This randomized clinical trial assesses the effect of a multispecies probiotic on the risk of antibiotic-associated diarrhea in children.
Key Points
Question
What is the efficacy of a multispecies probiotic in the prevention of antibiotic-associated diarrhea in children?
Findings
In this placebo-controlled randomized clinical trial of 350 children, a multispecies probiotic had no significant effect on the risk of antibiotic-associated diarrhea caused by Clostridioides difficile or of unknown etiology, but it reduced the overall risk of diarrhea regardless of the etiology from 32% to 20%, a statistically significant difference.
Meaning
The use of the studied probiotic formulation may be considered for diarrhea prevention during antibiotic treatment in children.
Abstract
Importance
The efficacy of multispecies probiotic formulations in the prevention of antibiotic-associated diarrhea (AAD) remains unclear.
Objective
To assess the effect of a multispecies probiotic on the risk of AAD in children.
Design, Setting, and Participants
This randomized, quadruple-blind, placebo-controlled trial was conducted from February 2018 to May 2021 in a multicenter, mixed setting (inpatients and outpatients). Patients were followed up throughout the intervention period. Eligibility criteria included age 3 months to 18 years, recruitment within 24 hours following initiation of broad-spectrum systemic antibiotics, and signed informed consent. In total, 646 eligible patients were approached and 350 patients took part in the trial.
Interventions
A multispecies probiotic consisting of Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Lactobacillus acidophilus W37, L acidophilus W55, Lacticaseibacillus paracasei W20, Lactiplantibacillus plantarum W62, Lacticaseibacillus rhamnosus W71, and Ligilactobacillus salivarius W24, for a total dose of 10 billion colony-forming units daily, for the duration of antibiotic treatment and for 7 days after.
Main Outcomes and Measures
The primary outcome was AAD, defined as 3 or more loose or watery stools per day in a 24-hour period, caused either by Clostridioides difficile or of otherwise unexplained etiology, after testing for common diarrheal pathogens. The secondary outcomes included diarrhea regardless of the etiology, diarrhea duration, and predefined diarrhea complications.
Results
A total of 350 children (192 boys and 158 girls; mean [range] age, 50 [3-212] months) were randomized and 313 were included in the intention-to-treat analysis. Compared with placebo (n = 155), the probiotic (n = 158) had no effect on risk of AAD (relative risk [RR], 0.81; 95% CI, 0.49-1.33). However, children in the probiotic group had a lower risk of diarrhea regardless of the etiology (RR, 0.65; 95% CI, 0.44-0.94). No differences were observed between the groups for most of the secondary outcomes, including adverse events.
Conclusions and Relevance
A multispecies probiotic did not reduce the risk of AAD in children when analyzed according to the most stringent definition. However, it reduced the overall risk of diarrhea during and for 7 days after antibiotic treatment. Our study also shows that the AAD definition has a significant effect on clinical trial results and their interpretation.
Trial Registration
ClinicalTrials.gov Identifier: NCT03334604
Introduction
Antibiotic-associated diarrhea (AAD) is a common complication of antibiotic treatment.1,2 Several different definitions of AAD have been proposed, including “diarrhea that occurs in relation to antibiotic treatment with the exclusion of other etiologies.”3,4 In clinical practice and in most clinical trials, microbiological tests are not routinely performed to exclude an infectious origin of AAD, confirming its etiology.5 AAD is considered to result from gut dysbiosis by antibiotics, which may provoke overgrowth of specific pathogens, most prominently Clostridioides difficile, and lead to altered function of the microbiota.6,7
The most thoroughly studied preventive intervention for AAD is the administration of probiotics, defined as “live microorganisms, that when administered in adequate amounts, confer a health benefit on the host.”8 According to a 2019 Cochrane review,2 probiotics as a group have a moderate protective effect on the prevention of pediatric AAD. The results of individual studies in this review varied depending on the dose of probiotic, with higher doses of 5 billion colony-forming units (CFU) or more per day demonstrating a better effect. Among the 33 included studies, only 6 randomized clinical trials (RCTs) of limited size investigated combinations of more than 3 probiotic strains, with varied results.9,10,11,12,13,14 Thus, the effect of multispecies probiotic supplementation on AAD incidence in children remains in question. In adult patients, one of the previously studied multispecies probiotics consisted of 9 bacterial species.15,16 In the current study, we aimed to assess the efficacy of a comparable multispecies probiotic mixture in the prevention of AAD in a pediatric population.
Methods
Study Design
A parallel-group, randomized, quadruple-blind placebo-controlled RCT (trial protocol can be found in Supplement 1) was conducted in pediatric clinical and outpatient wards of 3 Dutch and 2 Polish hospitals (eTable 1 in Supplement 2). The study was prospectively registered in ClinicalTrials.gov database (NCT03334604), and the protocol was published in a peer-reviewed journal.17 Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed for reporting trial results.18
Ethics
The study was approved by the Bioethics Committees of the Medical University of Warsaw (KB/198/2017) and Amsterdam UMC (2019.227). Written informed consent was obtained by the parents or the legal guardians of all participants. During the study, 2 changes in the study protocol were introduced in response to an unsatisfactory inclusion rate. First, recruitment in additional centers was started, as planned in the study protocol. Second, the lower age limit of the participants was adjusted from 6 months to 3 months.
Participants
Eligibility criteria included age from 3 months to 18 years, recruitment within 24 hours following initiation of broad-spectrum oral or intravenous antibiotic therapy, and signed informed consent. The exclusion criteria were as follows: use of antibiotics within the previous 4 weeks; use of probiotics, proton pump inhibitors, laxatives, or antidiarrheal drugs within the previous 2 weeks; severe infection or life-threatening illness at recruitment (ie, indicated or probable admission to an intensive care unit); preexisting diarrhea within the previous 4 weeks based on patient’s or caregiver’s report; severe chronic disease (eg, cancer, inflammatory bowel disease, short-bowel syndrome); diagnosed primary or secondary immune deficiency; required tube-feeding; exclusive breastfeeding; and known allergy or hypersensitivity to any component of the study product.
Randomization and Masking
A block randomization in blocks of 4 was performed centrally in a 1:1 ratio by Winclove Probiotics B.V. with use of a computer random-sequence generator, by a person not otherwise involved in the study. The randomization lists were stored in sealed, opaque envelopes at the study centers. The participants, caregivers, and all investigators, including data collectors and outcomes assessors, were blinded until the primary data analysis was performed. Probiotic and placebo were packed identically and had the same appearance, taste, and smell.
Procedures and Interventions
The parents were instructed to administer 2 sachets of the study product daily to their children for the duration of antibiotic treatment and for 7 days after, up to a maximum of 17 days, starting within 24 hours of the first antibiotic dose. The multispecies probiotic (Ecologic AAD 612; Winclove Probiotics B.V.) contained 8 bacterial strains: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Lactobacillus acidophilus W37, L acidophilus W55, Lacticaseibacillus paracasei W20, Lactiplantibacillus plantarum W62, Lacticaseibacillus rhamnosus W71, and Ligilactobacillus salivarius W24 (formerly known as Lactobacillus salivarius W24), for a total dose of 5 billion CFU per sachet (10 billion CFU daily).
The data on outcomes were collected using study diaries during antibiotic treatment and for 7 additional days. The consistency was reported according to the Amsterdam Infant Stool Scale (AISS)19 or Bristol Stool Form Scale (BSFS),20 depending on participant’s age. In case of diarrhea occurrence, the participants’ caregivers were requested to provide stool samples for testing for rotavirus, adenovirus, and norovirus by immunoassay; Campylobacter species, Salmonella species, Shigella species, and Yersinia species by isolation from stool cultures; and C difficile in children older than 1 year by detection of glutamate dehydrogenase in conjunction with toxins A and B with immunoassay. Additionally, stool samples for microbiota and metabolomics analysis were collected from a subset of patients at 4 timepoints: at baseline, on the day of antibiotic discontinuation, at the end of the intervention period, and 1 month after the intervention period. The results of microbiota and metabolomics analysis will be reported in a separate publication.
Outcome Measures
The primary outcome measure was AAD, defined as 3 or more loose or watery stools (a score of A on the AISS or 5-7 on the BSFS) per day in a 24-hour period, caused either by C difficile or of otherwise unexplained etiology, after testing for common, predefined diarrheal pathogens. Secondary outcomes included diarrhea, defined as 3 or more loose or watery stools per day in a 24-hour period regardless of the etiology, mild AAD, defined as 2 or more loose or watery stools per day for a minimum of a 24-hour period caused by C difficile or of otherwise unexplained etiology, severe AAD defined as 3 or more loose or watery stools per day for a minimum of a 48-hour period caused by C difficile or of otherwise unexplained etiology, diarrhea duration, defined as the interval until normalization of stool consistency according to the BSFS (1, 2, 3, or 4) or AISS (B, C, or D) and the presence of normal stools for 48 hours, diarrhea caused by C difficile, discontinuation of the antibiotic treatment owing to diarrhea, hospitalization caused by diarrhea, need for intravenous rehydration owing to diarrhea, and adverse events.
Sample Size Calculation
Based on the pooled risks of AAD determined from the previous studies conducted at the Medical University of Warsaw,21,22 as well as those reported in a Cochrane review,2 we expected that the incidence of AAD would be 16% among children receiving placebo. To detect a difference of 11% between the arms at a 5% significance level and with 80% power, we determined that 350 participants (175 in each arm) were needed assuming potential loss to follow-up of 20%.
Statistical Analysis
Descriptive statistics were used to present the participants’ characteristics. For the dichotomous outcomes, relative risk (RR) was calculated with 95% CIs, along with number needed to benefit (NNTB), if appropriate. Presented P values were derived from χ2 test or Fisher exact test where appropriate. For the continuous outcome, Man Whitney U test was performed. All of the statistical tests were 2-tailed and performed with a 5% level of significance. The primary outcome was also analyzed by logistic regression, controlling for 5 prespecified potential risk factors for AAD (age, sex, antibiotic type, duration of antibiotic treatment, and duration of hospital stay). Intention-to-treat (ITT) analysis was performed on the available participants. Owing to the completeness of our baseline data, no imputation methods were used in ITT analysis.23 Sensitivity analyses with plausible assumptions regarding patients lost to follow-up as described by Akl et al24 were performed. Additionally, per-protocol analysis was performed on the participants who ingested at least 75% of the study formula based on caregivers’ reports and the counting of unused sachets. For the all of the calculations, StatsDirect, version 3.3.5 (StatsDirect Ltd) was used.
Results
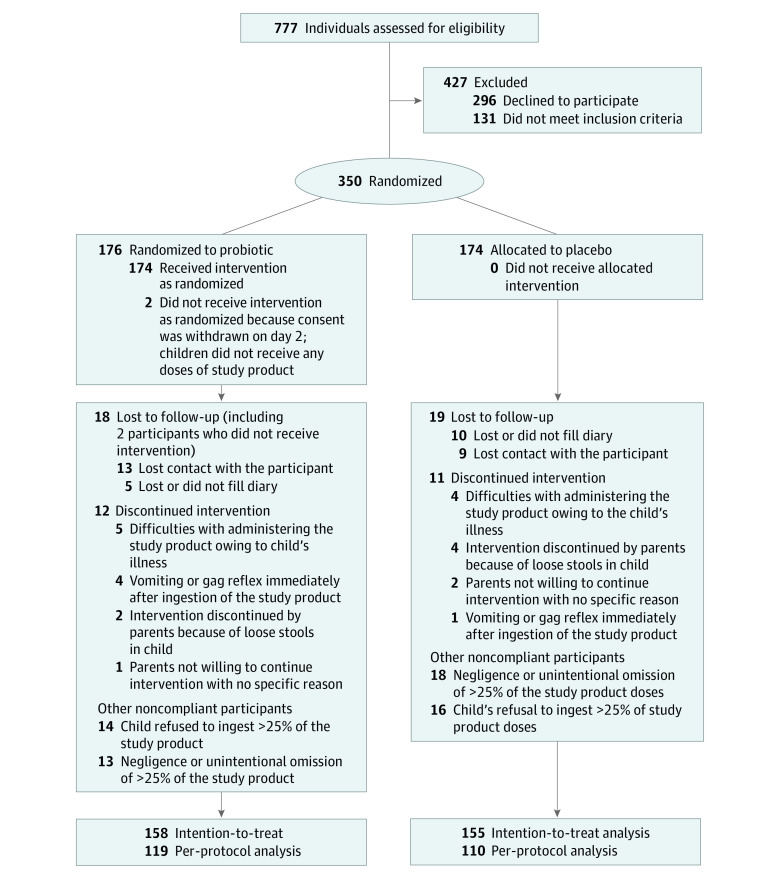
Between February 2018 and May 2021, 350 participants (192 boys and 158 girls; median age: 28 months; mean [range] age, 50 [3-212] months) were consecutively enrolled. Among them, 202 participants were included in Poland and 148 in the Netherlands. Available case analysis was carried out in 313 participants and per-protocol analysis in 229 compliant participants (Figure). Participants’ characteristics were comparable between the 2 groups (Table 1). Patients from the Netherlands differed from the Polish patients mainly in terms of class of used antibiotics, antibiotic administration route, and setting. Also, loss to follow-up frequency in Poland was almost 4 times higher than in the Netherlands (15.1% vs 4.1%, respectively) (eTable 2 in Supplement 2). The characteristics of the patients lost to follow-up were similar in the placebo and probiotic groups (eTable 3 in Supplement 2) and similar to characteristics of the remaining study participants (Table 1).
Figure. CONSORT 2010 Flow Diagram.
Table 1. Characteristics of Participants.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Placebo (n = 174) | Probiotic (n = 176) | Total (N = 350) | |
| Age, median (range), mo | 27 (3-204) | 32 (3-212) | 28 (3-212) |
| Sex | |||
| Female | 76 (43.7) | 82 (46.6) | 158 (45.1) |
| Male | 98 (56.3) | 94 (53.4) | 192 (54.9) |
| Setting | |||
| Inpatient | 135 (77.6) | 136 (77.3) | 271 (77.4) |
| Outpatient | 39 (22.4) | 40 (22.7) | 79 (22.6) |
| Reason for antibiotic treatment | |||
| Lower respiratory tract infection | 54 (31) | 56 (31.8) | 110 (31.4) |
| Upper respiratory tract infection | 52 (29.9) | 49 (27.8) | 101 (28.9) |
| Urinary tract infection | 35 (20.1) | 24 (13.6) | 59 (16.9) |
| Skin infection | 8 (4.6) | 16 (9.1) | 24 (6.9) |
| Lymphadenitis | 6 (3.4) | 7 (4) | 13 (3.7) |
| Nervous system infection | 3 (1.7) | 4 (2.3) | 7 (2.0) |
| Gastrointestinal infection | 5 (2.9) | 5 (2.8) | 10 (2.9) |
| Joint infection | 3 (1.7) | 2 (1.1) | 5 (1.4) |
| Other | 8 (4.6) | 13 (7.4) | 21 (6.0) |
| Antibiotic administration route | |||
| Only oral | 71 (40.8) | 73 (41.5) | 144 (41.1) |
| Only intravenous | 25 (14.4) | 28 (15.9) | 53 (15.1) |
| Intravenous followed by oral | 78 (44.8) | 75 (42.6) | 153 (43.7) |
| Antibiotic type | |||
| Cephalosporin | |||
| Second generation | 25 (14.4) | 26 (14.8) | 51 (14.6) |
| Third generation | 33 (19) | 36 (20.5) | 69 (19.7) |
| Aminopenicillin | 69 (39.7) | 71 (40.3) | 140 (40) |
| Amoxicillin with clavulanic acid | 67 (38.5) | 55 (31.3) | 122 (34.9) |
| Clindamycin | 14 (8) | 17 (9.7) | 31 (8.9) |
| Cloxacillin/flucloxacillin | 0 | 6 (3.4) | 6 (1.7) |
| Gentamicin | 1 (0.6) | 3 (1.7) | 4 (1.1) |
| Other | 6 (3.4) | 6 (3.4) | 12 (3.4) |
| Two concomitant antibiotics | 15 (8.6) | 24 (13.6) | 39 (11.1) |
| Change of antibiotic class | 26 (14.9) | 20 (11.4) | 46 (13.1) |
| Treatment duration, median (range), d | 10 (2-21) | 10 (1-36) | 10 (1-36) |
| Hospital stay duration, median (range), d | 5 (1-35) | 5 (1-45) | 5 (1-45) |
Among 83 patients who developed diarrhea, stools from 10 children tested positive for rotavirus, 3 for norovirus, 1 for adenovirus, and 1 for Salmonella enterica; 6 patients in the probiotic group and 11 patients in the placebo group did not provide a stool sample for the etiology testing. The reasons for the stool sampling failures were difficulties in communicating with patients after discharge from the hospital. All of these patients were not qualified as AAD cases for the primary outcome measure. In the ITT analysis (Table 2), AAD incidence was comparable between the probiotic and placebo groups (23 of 158 [14.6%] vs 28 of 155 [18.1%,] respectively; RR, 0.81; 95% CI, 0.49-1.33). The frequency of AAD according to the alternative definitions (mild, severe) was also similar between both study groups. The patients in the probiotic group had a significantly lower risk of developing diarrhea than those in the placebo group when analyzed regardless of its etiology (33 of 158 [20.9%] vs 50 of 155 [32.3%], respectively; RR, 0.65; 95% CI, 0.44-0.94; NNTB = 9; 95% CI, 5-64; P = .02); they were also less likely to require intravenous rehydration owing to diarrhea (0 of 158 [0%] vs 5 of 155 [3.2%], respectively; NNTB = 32; 95% CI, 14-125; P = .03). We found no significant difference between the groups in the other outcomes. Effect sizes in the per-protocol analysis were similar to the ones observed in the ITT analysis; however, because of a smaller sample size, they were not statistically significant (eTable 4 in Supplement 2).
Table 2. Main Results of the Available Case Analysis.
| Outcome | Events, No. (%) | Relative risk (95% CI) | Absolute risk reduction, % | NNTB (95% CI) | |
|---|---|---|---|---|---|
| Probiotic group | Placebo group | ||||
| AAD | 23 (14.6) | 28 (18.1) | 0.81 (0.49-1.33) | 3.5 | NA |
| Severe AAD | 18 (11.4) | 19 (12.3) | 0.93 (0.51-1.69) | 0.9 | NA |
| Mild AAD | 40 (25.3) | 38 (24.5) | 1.03 (0.7-1.52) | −0.8 | NA |
| Diarrheab | 33 (20.9) | 50 (32.3) | 0.65 (0.44-0.94)a | 11.4 | 9 (5-64)a |
| Clostridioides difficile diarrhea | 1 (0.6) | 3 (1.9) | 0.33 (0.05-2.26) | 1.3 | NA |
| Hospitalization owing to diarrhea | 1 (0.6) | 2 (1.3) | 0.49 (0.06-3.71) | 0.7 | NA |
| Antibiotic cessation owing to diarrhea | 0 | 0 | NA | 0 | NA |
| Intravenous rehydration owing to diarrhea | 0 | 5 (3.2) | NA | 3.2 | 32 (14-125)a |
| Adverse eventsc | 16 (10.1) | 10 (6.5) | 1.57 (0.75-3.3) | −3.6 | NA |
Abbreviations: AAD, antibiotic-associated diarrhea; NA, not applicable; NNTB, number needed to benefit.
P < .05.
Diarrhea duration in days, median (IQR) for probiotic group (5 [3-7]) and placebo group (4 [3-7]).
Including readmission to hospital owing to reasons other than diarrhea (5 in probiotic group; 4 in placebo group), rash (2 in probiotic group; 3 in placebo group), vomiting (3 in probiotic group; 1 in placebo group), gag reflex (2 in probiotic group), abdominal pain (3 in probiotic group; 2 in placebo group), trace of blood in the stool (1 in probiotic group).
To investigate whether the country-related differences might have had an effect on the results, we performed a subgroup analysis. The effect sizes for AAD, diarrhea, and diarrhea duration were similar in Poland and in the Netherlands, and only small differences were observed in the effect sizes for mild AAD and severe AAD outcomes between the countries. None of these differences between groups were statistically significant (eTable 5 in Supplement 2).
To examine which subgroup(s) of patients contributed to the difference between the effect sizes for AAD and diarrhea outcomes, we performed sensitivity analyses with modified outcomes: (1) patients with AAD combined with the patients with diarrhea who did not provide a stool sample, (2) infectious diarrhea with the exclusion of C difficile diarrhea, and (3) infectious diarrhea caused by specific pathogens (eTable 6 in Supplement 2). For all of these outcomes, the effect size was larger than that for the AAD outcome, especially for rotaviral diarrhea (RR, 0.11; 95% CI, 0.02-0.65; NNTB = 19; 95% CI, 10-63; P = .01). In the sensitivity analysis with plausible assumptions about missing data, the effect size for the diarrhea outcome was either no longer significant, of borderline significance, or statistically significant depending on the assumed risk of diarrhea among patients lost to follow-up (eTable 6 in Supplement 2). In the logistic regression, AAD was associated with younger age and diarrhea was associated with allocation to the placebo group, younger age, and use of amoxicillin with clavulanic acid (eTable 7 in Supplement 2).
Discussion
In this RCT, a multispecies probiotic did not significantly reduce the risk of AAD when analyzed according to the most stringent definition. However, the participants in the probiotic group had a significantly lower overall risk of diarrhea during the antibiotic treatment and 7 days after when the groups were analyzed regardless of diarrhea etiology. The studied probiotic did not demonstrate a beneficial effect on most other secondary outcomes, with the exception of the need for intravenous rehydration due to diarrhea, which was less common in the probiotic group. In the per-protocol analysis, the results were similar to those in the ITT analysis. Our results did not change after an adjustment for potential AAD risk factors.
It remains unclear why the studied probiotic had no significant effect on the AAD outcome, despite its beneficial effect in the prevention of diarrhea when analyzed regardless of the etiology. One could speculate that a trial involving a larger group might have shown significant results for the primary outcome. Nevertheless, considering the satisfactory incidence of AAD in the placebo group, our study was adequately powered to detect a clinically significant difference in this outcome and even more than adequately powered for assessing the diarrhea outcome. In the sensitivity analyses, we investigated which subgroup(s) of patients contributed to this difference in outcome effect sizes to the highest extent. We found that the effect was highest for viral gastroenteritis, especially caused by rotavirus. Another significant result, ie, the number of children requiring intravenous rehydration due to diarrhea, was also related to this finding, as all of these patients received intravenous fluids owing to rotavirus infection. There is evidence supporting a role of the microbiota in rotavirus infection,25,26 as well as for a preventive effect of certain probiotics.27 One could speculate that our study detected a similar effect of the studied probiotic on diarrhea caused by rotavirus. However, caution is needed when interpreting this finding, as this trial was not designed to answer this specific research question. Moreover, since the participants were not tested for the presence of diarrheal pathogens at baseline, some of them might have already been within the incubation period of infectious diarrhea on hospital admission.
In our study, we used a rather stringent definition of AAD, which allowed us to differentiate between clinically relevant conditions and clinically unimportant changes in the consistency of stools. It also considered the most common etiology of diarrhea related to antibiotic administration and assumed that common nosocomial infections, such as norovirus or rotavirus gastroenteritis,28,29 are not directly associated with antibiotic treatment. However, the definitions of AAD in published studies vary, and in many studies it was similar to the definition of diarrhea, as applied in current study. To illustrate, a 2020 review found that microbiological tests were not performed to identify AAD outcomes in 28 of 33 previous studies on probiotic supplementation during antibiotic treatment in children.5 While this approach may pose a question as to whether the researchers really measured AAD or rather diarrhea during antibiotic treatment regardless of the etiology, it also represents a much more pragmatic point of view. Etiology testing is not routinely recommended for cases of acute diarrhea in children,30 and for both the patient and the physician, what caused the diarrhea may not be relevant as long as the preventive intervention is effective.
Why the effect sizes in the ITT analysis were similar to those observed in the per-protocol analysis is unclear. This finding may reflect misclassification of compliance data, as it was collected only by indirect methods, ie, study diaries and counting of unused sachets. Another possible explanation is that the studied probiotic is effective even if not taken regularly. Additionally, participants deemed as overall noncompliant might have been compliant during a specific time period crucial for diarrhea, eg, during the first days of antibiotic therapy.
Strengths and Limitations
Our study had a number of strengths. To our knowledge, this is the largest trial investigating the effect of a probiotic containing more than 3 species of microorganisms on the incidence of AAD in children. The number of participants is almost 3 times higher than that in the second largest study of which we are aware.11 It was designed with an intent to answer an unambiguous research question with a choice of clearly predefined outcomes. The study was conducted in settings of international cooperation, which enabled verification of the collected data by comparison between the different populations and recruitment centers. However, there are also some limitations. Loss to follow-up was relatively high, which is reflected by the range of uncertainty demonstrated in analyses with plausible assumptions about missing data. To search for indications of imbalances between the trial arms owing to selective missing data,31 we investigated the number and characteristics of participants lost to follow-up in both arms. We found them to be comparable with each other, as well as with the rest of the study participants. We also compared the outcome data between the Polish and the Dutch participants, who differed greatly in terms of loss to follow-up, and we found mostly similar effect sizes. We assume that the missing data were unlikely to have introduced a significant bias to our study; nevertheless, no method of testing can rule out such a possibility completely.32 As mentioned, there was a puzzling difference between loss to follow-up in Poland and in the Netherlands. All but 4 of the participants were recruited and followed-up by 3 researchers (J.Ł., T.D., and T.d.M.) who were in a regular contact with each other to standardize the study conduct. Therefore, this difference may be explained by country-specific attitudes of patients and overlooked differences in the researchers’ practice. Another study limitation is a potential misclassification between the AAD and diarrhea outcomes, owing to the limited diagnostic accuracy of immunoassay tests,33 the limited number of diarrheal pathogens tested, and the number of patients who failed to provide stool samples. Additionally, the limited study follow-up duration might have led to an omission of some diarrhea cases occurring later than a week after antibiotic cessation.7
Conclusions
The multispecies probiotic used in this trial did not reduce the risk of AAD when analyzed according to the most stringent definition. However, we found a beneficial effect of the formulation on the overall risk of diarrhea during and 7 days after antibiotic therapy (NNTB = 9). The latter outcome corresponds well with the standard approach to AAD in clinical practice. Therefore, the use of the studied probiotic may be considered for diarrhea prevention during antibiotic treatment in children. Our study also shows that the AAD outcome definition has a significant effect on clinical trial results and their interpretation.
Protocol for Bioethics Committee Netherlands
eTable 1. Recruitment centres
eTable 2. Patient characteristics depending on the country of recruitment
eTable 3. Characteristics of patients lost to follow-up
eTable 4. Results of the per protocol analysis including 119 patients in probiotic group and 110 patients in placebo group
eTable 5. Available case analysis by the country of recruitment
eTable 6. Sensitivity analyses
eTable 7. Results of logistic regression analysis
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic-associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37(1):22-26. doi: 10.1097/00005176-200307000-00004 [DOI] [PubMed] [Google Scholar]
- 2.Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4(4):CD004827. doi: 10.1002/14651858.CD004827.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szajewska H, Canani RB, Guarino A, et al. ; ESPGHAN Working Group for ProbioticsPrebiotics . Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62(3):495-506. doi: 10.1097/MPG.0000000000001081 [DOI] [PubMed] [Google Scholar]
- 4.Liao W, Chen C, Wen T, Zhao Q. Probiotics for the prevention of antibiotic-associated diarrhea in adults: a meta-analysis of randomized placebo-controlled trials. J Clin Gastroenterol. 2021;55(6):469-480. doi: 10.1097/MCG.0000000000001464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Łukasik J, Guo Q, Boulos L, Szajewska H, Johnston BC. Probiotics for the prevention of antibiotic-associated adverse events in children-a scoping review to inform development of a core outcome set. PLoS One. 2020;15(5):e0228824. doi: 10.1371/journal.pone.0228824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42(3):1203-1206. doi: 10.1128/JCM.42.3.1203-1206.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3(5):563-578. doi: 10.2217/17460913.3.5.563 [DOI] [PubMed] [Google Scholar]
- 8.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506-514. doi: 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 9.Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23(1):79-84. [PMC free article] [PubMed] [Google Scholar]
- 10.Saneeyan H, Layegh S, Rahimi H. Effectiveness of probiotic on treatment of Helicobacter pylori infection in children. Majallah-i Danishkadah-i Pizishki-i Isfahan. 2011;146(29):882-889. [Google Scholar]
- 11.Merenstein DJ, Foster J, D’Amico F. A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: the Measuring the Influence of Kefir (MILK) study. Arch Pediatr Adolesc Med. 2009;163(8):750-754. doi: 10.1001/archpediatrics.2009.119 [DOI] [PubMed] [Google Scholar]
- 12.Conway S, Hart A, Clark A, Harvey I. Does eating yogurt prevent antibiotic-associated diarrhoea? a placebo-controlled randomised controlled trial in general practice. Br J Gen Pract. 2007;57(545):953-959. doi: 10.3399/096016407782604811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharnai S, Nirmala P, Ramanathan R, Vanitha S. Comparative study of efficacy and safety of azithromycin alone and in combination with probiotic in the treatment of impetigo in children. Int J Curr Pharm Res. 2017;9(6):52-55. doi: 10.22159/ijcpr.2017v9i6.23429 [DOI] [Google Scholar]
- 14.Zakordonets L, Tolstanova G, Yankovskiy D, Dyment H, Kramarev S. Different regimes of multiprobiotic for prevention of immediate and delayed side effects of antibiotic therapy In children. Res J Pharm Biol Chem Sci. 2016;7(3):2194-2201. [Google Scholar]
- 15.Koning CJ, Jonkers DM, Stobberingh EE, Mulder L, Rombouts FM, Stockbrügger RW. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am J Gastroenterol. 2008;103(1):178-189. doi: 10.1111/j.1572-0241.2007.01547.x [DOI] [PubMed] [Google Scholar]
- 16.Koning CJ, Jonkers D, Smidt H, et al. The effect of a multispecies probiotic on the composition of the faecal microbiota and bowel habits in chronic obstructive pulmonary disease patients treated with antibiotics. Br J Nutr. 2010;103(10):1452-1460. doi: 10.1017/S0007114509993497 [DOI] [PubMed] [Google Scholar]
- 17.Łukasik J, Szajewska H. Effect of a multispecies probiotic on reducing the incidence of antibiotic-associated diarrhoea in children: a protocol for a randomised controlled trial. BMJ Open. 2018;8(5):e021214. doi: 10.1136/bmjopen-2017-021214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanma A, Puttemans K, Deneyer M, Benninga MA, Vandenplas Y. Amsterdam infant stool scale is more useful for assessing children who have not been toilet trained than Bristol Stool Scale. Acta Paediatr. 2014;103(2):e91-e92. doi: 10.1111/apa.12422 [DOI] [PubMed] [Google Scholar]
- 20.Lewis SJ, Heaton KW. Stool Form Scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920-924. doi: 10.3109/00365529709011203 [DOI] [PubMed] [Google Scholar]
- 21.Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008;28(1):154-161. doi: 10.1111/j.1365-2036.2008.03714.x [DOI] [PubMed] [Google Scholar]
- 22.Kotowska M, Albrecht P, Szajewska H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2005;21(5):583-590. doi: 10.1111/j.1365-2036.2005.02356.x [DOI] [PubMed] [Google Scholar]
- 23.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akl EA, Briel M, You JJ, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344:e2809. doi: 10.1136/bmj.e2809 [DOI] [PubMed] [Google Scholar]
- 25.Gozalbo-Rovira R, Rubio-Del-Campo A, Santiso-Bellón C, et al. Interaction of intestinal bacteria with human rotavirus during infection in children. Int J Mol Sci. 2021;22(3):1010. doi: 10.3390/ijms22031010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis. 2014;210(2):171-182. doi: 10.1093/infdis/jiu037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hojsak I, Szajewska H, Canani RB, et al. ; ESPGHAN Working Group for Probiotics/Prebiotics . Probiotics for the prevention of nosocomial diarrhea in children. J Pediatr Gastroenterol Nutr. 2018;66(1):3-9. doi: 10.1097/MPG.0000000000001637 [DOI] [PubMed] [Google Scholar]
- 28.Załęski A, Banasiuk M, Karpierz K, Kuchar E, Podsiadły E. The clinical course of gastroenteritis due to nosocomial and community acquired norovirus infections in immunocompromised and immunocompetent children—single center experience. Przegl Epidemiol. 2020;74(1):23-31. [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis. 2012;12:62. doi: 10.1186/1471-2334-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-152. doi: 10.1097/MPG.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 31.Groenwold RH, Moons KG, Vandenbroucke JP. Randomized trials with missing outcome data: how to analyze and what to report. CMAJ. 2014;186(15):1153-1157. doi: 10.1503/cmaj.131353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. doi: 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby A, Iturriza-Gómara M. Norovirus diagnostics: options, applications and interpretations. Expert Rev Anti Infect Ther. 2012;10(4):423-433. doi: 10.1586/eri.12.21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for Bioethics Committee Netherlands
eTable 1. Recruitment centres
eTable 2. Patient characteristics depending on the country of recruitment
eTable 3. Characteristics of patients lost to follow-up
eTable 4. Results of the per protocol analysis including 119 patients in probiotic group and 110 patients in placebo group
eTable 5. Available case analysis by the country of recruitment
eTable 6. Sensitivity analyses
eTable 7. Results of logistic regression analysis
Nonauthor Collaborators
Data Sharing Statement