Abstract
Background
Soluble urokinase-type plasminogen activator receptor (suPAR), a marker of immune activation, was shown to be associated with outcomes and kidney disease among various patient populations. The prognostic role of circulating suPAR levels in patients with chronic kidney disease (CKD) needs to be investigated in a cohort with large sample size of renal diseases.
Methods
We measured serum suPAR concentration in 2391 CKD patients in the multicenter Chinese Cohort Study of Chronic Kidney Disease, and investigated the association of serum suPAR with the prespecified endpoint event, end-stage renal disease (ESRD), using Cox proportional hazards regression model.
Results
Altogether, 407 ESRD events occurred during the median follow-up of 54.8 (interquartile range: 47.5–62.2) months. The higher levels of serum suPAR were independently associated with increased risk of incident ESRD after adjusting for potential confounders including the baseline estimated glomerular filtration rate categories, with the hazard ratios (HRs) of 1.53 [95% confidence intervals (CIs) 1.10–2.12] for the top tertile (≥3904 pg/mL) compared with the bottom tertile (<2532 pg/mL). When stratified by the etiologies of CKD, among patients with glomerulonephritis (GN), serum suPAR levels were also independently associated with the higher risk of ESRD, with an HR of 1.61 (95% CI 1.03–2.53) in the top tertile compared with the bottom tertile.
Conclusions
Circulating suPAR level was independently associated with an increased risk of progression to ESRD in Chinese CKD patients, especially in those with an etiology of GN.
Keywords: chronic kidney disease, ESRD, outcomes, prospective study, suPAR
INTRODUCTION
Chronic kidney disease (CKD) is one of the leading public health problems in China. A recent national survey demonstrated that the prevalence of CKD reached 10.8% [1]. CKD is strongly associated with an increased risk of progression to end-stage renal disease (ESRD), cardiovascular diseases (CVD) and mortality [2, 3]; and the outcomes of CKD patients are highly heterogeneous. It is thus particularly important to identify high-risk patients with CKD. However, ideal biomarkers for assessing the outcomes of CKD patients are still lacking.
suPAR is a soluble form of urokinase-type plasminogen activator receptor (uPAR), derived from the proteolytic cleavage of uPAR at its glycosylphosphatidylinositol (GPI) anchor site [4]. Previous studies found that elevated suPAR levels were prognostic in a variety of populations including those with infectious diseases, cancer, type 1 diabetes, arthritis and CVD [5–10]. Recently, suPAR levels were shown to be a predictor for incident CKD and were also known to predict cardiovascular outcomes in mild-to-moderate CKD patients, as well as in patients with ESRD [11–13]. Whether high suPAR levels are associated with incident ESRD is not yet fully clear, as previous studies only examined estimated glomerular filtration rate (eGFR) decline as the primary outcomes [11, 14, 15]. We sought to investigate the association of circulating suPAR with incident ESRD in a large, multicenter prospective cohort of Chinese patients with CKD, the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE).
MATERIALS AND METHODS
Participants
The C-STRIDE is a multicenter prospective cohort of CKD, comprising 39 clinical centers in different geographic regions of China. The design of C-STRIDE has been described in detail elsewhere [16]. In brief, participants met the following criteria to be eligible for enrollment: (i) aged between 18 and 74 years and (ii) specified the eGFR range according to different CKD etiologies. For patients with glomerulonephritis (GN), the eGFR should be ≥15 mL/min/1.73 m2. For patients with diabetic kidney disease (DKD), the defining eligibility was 15 mL/min/1.73 m2 ≤eGFR <60 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 with ‘nephrotic range’ proteinuria, which is defined as 24-h urinary protein ≥3.5 g or urinary albumin creatinine ratio (uACR) ≥2000 mg/g or corresponding values of urine dipstick test or urinary protein creatinine ratio. For non-GN and non-DKD patients, 15 mL/min/1.73 m2 ≤eGFR <60 mL/min/1.73 m2 is the range for enrollment. The type of chronic GN included minimal change disease, membranous nephropathy, focal segmental glomerulosclerosis, membranoproliferative GN, IgA nephropathy, Henoch–Schönlein purpura nephritis and others without renal biopsy. The initial design was to recruit 3000 outpatients with CKD. Considering the loss to follow-up, eventually 3499 outpatients were enrolled from November 2011 to 30 June 2016. Among them, 686 were excluded due to missing values of serum creatinine and/or loss of follow-up data. Altogether, 2813 patients have the completed baseline and follow-up data. Due to the availability of the biosamples for measuring serum suPAR, 2391 patients were included in the present study.
Study design
We measured baseline serum suPAR concentration in 2391 CKD patients from C-STRIDE who are not yet on dialysis, and reported the associations of serum suPAR levels with the baseline clinical characteristics of these patients and the prespecified endpoint event of CKD, i.e. ESRD. The median follow-up time for the adverse outcomes was 54.8 [interquartile range (IQR): 47.5–62.2] months. The baseline data included detailed demographics, underlying disease, behavioral habits, medical and medication history, anthropometric measurements [height, weight and resting blood pressure (BP)], serum chemistry indexes [triglyceride, high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), prealbumin, serum creatinine, high-sensitivity C-reactive protein (hsCRP)] and uACR. ESRD events of CKD patients were collected before 30 June 2017. Informed consent was obtained from all patients. The study was approved by the Ethics Committee of Peking University First Hospital and was in adherence with the Declaration of Helsinki.
Detection of serum suPAR by enzyme-linked immunosorbent assay
Fasting venous blood samples were obtained at the study visit. All participants’ blood samples were transported by cold chain to the Central Laboratory of Peking University First Hospital and stored at −80°C until use. Serum suPAR was measured in batches from stored material in the Central Laboratory of Peking University First Hospital from June 2016 to September 2016. Serum suPAR was measured by enzyme-linked immunosorbent assay using a commercial kit (ViroGates, Blokken 45, DK-3460 Birkerød, Denmark) according to the manufacturer’s instructions. In the assay, the suPAR standards, curve control and patient specimens were mixed with peroxidase-conjugated anti-suPAR antibody in the white microwell mixing plate. This solution was then transferred from the white to the optically clear microwell plate, which is precoated with anti-suPAR antibody. During a 1-h incubation period, a sandwich was formed consisting of solid-phase antibody, suPAR and the peroxidase-conjugated antibody. After following a washing step, chromogenic substrate was added to the wells. After 20 min incubation in the dark, the color development was stopped with the addition of sulfuric acid that changes the color in the wells to yellow. The absorbance at 450 nm was measured using a microtiter plate reader. The detection limit of the assay was estimated to be 0.1 ng/mL. The intra-assay coefficient of variation was 3.5% at a mean concentration of 2.3 ng/mL, 1.3% at 3.7 ng/mL and 2.1% at 5.4 ng/mL. The inter-assay coefficient of variation was 5.1% at a mean concentration of 2.3 ng/mL, 2.3% at 3.7 ng/mL and 2.2% at 5.4 ng/mL.
Measurement of covariates
All blood and urine samples were analyzed in the Central Laboratory of Peking University First Hospital to avoid the variation of testing values between laboratories. Serum HDL-C and triglycerides were measured using commercially available reagents. hsCRP was detected by immunoturbidimetric assay following the instructions (Beckman DXC800, USA). Urinary albumin and creatinine were measured from a fresh morning spot urine sample or morning urine sample stored at 4°C for <1 week. Albuminuria was measured with immunoturbidimetric tests. Urinary creatinine was measured with the ammonia iminohydrolase method. The uACR (mg/g creatinine) was calculated. Patients with uACR >30 mg/g were defined as having albuminuria. Serum creatinine was measured using the same methods as urinary creatinine. The eGFR was evaluated by the equation developed by adaptation of the modification of diet in renal disease equation on the basis of data from the Chinese CKD participants: eGFR = 175 × [serum creatinine (in μmol/L)/88.4]−1.234 × age−0.179 × (if female, × 0.79) [17]. All eGFR values of >120 mL/min/1.73 m2 were set at 120 mL/min/1.73 m2.
Body mass index (BMI) was calculated by using the following formula: weight (kg)/height2 (m2). BP was measured three times at 5-min intervals by sphygmomanometer. The mean value of the three readings was calculated unless the difference between the readings was >10 mmHg, in which case the mean value of the two closest measurements was used. Hypertension was defined as a systolic BP ≥140 mmHg and/or a diastolic BP ≥90 mmHg, or a self-reported history of hypertension. Diabetes was defined as fasting plasma glucose of ≥7.0 mmol/L, or the use of hypoglycemic agents or a self-reported history of diabetes.
Study outcomes
ESRD is defined as the initiation of chronic dialysis or renal transplantation or irreversible development of eGFR <15 mL/min/1.73 m2. The ultimate ascertainment of eGFR is based on the value of the central laboratory. The doctors at the clinical center were requested to submit the related clinical data to the Renal Institute of Peking University via email. ESRD events were adjudicated by an independent committee consisting of relevant specialist physicians.
Statistical analysis
All CKD patients were stratified according to tertiles of baseline serum suPAR levels. Continuous variables are presented as the means and standard deviations except for highly skewed variables that are shown as median (IQRs), and categorical variables are described as proportions. We used one-way analysis of variance (ANOVA) to compare continuous variables and Chi-square tests to compare categorical variables. Multivariable Cox proportional hazards regression models were used to assess associations of serum suPAR levels with incident ESRD. Covariates for the models were selected based on prior knowledge about the factors that could be potential confounders of the associations of serum suPAR with ESRD. A series of sequential models were fit to evaluate the effect of adding certain sets of covariates. The potential confounders including age (continuous), gender (male versus female), BMI (continuous), current smoker (yes versus no), clinical characteristics [CVD (yes versus no), hypertension (yes versus no), diabetes (yes versus no)], log-transformed HDL-C (continuous) [18], log-transformed triglyceride (continuous), log-transformed hsCRP (continuous), statin use (yes versus no), log-transformed uACR (continuous) and eGFR categories [<30, 30–44, 45–59, 60–89 versus ≥90 mL/min/1.73 m2 (reference)]. Proportional hazards assumptions were verified by testing the interaction with time using the likelihood ratio test, which yielded nonsignificant P-values. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). P-values for trend were given by treating tertiles of suPAR as a continuous variable. We hypothesized that the effect of serum suPAR might be modified by the etiologies of CKD. The interaction terms were generated between suPAR (continuous variables) and each of the etiologies (DKD versus all others, GN versus all others). A stratified analysis in the prediction of ESRD events was conducted by the etiologies of CKD. Statistical analyses were performed using SAS software (version 9.4, SAS institute, CA, USA). P < 0.05 (two-sided) was considered statistically significant.
RESULTS
Baseline characteristics of the patients
Among the total 2391 CKD patients from C-STRIDE, 1402 (58.64%) were male and 989 (41.36%) were female, with an average age of 48.18 ± 13.78 years at sampling. At the baseline, 752 (31.45%) patients had an eGFR >60 mL/min/1.73 m2; 379 (15.85%), 555 (23.21%) and 705 (29.49%) patients were in CKD Stages 3a, 3b and 4, respectively. The cause of CKD consisted of GN (60.59%), DKD (14.12%) and other renal diseases (25.29%). The serum suPAR levels among patients with DKD, GN and other renal diseases were 4101.53 ± 1651.81, 3101.28 ± 1526.27 and 4011.11 ± 1746.32 pg/mL, respectively (all P-values for ANOVA <0.001).
The serum suPAR level was 3486.34 ± 1677.66 pg/mL in 2391 CKD patients. Baseline demographics and biochemical measurements of CKD patients according to tertiles of baseline serum suPAR levels are summarized in Table 1. Baseline higher suPAR levels were associated with older age, higher BP, higher levels of serum triglyceride, hsCRP and uACR, but lower levels of eGFR.
Table 1.
Baseline participant characteristics by tertiles of serum suPAR
| Characteristics | Total (N = 2391) | Serum suPAR tertiles (pg/mL) |
P-value | ||
|---|---|---|---|---|---|
| <2532 (N = 798) | ≥2532–3904 (N = 797) | ≥3904 (N = 796) | |||
| Age, years | 48.18 ± 13.78 | 42.57 ± 13.17 | 48.77 ± 13.10 | 53.21 ± 12.96 | <0.001 |
| Male, n (%) | 1402 (58.64) | 498 (62.41) | 478 (59.97) | 426 (53.52) | 0.001 |
| BMI, kg/m2 | 24.40 ± 3.61 | 24.30 ± 3.52 | 24.47 ± 3.68 | 24.43 ± 3.64 | 0.64 |
| Systolic BP, mmHg | 130.21 ± 19.37 | 124.69 ± 16.53 | 131.48 ± 18.56 | 134.87 ± 21.50 | <0.001 |
| Diastolic BP, mmHg | 81.31 ± 11.68 | 79.46 ± 10.75 | 82.63 ± 11.89 | 81.96 ± 12.18 | <0.001 |
| Smoking status, n (%) | 877 (37.79) | 283 (36.42) | 288 (37.45) | 306 (39.48) | 0.45 |
| Diabetes, n (%) | 483 (20.22) | 96 (12.03) | 173 (21.73) | 214 (26.92) | <0.001 |
| Hypertension, n (%) | 820 (34.79) | 196 (24.94) | 290 (36.80) | 334 (42.66) | <0.001 |
| CVD, n (%) | 287 (12.21) | 60 (7.64) | 91 (11.59) | 136 (17.41) | <0.001 |
| Triglyceride, mmol/L | 1.27 (2.57–2.57) | 1.65 (1.16–2.39) | 1.81 (1.32–2.62) | 1.85 (1.36–2.71) | <0.001 |
| HDL-C, mmol/L | 0.89 (1.31–1.30) | 1.14 (0.94–1.38) | 1.04 (0.89–1.25) | 1.04 (0.86–1.29) | <0.001 |
| Statins use, n (%) | 432 (18.07) | 134 (16.79) | 123 (15.43) | 175 (21.98) | 0.002 |
| FBG, mmol/L | 4.38 (5.55–5.56) | 4.84 (4.38–5.34) | 4.89 (4.4–5.61) | 5.00 (4.39–5.77) | <0.001 |
| Prealbumin, g/L | 325.87 ± 83.63 | 314.01 ± 77.74 | 329.49 ± 83.73 | 333.86 ± 87.84 | <0.001 |
| hsCRP, mg/L | 0.50 (3.00–2.30) | 1.00 (0.37–2.50) | 1.34 (0.61–3.10) | 1.61 (0.61–3.50) | <0.001 |
| uACR, mg/g | 140.75 (1055.08–993.36) | 379.49 (113.77–827.39) | 531.38 (174.30–1090.27) | 572.13 (153.70–1275.42) | <0.001 |
| Creatinine, μmol/L | 170.81 ± 122.71 | 119.82 ± 63.20 | 175.41 ± 130.39 | 217.34 ± 139.43 | <0.001 |
| eGFR, mL/min/1.73 m2 | 50.89 ± 29.89 | 71.10 ± 30.31 | 47.02 ± 25.76 | 34.50 ± 20.16 | <0.001 |
| eGFR group, mL/min/1.73 m2 | <0.001 | ||||
| ≥90 | 327 (13.68) | 235 (29.45) | 71 (8.91) | 21 (2.64) | |
| 60–89 | 425 (17.77) | 249 (31.20) | 121 (15.18) | 55 (6.91) | |
| 45–59 | 379 (15.85) | 123 (15.41) | 151 (18.95) | 105 (13.19) | |
| 30–44 | 555 (23.21) | 112 (14.04) | 233 (29.23) | 210 (26.38) | |
| 15–29 | 705 (29.49) | 79 (9.90) | 221 (27.73) | 405 (50.88) | |
Missing counts: BMI: 222; systolic BP: 324; diastolic BP: 324; smoking status: 70; diabetes: 2; hypertension: 34; CVD: 40; triglyceride: 49; HDL-C: 64; FBG: 80; prealbumin: 126; hsCRP: 390; uACR: 124.
The incidence rates of ESRD events according to levels of serum suPAR
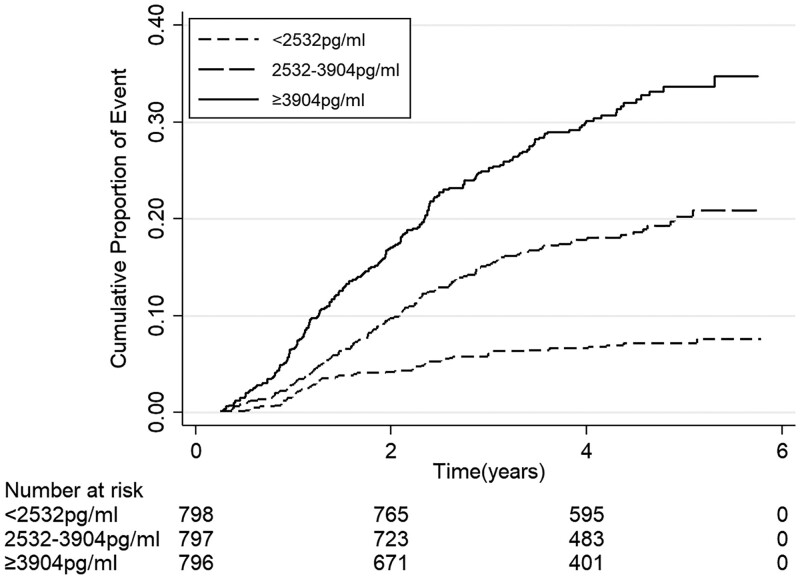
The incidence rates of ESRD events according to tertiles of serum suPAR levels are shown in Table 2. During the median follow-up of 54.8 (IQR: 47.5–62.2) months, 407 ESRD events occurred. ESRD rates were 4.17 per 100 person-years. Higher incidence rates of ESRD events were seen with the increased levels of serum suPAR (Figure 1; P for log-rank test < 0.001).
Table 2.
Relationship between serum suPAR levels and ESRD events rates
| Serum suPAR tertiles (pg/mL) | Number of events (%) | Events per 100 person-years | P for log-rank |
|---|---|---|---|
| ESRD events | <0.001 | ||
| <2532 (N = 798) | 55 (6.89) | 1.55 | |
| ≥2532–3904 (N = 797) | 137 (17.19) | 4.25 | |
| ≥3904 (N = 796) | 215 (27.01) | 7.20 | |
| Total | 407(17.02) | 4.17 |
FIGURE 1.
Kaplan–Meier curve for ESRD events according to tertiles of serum suPAR.
Associations between serum suPAR levels and ESRD
The associations between serum suPAR and ESRD are shown in Table 3. After the adjustment for demographic and traditional CVD risk factors, as well as the baseline eGFR categories, baseline higher serum suPAR levels were independently associated with the occurrence of ESRD, with an HR of 1.30 (95% CI 0.93–1.80) in the middle tertile compared with the bottom tertile and 1.53 (95% CI 1.10–2.12) in the top tertile compared with the bottom tertile (P for trend = 0.009).
Table 3.
Association of serum suPAR with ESRD events among total CKD patients and stratified by etiologies of CKD
| Serum suPAR tertiles (pg/mL) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Total CKD patients (N = 2391) | |||
| <2532 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥2532–3904 | 2.80 (2.04–3.84) | 2.13 (1.54–2.95) | 1.30 (0.93–1.80) |
| ≥3904 | 4.96 (3.65–6.75) | 3.35 (2.44–4.61) | 1.53 (1.10–2.12) |
| P for trend | <0.001 | <0.001 | 0.009 |
| Diabetic kidney disease (N = 330) | |||
| <2532 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥2532–3904 | 2.05 (0.91–4.64) | 1.66 (0.71–3.88) | 1.35 (0.58–3.17) |
| ≥3904 | 2.94 (1.34–6.49) | 2.22 (0.98–5.07) | 1.56 (0.67–3.62) |
| P for trend | 0.003 | 0.03 | 0.29 |
| GN (N = 1416) | |||
| <2532 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥2532–3904 | 3.25 (2.11–5.00) | 2.26 (1.45–3.53) | 1.22 (0.77–1.91) |
| ≥3904 | 7.12 (4.68–10.85) | 4.24 (2.74–6.59) | 1.61 (1.03–2.53) |
| P for trend | <0.001 | <0.001 | 0.02 |
| Other types (N = 591) | |||
| <2532 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥2532–3904 | 1.66 (0.89–3.11) | 1.41 (0.74–2.69) | 1.18 (0.62–2.28) |
| ≥3904 | 2.59 (1.44–4.66) | 1.93 (1.05–3.55) | 1.40 (0.75–2.60) |
| P for trend | <0.001 | 0.02 | 0.25 |
There are 54 cases missing their information of CKD etiology. Model 1: adjusted for age, gender. Model 2: Model 1 + current smoker, BMI, diabetes, hypertension, CVD history, log-transformed triglyceride, log-transformed HDL-C, statin use or not, prealbumin, log-transformed high-sensitivity C-reactive protein and log-transformed urinary albumin/creatinine ratio. Model 3: Model 2 + categories of estimated glomerular filtration rate.
Additionally, we detected significant interaction between suPAR and the dummy variable for GN in Models 1 and 2 (P for interaction = 0.007 and 0.019, respectively); however, the interaction failed to reach statistical significance in Model 3 (P for interaction > 0.05). We further analyzed the association of serum suPAR and incident ESRD stratified by the etiologies of CKD. Among patients with GN, the association between serum suPAR levels and occurrence of ESRD was greatly attenuated, while still significant after the adjustment of eGFR categories, with an HR of 1.22 (95% CI 0.77–1.91) in the middle tertile compared with the bottom tertile and 1.61 (95% CI 1.03–2.53) in the top tertile compared with the bottom tertile (P for trend = 0.02). No significant associations were detected among patients with DKD and other renal diseases.
DISCUSSION
The current study found that baseline higher levels of serum suPAR were associated with an increased risk of incident ESRD in predialysis patients with CKD, which was independent of traditional risk factors of CVD and CKD including uACR and eGFR. Furthermore, the association was stronger among CKD patients with an etiology of GN.
uPAR is a membrane-linked protein expressed in many cell types such as immune cells, vascular endothelial cells [19], tubular epithelial cells and podocytes [20], participating in cell migration, proliferation and survival [4]. suPAR is a soluble form of uPAR, released from proteolytic cleavage of uPAR at its GPI anchor site to body fluids [21]. It presents at low levels in healthy individuals [4], but at high levels under various disease conditions [5–10]. It was found that circulating suPAR participates in the development of focal segmental glomerulosclerosis [20, 22]. Circulating suPAR was also found to be associated with the new-onset cardiovascular events in CKD patients [12, 13] and a decline in the eGFR of CVD patients [11]. However, the prognostic value of circulating suPAR levels in adult patients with CKD needs to be verified in a cohort with large sample size and various etiologies of renal diseases.
To the best of our knowledge, there was only one study investigating the association between serum suPAR and ESRD, as well as 50% loss of eGFR in children with CKD [23]. Previous studies have not focused on this particular association of suPAR levels in CKD patients with the endpoint of ESRD [11, 14, 15]. Our study is the first to show that the higher levels of serum suPAR were an independent predictor of incident ESRD in adult patients with CKD, based on a large multicenter prospective cohort with a broad spectrum of eGFR distributions and measurement of urinary protein. The result was, to some extent, in line with the studies by Hayek et al. [11] and Schulz et al. [15], who found that high suPAR levels may predict a decline in renal function in a CVD population and middle-aged healthy participants, respectively. However, urinary protein was not included in the study by Schulz et al. [15] and was only found in a small number of patients in the study by Hayek et al. [11]. Because urinary protein was previously reported to be associated with circulating suPAR level [24, 25], it is a potential confounder. We adjusted uACR in the present study by using ESRD as the endpoint. Furthermore, we extended the previous observations in a Chinese population with CKD [11, 15, 23].
In the subgroup analysis, we do not have a sufficient sample size for the association in DKD and other types of CKD; however, the relatively large sample size promised a significant finding in the subgroup of GN. There is an underlying mechanism of circulating suPAR in proteinuric kidney diseases, which might explain this association. Circulating suPAR has been reported to affect the structure and function of podocytes by binding to and activating podocyte αvβ3 integrin on the podocyte membrane [20, 22, 26], which results in foot process migration and apoptosis. Moreover, the functional association of suPAR and αvβ3 integrin may be modified and modulated by sphingomyelinase-like phosphodiesterase 3b [27] and CD40 autoantibodies [28]. More recently, apolipoprotein L1 protein variants G1 and G2 were identified to synergize with suPAR in the activation of αvβ3 integrin on podocytes [29]. Additionally, the role of circulating suPAR in different forms of kidney disease might involve its different isoforms and potentially both its proteolytic and signaling functions [14, 19, 20, 26, 27] by different pathophysiological mechanisms, which needs further investigation in humans.
There are some limitations of this study. First, although most well-established risk factors of CKD progression were included in our multivariable regression models, the possibility of residual confounding still exists. Second, this study cannot distinguish whether circulating suPAR is causally related to incident ESRD of CKD patients.
In conclusion, circulating suPAR can independently predict the onset of ESRD in predialysis CKD patients. Circulating suPAR added valuable prognostic information to well-established clinical and biochemical prognostic markers in this setting, and could be used for improving risk stratification of patients with CKD. Future studies are warranted to see if CKD patients with an elevated suPAR level are benefiting from early renal therapies and interventions.
FUNDING
This study was supported by two grants from the National Key Research and Development Program (No. 2016YFC1305405 and 2011BAI10B01), two grants from the National Natural Science Fund (No. 81425008 and 81621092) and a grant by Peking University Health Science Center (No. BMU2017CJ002), a grant by the University of Michigan Health System and Peking University Health Sciences Center Joint Institute for Translational and Clinical Research.
AUTHORS’ CONTRIBUTIONS
M.H.Z., L.X.Z., M.C., Z.C. and F.W. were involved in the research idea and study design; L.W., L.L. and J.W.W. made contributions to the acquisition of data; M.H.Z., L.X.Z., M.C., Z.C., J.W.W., L.W., F.W. and L.L. conducted the analysis and interpretation of data; J.W.W., L.L., K.H., M.C. and L.X.Z. performed statistical analysis; J.W.W., L.X.Z. and M.C. were responsible for supervision or mentorship; L.L. participated in drafting of the manuscript; J.W.W., L.X.Z., C.L.W., S.S.H., J.R., K.H. and M.C. performed revisions of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
J.R. is a cofounder and shareholder of TRISAQ, a bio-pharmaceutical company that develops suPAR-related products. The other authors have no conflicts of interest to declare.
Contributor Information
Li Lv, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China; The First Affiliated Hospital of Baotou Medical College, Baotou, China.
Fang Wang, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
Liang Wu, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
Jin-Wei Wang, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
Zhao Cui, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
Salim S Hayek, Department of Medicine, Rush University Medical Center, Chicago, IL, USA.
Changli Wei, Department of Medicine, Rush University Medical Center, Chicago, IL, USA.
Jochen Reiser, Department of Medicine, Rush University Medical Center, Chicago, IL, USA.
Kevin He, Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Luxia Zhang, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China; Center for Data Science in Health and Medicine, Peking University, Beijing, China.
Min Chen, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
Ming-Hui Zhao, Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China; Institute of Nephrology, Peking University, Beijing, China; Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China.
REFERENCES
- 1. Zhang L, Wang F, Wang L et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822 [DOI] [PubMed] [Google Scholar]
- 2. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 3. van der Velde M, Matsushita K, Coresh J et al. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79: 1341–1352 [DOI] [PubMed] [Google Scholar]
- 4. Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3: 932–943 [DOI] [PubMed] [Google Scholar]
- 5. Florquin S, van den Berg JG, Olszyna DP et al. Release of urokinase plasminogen activator receptor during urosepsis and endotoxemia. Kidney Int 2001; 59: 2054–2061 [DOI] [PubMed] [Google Scholar]
- 6. Outinen TK, Tervo L, Mäkelä S et al. Plasma levels of soluble urokinase-type plasminogen activator receptor associate with the clinical severity of acute Puumala hantavirus infection. PLoS One 2013; 8: e71335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theilade S, Lyngbaek S, Hansen TW et al. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J Intern Med 2015; 277: 362–371 [DOI] [PubMed] [Google Scholar]
- 8. Slot O, Brunner N, Locht H et al. Soluble urokinase plasminogen activator receptor in plasma of patients with inflammatory rheumatic disorders: increased concentrations in rheumatoid arthritis. Ann Rheum Dis 1999; 58: 488–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyngbæk S, Marott JL, Møller DV et al. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol 2012; 110: 1756–1763 [DOI] [PubMed] [Google Scholar]
- 10. Lyngbaek S, Andersson C, Marott JL et al. Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin Chem 2013; 59: 1621–1629 [DOI] [PubMed] [Google Scholar]
- 11. Hayek SS, Sever S, Ko YA et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2015; 373: 1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meijers B, Poesen R, Claes K et al. Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int 2015; 87: 210–216 [DOI] [PubMed] [Google Scholar]
- 13. Drechsler C, Hayek SS, Wei C et al. Soluble urokinase plasminogen activator receptor and outcomes in patients with diabetes on hemodialysis. CJASN 2017; 12: 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayek SS, Ko YA, Awad M et al. Cardiovascular disease biomarkers and suPAR in predicting decline in renal function: a prospective Cohort Study. Kidney Int Rep 2017; 2: 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulz CA, Persson M, Christensson A et al. Soluble urokinase-type plasminogen activator receptor (suPAR) and impaired kidney function in the population-based Malmö Diet and Cancer Study. Kidney Int Rep 2017; 2: 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao B, Zhang L, Wang H et al. Chinese cohort study of chronic kidney disease: design and methods. Chin Med J (Engl ) 2014; 127: 2180–2185 [PubMed] [Google Scholar]
- 17. Ma YC, Zuo L, Chen JH et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944 [DOI] [PubMed] [Google Scholar]
- 18. Myers GL, Cooper GR, Winn CL et al. The Centers for Disease Control-National Heart, Lung and Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med 1989; 9: 105–135 [PubMed] [Google Scholar]
- 19. Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers 2009; 27: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei C, El Hindi S, Li J et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 2011; 17: 952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodges GW, Bang CN, Wachtell K et al. SuPAR: a new biomarker for cardiovascular disease? Can J Cardiol 2015; 31: 1293–1302 [DOI] [PubMed] [Google Scholar]
- 22. Wei C, Trachtman H, Li J et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 2012; 23: 2051–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaefer F, Trachtman H, Wühl E et al. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr 2017; 171: e172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spinale JM, Mariani LH, Kapoor S et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int 2015; 87: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Liu L, Huang J et al. Plasma soluble urokinase receptor level is correlated with podocytes damage in patients with IgA nephropathy. PLoS One 2015; 10: e0132869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dande RR, Peev V, Altintas MM et al. Soluble urokinase receptor and the kidney response in diabetes mellitus. J Diabetes Res 2017; 2017: 3232848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo TH, Pedigo CE, Guzman J et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol 2015; 26: 133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delville M, Sigdel TK, Wei C et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 2014; 6: 256ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayek SS, Koh KH, Grams ME et al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 2017; 23: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]