The hippocampus has previously been implicated in both cognitive and endocrine functions1–15. We simultaneously measured electrophysiological activity from the hippocampus and interstitial glucose concentrations in the body of freely behaving rats to identify an activity pattern that may link these disparate functions of the hippocampus. Here we report that clusters of sharp wave-ripples recorded from the hippocampus reliably predicted a decrease in peripheral glucose concentrations within about 10 min. This correlation was not dependent on circadian, ultradian or meal-triggered fluctuations, could be mimicked with optogenetically induced ripples in the hippocampus (but not in the parietal cortex) and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum, which is the major conduit between the hippocampus and the hypothalamus. Our findings demonstrate that a function of the sharp wave-ripple is to modulate peripheral glucose homeostasis, and offer a mechanism for the link between sleep disruption and blood glucose dysregulation in type 2 diabetes16–18.
Evolutionary demands on the nervous system have simultaneously applied pressure on the regulation of internal homeostatic requirements and on the interaction of the organism with the external environment. Thus, seemingly disparate neural computations—such as metabolic allostasis and memory-guided behaviours—have co-evolved within the same brain circuits. The hippocampal formation is a prominent candidate for serving such a dual role in allostatic and cognitive computations1. The hippocampus receives abundant inputs from external sensory systems2, and expresses the endocrine and hormonal receptors that are necessary to integrate bodily states8,9. The efferent connections of the hippocampus also imply a dual role, in projecting to both neocortical and subcortical regions19,20.
The route by which hippocampal outputs affect endocrine and hormonal systems may be through hypothalamic hormone-releasing neurons13–15 or autonomic control of peripheral organs3–5,21.
Because hippocampal output is strongest during sharp wave-ripples (SPW-Rs)22, we hypothesized that SPW-Rs may either be an electrophysiological read-out of current or past hormonal state or, alternatively, have a role in modulating future hormonal states. As a first step, we examined the relationship between the occurrence of SPW-Rs and fluctuations of interstitial glucose levels.
SPW-Rs phase reset glucose oscillations
We used an amperometric glucose monitor to continuously measure glucose concentrations in interstitial fluid (Fig. 1a, b, Extended Data Fig. 1a–d) and recorded electrophysiological signals from the dorsal hippocampal CA1 region (Fig. 1c–f, Extended Data Fig. 2) of rats while in their home cages with ad libitum food and water, and a regular 12-h light–dark cycle (Supplementary Information). We observed a high degree of variance in glucose concentrations at several time-scales23, which could be partially explained by circadian, ultradian and food-related fluctuations (Extended Data Fig. 1e–i). Similarly, comparison of the SPW-R rate across these extended timescales revealed that a portion of the variance could be explained by circadian and ultradian mechanisms (Extended Data Fig. 3).
Fig. 1 |. Chronic recording of interstitial glucose dynamics and CA1 in freely moving rats.

a, Diagram of the glucose monitor, which was placed behind the shoulder blades on rats. b, Top, raw current values recorded by the monitor over a five-day period. Bottom, high-pass-filtered current fluctuations over the same period. c, Example recording site from CA1. d, Example CA1 SPW-Rs from day 1 (left) and day 80 (right). e, Average high-pass-filtered local field potential (100 Hz cut-off) from ripples on day 1 (top) and day 80 (bottom). f, Ripple rate over about five days. Grey dashed lines mark 00:00 h. Rats were allowed ad libitum standard chow and water throughout.
Notably, SPW-R rates were negatively correlated with future changes in glucose concentrations (Fig. 2a–f) (R = −0.21 ± 0.08, n = 30 rats, temporal lag of 10.7 ± 4.5 min). Different features of SPW-Rs modulated the magnitude of this correlation. The most impactful of these was the inter-SPW-R interval (that is, the degree of clustering of SPW-Rs). Isolated SPW-Rs had little or no effect, whereas clustered events with more than 30 SPW-Rs per minute induced a several-fold-larger decrease in peripheral glucose levels (Fig. 2g). The second most influential feature was the amplitude of SPW-Rs, followed by SPW-R duration; the frequency of the ripple oscillation had the least effect (Extended Data Fig. 4a–c).
Fig. 2 |. SPW-R rate predicts future glucose fluctuations.
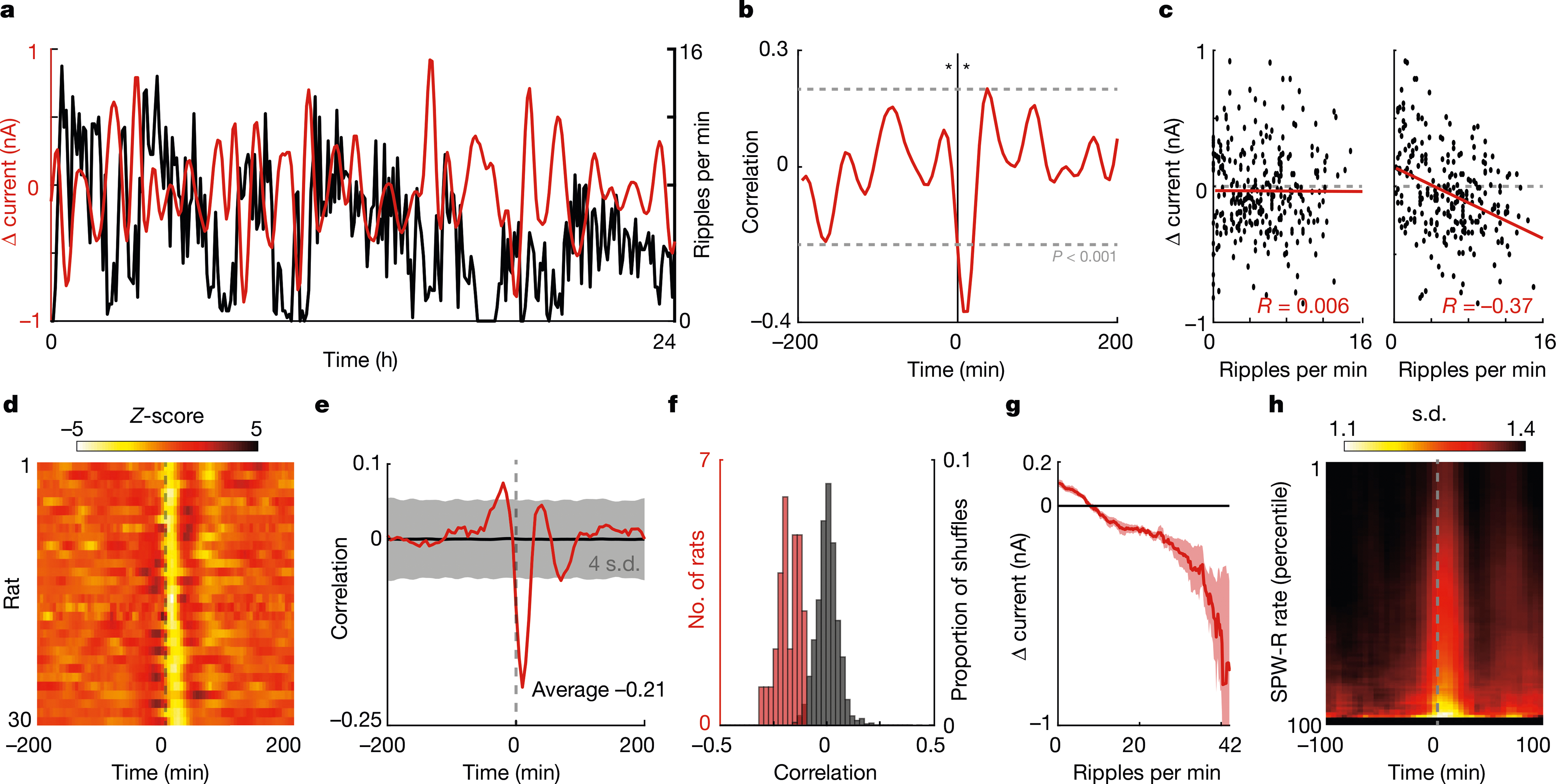
a, Example data from one 24-h period, for rat no. 7. Red trace is the derivative of the interstitial glucose concentration (shown as the change in current (Δ current)). Black trace is the rate of SPW-Rs per minute. b, Cross-correlogram for the data shown in a, centred on SPW-R rate. Asterisks indicate the 10-min offset time points shown in c. c, Scatter plots of the ripple rate and change in current with −10-min (left) and 10-min (right) lags. d, Summary cross-correlograms between SPW-R rate and change in current for all rats. e, Median cross-correlogram across rats (red) (n = 30) and median circularly shuffled cross-correlograms (black). f, Histograms of the correlation between SPW-R rate and current flux at 10 min (red) and the correlation observed when SPW-R rate is circularly permuted (black) (100 iterations per rat). g, Average change in current on the glucose monitor at 10-min time lag for 5-min epochs with different numbers of SPW-Rs. h, Heat map of the circular s.d. of glucose phase angles over time, triggered on peaks in the SPW-R rate.
Because interstitial glucose fluctuations displayed an approximately 60-min oscillation (Fig. 2a, Extended Data Fig. 1g–i), we wondered whether clusters of SPW-Rs might provide a phase-resetting signal. We observed significant power–power and phase–power relationships in the 15–75-min range (Extended Data Fig. 5), which suggests that a faster SPW-R frequency (about 40 min) modulates a slower (about 60 min) glucose rhythm (Extended Data Fig. 5d). Furthermore, the phase dispersion of the glucose rhythm decreased after SPW-Rs, and the magnitude of this decrease was proportional to SPW-R rate (Fig. 2h). In summary, clusters of SPW-Rs with larger amplitudes and shorter durations were associated with the largest decrease in future glucose concentrations, owing to phase-resetting of the glucose rhythm. This relationship could not be explained by covariance with satiation, brain state or the circadian rhythm (Extended Data Fig. 4d–l).
In addition to SPW-R rates, we also assessed the potential contribution of theta power, discretized brain state (non-rapid eye movement (NREM) sleep, or wake), continuous measures of brain state (power spectrum slope and theta/delta ratio), movement (head accelerometer), electromyogram, and linear combinations of these variables (Supplementary Information), using several predictive modelling approaches. In all cases, SPW-R rate was the most predictive variable (Extended Data Fig. 6).
Induced ripples decrease glucose levels
To further disentangle SPW-Rs from the web of correlated and hidden behavioural and physiological variables, we optogenetically induced ‘synthetic ripples’ that we applied independently from other physiological variables. We injected CamKII-ChR2-EYFP bilaterally into the dorsal hippocampi of eight rats. Six to eight weeks later, we implanted recording electrodes and 200-μm-diameter optical fibres coupled to laser diodes immediately dorsal to the viral injection site and induced ripples with ramping light pulses (Fig. 3a, b). We chose a stimulation protocol (Fig. 3c) in which the induced ripple rate was randomly chosen for every five-minute window, and thus was independent from ultradian and circadian timescales (Extended Data Fig. 7a, b). As in the intact rats, the optogenetically induced ripple events were followed by a negative peak in the cross-correlogram with glucose fluctuations (Fig. 3d, Extended Data Fig. 7c, d). The magnitude of the induced glucose effect corresponded to 63% of the effect size of naturally occurring SPW-Rs (peri-SPW-R, 0.094 nA; peri-stimulation, 0.059 nA). In seven of the eight rats, the effect on glucose concentrations was larger at higher stimulation rates (Fig. 3e, f) (median R = − 0.11, P < 0.01). The same intensity optogenetic stimulation in the posterior parietal area did not induce a measurable change in glucose concentrations (Extended Data Fig. 7e–h). Thus, the negative correlation between SPW-R rate and interstitial glucose concentration changes can be mimicked with artificially produced ripples in a manner that was decoupled from ongoing changes in vigilance, brain state and behaviour (Extended Data Fig. 7i–m).
Fig. 3 |. Optogenetic induction of ripples mimics negative correlation with glucose fluctuations.

a, Viral transfection and schematic of experiment design. CamKII-ChR2-EYFP was injected bilaterally in the hippocampus. Probes and optical fibres were implanted above the injection site. b, Blue shading is the current waveform applied to a head-mounted laser diode. Black trace is an evoked ripple event. c, Stimulation protocol. The rate of optogenetic stimulation over time. The stimulation protocol was designed to mimic the average rate of naturally occurring ripples and the dynamic range (0–40 ripples per minute). d, Top, cross-correlograms between stimulation rate and change in current for each rat. Bottom, median cross-correlogram across rats (red) and median circularly shuffled cross-correlograms (black). e, Dose response for stimulation rate. Relationship between stimulation rate and change in current at 10 min. With higher stimulation rates (x axis), we observed larger decreases in interstitial glucose concentrations. f, Histograms of the correlation between stimulation rate and current flux at 10 min (red) and the correlation observed when stimulation rate is circularly permuted (black) (100 iterations) (P = 0.00015, using a two-sample Kolmogorov–Smirnov test).
Lateral septum relays SPW-Rs downstream
We reasoned that these effects probably involve the hypothalamus either through (1) a direct projection from ventral hippocampus20 and/or (2) a disynaptic hippocampal–lateral septal–hypothalamic circuit24 (Extended Data Fig. 8a). To assess the first proposed circuit, we simultaneously recorded SPW-Rs from the dorsal and ventral CA1 pyramidal layer (Extended Data Fig. 9a–c). SPW-R events were only weakly correlated between septal and temporal poles of the hippocampus25 (Extended Data Fig. 9d). Ventral SPW-Rs were less strongly correlated with interstitial glucose fluctuations than were SPW-Rs recorded from the dorsal CA1 region (Extended Data Fig. 9e, f). To assess the second proposed circuit, we compared the effect of different hippocampal synchrony levels on lateral septum neurons during theta oscillations and SPW-Rs26 (Extended Data Fig. 8b). The magnitude of the hippocampal population firing rate, defined as the average of z-scored firing rates of all hippocampal neurons in successive 25-ms bins, was chosen as a metric that effectively separated the effect of these network patterns. Theta cycles occurred primarily at lower population firing rates, whereas SPW-Rs primarily occurred at the highest percentiles (Extended Data Fig. 8c). Lateral septal neuronal populations fired at a semi-constant rate across theta cycles with different levels of hippocampal synchrony, but increased linearly with SPW-Rs26 (Extended Data Fig. 8c). This rectified-linear response profile suggests that the circuit computations that occur during these two brain states—theta oscillations and SPW-Rs—are fundamentally different.
On the basis of this nonlinear filtering, we hypothesized that blocking the spike transmission in the lateral septum may decouple hippocampal SPW-Rs from interstitial glucose fluctuations. To this end, we expressed the inhibitory designer receptor exclusively activated by designer drug (DREADD) hM4Di in the lateral septum in six rats (Fig. 4a), and an additional seven rats served as clozapine N-oxide (CNO) controls that did not receive viral injections. Each day rats received one injection of vehicle (0.4 ml) or CNO (5 mg per kg body weight; 0.4 ml) at 09:00 h, while both SPW-Rs from CA1 and interstitial glucose levels were recorded. We then calculated cross-correlograms between SPW-R rate and glucose fluctuations in a two-hour window after injection for all days (Fig. 4b). When CNO was injected into control rats, the SPW-R correlation with glucose fluctuations was not significantly different from vehicle days (Fig. 4b–d, blue and magenta). However, when CNO was injected in rats that expressed hM4Di in the lateral septum, the SPW-R correlation with glucose fluctuations was significantly decreased compared to vehicle days and control rats on CNO days (Fig. 4b–d, Extended Data Fig. 10c, d). This difference was largest between 50 min and 190 min after injection (Fig. 4e).
Fig. 4 |. Pharmacogenetic decoupling of SPW-Rs from glucose dynamics via the lateral septum.
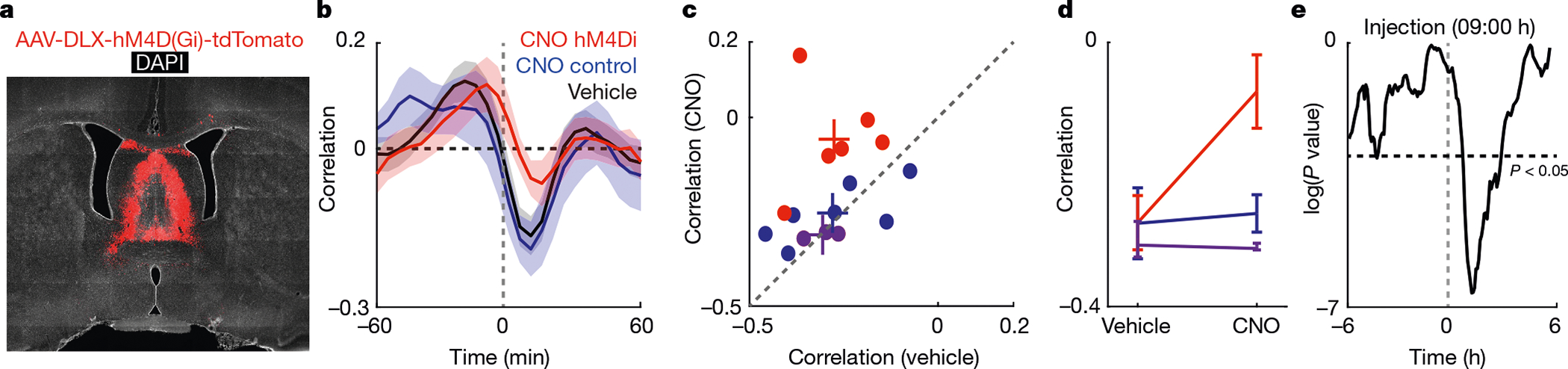
a, Viral transfection of the septum. b, Cross-correlograms using a 2-h window after injection of CNO (red for hM4Di rats; blue for control rat) or vehicle (black). Bounds are one s.e. c, Scatter plot of the effects of CNO and vehicle injections on the correlation between SPW-Rs and glucose fluctuations for each rat. Coloured ‘+’ symbols indicate the average for each cohort. As a final control, we also tested three rats (magenta dots) with a viral construct (AAV2-hSyn-hM4Di-mCherry) that selectively targeted the medial septum (Extended Data Fig. 10b). d, Summary of effects for CNO versus vehicle for each cohort. Bounds are one s.e. e, P values (two-way t-test) of the 10-min offset correlation between SPW-R rate and glucose fluctuations using a 90-min sliding window. Maximal effect of CNO was observed about 1.2 h after the injection of CNO.
Discussion
Maintaining physiological levels of blood glucose represents one of the most vital homeostatic control loops. Our findings suggest that the hippocampal bursting output, in the form of SPW-Rs, is part of such a loop (for a more detailed discussion, see Supplementary Information). SPW-Rs may exert a metabolic effect on peripheral glucose levels via the direct sympathetic and parasympathetic innervation of the pancreas and liver21,27,28. An alternate or parallel pathway may be through the regulation of glucocorticoids and growth hormones, via the hypothalamus29. Both of these hormones are known to affect insulin release and glucose homeostasis30,31. For either candidate pathway to whole-body glucose regulation, multiple hypothalamic nuclei are probably involved32. Hypothalamic neurons may be affected by direct ventral hippocampal–subicular projections20 or indirectly via the GABAergic lateral septum24. The latter pathway appears more likely given that dorsal CA1 SPW-Rs were more strongly correlated with glucose fluctuations and that pharmacogenetic inactivation of lateral septum breaks this correlation.
The effect of hippocampal output on whole-body metabolism may have feedback components. Insulin-sensitive glucose transporters, insulin receptors and GLP1 receptors are highly expressed throughout the hippocampus and lateral septum, where they may gate the production or transduction of SPW-Rs to subcortical structures33–36.
The majority of SPW-Rs occur during NREM sleep, which has been associated with changes in autonomic nervous tone37 and activity of the hypothalamic–pituitary–adrenal axis16,18,38. Despite the large number of studies that connect sleep quality and type 2 diabetes16–18, the mechanisms by which brain states affect body function has not been clarified17. One of the (presumably several) mechanisms by which sleep performs its regulatory role on glucose metabolism may be clusters of hippocampal SPW-Rs.
Finding a relationship between SPW-Rs and glucose metabolism assigns this hippocampal activity pattern a dual role. It has previously been tacitly assumed that sequences of spiking within SPW-Rs are the ‘signals’ to be transmitted and that they are computed upon or read by downstream cortical structures39. By contrast, when a target structure receives highly convergent input (such as the lateral septum), the exact sequential patterns become less important than the large degree of synaptic depolarization per unit time. In this latter case, the rate and magnitude of SPW-Rs become the signals to be read out26,40.
Mnemonic and metabolic processes are regulated simultaneously within an organism. Single events or patterns that occur at reliable behavioural time points may have been selected for—at evolutionary timescales—to act as drivers of these multiple functions. Our experiments demonstrate that two seemingly distinct processes—cognition and whole-body metabolism—are linked together by hippocampal SPW-Rs.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-021-03811-w.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Rat details
A total of 45 adult Long Evans rats were used in this study. Five were used for the hippocampal and lateral septal in vivo recordings26. Ten rats were used for chronic electrophysiological recordings paired with glucose monitoring. Eight rats were used for optogenetic induction of SPW-Rs and glucose monitoring. Six rats were used for the DREADD experiment, and seven rats were used as controls for this experiment. Three additional rats were used for simultaneous dorsal and ventral hippocampus recordings. Six additional rats were used for the posterior parietal cortex (PPC) optogenetic stimulation control experiments. Sample sizes were selected to match cohort sizes where applicable.
Recording and data processing
Recordings were conducted using the Intan RHD2000 interface board, sampled at 20 kHz. Amplification and digitization were done on the head stage. Data were visualized with Neurosuite software. All local field potential (LFP) analyses (ripple detection, state scoring and so on) were conducted on the 1,250-Hz down-sampled signal.
Chronic electrophysiological recordings
Rats were housed in passive airflow cages throughout the experiment. Rats were provided access to ad libitum food and water. Lighting in the apparatus was set on a timer to the normal 24-h light–dark cycle that rats experienced in the vivarium. The tops of the cages were extended vertically, and a small hole was added in the cage cover to pass electrophysiology cabling through. Cabling was wired through a commutator, allowing for the rat to turn in either direction without the cabling getting twisted. With the vertical extension of the cage walls, rats were provided a larger range of motion than currently exists in the vivarium housing and the cabling used for recording did not impede or restrict this range of motion. Rats were handled and acclimated to this home cage and recording room for 7 days before any experiments. During the experiment, rats were recorded continuously for up to 7 days in the modified home cage. All experiments were approved by the Institutional Animal Care and Use Committee at New York University Medical Center.
SPW-R detection
A modified version of a previously reported detection algorithm was used for hippocampal SPW-R41. Input parameters for the algorithm are stored in each of the MATLAB data structures storing ripple events. The code is available at https://github.com/buzsakilab/buzcode (bz_FindRipples.m). Ripples were detected by filtering (120–200 Hz; Butterworth; order = 3) the raw LFP (1.25 kHz) and calculating the normalized squared signal (NSS). The NSS was then used to identify peaks above a threshold merging neighbouring events and discarding events with excessive duration. Thresholds were computed as multiples of the s.d. of the NSS. The peak power threshold was set to five s.d. and the beginning and end cut-offs were set to two s.d. above the mean NSS. The duration limits were set to 15 and 250 ms. An additional ‘noise’ channel was provided to the algorithm that was not anatomically near CA1 (and did not have ripples in the LFP signal). This channel was used to exclude electromyogram (EMG)-related artefacts that had power in the 120–200 Hz range. High-power 120–200 Hz events occurring on this noise channel were used to identify and exclude simultaneously occurring 120–200-Hz high-power events on the ‘ripple’ channel. The same 5 s.d. threshold was applied to the NSS of this channel for detecting events. If such noise events occurred within the start and stop times for an event detected on the ripple channel, it was excluded as noise. For recordings without a ‘non-ripple’ channel, the estimated EMG was used for this exclusion criteria42.
Start and stop times for each ripple were considered the first timestamp at which the 120–200-Hz power dropped below 2 s.d. before, and following, the ripple peak (maximum power value >5 s.d.). Ripple duration was calculated as the difference between stop and start times. The instantaneous frequency for each ripple was calculated by (1) applying the Hilbert transform to the bandpass-filtered (120–200 Hz) LFP, (2) unwrapping these phase angles and median filtering (±6 bins) and (3) taking the difference between samples nearest the peak power bin of the ripple and dividing by two pi. The ripple amplitude was taken as the maximum NSS value between start andstop times for each ripple.
State scoring
A previously described semi-automated sleep scoring algorithm was used43,44. It calculates a spectrogram from the raw LFP (1,250 Hz) using a 10-s window fast Fourier transform, sliding at 1-s intervals, at logarithmically spaced frequencies from 1 to 100 Hz. In brief, this algorithm then uses a set of heuristics when examining the EMG, theta band ratio (4–9 Hz divided by 2–16 Hz) and broadband LFP. The estimated EMG is the summed pairwise zero-lag correlation between non-neighbouring electrodes (separate shanks; >200 μm distance) using the bandpass-filtered (300–600 Hz; 3th order Butterworth) LFP42. The theta band ratio is the 4–9-Hz power from the spectrogram, dividing by the 2–16-Hz power. The broadband LFP spectrogram was then compressed using principal components analysis (principal component 1 always corresponding to <20-Hz power).
The algorithm then uses these data in a sequence of separations, finding troughs that maximally split peaks in each distribution. In all recordings, manual inspection of scoring was conducted. In some cases, manual curation of algorithm parameters or specific segments of recordings was conducted to best identify brain state.
Continuous glucose monitor placement
A US Food and Drug Administration-approved device (Medtronic iPro2) for human use was adapted to measure glucose concentrations in rats. It is a 5-g device about 20 mm in diameter, typically worn on the abdomen for humans and is fixed to the skin with a tegaderm bandage. This device uses an amperometric method with an enzyme-coated electrode. The enzyme (glucose oxidase) catalyses glucose into gluconic acid and hydrogen peroxide. The electrochemical measurement of hydrogen peroxide allows for a current read out that is proportional to the concentration of glucose45.
To adapt this for use in rats, rats were anaesthetized using isoflurane and a small patch of skin on the back—between the shoulder blades—was exposed by shaving the fur. Skin was cleaned and disinfected with iodine and alcohol swabs. The iPro2 device was then positioned and the electrodes were inserted under the skin into the interstitial space. The iPro2 applicator consists of a spring-loaded needle that quickly and painlessly inserts the measurement electrodes below the skin, while the rest of the device is external and rests on the back of the rat (Fig. 1a). Once placed, a tegaderm cover holds the iPro2 onto surrounding skin, keeping the device fixed in place.
Glucose sensor calibration
A single tail vein blood sample was collected (approximately 5 μl) after sensor placement and stabilization. The blood drop was absorbed onto a test strip for the Contour Next Ez glucose monitor and a blood glucose reading was taken. Using this measurement, and the closest iPro2 current reading (±2.5 min) a conversion factor was calculated such that the iPro2 current reading (nA) multiplied by this factor matched the glucose measurement (mg dl−1).
Sources of temporal delay between SPW-Rs and glucose fluctuations
We observed an approximately 15-min temporal lag in correlation between SPW-R occurrence and glucose concentration changes. A large part of this delay is probably artefactual. The effect of insulin on regulating glucose uptake and the diffusion of glucose into the interstitial space may take tens of seconds to minutes depending on blood flow and the diffusion rate across the capillary epithelium46,47. In addition to this physiological delay, the continuous glucose monitor we employed has a temporal lag of 5–15 min owing to the diffusion kinetics of glucose over the sensor pore, and this delay can vary as a function of the rate-of-change in glucose concentrations48–50.Taking into consideration these delays, hippocampal SPW-R signalling to the pancreas may be quite fast.
Leave-one-out and pairwise model comparisons
For both modelling approaches in Fig. 3, data from each rat were examined separately. Data for each single rat were split into two-thirds for model training and one-third for model testing (all models were rerun 100 times with random splits). The predictors we were able to measure and examine were (1) SPW-R rate, (2) binarized brain state (NREM or wake)43, (3) high-pass coherence (EMG proxy)42, (4) power spectrum slope51, (5) theta residual from spectrum slope fit (that is, theta power), (6) theta/delta ratio and (7) movement from a head-mounted accelerometer.
For the first modelling approach (leave-one-out), all seven predictors were given to a linear regression model (fitlm.m) and the mean squared error was calculated on the withheld testing data. A second model was then created that used six out of the seven predictors (with the same train and test split), and a second mean squared error value was calculated. This dual-model approach was conducted for all temporal offsets (100 to −100 min). For each temporal offset (100 to −100 min) and withheld predictor (1–7) the difference between these two mean squared error values was taken. Extended Data Figure 6a shows the average differences for 100 iterations, and Extended Data Fig. 6b shows the difference for each iteration (black dots) and withheld predictor (labelled columns) at the 10-min offset.
For the second modelling approach (pairwise), each predictor was used in a separate linear regression model. For each train and test split, mean squared error values were calculated for each predictor and all pairwise comparisons of mean squared error values were calculated. Extended Data Figure 6c shows the average mean squared error difference (n = 100 iterations). Extended Data Figure 6d shows the number of rats, for each comparison, for which the mean squared error values were lower (students t-test; P < 0.05) for the column predictor when compared to the row predictor.
Viral injections, optrode implantation and optogenetic induction of ripples
For the experiments in which optogenetic induction of hippocampal ripples was conducted, rat were bilaterally injected with AAV-CamKII-ChR2-EYFP in either the CA1 (n = 5) or CA3 (n = 3) regions of the hippocampus. A total injection volume of 300 nl was injected into each hemisphere. Rat were allowed a minimum of six weeks recovery before implantation of recording electrodes and optical fibres.
For each hemisphere, a microdrive was assembled that consisted of three 50-μm tungsten wires offset by 200–400 μm along the dorsal-ventral axis (spanning 600–1,200 μm in total). A 200-μm optic fibre was attached to this microdrive such that the fibre tip was 0–200 μm above the most dorsal wire. These devices were implanted using the same surgical procedures previously described52, at a depth of 1–1.5 mm. After recovery from implantation surgery, each electrode and fibre bundle was moved ventrally (76-μm increments) until it reached the target region (either CA1 or CA3).
Calibration of light stimulation to induce SPW-Rs was done as previously described53,54. In brief, light intensity was calibrated for each hemisphere independently starting at zero, and light pulses of increasingly larger amplitudes where delivered while recording electrophysiological responses. The intensity value that elicited a ripple oscillation of the same magnitude as normally occurring ripples on the same channel was selected. Once selected, this value was held constant for the duration of the experiment.
Pharmacogenetic experiments
For the experiments in which pharmacogenetic inhibition of lateral septum was conducted, rat were bilaterally injected with either AAV1-hDlx-GiDREADD-dTomato-Fishell or AAV2-hSyn-hM4D(Gi)-mCherry. Three injections (100 nl each) were performed in each hemisphere (anterior–posterior, 0.5; medial–lateral, 0.5; dorsal–ventral, 5.4, 4.6, 3.8), for a total injection volume of 300 nl per hemisphere. The AAV2 construct showed a strong tropism for transfecting medial, but not lateral septum., whereas the AAV1 construct transfected both regions (Extended Data Fig. 10a, b). Rat were allowed a minimum of two weeks recovery before implantation of recording electrodes and further experiments.
CNO was dissolved in DMSO at a 40 mg ml−1 concentration to create a stock solution. Each day, stock solution was diluted in 300 μl of saline for injection. On alternating days, all rats received one intraperitoneal injection of either 5 mg kg−1 of CNO or vehicle. Injections were conducted each day between 08:00 h and 09:00 h. Rats were allowed ad libitum food and water throughout the experiment and were on the same 12-h light–dark cycle as described in ‘Chronic electrophysiological recordings’ (07:00 h lights on).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The datasets used during this study are available from https://buzsakilab.nyumc.org/datasets/TingleyD/.
Code availability
The code used in this study is available from https://github.com/buzsakilab/buzcode.
Extended Data
Extended Data Fig. 1 |. Continuous glucose monitor quality control, circadian, mealtime and spectral properties.

a, For a subset of experiments (n = 43 sessions in 8 rats) in which a continuous glucose monitor was implanted, the raw data are shown. Sorted by duration of patency. b, Average current readings (y axis) over time (x axis) after implantation. Note the slow decay over days, an indication of glucose oxidase enzyme breakdown on the electrode surface. c, Derivative (Δ current) of the data shown in a, showing faster time-scale fluctuations in glucose concentrations. d, Absolute value of the average Δ current (y axis) over time (x axis) after implantation. Note the relatively stable readings over days, and that the slow decay in absolute current readings is no longer present. e, Interstitial glucose concentrations are modulated over the circadian timescale. f, Interstitial glucose concentrations increase during the postprandial period (n = 36 meals, collected from 4 rats). Meal size was one 3-g pellet of standard chow. g, Average power spectral density plot across rats (n = 8) of the Δ current signal. Red line is a linear best fit to the data. h, Subtraction of the linear fit in c from the observed power at each frequency band. Note the elevated power in the 15-min to 90-min range. Note also that the ultradian cycling of SPW-R and glucose levels are different (compare with Extended Data Fig. 3). i, Average auto-correlogram of glucose fluctuations across eight rats. Red dashed lines are the 99% confidence interval for each rat.
Extended Data Fig. 2 |. SPW-R detection quality control.
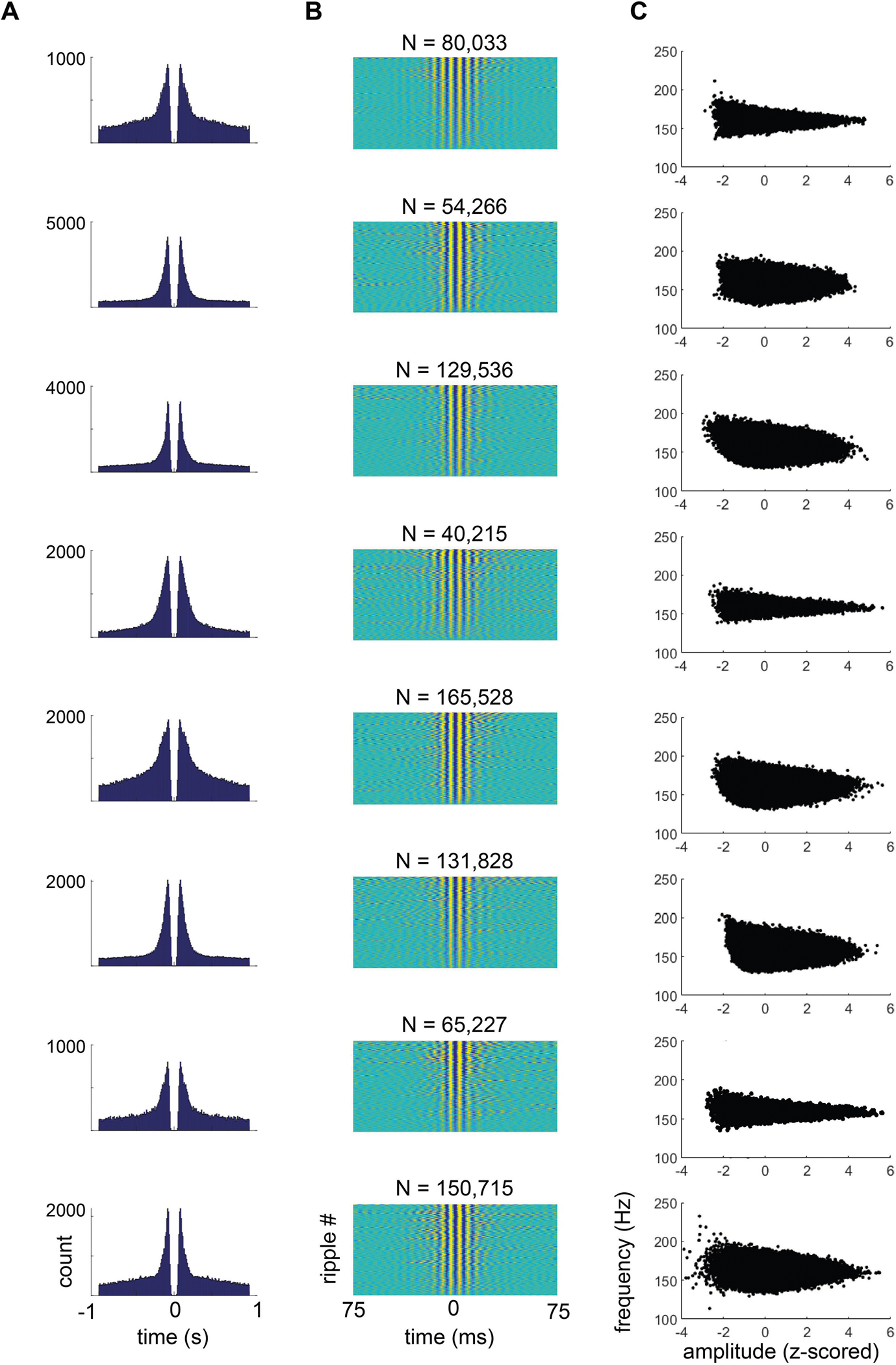
a, Auto-correlograms of all inter-ripple intervals. Each row is data from an individual rat. Note the peaks in the histogram from bursts of ripples, and the drop to zero, representing an approximately 20-ms refractory period between ripples. b, Heat map of the high-pass-filtered (>100 Hz) LFP for every SPW-R, sorted by peak amplitude of the event. c, Scatter plots of the z-scored ripple amplitude (x axis) and ripple frequency (y axis) for all ripples (black dots) and rats (rows).
Extended Data Fig. 3 |. Circadian and ultradian rhythms in SPW-R rate.
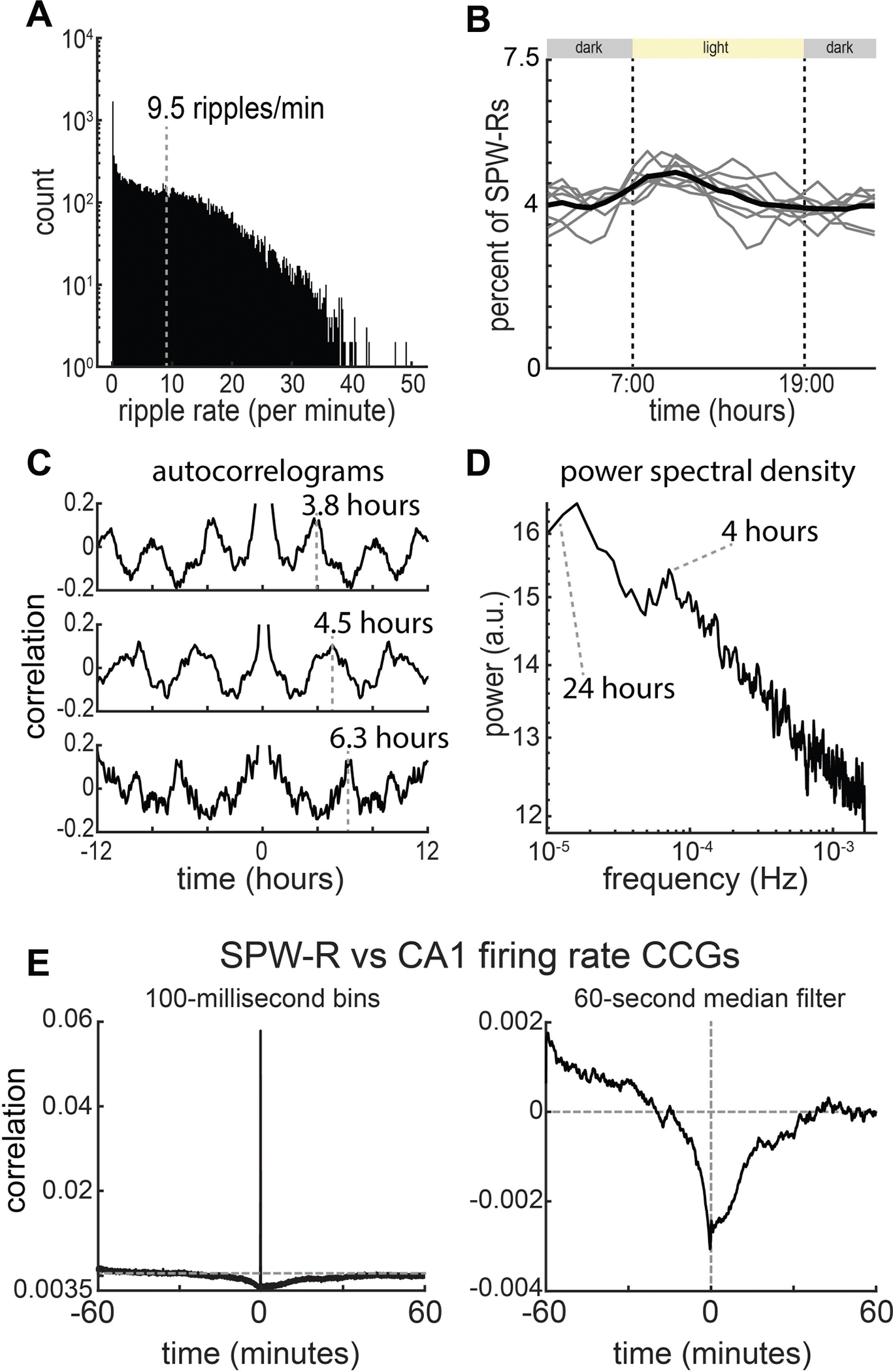
a, Histogram of SPW-R rate for data from eight rats and sessions (n = 1,568.5 recording hours from 8 rats; mean = 196 ± 84 h). b, Modulation of the SPW-R rate across the circadian timescale. Grey lines are individual rats, black line is the population average. c, Example auto-correlograms (±12 h) of SPW-R rate for three rats. d, Average power spectral density plot (n = 8 rats) of ripple rate. Note two peaks at about 24 h and about 4 h. e, Cross-correlograms of detected SPW-Rs and CA1 population firing rates at two temporal scales. Left, 100-ms bins; right, 60-s median filter. At slow temporal scales (>250 ms), CA1 SPW-Rs are associated with an overall decrease in CA1 firing rates.
Extended Data Fig. 4 |. SPW-R features and states that affect the correlation with glucose fluctuations.

a, Histograms of duration, amplitude, frequency and inter-ripple intervals across all detected SPW-Rs. b, Average effect of SPW-R features on peri-SPW-R glucose fluctuations. The y axes are time (±100 min) and the x axes are the percentile of each distribution (or time in milliseconds for ripple duration). White lines are the average effect at +10-min temporal offset. Red and blue asterisks indicate the column used for the plots in c. c, Red and blue traces highlight the effect on peri-SPW-R glucose fluctuations at two different positions in the distributions of ripple features in b. d, Cross-correlograms for four rats calculated on 4-h blocks of time in which the rat was fasted or fed. e, A sliding 2-h window of time was taken to calculate the cross-correlogram between SPW-Rs and glucose fluctuations before, during and after a 3-g meal was provided to fasted rats. f, The SPW-R–glucose fluctuation cross-correlogram was not different between fasting and fed states. g, Cross-correlograms for eight rats calculated using blocks of time in which the rat was in NREM sleep or awake. h, A sliding 2-h window of time was taken to calculate the cross-correlogram between SPW-Rs and glucose fluctuations, relative to brain state transitions. The negative correlation did not change in amplitude across either wake-to-NREM or NREM-to-wake state transitions. Bounds are one s.d. i, The SPW-R–glucose fluctuation cross-correlogram was not different between NREM and waking states. j, Average cross-correlograms between SPW-R rate and Δ current for 4-h windows centred at 06:00 h (blue), 12:00 h (red) and 20:00 (orange). k, Integrated cross-correlograms (5–20 min) over the 24-h light–dark cycle. l, Same line as in j, without s.d. bounds and zoomed in on the y axis. Note the cumulative effect size is only slightly larger during the dark period. Across rats, no hour of the day had a significantly different correlation than any other hour of the day (Student’s t-test; minimum P = 0.53). Note also that the circadian fluctuation of glucose level is not a mirror image of SPW-R rate (compare Extended Data Fig. 3b).
Extended Data Fig. 5 |. Reverse correlation and oscillatory coupling of glucose fluctuations with SPW-R rate.

a, For each experiment peaks and troughs in glucose fluctuations were identified (red or black dotted lines; >1 s.d.). b, The average rate of SPW-Rs is shown when triggered on peaks in the glucose derivative (black line) and troughs in the glucose derivative (red line). Shaded area shows three s.d. of a randomly circularly permuted null distribution (100 iterations). c, The average rate of stimulations is shown when triggered on peaks in the glucose derivative (black line) and troughs in the glucose derivative (red line). Shaded area, three s.d. of a randomly circularly permuted null distribution (100 iterations). d, Average power–power comodulogram at different frequencies of the glucose signal (y axis) and SPW-R rate signal (x axis) for 30 rats. Note that the patch of high correlations (R > 0.2) from 15–60 min is shifted upward, off of the diagonal. This indicates the SPW-R rate at slightly higher frequencies modulates glucose fluctuations at a slightly lower frequency, a hall-mark of phase-resetting mechanisms in biological systems. P < 1−24 using a Student’s t-test with circularly shuffled data. e, Top, phase–amplitude coupling for each rat when using the phase angle of the glucose fluctuation signal (x axis) and SPW-R rate as the amplitude (colour; each rat is z-scored). Bottom, average phase–amplitude coupling across all rats. P < 1−18 using the Rayleigh test for non-uniformity.
Extended Data Fig. 6 |. SPW-R rate is the best predictor of glucose concentration changes.
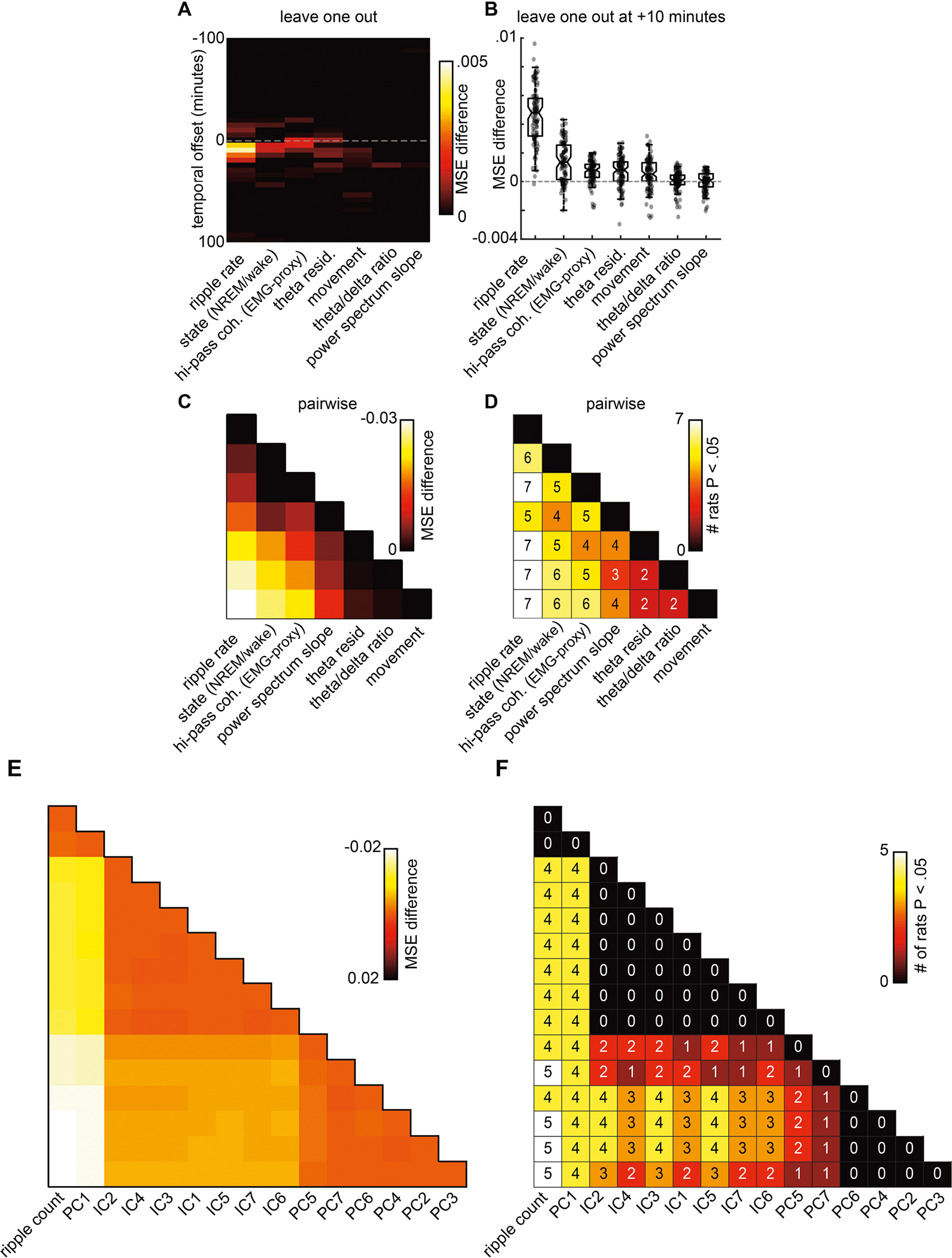
a, Leave-one-out prediction of glucose dynamics. Each heat map column represents the mean squared error difference between two models, one model with all predictors and a second with all predictors but one (leave-one-out analysis). The y axis is the temporal offset between predictors and glucose fluctuations (±100 min in 5-min increments). The heldout predictor for each column is labelled along the x axis. b, Mean squared error difference for each model pair at +10-min offset. Each grey dot represents a single model pair with a random train and test split of the data (100 iterations). Note the averages for each model pair are equivalent to the +10 y axis of a. Box represents median, and 25th and 75th percentiles. c, Pairwise comparison of models using different predictors. For each pair of models using different predictors, the mean squared error values were subtracted. Each bin colour indicates the average mean squared error difference across 100 iterations with random train and test splits. d, For each pairwise predictor comparison, a two-sample t-test was performed across the 100 iterations with different train and test splits. The number and colour in each bin indicate the number of rats for which the mean squared error for the predictor column was significantly (P < 0.05) lower than the mean squared error for the predictor row. For example, the upper leftmost value of 6 indicates that ripple rate was a better predictor than brain state in 6 of the 8 rats. e, Latent variables do not predict glucose fluctuations more strongly than SPW-R rate. The same pairwise approach was taken as in c, d, using a generalized linear model with two predictors to estimate glucose fluctuations. The predictors used were the principal components, or independent components, of all predictors listed in Fig. 3. SPW-R rate, by itself, was also included as an additional predictor. Columns are sorted by best prediction. For each pairwise comparison SPW-R rate allowed for better (or equivalent, for principal component 1) prediction. f, For each pair of models, the number of rats for which where one model performed significantly better (two-way t-test P < 0.05) is shown. SPW-R rate and PC1 were similar in all rats, all other principal components and independent components performed worse for at least 4 out of 8 rats.
Extended Data Fig. 7 |. Stimulation protocol, statistics of optogenetic stimulation effect and control PPC experiments.
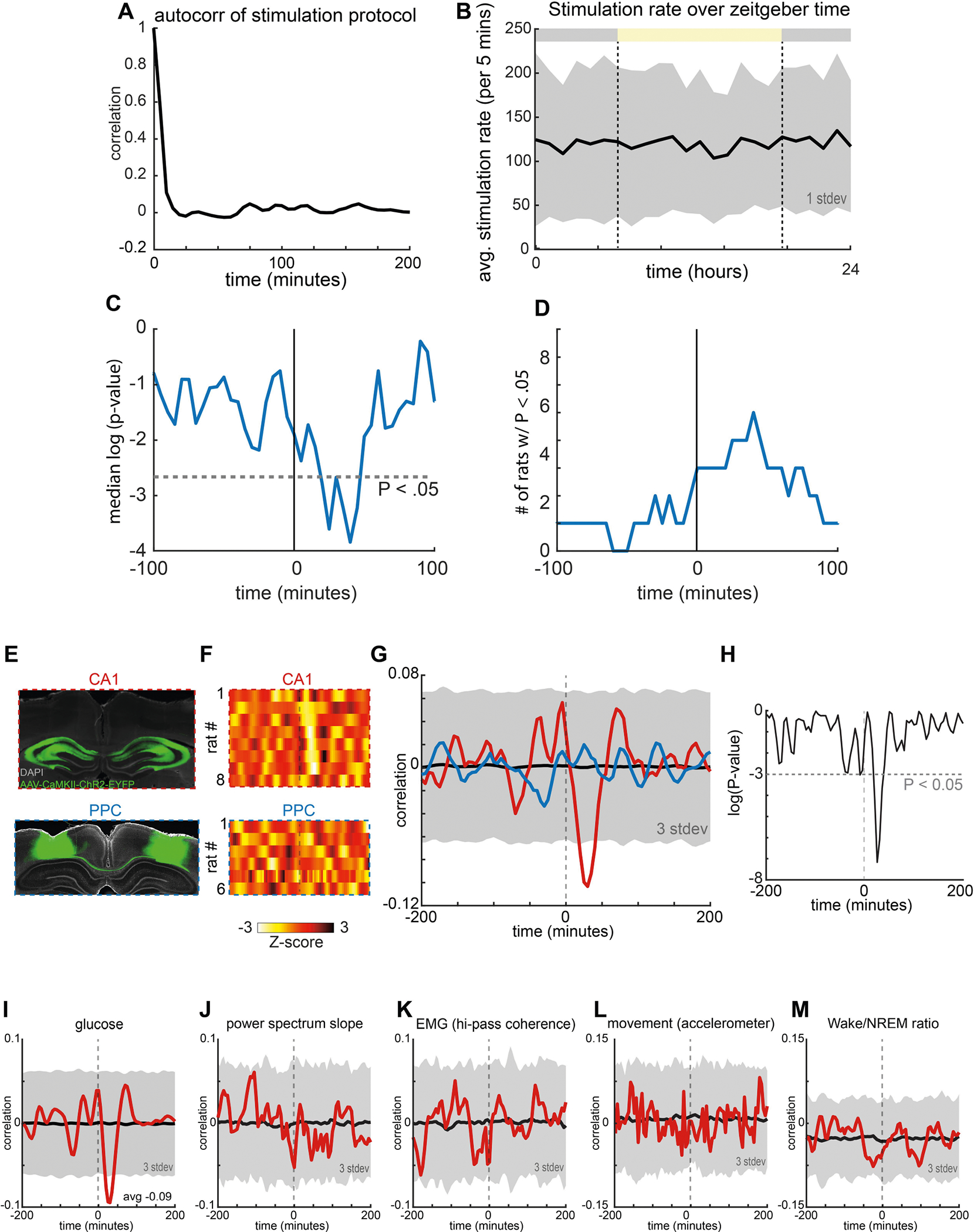
a, Autocorrelation of stimulation protocol showing no ultradian structure. b, Average stimulation rate was not different across the circadian light–dark cycle. c, Average P value of correlation between stimulation rate and Δ current at different temporal offsets (x axis) across all 8 rats. d, Number of rats (8 total) with P < 0.05 correlations between stimulation rate and Δ current at different temporal offsets (x axis). All tests were two-way t-tests. e, Optogenetic induction of population bursts in posterior parietal cortex does not induce decreases in glucose. Top, example histology. Bottom, example histology from the same experiment conducted in the PPC in 5 rats. f, Top, stimulation effect from CA1 experiments (as in Fig. 4). Bottom, stimulation effect from the same stimulation protocol in PPC. g, Red line is the average cross-correlogram between CA1 artificial ripples and glucose fluctuations. Blue line is the average cross-correlogram between PPC artificial ripples and glucose fluctuations. h, P values from a two-way t-test when comparing the cross-correlograms from the CA1 and PPC cohorts of rats. i, For reference, the cross-correlogram between artificial ripples and interstitial glucose fluctuations are shown (average from 8 rats). j, Average cross-correlogram between artificial ripples and the power spectrum slope of the LFP recorded in CA1. k, Average cross-correlogram between artificial ripples and the high-pass-filtered coherence (a proxy for EMG42). l, Average cross-correlogram between artificial ripples and movement as detected by an accelerometer on the head of the rat. m, Average cross-correlogram between artificial ripples and the ratio of wake to NREM sleep in each 5-min bin.
Extended Data Fig. 8 |. Nonlinear rectified response of lateral septum neurons to hippocampal synchrony.

a, Dorsal CA1 and CA3 projections converge within the lateral septum. Top, injection sites. Bottom, anterograde projections to the lateral septum. b, Left, black trace is one second of raw LFP recorded from CA1 pyramidal layer during running on a maze. Each row in the black raster plot indicates the action potentials from a single neuron. Grey dashed lines indicate the separation of theta cycles. The numbers below indicate the number of cells participating, and number of action potentials occurring, during each theta cycle. Right, three example SPW-Rs during sleep from the same session. In all example traces, neurons are sorted by the ordering of place fields on the maze (same sorting as left). c, Top, the proportion of theta cycles (black) and SPW-Rs (red) as a function of the hippocampal synchrony distribution. Dashed black and red vertical lines mark the position along the population synchrony axis at which 75% of all theta cycles (from left) or 75% of all SPW-Rs (from right) occurred. Bottom, the average z-scored firing rate across the lateral septum population (minimum threshold of 15 neurons) is shown as a function of the percentile of hippocampal synchrony distribution (n = 38 recordings from 5 rats). Bounds are one s.d.
Extended Data Fig. 9 |. Dorsal CA1 SPW-Rs more strongly correlate with glucose fluctuations than do ventral CA1 SPW-Rs.

a, Example histological verification of dorsal and ventral CA1 recording sites. Red triangles indicate the approximate location of the CA1 SPW-R detection channels for each region. Grey triangle indicates dorsal CA3. b, Example traces of raw LFP and bandpass-filtered LFP across both recording sites (top, ventral; bottom, dorsal). For each region, one shank with uniformly distributed recording sites along the dorsal–ventral axis is shown. c, Example LFP from channels used for ripple detection. d, Average cross-correlogram between dorsal CA1 and ventral CA1 SPW-Rs at slow timescales. Inset, average cross-correlogram between dorsal CA1 and ventral CA1 SPW-Rs at fast timescales. e, Across the three rats with simultaneous dorsal and ventral hippocampal recordings, dorsal SPW-Rs were equivalently correlated with glucose fluctuations as the previous cohorts of rats with only dorsal CA1 recordings (n = 30). Ventral SPW-Rs had a significantly (two-way t-test) weaker correlation with peripheral glucose fluctuations. f, For each rat, ventral CA1 SPW-Rs were more weakly correlated with glucose dynamics and dorsal CA1 SPW-Rs.
Extended Data Fig. 10 |. Histology from DREADD and CNO experiments.

a, Top, histology from the cohort of rats with AAV1-hDlx-GiDREADD-dTomato-Fishell injected into lateral septum. Bottom, example cross-correlograms between SPW-R and glucose fluctuations on vehicle (blue) and CNO (black) days. b, Top, histology from the cohort of rats with AAV2-hSyn-hM4D(Gi)-mCherry injected into medial septum. Bottom, example cross-correlograms between SPW-R and glucose fluctuations on vehicle (blue) and CNO (black) days. c, Comparison of effects for individual rats across vehicle versus CNO days for each cohort. d, P values (two-way t-test) for each part of the cross-correlograms shown in e when comparing within hM4Di rats (red; CNO versus vehicle) or across cohorts of rats (blue; CNO–hM4Di vs CNO–control). e, Ten colleagues who were not involved with these experiments were provided histology from all 9 rats (6 lateral septum; 3 medial septum) and asked to rate the degree of viral transfection in each subregion of the septum. Each rat had three images taken along the anterior–posterior axis and the individuals were blinded as to which virus had been injected and the effects of CNO on the SPW-R–glucose correlation. For each rat and subregion, the average score from these 10 individuals is shown in the table. For each subregion, the correlation between these scores and the effect of CNO was calculated and is shown in bold below. Red values had a P values < 0.05. f, Scatter plot of the SPW-R–glucose correlation after CNO injection (x axis) and the average expression score given by these rates (y axis) for the dorsal lateral septum. g, Scatter plot of the SPW-R–glucose correlation after CNO injection (x axis) and the average expression score given by these rates (y axis) for the medial septum.
Supplementary Material
Acknowledgements
We thank M. Elmaleh, D. Nitz, A. Fernandez-Ruiz and A. Maurer for feedback on an early version of the manuscript. Funding was from NIH MH122391, U19 NS104590 and U19NS107616.
Footnotes
Competing interests The authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41586-021-03811-w.
Peer review information Nature thanks H. Freyja Olafsdottir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Reprints and permissions information is available at http://www.nature.com/reprints.
References
- 1.Kanoski SE & Grill HJ Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavenex P & Amaral DG Hippocampal–neocortical interaction: a hierarchy of associativity. Hippocampus 10, 420–430 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Seto K, Saito H, Otsuka K & Kawakami M Influence of electrical stimulation of the limbic structure on insulin level in rabbit’s plasma. Exp. Clin. Endocrinol. 81, 347–349 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Endroczi E, Lissak K, Bohus B & Kovacs S The inhibitory influence of archicortical structures on pituitary-adrenal function. Acta Physiol. Acad. Sci. Hung. 16, 17–22 (1959). [Google Scholar]
- 5.Rubin RT, Mandell AJ & Crandall PH Corticosteroid responses to limbic stimulation in man: localization of stimulus sites. Science 153, 767–768 (1966). [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL & Gold PE Hormonal Modulation of Memory. Psychoendocrinology (Academic, 1989). [Google Scholar]
- 7.Gold PE & van Buskirk R Effects of posttrial hormone injections on memory processes. Horm. Behav. 7, 509–517 (1976). [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS, Weiss JM & Schwartz LS Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 16, 227–241 (1969). [DOI] [PubMed] [Google Scholar]
- 9.Lathe R Hormones and the hippocampus. J. Endocrinol. 169, 205–231 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Scoville WB & Milner B Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohus B in The Hippocampus: Volume 1: Structure and Development (eds Isaacson RL & Pribram KH) 323–353 (Springer, 1975). [Google Scholar]
- 12.Jacobson L & Sapolsky R The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118–134 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Hsu TM et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 4, e11190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saphier D & Feldman S Effects of septal and hippocampal stimuli on paraventricular nucleus neurons. Neuroscience 20, 749–755 (1987). [DOI] [PubMed] [Google Scholar]
- 15.Prutskova NP & Petrov, Yu. A Electrophysiological investigation of the hippocampal projections to the neurosecretory cells of the supraoptic nucleus of the rat hypothalamus. Neurosci. Behav. Physiol. 20, 194–200 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Tasali E, Leproult R, Ehrmann DA & Van Cauter E Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl Acad. Sci. USA 105, 1044–1049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappuccio FP, D’Elia L, Strazzullo P & Miller MA Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R & Van Cauter E Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Amaral DG & Lavenex P in The Hippocampus Book (eds. Anderson P et al.) 45–66 (Oxford Univ. Press, 2006). [Google Scholar]
- 20.Cenquizca LA & Swanson LW Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J. Comp. Neurol. 497, 101–114 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimazu T Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab. Rev. 3, 185–206 (1987). [DOI] [PubMed] [Google Scholar]
- 22.Buzsáki G Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen K Oscillations in the blood sugar in fasting normal persons. Acta Med. Scand. 57, 27–32 (1923). [Google Scholar]
- 24.Risold PY & Swanson LW Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev. 24, 115–195 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Sosa M, Joo HR & Frank LM Dorsal and ventral hippocampal sharp-wave ripples activate distinct nucleus accumbens networks. Neuron 105, 725–741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tingley D & Buzsáki G Routing of hippocampal ripples to subcortical structures via the lateral septum. Neuron 105, 138–149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niijima A Neural control of blood glucose level. Jpn. J. Physiol. 36, 827–841 (1986). [DOI] [PubMed] [Google Scholar]
- 28.Britton SW Studies on the conditions of activity in endocrine glands: XVII. The nervous control of insulin secretion. Am. J. Physiol. 74, 291–308 (1925). [Google Scholar]
- 29.Corkill AB, Marks HP & White WE Relation of the pituitary gland to the action of insulin and adrenaline. J. Physiol. 612, 193–205 (1933). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo T, McQueen A, Chen T-C & Wang J-C Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 872, 99–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SH & Park M-J Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 22, 145–152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Güemes A & Georgiou P Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron. Med. 4, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks WA & Kastin AJ Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19, 883–889 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Schulingkamp RJ, Pagano TC, Hung D & Raffa RB Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 24, 855–872 (2000). [DOI] [PubMed] [Google Scholar]
- 35.O’Malley D, Shanley LJ & Harvey J Insulin inhibits rat hippocampal neurones via activation of ATP-sensitive K+ and large conductance Ca2+-activated K+ channels. Neuropharmacology 44, 855–863 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Harasta AE et al. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology 40, 1969–1978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers VK, Dyken ME, Mark AL & Abboud FM Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 328, 303–307 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Alford FP et al. Temporal patterns of integrated plasma hormone levels during sleep and wakefulness. I. Thyroid-stimulating hormone, growth hormone and cortisol. J. Clin. Endocrinol. Metab. 37, 841–847 (1973). [DOI] [PubMed] [Google Scholar]
- 39.Logothetis NK et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Swanson RA, Levenstein D, McClain K, Tingley D & Buzsáki G Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Curr. Opin. Behav. Sci. 32, 126–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csicsvari J, Hirase H, Czurkó A, Mamiya A & Buzsáki G Fast network oscillations in the hippocampal CA1 region of the behaving rat. J. Neurosci. 19, RC20 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schomburg EW et al. Theta phase segregation of input-specific gamma patterns in entorhinal–hippocampal networks. Neuron 84, 470–485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson BO, Levenstein D, Greene JP, Gelinas JN & Buzsáki G Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levenstein D, Watson BO, Rinzel J & Buzsáki G Sleep regulation of the distribution of cortical firing rates. Curr. Opin. Neurobiol. 44, 34–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C et al. Recent advances in electrochemical glucose biosensors: a review. RSC Advances 3, 4473–4491 (2013). [Google Scholar]
- 46.Basu A et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 62, 4083–4087 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovatchev BP, Shields D & Breton M Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol. Ther. 11, 139–143 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker DA & Gough DA Dynamic delay and maximal dynamic error in continuous biosensors. Anal. Chem. 68, 1292–1297 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Davey RJ, Low C, Jones TW & Fournier PA Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J. Diabetes Sci. Technol. 4, 1393–1399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keenan DB, Mastrototaro JJ, Voskanyan G & Steil GM Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J. Diabetes Sci. Technol. 3, 1207–1214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donoghue T et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tingley D & Buzsáki G Transformation of a spatial map across the hippocampal–lateral septal circuit. Neuron 98, 1229–1242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark E et al. Pyramidal cell–interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Ruiz A et al. Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during this study are available from https://buzsakilab.nyumc.org/datasets/TingleyD/.


