Abstract
Cardiorenal syndrome is the term that describes the interaction between the heart and kidney that leads to diuretic resistance and worsening renal function. Prolonged anuria generally represents irreversible renal failure, and recovery of renal function after prolonged anuria in patients with heart failure has rarely been reported. Moreover, increased central venous pressure including heart failure is associated with impaired renal function. We herein report a rare case of a 46-year-old man with dilated cardiomyopathy who presented with dyspnea and generalized edema. His body weight increased from 90 kg to 128 kg in one year and he was hospitalized. Central venous pressure (CVP) on admission was 33 mmHg. Intravenous catecholamines were not effective; thus, he was started on continuous hemodiafiltration. Anuria occurred after hemodiafiltration due to heart failure, sepsis, and antibiotics use. However, he experienced weight reduction of over 70 kg, under hemodialysis guided by central venous pressure measurement, and renal function recovery after 87 days of anuria. His CVP had improved to 5 mmHg at discharge. This case showed continuous trial to reduce the CVP and raise cardiac output could result in the recovery of impaired renal function even in the presence of prolonged anuria.
<Learning objective: We report a rare case of a patient with dilated cardiomyopathy who experienced weight reduction of over 70 kg under hemodialysis guided by central venous pressure (CVP) measurement and renal function recovery after 87 days of anuria due to chronic heart failure. Even in the presence of prolonged anuria, continuous trial to reduce the CVP and raise cardiac output could result in the recovery of impaired renal function.>
Keywords: Anuria, Acute kidney injury, Cardiorenal syndrome, Central venous pressure, Ivabradine, Renal replacement therapy
Introduction
Cardiorenal syndrome is the term that describes the interaction between the heart and kidney that leads to diuretic resistance and worsening renal function [1]. A urine output of less than 100 mL per day is called anuria, and prolonged anuria generally represents irreversible renal failure [2]. Although cardiorenal syndrome could cause acute kidney injury or even anuria, renal function recovery after prolonged anuria in patients with heart failure has rarely been reported to date.
Case report
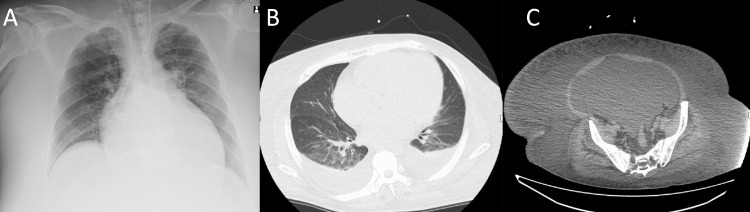
A 46-year-old man presented with dyspnea and generalized edema. His first hospitalization for heart failure was at the age of 36 years, when his body weight was 70 kg, and his brain-type natriuretic peptide (BNP) was 742 pg/mL. Moreover, his left ventricular ejection fraction (LVEF) was 10% and no coronary stenosis was noted. The patient was placed on an angiotensin-converting enzyme inhibitor, a beta-blocker, and mineralocorticoid-receptor antagonists. After these treatments, his regular checkup in outpatients revealed systolic blood pressure of 90 to 120 mmHg, heart rate of 50 to 80 beats per minute (bpm), NT-proBNP of 33 to 903 pg/mL, serum creatinine of 1.01 to 1.33 mg/dL, and New York Heart Association Class II. However, his body weight rapidly increased from 90 kg to 128 kg in one year (Fig. 1A), and he was hospitalized at another hospital. Catecholamine and intravenous diuretics were started; however, his symptoms persisted, prompting referral to our hospital for further evaluation and treatment. His past medical history included left renal infarction due to a left ventricular thrombus from the reduced LVEF. His medications included carvedilol (10 mg), candesartan (4 mg), spironolactone (25 mg), tolvaptan (15 mg), warfarin, and intravenous furosemide. His body weight on admission was 126 kg, and his body mass index was 49.2 kg/m2. The patient was afebrile with a heart rate of 104 bpm, blood pressure of 80/60 mm Hg, and oxygen saturation of 90% on room air. Physical examinations showed bilateral coarse crackles, an S3 gallop, and prominent generalized edema. Chest radiography revealed cardiomegaly with a cardiothoracic ratio of 63% and pleural effusion, with slight pulmonary congestion. Chest computed tomography scan revealed pleural effusions, ascites, and prominent subcutaneous edema (Fig. 2, Online Figs 1,2, and Video 1). Abdominal computed tomography indicated slight atrophy of the kidneys of 95.4 × 59.3 mm (right) and 97.7 × 47.9 mm (left), respectively. An electrocardiogram showed sinus tachycardia with low voltage. Online Table 1 shows laboratory studies. The LVEF was 17% with diffuse severe hypokinesis and a dilated left ventricle, and no valvular abnormalities were noted on transthoracic echocardiography. Central venous pressure (CVP) was 33 mmHg. Intravenous catecholamines were started; however, his urine emission was still insufficient. Thus, we considered auxiliary circulation, which could however not be introduced due to the lack of an access site because of the prominent generalized edema. Since he already developed oliguria and further deteriorations of his renal function as well as fluid retention were predicted, he was started on continuous hemodiafiltration (CHDF).
Fig. 1.
Physical appearance at one month before admission with 128 kg of body weight (A) and at discharge with 55 kg of body weight (B).
Fig. 2.
Chest radiography revealing bilateral pulmonary congestion and pleural effusion with a cardiothoracic ratio of 63% (A), and computed tomography scans showing pleural effusions (B), ascites, and prominent subcutaneous edema (C).
Fig. 3 shows the clinical course of this patient. After the initiation of CHDF, his diuresis was temporarily increased; however, anuria occurred on day 11. On the same day, he had fever and methicillin-sensitive Staphylococcus aureus grew in his blood culture. Our diagnosis was a catheter-related blood stream infection (CRBSI), and we started teicoplanin first and then de-escalated to cefazolin for a total of four weeks. CHDF was switched to intermittent hemodialysis on day 15. Right heart catheterization (RHC) performed on day 26, when his body weight reduced to 80 kg, revealed his CVP was still high (23 mmHg), which suggested further volume reduction was required. Other parameters were mean pulmonary artery pressure (mPAP), 36 mmHg; pulmonary capillary wedge pressure (PCWP), 25 mmHg; mixed venous oxygen saturation (SvO2), 57.6%; and thermodilution-derived cardiac index (CI), 2.1 × L/min/m2. Coronary angiography showed no significant stenosis and myocardial biopsy revealed no significant findings. Unfortunately, on day 48, he had Stenotrophomonas maltophilia-induced CRBSI, and was placed on sulfamethoxazole/trimethoprim for 2 weeks. As he had tachycardia and his blood pressure deteriorated easily during hemodialysis, we placed him on ivabradine from day 79. The dosage of ivabradine was increased after 2 weeks. After initiating ivabradine, the heart rate went down to 60 to 80 bpm (Fig. 3). The E-wave and A-wave separated from the echocardiographic index before and after administrating ivabradine (Online Fig. 3). His velocity time integral multiplied by heart rate on transthoracic echocardiography was increased from 8.4 (cm) × 90 (bpm) before to 19.2 (cm) × 65 (bpm) on administering ivabradine. Surprisingly, he began to emit urine from day 98, and increased to a sufficient volume with furosemide and tolvaptan. RHC on day 107 demonstrated the CVP had improved to 5 mmHg along with an mPAP of 18 mmHg, a PCWP of 6 mmHg, an SvO2 of 71.9%, and a CI of 2.7 L/min/m2.
Fig. 3.
Clinical course., BNP, brain-type natriuretic peptide; BP, blood pressure; BW, body weight; CHDF, continuous hemodiafiltration; CI, cardiac index; Cre, creatinine; CVP, central venous pressure; DOA, dopamine; DOB, dobutamine; HD, hemodialysis; HR, heart rate; mPAP, mean pulmonary artery pressure; NAD, noradrenaline; PCWP, pulmonary capillary wedge pressure; γ, µg/kg/min.
Ivabradine was discontinued due to eosinophil elevation (6,596 /μL), and in its place, the dosage of carvedilol was increased from 10 mg to 15 mg. The patient was discharged without dialysis on day 120 with a creatinine level of 1.5 mg/dL and a body weight of 55 kg (Fig. 1B). The LVEF was 23% with diffuse severe hypokinesis at the discharge (Online Video 2). At nine-month follow-up, the patient was treated with sacubitril valsartan, eplerenone, carvedilol, dapagliflozin, furosemide, and tolvaptan without eosinophilia, and laboratory findings showed a creatinine level of 1.4 mg/dL and BNP of 25 pg/mL.
Discussion
To the best of our knowledge, this is the first reported case of a weight loss of over 70 kg under hemodialysis and renal function recovery from prolonged anuria in a patient with chronic heart failure. In addition, this is the second longest prolonged anuria recovery in a patient with heart failure. The longest case was a patient with dilated cardiomyopathy who experienced gender- and weight-mismatch heart transplantation with iatrogenic anuria requiring dialysis in which the patient experienced renal function recovery after 315 days [2].
A previous study showed prolonged anuria was an independent risk factor for incomplete renal function recovery after continuous renal replacement therapy [3]. Coexisting renal dysfunction often complicates the treatment course of heart failure, and the use of diuretics sometimes alleviates congestion but worsens renal function [4]. Some studies showed renal venous congestion, rather than impairment of cardiac output, is the most important hemodynamic factor driving worsening renal function in decompensated patients with advanced heart failure [4]. In addition, increased CVP is associated with impaired renal function and independently related to all-cause mortality in a broad spectrum of patients with cardiovascular disease because greater CVP levels will then decrease renal perfusion pressure [5], [6], [7].
We consider our patient's renal failure was caused by multiple factors such as repeated sepsis, medications including antibiotics or diuretics, prerenal renal failure due to decreased cardiac output, and elevated renal venous pressure in addition to the original chronic kidney disease due to renal infarction in the past and heart failure itself. We thought the first trigger of anuria was sepsis because the timing of decreasing urinary output and sepsis showed a coincidence. In terms of the improvement of renal function, both improved cardiac output and reduced renal venous pressure contributed the most since the trend after initiating of ivabradine showed a gradual improvement in serum creatinine level. On admission, his CVP was 33 mmHg and it was still as high as 23 mmHg when his body weight was 80 kg. CVP was a critical guiding parameter for us and for deciding if volume reduction should be continued or not, because his body weight was 90 kg before exacerbation of heart failure this time. Finally, his CVP was improved up to 5 mmHg and the body weight was 55 kg; consequently, we continued volume reduction of even more than 70 kg. Thus, we propose hemodialysis guided by CVP measurement may be a useful way for patients with heart failure to decide the range of volume reduction.
Patients with heart failure with reduced ejection fraction are recommended to have combined treatment including angiotensin receptor/neprilysin inhibitor (ARNI), beta-blocker, mineralocorticoid receptor antagonist, and sodium–glucose co-transporter 2 inhibitor. Ivabradine was also the key drug responsible for the improvement in renal failure, although we had to stop its administration due to eosinophilia [8]. Considering the result of J-systolic heart failure treatment with the inhibitor ivabradine (J-SHIFT) trial, ivabradine may maintain or rather increase the systemic blood pressure, probably due to an increased cardiac output driven by heart rate reduction [9,10]. In this case, ivabradine helped increase cardiac output due to heart rate optimization, and the withdrawal of hemodialysis contributed to the removal of blood pressure fluctuation during hemodialysis that could eventually lead to the uptitration of the beta-blocker.
In conclusion, we report a rare case of a patient with dilated cardiomyopathy who experienced weight reduction of over 70 kg under hemodialysis guided by CVP measurement and renal function recovery after 87 days of anuria. Even in the presence of prolonged anuria, continuous trial to reduce the CVP and raise cardiac output could result in the recovery of impaired renal function.
Declaration of Competing Interest
The authors declare there is no conflict of interest.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2022.01.013.
Appendix. Supplementary materials
References
- 1.Nohria A., Hasselblad V., Stebbins A., Pauly D.F., Fonarow G.C., Shah M., Yancy C.W., Califf R.M., Stevenson L.W., Hill J.A. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y.H., Yu X.M., Yan C., Luo L., Li T.S., Xiao J. Recovery of renal function in a heart transplantation recipient with over 300 days of iatrogenic anuria: A case report. Medicine (Baltimore) 2018;97:e0451. doi: 10.1097/MD.0000000000010451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung H.Y., Lee J.H., Park Y.J., Kim S.U., Lee K.H., Choi J.Y., Park S.H., Kim C.D., Kim Y.L., Cho JH. Duration of anuria predicts recovery of renal function after acute kidney injury requiring continuous renal replacement therapy. Korean J Intern Med. 2016;31:930–937. doi: 10.3904/kjim.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullens W., Abrahams Z., Francis G.S., Sokos G., Taylor D.O., Starling R.C., Young J.B., Tang W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damman K., van Deursen V.M., Navis G., Voors A.A., van Veldhuisen D.J., Hillege H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 6.Patel A., Nguyen P. Acute kidney injury in cardiogenic shock. Methodist Debakey Cardiovasc J. 2020;16:68. doi: 10.14797/mdcj-16-1-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Akker J.P.C., Bakker J., Groeneveld A.B.J., den Uil C.A. Risk indicators for acute kidney injury in cardiogenic shock. J Crit Care. 2019;50:11–16. doi: 10.1016/j.jcrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Patel P.A., Ali N., Roy A., Pinder S., Cubbon R.M., Kearney M.T., Witte KK. Effects of ivabradine on hemodynamic and functional parameters in left ventricular systolic dysfunction: a systematic review and meta-analysis. J Gen Intern Med. 2018;33:1561–1570. doi: 10.1007/s11606-018-4578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori M., Imamura T., Nakamura M., Kinugawa K. Implication of ivabradine in up-titrating beta-blocker in a patient with advanced heart failure. Intern Med. 2021;60:897–900. doi: 10.2169/internalmedicine.6061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsui H., Momomura S.I., Yamashina A., Shimokawa H., Kihara Y., Saito Y., Hagiwara N., Ito H., Yano M., Yamamoto K., Ako J., Inomata T., Sakata Y., Tanaka T., Kawasaki Y., et al. Efficacy and safety of ivabradine in Japanese patients with chronic heart failure- J-SHIFT Study. Circ J. 2019;83:2049–2060. doi: 10.1253/circj.CJ-19-0227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.