Abstract
Natural products have been used to prevent and treat human diseases for thousands of years, especially the extensive natural small molecules (NSMs) such as terpenoids, steroids and glycosides. A quantity of studies are confined to concern about their chemical structures and pharmacological activities at the monomolecular level, whereas the spontaneous assemblies of them in liquids yielding supramolecular structures have not been clearly understood deeply. Compared to the macromolecules or synthetic small molecular compounds, NSMs have the inherent advantages of lower toxicity, better biocompatibility, biodegradability and biological activity. Self-assembly of single component and multicomponent co-assembly are unique techniques for designing supramolecular entities. Assemblies are of special significance due to their range of applications in the areas of drug delivery systems, pollutants capture, materials synthesis, etc. The assembled mechanism of supramolecular NSMs which are mainly driven by multiple non-covalent interactions are summarized. Furthermore, a new hypothesis aimed to interpret the integration effects of multi-components of traditional Chinese medicines (TCMs) inspired on the theory of supramolecular assembly is proposed. Generally, this review can enlighten us to achieve the qualitative leap for understanding natural products from monomolecule to supramolecular structures and multi-component interactions, which is valuable for the intensive research and application.
Keywords: Supramolecular assemblies, Self-assembly, Co-assembly, Natural small molecules (NSMs), Traditional Chinese medicines (TCMs)
Graphical abstract

1. Introduction
Supramolecular assemblies are well-organized entities that result from the spontaneous association of a non-defined number of chemical species, which usually form into specific macroscopic systems like gel, liposome, micelle, mesocrystal, host-guest composite, protein or enzyme, biomembrane, etc. with well-defined microscopic architectures (Fig. 1) [1]. The construction and development of them is relying on the progress of several subjects, involving biological science, materials science, pharmacy, pharmacochemistry, and physical chemistry. It also usually be used as a design and synthesis strategy to produce various types of self- or co-assembling prodrugs having supramolecular nanostructures of high reproducibility [2]. Some macromolecular substances including peptides, nucleic acids, lignin, cellulose, chitin, oligo- and polysaccharides having a strong tendency to aggregate in supramolecular patterns are important issues which people focus on and research. A large number of supramolecular assemblies based on them were constructed with a realization of the translation of one-dimensional carbohydrate polymers into various kinds of tunable, multifunctional, renewable and bioactive materials for application in optical components, drug delivery systems, regenerative medicine and tissue engineering. However, most of these macromolecular polymers without biofunctional modifications could only act as vehicles and carriers with insufficient bioactivities [3]. On the other hand, more and more synthetic and NSMs exhibiting intrinsic self-assembly properties have also attracted much attention. Thousands of synthetic small molecules such as alkaloids, steroids, terpenes, lignans and glycosides as building units of the self-assembly have been designed that containing both hydrophobic and hydrophilic segments to form into discrete nano- or microscale entities spontaneously in a certain solvent [4]. By contrast, there are only a few dozen of supramolecular self-assembled NSMs reported up to now and most are terpenoids. Although the discovery of supramolecular self-assembled NSMs is full of changeability and uncertainty, their great potential application in functionalized materials have brought about the widespread attention in the field of medicines and material science, due to more excellent safety, biodegradability, biocompatibility and lower toxicity of them. Among them, natural materials like natural small molecules (NSMs) are regarded as currently approved or promising pre-new drug application resources owing to their wide range of pharmacological activity.
Fig. 1.
Schematic representation of various supramolecular assembly systems.
Historically, natural-occurring small molecules, especially from plants and animals, were always regarded as rich sources of leads for new medicines owing to their wide range of structures and pharmacological activities [5]. Currently, the utilization of NSMs and their synthetic derivatives for discovering and developing new drugs is still an alive and well measure to conquer various diseases. Taking new antitumor drugs approved by the US Food and Drug Administration (FDA) as an example, over the time frame from 1981 to 2019, unaltered natural products or natural product derivatives account for nearly 33.5% of the all 185 small molecules [6]. Of particular note is that traditional Chinese medicine (TCM) is a major source of NSMs, and therein lies plenty of new chemical entities and drug leads. Some like paclitaxel (PTX), camptothecin, homoharringtonine, arteannuin, berberine etc. all as star molecules from TCM have entered clinical trials or to provide leads for new drugs [[7], [8], [9], [10], [11]]. Every year, numerous novel small molecules from nature have isolated and identified on account of natural pharmaceutical chemistry theory and conventional separation techniques by the effort of medicinal chemists. However, a major obstacle is that over 90% of the natural compounds isolated from every organism are abandoned in new drug screening owing to their poor, solubility, stability or pharmacokinetics. Whilst it has been researched deeply and has relatively mature practice and theoretical basis in structural determination and pharmacodynamic study of monomolecular compounds, the spontaneous assemblies of NSMs in liquids yielding supramolecular structures of nano-to micro-meter dimensions like vesicles, fibers, spheres, tubules, etc. as well as the relative action mode have not been clearly understood. Natural metabolites possessing diversified molecular frameworks and functional groups offer new opportunities for the study of their assemblies because of their availability in renewable supply. It also should be noted that integration effects of multi-components of TCM may be attributed to assembly properties of some NSMs. Previous studies have demonstrated that colloidal aggregates formed in TCM played a key role in the interaction with multiple targets and absorption in body [12,13]. In this review, a new hypothesis will be proposed to help uncovering the mysterious veil of the mode of action of TCM from the view of the supramolecular assembly mechanism of NSMs in TCM.
Through physical phase states interconversion of various NSMs to form nanoparticle (NP), gel, micelle, liposome etc. macroscopically in certain condition, it can be deduced that new supramolecular entities are constructed by self-assembly of single component or co-assembly of multicomponent from nature [14]. Based on NSMs with suitable size and structure, phase-behavior property of supramolecular assemblies are also usually determined by pH, concentration, temperature, type of solvents and ultrasonic frequency. Regretfully, there is no clear principle to explore the phase transition behavior of NSMs at present. For example, the preparation of natural product gels (NPGs) derived from self-assembled NSMs has been described as an empirical science because it is extremely difficult to predict whether a molecule is capable of forming a gel or not according to structural similarity [15,16]. Despite superior performance, it has already become one of the important factors of restricting development and application of NPGs.
Avoiding redundant additives and complex chemical modification, supramolecular assemblies can be established by the NSMs only via non-covalent interactions, including electrostatic interactions, hydrogen bonding, hydrophobic interactions, π-π stacking interactions and van der Waals interactions [17]. Dynamic and stimulus-responsive characteristics produced by these non-covalent interactions make the supramolecular assemblies more conducive to interface with biological systems and particularly use in the field of medicine. Researchers have realized gradually that some supramolecular NSMs such as glycyrrhizic acid could be induced by solvents to self-assemble to form gel or micelle used as drug delivery carrier with high-efficient and low-toxicity [[18], [19], [20], [21]]. In addition, the co-assembled NPs formed by the combination of glycyrrhetinic acid and liquidambaric acid for constructing carrier-free nanodrugs showed the stronger therapeutic effect with reliable biosafety comparing with each single-component [22]. Importantly, the supramolecular assemblies highlight the distinctive advantages of NSMs in terms of bioactivity and biosafety compared to the monomers. As the proverb goes, unity is strength. Therefore, supramolecular assembly may be an innate and effective mode of action of NSMs, which is of great significance in the research of natural products.
2. Diversity of self-assembled natural molecular structures
2.1. Terpenoids
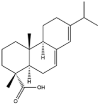
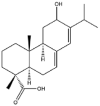
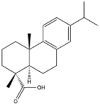
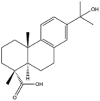
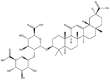
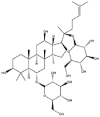
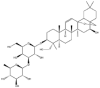
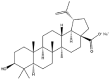
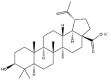
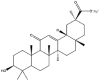
As one of the most abundant secondary metabolites in nature, terpenoid natural products possess various critical biological activities and unique self-assembled behaviors owing to the structure of multiple of isoprene units. The first self-assembly study of NSMs, betulinic acid (BA), was reported to have ability to self-assemble in nineteen organic liquids and alcohol-water mixtures to afford strong gels which revealed a fibrillar network microcosmically in 2011 [23]. Being an oxidation product of betulin, BA is a naturally 6-6-6-6-5 pentacyclic triterpenic acid widely distributed in the plant kingdom like Ziziphus jujubae and Eucommia ulmoides. In a study to evaluate the potency of self-assembled betulinic acid (SA-BA) against doxorubicin (DOX) induced cytotoxic effects in human peripheral blood lymphocytes (PBLs), pre-treatment with SA-BA could protect the PBLs from DOX induced inflammation and oxidative stress, which confirmed SA-BA can be used to ameliorate chemotherapeutic toxicity from DOX in cancer patients [24]. Comparing with non-assemble BA, SA-BA also could facilitate reactive oxygen species (ROS) and TNF-α mediated leukemic cell death, thus producing higher efficacy toward human leukemic cell lines [25]. As a triterpenoid having the same pentacyclic skeleton with BA, betulin from Betula papyrifera, liquidambaric acid from Liquidambar formosana, and lupeol from Taraxacum mongolicum all could self-assemble spontaneously in various media exhibiting different micromorphology and similar rheological behaviors [16,17,26]. Among rigid 6-6-6-6-6 pentacyclic triterpenoid compounds, arjunolic acid extracted from Terminalia arjuna was reported to self-assemble hierarchically to yield vesicular structures in aqueous solvents, accompanied by its derived compounds including crown ether, esters, ketals and arjunabromolactone affording gels [[27], [28], [29], [30], [31]]. Likewise, maslinic acid, corosolic acid, and oleanolic acid (OA) present in the fruits of Olea europaea, leaves of Psidium guajava, respectively, both underwent self-assembly especially in aqueous binary liquids giving supramolecular gels with vesicular morphology [[32], [33], [34]]. Moreover, in different liquids, self-assembled glycyrrhetinic acid from Glycyrrhiza glabra and OA from Lantana camara produced spherical objects and vesicles or fibrils to form supramolecular gels, respectively [34,35]. Besides, sumaresinolic acid from Melastoma candidum and echinocystic acid from Albizzia julibrissin had been identified as spherical or irregular NPs [36,37]. Ursolic acid (UA) presented in the leaves of Plumeria rubra were proved to aggregate into stable self-assembly in the form of supramolecular gel or nanocrystal which could improve its solubility and anticancer activity in vitro [[38], [39], [40]]. Subsequential experiments in vivo showed that self-assembled UA NPs enabled the population of CD4+ T-cells to increase significantly in the mice with the A549 cell xenograft tumor, and had the liver protection effect when inhibit the tumor growth [41]. Erythrodiol from the dried leaves of Olea europia was found to self-assemble for flowers- and grass-like fibrillar architectures [42]. The latest study revealed that pomolic acid from Rosa cymosa was capable of forming supramolecular gel with micromorphology of stacked layer in mixed solvent of DMSO and H2O [43]. Tetracyclic triterpenes such as dehydrotrametenolic acid (DTA), dehydrotumulosic acid and tricyclic triterpenes like poricoic acid A which all originated from Poria cocos also were reported to self-assemble with mixed solvents to yield gels consisted of regular nanoscale fibers (Table 1) [17,44].
Table 1.
Reported self-assembled triterpenoid NSMs.
| Compound | Structural formula | Micromorphology | Macroscopic Phase | Ref. |
|---|---|---|---|---|
| Betulinic acid | 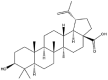 |
Fibrillar network | Gel | [23] |
| Betulin | 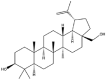 |
Flower-like fibrillary network | Gel/colloidal suspension | [26] |
| Liquidambaric acid | 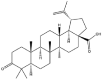 |
Fibrillar network | Gel | [17] |
| Lupeol | 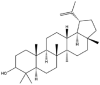 |
Rod/lamellae/fibrillar network | Gel | [16] |
| Arjunolic acid | 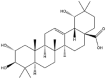 |
Vesicle | Gel | [31] |
| Maslinic acid | 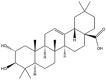 |
Vesicle | Viscous suspension | [33] |
| Corosolic acid |  |
Vesicle | Gel/colloidal suspension | [32] |
| Oleanolic acid | 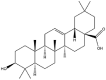 |
Vesicle/fiber | Gel | [34] |
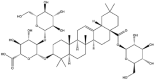
| Glycyrrhetinic acid | 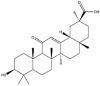 |
Sphere/petal | Gel | [35] |
| Sumaresinolic acid | 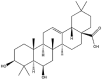 |
Sphere | NP | [36] |
| Echinocystic acid | 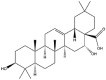 |
Irregular | NP | [37] |
| Ursolic acid | 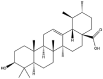 |
Vesicles/tubes/fibers/flowers/sphere/layer | Gel/colloidal suspension/nanocrystal/NP | [[38], [39], [40], [41]] |
| Erythrodiol | 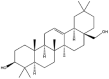 |
Flower/grass-like fibrillary network | Colloidal suspension | [42] |
| Pomolic acid | 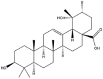 |
Layer | Gel | [43] |
| Dehydrotrametenolic acid | 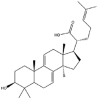 |
Fibrillar network | Gel | [16] |
| Dehydrotumulosic acid | 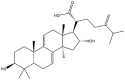 |
Fibrillar network | Gel | [17] |
| Poricoic acid A | 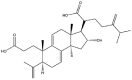 |
Fibrillar network | Gel | [16,17,44] |
Self-assembled natural diterpene acids prepared by standard emulsion-solvent evaporation method, including abietic acid (AA), 12-hydroxyabietic acid, dehydroabietic acid, and 15-hydroxy-dehydroabietic acid from Pinus koraiensis, were discovered as NPs with different morphologies (Table 2) [45].
Table 2.
Reported self-assembled diterpenoid NSMs.
2.2. Sterols
Steroids, a widespread class of NSMs containing the cyclopentano-perhydrophenanthrene steroid nucleus, have presented multiple medical value and health function, encompassing C21-steroids, phytosterols, bile acids and so forth. Phytosterols are natural bioactive constituents originated from plants, whose aliphatic side chain at C-17 often consist of 9 or 10 carbon atoms, which play major roles in stabilizing the phospholipid bilayers in cell membranes similar to cholesterol. Various products based on them have been widely applied in the areas of pharmaceuticals, nutrition, and cosmetics [46]. In the studies on chemical constituents of different plants, diosgenin from Dioscorea Nipponica, ergosterol (Ergo) from Pleurotus ostreatus, stigmasterol from Taxus chinensis and β-sitosterol from Arisaema heterophyllum Blume were found to have striking self-assembled abilities to form nanostructures [16,47].
Bile acids, a cholesterol-derived facial amphiphiles, usually present in the bile of animals with salts formation responsible for the digestion of lipids in the body, having the side chain of valeric acids at C-17 [48]. Since early 20th century, the micellization or gelation from self-assembly of bile salts in aqueous solution has been extensively investigated. It has been reported that sodium lithocholate (monohydroxy bile salt) and sodium deoxycholate (dihydroxy bile salt) can yield self-assembled helical ribbons or nanotubes to further form hydrogels in certain conditions [[49], [50], [51], [52], [53]]. Inexplicably, sodium cholate have not been confirmed to exhibit self-assembled property as a trihydroxy bile salt [53]. It is worth mentioning that cholesterol, known as a ubiquitous sterol molecule in biological membrane systems and a precursor to bile acids, vitamin D, and steroidal hormones, has been extensively exploited as hydrophobic building block to construct kinds of self-assembly systems in supramolecular and materials chemistry. In the solvent of isopropanol, cholesterol could be transformed into gel state in lower temperature spontaneously (Table 3) [54]. A number of fascinating cholesterol modified self-assemblies were created to give liposome anchors, liquid crystals, lipoprotein delivery vehicles, endosomal membrane disrupting vehicles, etc [55].
Table 3.
Reported self-assembled steroid NSMs.
| Compound | Structural formula | Micromorphology | Macroscopic Phase | Ref. |
|---|---|---|---|---|
| Diosgenin | 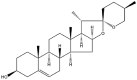 |
Fiber/pellet/pleat/rod | Gel | [16] |
| Ergosterol | 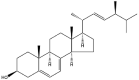 |
Lamellae/rod/pleat/clavate | Gel/NP | [16,47] |
| Stigmasterol | 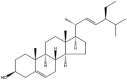 |
Rod | NP | [47] |
| β-Sitosterol | 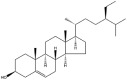 |
Clavate/belt/sheet/fiber/honeycomb/lamellae | Gel/NP | [47] |
| Cholesterol | 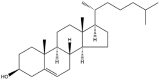 |
-a) | Gel | [54] |
“-” means no reported.
2.3. Glycosides
Theoretically, numerous hydroxyl groups residing in the glycosides will contribute to forming more hydrogen bonds, which can promote this class of compounds easy to self-assemble. And there are a lot of reports associated with self-assemblies based on saponin glycosides. The amphiphilic characteristic structure endows saponins with excellent self-assembly property and surface activity. Considering the molecular weight (MW) of most saponins is usually larger than 1000 and they are one of the well-known plant-derived biosurfactants like escin [56], tea saponin [57], Quillaja saponin [58], platycodin [59,60], ginsenosides [61] and so forth, several typical saponins with MW<1000 will be emphasized in this review.
As a representative triterpenoid saponin, the glycyrrhizic acid is a principal active ingredient in licorice, and also acknowledged as a specific ligand for targeting nanosystems. According to pioneer research, the amphiphilic nature of glycyrrhizic acid, the hydrophilic diglucuronic unit and the hydrophobic glycyrrhetic acid residues, endows it with micelle-forming capability and aggregation behavior in aqueous and non-aqueous media [62]. In recent years, it was found to self-assemble into a moldable hydrogel with microstructure of nanocluster fibrillar networks in physiological phosphate buffered saline (PBS) or pure water [18,63]. Superior self-assembled performance lead it to become the most frequently used amphiphilic molecule applying in diverse multifunctional biomaterials. Another group of dammarane-type triterpenoid saponin, ginsenosides with remarkable self-assembled performances from Panax ginseng attract researcher's attention all the time. It was reported that the amphiphilic molecule, ginsenoside Rg1, was prone to form spherical micelles or core-shell structure NP whose hydrophilic glycosyl side chains toward the outside and the hydrophobic triterpenes aglycones in the center. Nevertheless, ginsenoside Ro molecule self-assembled to form vesicles in aqueous solution with diameters of 30–50 nm [64]. Furthermore, saikosaponin A was also found to have self-assembly behavior similar to ginsenoside Rg1 [65].
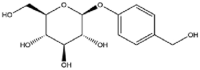
It is worth noting that gastrodin extracted from Gastrodia elata is the only phenolic glycoside consisting of benzene ring and monosaccharide simply which can gel with acetonitrile, acetone, and ethyl acetate [16]. Structurally similar molecules were abundant in nature so that we can discovered more to make up for the lack of natural low-molecular-weight gelators. Above structural formulae of self-assembled glycosides are shown in Table 4.
Table 4.
Reported self-assembled glycosides NSMs.
2.4. Others
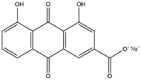
Zheng et al. reported on rhein induced by temperature and pH to self-assemble directly into sustained release supramolecular hydrogels, which could enter cells easily and facilitate to bind to the active site of toll-like receptor 4 so as to exert better anti-neuroinflammatory effects than free-drugs with almost no cytotoxicity [66]. This is to date the only naturally occurring anthraquinone having a strong capacity of self-assembly. By contrast, physcion, aloe-emodin, emodin, and chrysophanol could not form hydrogels, suggesting that the carboxyl group in anthraquinones played a key role in the gelation process [66]. Besides, sodium/potassium betulinates and sodium glycyrrhetinate had been also confirmed as excellent gelators of aqueous solvent mixtures [67,68]. The salts of NSMs is particularly conducive in fabricating water-soluble supramolecular architectures (Table 5). Therefore, supramolecular assemblies approach based on salts of NSMs should be considered to construct new host−guest complexation engaging in building enzyme-triggered drug delivery systems because of their intrinsic striking biocompatibility. There were some other biological small molecules noticed to building diverse supramolecular systems, for instance, self-assembled D-sorbitol organogel and vitamin B2 nanocrystals, which would help us to give insight into the structure-property relationship of self-assembled NSMs.
Table 5.
Reported self-assembled ionic NSMs.
3. Directly assembly mechanism of supramolecular natural products
3.1. Main driving forces of self-assembly
Structural diversities of NSMs and their multi-target differential physiological activity provide a broad foresight to screening of appropriate molecules with self-assembled characteristic and preparation of complex supramolecular systems because they are renewable resources without convoluted synthetic effort. Regrettably, in terms of self-assembled NSMs, the quantity found is not significant up to now and the vast majority of them were triterpenes. Although it was reported that a general method was proposed to screen new natural product gels for obtaining self-assembled NSMs through testing the gelation ability constantly in the whole process of extraction and separation of organisms [16], the structure-property relationship studies of them fell far behind the biological application and pharmacological research. Currently, there are hardly any other studies to focus on how to discover new self-assembled NSMs. The path to exploring more self-assembled NSMs is full of randomness and uncertainty. Therefore, summarizing the relationship between structures and self-assembled properties is needed urgently for predicting more and better correlative NSMs to expand their functional applications.
It is widely believed that the self-assembly property leads these NSMs to form micro- or nano-entities spontaneously through intermolecular noncovalent interactions such as hydrophobic interaction, hydrogen bonding, van der Waals forces, π-π interaction and others, as well as Host-Guest interaction, which determine mutually the packing patterns of molecular assembly (Fig. 2) [69]. In general, disordered molecules self-assemble to form dimers via hydrogen bonding firstly, and then stacking axially or helically to fabricate the higher-order aggregates, or in other words one-dimensional nanosturctures through non-covalent forces generated by molecular skeletons like van der Waals force. Terpenoids and steroids are two typical type of NSMs which deserve to analyze self-assembly performances emphatically. Similar rather rigid and quasi-planar backbone constituted by polycyclic alkane system with polar hydroxyl groups and nonpolar methyl groups promotes them to be preferable self-assembly building units. The number of carbon rings and substituent groups as well as their spatial dispositions may exert great influence upon the self-assembly property of them with distinguishing morphologies. Through analysis of their molecular structures, it can be deduced that polycyclic alkane skeleton, oxygen-containing functional groups, and olefin moieties constitute the three important elements of self-assembled terpenoids and steroids. Oxygen-containing functional groups generally include hydroxyl and carboxyl groups, which are acknowledged as hydrogen bonding donors/acceptors. In supramolecular self-assembly, hydrogen bond plays the dominant role in interconnection of single molecule for forming dimer. Despite being defined as a kind of noncovalent interaction, the energy of a hydrogen bond can achieve the dynamic changes from 0.5 to 40 kcal/mol relying on the nature of donators and acceptors so that it can become either strong enough to impel molecules closer together and aligning orientationally or weak sufficiently to allow molecules association disintegrating and moving chaotically at normal temperatures [70]. It has electrostatic and van der Waals origins, along with partly covalent and polarized character, which have been validated to exist between molecules as a real-space visualized non-covalent interaction force [71]. Accordingly, the hydroxyl or carboxyl groups present in different positions and orientations in self-assembled NSMs are indispensable which can contribute to providing hydrogen bond linked sites.
Fig. 2.
Schematic illustration of multiple non-covalent interactions (hydrogen bonding, van der Waals force, π−π, hydrophobic, electrostatic and dipole−dipole interactions, etc.) in the formation of the supramolecular structure.
In addition, the polycyclic alkane skeleton with distributing multiple methyl groups of terpenoids and steroids are particularly advantageous in molecular assembly via van der Waals forces and hydrophobic interaction. Molecular dynamics (MD) simulation and experimental methods such as 2D NMR NOESY, IR etc. both had been exploited to demonstrate that the van der Waals force, principally considered as dispersive interaction like σ-σ and π-σ dispersion, was the critical driving force in self-assembly of NSMs [17,35,38,43]. The van der Waals force is a kind of weak electrical attraction existing commonly between neutral molecules or atoms to maintain charge fluctuations with each other [72]. Zhi et al. had supposed that the van der Waals force occurring in self-assembled triterpenoid molecule made a contribution mainly by the formation of repulsive force [17]. The hydrophobic polycyclic alkane skeleton also will be constrained to gather together for avoiding water molecules. The self-assembly performances of most of reported NSMs in aqueous solutions suggested the hydrophobic interaction could not be neglected. Parts of hydrophilic groups like hydroxyl or carboxyl can make the molecular aggregates in aqueous solution achieving a fine hydrophilic/hydrophobic balance, thereby forming macroscopic supramolecular systems, for example, gel or nano-emulsion. Despite the amphiphilic structural characteristics of terpenoids and steroids are not obvious comparing with that of corresponding saponins, they are still proven to be fascinating supramolecular building units.
According to the analysis of the molecular formula comparatively in Table 1, Table 2, Table 3, Table 4, we found that there were a more or less number of olefin moieties encompassing carbonyl double bonds (C O) and olefinic double bonds (C C) in these structures, which meant that π-π interaction would play a certain role in molecular assembly. UV spectrum was used to determine that only the C C exhibited its π-π interaction in the self-assembly of NSMs, while C O participated in the formation of hydrogen bonding as receptor [17]. Despite relatively weak in nature, π−π interactions between aromatic rings are known to produce stacking effect with significant impact on molecular spatial arrangement owing to the extended π conjugation [73]. The results of MD simulation of dehydroabietic acid and 15-hydroxy-dehydroabietic acid suggested that offset π−π stacking dominated the monomer molecule to form dimers in a coplanar arrangement [45]. In rhein hydrogel, π−π interactions produced by the anthraquinone structure induced the aggregate conformational transformation from H-type to J-type aggregation in the process of self-assembly [66]. Hence, natural compounds containing aromatic structures are prone to bring about π−π stacking interactions, exampled with naphthalene, phenanthrene, anthracene, etc.
Out of these, some prominent low-molecular-weight hydrogelators based on synthetic alkali metal salt of NSMs had attracted our attention (Table 4). Apparently, the introduction by alkali metal salt can make the molecular self-assembly regulated by the pH of solution systems [66]. In the study of self-assembled sodium glycyrrhetinate hydrogel, the results of gelling critical temperature change with solution pH revealed that the hydrogel would became more stable as pH increasing. Comparing with sodium 3-acetyl-glycyrrhetinate, it was confirmed that 3-OH group had no impact on the molecular self-assembly. Therefore, the electrostatic and dipole–dipole interactions between alkali metal carboxylates have turned into the dominant driving forces in the process of self-assembly [67,68]. In general, there is a strong attractive force between the positive and the negative charges which endows molecules with a high dipole moment to draw them assembly in an orderly way through electrostatic and dipole–dipole interactions [74].
Even though the strength of a single type of noncovalent interaction is much weaker than covalent bonds, multiple noncovalent interactions can elicit dramatic effects on steering molecular assembly directly so as to construct steady supramolecular systems when occurring en masse. According to the relationships between noncovalent interactions and molecular structures, it is hopeful that more and more appropriate NSMs are found to self-assemble as high-performance materials using in reality.
3.2. Diverse packing patterns of self-assembled NSMs
Spontaneous self-assembly of NSMs in different liquids will yield supramolecular microstructures such as fibers, vesicles, tubules, spheres, etc. with nano-to micro-meter dimensions, accompanied by phase change behaviors macroscopically under certain conditions to afford gel, liquid crystal, NP, and so forth. The microstructures are dependent on molecular packing patterns of self-assembly, and also closely associated with the mechanism of action of these natural supermolecules. Therefore, it will be quite necessary to summarize the packing patterns of known self-assembled NSMs, which can be beneficial to make use of them in versatile application.
The NSMs were often self-assembled into the uniform spherical structures obtained by reprecipitation or standard emulsion–solvent evaporation method, which were comprised of the inward hydrophobic backbones and the outward hydrophilic hydroxyl and carboxyl groups [41,45,75]. Vesicle is also one rare and valuable kind of spherical manifestation with distinct membrane structure in self-assemblied NSMs, which can be formed in aqueous medium spontaneously. Typically, arjunolic acid, maslinic acid, corosolic acid, OA and UA could self-assemble to yield vesicles, whose diameters were in the nanometer to the micron level [[31], [32], [33], [34],39,76]. The vesicles usually can retain so robust spherical shapes that observed in the experimental conditions. The identical alkyl chained skeleton having one or two hydroxyl groups at one end and the free carboxyl group on the other end in these pentacyclic triterpenic acids have remained the cornerstone to construct bilayered vesicular structures. And the thickness of bilayered membrane obtained by electron microscopes exactly matches with the length of the dimers with optimal configuration [77,78].
Fibrillary microstructure can be found commonly in the self-assemblies of most NSMs, such as BA, liquidambaric acid, poricoic acid A and so forth [17,23,44,66]. Generally, the one-dimensional nanofibers can crosslink with the solvent molecules to form three-dimensional networks relying on polar groups. Self-assembled fibrillar networks are even so strong that hindering the mobility of solvent molecules to generate gel-like materials.
In some other cases, the microstructure of self-assemblies can be characterized by lamellae or sheet. As a typical of lamellar structure, the self-assembly mechanism of pomolic acid suggested that numerous monomers were assembled directly to form monolayer architecture and crosslinked with solvent molecules into laminated flake-like network successively [43]. Nevertheless, other structurally similar NSMs like betulin could be erected as porous nanosheets initially, and further form three dimensional flower-like superstructures which provided highly accessible surfaces to complete the fast adsorption of some ions or electrolytes [26,79]. The intermolecular “head-to-tail” arrangement also was confirmed by X-ray single crystal diffraction analysis [80,81]. The anisotropic molecular arrangement of NSMs brings about different assemblies with all sorts of microstructures, and further forming network structures via interacting with solvent molecules when the concentration is high enough [82]. Undeniably, the solvent has a dramatic effect on the supramolecular assembly modes. In the mixed solvent of ethanol–water, spherical self-assemblies of UA had been observed, while mesitylene, toluene and chlorobenzene could make the formation of flower-like superstructure [39]. And with respect to β-sitosterol, belt-like, sheet and fiber morphology exist in the solvent of methanol, DMSO and cyclohexane, respectively [83]. All in all, diverse packing patterns of self-assemblies can throw some light into the discovery of more similar NSMs, and the schematic representation for the formation of various self-assemblies in NSMs is depicted in Fig. 3.
Fig. 3.
Several self-assembly process of NSMs for several representative microstructures, including (a) spheres; Reproduced with permission [76]. Copyright 2020, American Chemical Society. (b) vesicles; Reproduced with permission [30]. Copyright 2013, American Chemical Society. (c) fibrillary networks; Reproduced with permission [76]. Copyright 2020, American Chemical Society. (d) laminated flake-like networks; Reproduced with permission [43]. Copyright 2022, Elsevier. (e) flower-like networks; Reproduced with permission [26]. Copyright 2015, American Chemical Society.
4. Multi-components assembled systems based on NSMs
Multi-components assembled system is not a definite theoretical concept in pharmacy and chemistry. It could be classified into two types, namely, multi-components directed self-assemblies and NSMs-tuned co-assemblies according to their assembly modes. Herein, the multi-components directed self-assemblies are designated as the new assembled micro- or nano-entities obtained by combining different chemical compositions without self-assembled ability in themselves. And the NSMs-tuned co-assemblies refers to novel multicomponent coordinated hybrid systems assembled usually including and depending on at least one component possessing strong capacity of self-assembly. Of particular note is that no matter what assembly modes, the intermolecular non-covalent interaction among diverse components is the driving force in the construction of multi-components assembled system in nature, thus no strict distinction exist in practice. Whilst the class and number of components were no limited conceptually, most of related research narrowly focus on a mixture of two components so far, probably due to complicated ratio and imprecise concentration of each component. Generally speaking, both of them designated as synthetic strategies are designed and developed to acquire effective, safe, renewable and multifunctional materials. They are especially common in reports associated with polymers, proteins, coupled antibodies etc. With the increase in the quantity of self-assembled NSMs, researchers have made a series of tentative explorations on multicomponents assembled systems based on NSMs and achieved some preliminary success.
4.1. Multi-components directed self-assemblies
In mining compatibility of TCM, self-precipitation and colloid of compounds induced by decocting has drawn a widespread attention. During the decocting of Huang-Lian-Jie-Du-Tang (HLJDT), one traditional Chinese medicinal prescription composed of Coptidis Rhizoma, Gardeniae Fructus, Radix Scutellariae, and Phellodendri Cortex in 3:3:2:2 proportions, naturally occurring precipitate were detected and identified as baicalin-berberine self-assembled complex with uniform spherical NPs microcosmically [[84], [85], [86]]. Inspired by that, two or three main bioactive compositions from TCM were integrated into a new self-assembled entity via intermolecular interactions. Baicalin and wogonoside, two main bioactive components in Scutellariae radix, were self-assembled with berberine to produce baicalin-berberine NPs and wogonoside-berberine nanofibers, respectively, governed by hydrophobic and electrostatic interactions. Different self-assembly processes and spatial structures of NPs and nanofibers determined the distinguishing bacteriostatic properties. The enhanced antibacterial activity was ascribed to the stronger affinity of external hydrophilic glucuronic acid in baicalin-berberine NPs to bacteria comparing with wogonoside-berberine nanofibers [87]. Similarly, a great deal of evidence supported that variety of phytochemicals rhein from Rheum palmatum L, cinnamic acid from Cinnamomi cortex, tannic acid from Rhei radix et rhizome, 3,4,5-methoxycinnamic acid from Polygalae radix and aristolochic acid in Asarum and Aristolochia all could be bound with berberine to form new self-assembled supramolecular systems in order to attain the goal of reducing the toxicity and increasing the effect of medicine [[88], [89], [90], [91], [92]]. Hence one can see that, berberine is an excellent model compound suppling electronic acceptor rest on N+, in correspondence with electronic donors such as carboxyl and phenolic hydroxyl groups inevitably resulting in electrostatic attraction. It could be confirmed further that the similar salts obtained by neurtralization of OA and different organic cations were promising assembly scaffolds in the formation of low molecular-weight hydrogels [93]. Electrostatic interaction is usually regarded as a strong noncovalent driving force because of the ability of charges to trigger toward a higher and more stable level of assembly [94]. Nevertheless, it is far from enough to self-assemble through individual electrostatic attraction. On the whole, electrostatic attraction between cations and anions had drove the formation of primary building units. Thereafter, hydrogen bonds, van der Waals forces, π−π stacking, and hydrophobic interaction etc. as the main driving forces facilitated these primary building units to form the last well-ordered assemblies and maintain the stability. Sufficient thermodynamic evidence suggested that the assembly process of them was an enthalpy-driven reaction occurring spontaneously [91]. It can be speculated that deprotonation of electron-donating groups such as carboxyl would affect the metabolic pathways, thus resulting in the offset of toxicity, meanwhile, the antimicrobial activity was enhanced due to the synergistic effect of berberine and another compound after assembly.
There are also other examples of multicomponents directed self-assemblies without relying on anion-cation complex in constructing primary building units. Wu et al. utilized two natural acids, L-(−)-malic acid or citric acid to replace water to prepared supramolecular adhesive materials with D-(+)-trehalose, D-(+)-glucose, D-(+)-fructose, and sucrose, respectively. The formation of hydrogen bond networks between natural low-molecular-weight acids and sugars by mixing and heating simply led to polymerize intensively so as to elicit the strong cohesion effects [95]. Lan et al. had designed a three multicomponent carrier-free nanodrug assembled based on natural gambogic acid, folic acid and chlorin e6 (Ce6) through π-π stacking, electrostatic and hydrophobic interactions with each other to offer a potent anticancer function via depleting glutathione (GSH) and generating reactive oxygen species (ROS) [96].
In summary, the multicomponents directed self-assemblies strategy may provide a new supramolecular mode to achieve the combined utilization of different NSMs for further application of materials and medical treatment.
4.2. Bioactive NSMs-tuned co-assemblies
For years, supramolecular co-assembly methods have been acknowledged as a fascinating synthetic strategy of new nanodrugs owing to their practicality and simplicity, especially in application of cancer treatment [97,98]. With the increase of unmodified self-assembled NSMs, constructing new NSM co-assembled nanodrugs or nanocarriers with multi-pharmacological activities have been made possible. In a word, supramolecular co-assemblies based on NSMs could not only retain and exert the unique pharmacological activities of each constituent simultaneously, but also regulate the self-assembly morphology and sizes of the constitutional units so as to make them particularly advantageous in combination nanotherapy.
Distinguishing from multicomponents directed self-assemblies mentioned above, supramolecular co-assemblies are new chemical entities on account of multiple non-covalent interactions apart from acid-base neutralization via combining different components, which can be used as nanodrugs or nanocarriers. Wang et al. have observed the formation of new morphological co-assembled NPs such as glycyrrhetinic acid and OA or betulin differentiated from that of each self-assembled NPs through simple co-dissolved method, then investigated the co-assembly mechanism by means of molecular dynamics (MD) simulation and experiment methods. More results of hybridization suggested that OA/BA, glycyrrhetinic acid/liquidambaric acid and liquidambaric acid/lupeol could be co-assembled to form new uniform NPs mainly driven by hydrophobic interaction and hydrogen bond. Further studies in vitro and in vivo have substantiated the existence of synergistic effects in the co-assemblies in tumor treatment [22,37]. However, not every effort of co-assembly would be productive. It had been corroborated that there were no co-assembled morphologies observed in some combination like UA/BA, glycyrrhetinic acid/lupeol and UA/liquidambaric acid. By comparison, MD simulation implied that whether to co-assembly might be dominated by intermolecular spatial configuration, which stacked parallel planes and approached hydrophilic groups were most favorable to the formation of hydrogen bond and hydrophobic interaction. Consequently, MD simulation has become a practical method providing guidance theoretically for predicting whether the two NSMs are able to co-assemble [22]. Furthermore, co-assembly study between PTX and betulonic acid or UA showed that supramolecular co-assembled NPs based on them exhibited striking synergistic improvement of anti-cancer efficacy through their different mechanisms individually with impressive biosafety [76,99].
In addition, NSMs were often used to co-assemble with other unnatural constituents to build up ideal drug systems. For example, eleven NSMs, encompassing betulonic acid, BA, UA and so forth, were identified to co-assemble with Ce6, a widely used photosensitizer in tumor therapy, which could ensure tumor ablation efficacy of 93.6% through a type I photoreaction mechanism [100]. Zhang et al. had established a novel carrier-free nanosystem with structure of the “core‒shell” employing co-assembled UA and polyphenol originated from food or plant, which was modified by EpCAM-aptamer for hepatocellular carcinoma synergistic immunotherapy by activating the innate and acquired immunity [101]. Taken together, the co-assembled strategy highlight the integrated superiority of different NSMs and create a new approach towards wide medical applications of natural bioactive substances.
5. Applications of supramolecular assemblies based on NSMs
Various kinds of supramolecular architectures produced by the self-assembly of NSMs have been applied in an attempt to the fields of delivering drugs, capturing pollutants, synthetizing nanomaterials, and so on (Fig. 4). Particularly, self-assembly microstructures make them containing a number of merits in pharmaceutical field owing to the complexity of modifying drugs, which can enrich the potency of known NSMs greatly [102]. Increasing evidences of self-assembled NSMs have revealed that this was an emerging trend for using assemblies of NSMs in clinic therapy.
Fig. 4.
Representative applications of supramolecular assemblies based on NSMs in the fields of delivering drugs, capturing pollutants and synthetizing nanomaterials.
One of the most common uses of functional nanomaterials is to encapsulate and deliver drugs to target site via formation of NPs. To overcome a series of obstacles of known drug nanocarriers, such as poor solubility, low drug loading capacity and no effectiveness by themselves, preparation of NPs of self-assembled NSMs was regarded as a new and simple approach to acquire carrier-free nanodrugs [41,45,97]. Different self-assembled terpenoids had been investigated that how to release active ingredients as drug delivery vehicles. Deng et al. established a new formulation of glyburide-loaded BA NPs, which provided antioxidant and anti-edema combination therapy and might be translated into treatment for ischemic stroke in clinic. It had been confirmed that BA could form NPs to penetrating the ischemic brain via interaction with cannabinoid receptor 1 (CB1), and increasing the delivery efficacy of glyburide to reduce brain edema and blood-brain barrier leakage significantly. The suitable sizes and shapes determined that BA NPs could keep longer retention in brain tissue and provide controlled release of 91% of glyburide over three days. The formulation of glyburide-loaded BA NPs also could avoid the risk of exposing glyburide to the circulatory system and inducing hypoglycemia. Moreover, BA NPs could also exert an antioxidant effect through regulation of the Nrf2 signaling pathway to improve stroke recovery [103]. Yang et al. identified and determined that self-assembled DTA NPs were capable of penetrating the gastrointestinal tract to achieve oral drugs delivery for the treatment of diseases. After oral administration, DTA NPs were found to be accumulated mainly in tumors which was likely owing to the enhanced permeability and retention (EPR) effect. Therefore, they were employed to encapsulate and deliver PTX to enhance its potency and bioavailability. Further mechanism studies showed that majority of DTA NPs could survive in the ileum, and the apical sodium-dependent bile transporter (ABST) highly expressed on the apical membrane of enterocytes account for the uptake of DTA NPs probably because of structural similarity between DTA and bile acids [36]. Wang et al. constructed a new “self-contained bioactive nanocarrier” utilizing self-assembled UA to form a stable nanocomposite with PTX, which could significantly prolong the plasma half-life, improve the antitumor effects and alleviate PTX-induced liver injury. The optimal hydrogen bonding sites and spatial conformations endows UA NPs with high drug-loading content and excellent stability. It was been speculated that UA NPs might dissociate gradually through interaction with lipids in cell membranes and release active components after entering the cells [76]. Cheng et al. selected self-assembled AA molecules as the synergistic antitumor drug to prepare AA–PTX NPs by co-administration with PTX mainly via hydrogen bonding, which led to a highly efficient anticancer efficacy and excellent biosafety. In the acidic environment, it was obtained that half of PTX was released slowly, which might be attributed to the destruction of hydrogen bonding in AA–PTX NPs by H+. This pH-responsive behavior may be conductive to targeted release of drugs in tumor tissue due to the weakly acidic tumor microenvironment. Moreover, it was indicated that lysosome acidification mediated internalization pathway, clathrin and caveolae-mediated endocytosis were main mechanisms in the cellular uptake of AA–PTX NPs [45]. Liu et al. used self-assembled bioactive OA NPs to encapsulate hydrophobic β-carotene (Car) to improve its stability and water dispersibility so as to achieve delayed and controlled release of it in simulated gastrointestinal fluid. The hydrophobic interaction between Car and OA molecules was regarded as the crucial force driving the encapsulation. The in vitro release profiles of OA–Car NPs denoted that the Car would be released preferentially in simulated intestinal fluid, which could prevent it from early degradation in simulated gastric fluid. It was suggested that the release of OA–Car NPs significantly associated with the release medium [75]. Cheng et al. proposed a promising photodynamic therapy strategy against cancers based on supramolecular photosensitizer Ce6 combined with self-assembled Ergo, namely Ergo–Ce6 NPs. The improved electron-donating abilities from intermolecular π−π stacking interactions might be responsible for promoting type I photoreactions in Ergo–Ce6 NPs induced photodynamic therapy. The remarkable water solubility, and stability as well as EPR effect of Ergo–Ce6 NPs were of great benefit to prolonging blood circulation and accumulating selectively at the tumor sites [47]. And even total ginsenosides (GS) were used to self-assemble to form NPs to increase transdermal penetration of insulin (INS). The hydrogen bonding, hydrophobic and electrostatic interaction between GS and the amino acid residues of INS made the GS NPs encapsulate INS to construct INS@GS NPs with the core/shell structure successfully, which also protected INS from degradation by body enzymes. It was inferred that the amphiphilicity of GS NPs might disturb the lipid bilayers of the stratum corneum, thereby promoting skin permeability of INS@GS NPs markedly. The skin was acted as a drug depot that realized the storage of INS@GS NPs in body for a long time and the sustained release in vivo, which also could avoid peak-valley effect and reduce toxicity [104]. Additionally, OA/glycyrrhetinic acid and glycyrrhetinic acid/liquidambaric acid co-assemblies could be used to load with PTX for improving antitumor activity and eliminating toxicity as bioactive nanocarriers possessing unique anti-inflammatory and hepatoprotective effects simultaneously. The hydrophobic cavities produced by bimolecular aggregation in these co-assemblies could interact with the benzene ring of PTX to evoke strong hydrophobic interaction. Meanwhile, hydrogen bonding interactions between drugs and carriers also ensured that PTX could be loaded in the co-assemblies efficiently to form shell-free solid NPs. After being endocytosed by the cells, the NPs would dissociate gradually and release continuously PTX through hydrophobic competition with the lipids in the cell membrane [22,37]. Gel is also used as intelligent scaffolds for drug delivery extensively, whose fibrillary networks have more durable and stable releasing capacity for preventing premature degradation, thus prolonging the circulation time and exhibiting lower cytotoxicity [2,105]. Moreover, the fibrillary networks can facilitate NSMs to be uptaken easily by cells and bind to corresponding receptors, thereby improving the therapeutic effects [66,106,107]. Particularly, bioactive injectable gel scaffolds is the first choice to enter the lesion for treatment of the disease. Zhi et al. had developed a series of injectable gel scaffolds to deliver and release DOX continuously so as to improve the antitumor effect through synergistic action in vitro and in vivo, which were established by self-assembled poricoic acid A, dehydrotumulosic acid, and liquidambaric acid [17,44]. It has been observed by many researchers that the loaded anticancer drug, DOX, could be released slowly from self-assembled arjunolic acid, OA, betulin, UA, maslinic acid, corosolic acid and sodium deoxycholate self-assemblies to the buffer solutions under physiological conditions [26,[31], [32], [33], [34],39,53]. In brief, the NSMs self-assembled to produce different one-dimensional microscopic patterns via non-covalent interactions, and further crosslink the solvents to form gel having three-dimensional network structures. And the anticancer drugs like DOX were encapsulated into the network structures by incorporation to maintain long-term storage and sustained release. Similarly, the weakly acidic environment in the tumor issues were also beneficial for the release of drugs from gel scaffolds. Besides, the self-assembly of glycyrrhizic acid, as one of the most frequently used drug delivery systems, could form water soluble “host-guest” complexes with a wide range of lipophilic drugs such as buspirone, simvastatin, lappaconitine, nifedipine etc. to strengthen the therapeutic activities and reduce side effects. In aqueous solutions, the “host” molecules, glycyrrhizic acid, self-assembled to form cyclic multimers with torus type. As “guest” molecules, the active ingredients could be located in the torus to form complexes with glycyrrhizic acid in certain proportion. The process of complexation was attributed to the multiple noncovalent interactions. After entering the body, glycyrrhizic acid might affect the ordering of lipids and enhance the fluidity and permeability of cell membrane via incorporating and/or extracting the membrane cholesterol, thus promoting the active ingredients penetration into the cell [[108], [109], [110], [111], [112]].
The fibrillar network, vesicular and porous structures induced by self-assembly with stronger electrostatic interaction and larger surface area allows greater adsorption so as to entrap toxic rhodamine-B, rhodamine 6G, acridine yellow, crystal violet, methylene blue, cresol red and other carcinogenic dyes used in the textile and paint industries [26,31,32,42,67,68]. Hence, it is likely that the self-assembled NSMs will be designed and developed as new kinds of effective and environmentally-friendly adsorbents to prevent the contamination of water from toxic dyes in the future.
In the development of new functional materials, NSMs can be used as ample raw material for their nontoxicity, low cost, long-term stability, good renewability, and biodegradability. Gel–gold NP as an emerging hybrid material could be in situ prepared by exploiting sodium/potassium betulinates, or arjunolic acid under mild conditions, which applied in the development of rapid test strip for biological labelling [31,67]. A family of natural acid–sugar adhesive materials with organic solvent-resistant and tough adhesion effects were developed dramatically as prospective alternatives to polymer adhesives materials [95]. The self-assembled fiber structures of glycyrrhetinic acid had been employed for the templated growth of semiconductor NPs like CdS, which was particularly advantageous in applying in light emitting diodes, photoelectronic devices, and other industry areas [35]. Wan et al. used glycyrrhizic acid nanofibrils to impel to transform liquid oil into smart responsive soft-solid structured materials with a promisingly applying prospect in the fields of cosmetic, pharmaceutical, or food [113]. And developing a biocompatible and ultrastable pesticide formulation is of great significance to environmental safety and agricultural production. A research revealed that on account of admirable assembled behavior of natural glycyrrhizic acid, 1 wt% of it could emulsify and stabilize 80 wt% agricultural oil, thus obtaining gel-like Pickering emulsion as eco-friendly pesticide formulation [114]. And with the outstanding rheological and atomization characteristics, it was even considered as gel propellant in rocket engines [115]. The technological innovation and development of functional materials can fully unleash the power of reproducible NSMs, which also closely tie with every aspect of our daily lives.
6. New supramolecular system hypothesis of TCMs
Since the discovery of self-assembled NSMs, they almost without exception were considered as raw resources for new chemical materials all long. However, their roles in natural medicine were ignored, to a certain extent. It is likely to provide new perspectives and technical methods to give insight into the mode of action of TCMs, because of majority of them as such extracted and isolated from TCMs. As invaluable sources and powerful weapons against diseases, TCMs has embodied the great wisdom of the Chinese ancestors in the evolution over thousands of years. During the pandemic by coronavirus disease 2019 (Covid-19) globally, the TCMs has been widely advocated to repurpose for the clinical treatment of Covid-19, such as Shuanghuanglian preparation and Lianhuaqingwen capsules [[116], [117], [118], [119]]. Nevertheless, the use of some TCMs is controversial owing to the indefinite chemical composition and mode of action, thus resulting being wrongly acknowledged and lack of entirely application internationally [120]. In recent centuries, a wealth of research on the material foundation of TCMs had illuminated the structures and pharmacological action of tens of thousands of NSMs. With the continuous improvement of isolation and purification techniques, there are constantly new NSMs being discovered from TCMs even with very low content. In terms of TCMs, they are by no means confined only as simple mixtures of various chemical compositions. Inspired by the theory of supramolecular chemistry, we will put forward a new hypothesis that the spontaneous formation of multicomponent assemblies in TCMs is responsible for their therapeutic effects, which will help scientists unravel the mystery of active substances, compositions and action mechanisms of TCMs (Fig. 5).
Fig. 5.
Schematic illustration of the new supramolecular system hypothesis of TCMs.
Over the last decade, some researchers had found the phase state interconversion phenomenon in the extraction of TCMs many times already. Zhuang et al. had observed the aggregates in all detected eighty-four TCM solutions via dynamic light scattering (DLS), and demonstrated that the biological activities of two TCM formulae were highly correlated with the aggregates which could survive in the gastro-intestinal environment and transport across the monolayer of the cells [13]. The bioactive compounds like genistein, daidzin, daidzein and puerarin were further identified in the aggregates of Pueraria lobata (Willd.) Ohwi [121]. In Bai-Hu-Tang decoction, a classic TCM preparation, nanometer aggregates consisting of mangiferin, neomangiferin, glycyrrhizic acid, ammonium glycyrrhizinate and other inorganic elements were isolated and characterized with better antipyretic activity than other dispersion phases [122]. Another study suggested that NPs isolated form broccoli had ability to adjust intestinal immune homeostasis through targeting dendritic cells [123]. Ephedrine, a well-known medicinal phytochemical, was found to be the main active constituent of colloidal NPs in Ma-Xing-Shi-Gan-Tang decoction, a TCM formula containing Ephedra sinica Stapf [124]. Pharmacological studies in vivo involving Ge-Gen-Qin-Lian-Tang decoction had showed that the constitutive aggregates of the herbal decoction possessed stronger antioxidant and hypoglycemic activities than that of soluble compositions [125]. These above achievements suggested that the decoction of TCMs were complex multicomponent dispersion systems. Colloid, emulsions, aggregates, precipitates, crystallizations, gelations and other phase state interconversion phenomenon emerging in the extracting process were attributed to the exist of parts of phytochemicals with assembly property. They either by themselves or gathering with other active substances have assembled spontaneously to construct supramolecular architectures. Into which supramolecular structure phytochemicals are formed determines their effects, delivery and metabolism. With regard to the special influence of supramolecular chemistry on the theory of TCMs, standing on the shoulders of our predecessors, we hypothesized that, TCM was a complex supramolecular systems in nature, in which a certain amount of self-assembled NSMs may bear the responsibility to deliver main active ingredients into the target organs through co-assembly or encapsulation so as to elicit the multi-effects via multiple targets, levels and pathways in the bodies and finally treat the diseases [[126], [127], [128], [129], [130]]. Notably, some NSMs not only served as the carriers for encapsulating and delivering, but also played therapeutic effects such as terpenoids and steroids, which were known to the scientists for their anticancer, anti-inflammatory and antioxidant activities [[131], [132], [133], [134], [135], [136]]. It is also an important manifestation of TCMs-“the unification of drug and adjuvant” [137]. And it is likely that the self-assembly properties determined their roles of both drug and adjuvant. Moreover, according with basic theory of thermodynamics, hot refluxing and decoction with water have inadvertently created appropriate conditions for the formation of natural assemblies, which was the cause of the majority of self-assembled NSMs discovered by the formation of thermo-sensitive gel [138,139].
Indefiniteness of effective constituents and uncertainty of synergetic mechanism of multiple components are the tremendous obstacles for clinical application of TCMs. Current research about supramolecular assembly is mostly limited in the coordination of two components owing to the simplicity in selecting optimal composite ratio of two parts. With respect to the multicomponent supramolecular systems of TCMs, we suggested to identify and isolate main supramolecular components by HPLC-MS combined with superparamagnetic iron oxide (SPIO) nanodots. The HPLC-MS technique is a conventional tool for the study of chemical substance in TCMs [140]. And the SPIO nanodot method developed by Zhou et al. was a chemical approach to directionally separate nano-NSMs utilizing SPIO as a magnetic cargo agent [36,103,[141], [142], [143]]. Alternatively, a general isolating method with the gelation ability as guidance promptly in the process of extraction and separation is convenient and effective to obtain gelling self-assembled NSMs [16]. Then according to the results of the HPLC-MS analysis, the proportion of the components will be determined and multicomponent hybrid model can be configured. Noncovalent intermolecular interactions dominate multicomponent co-assembly performance, which can bring about the increased efficacy and lower toxicity, thus highlighting the integral regulating function of TCMs. On the one hand, the multicomponent supramolecular assemblies can straightforwardly promote the internal absorption and tissue-penetrating of active constituents to produce synergistic efficacy, via improving solubility, increasing cellular membrane permeability, inhibiting intestinal metabolism, and opening paracellular tight junction between enterocytes [144]. And on the other hand, the assembly mode may block the toxic site of some toxins and hinder their metabolism, or regulating key signaling pathways to reduce the risk of tissues damage and slow down the side effects [37,76,88,99,145]. Taken together, supramolecular assembly is supposed as the underlying mechanism to endow TCMs with impressive bioactivity and biosafety. This hypothesis has provided new perspectives and techniques to help researchers understand and investigate the sophisticated nature of TCMs in the future. The traditional Chinese herbal medicine like licorice, ginseng, Radix Bupleuri, Poria cocos etc. which are rich in known self-assembled terpenoids can is used as a representative starting point of research to test it [[146], [147], [148], [149]].
7. Conclusions and prospects
From immense cosmos to living organisms, assembly is the essential law for the formation of every entity. We generally consider assembly is the process on the certain condition of building units spontaneous form well-order structure. The assemblies of biomolecules in situ forming in biological systems have opened up a new era of life science. As a consequence, supramolecular assembly chemistry is an advanced research field with unlimited vitality and good development prospects in chemistry. The discovery of self-assembled NSMs was regarded as a breakthrough in the research of natural products. After several centuries of rapid growing, natural product chemistry is undergoing a bottleneck period for development. The traditional strategy of drugs screening relying on the novel natural products began to face more and more difficulties and problems. In order to fully exploit natural resources, more and more researchers have turned the spotlight on the functionality of NSMs. With the development of supramolecular chemistry including host−guest system and directly molecular self-assembly, more and more NSMs were identified and used for preparation of functional materials [83,150]. Spontaneous assembly characteristics endow some NSMs extraordinary microstructures, functions, uses, and superiority, which have managed to get rid of the bondage of toxic additives and complicate synthesis. It becomes not only an effective strategy to design new prodrugs and discover new uses of old drugs, but also a convenient approach to prepare functional materials for practical applications in various fields of environmental protection, electromechanical industry, agricultural production and so on. Hence, it is of great significance to realize transformation from monomers to supermolecular level, which will help us understand the essence of nature in depth.
Despite the self-assembled NSMs are becoming a hotspot in the field of research, many theoretical and technological hurdles are yet to be overcome. Firstly, more novel NSMs with self-assembled property must be identified and characterized. From the view of structural chemistry, a sufficient amount of these natural compounds is the keystones of the further frame, synthesis, structural relation, pharmacological action and degradation metabolization of self-assemblies. Nevertheless, the reported numbers and structural types of them are too weak to support further research. The introduction of different functional groups in small molecule will lead to specific non-covalent forces. Essentially, the superposition of multiple non-covalent interactions between molecules is the primary cause of organized supramolecular assembly. However, it's really hard to figure out the relationship between the additive functional groups and assembling modes owing to the weakness and dynamics of non-covalent forces. There will still be efforts to clarify molecular assembling principles in different phase states.
As excellent natural resources, self-assembled NSMs are believed to have wide application prospect, especially in pharmaceutical and material industry. Related studies reported previously were so shallow that make them unable to be really applied into actual production. Actually, self-assembled NSMs may be better suited to any application systems than synthetic products because of their own characteristics such as excellent biocompatibility, renewability, biodegradability inherent bioactivities and so on. They can be used as NP platform to achieve independent delivery of small interfering RNA across the blood-brain barrier for the treatment of traumatic brain injury [151]. Vesicular self-assemblies of NSMs are expected to mimic cellular membranes for delivering drugs better and catalyze nano-reactions as microreactors more efficiently [77,78]. The gels naturally formed by self-assembled NSMs, especially glycoside, can be redesigned as bioadhesive patch in place of handsewn closure of gastrointestinal defects or medical dressing to enhance tendon or wound healing [3,[152], [153], [154]]. Compared to synthetic substrates, natural hydrogels may be better suited to simulate the internal environment for 3D cell culture and organogenesis [155,156]. The antitumor, antimicrobial, antiviral and many other biologic activities will be improved by self-assembly when manufacturing the corresponding natural biomaterials [45,[157], [158], [159]]. In other ways, the reversible and weak non-covalent interactions will make supramolecular materials highly responsive to stimuli such as pH, heat, light, ions, and electrical fields, causing a change of mechanical properties. Particularly, the electricity-, light-responsive supramolecular assemblies based on NSMs were not found yet. Hence, whether or not the natural supramolecular systems can be designed as catalytic agent converting renewable energy to fuels, reversible batteries storing ion energy and light-regulated smart materials are still subjects in need of further research [[160], [161], [162], [163]]. Based on these stimuli-responsiveness and self-healing properties, assemblies of NSMs may be more prone to control dynamic stereochemistry and accomplish dynamic covalent bonds-mediated reversible polymerization in designing multifunctional smart materials [[164], [165], [166], [167]]. Hence, it will have the great potential to be used to create more new materials.
On the basis of self-assembled NSMs, the strategy of multicomponent co-assembly was adopted to obtain more new natural materials aimed to improve the efficiency. Whilst many bicomponent co-assemblies have been confirmed to be potential drug carriers, that of three or more components have been restricted because of their complexity. Inspired by that, we proposed a new supramolecular system hypothesis of TCMs for interpreting the mode of action of TCMs from the viewpoint of multicomponent co-assembly. It is widely known that TCMs is a major source of NSMs. And with respect to the known NSMs having self-assembled properties at the moment, almost all of them were identified and isolated from TCMs. According to this, TCMs could be seen as a huge and complex supramolecular system, in which a certain of constituents having assembling properties acted as transporters to delivery other active ingredients. Certainly, the hypothesis must be validated by more results of the experiment. A combination of theories and technologies between traditional Chinese medicinal chemistry and supermolecular assembly science will be a new research direction. In summary, supramolecular assemblies based on natural small molecules are at the head of the development of chemistry as well as material science, which will be immensely valuable for examining nature of the molecular interaction and exploiting new materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research was financially supported by the General Program of the Natural Science Foundation of Beijing (No. 7202134), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1–071), the National Natural Sciences Foundation of China (No. 82073718) and the Yunnan Key Laboratory of Southern Medicine Utilization (202105AG070011).
Contributor Information
Xudong Xu, Email: xdxu@implad.ac.cn.
Guoxu Ma, Email: mgxfl8785@163.com.
References
- 1.Lehn J.M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 1988;27:89–112. [Google Scholar]
- 2.Cheetham A.G., Chakroun R.W., Ma W., Cui H. Self-assembling prodrugs. Chem. Soc. Rev. 2017;46:6638–6663. doi: 10.1039/c7cs00521k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun X., Jia P., Zhang H., Dong M., Wang J., Li L., Bu T., Wang X., Wang L., Lu Q., Wang J. Green regenerative hydrogel wound dressing functionalized by natural drug-food homologous small molecule self-Assembled nanospheres. Adv. Funct. Mater. 2021 2021. [Google Scholar]
- 4.Lin R., Cui H. Supramolecular nanostructures as drug carriers. Curr. Opin. Chem. Eng. 2015;7:75–83. [Google Scholar]
- 5.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 7.Rowinsky E.K., Donehower R.C. Paclitaxel (taxol), new. Engl. J. Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 8.Martino E., Della Volpe S., Terribile E., Benetti E., Sakaj M., Centamore A., Sala A., Collina S. The long story of camptothecin: from traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017;27:701–707. doi: 10.1016/j.bmcl.2016.12.085. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H.M., Talpaz M., Santini V., Murgo A., Cheson B., O'Brien S.M. Homoharringtonine: history, current research, and future direction. Cancer. 2001;92:1591–1605. doi: 10.1002/1097-0142(20010915)92:6<1591::aid-cncr1485>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Efferth T. From ancient herb to modern drug: artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Xun K., Wang Y., Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti Cancer Drugs. 2009;20:757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- 12.Duan D., Doak A.K., Nedyalkova L., Shoichet B.K. Colloidal aggregation and the in vitro activity of traditional Chinese medicines. ACS Chem. Biol. 2015;10:978–988. doi: 10.1021/cb5009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang Y., Yan J., Zhu W., Chen L., Liang D., Xu X. Can the aggregation be a new approach for understanding the mechanism of traditional Chinese medicine? J. Ethnopharmacol. 2008;117:378–384. doi: 10.1016/j.jep.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Wang X., Wang T., Liu M. Tuning soft nanostructures in self-assembled supramolecular gels: from morphology control to morphology-dependent functions. Small. 2015;11:1025–1038. doi: 10.1002/smll.201402075. [DOI] [PubMed] [Google Scholar]
- 15.Draper E.R., Adams D.J. Low-molecular-weight gels: the state of the art. Chem. 2017;3:390–410. [Google Scholar]
- 16.Zhi K., Zhao H., Yang X., Zhang H., Wang J., Wang J., Regenstein J.M. Natural product gelators and a general method for obtaining them from organisms. Nanoscale. 2018;10:3639–3643. doi: 10.1039/c7nr08368h. [DOI] [PubMed] [Google Scholar]
- 17.Zhi K., Wang J., Zhao H., Yang X. Self-assembled small molecule natural product gel for drug delivery:a breakthrough in new application of small molecule natural products. Acta Pharm. Sin. B. 2020;10:913–927. doi: 10.1016/j.apsb.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X., Zhang H., Gao Y., Lin Y., Hu J. A simple injectable moldable hydrogel assembled from natural glycyrrhizic acid with inherent antibacterial activity. ACS Appl. Bio Mater. 2019;3:648–653. doi: 10.1021/acsabm.9b01007. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Xue Y., Chen T., Du Q., Zhu Z., Wang Y., Wu Y., Zeng Q., Shen C., Jiang C., Yang Z., Zhu H., Liu L., Liu Q. Glycyrrhiza acid micelles loaded with licochalcone A for topical delivery: co-penetration and anti-melanogenic effect. Eur. J. Pharmaceut. Sci. 2021;167 doi: 10.1016/j.ejps.2021.106029. [DOI] [PubMed] [Google Scholar]
- 20.Shen C., Shen B., Zhu J., Wang J., Yuan H., Li X. Glycyrrhizic acid based self assembled micelles for improving oral bioavailability of paeoniflorin. Drug Dev. Ind. Pharm. 2020;47:207–214. doi: 10.1080/03639045.2020.1862178. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Zhao B., Wang S., Liang Q., Cai Y., Yang F., Li G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016;23:1623–1635. doi: 10.3109/10717544.2015.1135489. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Qiao W., Li X., Zhao H., Zhang H., Dong A., Yang X. A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy. J. Mater. Chem. B. 2021;9:1040–1048. doi: 10.1039/d0tb02071k. [DOI] [PubMed] [Google Scholar]
- 23.Bag B.G., Dash S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale. 2011;3:4564–4566. doi: 10.1039/c1nr10886g. [DOI] [PubMed] [Google Scholar]
- 24.Dash S.K., Chattopadhyay S., Ghosh T., Dash S.S., Tripathy S., Das B., Bag B.G., Das D., Roy S. Self-assembled betulinic acid protects doxorubicin induced apoptosis followed by reduction of ROS-TNF-alpha-caspase-3 activity. Biomed. Pharmacother. 2015;72:144–157. doi: 10.1016/j.biopha.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Dash S.K., Chattopadhyay S., Dash S.S., Tripathy S., Das B., Mahapatra S.K., Bag B.G., Karmakar P., Roy S. Self-assembled nano fibers of betulinic acid: a selective inducer for ROS/TNF-alpha pathway mediated leukemic cell death. Bioorg. Chem. 2015;63:85–100. doi: 10.1016/j.bioorg.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Bag B.G., Dash S.S. Hierarchical self-assembly of a renewable nanosized pentacyclic dihydroxy-triterpenoid betulin yielding flower-like architectures. Langmuir. 2015;31:13664–13672. doi: 10.1021/acs.langmuir.5b03730. [DOI] [PubMed] [Google Scholar]
- 27.Bag B.G., Dey P.P., Dinda S.K., Sheldrick W.S., Oppel I.M. A simple route for renewable nano-sized arjunolic and asiatic acids and self-assembly of arjuna-bromolactone. Beilstein J. Org. Chem. 2008;4:24. doi: 10.3762/bjoc.4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bag B.G., Dinda S.K. Arjunolic acid: a renewable template in supramolecular chemistry and nanoscience, Pure. Appl. Chem. 2007;79:2031–2038. [Google Scholar]
- 29.Bag B.G., Dinda S.K., Dey P.P., Mallia V.A., Weiss R.G. Self-assembly of esters of arjunolic acid into fibrous networks and the properties of their organogels. Langmuir. 2009;25:8663–8671. doi: 10.1021/la8042796. [DOI] [PubMed] [Google Scholar]
- 30.Bag B.G., Majumdar R., Dinda S.K., Dey P.P., Maity G.C., Mallia V.A., Weiss R.G. Self-assembly of ketals of arjunolic acid into vesicles and fibers yielding gel-like dispersions. Langmuir. 2013;29:1766–1778. doi: 10.1021/la304485e. [DOI] [PubMed] [Google Scholar]
- 31.Bag B.G., Majumdar R. Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: study of entrapment properties and in situ generation of gel–gold nanoparticle hybrid material. RSC Adv. 2014;4:53327–53334. [Google Scholar]
- 32.Bag B.G., Garai C., Ghorai S. Vesicular self-assembly of a natural ursane-type dihydroxy-triterpenoid corosolic acid. RSC Adv. 2019;9:15190–15195. doi: 10.1039/c9ra02801c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bag B.G., Hasan S.N., Ghorai S., Panja S.K. First self-assembly of dihydroxy triterpenoid maslinic acid yielding vesicles. ACS Omega. 2019;4:7684–7690. [Google Scholar]
- 34.Bag B.G., Paul K. Vesicular and fibrillar gels by self-assembly of nanosized oleanolic acid. Asian. J. Org. Chem. 2012;1:150–154. [Google Scholar]
- 35.Bag B.G., Majumdar R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012;2:8623–8626. [Google Scholar]
- 36.Yang X., Ma C., Chen Z., Liu J., Liu F., Xie R., Zhao H., Deng G., Chen A.T., Gong N., Yao L., Zuo P., Zhi K., Wang J., Gao X., Wang J., Fan L., Zhou J. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019;12:2468–2476. doi: 10.1007/s12274-019-2470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Zhao H., Qiao W., Cheng J., Han Y., Yang X. Nanomedicine-cum-carrier by co-assembly of natural small products for synergistic enhanced antitumor with tissues protective actions. ACS Appl. Mater. Interfaces. 2020;12:42537–42550. doi: 10.1021/acsami.0c12641. [DOI] [PubMed] [Google Scholar]
- 38.Lu J., Wu X., Liu L., Chen H., Liang Y. First organogelation study of ursolic acid, a natural ursane triterpenoid. Chem. Lett. 2016;45:860–862. [Google Scholar]
- 39.Bag B.G., Das S., Hasan S.N., Chandan Barai A. Nanoarchitectures by hierarchical self-assembly of ursolic acid: entrapment and release of fluorophores including anticancer drug doxorubicin. RSC Adv. 2017;7:18136–18143. [Google Scholar]
- 40.Song J., Wang Y., Song Y., Chan H., Bi C., Yang X., Yan R., Wang Y., Zheng Y. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. AAPS PharmSciTech. 2014;15:11–19. doi: 10.1208/s12249-013-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan L., Zhang B., Xu A., Shen Z., Guo Y., Zhao R., Yao H., Shao J.W. Carrier-free, pure nanodrug formed by the self-assembly of an anticancer drug for cancer immune therapy. Mol. Pharm. 2018;15:2466–2478. doi: 10.1021/acs.molpharmaceut.8b00444. [DOI] [PubMed] [Google Scholar]
- 42.Panja S.K., Bag B.G. Flower- and grass-like self-assemblies of an oleanane-type triterpenoid erythrodiol: application in the removal of toxic dye from water. ACS Omega. 2020;5:30488–30494. doi: 10.1021/acsomega.0c04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Y., Chen M., Ruan H., Sun Z., Wu H., Xu X., Yang J., Ma G., Zhou X. A new supramolecular natural product gel based on self-assembled pomolic acid from traditional Chinese medicine. Colloid Interface Sci. 2022;46 [Google Scholar]
- 44.Zhi K., Sun Y., Zhao H., Zhang C., Peng H., Yang X. Self-assembled supramolecular material derived from traditional Chinese medicine: injectable self-assembled natural product gel for drug delivery with biological activity. Mater. Today Commun. 2020;23 [Google Scholar]
- 45.Cheng J., Fu S., Qin Z., Han Y., Yang X. Self-assembled natural small molecule diterpene acids with favorable anticancer activity and biosafety for synergistically enhanced antitumor chemotherapy. J. Mater. Chem. B. 2021;9:2674–2687. doi: 10.1039/d0tb02995e. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes P., Cabral J.M. Phytosterols: applications and recovery methods. Bioresour. Technol. 2007;98:2335–2350. doi: 10.1016/j.biortech.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Cheng J., Zhao H., Yao L., Li Y., Qi B., Wang J., Yang X. Simple and multifunctional natural self-assembled sterols with anticancer activity-mediated supramolecular photosensitizers for enhanced antitumor photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:29498–29511. doi: 10.1021/acsami.9b07404. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S., Maitra U. Chemistry and biology of bile acids. Curr. Sci. 2004;87:1666–1683. [Google Scholar]
- 49.Terech P., Geyer A., Struth B., Talmon Y. Self-assembled monodisperse steroid nanotubes in water. Adv. Mater. 2002;14:495–498. [Google Scholar]
- 50.Terech P., S K.P.V., Pernot P., Wiegart L. Salt effects in the formation of self-assembled lithocholate helical ribbons and tubes. J. Phys. Chem. B. 2012;116:11344–11355. doi: 10.1021/jp305365m. [DOI] [PubMed] [Google Scholar]
- 51.Rich A., Blow D.M. Formation of a helical steroid complex. Nature. 1958;182:423–426. doi: 10.1038/182423a0. [DOI] [PubMed] [Google Scholar]
- 52.Blow D.M., Rich A. Studies on the formation of helical deoxycholate complexes. Minerva Ginecol. 1968;20:1004–1010. [Google Scholar]
- 53.Liang W., Guman-Sepulveda J.R., He S., Dogariu A., Fang J.Y. Microrheology and release behaviors of self-assembled steroid hydrogels. J. Mater. Sci. Chem. Eng. 2015;3:6–15. [Google Scholar]
- 54.Acree W.E., Bertrand G.L. A cholesterol–isopropanol gel. Nature. 1977;269:450. doi: 10.1038/269450a0. [DOI] [PubMed] [Google Scholar]
- 55.Ercole F., Whittaker M.R., Quinn J.F., Davis T.P. Cholesterol modified self-assemblies and their application to nanomedicine. Biomacromolecules. 2015;16:1886–1914. doi: 10.1021/acs.biomac.5b00550. [DOI] [PubMed] [Google Scholar]
- 56.Tsibranska S., Ivanova A., Tcholakova S., Denkov N. Self-assembly of escin molecules at the air-water interface as studied by molecular dynamics. Langmuir. 2017;33:8330–8341. doi: 10.1021/acs.langmuir.7b01719. [DOI] [PubMed] [Google Scholar]
- 57.Xu M., Wan Z., Yang X. Recent advances and applications of plant-based bioactive saponins in colloidal multiphase food systems. Molecules. 2021;26:6075. doi: 10.3390/molecules26196075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X.W., Sun S.D., Ma C.G., Yang X.Q. Oil-water interfacial-directed spontaneous self-assembly of natural Quillaja saponin for controlling interface permeability in colloidal emulsions. J. Agric. Food Chem. 2020;68:13854–13862. doi: 10.1021/acs.jafc.0c04431. [DOI] [PubMed] [Google Scholar]
- 59.Sun H., Chen L., Wang J., Wang K., Zhou J. Structure-function relationship of the saponins from the roots of Platycodon grandiflorum for hemolytic and adjuvant activity. Int. Immunopharm. 2011;11:2047–2056. doi: 10.1016/j.intimp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Dai X., Ding H., Yin Q., Wan G., Shi X., Qiao Y. Dissipative particle dynamics study on self-assembled platycodin structures: the potential biocarriers for drug delivery. J. Mol. Graph. Model. 2015;57:20–26. doi: 10.1016/j.jmgm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Liao Y., Li Z., Zhou Q., Sheng M., Qu Q., Shi Y., Yang J., Lv L., Dai X., Shi X. Saponin surfactants used in drug delivery systems: a new application for natural medicine components. Int. J. Pharm. 2021;603 doi: 10.1016/j.ijpharm.2021.120709. [DOI] [PubMed] [Google Scholar]
- 62.Stecanella L.A., Bitencourt A.P.R., Vaz G.R., Quarta E., Silva Junior J.O.C., Rossi A. Glycyrrhizic acid and its hydrolyzed metabolite 18beta-glycyrrhetinic acid as specific ligands for targeting nanosystems in the treatment of liver cancer. Pharmaceutics. 2021;13:1792. doi: 10.3390/pharmaceutics13111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saha A., Adamcik J., Bolisetty S., Handschin S., Mezzenga R. Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features. Angew. Chem. Int. Ed. Engl. 2015;54:5408–5412. doi: 10.1002/anie.201411875. [DOI] [PubMed] [Google Scholar]
- 64.Dai X., Shi X., Wang Y., Qiao Y. Solubilization of saikosaponin a by ginsenoside Ro biosurfactant in aqueous solution: mesoscopic simulation. J. Colloid Interface Sci. 2012;384:73–80. doi: 10.1016/j.jcis.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 65.Dai X., Shi X., Yin Q., Ding H., Qiao Y. Multiscale study on the interaction mechanism between ginsenoside biosurfactant and saikosaponin a. J. Colloid Interface Sci. 2013;396:165–172. doi: 10.1016/j.jcis.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 66.Zheng J., Fan R., Wu H., Yao H., Yan Y., Liu J., Ran L., Sun Z., Yi L., Dang L., Gan P., Zheng P., Yang T., Zhang Y., Tang T., Wang Y. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019;10:1604. doi: 10.1038/s41467-019-09601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bag B.G., Dash S.S. Self-assembly of sodium and potassium betulinates into hydroand organo-gels Entrapment and removal studies of fluorophores. RSC Adv. 2016;6:17290–17296. [Google Scholar]
- 68.Wu J., Lu J., Hu J., Gao Y., Ma Q., Ju Y. Self-assembly of sodium glycyrrhetinate into a hydrogel: characterisation and properties. RSC Adv. 2013;3:24906–24909. [Google Scholar]
- 69.Kumar S., Bajaj A. Advances in self-assembled injectable hydrogels for cancer therapy. Biomater. Sci. 2020;8:2055–2073. doi: 10.1039/d0bm00146e. [DOI] [PubMed] [Google Scholar]
- 70.Desiraju G.R. A bond by any other name. Angew. Chem. Int. Ed. Engl. 2011;50:52–59. doi: 10.1002/anie.201002960. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Chen P., Yuan B., Ji W., Cheng Z., Qiu X. Real-space identification of intermolecular bonding with atomic force microscopy. Science. 2013;342:611–614. doi: 10.1126/science.1242603. [DOI] [PubMed] [Google Scholar]
- 72.Lee K., Murray É.D., Kong L., Lundqvist B.I., Langreth D.C. Higher-accuracy van der Waals density functional. Phys. Rev. B. 2010;82 [Google Scholar]
- 73.Umadevi D., Panigrahi S., Sastry G.N. Noncovalent interaction of carbon nanostructures. Acc. Chem. Res. 2014;47:2574–2581. doi: 10.1021/ar500168b. [DOI] [PubMed] [Google Scholar]
- 74.Wang L., Gao G., Zhou Y., Xu T., Chen J., Wang R., Zhang R., Fu J. Tough, adhesive, self-Healable, and transparent ionically conductive zwitterionic nanocomposite hydrogels as skin strain sensors. ACS Appl. Mater. Interfaces. 2019;11:3506–3515. doi: 10.1021/acsami.8b20755. [DOI] [PubMed] [Google Scholar]
- 75.Liu S., Zhang J., Fu R., Feng H., Chu Y., Huang D., Liu H., Li C., Ma C., Abd El-Aty A.M. Improved stability and aqueous solubility of beta-carotene via encapsulation in self-assembled bioactive oleanolic acid nanoparticles. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131498. [DOI] [PubMed] [Google Scholar]
- 76.Wang J., Zhao H., Zhi K., Yang X. Exploration of the natural active small-molecule drug-loading process and highly efficient synergistic antitumor efficacy. ACS Appl. Mater. Interfaces. 2020;12:6827–6839. doi: 10.1021/acsami.9b18443. [DOI] [PubMed] [Google Scholar]
- 77.Kundu N., Banik D., Sarkar N. Self-assembly of amphiphiles into vesicles and fibrils: investigation of structure and dynamics using spectroscopy and microscopy techniques. Langmuir. 2018;34:11637–11654. doi: 10.1021/acs.langmuir.7b04355. [DOI] [PubMed] [Google Scholar]
- 78.Dutta R., Kundu S., Sarkar N. Ionic liquid-induced aggregate formation and their applications, Biophys. Rev. 2018;10:861–871. doi: 10.1007/s12551-018-0408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang J., Chen S., Xie M., Wang Y., Guo X., Guo X., Ding W. Expeditious fabrication of flower-like hierarchical mesoporous carbon superstructures as supercapacitor electrode materials. J. Mater. Chem. A. 2014;2:16884–16891. [Google Scholar]
- 80.Boryczka S., Michalik E., Jastrzebska M., Kusz J., Zubko M., Bębenek E. X-ray crystal structure of betulin–DMSO solvate. J. Chem. Crystallog. 2011;42:345–351. [Google Scholar]
- 81.Drebushchak T.N., Mikhailenko M.A., Brezgunova M.E., Shakhtshneider T.P., Kuznetsova S.A. Crystal structure of betulin ethanol solvate. J. Struct. Chem. 2010;51:798–801. [Google Scholar]
- 82.Estroff L.A., Hamilton A.D. Water gelation by small organic molecules. Chem. Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- 83.He N., Zhi K., Yang X., Zhao H., Zhang H., Wang J., Wang Z. Self-assembled fibrillar networks induced by two methods: a new unmodified natural product gel. New J. Chem. 2018;42:14170–14178. [Google Scholar]
- 84.Chen M., Wang P., Li T., Li L., Li J., Bai H., Lei H., Ma Q. Comprehensive analysis of Huanglian Jiedu decoction: revealing the presence of a self-assembled phytochemical complex in its naturally-occurring precipitate. J. Pharm. Biomed. Anal. 2021;195 doi: 10.1016/j.jpba.2020.113820. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C., Zhao R., Yan W., Wang H., Jia M., Zhu N., Zhu Y., Zhang Y., Wang P., Lei H. Compositions, formation mechanism, and neuroprotective effect of compound precipitation from the traditional Chinese prescription Huang-Lian-Jie-Du-Tang. Molecules. 2016;21:1094. doi: 10.3390/molecules21081094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H., Li T., Xiang H., Zhang X., Fang K., Wu G., Yan M., Xue N., Chen M., Xie T., Zhang Y., Wang P., Lei H. Origin and formation mechanism investigation of compound precipitation from the traditional Chinese prescription Huang-Lian-Jie-Du-Tang by isothermal titration calorimetry. Molecules. 2017;22:1456. doi: 10.3390/molecules22091456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li T., Wang P., Guo W., Huang X., Tian X., Wu G., Xu B., Li F., Yan C., Liang X.J., Lei H. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano. 2019;13:6770–6781. doi: 10.1021/acsnano.9b01346. [DOI] [PubMed] [Google Scholar]
- 88.Wang P., Guo W., Huang G., Zhen J., Li Y., Li T., Zhao L., Yuan K., Tian X., Huang X., Feng Y., Lei H., Xu A. Berberine-based heterogeneous linear supramolecules neutralized the acute nephrotoxicity of aristolochic acid by the self-assembly strategy. ACS Appl. Mater. Interfaces. 2021;13:32729–32742. doi: 10.1021/acsami.1c06968. [DOI] [PubMed] [Google Scholar]
- 89.Tian X., Wang P., Li T., Huang X., Guo W., Yang Y., Yan M., Zhang H., Cai D., Jia X., Li F., Xu B., Ma T., Yan C., Lei H. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm. Sin. B. 2020;10:1784–1795. doi: 10.1016/j.apsb.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang X., Wang P., Li T., Tian X., Guo W., Xu B., Huang G., Cai D., Zhou F., Zhang H., Lei H. Self-assemblies based on traditional medicine berberine and cinnamic acid for adhesion-induced inhibition multidrug-resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces. 2020;12:227–237. doi: 10.1021/acsami.9b17722. [DOI] [PubMed] [Google Scholar]
- 91.Chen S., Chen Z., Wang Y., Hao W., Yuan Q., Zhou H., Gao C., Wang Y., Wu X., Wang S. Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis. J. Adv. Res. 2021 doi: 10.1016/j.jare.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han N., Huang X., Tian X., Li T., Liu X., Li W., Huo S., Wu Q., Gu Y., Dai Z., Xu B., Wang P., Lei H. Self-assembled nanoparticles of natural phytochemicals (berberine and 3,4,5-methoxycinnamic acid) originated from traditional Chinese medicine for inhibiting multidrug-resistant Staphylococcus aureus. Curr. Drug Deliv. 2021;18:914–921. doi: 10.2174/1567201817666201124121918. [DOI] [PubMed] [Google Scholar]
- 93.Fan J.P., Zhong H., Zhang X.H., Yuan T.T., Chen H.P., Peng H.L. Preparation and characterization of oleanolic acid-based low-molecular-weight supramolecular hydrogels induced by heating. ACS Appl. Mater. Interfaces. 2021;13:29130–29136. doi: 10.1021/acsami.1c05800. [DOI] [PubMed] [Google Scholar]
- 94.Babut T., Semsarilar M., Rolland M., Quemener D. Nano-fibrous networks from co-assembly of amphiphilic peptide and polyelectrolyte. Polymers. 2021;13:3983. doi: 10.3390/polym13223983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu S., Cai C., Li F., Tan Z., Dong S. Supramolecular adhesive materials from natural acids and sugars with tough and organic solvent-resistant adhesion. CCS. Chemistry. 2021;3:1690–1700. [Google Scholar]
- 96.Lan J.S., Liu L., Zeng R.F., Qin Y.H., Hou J.W., Xie S.S., Yue S., Yang J., Ho R.J.Y., Ding Y., Zhang T. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021;407 [Google Scholar]
- 97.Wen Y., Zhang W., Gong N., Wang Y.F., Guo H.B., Guo W., Wang P.C., Liang X.J. Carrier-free, self-assembled pure drug nanorods composed of 10-hydroxycamptothecin and chlorin e6 for combinatorial chemo-photodynamic antitumor therapy in vivo. Nanoscale. 2017;9:14347–14356. doi: 10.1039/c7nr03129g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S., Zou Q., Li Y., Yuan C., Xing R., Yan X. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly. J. Am. Chem. Soc. 2018;140:10794–10802. doi: 10.1021/jacs.8b04912. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Qiao W., Zhao H., Yang X. Paclitaxel and betulonic acid synergistically enhance antitumor efficacy by forming co-assembled nanoparticles. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114232. [DOI] [PubMed] [Google Scholar]
- 100.Cheng J., Zhao H., Wang J., Han Y., Yang X. Bioactive natural small molecule-tuned coassembly of photosensitive drugs for highly efficient synergistic and enhanced type I photochemotherapy. ACS Appl. Mater. Interfaces. 2020;12:43488–43500. doi: 10.1021/acsami.0c13164. [DOI] [PubMed] [Google Scholar]
- 101.Zhang B., Jiang J., Wu P., Zou J., Le J., Lin J., Li C., Luo B., Zhang Y., Huang R., Shao J. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B. 2021;11:246–257. doi: 10.1016/j.apsb.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pradeesh K., Rao Kotla N., Ahmad S., Dwivedi V.K., Prakash G.V. Naturally self-assembled nanosystems and their templated structures for photonic applications. J. Nanoparticles. 2013:1–13. 2013. [Google Scholar]
- 103.Deng G., Ma C., Zhao H., Zhang S., Liu J., Liu F., Chen Z., Chen A.T., Yang X., Avery J., Zou P., Du F., Lim K.P., Holden D., Li S., Carson R.E., Huang Y., Chen Q., Kimberly W.T., Simard J.M., Sheth K.N., Zhou J. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics. 2019;9:6991–7002. doi: 10.7150/thno.35791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zou J.J., Le J.Q., Zhang B.C., Yang M.Y., Jiang J.L., Lin J.F., Wu P.Y., Li C., Chen L., Shao J.W. Accelerating transdermal delivery of insulin by ginsenoside nanoparticles with unique permeability. Int. J. Pharm. 2021;605 doi: 10.1016/j.ijpharm.2021.120784. [DOI] [PubMed] [Google Scholar]
- 105.Cui H., Xu B. Supramolecular medicine. Chem. Soc. Rev. 2017;46:6430–6432. doi: 10.1039/c7cs90102j. [DOI] [PubMed] [Google Scholar]
- 106.Cai Y., Shi Y., Wang H., Wang J., Ding D., Wang L., Yang Z. Environment-sensitive fluorescent supramolecular nanofibers for imaging applications. Anal. Chem. 2014;86:2193–2199. doi: 10.1021/ac4038653. [DOI] [PubMed] [Google Scholar]
- 107.Yang C., Wang Z., Ou C., Chen M., Wang L., Yang Z. A supramolecular hydrogelator of curcumin. Chem. Commun (Camb). 2014;50:9413–9415. doi: 10.1039/c4cc03139c. [DOI] [PubMed] [Google Scholar]
- 108.Polyakov N.E., Leshina T.V., Salakhutdinov N.F., Kispert L.D. Host-guest complexes of carotenoids with beta-glycyrrhizic acid. J. Phys. Chem. B. 2006;110:6991–6998. doi: 10.1021/jp056038l. [DOI] [PubMed] [Google Scholar]
- 109.Tolstikova T.G., Khvostov M.V., Bryzgalov A.O., Dushkin A.V., Meteleva E.S. Improvement of the pharmacological activity of nifedipine by mechanochemical complexation with glycyrrhizic acid. Biomed. Khim. 2010;56:187–194. doi: 10.18097/pbmc20105602187. [DOI] [PubMed] [Google Scholar]
- 110.Vavilin V.A., Salakhutdinov N.F., Ragino Y.I., Polyakov N.E., Taraban M.B., Leshina T.V., Stakhneva E.M., Lyakhovich V.V., Nikitin Y.P., Tolstikov G.A. The cholesterol lowering properties of the complex compound simvastatin with glycyrrhizic acid (simvaglyzin) in experimental models. Biochem. Mosc- Suppl. S. 2008;2:373–380. [PubMed] [Google Scholar]
- 111.Polyakov N.E., Khan V.K., Taraban M.B., Leshina T.V., Salakhutdinov N.F., Tolstikov G.A. Complexation of lappaconitine with glycyrrhizic acid stability and reactivity studies. J. Phys. Chem. B. 2005;109:24526–24530. doi: 10.1021/jp053434v. [DOI] [PubMed] [Google Scholar]
- 112.Selyutina O.Y., Polyakov N.E., Korneev D.V., Zaitsev B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: perspectives for drugs delivery. Drug Deliv. 2016;23:858–865. doi: 10.3109/10717544.2014.919544. [DOI] [PubMed] [Google Scholar]
- 113.Wan Z., Sun Y., Ma L., Guo J., Wang J., Yin S., Yang X. Thermoresponsive structured emulsions based on the fibrillar self-assembly of natural saponin glycyrrhizic acid. Food Funct. 2017;8:75–85. doi: 10.1039/c6fo01485b. [DOI] [PubMed] [Google Scholar]
- 114.Ma Y., Gao Y., Zhao X., Zhu Y., Du F., Hu J. A natural triterpene saponin-based Pickering emulsion. Chemistry. 2018;24:11703–11710. doi: 10.1002/chem.201801619. [DOI] [PubMed] [Google Scholar]
- 115.Sun H., Jiang J., Zhang L., Yuan C., Jiang Y., Liu P. Rheological and atomization behavior of glycyrrhizic acid based supramolecular gel propellant simulant. Colloid. Surface. A. 2022;640 [Google Scholar]
- 116.Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., Xie H., Ke C.Q., Hu H.C., Gao M.N., Yu K.Q., Liu H., Shen J.S., Tang W., Zhang L.K., Xiao G.F., Ni L., Wang D.W., Zuo J.P., Jiang H.L., Bai F., Wu Y., Ye Y., Xu Y.C. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ni L., Wen Z., Hu X., Tang W., Wang H., Zhou L., Wu L., Wang H., Xu C., Xu X., Xiao Z., Li Z., Li C., Liu Y., Duan J., Chen C., Li D., Zhang R., Li J., Yi Y., Huang W., Chen Y., Zhao J., Zuo J., Weng J., Jiang H., Wang D.W. Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front. Med. 2021;15:704–717. doi: 10.1007/s11684-021-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cyranoski D. China is promoting coronavirus treatments based on unproven traditional medicines. Nature. 2020 doi: 10.1038/d41586-020-01284-x. [DOI] [PubMed] [Google Scholar]
- 121.Hu J., Wu Z., Yan J., Pang W., Liang D., Xu X. A promising approach for understanding the mechanism of Traditional Chinese Medicine by the aggregation morphology. J. Ethnopharmacol. 2009;123:267–274. doi: 10.1016/j.jep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Lu S., Su H., Sun S., Guo Y., Liu T., Ping Y., Li Y. Isolation and characterization of nanometre aggregates from a Bai-Hu-Tang decoction and their antipyretic effect. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-30690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., Samykutty A., Zhang L., Yan J., Miller D., Suttles J., Zhang H.G. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou J., Gao G., Chu Q., Wang H., Rao P., Ke L. Chromatographic isolation of nanoparticles from Ma-Xing-Shi-Gan-Tang decoction and their characterization. J. Ethnopharmacol. 2014;151:1116–1123. doi: 10.1016/j.jep.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 125.Lin D., Du Q., Wang H., Gao G., Zhou J., Ke L., Chen T., Shaw C., Rao P. Antidiabetic micro-/nanoaggregates from Ge-Gen-Qin-Lian-Tang decoction increase absorption of baicalin and cellular antioxidant activity in vitro. BioMed Res. Int. 2017 doi: 10.1155/2017/9217912. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu-yuan H., Yi-qun Z., Kai-wen D., Jun-lin D., Ji-lian S., Wen-long L., Yan-tao Y., Yu T., Zhigang L. Special impact of supramolecular chemistry on Chinese medicine theories. China J. Chin. Mater. Med. 2014;39:1534–1543. [PubMed] [Google Scholar]
- 127.Xiao-hui Z., Pu J., Ya-jun B. Research strategy for combination of traditional Chinese medicine molecular chemistry based on the traditional theory of “Jun-Chen-Zuo-Shi”. J. Northwest. U. (Nat. Sci. Ed). 2015;45:405–412. [Google Scholar]
- 128.Lei W., Xue-xiao C., Huan-huan L., Meng W., Xiao-liang R. Application of molecular recognition and self-assembly of chemical components in study of Chinese materia medica. Chin. Tradit. Herb. Drugs. 2020;51:516–521. [Google Scholar]
- 129.Shen C.Y., Hu F., Zhu J.J., Li X.F., Shen B.D., Yuan H.L. Advances in formation and application of self-assembled nanoparticles from traditional Chinese medicine. China J. Chin. Mater. Med. 2021;46:4875–4880. doi: 10.19540/j.cnki.cjcmm.20210528.603. [DOI] [PubMed] [Google Scholar]
- 130.Hong-zhi Q., Liu-qing D., Qi-neng P., Li-hong H. Structural Chinese medicine: new research field on pharmacodynamics substance basis of traditional Chinese medicine. China J. Chin. Mater. Med. 2021;46:2443–2448. doi: 10.19540/j.cnki.cjcmm.20210129.601. [DOI] [PubMed] [Google Scholar]
- 131.Xiao J., Gao M., Fei B., Huang G., Diao Q. Nature-derived anticancer steroids outside cardica glycosides. Fitoterapia. 2020;147 doi: 10.1016/j.fitote.2020.104757. [DOI] [PubMed] [Google Scholar]
- 132.El-Baba C., Baassiri A., Kiriako G., Dia B., Fadlallah S., Moodad S., Darwiche N. Terpenoids' anti-cancer effects: focus on autophagy. Apoptosis. 2021;26:491–511. doi: 10.1007/s10495-021-01684-y. [DOI] [PubMed] [Google Scholar]
- 133.Morikawa T., Matsuda H., Yoshikawa M. A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. J. Oleo Sci. 2017;66:805–814. doi: 10.5650/jos.ess16149. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y.Y., Wang T., Yang R.X., Tang H.X., Qiang L., Liu Y.P. Anti-inflammatory steroids from the fruits of Artocarpus heterophyllus. Nat. Prod. Res. 2021;35:3071–3077. doi: 10.1080/14786419.2019.1693562. [DOI] [PubMed] [Google Scholar]
- 135.Mooradian A.D. Antioxidant properties of steroids. J. Steroid Biochem. Molec. Biol. 1993;45:509–511. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 136.González-Burgos E., Gómez-Serranillos M.P. Terpene compounds in nature a review of their potential antioxidant activity. Curr. Med. Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 137.You X., Yaqi W., Yanjun H., Zhenfeng W., Pengfei Y., Qin Z., Ming Y. A traditional and modern application research on “the unification of drug and adjuvant”. World. Sci. Technol. 2016;18:1765–1770. [Google Scholar]
- 138.Zhi K., Zhao H., Yang X., Zhang H., Wang J., Wang Z. Solvent-induced gel formation hypothesis for natural product gelators with polycyclic structures. Chempluschem. 2018;83:797–803. doi: 10.1002/cplu.201800334. [DOI] [PubMed] [Google Scholar]
- 139.Panja S., Adams D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021;50:5165–5200. doi: 10.1039/d0cs01166e. [DOI] [PubMed] [Google Scholar]
- 140.Ganzera M., Sturm S. Recent advances on HPLC/MS in medicinal plant analysis-An update covering 2011-2016. J. Pharm. Biomed. Anal. 2018;147:211–233. doi: 10.1016/j.jpba.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 141.Strohbehn G., Coman D., Han L., Ragheb R.R., Fahmy T.M., Huttner A.J., Hyder F., Piepmeier J.M., Saltzman W.M., Zhou J. Imaging the delivery of brain-penetrating PLGA nanoparticles in the brain using magnetic resonance. J. Neuro Oncol. 2015;121:441–449. doi: 10.1007/s11060-014-1658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou J., Liu J., Cheng C.J., Patel T.R., Weller C.E., Piepmeier J.M., Jiang Z., Saltzman W.M. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat. Mater. 2011;11:82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han L., Kong D.K., Zheng M.Q., Murikinati S., Ma C., Yuan P., Li L., Tian D., Cai Q., Ye C., Holden D., Park J.H., Gao X., Thomas J.L., Grutzendler J., Carson R.E., Huang Y., Piepmeier J.M., Zhou J. Increased nanoparticle delivery to brain tumors by autocatalytic priming for improved treatment and imaging. ACS Nano. 2016;10:4209–4218. doi: 10.1021/acsnano.5b07573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao Q., Luan X., Zheng M., Tian X.H., Zhao J., Zhang W.D., Ma B.L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: focus on natural occurring nanoparticles. Pharmaceutics. 2020;12:128. doi: 10.3390/pharmaceutics12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang W., Yang J., Zhao Z., Lian Z., Liang G. Intracellular coassembly boosts the anti-inflammation capacity of dexamethasone. Nanoscale. 2017;9:17717–17721. doi: 10.1039/c7nr07197c. [DOI] [PubMed] [Google Scholar]
- 146.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y., Zhang H., Dai X., Zhu R., Chen B., Xia B., Ye Z., Zhao D., Gao S., Orekhov A.N., Zhang D., Wang L., Guo S. A comprehensive review on the phytochemistry, pharmacokinetics, and antidiabetic effect of Ginseng. Phytomedicine. 2021;92 doi: 10.1016/j.phymed.2021.153717. [DOI] [PubMed] [Google Scholar]
- 148.Yang F., Dong X., Yin X., Wang W., You L., Ni J. Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Res. Int. 2017 doi: 10.1155/2017/7597596. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nie A., Chao Y., Zhang X., Jia W., Zhou Z., Zhu C. Phytochemistry and pharmacological activities of wolfiporia cocos (F.A. Wolf) ryvarden & gilb. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.505249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qu D.H., Wang Q.C., Zhang Q.W., Ma X., Tian H. Photoresponsive host-guest functional systems. Chem Rev. 2015;115:7543–7588. doi: 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- 151.Li W., Qiu J., Li X.L., Aday S., Zhang J., Conley G., Xu J., Joseph J., Lan H., Langer R., Mannix R., Karp J.M., Joshi N. BBB pathophysiology–independent delivery of siRNA in traumatic brain injury. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu J., Yuk H., Sarrafian T.L., Guo C., Griffiths L.G., Nabzdyk C.S., Zhao X. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abh2857. [DOI] [PubMed] [Google Scholar]
- 153.Freedman B.R., Kuttler A., Beckmann N., Nam S., Kent D., Schuleit M., Ramazani F., Accart N., Rock A., Li J., Kurz M., Fisch A., Ullrich T., Hast M.W., Tinguely Y., Weber E., Mooney D.J. Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity. Nat. Biomed. Eng. 2022 doi: 10.1038/s41551-021-00810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu X.L., Chu L., Dong X., Chen G.R., Tang T., Chen D., He X.P., Tian H. Multivalent glycosheets for double light-driven therapy of multidrug-resistant bacteria on wounds. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 155.Liu Z., Tang M., Zhao J., Chai R., Kang J. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv. Mater. 2018;30 doi: 10.1002/adma.201705388. [DOI] [PubMed] [Google Scholar]
- 156.Chrisnandy A., Blondel D., Rezakhani S., Broguiere N., Lutolf M.P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 2021 doi: 10.1038/s41563-021-01136-7. [DOI] [PubMed] [Google Scholar]
- 157.Song J., Yuan C., Jiao T., Xing R., Yang M., Adams D.J., Yan X. Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small. 2020;16 doi: 10.1002/smll.201907309. [DOI] [PubMed] [Google Scholar]
- 158.Lv X., Yuan M., Pei Y., Liu C., Wang X., Wu L., Cheng D., Ma X., Sun X. The enhancement of antiviral activity of chloroinconazide by aglinate-based nanogel and its plant growth promotion effect. J. Agric. Food Chem. 2021;69:4992–5002. doi: 10.1021/acs.jafc.1c00941. [DOI] [PubMed] [Google Scholar]
- 159.Zheng D.W., Deng W.W., Song W.F., Wu C.C., Liu J., Hong S., Zhuang Z.N., Cheng H., Sun Z.J., Zhang X.Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat. Biomed. Eng. 2022;6:32–43. doi: 10.1038/s41551-021-00807-9. [DOI] [PubMed] [Google Scholar]
- 160.Verma P., Singh A., Rahimi F.A., Sarkar P., Nath S., Pati S.K., Maji T.K. Charge-transfer regulated visible light driven photocatalytic H2 production and CO2 reduction in tetrathiafulvalene based coordination polymer gel. Nat. Commun. 2021;12:7313. doi: 10.1038/s41467-021-27457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qiu X., Wang X., He Y., Liang J., Liang K., Tardy B.L., Richardson J.J., Hu M., Wu H., Zhang Y., Rojas O.J., Manners I., Guo J. Superstructured mesocrystals through multiple inherent molecular interactions for highly reversible sodium ion batteries. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abh3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Z., Chen X., O'Neill S.J.K., Wu G., Whitaker D.J., Li J., McCune J.A., Scherman O.A. Highly compressible glass-like supramolecular polymer networks. Nat. Mater. 2022;21:103–109. doi: 10.1038/s41563-021-01124-x. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Q., Qu D.H., Tian H. Photo-regulated supramolecular polymers: shining beyond disassembly and reassembly. Adv. Opt. Mater. 2019;7 [Google Scholar]
- 164.Zhang Q., Crespi S., Toyoda R., Costil R., Browne W.R., Qu D.H., Tian H., Feringa B.L. Stereodivergent chirality transfer by noncovalent control of disulfide bonds. J. Am. Chem. Soc. 2022;144:4376–4382. doi: 10.1021/jacs.1c10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Q., Qu D.H., Feringa B.L., Tian H. Disulfide-mediated reversible polymerization toward intrinsically dynamic smart materials. J. Am. Chem. Soc. 2022;144:2022–2033. doi: 10.1021/jacs.1c10359. [DOI] [PubMed] [Google Scholar]
- 166.Ma X., Tian H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 2014;47:1971–1981. doi: 10.1021/ar500033n. [DOI] [PubMed] [Google Scholar]
- 167.Yin Y., Rogers J.A. Introduction: smart materials. Chem. Rev. 2022;122:4885–4886. doi: 10.1021/acs.chemrev.2c00074. [DOI] [PubMed] [Google Scholar]