Abstract
Myocarditis is a rare extra-intestinal complication of inflammatory bowel disease (IBD), in particular, ulcerative colitis.
We report a case of acute myocarditis as first manifestation of severe ulcerative colitis. A 22-year-old man was admitted with fever, bloody diarrhea, and fatigue. He had suffered from frequent bloody diarrhea, abdominal pain, fatigue, and weight loss for one month. A 12-lead-electrocardiogram showed sinus rhythm with QRS fragmentation and T waves inversion. High sensitivity troponin-I was elevated and the echocardiogram showed a mild pericardial effusion and inferior hypokinesia with normal ejection fraction. Cardiac magnetic resonance disclosed late enhancement in the inferior wall, corroborating the hypothesis of myocarditis. One week later, a colonoscopy revealed severe ulcerative extensive colitis (Mayo subscore 3). 5-aminosalicylic acid (mesalazine) and systemic steroid were started with good clinical and biochemical response. The following days the patient developed mesalazine hepatic and pancreatic induced toxicity requiring drug discontinuation and strict multi-disciplinary follow-up. At 7 months follow-up intestinal symptoms were well controlled with complete normalization of liver and pancreatic enzymes. Transthoracic echocardiography showed normal biventricular function and pericardial effusion resolution.
This case underscores the importance of a high suspicion for extra intestinal involvement in patients with IBD. These complications may be multifactorial and need multidisciplinary management.
Learning objective
-
•
When a patient was first-time diagnosed with a severe form of inflammatory bowel disease or has a disease relapse, bear in mind myocarditis as possible extra intestinal manifestation.
-
•
Multidisciplinary management is crucial to ensure the best level of care and follow-up in a such challenging and insidious clinical picture.
Keywords: Myocarditis, Inflammatory bowel disease, Electrocardiography, Emergency medicine, Mesalazine
Introduction
Ulcerative colitis is an idiopathic inflammatory bowel disease (IBD) of the colonic mucosa, which starts in the rectum and generally extends proximally through part of, or the entire, colon [1].
IBD may be complicated with extraintestinal manifestations. Cardiac involvement, especially myo-pericarditis, occurs more frequently in association with ulcerative colitis [2].
Myocarditis can be occasionally the initial manifestation, independently of bowel disease activity [3]. Alternatively, it can be triggered by a mesalazine cardiotoxicity [4,5].
However, the pathophysiological mechanisms underlying cardiac involvement in IBD are incompletely understood [6].
We report a case of a 22-year-old man with acute myocarditis as first manifestation of a severe ulcerative colitis. This patient had hepatic and pancreatic toxicity induced by mesalazine, further complicating the clinical picture that required multi-disciplinary management.
Case report
A 22-year-old man was admitted with persistence of fever (up to 39 °C), bloody diarrhea, and severe weakness since the previous 2 weeks. He was a mechanical worker, non-smoker, with no specific past medical and travel history.
One month before admission he started to suffer from worsening bloody diarrhea, fatigue, and weight loss (about 7 kg). When he presented for the first time to the emergency department (ED), no major pathologic findings were found, apart from leucocytosis (white blood cell count 12.37 10^9/L) and increased C-reactive protein (CRP): 8.8 mg/dL (normal value <1 mg/dL). He was therefore discharged to home after an observation of 12 h with antibiotics (amoxicillin+clavulanic acid three times per day) and probiotics.
After two weeks he presented again to ED with fever, severe bloody diarrhea, and weakness. On admission his vital signs were stable: blood pressure of 130/60 mm Hg, pulse rate 110 beats/min, and body temperature 38.7 °C. The abdomen was soft and non-tender, without palpable mass, but diffusely sore at palpation. Laboratory findings showed a white blood cell count of 18.23 10^9/L with 92% neutrophil, hemoglobin 125 g/L, platelet 432,000/mL, creatinine 1.1 mg/dL, and CRP 5.7 mg/L. Coronavirus disease 2019 swab was negative.
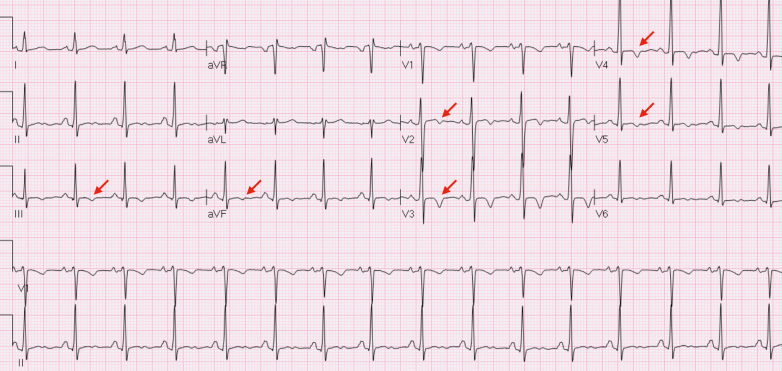
Twelve lead electrocardiogram showed sinus rhythm, 90 bpm, with T waves inversion in inferior-lateral leads and subtle PR depression (Fig. 1). High sensitivity troponin I was elevated (64 ng/L). A trans-thoracic echocardiogram showed a normal ejection fraction with inferior and septal hypokinesia, no valvular defects, and a mild pericardial effusion (5–6 mm). Due to the absence of cardiovascular risk factors, according to our institutional diagnostic algorithm, coronary angiography was not performed.
Fig. 1.
Electrocardiogram at presentation in the emergency department showing T waves inversion in inferior-lateral leads.
The chest radiograph and complete abdomen ultrasound were unremarkable.
He was admitted to the cardiology ward. During the admission the patient remained febrile with persistence of liquid bloody stools.
The blood cultures and stool examinations were not diagnostic (negative for Shigella, Salmonella, Campylobacter, and Clostridium difficile). Human immunodeficiency virus antibody, hepatitis B surface antigen (HBsAg), and hepatitis C virus antibody were all negative. Autoantibody panel was: Ag antitransglutaminase IgA 1 U/mL, IgG 3.3 AU, antinuclear antibody negative, antimitochondrial antibody negative, anti-smooth muscle antibody negative, antibodies to extractable nuclear antigens negative. Cytomegalovirus (CMV) with positive IgG and IgM, polymerase chain reaction (PCR)-DNA (real-time PCR) on day 8 was 192 UI/ml (Table 1).
Table 1.
Summary of laboratory and microbiology findings.
| Examination | Value | Normal reference |
|---|---|---|
| White blood cells | 18.23 10^9/L | <10.00 10^9/L |
| C-reactive protein | 8.8 mg/dL | <1 mg/dL |
| Hemoglobin | 125 g/L | 120–180 g/L |
| Platelets | 432,000/mL | 150–400 |
| Creatinine | 1,1 mg/dL | 0,30–1,30 mg/dL |
| Troponin-I HS | 64 ng/L | <54 ng/L |
| Aspartate aminotransferase | 130 U/I | 8–48 U/I |
| Alanine aminotransferase | 202 U/I | 7–45 U/I |
| Gamma glutamyl transferase | 66 U/I | 9–31 U/I |
| Amylase | 255 U/l | 26–102 U/l |
| Lipase | 187 U/l | 8–57 U/l |
| Fecal calprotectin | 2954 mg/kg | 0–50 mg/kg |
| Cytomegalovirus | ||
| - IgG | Positive (>500,00 U/mL) | Negative |
| - IgM | Positive (0.94 U/mL) | Negative |
| - Polymerase chain reaction (PCR)-DNA (real-time PCR) | 379 UI/mL | Negative |
| Stool cultures | ||
| - Shigella | Negative | Negative |
| - Salmonella | Negative | Negative |
| - Campylobacter | Negative | Negative |
| - Clostridium difficile | Negative | Negative |
| Viral antibodies | ||
| - Epstein Barr virus (IgG, IgM) | Positive (55 U/mL, 43 U/mL) | Negative |
| - Human immunodeficiency virus antibody | Negative | Negative |
| - Hepatitis B surface antigen (HBsAg) | Negative | Negative |
| - Hepatitis C virus antibody | Negative | Negative |
| Autoantibody panel | ||
| - Ag antitransglutaminase (IgA, IgG) | 1 U/mL, 3 AU | 0–20 U/mLL, 0–20 UA |
| - ANA (antinuclear antibodies) | Negative | Negative <1/160 |
| - AMA (anti-mitochondrial antibodies) | Negative | Negative |
| - ASMA (anti-smooth muscle antibodies) | Negative | Negative <1:40 |
| - ENA (extractable nuclear antigen) | Negative | Negative |
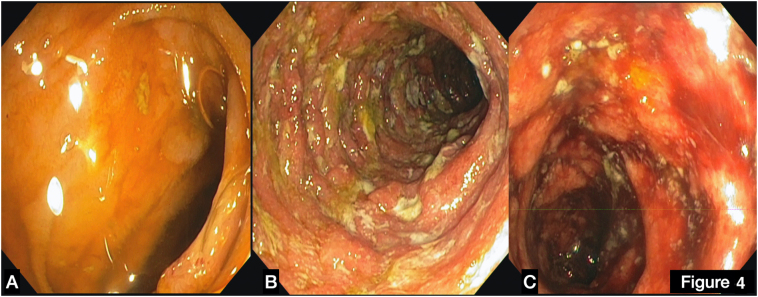
In the light of clinical presentation empirical therapy with ciprofloxacin was started. At day 12 a colonoscopy revealed severe ulcerative colitis (Mayo score III) (Online Fig. 1), therefore therapy with 5-aminosalicylic acid (ASA) (mesalazine) 1600 mg twice a day, and systemic steroid (prednisone 40 mg iv once/day) was started with good clinical and biochemical response with reduction of inflammatory markers.
Online Fig. 1.
(A) Terminal ileum, normal mucosa without ulcerations. (B) Descending colon, large ulcers surrounded by hyperemic and edematous mucosa. (C) Sigma large ulcers surrounded by hyperemic and edematous mucosa.
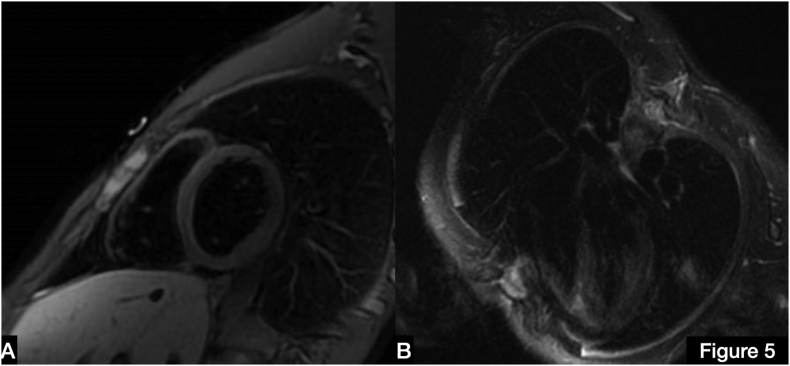
Due to the suspicion of myocardial involvement, cardiac magnetic resonance (CMR) was performed on day 7 and showed subepicardial late gadolinium enhancement (LGE) of the lateral ventricular wall without evidence of edema and normal systolic function and volumes (Fig. 2 and Online Fig. 2).
Fig. 2.
(A) Short-axis cardiac magnetic resonance (CMR) image showing nuanced late gadolinium enhancement (LGE) in the inferior and lateral ventricular wall (white arrows). (B) Long-axis CMR image with evidence of inferior LGE (white arrow).
Online Fig. 2.
Black blood stir T2 images of CMR without evidence of edema. (A) Short-axis, (B) four-chamber view.
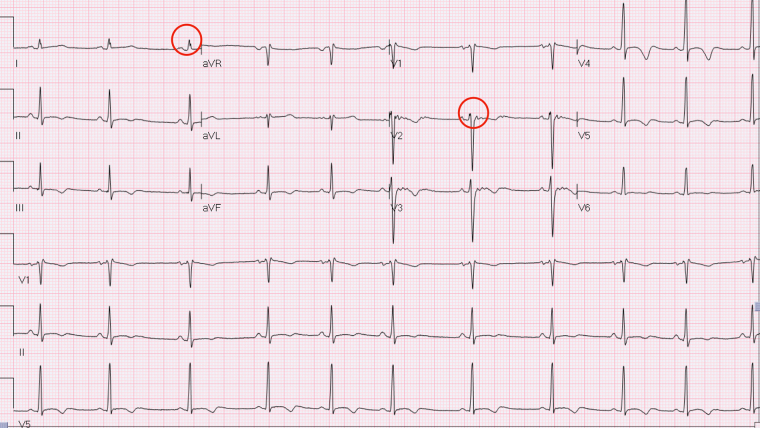
Electrocardiogram (ECG) evolution with mild QRS fragmentation in DI and V2 further supported the diagnosis of myocarditis (Fig. 3).
Fig. 3.
Electrocardiogram shows evolution with mild QRS fragmentation in DI and V2 (red circles).
At day 20, laboratory tests revealed an increase of transaminases and pancreatic enzymes (aspartate aminotransferase 130 U/L, alanine aminotransferase 202 U/L, gamma glutamyl transferase 66 U/L, amylase 255 U/L, lipase 187 U/L) with only a slight increase in CMV-DNA compared to pre-steroid baseline (192 > 379 UI/mLL). Suspecting hepatic and pancreatic toxicity, 5-ASA was suspended, with progressive reduction of cytolysis markers.
We also observed a decrease of fecal calprotectin from 2954 to 1376 mg/kg of day 8 and 22, respectively. The patient remained asymptomatic and liquid stool discharges were reduced in frequency and with fewer traces of blood.
On day 24 he was discharged home in good condition, on weaning prednisone course, vitamins and iron supplementation, and proton pump inhibitors. The weight was 68 kg (ideal weight about 80 kg for 185 cm of height).
Other suggestions were a slag-free diet until diarrhea is resolved then free diet and refraining from physical exertion and sports for at least 6 months. A close multidisciplinary follow-up has been set.
Follow-up
Two weeks after discharge, the patient's clinical conditions and weight recovered, reaching 74 kg. Sagittal sonogram of descending colon revealed only subtle thickening of bowel wall (3.2-mm-thick submucosa) with preserved stratification and normal echogenicity of adjacent mesenteric fat.
Laboratory tests showed normalization in liver and pancreatic enzymes, reduction of inflammatory markers, and fecal calprotectin decreased to 210 mg/kg. Steroid therapy was tapered and stopped in 3 months and azathioprine was up titrated to 75 mg once daily associated with rectal beclomethasone dipropionate and iron and D vitamin supplementation.
Seven months after discharge, the patient was feeling well, without abdominal pain and regular bowel movements, fecal calprotectin was 223 mg/kg. Transthoracic echocardiogram disclosed a normal biventricular function and resolution of pericardial effusion. ECG was also described as completely normalized. Based on these findings the gastroenterologist decided to keep on with azathioprine 75 mg twice a day and annual follow-up.
Discussion
Extraintestinal manifestations of IBD occur in 42% of patients with colonic disease and 23% of patients with small bowel involvement. The main sites of extraintestinal involvement are the joints, the skin, the eyes, and the oral cavity [7].
Another extraintestinal complication of IBD might be myocarditis, with a not negligible percentage of giant cell variant [4,8].
In a Danish cohort study, the risk ratio of myocarditis was 8.3 for Crohn's disease and 2.6 for ulcerative colitis [3].
Myocarditis may be more common at the onset of IBD exacerbation but there is no direct relationship between disease activity and severity of myocardial involvement [9]. In this case ECG showed evolutive changes as previously reported in inflammatory heart disease, suggesting a role for ECG as first-line screening tool [10]. In our patient clinical myocarditis was confirmed by the presence of LGE at CMR. Although diagnostic accuracy of LGE in the absence of edema may be reduced, it should be underscored that CMR was not performed in the acute phase, therefore myocardial edema might have already disappeared [11].
Mesalazine is an established first-line therapy for IBD and is a common prescription due to its safety and efficacy profile. Adverse side effects are rare but include agranulocytosis, hepatotoxicity, pancreatitis, peripheral neuropathy, and cardiac inflammation. Cardiotoxicity following administration of mesalazine is a potentially fatal complication with a reported incidence up to 0.3% [5].
Differential diagnosis of hepatic and pancreatic toxicity includes CMV reactivation.
The prevalence of CMV intestinal disease in IBD varies from 2% to 38%. This wide range is mainly due to a wide heterogeneity of the definitions of CMV infection in IBD [12]. An association of CMV with corticosteroid use, corticosteroid-refractory ulcerative colitis, and leukopenia has been described.
Therefore, it is clinically relevant to exclude CMV infection as it may contribute to extraintestinal complications and is associated with a more severe colitis. In our case the low serum viral load, the absence of histologic findings of CMV infection at biopsies, the optimal response to steroid therapy ruled out a CMV reactivation.
Conclusions
When a patient is diagnosed for the first time with a severe form of IBD or has a recrudescence of the disease, even if he/she has no overt cardiac symptoms, it is advisable to perform cardiac investigation and have in mind myocarditis as possible extraintestinal, subtle, manifestation. The possibility of CMV reactivation and mesalazine cardiotoxicity further complicate the possible etiopathogenetic interplay and the practical management.
Besides having a high grade of suspicion for extraintestinal complications of IBD and drug toxicity a multidisciplinary management is crucial to ensure the most effective and safe treatment as well as an adequate follow-up surveillance.
The following are the supplementary data related to this article.
Consent
The author confirms that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with the journal and COPE guidance.
Funding
None declared.
Declaration of competing interest
None declared.
References
- 1.Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Becker S.A., Wishnitzer R., Botwin S., Eliraz A., Bass D.D. Myopericarditis associated with inflammatory bowel disease. J Clin Gastroenterol. 1981;3:267–270. doi: 10.1097/00004836-198109000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen H.T., Fonager K.M. Myocarditis and inflammatory bowel disease. A 16-year Danish nationwide cohort study. Dan Med Bull. 1997;44:442–444. [PubMed] [Google Scholar]
- 4.Shergill S. Mesalazine-induced myopericarditis: a case report. Eur Heart J Case Rep. 2020;5:ytaa508. doi: 10.1093/ehjcr/ytaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taha M.E., Abdalla A., Al-Khafaji J., Malik S. Mesalamine-induced myopericarditis: a case report and literature review. Cardiol Res. 2019;10:59–62. doi: 10.14740/cr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlot B., Harries I., Bucciarelli-Ducci C. Connection between the heart and the gut. Heart. 2019;105:1148–1196. doi: 10.1136/heartjnl-2019-314832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenstein A.J., Janowitz H.D., Sachar D.B. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Razzano D., Fallon J.T. Myocarditis: somethings old and something new. Cardiovasc Pathol. 2020;44:107155. doi: 10.1016/j.carpath.2019.107155. [DOI] [PubMed] [Google Scholar]
- 9.Oh I.S., Choi C.H., Park J.H., Kim J.W., Cha B.K., Do J.H., et al. A case of acute myocarditis as the initial presentation of Crohn’s disease. Gut Liver. 2012;6:512–515. doi: 10.5009/gnl.2012.6.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero P., Piazza I., Kühl U., Grosu A., Tschöpe C., Senni M. QRS fragmentation as a possible electrocardiographic diagnostic marker in patients with acute myocarditis: preliminary histopathological validation. ESC Heart Fail. 2020;7:2527–2533. doi: 10.1002/ehf2.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T., et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mourad F.H., Hashash J.G., Kariyawasam V.C., Leong R.W. Ulcerative colitis and cytomegalovirus infection: from A to Z. J Crohns Colitis. 2020;14:1162–1171. doi: 10.1093/ecco-jcc/jjaa036. [DOI] [PubMed] [Google Scholar]