Abstract
We have synthesized a new series of 1,2,3-triazolo piperazine and piperidine carboxylate derivatives using a simple and one-pot click chemistry with significantly reduced reaction times (~5 min) and enhanced reaction yields (~95–98%). The fourteen novel compounds thus synthesized were tested for ability to target GPR119, a G-protein coupled target receptor that plays critical role in regulation of type-2 diabetes mellitus. Four analogs (3e, 3g, 5e and 5g) demonstrated similar or better EC50 values over previously reported AR231453 activity towards GPR119.
Graphical Abstract

Triazoles form an important class of heterocyclic compounds and have recently attracted considerable attention amongst multiple pharmacological and medicinal chemistry groups for their potential applications of therapeutics. The broad swathe of applicability and potency of triazole nucleus stems from their ability to act as antiviral, antibacterial, antifungal, anticonvulsant, antidepressant, anti-inflammatory, and anticancer molecules.1 Triazoles have also been reported to inhibit glycogen synthase kinase-3,2 acts as antagonists of GABA receptors, 3 4 agonists of muscarine receptors,5 and as a neuroleptic,6 and these class of compounds also show anti-HIV-1,7 cytotoxic,8 antihistaminic,9 and antiproliferative activities.10–13 Although several synthetic methods have been reported to design and synthesize pharmacologically active triazole derivatives, they are either too tedious and/or arduous and have low reaction yields. 14–16 Thus, advancements in design and synthesis aspects of novel triazole derivatives would significantly enhance the broader pharmacological applications.
GPR119, a G-protein coupled target receptor plays a critical role in the potential therapeutic applications of type 2-diabetes mellitus, cancer and obesity.17 Triazole derivatives were recently investigated for their agonistic activity on GPR119, based on the hypothesis that such agonists will advance understandings of (a) molecular mechanisms and (b) structural contributions of novel derivatives for GPR119, especially in pathogenesis and treatment of obesity and type-2 diabetes mellitus (T2DM). Though multiple approaches are being investigated to synthesize triazole entities for use against GPR119, a simple one-step synthetic route will significantly advance the field to identify more biologically active GPR119 analogs. Herein we describe a simple one-pot synthetic method for novel tert-butyl-4-subsituted phenyl-1H-1,2,3-triazolo piperazine/piperidine carboxylate derivatives using Cu(I)-assisted click chemistry resulting in high chemical yields and purities. Furthermore, we have demonstrated their preliminary in vitro biological potency towards binding of GPR119 in human embryonic kidney (HEK) 293 cells transfected with GPR119.
AR231543 is one of the early reported, highly potent small-molecule GPR119 agonist (Arena Pharmaceuticals).18 Oral demonstration of AR231543 in rats significantly improved the circulating levels of insulin, and glucagon-like peptide 1 (GLP-1). AR231543 lowered the blood glucose concentrations in mouse islets in murine models of T2DM.19 ZSY-13 is a [1,2,4] triazole [4,3-b] pyridazine-based analog reported to be a potent GPR agonist that activates GPR119-mediated signaling pathway in T2DM.17 We used the core structure of AR231543 and ZSY-13 as our leads to design and synthesize 14 derivatives involving both piperazine- and piperidine-based triazole units (Figure 1).
Figure 1:
Structures of A. AR231453 and B. ZSY-13
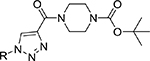
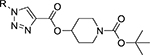
Tert-butyl 4-propioloylpiperazine-1-carboxylate (1) was reacted with aryl/alkyl substituted azides (1.0 eq) (2) in the presence of CuI (10 mol%), DIPEA (1.5 eq) in DMF (5 mL) at 0 °C for 5 min. The crude reaction mixture was quenched with ice cold water and the resultant solid was filtered, dried under vacuum and washed with anhydrous diethyl ether to produce final tert-butyl-1H-1,2,3-triazole-4-carbonyl piperazine-1-carboxylates (3a-g) in >95% purity and ~90–97% isolated yields. Similarly, tert-butyl 4-(propioloyloxy)piperidine-1-carboxylate (4) was reacted with alkyl/aryl-azides (2) under similar conditions as above to obtain the corresponding tert-butyl-1H-1,2,3-triazole-4-carbonyloxy piperidine-1-carboxylates (5a-g) in >96% purity and ~92–97% isolated yields as shown in Scheme 1. Both the reactions follow typical click chemistry reaction mechanisms.20
Scheme.1:
One-pot synthesis of 1, 2, 3-triazolo piperazine and piperidine carboxylates
The fourteen new compounds and their isolated yields were shown in Table.1. Structures of the newly synthesized compounds were completely characterized using (a) elemental analyses [C, H, N], (b) Infra-red spectroscopy, and (c) 1H and 13C NMR, and (d) Mass spectral studies (see supplementary material).
Table 1:
Library of novel triazole analogs synthesized from the one-pot synthesis, shown in Scheme 1 and their EC50 values
|
|
|||||
|---|---|---|---|---|---|---|
| R | Compound | Isolated yield (%) | EC50 (nM) | Compound | Isolated yield (%) | EC50 (nM) |
|
3a | 95 | 12.34±3.12 | 5a | 92 | 12.31 ±2.11 |

|
3b | 94 | 13.56±2.45 | 5b | 94 | 10.43±2.11 |

|
3c | 95 | 8.78±1.23 | 5c | 93 | 11.12±3.54 |
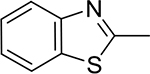
|
3d | 97 | 7.12±1.12 | 5d | 97 | 8.17±2.11 |

|
3e | 92 | 4.21±0.13 | 5e | 93 | 3.59±1.01 |

|
3f | 91 | 12.43±1.63 | 5f | 95 | 13.98±2.76 |
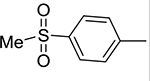
|
3g | 97 | 3.12±0.42 | 5g | 96 | 3.67± 1.01 |
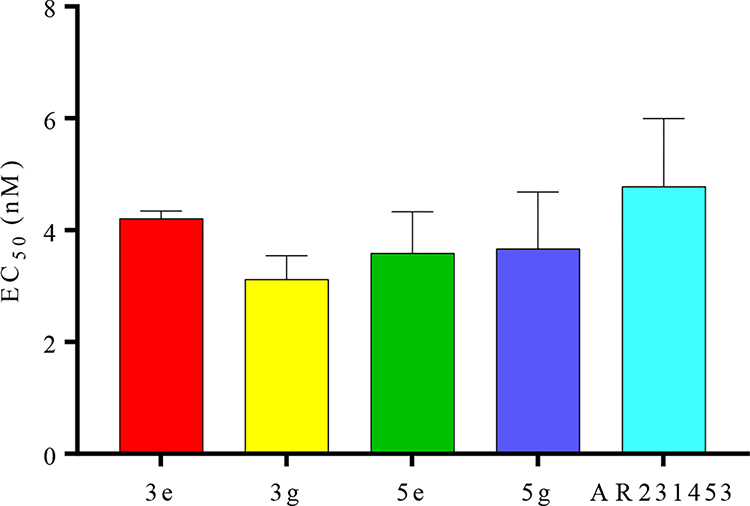
All the fourteen synthesized derivatives were tested for their efficacy to facilitate glucose-stimulated insulin secretion (GSIS) using human embryonic kidney HEK-293 cells transfected with plasmids encoding GPR119 and pCRE-Luc, a reporter designed to monitor cAMP-mediated signal transduction (HEK-293/GPR119/pCRE-luc). Previous work established that after treatment with agonists, HEK-293/GPR119/pCRE-luc cells demonstrated enhanced bioluminescence, which corresponded to increased levels of cAMP on western blot analyses, as GPR119 stimulates insulin secretion in a glucose-dependent manner.17 HEK-293/GPR119/pCRE-luc cells was therefore used a cell-based screening assay for our library of compounds. 17 AR231453 was used as the reference standard. Briefly, cells expressing GPR119 and CRE-luc were plated at a density of 10,000 cells per well in a 96-well plate. After 24 h in culture, all 14 compounds at various concentrations (0.1 nM to 1mM) were added. DMSO was used as a negative control. After an additional 24 h, luciferase activities were measured using the Steady-Glo luciferase assay system and an EnVision microplate reader according to the reported assays and manufacturer’s instructions. 17 Resultant EC50 values through the luciferase activities were calculated (Table 1). From the fourteen novel compounds tested, we prioritized four analogs i.e., 3e, 3g: piperazine- and 5e, 5g: piperidine-based carboxylates, based on the fact that they exhibit a >3-fold increase in the luciferase activity for further validation. The four analogs, i.e., 3e, 3g, 5e and 5g displayed EC50 values in the range of standard AR231543’s value of 4.78 nM (Figure 2). This initial screening suggests that (a) triazole substituted piperidine and piperazine carboxylate derivatives could serve as a potential avenue for GPR119 units and (b) among those substituents, –PhCN and –PhSO2CH3 groups may enhance the binding affinity of the analogs. Additionally, these analogs synthesized by one-pot click chemistry in short time, demonstrate comparable agonistic activity as AR231543 towards GPR119. We are currently conducting additional in vitro and ex vivo assessments on these derivatives with respect to their efficacy on activating GLP-1, including in vitro analysis for cGMP activations, desensitization, and insulin-regulated induction experiments.
Figure 2:
EC50 values of 3e, 3g, 5e and 5g in HEK-293 cells (triplicate) with AR231543 as reference standard, with p*>0.05 as statistically significant
In the present study, we have demonstrated a simple, one-pot synthetic strategy to synthesize novel tert-butyl-4-subsituted phenyl-1H-1,2,3-triazolo piperazine/piperidine carboxylate derivatives in high reaction yields and chemical purities. We also identified four novel potential GPR119 agonists and further biological evaluations including, extensive GLP-1 binding characterizations are currently explored in the lab. The four analogs with strong binding affinity could serve as promising GLP-1 candidates for the treatment of T2DM.
Supplementary Material
Acknowledgements:
The authors acknowledge financial support for these studies provided by the Translational Imaging Program at the Wake Forest School of Medicine, CTSA ULTR001420 (pilot funds to K.K.S.S), P30 ADRC grant 5P30AG049638–03 (to S. Craft), and startup funds from Wake Forest School of Medicine (to K.K.S.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kharb R, Sharma PC, Yar MS. Pharmacological significance of triazole scaffold. Journal of Enzyme Inhibition and Medicinal Chemistry. 2011;26(1): 1–21. [DOI] [PubMed] [Google Scholar]
- 2.Olesen PH, Sørensen AR, Ursø B, et al. Synthesis and in Vitro Characterization of 1-(4-Aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic Acid Derivatives. A New Class of Selective GSK-3 Inhibitors. Journal of Medicinal Chemistry. 2003;46(15): 3333–3341. [DOI] [PubMed] [Google Scholar]
- 3.Holden-Dye L, Krogsgaard-Larsen P, Nielsen L, Walker RJ. GABA receptors on the somatic muscle cells of the parasitic nematode, Ascaris suum: stereoselectivity indicates similarity to a GABAA-type agonist recognition site. British journal of pharmacology. 1989;98(3): 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biagi G, Giorgi I, Livi O, Lucacchini A, Martini C, Scartoni V. Studies on specific inhibition of benzodiazepine receptor binding by some C-benzoyl-1,2,3-triazole derivatives. Journal of Pharmaceutical Sciences. 1993;82(9): 893–896. [DOI] [PubMed] [Google Scholar]
- 5.Moltzen EK, Pedersen H, Boegesoe KP, et al. Bioisosteres of Arecoline: 1,2,3,6-Tetrahydro-5-pyridyl-Substituted and 3-Piperidyl-Substituted Derivatives of Tetrazoles and 1,2,3-Triazoles. Synthesis and Muscarinic Activity. Journal of Medicinal Chemistry. 1994;37(24): 4085–4099. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti JK, Hotten T, Pullar I, Steggles D. Synthesis and pharmacological evaluation of CNS activities of [1, 2, 3] triazolo [4, 5-b][1, 5]-, imidazolo [4, 5-b][1, 5]-, and pyrido [2, 3-b][1, 5] benzodiazepines. 10-Piperazinyl-4H-1, 2, 3-triazolo [4, 5-b][1, 5] benzodiazepines with neuroleptic activity. Journal of medicinal chemistry. 1989;32(10): 2375–2381. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez R, Velazquez S, San-Felix A, et al. 1, 2, 3-Triazole-[2, 5-Bis-O-(tert-butyldimethylsilyl)-. beta.-D-ribofuranosyl]-3’-spiro-5”-(4”-amino-1”, 2”-oxathiole 2”, 2”-dioxide)(TSAO) Analogs: Synthesis and Anti-HIV-1 Activity. Journal of medicinal chemistry. 1994;37(24): 4185–4194. [DOI] [PubMed] [Google Scholar]
- 8.Sanghvi YS, Bhattacharya BK, Kini GD, et al. Growth inhibition and induction of cellular differentiation of human myeloid leukemia cells in culture by carbamoyl congeners of ribavirin. Journal of medicinal chemistry. 1990;33(1): 336–344. [DOI] [PubMed] [Google Scholar]
- 9.Buckle DR, Rockell CJ, Smith H, Spicer BA. Studies on 1, 2, 3-triazoles. 13.(Piperazinylalkoxy)-[1] benzopyrano [2, 3-d]-1, 2, 3-triazol-9 (1H)-ones with combined H1-antihistamine and mast cell stabilizing properties. Journal of medicinal chemistry. 1986;29(11): 2262–2267. [DOI] [PubMed] [Google Scholar]
- 10.Hupe DJ, Boltz R, Cohen C, et al. The inhibition of receptor-mediated and voltage-dependent calcium entry by the antiproliferative L-651,582. Journal of Biological Chemistry. 1991;266(16): 10136–10142. [PubMed] [Google Scholar]
- 11.Li A, Mishra Y, Malik M, et al. Evaluation of N-phenyl homopiperazine analogs as potential dopamine D3 receptor selective ligands. Bioorganic & medicinal chemistry. 2013;21(11): 2988–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayatshahi HS, Xu K, Griffin SA, et al. Analogues of Arylamide Phenylpiperazine Ligands To Investigate the Factors Influencing D3 Dopamine Receptor Bitropic Binding and Receptor Subtype Selectivity. ACS Chemical Neuroscience. 2018;9(12): 2972–2983. [DOI] [PubMed] [Google Scholar]
- 13.Peng X, Wang Q, Mishra Y, et al. Synthesis, pharmacological evaluation and molecular modeling studies of triazole containing dopamine D3 receptor ligands. Bioorganic & medicinal chemistry letters. 2015;25(3): 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B-L, Zhang L-Y, Zhan Y-Z, et al. Synthesis and biological activities of novel 1,2,4-triazole thiones and bis(1,2,4-triazole thiones) containing phenylpyrazole and piperazine moieties. Journal of Fluorine Chemistry. 2016;184: 36–44. [Google Scholar]
- 15.Anil Kumar BSP, Harsha Vardhan Reddy K, Satish G, Uday Kumar R, Nageswar YVD. Synthesis of β-hydroxy-1,4-disubstituted-1,2,3-triazoles catalyzed by copper ferrite nanoparticles in tap water using click chemistry. RSC Adv, 2014;4(105): 60652–60657. [Google Scholar]
- 16.Punidha S, Sinha J, Kumar A, Ravikanth M. First Triazole-Bridged Unsymmetrical Porphyrin Dyad via Click Chemistry. The Journal of Organic Chemistry. 2008;73(1): 323–326. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S-y, Li J, Xie X. Discovery and characterization of novel small molecule agonists of G protein-coupled receptor 119. Acta pharmacologica Sinica. 2014;35(4): 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah U, Kowalski TJ. Chapter Sixteen - GPR119 Agonists for the Potential Treatment of Type 2 Diabetes and Related Metabolic Disorders. In: Litwack G, ed. Vitamins & Hormones. Vol 84. Academic Press; 2010:415–448. [DOI] [PubMed] [Google Scholar]
- 19.Hassing HA, Fares S, Larsen O, et al. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochemical Pharmacology. 2016;119: 66–75. [DOI] [PubMed] [Google Scholar]
- 20.Pachón LD, van Maarseveen JH, Rothenberg G. Click Chemistry: Copper Clusters Catalyse the Cycloaddition of Azides with Terminal Alkynes. Advanced Synthesis & Catalysis. 2005;347(6): 811–815. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.