Abstract
We report our experience with a 69-year-old man who had different types of Kounis syndrome over a short time frame, caused by two episodes of bee sting anaphylaxis. After his first allergic reaction to a bee sting, he experienced a non–ST-segment elevation myocardial infarction; he was treated with percutaneous coronary intervention for near-occlusion of his right coronary artery. This episode was deemed type 2 Kounis syndrome. Four weeks later, we electively treated the nonculprit residual stenosis in his left anterior descending artery. Unfortunately, 2 weeks after this elective procedure, he experienced anaphylactic shock due to a second bee sting. Electrocardiography showed ST elevation in the anterior leads, and emergent coronary angiography showed thrombotic occlusion of the newly implanted stent in the left anterior descending artery. This second episode was deemed type 3 Kounis syndrome.
Learning objectives
This is a rare example of different types of Kounis syndrome resulting from repeated exposures to an allergic source, an example that deepens our understanding of Kounis syndrome. This patient's experience illustrates the need for careful evaluation of the indications for revascularization of nonculprit lesions in patients with a history of Kounis syndrome.
Keywords: Kounis syndrome, ST elevation myocardial infarction, Stent thrombosis, Case report
Introduction
Kounis syndrome (KS) is an acute myocardial infarction that is triggered by an allergic reaction. It is classified into 3 variants: spasm of a normal vessel (type 1), rupture of an existing plaque (type 2), and stent thrombosis (type 3) [1]. A transient disease, KS has a relatively good prognosis if allergen exposure can be avoided [2]. We encountered a patient with two different types of KS caused by two episodes of bee sting anaphylaxis over a short period of time frame.
Case report
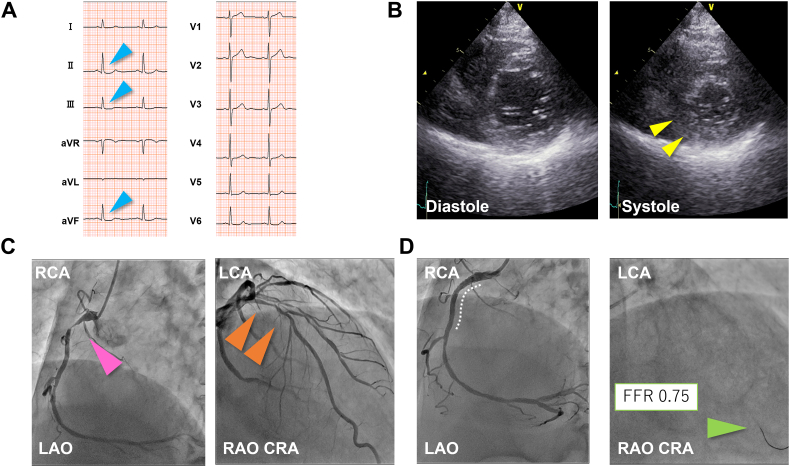
A 69-year-old man with a longstanding history of hypertension was treated for allergic symptoms following a bee sting, including facial and neck swelling and redness. The next day, he had new-onset exertional angina. He underwent electrocardiography (ECG) and echocardiography and was diagnosed with a non–ST-segment elevation myocardial infarction (NSTEMI) (Fig. 1A, B). Emergent coronary angiography (CAG) showed near-occlusion in the proximal right coronary artery (RCA) and moderate stenosis in the proximal left anterior descending artery (LAD) (Fig. 1C). We performed emergent percutaneous coronary intervention (PCI) and inserted a drug-eluting stent (DES) measuring 3.5 × 23 mm (Xience Skypoint; Abbott Vascular, Santa Clara, CA, USA) into the RCA (Fig. 1D). He was discharged on the following daily medications: 100 mg aspirin, 3.75 mg prasugrel, 10 mg vonoprazan fumarate, 10 mg rosuvastatin, 5 mg enalapril, and 2.5 mg bisoprolol fumarate.
Fig. 1.
Electrocardiography (ECG), echocardiography, coronary angiography (CAG) during the first episode. (A) ECG on admission shows slight ST depression in the inferior leads (blue arrowheads). (B) Parasternal short-axis view of the left ventricle on echocardiography shows hypokinesis in the inferior wall (yellow arrowheads). (C) Initial CAG shows near-occlusion in the proximal right coronary artery (RCA) and moderate stenosis in the proximal left anterior descending artery. (D) Final CAG shows recanalization of the culprit lesion with a drug-eluting stent in place (white dotted curve); the moderate stenosis is assessed using a fractional flow reserve wire (green arrowheads).
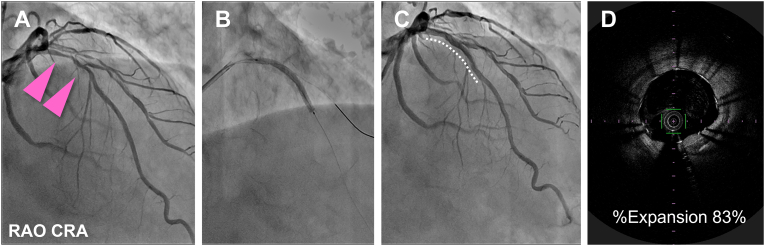
Four weeks later, we performed elective treatment of the proximal LAD lesion because of the low fractional flow reserve value (0.75) of the artery. We deployed a 2.5 × 33 mm DES (Ultimaster Tansei; Terumo Corporation, Tokyo, Japan) under optical frequency domain imaging (OFDI) guidance (Lunawave; Terumo Corporation) (Fig. 2).
Fig. 2.
Elective percutaneous coronary intervention for the proximal left anterior descending lesion, 4 weeks after the first event. (A) Initial coronary angiography (CAG) for the left coronary artery. (B) After predilation, a drug-eluting stent is deployed in the proximal left anterior descending artery (LAD). (C) Final CAG shows that the LAD lesion has good coronary flow. Stents are shown with white dotted curves. (D) Final optical frequency domain imaging shows adequate stent expansion.
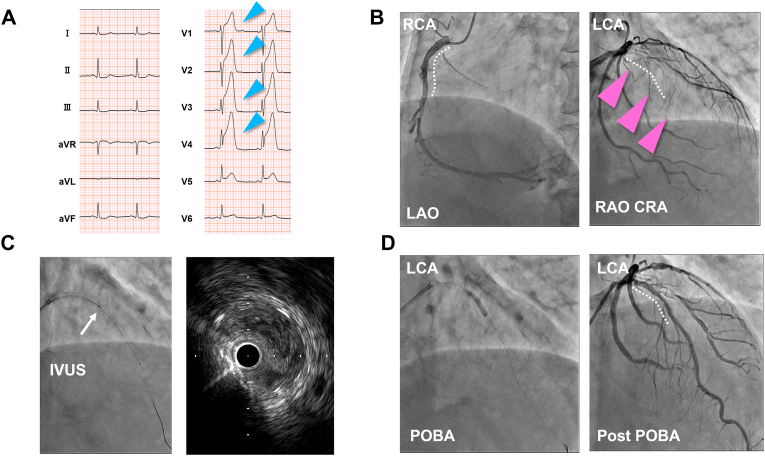
After this second procedure, the patient was asymptomatic for 2 weeks. He was then emergently transferred to a primary care clinic because of anaphylactic shock due to another bee sting. While he was being treated for anaphylaxis, he experienced sudden chest pain. His ECG showed ST-segment elevation in the anterior lead (Fig. 3A), and echocardiography showed hypokinesis in the anteroseptal wall of the left ventricle. He was diagnosed with an ST-segment elevation myocardial infarction (STEMI) and was transferred to our hospital. Emergent CAG revealed occlusion of the DES deployed 2 weeks prior in the proximal LAD. The DES in the proximal RCA, deployed 6 weeks prior, was patent (Fig. 3B). During emergent PCI, intravascular ultrasonography showed thrombi within the LAD stent (Fig. 3C). Flow of the LAD was recovered using repetitive balloon dilation within the stent (Fig. 3D). Since some thrombi remained within the stent, based on CAG and intravascular ultrasonography, intraaortic balloon pumping (IABP) was implemented after the procedure. Our patient was transferred to the intensive care unit, where he was treated with heparin. The next day, the IABP was removed after we confirmed persistent coronary flow using CAG.
Fig. 3.
Emergent percutaneous coronary intervention for left anterior descending stent thrombosis, 6 weeks after the first event. (A) Electrocardiography on admission for the second event shows ST elevation in the anterior leads (blue arrowheads). (B) Initial coronary angiography (CAG) shows occlusion of the left anterior descending artery (LAD) stent (pink arrowheads) and a patent right coronary artery (RCA) stent. (C) Intravascular ultrasonography (white arrow) shows thrombus occlusion of the LAD stent. (D) Final CAG shows recanalization of the LAD stent after plain old balloon angioplasty (POBA). Stents are shown with white dotted curves.
At the time of his second admission, our patient's platelet count was 209 × 103/L, and the blood eosinophil percentage was 1.2%; these values did not change significantly over the course of his hospitalization. His blood immunoglobulin E level was within normal limits, but sensitivity to bees on radioallergosorbent testing was positive. After the emergent procedure, his peak creatinine kinase and creatine kinase MB levels were 1777 U/L and 136 U/L, respectively.
To prevent another allergic reaction, we added histamine type-1 and type-2 receptor antagonists to his regimen. The patient's clinical course was uneventful afterward. On day 13, we performed an acetylcholine spasm provocation test to rule out coronary spasm and determine whether vasodilator therapy was indicated. Repeat CAG showed patency of both stents, and the provocation test was negative. The patient was discharged on day 14 after completing cardiac rehabilitation.
By the time of his discharge, the season for bees in the area had passed; he had no complaints at his 3-month follow-up visit.
Discussion
We encountered a patient who had two types of allergic acute coronary syndrome caused by two episodes of bee sting anaphylaxis. The first event happened in the native RCA and was a type 2 KS. The second event was a subacute stent thrombosis in the LAD, representing type 3 KS.
This patient taught us several lessons. First, it is sometimes impossible to avoid repeat exposure to an allergen, even though a second exposure can be fatal for patients with KS. We also learned to be more cautious when considering PCI for a nonculprit lesion in a patient with KS.
There is ongoing discussion on whether nonculprit lesions should be treated with PCI in patients with acute coronary syndrome [3], and how this decision should be made [4]. In our patient, we made the decision to perform PCI for moderate stenosis in the nonculprit LAD based on the fractional flow reserve value. However, we should have confirmed that our patient would be able to avoid any potential exposure to his allergen before performing an additional revascularization procedure.
Early stent thrombosis, defined as stent thrombosis within 30 days after stent implantation, is related to procedural variables—including major-edge dissections and stent underexpansion [5]—and postprocedural factors, such as cessation of dual antiplatelet therapy [6]. In our patient, we used OFDI to guide the elective procedure. We selected the stent size based on the OFDI findings, and there were no major-edge dissections. Our patient continued all his medications, including dual antiplatelet therapy, without interruption. However, the lesion in the LAD was diffuse and long, with severe calcification in the proximal part; this may indicate a higher risk of early stent thrombosis [7]. In addition, the patient was in a state of shock after the second bee sting; this may have caused stagnation of coronary flow and led to stent thrombosis. Although our patient's acetylcholine spasm provocation test was negative, we could not completely rule out coronary spasm due to allergic reaction. We speculate that the early stent thrombosis in our patient was caused by a combination of the factors listed above.
Our patient experienced occlusion of the LAD stent only, not the RCA stent. At the time of his second coronary event, it had been 6 weeks since the RCA stent was implanted but only 2 weeks since the LAD stent. In the current DES era, a shortened duration (1 month) of dual antiplatelet therapy is considered acceptable [8]. Although it was only 6 weeks old, the RCA stent had some endothelial coverage that may have allowed it to avoid occlusion.
Our patient's acetylcholine spasm provocation test was negative, but it is not clear what this test means in patients with KS [9]. The histamine released during the allergic reaction is believed to induce acute coronary syndrome [10], but we considered it unethical to perform a histamine provocation test in this patient.
Conclusion
We encountered a patient who had different types of KS over a short period of time caused by two episodes of bee sting anaphylaxis. This patient's case provides new insights into how revascularization should be performed in a patient with a history of KS.
Declaration of competing interest
The authors declare that they have no competing interests.
References
- 1.Kounis N.G., Mazarakis A., Tsigkas G., Giannopoulos S., Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7:805–824. doi: 10.2217/fca.11.63. [DOI] [PubMed] [Google Scholar]
- 2.Abdelghany M., Subedi R., Shah S., Kozman H. Kounis syndrome: a review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol. 2017;232:1–4. doi: 10.1016/j.ijcard.2017.01.124. [DOI] [PubMed] [Google Scholar]
- 3.Mehta S.R., Wood D.A., Storey R.F., Mehran R., Bainey K.R., Nguyen H., et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. doi: 10.1056/NEJMoa1907775. [DOI] [PubMed] [Google Scholar]
- 4.Puymirat E., Cayla G., Simon T., Steg P.G., Montalescot G., Durand-Zaleski I., et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N Engl J Med. 2021;385:297–308. doi: 10.1056/NEJMoa2104650. [DOI] [PubMed] [Google Scholar]
- 5.Holmes D.R., Kereiakes D.J., Garg S., Serruys P.W., Dehmer G.J., Ellis S.G., et al. Stent thrombosis. J Am Coll Cardiol. 2010;56:1357–1365. doi: 10.1016/j.jacc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Mehran R., Baber U., Steg P.G., Ariti C., Weisz G., Witzenbichler B., et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 7.Kuramitsu S., Ohya M., Shinozaki T., Otake H., Horie K., Kawamoto H., et al. Risk factors and long-term clinical outcomes of second-generation drug-eluting stent thrombosis. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.119.007822. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H., Domei T., Morimoto T., Natsuaki M., Shiomi H., Toyota T., et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinkiewicz W., Sobanski P., Bartuzi Z. Allergic myocardial infarction. Cardiol J. 2008;15:220–225. [PubMed] [Google Scholar]
- 10.Kounis N.G., Koniari I., Velissaris D., Tzanis G., Hahalis G. Kounis syndrome—not a single-organ arterial disorder but a multisystem and multidisciplinary disease. Balkan Med J. 2019;36:212–221. doi: 10.4274/balkanmedj.galenos.2019.2019.5.62. [DOI] [PMC free article] [PubMed] [Google Scholar]