Oral H, Chugh A, Özaydın M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114(8):759-765. doi: 10.1161/CIRCULATIONAHA.106.641225 [DOI] [PubMed] [Google Scholar]
- 39.Lee DS, Dorian P, Downar E, et al. Thrombogenicity of radiofrequency ablation procedures: what factors influence thrombin generation? Europace. 2001;3(3):195-200. doi: 10.1053/eupc.2001.0167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Nonauthor Collaborators
eAppendix. Committees: Steering Committee, Data Safety and Monitoring Board, End Point Adverse Event Committee, Data Coordinating Center
eFigure 1. Examples of Ablation Points Targeting Fibrosis and Scar-Coverage/Encirclement of Fibrosis at 3 Months in Each Level on the 5-Level Scale as Assessed on 3-Month MRI
eFigure 2. Completeness of ECG Transmissions in Both Treatment Arms
eFigure 3. Primary End Point by Fibrosis Stages <20% and ≥20%
eTable 1. Eligibility Criteria
eTable 2. Descriptive Statistics of Mean Fibrosis Covered/Encircled and Mean Fibrosis Targeted in Each Treatment Arm
eTable 3. Sensitivity Analyses for Estimating the Hazard Ratio Comparing the Primary Atrial-Arrhythmia Recurrence Composite Outcome Between the MRI-Guided and PVI-Only Treatment Groups
eTable 4A. Effects of Randomized Interventions on Quality of Life Outcomes: Prespecified Analysis
eTable 4B. Effects of Randomized Interventions on Quality of Life Outcomes: Post Hoc Sensitivity Analysis Including Site as a Random Effect
eTable 5. Efficacy Outcomes by Fibrosis Stages: <20% and ≥20%
eTable 6. Details of Strokes in First 30 Days
eTable 7. Safety Outcomes by Fibrosis Stages
Data Sharing Statement