Abstract
Objective
To examine the associations of infertility, recurrent miscarriage, and stillbirth with the risk of first non-fatal and fatal stroke, further stratified by stroke subtypes.
Design
Individual participant pooled analysis of eight prospective cohort studies.
Setting
Cohort studies across seven countries (Australia, China, Japan, Netherlands, Sweden, the United Kingdom, and the United States) participating in the InterLACE (International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events) consortium, which was established in June 2012.
Participants
618 851 women aged 32.0-73.0 years at baseline with data on infertility, miscarriage, or stillbirth, at least one outcome event (non-fatal or fatal stroke), and information on covariates were included; 93 119 women were excluded. Of the participants, 275 863 had data on non-fatal and fatal stroke, 54 716 only had data on non-fatal stroke, and 288 272 only had data on fatal stroke.
Main outcome and measures
Non-fatal strokes were identified through self-reported questionnaires, linked hospital data, or national patient registers. Fatal strokes were identified through death registry data.
Results
The median follow-up for non-fatal stroke and fatal stroke was 13.0 years (interquartile range 12.0-14.0) and 9.4 years (7.6-13.0), respectively. A first non-fatal stroke was experienced by 9265 (2.8%) women and 4003 (0.7%) experienced a fatal stroke. Hazard ratios for non-fatal or fatal stroke were stratified by hypertension and adjusted for race or ethnicity, body mass index, smoking status, education level, and study. Infertility was associated with an increased risk of non-fatal stroke (hazard ratio 1.14, 95% confidence interval 1.08 to 1.20). Recurrent miscarriage (at least three) was associated with higher risk of non-fatal and fatal stroke (1.35, 1.27 to 1.44, and 1.82, 1.58 to 2.10, respectively). Women with stillbirth were at 31% higher risk of non-fatal stroke (1.31, 1.10 to 1.57) and women with recurrent stillbirth were at 26% higher risk of fatal stroke (1.26, 1.15 to 1.39). The increased risk of stroke (non-fatal or fatal) associated with infertility or recurrent stillbirths was mainly driven by a single stroke subtype (non-fatal ischaemic stroke and fatal haemorrhagic stroke), while the increased risk of stroke (non-fatal or fatal) associated with recurrent miscarriages was driven by both subtypes.
Conclusion
A history of recurrent miscarriages and death or loss of a baby before or during birth could be considered a female specific risk factor for stroke, with differences in risk according to stroke subtypes. These findings could contribute to improved monitoring and stroke prevention for women with such a history.
Introduction
Globally, stroke is one of the leading causes of mortality and disability in women.1 In 2019, around three million women died from stroke, and women lost over 10 million years of healthy life due to disability caused by stroke—44% higher than the number for men.2 Current stroke prevention guidelines have identified some risk factors, such as obesity, hypertension, and diabetes, but these are insufficient to explain the difference in risk of stroke between women and men. Female specific risk factors might be needed to identify women at higher risk of stroke.
To date, multiple studies have generated an expanding body of evidence on the association between pregnancy complications (eg, gestational diabetes and preeclampsia) and the long term risk of stroke, but studies on associations with infertility, miscarriage, or stillbirth have produced mixed evidence.3 4 Infertility, miscarriage, and stillbirth could increase the risk of stroke through the background of endocrine disorders (such as, low oestrogen or insulin resistance), systematic inflammation, endothelial dysfunction, psychological disorders, unhealthy behaviours (eg, smoking), and obesity.5 6 7 8 9 The inconclusive findings could be owing to methodological differences and limitations, such as inadequate follow-up, inconsistent definitions for outcomes, and lack of adjustment for residual confounders. Additionally, little evidence is available on whether the relations differ by non-fatal and fatal stroke, or by stroke subtypes. Research has suggested that risk factors, such as smoking, blood pressure, atrial fibrillation, and diabetes, are higher for fatal stroke than for non-fatal stroke, and such differences might also exist for the associations with infertility, miscarriage, and stillbirth.10 Stroke subtypes have divergent pathophysiology (brain vessel obstruction or bleeding) and would be linked through different mechanisms. Analysis by stroke subtypes would provide basic information for future studies on underlying mechanisms.
This study used pooled individual participant data from studies contributing to the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events (InterLACE) consortium.11 The aim was to assess the association of infertility, recurrent miscarriage, and stillbirth with the risk of first non-fatal and fatal stroke, further stratified by stroke subtypes.
Methods
Study participants
We analysed data from the InterLACE consortium, which was established in June 2012 and provides pooled individual level data on reproductive health and chronic disease.11 Currently, InterLACE is composed of 27 observational studies with over 850 000 women from 12 countries. The design and data harmonisation used with InterLACE have been reported previously.12 Eight studies from seven countries (Australia, China, Japan, Netherlands, Sweden, the United Kingdom, and the United States) that collected data on infertility, miscarriage, or stillbirth were included (n=711 970): Australian Longitudinal Study on Women’s Health 1946-51 cohort (ALSWH-mid), China Kadoorie Biobank, Japan Nurses’ Health Study (JNHS), UK MRC National Survey of Health and Development (NSHD), the Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherlands (Prospect-EPIC), US Study of Women’s Health Across the Nation (SWAN), UK Biobank, and the Swedish Women’s Lifestyle and Health Study (WLH; table S1).
All studies began between 1990 and early 2000, with the exception of NSHD (1946 British birth cohort), in which participants were recruited at birth in 1946.13 For the present analysis, baseline was considered as the first time when infertility, miscarriage, or stillbirth was determined, except for NSHD. NSHD first collected information on stroke in 1982 (when participants were aged 36), and the history of infertility and miscarriage was retrospectively collected in 1989 (aged 43); therefore, 1982 was used as the baseline year for this analysis. Women with data on at least one of infertility, miscarriage, or stillbirth, at least one outcome (first non-fatal stroke or fatal stroke), and covariates (race or ethnicity, body mass index, smoking status, education level, and hypertension) were included (fig S1). Women with non-fatal stroke before the age of 40 were excluded because they might have experienced stroke before a history of infertility, miscarriage, or stillbirth could be fully established.
Infertility, miscarriage, and stillbirth
Information on infertility, miscarriage, and stillbirth was mostly obtained through questionnaires at baseline, or in some studies at repeated follow-up surveys (table S2). Women provide information on their reproductive history according to their understanding, previous diagnosis, or treatment by a physician. Questions related to infertility were asked, such as whether the woman had tried to become pregnant during a period of 12 months or more without success, consulted a doctor for infertility, had a diagnosis of infertility from a doctor, or been treated for infertility. Women with any of the above experiences were identified as having experienced infertility. For miscarriage and stillbirth, the outcome of each pregnancy (livebirth, miscarriage, or stillbirth), number of miscarriages, and number of stillbirths were recorded. The numbers of miscarriages and stillbirths were categorised into four categories (0, 1, 2, and ≥3) and three categories (0, 1, and ≥2), respectively.14 15 Recurrent miscarriages was defined as three or more miscarriages, and recurrent stillbirths was defined as two or more stillbirths, which could be interspersed with livebirths.
First non-fatal and fatal stroke
The first non-fatal stroke event was identified through self-reported questionnaires (physician diagnosed or treated) or linked hospital data (table S2). All studies provided survey data on stroke; additionally, ALSWH-mid, Prospect-EPIC, UK Biobank, and WLH included linked hospital data, coded according to the 9th or 10th versions of the international classification of diseases (ICD-9 and ICD-10). Fatal stroke was identified through death registries in five studies (ALSWH-mid, China Biobank, Prospect-EPIC, JNHS, and UK Biobank; table S2), using ICD-9 or ICD-10 codes.
The following ICD codes were used to define stroke from hospital records and death registries: ICD-9 (430, 431, 433, 434, 436) and ICD-10 (I60, I61, I63, I64, I69.0, I69.1, I69.3, I69.4).16 Subtypes of stroke were classified as haemorrhagic stroke (ICD-9: 430, 431; ICD-10: I60, I61, I69.0, I69.1) and ischaemic stroke (ICD-9: 433, 434, 436; ICD-10: I63, I64, I69.3, I69.4).16 Only JNHS, in which participants were registered nurses, collected self-reported data on stroke subtypes (subarachnoid haemorrhage, cerebral haemorrhage, and cerebral infarction). Any stroke event, not specified as haemorrhagic or ischaemic stroke, was classified as unspecified stroke.
Covariates
Information on covariates was collected at baseline. For women who were not Asian, body mass index was categorised into underweight (<18.5), normal (18.5-24.9), overweight (25-29.9), and obese (≥30).17 For Asian women, the categories were defined as underweight (<18.5), normal (18.5-22.9), overweight (23-27.4), and obese (≥27.5).18 Other covariates included race or ethnicity (white, Asian, or others), current smoking status (yes or no), education level (≤10, 11-12, or >12 years), hypertension (yes or no), and diabetes mellitus (yes or no).
Statistical analysis
Baseline characteristics were presented as medians and interquartile ranges for continuous variables, and as numbers and percentages for categorical variables. Kaplan-Meier survival curves were drawn to show the probability of stroke among women with and without infertility, miscarriage, or stillbirth. We fit Cox proportional hazards survival time models to estimate the associations between infertility, miscarriage, stillbirth, and outcomes (first non-fatal stroke, fatal stroke, or subtypes of stroke), providing hazard ratios and 95% confidence intervals. The proportional hazards assumption was tested using Schoenfeld residuals. We adjusted the survival time models for race or ethnicity, body mass index, smoking status, and education level. To account for the time varying effects of hypertension, a stratified analysis was conducted through an option of strata in the proportional hazards regression procedure.19 However, because the proportion of women with diabetes was too low for stratified analysis, diabetes was taken into consideration in the sensitivity analyses.19 In the stratified proportional hazards model, the regression coefficients were assumed to be the same for each stratum, and the baseline hazard functions might be different. We took study variability into account by including an indicator for study as a covariate, and robust variance estimators were used to account for potential within study correlation.20 In the analysis of non-fatal stroke, survival times were age at first non-fatal stroke or age at last update of non-fatal data, and women without stroke were censored. In the analysis of fatal stroke, survival times were age at death or age at last update of death data, and women who had not died were censored. We restricted analyses for miscarriage and stillbirth to women who had ever been pregnant.
To account for the possible bias arising from competing risks across groups, we also calculated Fine-Gray subdistribution hazards for subtypes of non-fatal stroke, fatal stroke, and subtypes of fatal stroke.21 When one subtype of non-fatal stroke was the event of interest, other subtypes of non-fatal stroke were incorporated as competing events. When fatal stroke or one subtype of fatal stroke was the event of interest, deaths of other causes were treated as competing events. Because of insufficient outcome events, neither the association of fatal stroke (n=204) with infertility nor the associations of unspecified fatal stroke (n=161) with miscarriage and stillbirth were assessed.
We conducted several sensitivity analyses to examine the robustness of findings. Firstly, data from a subset of studies (ALSWH-mid, Prospect-EPIC, UK Biobank, and WLH) were analysed, in which linked hospital data were used to identify non-fatal stroke events. This resulted in the exclusion of 19 114, 15 837, and 15 709 women in the analysis for infertility, miscarriage, and stillbirth, respectively. Secondly, women with infertility who did not have a diagnosis of infertility, meet the definition of infertility (unsuccessfully trying to be pregnant for 12 months or more), or receive maternal treatment of infertility were excluded (n=32 737). Thirdly, the baseline of NSHD was changed from 1982 (when the women were aged 36) to 1989 (aged 43), so that the women’s age was more comparable to the data in other included studies. Because fewer data were missing in the 1989 survey, 250, 113, and 88 more women were included in the analysis for infertility, miscarriage, and stillbirth, respectively.
Fourthly, the survival time models were additionally adjusted for a history of the oral contraceptive pill use (yes or no) and of hormone replacement therapy (yes or no) at baseline; these data were not available in the JNHS and China Biobank studies. This resulted in the exclusion of 63 354, 23 597, and 13 317 women before assessing the associations of infertility, miscarriage, and stillbirth with the risk of non-fatal stroke, and 310 828 and 300 593 women before estimating the associations between miscarriage, stillbirth, and fatal stroke. Fifthly, data from women without a history of diabetes were analysed to exclude the influence of diabetes. For non-fatal stroke, 1919, 9786, and 9490 women were excluded from the analysis of infertility, miscarriage, and stillbirth. For fatal stroke, 25 013 and 24 719 women were excluded from the analysis of miscarriage and stillbirth. Sixthly, associations of infertility, miscarriage, and stillbirth with combined stroke event (first non-fatal or fatal stroke) were explored. Finally, missing data on covariates were imputed using multiple imputation (10 times), and imputed datasets were used to assess the validity of results. We also assessed associations in single studies. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
Patient and public involvement
Because the study was a pooled analysis of pre-existing datasets, no patients were involved in setting the research questions or outcome measures, nor were they involved in developing the plan for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. However, their contributions to the respective studies are acknowledged.
Results
Study characteristics
Overall, 618 851 women from eight studies were included, aged from 32.0 to 73.0 years at baseline. The median follow-up for non-fatal stroke was 13.0 years (interquartile range 12.0-14.0), and for fatal stroke it was 9.4 years (7.6-13.0). In total, 275 863 women had data on non-fatal and fatal stroke, 54 716 women only had data on non-fatal stroke, and 288 272 only had data on fatal stroke. Among these, 9265 (2.8%) women experienced a first non-fatal stroke at a median age of 62.0 (interquartile range 54.0-70.0), and 4003 (0.7%) had fatal stroke at a median age of 71.0 (64.0-76.0). The proportion of women who experienced infertility, miscarriage, and stillbirth was 17.2%, 16.6%, and 4.6%, respectively (table 1). Table 1 presents the baseline characteristics of women with and without such reproductive histories. Less than 7.0% of women were excluded owing to missing data, and they are more likely to be underweight, current smokers, and less educated (tables S3, S4).
Table 1.
Baseline characteristics of study participants by history of infertility, miscarriage, and stillbirth. Data are numbers (%)
| Characteristics | Infertility (n=94 286) | Miscarriage (n=550 853) | Stillbirth (n=540 638) | |||||
|---|---|---|---|---|---|---|---|---|
| Never | Ever | Never | Ever | Never | Ever | |||
| Sample size | 78 055 (82.79) | 16 231 (17.21) | 459 284 (83.38) | 91 569 (16.62) | 515 765 (95.40) | 24 873 (4.60) | ||
| Race or ethnicity | ||||||||
| White | 63 738 (81.66) | 13 804 (85.05) | 177 722 (38.70) | 60 207 (65.75) | 221 459 (42.94) | 6710 (26.98) | ||
| Asian | 13 276 (17.01) | 2160 (13.31) | 275 992 (60.09) | 29 093 (31.77) | 287 215 (55.69) | 17 608 (70.79) | ||
| Others | 1041 (1.33) | 267 (1.65) | 5570 (1.21) | 2269 (2.48) | 7091 (1.37) | 555 (2.23) | ||
| Body mass index | ||||||||
| Underweight | 4051 (5.19) | 917 (5.65) | 13 566 (2.95) | 2490 (2.72) | 13 968 (2.71) | 1429 (5.75) | ||
| Normal | 46 223 (59.22) | 9914 (61.08) | 176 583 (38.45) | 34 955 (38.17) | 197 260 (38.25) | 9453 (38.01) | ||
| Overweight | 20 328 (26.04) | 3895 (24.00) | 186 293 (40.56) | 34 689 (37.88) | 208 466 (40.42) | 9653 (38.81) | ||
| Obese | 7453 (9.55) | 1505 (9.27) | 82 842 (18.04) | 19 435 (21.22) | 96 071 (18.63) | 4338 (17.44) | ||
| Current smoker | ||||||||
| No | 62 816 (80.48) | 12 649 (77.93) | 429 192 (93.45) | 82 966 (90.60) | 480 771 (93.22) | 23 060 (92.71) | ||
| Yes | 15 239 (19.52) | 3582 (22.07) | 30 092 (6.55) | 8603 (9.40) | 34 994 (6.78) | 1813 (7.29) | ||
| Education level | ||||||||
| ≤10 | 23 600 (30.24) | 4397 (27.09) | 320 770 (69.84) | 55 316 (60.41) | 350 177 (67.89) | 20 679 (83.14) | ||
| 11-12 | 18 534 (23.74) | 4113 (25.34) | 63 985 (13.93) | 11 763 (12.85) | 72 174 (13.99) | 1861 (7.48) | ||
| >12 | 35 921 (46.02) | 7721 (47.57) | 74 529 (16.23) | 24 490 (26.74) | 93 414 (18.11) | 2333 (9.38) | ||
| Hypertension | ||||||||
| No | 62 505 (80.08) | 13 373 (82.39) | 317 457 (69.12) | 63 644 (69.50) | 358 816 (69.57) | 14 254 (57.31) | ||
| Yes | 15 550 (19.92) | 2858 (17.61) | 141 827 (30.88) | 27 925 (30.50) | 156 949 (30.43) | 10 619 (42.69) | ||
| Diabetes mellitus | ||||||||
| No | 76 502 (98.06) | 15 862 (97.78) | 438 278 (95.50) | 87 260 (95.43) | 492 462 (95.57) | 23 160 (93.20) | ||
| Yes | 1511 (1.94) | 360 (2.22) | 20 649 (4.50) | 4176 (4.57) | 22 843 (4.43) | 1689 (6.80) | ||
Infertility: women from Australian Longitudinal Study on Women’s Health 1946-51 cohort (ALSWH-mid), Swedish Women’s Lifestyle and Health Study (WLH), United Kingdom MRC National Survey of Health and Development (NSHD), United States Study of Women’s Health Across the Nation (SWAN), Japan Nurses’ Health Study (JNHS), and Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherlands (Prospect-EPIC) were included. Miscarriage: women from NSHD, SWAN, UK Biobank, China Biobank, and Prospect-EPIC were included. Among the 550 583 women, 67 345 (12.23%) had one miscarriage, 16 101 (2.92%) had two miscarriages, and 8064 (1.46%) had three or more miscarriages. Stillbirth: women from NSHD, SWAN, UK Biobank, China Biobank, and Prospect-EPIC were included. Among the 540 638 women, 19 937 (3.69%) had one stillbirth, and 4924 (0.91%) had two or more stillbirths. Body mass index: for Asian women, the classifications of body mass index are <18.5 (underweight), 18.5-22.9 (normal), 23-27.4 (overweight), and ≥27.5 (obese); for others, the classifications of body mass index are <18.5 (underweight), 18.5-24.9 (normal), 25-29.9 (overweight), and ≥30 (obese).
Infertility
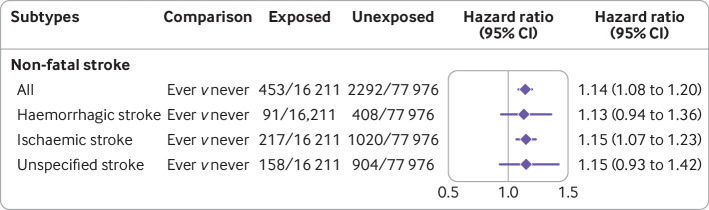
Women with a history of infertility were at higher risk of non-fatal stroke compared with women without infertility (hazard ratio 1.14, 95% confidence interval 1.08 to 1.20; table 2, fig S2). Analyses by subtypes of non-fatal stroke showed infertility was associated with an increased risk of ischaemic stroke (1.15, 1.07 to 1.23; fig 1).
Table 2.
Association of infertility, miscarriage, and stillbirth with non-fatal and fatal stroke
| Reproductive histories | Sample size | No of events | Incidence rate | Non-fatal stroke | Sample size | No of events | Incidence rate | Fatal stroke | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | |||||||
| Models with single reproductive histories | ||||||||||
| History of infertility* | ||||||||||
| Never | 77 976 | 2292 | 47.6 | Reference | Reference | 34 488 | 177 | 7.6 | — | — |
| Ever | 16 211 | 453 | 46.0 | 1.13 (1.05 to 1.21) | 1.14 (1.08 to 1.20) | 5168 | 27 | 7.8 | — | — |
| History of miscarriage† | ||||||||||
| Never | 197 303 | 5812 | 43.0 | Reference | Reference | 455 363 | 3255 | 11.3 | Reference | Reference |
| Ever | 65 315 | 2124 | 47.7 | 1.11 (1.08 to 1.15) | 1.11 (1.07 to 1.15) | 89 983 | 712 | 11.8 | 1.18 (1.04 to 1.35) | 1.17 (1.07 to 1.29) |
| No of miscarriages† | ||||||||||
| 0 | 197 303 | 5812 | 43.0 | Reference | Reference | 455 363 | 3255 | 11.3 | Reference | Reference |
| 1 | 46 681 | 1454 | 45.6 | 1.06 (1.04 to 1.09) | 1.07 (1.04 to 1.10) | 66 227 | 479 | 10.8 | 1.08 (0.91 to 1.28) | 1.08 (0.96 to 1.21) |
| 2 | 11 988 | 395 | 48.4 | 1.13 (1.07 to 1.18) | 1.12 (1.07 to 1.17) | 15 805 | 137 | 12.9 | 1.26 (1.18 to 1.36) | 1.26 (1.07 to 1.49) |
| ≥3 | 6588 | 271 | 58.1 | 1.44 (1.34 to 1.56) | 1.35 (1.27 to 1.44) | 7897 | 96 | 18.0 | 1.93 (1.72 to 2.16) | 1.82 (1.58 to 2.10) |
| History of stillbirth‡ | ||||||||||
| Never | 243 859 | 7070 | 42.4 | Reference | Reference | 510 586 | 3479 | 10.7 | Reference | Reference |
| Ever | 8547 | 411 | 69.5 | 1.46 (1.18 to 1.82) | 1.31 (1.10 to 1.57) | 24 673 | 451 | 27.1 | 1.13 (1.08 to 1.18) | 1.07 (1.00 to 1.13) |
| No of stillbirths‡ | ||||||||||
| 0 | 243 859 | 7070 | 42.4 | Reference | Reference | 510 586 | 3479 | 10.7 | Reference | Reference |
| 1 | 7310 | 349 | 68.9 | 1.46 (1.23 to 1.72) | 1.32 (1.15 to 1.51) | 19 749 | 275 | 20.8 | 1.02 (0.97 to 1.06) | 0.97 (0.91 to 1.03) |
| ≥2 | 1225 | 62 | 73.6 | 1.53 (0.93 to 2.53) | 1.29 (0.84 to 1.98) | 4914 | 176 | 51.1 | 1.38 (1.27 to 1.50) | 1.26 (1.15 to 1.39) |
| Model with miscarriage and stillbirth§ | ||||||||||
| No of miscarriages | ||||||||||
| 0 | 190 991 | 5554 | 42.5 | Reference | Reference | 449 055 | 3236 | 11.4 | Reference | Reference |
| 1 | 43 855 | 1321 | 44.2 | 1.04 (1.02 to 1.06) | 1.05 (1.02 to 1.08) | 63 509 | 469 | 11.1 | 1.07 (0.91 to 1.27) | 1.08 (0.96 to 1.21) |
| 2 | 11 147 | 350 | 46.2 | 1.08 (1.05 to 1.12) | 1.08 (1.04 to 1.12) | 14 988 | 132 | 13.1 | 1.23 (1.14 to 1.33) | 1.23 (1.04 to 1.46) |
| ≥3 | 6032 | 236 | 57.9 | 1.38 (1.27 to 1.50) | 1.31 (1.23 to 1.41) | 7351 | 91 | 18.3 | 1.85 (1.67 to 2.06) | 1.76 (1.53 to 2.03) |
| No of stillbirths | ||||||||||
| 0 | 243 589 | 7056 | 42.4 | Reference | Reference | 510 320 | 3477 | 10.7 | Reference | Reference |
| 1 | 7231 | 344 | 68.6 | 1.43 (1.21 to 1.68) | 1.29 (1.13 to 1.47) | 19 689 | 275 | 20.9 | 1.01 (0.96 to 1.05) | 0.96 (0.90 to 1.02) |
| ≥2 | 1205 | 61 | 73.7 | 1.45 (0.90 to 2.32) | 1.23 (0.81 to 1.85) | 4894 | 176 | 51.4 | 1.34 (1.23 to 1.45) | 1.23 (1.12 to 1.34) |
Incidence rate (per 100 000 person years). Crude hazard ratios (HRs) took the cluster and fixed effects of study into account. Adjusted HRs were additionally adjusted for race or ethnicity, body mass index, smoking status, and education level, and stratified by history of hypertension.
Six studies (Australian Longitudinal Study on Women’s Health 1946-51 cohort—ALSWH-mid, Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherlands—Prospect-EPIC, Japan Nurses’ Health Study—JNHS, United Kingdom MRC National Survey of Health and Development—NSHD, United States Study of Women’s Health Across the Nation—SWAN, and Swedish Women’s Lifestyle and Health Study—WLH) and two studies (ALSWH-mid and JNHS) were included for non-fatal and fatal stroke, respectively.
Six studies (ALSWH-mid, China Biobank, Prospect-EPIC, NSHD, SWAN, and UK Biobank) and four studies (ALSWH-mid, China Biobank, Prospect-EPIC, and UK Biobank) were included for non-fatal and fatal stroke, respectively.
Five studies (China Biobank, Prospect-EPIC, NSHD, SWAN, and UK Biobank) and three studies (China Biobank, Prospect-EPIC, and UK Biobank) were included for non-fatal and fatal stroke, respectively.
Five studies (China Biobank, Prospect-EPIC, NSHD, SWAN, and UK Biobank) and three studies (China Biobank, Prospect-EPIC, and UK Biobank) were included for non-fatal and fatal stroke, respectively.
Fig 1.
Association between infertility and first non-fatal stroke overall and by subtypes. Individual level data of 94 187 women were pooled from six studies (Australian Longitudinal Study on Women’s Health 1946-51 cohort—ALSWH-mid, Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherlands—Prospect-EPIC, Japan Nurses’ Health Study—JNHS, United Kingdom MRC National Survey of Health and Development—NSHD, United States Study of Women’s Health Across the Nation—SWAN, and Swedish Women’s Lifestyle and Health Study—WLH). Hazard ratios were adjusted for race or ethnicity, body mass index, smoking status, education level, and study, and stratified by history of hypertension
Miscarriage
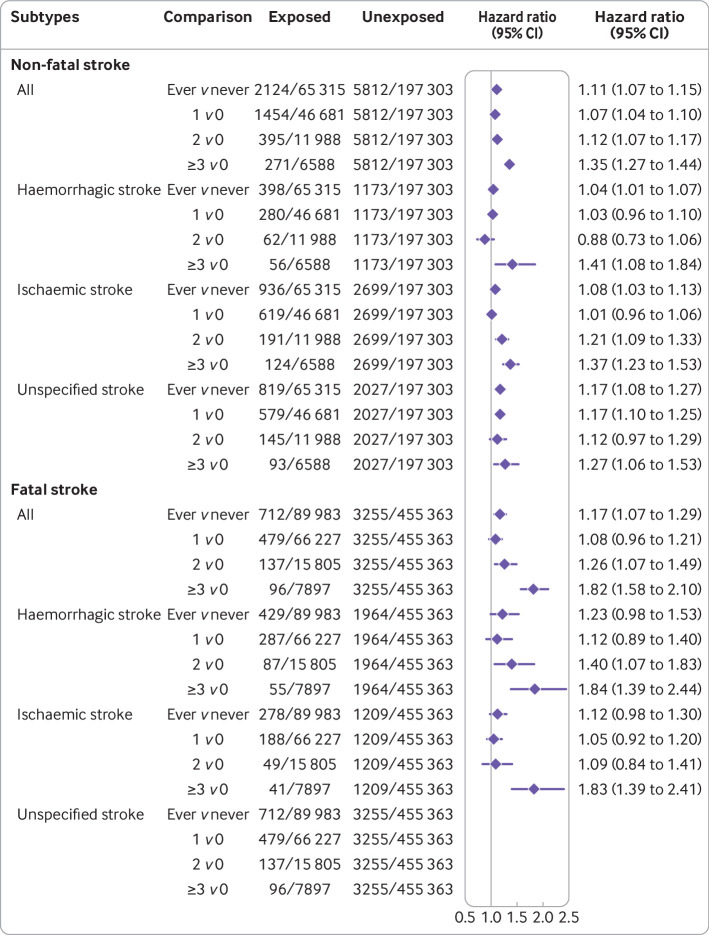
Among women who had ever been pregnant, a history of miscarriage was associated with 11% higher risk of non-fatal stroke compared with women without miscarriage (hazard ratio 1.11, 95% confidence interval 1.07 to 1.15; table 2, fig S3). The risk of non-fatal stroke increased with the number of miscarriages (1, 2, and ≥3), with adjusted hazard ratios of 1.07 (1.04 to 1.10), 1.12 (1.07 to1.17), and 1.35 (1.27 to 1.44), respectively (table 2, fig S4). Women with recurrent miscarriage (≥3) were more likely to experience ischaemic and haemorrhagic non-fatal stroke than women without miscarriage (1.37, 1.23 to 1.53, and 1.41, 1.08 to 1.84, respectively; fig 2).
Fig 2.
Association of miscarriage with first non-fatal and fatal stroke overall and by subtypes. Non-fatal stroke: individual level data of 262 618 women were pooled from six studies (Australian Longitudinal Study on Women’s Health 1946-51 cohort—ALSWH-mid, China Biobank, Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherlands—Prospect-EPIC, United Kingdom MRC National Survey of Health and Development—NSHD, United States Study of Women’s Health Across the Nation—SWAN, and UK Biobank). Fatal stroke: individual level data of 545 346 women were pooled from four studies (ALSWH-mid, China Biobank, Prospect-EPIC, and UK Biobank). Hazard ratios were adjusted for race or ethnicity, body mass index, smoking status, education level, and study, and stratified by history of hypertension
For fatal stroke, the results showed a similar pattern (table 2, figs S5, S6). Women with one or more miscarriages (1, 2, and ≥3) had a higher risk of fatal stroke compared with women without miscarriages, with adjusted hazard ratios of 1.08 (0.96 to 1.21), 1.26 (1.07 to 1.49), and 1.82 (1.58 to 2.10), respectively. Women with recurrent miscarriages were more likely to experience ischaemic and haemorrhagic fatal stroke (1.83, 1.39 to 2.41, and 1.84, 1.39 to 2.44, respectively; fig 2).
Stillbirth
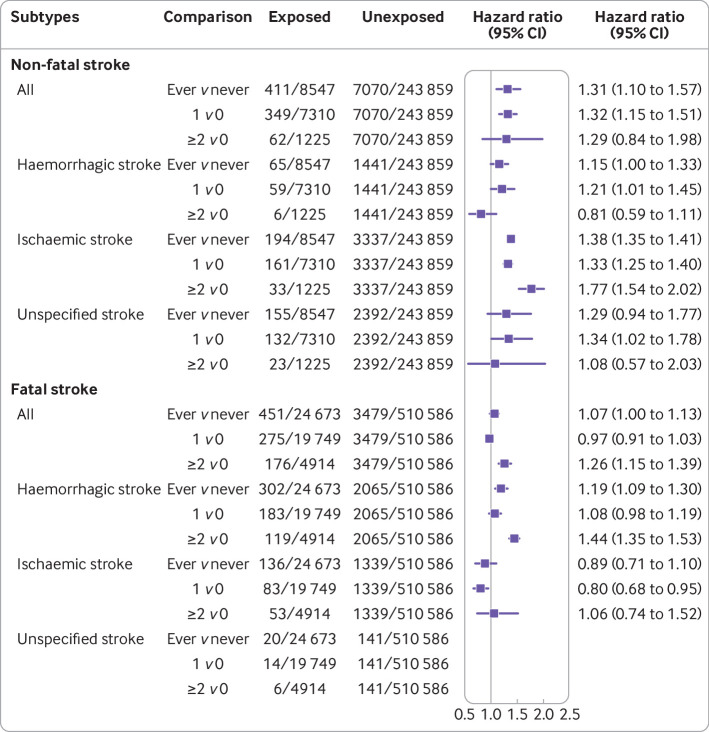
Among women who have ever been pregnant, a history of stillbirth was associated with 31% increased risk of non-fatal stroke compared with women without stillbirth (hazard ratio 1.31, 95% confidence interval 1.10 to 1.57; table 2, fig S7). Adjusted hazard ratios for single and recurrent stillbirths were 1.32 (1.15 to 1.51) and 1.29 (0.84 to 1.98), respectively, which are consistent with the Kaplan-Meier plot (table 2, fig S8). Women with recurrent stillbirths were more likely to experience ischaemic non-fatal stroke than women without stillbirth (1.77, 1.54 to 2.02; fig 3).
Fig 3.
Association of stillbirth with first non-fatal and fatal stroke overall and by subtypes. Non-fatal stroke: individual level data of 252 406 women were pooled from five studies (China Biobank, Utrecht contribution to the European Prospective Investigation into Cancer and Nutrition cohort, Netherland—Prospect-EPIC, United Kingdom MRC National Survey of Health and Development—NSHD, United States Study of Women’s Health Across the Nation—SWAN, and UK Biobank). Fatal stroke: individual level data of 535 259 women were pooled from three studies (China Biobank, Prospect-EPIC, and UK Biobank). Hazard ratios were adjusted for race or ethnicity, body mass index, smoking status, education level, and study, and stratified by history of hypertension
Women with stillbirth were also at higher risk of fatal stroke than women without stillbirth (hazard ratio 1.07, 95% confidence interval 1.00 to 1.13; table 2, fig S9), and the risk increased with the number of stillbirths (1 v 0: 0.97, 0.91 to 1.03; ≥2 v 0: 1.26, 1.15 to 1.39; table 2, fig S10). Women with recurrent stillbirths were more likely to have haemorrhagic fatal stroke (1.44, 1.35 to 1.53; fig 3).
In this study, miscarriage and stillbirth acted independently on the risk of non-fatal and fatal stroke. Including miscarriage and stillbirth as separate variables in the same model produced almost no changes in the hazard ratios for each variable (table 2). Additionally, models with an eight level variable that combined the history of miscarriage (0, 1, 2, ≥3) and stillbirth (0, ≥1; Akaike information criterion 164 358.7 for non-fatal stroke and 84 611.5 for fatal stroke) did not fit the data better than the models with separate variables of miscarriage and stillbirth (Akaike information criterion 164 355.7 for non-fatal stroke and 84 604.7 for fatal stroke).
Sensitivity analyses
Firstly, studies with hospital data on non-fatal stroke were included. Estimated hazard ratios generally remained unchanged, except for an increase for recurrent stillbirth (table S5). Secondly, a separate analysis that included studies determining infertility through a strict definition (physician diagnosis, maternal treatment, and having difficulty conceiving for 12 months or more) was conducted, and the results were consistent with the main analysis (table S6).22 Thirdly, redefining the baseline of NSHD had almost no influence on the results (table S7). Fourthly, additional adjustment for a history of oral contraceptive pill and hormone replacement therapy use did not change the associations, but the 95% confidence intervals of subtypes of fatal stroke became wider owing to the smaller sample size (tables S8, S9). Fifthly, restricting the analysis to women without diabetes had similar results to the main analysis (tables S10, S11). Sixthly, when the associations of infertility, miscarriage, and stillbirth with combined outcome (non-fatal or fatal stroke) were observed, recurrent miscarriages and stillbirths were still associated with the risk of stroke (table S12). Finally, estimates from the imputed datasets had almost no changes from the main analysis (tables S13, S14). The associations of infertility, miscarriage, and stillbirth with non-fatal and fatal stroke in each study separately are also provided in the supplement (figs S11-S15).
Examination of the Schoenfeld residuals did not support violation of the proportional hazard assumptions (table S15). Even though the residuals were correlated with survival time for some variables (eg, education level ≤10 years, obesity, and other races), the correlation coefficients were quite small (<0.08) though statistically significant owing to the large sample size; the inclusion of interaction terms with time did not improve the model fit.
Discussion
Summary of findings
This pooled analysis of 618 851 women (275 863 with data on non-fatal and fatal stroke, 54 716 with data on non-fatal stroke only, and 288 272 with data on fatal stroke only) showed that infertility, miscarriage, and stillbirth were all associated with increased risk of stroke, especially recurrent miscarriages and stillbirths. The increased risk of stroke associated with infertility or recurrent stillbirths was mainly driven by a single subtype of stroke (non-fatal ischaemic stroke or fatal haemorrhagic stroke, respectively), whereas the risk of stroke associated with recurrent miscarriages was driven by both subtypes.
Infertility and stroke
Previous studies on the association between infertility and stroke have been inconsistent. Three cohort studies from Canada, Sweden, and Denmark did not find evidence of an association when infertility was identified through fertility treatment.23 24 25 Another study using American Optum’s Clinformtics Data Mart showed 26% increased risk of cerebrovascular disease among women with a diagnosis of infertility or who had received treatment or testing for infertility.26 In this study, women with infertility diagnosis, fertility treatment, infertility consultation, or an experience of trying unsuccessfully to become pregnant for 12 months or more were considered as infertile. Compared with previous studies, the present study included women who met the definition of infertility but had not sought medical help.
In the present study, women with infertility were found to be at increased risk of non-fatal stroke, which was mainly driven by the ischaemic subtype. Infertility might be pathologically linked to stroke through a background of ovarian disorders.27 28 Polycystic ovary syndrome and premature ovarian insufficiency are two of the main ovarian disorders related to female infertility. Polycystic ovary syndrome is associated with a higher risk of insulin resistance, glucose intolerance, and an increased prothrombotic state, which would lead to vascular dysfunction and long term risk of stroke.5 Premature ovarian insufficiency is accompanied by decreased levels of oestrogen, and would increase the risk of stroke through the loss of potential neuroprotective and anti-inflammatory properties from oestrogen.6 29 Meanwhile, in the case of infertility due to polycystic ovary syndrome or premature ovarian insufficiency, a higher prevalence of thyroid autoimmunity has been documented, which would further increase the risk of ischaemic stroke through thyrotoxic atrial fibrillation and hypercoagulability state.28 30 Additionally, women with infertility are more likely to be smokers, have obesity, and experience anxiety and depression, which would increase future risk of stroke through the toxic effects of smoking, insulin resistance, increased inflammation, and enhanced platelet aggregation.4 8 31 32
Miscarriage, stillbirth, and stroke
Even though a few cohort studies from China, the UK, Denmark, and Sweden have shown miscarriage and stillbirth to be associated with increased risk of stroke,33 34 35 36 37 other studies have not.38 39 40 41 42 A recent meta-analysis pooled the results of 13 cohort studies and found associations between miscarriage, stillbirth, and the risk of stroke.43 However, the definitions of outcomes were inconsistent across the included studies (non-fatal stroke only, fatal stroke only, and both non-fatal and fatal stroke).43 In the present study, the associations for non-fatal and fatal stroke were explored separately. Women with stillbirth or miscarriage, especially recurrent miscarriages or stillbirths, were found to be at increased risk of non-fatal and fatal stroke.
Analysis by subtypes of stroke showed that women with recurrent miscarriages (at least three) were at higher risk of haemorrhagic and ischaemic stroke (non-fatal or fatal), and recurrent stillbirths (at least two) were associated with increased risk of ischaemic non-fatal stroke and haemorrhagic fatal stroke. An underlying mechanism for the risk of stroke associated with recurrent miscarriages or stillbirths would be endothelial dysfunction. Endothelial dysfunction might lead to pregnancy loss through placentation related defects, persist after a complicated pregnancy, and contribute to the development of stroke through reduced vasodilation, proinflammatory status, and prothrombic properties.7 44 Meanwhile, antiphospholipid antibodies, which are characterised by pregnancy loss attributed to thrombosis of placental vessels, cause stroke through the induction of a prothrombotic state.45 Binding by antiphospholipid antibodies to endothelial cells deranges normal endothelial anticoagulant function and promotes thrombosis, which would evolve in the development of ischaemic stroke.46 Women with recurrent pregnancy loss are also more likely to have unhealthy behaviours (eg, smoking), to have obesity, and to experience depression, which could contribute to later risk of stroke.8 47 48 49
Clinical implications
The findings from this pooled study provided robust evidence for future clinical practice and guidelines. Having a history of recurrent pregnancy loss might be considered a female specific risk factor for stroke. Compared with the overall rates of non-fatal and fatal stroke (2.8% and 0.7%), the rates among women with recurrent miscarriages (at least three) or stillbirths (at least two) increased up to five times (recurrent miscarriage 4.1% and 1.2%; recurrent stillbirth 5.1% and 3.6%). We suggest early monitoring of women with recurrent miscarriages or stillbirths for stroke risk factors (eg, raised blood pressure, blood sugar, blood lipids), and promoting a healthy lifestyle programme (eg, smoking cessation, maintaining healthy weight, exercise) to reduce their excess risk of stroke in later life.
Strengths
This study pooled individual level data from divergent geographical regions and racial groups to quantify the association between infertility, recurrent miscarriages, recurrent stillbirths, and stroke risk, which increases the generalisability of the study findings. All the variables were harmonised with common definitions and coding rules.12 This reduced potential bias from inconsistent classifications of reproductive histories or outcomes. Additionally, with a large sample size, sufficient power exists to detect the association of rare events (eg, recurrent miscarriages and recurrent stillbirths) with the risk of non-fatal and fatal stroke, further stratified by stroke subtypes, which is often not possible in any single study.
Limitations
Some limitations need to be considered in interpreting the findings. Information on infertility, miscarriage, and stillbirth was collected from questionnaires, which might induce recall bias. Previous studies have found that compared with medical records, self-reported infertility (sensitivity 72.0%, specificity 70.0%) and pregnancy loss (sensitivity 73.5%, specificity 99.4%) would be reliable, but stillbirth might be over reported (sensitivity 100.0%, specificity 30.0%).50 51 52 Therefore, it is plausible to assume that for this study the impact of recall bias was limited.
The definitions of infertility, miscarriage, and stillbirth might not be the same in each of the included studies because women reported according to their understanding of the definition of their reproductive history, previous diagnosis, or treatment by physicians. Additionally, the effects of different causes or treatments related to these reproductive histories were not explored owing to limited data. Only two studies (ALSWH-mid and WLH) and one study (JNHS) had data on infertility treatment and causes of infertility, respectively, and none of the studies had data on causes or treatment of pregnancy loss.
Among 12.1% of the included women, information on non-fatal stroke was collected through questionnaires only. Engstad and colleagues, and Jackson and colleagues have shown, however, that self-reported stroke was reliable (sensitivity 80%, specificity 99%; and sensitivity 78.6%, specificity 99.3%, respectively).53 54 In this study, sensitivity analysis using hospital data showed consistent results with the main analysis, which indicated that this limitation should have little influence on the results. Also, around 4.0% (161/4003) of fatal strokes and 33.0% (3053/9265) of non-fatal strokes were categorised as unspecified subtype because information on the subtypes of stroke was not available or missing for patients with stroke identified from questionnaires, derived hospital records, or death registry codes, which might affect the strength of association for ischaemic or haemorrhagic stroke.
Although the models were adjusted for a range of covariates, including hypertension and diabetes, some comorbidities of interest, such as thyroid disorders, endometriosis, and pelvic inflammatory disease, were not available in all studies. Finally, some women were excluded owing to missing data, which could lead to sample bias. The proportions of women with missing data were less than 7.0%, and the findings from multiple imputations were consistent with the main analysis.
Conclusions
Infertility and pregnancy loss, especially recurrent miscarriages, and stillbirths, were associated with women’s later risk of non-fatal and fatal stroke. The risk of stroke (non-fatal or fatal) associated with infertility or recurrent stillbirths was mainly driven by a single subtype of stroke (non-fatal ischaemic stroke or fatal haemorrhagic stroke, respectively), whereas the risk of stroke (non-fatal or fatal) associated with recurrent miscarriages was driven by both subtypes.
These findings extend our current knowledge on the associations of infertility, miscarriage, and stillbirth with stroke, and highlight the need for future studies on the underlying mechanisms, linking the subtype, severity, and prognosis of stroke. A history of recurrent miscarriages and death or loss of a baby before or during birth should be considered a female specific risk factor for stroke. Early monitoring of women with recurrent miscarriages or stillbirths and tailored healthy lifestyle interventions are recommended to lower the risk of stroke.
What is already known on this topic?
Evidence on the links of infertility, miscarriage, and stillbirth with stroke has been inconclusive
Limited evidence is available on the association of infertility, miscarriage, and stillbirth with stroke by subtype
What this study adds
Infertility and pregnancy loss, especially recurrent miscarriage (at least three) and recurrent stillbirth (at least two), increased women’s later risk of non-fatal and fatal stroke
The risk of non-fatal or fatal stroke associated with infertility or recurrent stillbirths was mainly driven by a single subtype of stroke (non-fatal ischaemic stroke or fatal haemorrhagic stroke); the risk of non-fatal or fatal stroke associated with recurrent miscarriages was driven by both subtypes
A history of recurrent pregnancy loss could be considered a female specific risk factor for stroke
Acknowledgments
The data on which this research is based were drawn from eight observational studies. The research included data from the Australian Longitudinal Study on Women’s Health (ALSWH), the University of Newcastle, Australia, and the University of Queensland, Australia. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. The authors acknowledge the Australian Government Department of Health for providing Aged Care data, and the Australian Institute of Health and Welfare (AIHW) as the integrating authority. The authors acknowledge the assistance of the Data Linkage Unit at the Australian Institute of Health and Welfare (AIHW) for undertaking the data linkage to the National Death Index (NDI). The authors acknowledge the following: Centre for Health Record Linkage (CHeReL), NSW Ministry of Health and ACT Health for the NSW Admitted Patients, and the ACT Admitted Patient Care Data Collections; Department of Health Western Australia, including the Data Linkage Branch, and the WA Hospital Morbidity Data Collection; SA NT Datalink, SA Health, and Northern Territory Department of Health for the SA Public Hospital Separations and NT Public Hospital Inpatient Activity-Data Collections; Department of Health Tasmania, and the Tasmanian Data Linkage Unit for the Public Hospital Admitted Patient Episodes Data Collection; Department of Health Victoria and Centre for Victorian Data Linkage for the Victorian Admitted Episodes Dataset.
Women’s Lifestyle and Health Study (WLHS) was funded by a grant from the Swedish Research Council (grant No 521-2011-2955). MRC National Survey of Health Development (NSHD) has core funding from the UK Medical Research Council (MC UU 12019/1). Baseline survey of the Japan Nurses’ Health Study (JNHS) was supported in part by a Grant-in-Aid for Scientific Research (B: 14370133, 18390195) from the Japan Society for the Promotion of Science, and by grants from the Japan Menopause Society. The China Kadoorie Biobank has grant support from the Kadoorie Charitable Foundation in Hong Kong, the Wellcome Trust in the UK (088158/Z/09/Z) and the Chinese Ministry of Science and Technology (2011BAI09B01). The UK Medical Research Council, the British Heart Foundation (BHF) and Cancer Research UK also provide core funding to the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University for the project. This research has been conducted using the UK Biobank resource under application 26629.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Ageing (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, principal investigator (PI) 2011-present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999-present; Robert Neer, PI 1994-9; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009-present; Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011-present, Rachel Wildman, PI 2010-11; Nanette Santoro, PI 2004-10; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994-2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Ageing, Bethesda, MD—Chhanda Dutta 2016-present; Winifred Rossi 2012-16; Sherry Sherman 1994-2012; Marcia Ory 1994-2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012-present; Kim Sutton-Tyrrell, PI 2001-12; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995-2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
All study teams would like to thank the participants for volunteering their time to be involved in the respective studies. The findings and views in this paper are not those from the original studies or their respective funding agencies.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organisation.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary tables and figures
Contributors: CL, H-FC, AJD, and GDM contributed to the conception, study design, and statistical methods. CL performed statistical analyses and drafted the manuscript. AJD, KH, YTvdS, DK, RH, CAD, SREK, IJ, SS, and EW contributed data and provided critical revision of the manuscript for important intellectual content. GDM is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and no others meeting the criteria have been omitted.
Funding: This study is funded by the Australian National Health and Medical Research Council Centres of Research Excellence (APP1153420). GDM is supported by an Australian National Health and Medical Research Council Investigator grant (APP2009577). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Australian National Health and Medical Research Council Centres of Research Excellence for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (GDM) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The dissemination plan targets a wide rangeing audience, including patients, public communities, health professionals, and experts in related fields through social media, websites, conferences, and scientific events. The results will be presented at conferences and disseminated through press releases and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Each study in the InterLACE consortium received ethics approval from the human research ethics committee or the institutional review board at each study institution. All the participants provided informed consent.
Data availability statement
No additional data available.
References
- 1. Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke 2017;12:13-32. 10.1177/1747493016676285. [DOI] [PubMed] [Google Scholar]
- 2.Leading causes of death and disability: a visual summary of global and regional trends 2000-2019. https://www.who.int/data/stories/leading-causes-of-death-and-disability-2000-2019-a-visual-summary (accessed 25 April 2022).
- 3. Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke risk factors unique to women. Stroke 2018;49:518-23. 10.1161/STROKEAHA.117.018415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bushnell C, McCullough LD, Awad IA, et al. American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council for High Blood Pressure Research . Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545-88. 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 2012;33:812-41. 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohrabji F, Okoreeh A, Panta A. Sex hormones and stroke: Beyond estrogens. Horm Behav 2019;111:87-95. 10.1016/j.yhbeh.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Germain AM, Romanik MC, Guerra I, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007;49:90-5. 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- 8. Williams LS. Depression and stroke: cause or consequence? Semin Neurol 2005;25:396-409. 10.1055/s-2005-923534. [DOI] [PubMed] [Google Scholar]
- 9. Virani SS, Alonso A, Benjamin EJ, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139-596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 10. McCarron P, Greenwood R, Elwood P, et al. The incidence and aetiology of stroke in the Caerphilly and Speedwell Collaborative Studies II: risk factors for ischaemic stroke. Public Health 2001;115:12-20. 10.1016/S0033-3506(01)00408-5. [DOI] [PubMed] [Google Scholar]
- 11. Mishra GD, Anderson D, Schoenaker DAJM, et al. InterLACE: A New International Collaboration for a Life Course Approach to Women’s Reproductive Health and Chronic Disease Events. Maturitas 2013;74:235-40. 10.1016/j.maturitas.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 12. Mishra GD, Chung HF, Pandeya N, et al. The InterLACE study: design, data harmonization and characteristics across 20 studies on women’s health. Maturitas 2016;92:176-85. 10.1016/j.maturitas.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: The 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006;35:49-54. 10.1093/ije/dyi201. [DOI] [PubMed] [Google Scholar]
- 14. Toth B, Würfel W, Bohlmann M, et al. Recurrent miscarriage: diagnostic and therapeutic procedures. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF registry number 015/050). Geburtshilfe Frauenheilkd 2018;78:364-81. 10.1055/a-0586-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royal College of Obstetricians and Gynaecologists. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage (Green-top Guideline No. 17). https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-investigation-and-treatment-of-couples-with-recurrent-miscarriage-green-top-guideline-no-17/ 2011 (accessed 25 April 2022).
- 16. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 2005;36:1776-81. 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 17. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med 1998;158:1855-67. 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 18. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19. Abbott RD, Curb JD, Rodriguez BL, et al. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol 2003;56:479-86. 10.1016/S0895-4356(02)00611-X. [DOI] [PubMed] [Google Scholar]
- 20. Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med 1994;13:2233-47. 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 22. Zegers-Hochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril 2017;108:393-406. 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 23. Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol 2013;62:1704-12. 10.1016/j.jacc.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 24. Westerlund E, Brandt L, Hovatta O, Wallén H, Ekbom A, Henriksson P. Incidence of hypertension, stroke, coronary heart disease, and diabetes in women who have delivered after in vitro fertilization: a population-based cohort study from Sweden. Fertil Steril 2014;102:1096-102. 10.1016/j.fertnstert.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 25. Bungum AB, Glazer CH, Arendt LH, et al. Risk of hospitalization for early onset of cardiovascular disease among infertile women: a register-based cohort study. Hum Reprod 2019;34:2274-81. 10.1093/humrep/dez154. [DOI] [PubMed] [Google Scholar]
- 26. Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of incident chronic medical conditions in infertile women: analysis of US claims data. Am J Obstet Gynecol 2019;220:473.e1-14. 10.1016/j.ajog.2019.01.214. [DOI] [PubMed] [Google Scholar]
- 27. Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA 2003;290:1767-70. 10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- 28. Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab 2011;25:861-73. 10.1016/j.beem.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 29. Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res 2013;4:390-401. 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Squizzato A, Gerdes VEA, Brandjes DPM, Büller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke 2005;36:2302-10. 10.1161/01.STR.0000181772.78492.07. [DOI] [PubMed] [Google Scholar]
- 31. Ramezanzadeh F, Aghssa MM, Abedinia N, et al. A survey of relationship between anxiety, depression and duration of infertility. BMC Womens Health 2004;4:9. 10.1186/1472-6874-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018;62:2-10. 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 33. Peters SAE, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart 2018;104:1069-75. 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 34. Peters SAE, Yang L, Guo Y, et al. China Kadoorie Biobank collaboration group . Pregnancy, pregnancy loss, and the risk of cardiovascular disease in Chinese women: findings from the China Kadoorie Biobank. BMC Med 2017;15:148. 10.1186/s12916-017-0912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ranthe MF, Andersen EAW, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation 2013;127:1775-82. 10.1161/CIRCULATIONAHA.112.000285. [DOI] [PubMed] [Google Scholar]
- 36. Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation 2011;124:2839-46. 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 37. Pell JP, Smith GCS, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am J Epidemiol 2004;159:336-42. 10.1093/aje/kwh064. [DOI] [PubMed] [Google Scholar]
- 38. Hall PS, Nah G, Vittinghoff E, et al. Relation of pregnancy loss to risk of cardiovascular disease among parous postmenopausal women (from the Women’s Health Initiative). Am J Cardiol 2019;123:1620-5. 10.1016/j.amjcard.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamada K, Iso H, Cui R, Tamakoshi A. Recurrent pregnancy loss and cardiovascular disease mortality in Japanese women: a population-based, prospective cohort study. J Stroke Cerebrovasc Dis 2017;26:1047-54. 10.1016/j.jstrokecerebrovasdis.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 40. Wagner MM, Bhattacharya S, Visser J, Hannaford PC, Bloemenkamp KW. Association between miscarriage and cardiovascular disease in a Scottish cohort. Heart 2015;101:1954-60. 10.1136/heartjnl-2015-307563. [DOI] [PubMed] [Google Scholar]
- 41. Parker DR, Lu B, Sands-Lincoln M, et al. Risk of cardiovascular disease among postmenopausal women with prior pregnancy loss: the women’s health initiative. Ann Fam Med 2014;12:302-9. 10.1370/afm.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population-based cohort study (EPIC-Heidelberg). Heart 2011;97:49-54. 10.1136/hrt.2010.202226. [DOI] [PubMed] [Google Scholar]
- 43. Liang C, Chung HF, Dobson AJ, Mishra GD. Infertility, miscarriage, stillbirth, and the risk of stroke among women: a systematic review and Meta-analysis. Stroke 2022;53:328-37. 10.1161/STROKEAHA.121.036271. [DOI] [PubMed] [Google Scholar]
- 44. Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci 2013;9:1057-69. 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brey RL, Stallworth CL, McGlasson DL, et al. Antiphospholipid antibodies and stroke in young women. Stroke 2002;33:2396-400. 10.1161/01.STR.0000031927.25510.D1. [DOI] [PubMed] [Google Scholar]
- 46. Coull BM, Levine SR, Brey RL. The role of antiphospholipid antibodies in stroke. Neurol Clin 1992;10:125-43. 10.1016/S0733-8619(18)30237-8 [DOI] [PubMed] [Google Scholar]
- 47. Cavalcante MB, Sarno M, Peixoto AB, Araujo Júnior E, Barini R. Obesity and recurrent miscarriage: a systematic review and meta-analysis. J Obstet Gynaecol Res 2019;45:30-8. 10.1111/jog.13799. [DOI] [PubMed] [Google Scholar]
- 48. Yao R, Ananth CV, Park BY, Pereira L, Plante LA, Perinatal Research Consortium . Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol 2014;210:457.e1-9. 10.1016/j.ajog.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 49. Kolte AM, Olsen LR, Mikkelsen EM, Christiansen OB, Nielsen HS. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Hum Reprod 2015;30:777-82. 10.1093/humrep/dev014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kristensen P, Irgens LM. Maternal reproductive history: a registry based comparison of previous pregnancy data derived from maternal recall and data obtained during the actual pregnancy. Acta Obstet Gynecol Scand 2000;79:471-7. 10.1080/j.1600-0412.2000.079006471.x [DOI] [PubMed] [Google Scholar]
- 51. Jung AM, Missmer SA, Cramer DW, et al. Self-reported infertility diagnoses and treatment history approximately 20 years after fertility treatment initiation. Fertil Res Pract 2021;7:7. 10.1186/s40738-021-00099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hure AJ, Chojenta CL, Powers JR, Byles JE, Loxton D. Validity and reliability of stillbirth data using linked self-reported and administrative datasets. J Epidemiol 2015;25:30-7. 10.2188/jea.JE20140032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Engstad T, Bønaa KH, Viitanen M. Validity of self-reported stroke : The Tromso Study. Stroke 2000;31:1602-7. 10.1161/01.STR.31.7.1602. [DOI] [PubMed] [Google Scholar]
- 54. Jackson CA, Mishra GD, Tooth L, Byles J, Dobson A. Moderate agreement between self-reported stroke and hospital-recorded stroke in two cohorts of Australian women: a validation study. BMC Med Res Methodol 2015;15:7. 10.1186/1471-2288-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary tables and figures
Data Availability Statement
No additional data available.