Abstract
Introduction:
Tenofovir alafenamide (TAF)-containing fixed-dose drug combinations (FDCs) are increasingly being used in managing pregnant women living with HIV. However, TAF is not currently recommended during pregnancy due to limited pharmacokinetic and safety data. TAF, a newer nucleotide phosphonamidate prodrug of tenofovir (TFV), achieves high levels of tenofovir-diphosphate in lymphoid cells and hepatocytes, and 90% lower systemic concentrations of TFV compared to tenofovir disoproxil fumarate (TDF), thereby maximizing TAF’s antiviral efficacy, potency and clinical safety.
Areas covered:
This review discusses the currently available information on the pharmacology of TAF in pregnant women living with HIV. Pharmacokinetic studies with TAF during pregnancy have yielded varying results compared to postpartum, but TAF exposures during pregnancy have been within the range of those typically observed in non-pregnant adults. The efficacy and safety of TAF in treatment-naïve pregnant women living with HIV is currently being evaluated in the VESTED study, a phase-III NIH randomized clinical trial.
Expert opinion:
Initial pregnancy data suggest that TAF-based FDCs have high efficacy and low risk of adverse effects during pregnancy. TAF is likely to become part of first-line regimens for use in pregnant women living with HIV once additional pregnancy data from phase III trials are available.
Keywords: Tenofovir Alafenamide (TAF), pregnancy, postpartum, Human Immunodeficiency Virus (HIV)
1. Introduction
The use of antiretroviral (ARV) medications in pregnant women living with human immunodeficiency virus (HIV) continues to be of critical public health importance [1,2]. Without treatment, about 15–40% of pregnant women living with HIV are at risk of transmitting the virus to their fetuses [3]. The 1994 landmark Pediatric AIDS Clinical Trials Group (PACTG) 076 study, the first study of antiretroviral safety and efficacy in pregnancy, showed that the administration of zidovudine (ZDV) to pregnant women living with HIV and to their neonates after birth, decreased the risk of perinatal HIV transmission by 68% (from 25.5% to 8.3%) [4]. Since 1994, combination ARV therapies with multiple potent HIV drugs have proven to be more effective than ZDV alone at preventing perinatal HIV transmission, changing the trajectory for the treatment of HIV during pregnancy and reducing perinatal transmission rates to negligible numbers, while improving maternal health immensely.
The World Health Organization (WHO) [5], United States Department of Health and Human Services (DHHS) [1] and the European AIDS Clinical Society (EACS) [6] HIV perinatal guidelines all recommend that pregnant women living with HIV receive a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with a third ARV from another class. Currently recommended regimens for most pregnant women include a two NRTI backbone of abacavir/lamivudine, tenofovir disoproxil fumarate (TDF)/lamivudine or TDF/emtricitabine, plus an integrase strand inhibitor (raltegravir or dolutegravir) or a boosted protease inhibitor (darunavir/ritonavir or atazanavir/ritonavir) [1]. Although TDF is commonly included in the NRTI backbone of ARV regimens currently used in pregnant women living with HIV [7], its use was associated with a higher risk of adverse pregnancy outcomes in comparison to ZDV-based regimens [8], though other studies and systematic reviews have deemed its use as safe [9,10]. TDF is associated with declines in renal function and greater bone density loss in non-pregnant adults in comparison to tenofovir alafenamide (TAF), especially when coadministered with ritonavir or cobicistat [11]. Despite these advantages, TAF is not currently recommended during pregnancy due to limited pharmacokinetic (PK) and safety data.
TAF is a newer TFV prodrug that is increasingly being used by women living with HIV across the United States and Europe [12-14]. TAF achieves high levels of tenofovir-diphosphate (TFV-DP) in lymphoid cells and hepatocytes, and ~90% lower systemic concentrations of TFV compared to TDF [15]. The ability of TAF to selectively concentrate in target cells and its greater affinity and distribution into lymphoid tissue maximizes its antiviral efficacy, potency and clinical safety [16]. These pharmacologic properties are critical, as the lower plasma concentrations of TFV from TAF are associated with a reduced risk of decline in glomerular filtration rate, renal tubular toxicity, and decreased bone mineral density with prolonged use compared to TDF [14,17].
Fixed dose combinations (FDCs) of ARVs provide single pill once daily dosing regimens that improve HIV management due to their convenience, enhanced safety profile and reduced cost, leading to improved adherence and reduced risk of HIV drug resistance and transmission [18-20]. Since FDCs that include TAF are increasingly being used in pregnancy to treat HIV infection and prevent perinatal transmission, there needs to be a clear understanding of its pharmacology, safety and efficacy in this population. The aim of this article is to provide a review of the currently available information on the pharmacology, clinical efficacy, and safety of TAF containing ART combinations during pregnancy.
2. TAF fixed dose combinations
Tenofovir must be administered as a prodrug to facilitate its absorption through the gastrointestinal tract. Tenofovir was first licensed as the prodrug TDF in 2001, and the newer prodrug TAF became available in 2016. TAF is more stable in the plasma compared to TDF, and is administered at approximately one-tenth the TDF dose, resulting in lower systemic TFV exposures [15]. TAF may be administered at a strength of 10 mg in combination with cobicistat for boosting or 25 mg either unboosted or with cobicistat or ritonavir boosting. TAF is available co-formulated in fixed dose combinations with several other ARV medications – Table 1. The once daily FDC of RPV/TAF(25 mg)/FTC (Odefsey®, Gilead Sciences) [21] is indicated for treating HIV-1 infections without known resistance mutations to NNRTIs, tenofovir or emtricitabine, and with a baseline viral load ≤ 100,000 copies/mL [21] The tablet must be taken with food. Since lower exposures of rilpivirine were observed in prior PK studies involving pregnant women on a rilpivirine based regimen [22,23], pregnant women on TAF/FTC/RPV should be monitored very closely for viral breakthrough. The BIC/TAF(25 mg)/FTC fixed dose combination (Biktarvy®, Gilead Sciences) [24] is approved for the treatment of adults and adolescents living with HIV-1 (Table 1), but this product is not recommended for use in pregnancy because there are currently no pregnancy PK or safety data. TAF comes co-formulated with the booster cobicistat as DRV/c/TAF(10 mg)/FTC (Symtuza®, Janssen) [25] and EVG/c/TAF(10 mg)/FTC (Genvoya®, Gilead Sciences) [26], but these products are not recommended for use in pregnancy [27-29] due to the failure of cobicistat to adequately boost protease or integrase inhibitors [30,31]. TAF is also available as the 2 drug combination TAF (25 mg)/FTC (Descovy®, Gilead Sciences) and as the single agent TAF (25 mg) (Vemlidy®, Gilead Sciences) for use in combination with additional ARVs.
Table 1.
Currently available Tenofovir Alafenamide (TAF) Fixed dose drug combinations and their use during pregnancy.
| Drug Name | Formulation | Company | FDA Approval date |
Relevant Clinical PK studies in pregnancy |
Use in Pregnancy |
|---|---|---|---|---|---|
| TAF/FTC/RPV (Odefsey) | TAF – 25 mg FTC – 200 mg RPV – 25 mg |
Gilead Sciences | March 2016 | *IMPAACT P1026 s (Momper et al.) [63] | Insufficient data to use during pregnancy. Monitor renal function in pregnancy |
| TAF/FTC (Descovy) | TAF – 25 mg FTC – 200 mg |
Gilead Sciences | April 2016 | *IMPAACT P1026 s (Brooks et al.) [64] | Insufficient data to use during pregnancy. Monitor renal function in pregnancy |
| TAF/FTC/EVG/COBI (Genvoya) | TAF – 10 mg FTC – 200 mg EVG – 25 mg COBI – 150 mg |
Gilead Sciences | November 2015 | IMPAACT P1026 s (Momper et al) [63] #PANNA (Schalkwijk et al.) [36] | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimester of pregnancy [33–37] |
| TAF/FTC/DTG | TAF – 25 mg FTC – 200 mg DTG – 50 mg |
Mylan | February 2018 | IMPAACT 1026s | Insufficient data to use during pregnancy. |
| TAF/FTC/DRV/COBI (Symtuza) | TAF – 10 mg FTC – 200 mg DRV – 800 mg COBI – 150 mg |
Janssen | July 2018 | IMPAACT P1026 | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimester of pregnancy[33–37] |
| TAF/FTC/BIC (Biktarvy) | TAF – 25 mg FTC – 200 mg BIC – 50 mg |
Gilead Sciences | March 2019 | IMPAACT 2026 s and Gilead GS-US-380-5310 (NCT03960645) | Insufficient data to use during pregnancy. Data to be studied in ^IMPAACT P2026s. Monitor renal function in pregnancy |
International Maternal Pediatric Adolescent AIDS Clinical Trials – IMPAACT P1026 s, an ongoing, non-randomized, open-label, multi-center study of antiretroviral PK in pregnant women living with HIV in the United States, Brazil, Thailand, and Africa.
International Maternal Pediatric Adolescent AIDS Clinical Trials – IMPAACT P2026 s, a non-randomized, open-label, multi-center study of antiretroviral PK in pregnant women living with HIV in the United States, Brazil, Thailand, and Africa. The PK study arms will open in 2020.
Pharmacokinetics of newly developed ANtiretroviral agents in HIV-positive pregNAnt women (PANNA), an ongoing, non-randomized, open-label, multi-center study of antiretroviral PK in pregnant women living with HIV in Europe.
3. Chemistry, pharmacokinetics profile of TAF
3.1. Chemistry and mechanism of action
TAF (GS-7340; C21H29N6O5P) is an acyclic purine nucleotide phosphonate prodrug of TFV [32]. Following oral administration and absorption, TAF enters the systemic circulation as a prodrug, and undergoes very little hydrolysis to TFV in plasma due to its unique molecular properties that confer stability to its chemical structure [33]. TAF subsequently enters cells passively, including lymphoid tissue and PBMCs [34], where it is hydrolyzed to TFV and phosphorylated by intracellular kinases to tenofovir-monophosphate (TFV-MP), and then its active metabolite, TFV-DP [35] – Figure 1. Cathepsin A (CatA), a lysosomal carboxypeptidase, is the key enzyme responsible for the first hydrolysis step of TAF in PBMC [36,37]. TFV-DP inhibits reverse transcriptase by competing with endogenous nucleotides (2’-deoxyadenosine triphosphate – dATP) for inclusion into HIV viral DNA, causing premature DNA chain termination. TFV-DP also has minimal effects on DNA polymerases alpha, beta, and mitochondrial DNA polymerase, and thus is associated with less mitochondrial toxicity than older NRTIs [36,38].
Figure 1.
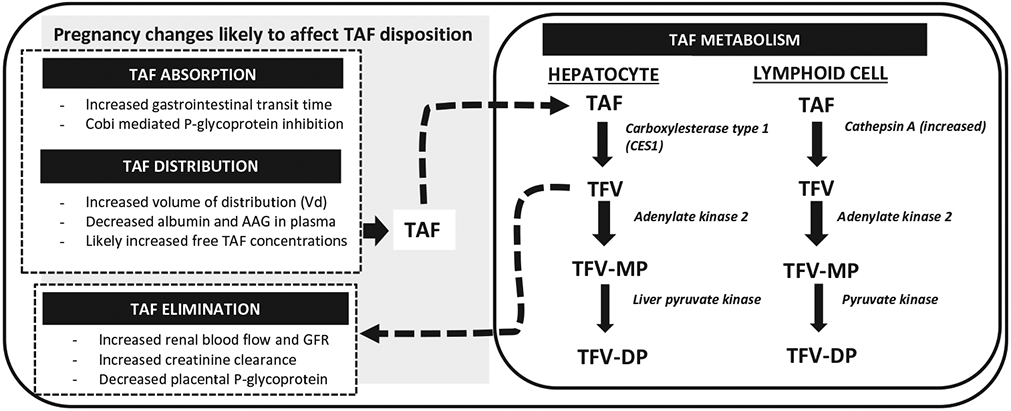
Tenofovir Alafenamide (TAF) absorption, metabolism and elimination.
3.2. Absorption, volume of distribution, drug transport, distribution and half-life
TAF is 80% protein bound to plasma proteins [39], with an apparent volume of distribution of over 100 liters [40] and a blood-to-plasma ratio of 1.0 [39]. TAF is rapidly absorbed from the gastrointestinal tract after oral administration, reaching peak concentrations between 0.5 to 2 hours post-dose [39]. TAF is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) [41]. Therefore, drugs that inhibit P-gp or BCRP (e.g., cobicistat or ritonavir) increase TAF absorption. Similarly, medications that induce P-gp or BCRP can decrease TAF absorption, resulting in decreased plasma concentrations and potential loss of therapeutic effect or resistance. After absorption, TAF is taken up into the liver via hepatic uptake organic anionic transporters family 1B1 and 1B3 (OATP1B1 and OATP1B3), and circulates largely intact in the blood where it loads PBMCs [42]. The half-life of TAF in plasma is ~30 minutes [15], while the half-life of its active anabolite, TFV-DP, in PBMCs is approximately 4 days [43,44]. TAF achieves ~2-7-fold higher intracellular TFV-DP concentrations in PBMCs than TDF due to its increased stability in blood and higher lipophilicity in comparison to the parent tenofovir form [14,16,45].
3.3. Metabolism and excretion
Over 80% of orally administered TAF is metabolized intracellularly in PBMCs and hepatocytes [12,46]. The cytochrome P450 enzymes (mainly CYP3A) minimally metabolize TAF. TAF is converted to tenofovir intracellularly in the liver by carboxylesterase type 1 (CES1) and in white blood cells (PBMCs and macrophages) by CatA, and then undergoes additional phosphorylation steps to TFV-DP [33]. TAF is excreted primarily in the feces (32%). TAF is not a substrate for the renal transporters OAT1 and OAT3, unlike tenofovir, and thus less than 1 percent is excreted via the kidneys [47]. Lower plasma TFV concentrations are achieved with TAF, and parent tenofovir is excreted through the kidneys. The lower plasma tenofovir levels achieved with TAF are responsible for the reduced renal and bone complications when compared to TDF [14]. Due to its low renal excretion and low plasma TFV exposures, TAF has been used safely in patients with chronic renal disease with glomerular filtration rates of <30 mL/min [48-52]. In addition, declines in renal function are less common with TAF than TDF [53].
4. Pharmacodynamics – antiviral activity of TAF and barrier to drug resistance
Pharmacodynamic variables of importance in measuring dose-response and viral susceptibility to TAF include concentrations that produce 50% or 90% of maximal antiviral activity (EC50 or EC90) [54]. The established plasma EC50 for HIV-1 for TAF and TDF are 0.005 μM, and 0.05 μM respectively [15,34], demonstrating that TAF is 10 times more potent than TDF [34]. These pharmacodynamic parameters help predict and define TAF’s potency, efficacy and barrier to resistance. Another way to assess TAF’s potency is by measuring the logarithmic decline in HIV-RNA levels (viral decay) when TAF is used in the management of HIV. Ascending TAF doses demonstrated median declines in HIV-1 RNA of 1.08 log10 copies/mL, 1.46 log10 copies/mL, and 1.73 log10 copies/mL from baseline to day 11 after treatment with 8 mg, 25 mg, or 40 mg of TAF, respectively, as compared with a viral decay of 0.97 log10 copies/mL with 300 mg TDF. The enhanced viral decays with TAF 25 mg and 40 mg doses was due to the higher intracellular concentrations of TFV-DP achieved with TAF versus TDF [16]. While TAF’s effective plasma concentration (EC50 or EC90) and viral decay as measures of potency are critically important, the duration of drug effect and barrier to antiviral resistance are also very crucial measures of antiviral effect. TAF was shown to have a more rapid attainment of protective levels in PBMC and, a longer duration of effect above the EC90 following drug discontinuation in comparison TDF (16 vs. 10 days, respectively) [55]. TAF has a higher genetic barrier to treatment-emergent resistance mutations such as K65 R, Q151 M, T69-insertion complex, and multiple thymidine analog resistance mutations (TAMs) in comparison to TDF, again owing to the high TFV-DP concentrations attained in PBMCs [56].
5. Physiological changes during pregnancy relevant to TAF disposition
Several physiologic changes during pregnancy may impact the PK of TAF through alterations in drug absorption, distribution, metabolism and elimination compared to the non-pregnant state (Figure 1). TAF absorption may be affected by increased residence time of food in the gastrointestinal tract and increased gut pH due to the smooth muscle relaxant effect of progesterone production by the placenta during pregnancy [57]. TAF is transported across the gastrointestinal wall by passive diffusion, but is also a substrate for the efflux transporters, P-gp and BCRP, which can decrease its absorption [41]. Inhibition of P-gp by cobicistat or ritonavir reduces P-gp–mediated TAF efflux, thereby increasing the fraction of TAF absorbed. While the absorption and absolute bioavailability of TAF have not been studied in pregnant women, these parameters may differ during pregnancy due to these collective physiologic changes.
Due to the increased plasma volume and reduced albumin and alpha-1-acid glycoprotein concentrations during pregnancy [58], the protein-binding of TAF is expected to be reduced during pregnancy, as approximately 80% of TAF is bound to plasma proteins, increasing the free fraction available for clearance. TAF is a substrate for hepatic OATP1B1 and OATP1B3 [42]. While the expression of OATP1B3 decreased in women with intrahepatic cholestasis of pregnancy relative to normal pregnancy, the expression of OATP1B1 was relatively unchanged [59]. Following hepatic uptake, TAF is hydrolyzed in hepatocytes by CES1 and metabolized by CYP3A to a smaller extent. Hence, the increased activity of CYP3A enzymes that occurs during pregnancy is not expected to have a significant effect on the clearance of TAF or its metabolites. Pregnancy PK studies of drugs metabolized by CES1 have demonstrated that CES1 activity is unchanged during pregnancy [60].
Expression of CatA, the enzyme that activates TAF in PBMCs and macrophages to TFV-DP, increases during pregnancy and postpartum [61]. Increased CatA levels during pregnancy would likely increase the conversion of TAF to TFV-DP. Renal elimination of TFV occurs through a combination of glomerular filtration and tubular secretion via uptake through OAT1 and OAT3 renal transporters [62,63] and efflux into urine via MRP-4 [64]. Pregnancy decreases plasma TFV concentrations with TDF in the second and third trimesters through increases in plasma volume, and increased glomerular filtration rates owing to increases in renal blood flow. In contrast to TFV, TAF is not eliminated by OAT1 and OAT3 kidney transporters. Hence, TAF does not concentrate within the proximal tubules of the kidneys [65]. However, TAF is the predominant moiety circulating in blood and loading PBMCs, and so the relevance of pregnancy-related increased TFV clearance is unclear. Placental expression of P-glycoprotein on the microvilli of syncytiotro-phoblasts decrease with increasing gestational age from placental studies, and this has implications for maternal to fetal transfer of TAF [66].
6. TAF pharmacokinetic studies during pregnancy
6.1. IMPAACT P1026 s PK study of TAF 25 mg unboosted and TAF 10 mg boosted with COBI [67]
The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1026 s study was a non-randomized, open-label, multi-center study that evaluated the PK of ARV medications in pregnant women living with HIV. The first PK data from TAF in pregnant women came from this study [67]. Steady state plasma PK profiles of TAF following once-daily dosing of either TAF/FTC/RPV (25/200/25 mg, Odefsey®) or TAF/FTC/EVG/COBI (10/200/150/150 mg, Genvoya®) were obtained during the 2nd and 3rd trimesters of pregnancy, and 6–12 weeks postpartum. Data were available in 31 women taking TAF 25 mg without boosting, and 27 women taking TAF 10 mg boosted with cobicistat. For the 25 mg unboosted arm, TAF exposures were 43% lower during the second trimester versus postpartum [(GMR 0.57 (90% CI 0.34–0.98)], and 34% lower during the third trimester compared to postpartum [(GMR 0.66 (90% CI 0.54–0.82)]. Of note, these postpartum exposures were higher than typical non-pregnant adult values – Table 2. For the 10 mg TAF cobicistat-boosted arm, TAF exposures were 21% lower during the second trimester versus postpartum [(GMR 0.79 (90% CI 0.50–1.27)], and 14% lower during the third trimester compared to postpartum [(GMR 0.86 (90% CI 0.66–1.12)], but these differences were not statistically significant – Table 2 [67]. Though pregnancy and postpartum differences in TAF levels were observed between the 2 dosing regimens, plasma TAF exposures during pregnancy and postpartum with both regimens were within the range of those typically observed in non-pregnant adults. Furthermore, 10/11 women (91%) in the TAF 25 mg arm (without boosting) and 24/27 women (89%) in the TAF 10 mg with cobicistat arm had suppressed viral loads (<50 copies of HIV-1 RNA/mL) at the time of delivery [67].
Table 2.
Steady state Pharmacokinetics of Once-Daily Oral Administration of Tenofovir Alafenamide (TAF): aTAF (25 mg unboosted) in pregnancy, bTAF (10 mg boosted) in pregnancy, cTAF(25 mg boosted) in pregnancy, dTAF (25 mg unboosted) in non-pregnant adults, and eTAF (10 mg boosted) in non-pregnant adults.
|
a25 mg unboosted |
b10 mg with COBI |
c25 mg with RTV or COBI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 2nd Trimester | 3rd Trimester | Postpartum | 2nd Trimester | 3rd Trimester | Postpartum | 2nd Trimester | 3rd Trimester | Postpartum |
d25 mg unboosted (Non-pregnant adults) |
e10 mg with COBI (Non-pregnant adults) |
| TFV AUCtau (ng•h/mL) | 162 (153–184) | 184 (147–350) | 390 (188–461) | 197 (145–354) | 209 (158–284) | 213 (169–304) | 133 (128–720) | 335 (192–549) | 507 (221–693) | 273 (227–319) | 271 (213–329) |
| TFV Cmax (ng/mL) | 69.7 (61.0–87.7) | 91 (50–105) | 157 (92–241) | 80.4 (57.5–121.0) | 93 (51–136) | 96 (74–110) | 44 (41–219) | 101 (78–119) | 164 (107–337) | 7.9 (6.4–9.5) | 7.4 (6–8.8) |
| TFV CL/F (L/hr) | 154 (136–163) | 136 (72–170) | 64 (55–133) | 127 (71–172) | 120 (88–158) | 118 (83–148) | 188 (35–195) | 75 (46–130) | 49 (37–123) | - | - |
| TFV T1/2 (hours) | 0.25 (0.24–0.27) | 0.28 (0.23–0.53) | 0.32 (0.30–0.43) | 0.36 (0.25–0.52) | 0.35 (0.27–0.48) | 0.30 (0.27–0.44) | 0.20 (0.20–0.33) | 0.32 (0.26–0.61) | 0.31 (0.25–0.66) | 0.40 (0.36–0.43) | 0.46 (0.38–0.53) |
Summary PK reported as median (IQR).
Source:
Momper JD, Best B, Wang J, et al. Tenofovir Alafenamide Pharmacokinetics With and Without Cobicistat in Pregnancy. 22nd International AIDS Conference; July 23–27, 2018, 2018; Amsterdam, the Netherlands.
Brooks K, Pinilla M, Shapiro D, et al. Pharmacokinetics of tenofovir alafenamide 25 mg with PK boosters during pregnancy and postpartum. Oral abstract presented at 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis, and Other Antiviral Drugs; 14–16 May 2019, 2019; Noordwijk, the Netherlands
Yamaha H, Yonemura T, Nemoto T et al. Pharmacokinetics of Tenofovir Alafenamide, Tenofovir and Emtricitabine following coformulated Emtricitabine/Tenofovir Alafenamide in Healthy Japanese Subjects. Clin Pharmacol Drug Dev, 2019; 8(4):511–520
Key to Acronyms: AUCtau = area under the curve over the dosing interval (i.e., 24 hours); CL/F = apparent oral clearance; Cmax = maximum plasma concentration; T1/2 = half life; TAF = tenofovir alafenamide
6.2. IMPAACT P1026 s PK study [68] of TAF 25 mg boosted with ritonavir (RTV) or COBI
The PK and safety of TAF 25 mg with ritonavir or cobicistat boosting were also examined in a separate arm of the P1026 s study [68]. Seventeen women were enrolled during the second or third trimesters of pregnancy, and were concomitantly taking either darunavir/cobicistat, darunavir/ritonavir, atazanavir/cobicistat, or atazanavir/ritonavir. PK data were available from six, fourteen, and eight women during the second trimester, third trimester, and postpartum respectively. TAF exposures did not significantly differ during the third trimester compared to postpartum [(GMR 0.94 (90% CI 0.38–1.33)] – Table 2 [68], and exposures were comparable to or higher than historical data in adults receiving TAF 10 mg with cobicistat (Table 2). Importantly, only two women had paired data available during the second trimester and postpartum, so GMR comparisons were limited to third trimester versus postpartum in a total of eight women while awaiting additional data. A total of 16/17 women (94.1%) had suppressed HIV-1 viral loads at the time of delivery. No major safety concerns were noted. The full manuscripts and publications of the three TAF in pregnancy PK studies are in progress.
7. Randomized clinical trials of TAF-based FDC during pregnancy and lactation
7.1. VESTED-IMPAACT 2010 randomized clinical trial [69]
The Virologic Efficacy and Safety of combined antiretroviral therapy with TAF/TDF, EFV, and DTG (VESTED) trial [69] is an ongoing National Institute of Health (NIH)/IMPAACT funded phase III randomized controlled trial (NCT03048422). IMPAACT 2010 is comparing the virologic efficacy and safety of three ARV regimens (TAF/FTC/DTG, TDF/FTC/DTG, and TDF/FTC/EFV) in over 600 ARV-naïve pregnant women living with HIV [69]. It is the first phase III clinical trial to randomize pregnant women specifically to a TAF-based regimen to study HIV virologic suppression. The study will also compare the safety of these ARV regimens among pregnant women and their infants. At study entry, pregnant women are randomly assigned 1:1:1 to receive one of three regimens: TAF/FTC/DTG (arm 1), TDF/FTC/DTG (arm 2), or TDF/FTC/EFV (arm 3) during pregnancy and through one year postpartum. The primary outcome of the trial is the proportion of pregnant women living with HIV with viral loads < 200 copies/mL at the time of delivery. The study will also compare rates of adverse pregnancy outcomes, including maternal and infant adverse events across all three arms. The current clinical sites include the United States, Botswana, Brazil, India, South Africa, Tanzania, Thailand, Uganda, and Zimbabwe. Estimated completion date is July 31st, 2020.
8. TAF drug-drug interactions
TAF plasma concentrations can be altered by concomitant administration of other medications. TAF is a substrate of BCRP and P-gp transporters, and absorption of TAF increases when it is co-administered with inhibitors of these efflux transporters (e.g., cobicistat). Conversely, use of TAF with a BCRP/P-gp inducer such as rifampicin may reduce TAF concentrations. Concomitant use of other ARVs can also affect the PK of TAF in non-pregnant adults [41]. TAF and TFV concentrations are unaffected after co-administration with RPV, and ~20% higher with DTG. Co-administration with BCRP/P-gp inhibitors such as cobicistat or ritonavir (as part of boosted protease inhibitor regimens) can result in marked increases in TAF and TFV exposure. However, the net effect of boosted PIs on TAF varies between the specific booster and PI used. Therefore, drug interactions between TAF and other ARVs, as well as drugs that induce or inhibit BCRP/P-gp are important to assess, as these drug interactions may further alter TAF exposures in this population.
9. Cobicistat boosting of TAF versus cobicistat boosting of protease/integrase inhibitors containing FDCs
Although cobicistat effectively boosts TAF exposure during pregnancy (section 6), it does not effectively boost exposures of the integrase inhibitor elvitegravir or the protease inhibitors darunavir or atazanavir (section 2) during pregnancy. There is a unique difference between the site and mechanism of action of cobicistat boosting of TAF and cobicistat boosting of protease and integrase inhibitors [28,29]. Cobicistat boosts TAF plasma exposures by inhibiting efflux transporters (P-gp and BCRP) in gut enterocytes, enhancing bioavailability. However, cobicistat boosts protease and integrase inhibitors mainly by selectively inhibiting CYP3A4 metabolism in the liver and intestinal tract, and by inhibiting efflux transporters (to a smaller extent) [70]. Cobicistat inhibition of CYP3A4 is dependent on plasma cobicistat concentrations, which are reduced in pregnancy. This difference in site and mechanism of action likely explains the difference in effectiveness of cobicistat boosting of TAF in pregnancy compared to protease and integrase inhibitors. Since TAF 10 mg with cobicistat is available only in FDCs with either darunavir, atazanavir or elvitegravir, the ability of cobicistat to effectively boost TAF 10 mg during pregnancy is not clinically relevant, as use of these FDCs in pregnancy is not recommended due to the low exposures of darunavir, atazanavir and elvitegravir during pregnancy. The IMPAACT P1026 s data suggest that the TAF exposures are adequate when TAF is used in pregnancy as 25 mg with or without boosting and 10 mg with cobicistat.
10. Intracellular TFV-DP data in pregnancy are limited
Intracellular TFV-DP data during pregnancy are currently limited to dried blood swab (DBS) assessments with TDF. DBS levels of TFV-DP with TDF were lower in pregnant women on HIV pre-exposure prophylaxis (PrEP) in comparison to the postpartum period [71]. A separate study in pregnant women taking TDF/FTC for PrEP under directly observed therapy is currently being conducted to establish DBS thresholds associated with 100% adherence in this population [72]. The Promoting Maternal Infant Survival Everywhere (PROMISE) [73] randomized clinical trial did not identify associations between higher TDF exposure, as measured by maternal TFV-DP concentrations in DBS, and adverse maternal, fetal and neonatal outcomes [73]. DBS levels measured in PROMISE were also lower than those measured in other studies in non-pregnant adults living with HIV [74]. Several studies have consistently demonstrated lower plasma TFV [14,15] and higher intracellular TFV-DP concentrations in PBMCs in non-pregnant adults on TAF-based regimens compared to TDF [14,16,55,75-77]. There are currently no data describing TFV-DP intracellular levels with PBMCs or DBS in pregnant, postpartum and lactating women on TAF. However, these assessments are planned in IMPAACT 2026. As discussed above (sections 6.1 and 6.2), although plasma TAF concentrations are lower during pregnancy than postpartum, they remain close to those found in non-pregnant women [67]. While the three different TAF dosing arms of IMPAACT 1026 s in pregnant women showed acceptable plasma TAF levels, viral load data at the time of delivery are sparse and challenging to interpret as they reflect efficacy of the entire ARV regimen and not just TAF. The majority of women – 91% (50/55) had HIV-1 viral suppression across all three P1026 s arms [67,68], which suggests that adequate TFV-DP levels in PBMCs are likely achieved. However, additional studies to establish intracellular TFV-DP concentrations in PBMCs and DBS with TAF in pregnant women are needed to inform efficacy, safety, and adherence assessments in this population.
11. TAF use and the risk of congenital anomalies and metabolic complications
There is a dose-response relationship between most drugs and the risk of adverse effects and congenital anomalies [78-80]. TDF exposure during pregnancy has been associated with reduced neonatal whole-body bone mineral density, decreased mean length-for-age Z-scores, and lower head circumference-for-age Z scores at one year of age in children enrolled in the Surveillance Monitoring for ART Toxicities (SMARTT) cohort [81], but these findings were most likely of uncertain significance. Hence, TDF is considered safe in pregnancy and still one of the ARV back-bones used during pregnancy. While TAF results in lower systemic concentrations of TFV, it produces higher intracellular concentrations of TFV-DP than TDF. It remains uncertain if higher intracellular TFV-DP concentrations would increase the risk of congenital anomalies and adverse pregnancy outcomes. The HIV Antiretroviral Pregnancy Registry (APR) is a project established to monitor prenatal ARV exposures and detect potential increases in the risk of teratogenicity [82]. While results from the APR are increasing bit by bit, there remains a paucity of data on TAF use during pregnancy and lactation, making this Registry an essential component of the ongoing program of epidemiologic studies of the safety of TAF-based FDCs [83]. In the IMPAACT P1026 s TAF 25 mg and 10 mg boosted with cobicistat study arms, congenital anomalies considered possibly related to study drugs included a ventral septal defect (VSD) in one infant and congenital pseudo-arthrosis of the left clavicle and neonatal compartment syndrome in another infant. Since this study arm involved a small number of women receiving TAF FDCs, it is difficult to conclude if these congenital anomalies were related to TAF or other ARVs in the FDCs, or were incidental findings. The number of cases related to TAF reported in the APR is insufficient to draw any reasonable conclusions on the association between TAF and any congenital anomalies at the current time. As more data become available, additional information on possible adverse effects and risk of teratogenicity will be gathered.
Recent studies in non-pregnant adults have linked TAF with increases in body weight and metabolic abnormalities compared to TDF [84,85]. Data from the AMBER randomized trial with cobicistat-boosted darunavir showed that participants randomized to TAF-based regimens had a greater increase in body weight compared to TDF-based regimens [86]. Other studies have shown similar findings of increased weight gain with TAF-based FDCs. Early data from the ADVANCE randomized controlled trial suggest that exposure to TAF-FDCs led to progressive increases in weight gain, serum lipid parameters (elevated triglycerides), low high-density lipoprotein (HDL) levels, hypertension, and hyperglycemia (metabolic syndrome) in patients during a 96-week period [87]. TAF-FDC associated metabolic syndrome was demonstrated in 9% of participants versus 3–5% of individuals taking TDF-based FDCs. This weight gain was more pronounced in women compared to men, with an average of 6 kg weight gain in men compared to 9 kg weight gain in women[87]. The impact of these findings and the implications during pregnancy remain unknown.
12. Conclusions
Plasma TAF exposures during pregnancy are within the typical range of those in non-pregnant adults taking similar doses, but higher than expected plasma exposures of TAF were noted postpartum for unclear reasons. No pregnancy data describing intracellular concentrations of TFV-DP, the active moiety of tenofovir, are available. Pending further PK, safety and efficacy studies, the US DHHS Perinatal Guidelines do not recommend TAF for use in pregnancy [7]. Prospective, comparative virologic response, intracellular PK, and safety data are needed to establish the role for this drug in pregnancy.
13. Expert opinion
Early pregnancy data from the PK studies as described in sections 6.1 and 6.2 above are suggestive that TAF based FDCs hold promise as part of first-line regimens for the treatment of pregnant women living with HIV and the prevention of perinatal HIV transmission due to their limited reports of adverse effects. Cobicistat (a P-gp inhibitor) may explain some of the differences in findings between boosted and unboosted TAF PK during pregnancy. There are several research gaps that, if filled, could aid our understanding of TAF use in pregnancy – the population PK of TAF during pregnancy (including sources of variability and transfer into and biotransformation in cells), placental drug efflux transporters in TAF disposition, and the utility of DBS and PBMCs to serve as measures of medication adherence and site-of-action drug exposure, respectively, during pregnancy.
Population PK of TAF use during pregnancy has not been described, as current TAF population PK models exclude pregnant women [88,89]. Pop-PK models are important because they incorporate data from sparse PK sampling and can explore the effects of multiple covariates (for example, maternal height, age, maternal and fetal weight, disease status, serum creatinine, hemoglobin concentrations, genetic polymorphisms and gestational age) on inter-individual and residual variability unexplained by non-compartmental pharmacokinetics [90]. A two-compartment population PK model of TDF in pregnant women demonstrated that only gestational age and serum creatinine significantly influenced plasma tenofovir disposition [91]. TAF population PK modeling in non-pregnant adults identified body weight and protein-binding as significant covariates on plasma TAF disposition [89]. A separate population PK study in non-pregnant women also linked plasma TDF and TAF with TFV-DP concentrations in various compartments, including PBMCs [88]. Just as the population PK models in non-pregnant adults demonstrated the effects of between-subject and unexplained residual variability on the PK of TAF and TFV-DP, similar approaches should be attempted in future pregnancy population PK models. The TDF population PK model in pregnant women (as with many pregnancy population PK models) was limited because it did not include the placental or fetal compartments, as fetal plasma drug concentrations can only be collected safely from the umbilical cord of term fetuses at the time of birth (and not earlier in pregnancy). In addressing these knowledge gaps, a future population PK study on TAF disposition during pregnancy, with appropriate covariate–parameter relationships and covariate stratification to prevent hidden biases, would be critically important to the prediction of TAF disposition across the different trimesters of pregnancy and postpartum. Gestational changes in placental drug transporter expression and activity remain an area of intense study [92], yet very little is known regarding their regulation during pregnancy. To address limitations of population PK in predicting in utero drug exposures, data on the expression of drug transporters in the placenta in combination with cord blood samples at birth could be incorporated into physiologically-based pharmacokinetic (PBPK) models [93]. It is of critical importance to further understand the role of placental P-gp, BCRP, as well as other drug transporters in regulating TAF, TFV and TFV-DP transfer to the developing fetus, and how these transporter functions are altered during pregnancy.
Given the recent data pointing toward metabolic concerns with TAF-based FDCs in non-pregnant adults, TAF use during pregnancy and the potential for metabolic changes need to be examined. Pregnancy is known to be associated with weight gain, especially during the second and third trimesters. It is unknown whether TAF use in pregnancy results in additional weight gain above the current Institute of Medicine (IOM) recommendations for pregnancy or an increased incidence of metabolic syndrome, which could increase adverse pregnancy outcomes in the short and long term. Understanding whether TAF-induced metabolic syndrome is modified during pregnancy by age, gestational age, parity, and race are critical research questions, especially in women with class III obesity (body mass index of ≥40 kg/m2). Answers to these questions are necessary before we can be confident that TAF can be used safely and effectively in pregnant women.
There remains a critical, unmet need for objective, quantitative measures of ARV adherence and drug levels at the site of action (i.e., PBMC) among women living with HIV in pregnancy. Non-pharmacologic adherence measures, such as patient self-report, calculation of proportion of pill days covered, pillbox checks, and use of electronic pill boxes/apps, have limitations and may over-estimate or under-estimate antiretroviral adherence [74]. To address these shortcomings, multiple pharmacologic adherence measures have been developed to objectively quantify antiretroviral medication adherence [94,95]. One approach is the use of DBS. TFV-DP has a long half-life in RBCs (~17 days) [43,96], and thus reflects cumulative medication adherence over the previous 2–3 months of therapy. TFV-DP in DBS has been used in several HIV pre-exposure prophylaxis (PrEP) [97–99] and treatment [100-103] studies to better understand adherence and several other treatment outcomes in the HIV field. Additionally, examining TFV-DP concentrations in PBMCs will provide critical insights as to whether changes in plasma levels of TAF or TFV result in clinically relevant changes in TFV-DP levels at the site of action. There are currently no data on TFV-DP concentrations in PBMCs during pregnancy. Intracellular TFV-DP data are limited to DBS concentrations with TDF in pregnant women living with HIV [73] and on PrEP [71,72]. Assessments of intracellular TFV-DP levels in DBS and PBMCs with TAF during pregnancy are developments likely to be clinically important in the future for understanding relationships with medication adherence and drug levels at the site of action.
Article Highlights.
There is a unique difference between the site and mechanism of action of cobicistat boosting of TAF and cobicistat boosting of protease and integrase inhibitors. This difference in site and mechanism of action likely explains the difference in effectiveness of cobicistat boosting of TAF in pregnancy compared to protease and integrase inhibitors.
Plasma TAF exposures during pregnancy are within the typical range of those in non-pregnant adults taking similar doses, but higher than expected plasma exposures of TAF were noted postpartum for unclear reasons.
TAF has a higher genetic barrier to treatment-emergent resistance mutations such as K65R, Q151M, T69-insertion complex, and multiple thymidine analog resistance mutations (TAMs) in comparison to TDF, owing to the high TFV-DP concentrations attained in PBMCs
TAF was shown to have a more rapid attainment of protective levels in PBMC and, a longer duration of effect above the EC90 following drug discontinuation in comparison TDF.
TAF, due to its potency and good safety profile, might form the cornerstone for management of pregnant women living with HIV
This box summarizes key points contained in the article.
Footnotes
Declaration of Interest
M Mirochnick has received research support from Merck, ViiV and Gilead, and has served as a consultant for ViiV and Gilead. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
A reviewer of this manuscript discloses receiving research support from Gilead Sciences (paid to institution) for an investigator-initiated study.
References
- 1.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. [cited 2019 Oct 11]. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf
- 2.Eke AC, McCormack SA, Best BM, et al. Pharmacokinetics of increased nelfinavir plasma concentrations in women during pregnancy and postpartum. J Clin Pharmacol. 2019;59(3):386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teasdale CA, Marais BJ Abrams EJ., Hiv: prevention of mother-to-child transmission. Bmj Clin Evid. 2011. Jan;17(2011):0909. [PMC free article] [PubMed] [Google Scholar]
- 4.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. [DOI] [PubMed] [Google Scholar]
- 5.Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. The World Health Organization (WHO). [cited 2019 Oct 11]. Available from: https://www.who.int/hiv/pub/mtct/guidelines/en/ [PubMed] [Google Scholar]
- 6.European AIDS Clinical Society (EACS) Guidelines. [cited 2019 Oct 11]. Available from: https://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- 7.Panel on treatment of pregnant women with HIV infection and prevention of perinatal transmission. Recommendations for use of antiretroviral drugs in transmission in the United States. 2018. [cited 2019 Nov 1]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf
- 8.Fowler MG, Qin M, Fiscus SA, et al. , Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachega JB, Uthman OA, Mofenson LM, et al. Safety of tenofovir disoproxil fumarate-based antiretroviral therapy regimens in pregnancy for HIV-infected women and their infants: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;76(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pintye J, Baeten JM, Celum C, et al. Maternal tenofovir disoproxil fumarate use during pregnancy is not associated with adverse perinatal outcomes among HIV-infected East African women: a prospective study. J Infect Dis. 2017;216(12):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill A, Hughes SL, Gotham D, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad. 2018;4(2):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Clercq E Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem Pharmacol. 2018;153:2–11. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Podany AT, Bares SH, Havens J, et al. , Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS. 2018;32(6):761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63–70. [DOI] [PubMed] [Google Scholar]
- 16.Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455. [DOI] [PubMed] [Google Scholar]
- 17.Baxi SM, Scherzer R, Greenblatt RM, et al. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS. 2016;30(4):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavra ZMM, de Medeiros FPM, da Silva RMF, et al. Formulation, development and scale-up of fixed-dose combination tablets containing zidovudine, lamivudine and nevirapine. Curr HIV Res. 2019. DOI: 10.2174/1570162X17666190927162155 [DOI] [PubMed] [Google Scholar]
- 19.Janelle JW, Kariyawasam V. Single-tablet combination therapy for HIV infection in pregnancy. Clin Obstet Gynecol. 2019;62(4):804–815. [DOI] [PubMed] [Google Scholar]
- 20.Eke AC, Stek AM, Wang J, et al. Darunavir pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2020;83(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossi P, Peytavin G, Ait-Mohand H, et al. GENOPHAR: a randomized study of plasma drug measurements in association with genotypic resistance testing and expert advice to optimize therapy in patients failing antiretroviral therapy. HIV Med. 2004;5(5):352–359. [DOI] [PubMed] [Google Scholar]
- 22.Tran AH, Best BM, Stek A, et al. Pharmacokinetics of rilpivirine in HIV-infected pregnant women. J Acquir Immune Defic Syndr. 2016;72(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eke AC, Chakhtoura N, Kashuba A, et al. Rilpivirine plasma and cervicovaginal concentrations in women during pregnancy and postpartum. J Acquir Immune Defic Syndr. 2018;78(3):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye ZW, Augustijns P, Annaert P. Cellular accumulation of cholyl-glycylamido-fluorescein in sandwich-cultured rat hepatocytes: kinetic characterization, transport mechanisms, and effect of human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36(7):1315–1321. [DOI] [PubMed] [Google Scholar]
- 25.Tam VH, Kabbara S, Yeh RF, et al. Impact of sample size on the performance of multiple-model pharmacokinetic simulations. Antimicrob Agents Chemother. 2006;50(11):3950–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang D, Schwartz JB, Verotta D. A sample size computation method for non-linear mixed effects models with applications to pharma-cokinetics models. Stat Med. 2004;23(16):2551–2566. [DOI] [PubMed] [Google Scholar]
- 27.Boyd SD, Sampson MR, Viswanathan P, et al. Cobicistat-containing antiretroviral regimens are not recommended during pregnancy: viewpoint. AIDS. 2019;33(6):1089–1093. [DOI] [PubMed] [Google Scholar]
- 28.Eke AC, Mirochnick MH. Cobicistat as a pharmacoenhancer in pregnancy and postpartum: progress to date and next steps. J Clin Pharmacol. 2019;59(6):779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eke AC, Mirochnick M. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expert Opin Drug Metab Toxicol. 2019;15(7):523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Momper JD, Best BM, Wang J, et al. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. AIDS. 2018;32(17):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schalkwijk S, Colbers A, Konopnicki D, et al. First reported use of elvitegravir and cobicistat during pregnancy. AIDS. 2016;30(5):807–808. [DOI] [PubMed] [Google Scholar]
- 32.De Clercq E The acyclic nucleoside phosphonates (ANPs): Antonin Holy’s legacy. Med Res Rev. 2013;33(6):1278–1303. [DOI] [PubMed] [Google Scholar]
- 33.Aloy B, Tazi I, Bagnis CI, et al. Is tenofovir alafenamide safer than tenofovir disoproxil fumarate for the kidneys? AIDS Rev. 2016;18(4):184–192. [PubMed] [Google Scholar]
- 34.Lee WA, He GX, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa E, Furusyo N, Nguyen MH. Tenofovir alafenamide in the treatment of chronic hepatitis B: design, development, and place in therapy. Drug Des Devel Ther. 2017;11:3197–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkus G, Bam RA, Willkom M, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkus G, Wang R, Liu X, et al. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob Agents Chemother. 2007;51(2):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkus G, Hajek M, Kramata P, et al. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerases alpha, delta, and epsilon*. Antimicrob Agents Chemother. 2002;46(5):1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deeks ED. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs. 2018;78(17):1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markowitz M, Zolopa A, Squires K, et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69(5):1362–1369. [DOI] [PubMed] [Google Scholar]
- 41.Begley R, Das M, Zhong L, et al. Pharmacokinetics of tenofovir alafenamide when coadministered with other HIV antiretrovirals. J Acquir Immune Defic Syndr. 2018;78(4):465–472. [DOI] [PubMed] [Google Scholar]
- 42.Murakami E, Wang T, Park Y, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59(6):3563–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifert SM, Chen X, Meditz AL, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses. 2016;32(10–11):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Custodio JGW, Callebaut C. The pharmacokinetics of tenofovir and tenofovir diphosphate following administration of tenofovir alafenamide versus tenofovir disoproxil fumarate (Abstract 6). Paper presented at: 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; 2015; Washington DC. [Google Scholar]
- 46.Atta MG, De Seigneux S, Lucas GM. Clinical Pharmacology in HIV Therapy. Clin J Am Soc Nephrol. 2019;14(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genvoya® (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets for oral use. [cited 2019 Oct 8]. Available from: https://www.gilead.com/-/media/files/pdfs/medicines/hiv/genvoya/genvoya_pi.pdf
- 48.Eron JJ, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3(4):e158–165. [DOI] [PubMed] [Google Scholar]
- 50.Mills A, Crofoot G Jr., McDonald C, et al. tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015;69(4):439–445. [DOI] [PubMed] [Google Scholar]
- 51.Orkin C, DeJesus E, Ramgopal M, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV. 2017;4(5):e195–e204. [DOI] [PubMed] [Google Scholar]
- 52.Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33(9):1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Lu X, Yang X, et al. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine (Baltimore). 2016;95(41):e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callebaut C, Stepan G, Tian Y, et al. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob Agents Chemother. 2015;59(10):5909–5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spinner C, Brunetta J, Shalit P, et al. DISCOVER study for HIV pre-exposure prophylaxis (PrEP): F/TAF has a more rapid onset and longer sustained duration of HIV protection compared with F/TDF. 10th IAS Conference on HIV Science (IAS 2019); 2019 Jul 21–24; Mexico City. Abstract TUAC0403LB. [Google Scholar]
- 56.Margot NA, Liu Y, Miller MD, et al. High resistance barrier to tenofovir alafenamide is driven by higher loading of tenofovir diphosphate into target cells compared to tenofovir disoproxil fumarate. Antiviral Res. 2016;132:50–58. [DOI] [PubMed] [Google Scholar]
- 57.Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am. 1992;21(4):751–776. [PubMed] [Google Scholar]
- 58.Sheffield JS, Siegel D, Mirochnick M, et al. Designing drug trials: considerations for pregnant women. Clin Infect Dis. 2014;59(Suppl 7):S437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Yan Z, Dong M, et al. Alteration in placental expression of bile acids transporters OATP1A2, OATP1B1, OATP1B3 in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet. 2012;285(6):1535–1540. [DOI] [PubMed] [Google Scholar]
- 60.Beigi RH, Han K, Venkataramanan R, et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurzatkowski W, Ostrowska H, Doroszko M. Serum cathepsin A activity in pregnant, parturient and puerperal patients. Zentralblatt fur Gynakologie. 1990;112(4):227–229. [PubMed] [Google Scholar]
- 62.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. [DOI] [PubMed] [Google Scholar]
- 63.Cihlar T, Ho ES, Lin DC, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):641–648. [DOI] [PubMed] [Google Scholar]
- 64.Imaoka T, Kusuhara H, Adachi M, et al. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2):619–627. [DOI] [PubMed] [Google Scholar]
- 65.Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687–692. [DOI] [PubMed] [Google Scholar]
- 66.Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R963–969. [DOI] [PubMed] [Google Scholar]
- 67.Momper JD, Best B, Wang J, et al. Tenofovir alafenamide pharmacokinetics with and without cobicistat in pregnancy. 22nd International AIDS Conference; 2018 Jul 23–27; Amsterdam, The Netherlands. [Google Scholar]
- 68.Brooks K, Pinilla M, Shapiro D, et al. Pharmacokinetics of tenofovir alafenamide 25 mg with PK boosters during pregnancy and postpartum. Oral abstract presented at 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis, and Other Antiviral Drugs; 2019 May 14–16; Noordwijk, The Netherlands. [Google Scholar]
- 69.Evaluating the efficacy and safety of dolutegravir-containing versus efavirenz-containing antiretroviral therapy regimens in HIV-1-Infected Pregnant Women and Their Infants (VESTED). Clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). Available from: https://clinicaltrials.gov/ct2/show/NCT03048422. [Google Scholar]
- 70.Tseng A, Hughes CA, Wu J, et al. Cobicistat versus ritonavir: similar pharmacokinetic enhancers but some important differences. Ann Pharmacother. 2017;51(11):1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pyra M, Anderson PL, Hendrix CW, et al. Tenofovir and tenofovir-diphosphate concentrations during pregnancy among HIV-uninfected women using oral preexposure prophylaxis. AIDS. 2018;32(13):1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.IMPAACT. 2009. Evaluating the pharmacokinetics, feasibility, acceptability, and safety of oral pre-exposure prophylaxis for HIV prevention during pregnancy and postpartum (IMPAACT 2009). [cited 2020 Feb 12] Available from: https://clinicaltrials.gov/ct2/show/NCT03386578
- 73.Aizire J, Brooks KM, Mirochnick M, et al. Antenatal intracellular concentrations of tenofovir diphosphate and emtricitabine triphosphate and associations between tenofovir diphosphate and severe adverse pregnancy outcomes: IMPAACT-PROMISE (1077BF) trial. J Acquir Immune Defic Syndr. 2020;83(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castillo-Mancilla JR, Haberer JE. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Curr HIV/AIDS Rep. 2018;15(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hare CB, Coll J, Ruane P, et al. The Phase 3 DISCOVER Study: Daily F/TAF or F/TDF for HIV Pre-exposure Prophylaxis Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI); 2019; Seattle, WA, USA. [Google Scholar]
- 76.Yager J, Brooks K, Castillo-Mancilla J, et al. Tenofovir-diphosphate in PBMC following increasing TAF vs. TDF dosing under directly observed therapy. 20th International Workshop on Clinical Pharmacology of HIV Hepatitis & Other Antiviral Drugs; 2019 May 14–16; Noordwijk, The Netherlands. [Google Scholar]
- 77.Ting SL, Zack J, Yan M et al. Enhanced Exposure of Tenofovir-diphosphate (TFV-DP) in Peripheral Blood Mononuclear Cells (PBMC) by Tenofovir Alafenamide (TAF) Compared with Tenofovir Disoproxil Fumarate (TDF). American Society of Microbiology (ASM) Microbe Conference; 2016 June 16–20; Boston, Massachussetts. [Google Scholar]
- 78.Berard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Research Part B, Dev Reprod Toxicol. 2007;80(1):18–27. [DOI] [PubMed] [Google Scholar]
- 79.Gilbert-Barness E Teratogenic causes of malformations. Ann Clin Lab Sci. 2010;40(2):99–114. [PubMed] [Google Scholar]
- 80.Eke AC, Dooley KE, Sheffield J. Pharmacologic research in pregnant women – time to get it right. N Engl J Med. 2019;380(14):1293–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zash RM, Williams PL, Sibiude J, et al. Surveillance monitoring for safety of in utero antiretroviral therapy exposures: current strategies and challenges. Expert Opin Drug Saf. 2016;15(11):1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James JS. HIV & AIDS treatment registry database: public registry now online. AIDS Treat News. 1999;326:3. [PubMed] [Google Scholar]
- 83.Tenofovir Alafenamide (TAF). [cited 2019 Oct 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208464Orig1s000TOC.cfm
- 84.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. [DOI] [PubMed] [Google Scholar]
- 85.Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad. 2019;5(1):41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orkin C, Eron J, Rockstroh J, et al. Efficacy and safety of the once-daily, darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) single-tablet regimen (STR) in antiretroviral treatment (ART)-naïve, HIV-1-infected adults: AMBER Week 96 results. HIV Glasgow; 2018; Glasgow, Scotland. [Google Scholar]
- 87.McCann K, Moorhouse M, Sokhela S, et al. The ADVANCE clinical trial: changes from baseline to week 96 in DXA-assessed body composition in TAF/FTC +DTG compared to TDF/FTC+DTG, and TDF/FTC/EFV. 17th European AIDS Conference; 2019; Basel, Switzerland. [Google Scholar]
- 88.Greene SA, Chen J, Prince HMA, et al. Population modeling highlights drug disposition differences between tenofovir alafenamide and tenofovir disoproxil fumarate in the blood and semen. Clin Pharmacol Ther. 2019;106(4):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ackaert O, McDougall D, Perez Ruixo C, H C. Population pharmacokinetic analysis for darunavir and tenofovir alafenamide in HIV-1-infected patients on the darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) single-tablet regimen (AMBER and EMERALD studies). 19th International Workshop on Clinical Pharmacology of Antiviral Therapy; 2018; Baltimore, Maryland,USA. [Google Scholar]
- 90.Ke AB, Rostami-Hodjegan A, Zhao P, et al. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol Toxicol. 2014;54:53–69. [DOI] [PubMed] [Google Scholar]
- 91.Shoji K, Best B, Mirochnick M, et al. Population pharmacokinetic assessment of factors associated with tenofovir clearance in pregnant and postpartum women with HIV infection in IMPAACT P1026s. 55th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2015; San Diego, California, USA. [Google Scholar]
- 92.Joshi AA, Vaidya SS, St-Pierre MV, et al. Placental ABC transporters: biological impact and pharmaceutical significance. Pharm Res. 2016;33(12):2847–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ke AB, Greupink R, Abduljalil K. Drug dosing in pregnant women: challenges and opportunities in using physiologically based pharmacokinetic modeling and simulations. CPT Pharmacometrics Syst Pharmacol. 2018;7(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castillo-Mancilla JR. Adherence to ART and PrEP: tARGETing the Ideal Measure. Clin Infect Dis. 2019. DOI: 10.1093/cid/ciz651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brooks KM, Anderson PL. Pharmacologic-based methods of adherence assessment in HIV prevention. Clin Pharmacol Ther. 2018;104(6):1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62(1):e0170–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seifert SM, Castillo-Mancilla JR, Erlandson K, et al. Brief report: adherence biomarker measurements in older and younger hiv-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr. 2018;77(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castillo-Mancilla JR, Searls K, Caraway P, et al. Short communication: tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses. 2015;31(4):428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis. 2019;68(8):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yager JL, Coyle RP, Coleman SS, et al. Moderately high tenofovir diphosphate in dried blood spots indicates drug resistance in viremic persons Living with HIV. J Int Assoc Provid AIDS Care. 2019;18:2325958219888457. [DOI] [PMC free article] [PubMed] [Google Scholar]


