Abstract
Context:
Beetroots have antioxidant and anti-inflammatory properties, which may help attenuate inflammation and oxidative stress, enhancing recovery from exercise-induced muscle damage (EIMD).
Objective:
To evaluate the effects of beetroot supplementation on oxidative stress, inflammation, and recovery after EIMD.
Data Sources:
SPORTDiscus, PubMed, Web of Science, and Scopus databases were searched, and hand-searching was performed by looking to relevant studies that were cited in other studies.
Study Selection:
For a study to be included in this review, the following inclusion criteria had to be met: (1) research conducted with human participants, (2) original articles in peer-reviewed publications, (3) original studies that had investigated beetroot supplementation intervention on muscle damage and recovery, (4) research conducted with 1 control/placebo group, and (5) articles published from inception to October 2020.
Study Design:
Systematic review using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
Level of Evidence:
Level 3.
Data Extraction:
Two of the 4 authors independently extracted data and assessed the methodological quality of the articles with the PEDro scale. All discrepancies were resolved through a consensus meeting.
Results:
A total of 9 studies were included in this review. The methodological quality of the included studies ranged from moderate to high. Most of the studies found a better recovery of functional variables and muscle soreness, but improvements in markers of muscle damage, inflammation, and oxidative stress were not reported.
Conclusion:
The existing evidence suggests that a short-term beetroot supplementation has the potential to accelerate recovery of functional measures and muscle soreness, but further research is needed to clarify if a longer supplementation period (with some days before exercise and some days after) could also promote recovery of markers of muscle damage, inflammation, and oxidative stress.
Keywords: inflammation, oxidative stress, functional performance, recovery, muscle damage
Strenuous and/or prolonged exercise produces exercise-induced muscle damage (EIMD), especially after eccentric muscle actions, which cause greater levels of muscle damage than concentric or isometric actions. 7 Prolonged exercise leads to the production of reactive oxygen species (ROS) that occurs primarily in skeletal muscle. 27 Possible sources of exercise-induced ROS are the mitochondrial electron transport chain in muscle cells or the capillary endothelium in contracting muscles as a result of the ischemia-reperfusion process during exercise. 2 This high level of ROS results in an imbalance between oxidants and antioxidants in favor of the oxidants. The impact of ROS on muscle force production depends on the level of ROS within the fiber. 42 For example, according to Reid, 44 in unfatigued muscles an optimum level of ROS is necessary for muscle fibers to generate 100% of their maximal isometric force production. Conversely, if the levels of ROS increase above a certain point, the muscles’ ability to generate force decreases. 41
Researchers have proposed that EIMD is developed as a 2-phase process26,40,49: The first phase is the initial mechanical and metabolic damage that occurs as a consequence of the mechanical work performed and the second phase is the tissue damage produced by the inflammatory response. In the first phase, the strenuous exercise disrupts sarcomeres in myofibrils and causes membrane damage and excitation-contraction coupling dysfunction.7,42 The mechanical stress produced in the primary phase triggers an inflammatory cascade, which is vital to clear damaged tissue and initiates tissue repair and adaptation, 10 but it increases production of ROS and can sustain inflammation, oxidative stress, and cellular damage.4,17 This secondary inflammatory response may, therefore, accentuate the loss of force production and the decreased range of motion and seems to be strongly involved in delayed-onset muscle soreness (DOMS), which ultimately affects exercise performance.6,19,26
One way to attenuate symptoms from EIMD and accelerate recovery could be an oral intake of anti-inflammatory or antioxidant drugs, 15 but because of the adverse effects of treatments including ibuprofen and nonsteroidal anti-inflammatory drugs, 26 there is growing interest in the consumption of anti-inflammatory and antioxidant food supplements to reduce inflammation and enhance recovery. 45 Supplementation with tart cherry and pomegranate has been widely used in sports not only to enhance performance 1 but also to attenuate symptoms from EIMD and accelerate recovery because tart cherries and pomegranates have potential antioxidant and anti-inflammatory properties due to their high content in phenolic compounds.19,31,45 Another natural product that is frequently used is curcumin, the main bioactive polyphenol of the spice herb turmeric, which possesses anti-inflammatory and antioxidant qualities. 39 Supplementation with curcumin has been associated with faster muscle recovery and reduction of inflammation and oxidative stress. 19
Consumption of nitrate-rich (NO3–) vegetables has also been proposed for enhancement of cardiovascular function. 24 Once ingested, some of the NO3– is reduced to nitrite (NO2–) by the action of anaerobic bacteria in the oral cavity and this NO2– is then partially reduced to nitric oxide (NO) in the stomach. 35 The remaining NO3– and NO2– is absorbed from the intestine into the circulation and, in conditions of low oxygen levels, will become bioactive NO in tissues and blood. 35 NO is fundamental in some cardiovascular and metabolic processes as blood flow regulation, muscle contractility improvements, and mitochondrial respiration. 38 Because of its vasodilatory action, NO reduces blood pressure and increases oxygen and nutrient delivery to the muscles 46 and sport-enhancing effects have been reported by a high number of studies.36,53
It has also been demonstrated that NO plays an important role on skeletal muscle regeneration after damage. 29 Essential processes in the skeletal muscle regeneration are myogenesis and angiogenesis. NO can act as a vasodilator in the injured skeletal muscle promoting rebuilding of the damage vessels, restoration of blood flow, and oxygen supply to the tissues.20,54 NO can also act as a signal molecule, which promotes activation of satellite cells that enter the cell cycle, divide, differentiate, and fuse with muscle fibers to repair damaged regions after injuries.20,54 However, there are very few studies investigating the role of NO on oxidative stress and muscle inflammation after muscle damage, with contradictory results. Radak et al 43 suggested that DOMS induced an increase in NO formation that could suppress force generation as a protective mechanism to prevent further damage produced by maximal contraction and Zembron-Lacny et al 54 conclude that NO could be 1 of the causes of inflammation and DOMS. However, according to Tidball, 48 NO is an important regulator of muscle inflammation and reduces muscle damage produced by inflammatory cells by increasing their apoptosis. NO can also reduce neutrophil-mediated lysis of muscle cells and decrease superoxide concentration forming less reactive intermediates. 47
Polyphenol-rich foods supplementation may reduce damage induced by oxidative stress and lipid peroxidation, leading to improvements in plasma antioxidant status and reducing cellular inflammation and muscle pain. 45 Apart from polyphenols, beetroots also have high concentrations of betalains, a group of phenolic secondary plant metabolites that gives them the intense red color. 52 Some studies have suggested that betalains have potential antioxidant and anti-inflammatory effects23,55 and supplementation with beetroot has been proposed not only to improve exercise performance but also to protect against oxidative stress. 39 The betalains, and betanins in particular, are thought to be the most potent antioxidant molecules and have shown to attenuate ROS production and to stimulate the expression of antioxidant enzyme genes. 18
Specific interest in beetroots has arisen in the last years because it is a rich source of polyphenolic compounds and NO3–. 39 Since NO3– may serve as a precursor of NO, supplementation with beetroots could have beneficial effects on sports performance. 54 Furthermore, because of the potential involvement of NO in the damage and repair processes in skeletal muscle, it has been suggested that beetroot supplementation could enhance recovery after EIMD. 15 Although positive effects of supplementation with beetroots on reduction of oxidative stress and inflammatory markers have not always been found,32,51 the effects of beetroot supplementation on recovery after exercise in humans generate increasing interest. Therefore, the aim of this systematic review is to summarize the effects of beetroot supplementation on oxidative stress, inflammation, and recovery after EIMD found in the existing literature.
Methods
The protocol for this systematic review was designed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 34
Inclusion and Exclusion Criteria
The studies included in this systematic review needed to fulfill the following inclusion criteria: (1) research conducted with human participants, (2) original articles in peer-reviewed publications, (3) original studies that had investigated beetroot supplementation intervention on muscle damage and recovery, (4) research conducted with 1 control/placebo group, and (5) articles published from inception to October 2020. Exclusion criteria were the following: (1) research conducted with animals, (2) non-English articles, and (3) systematic reviews or meta-analyses.
Search Strategy
Four electronic databases were searched: SPORTDiscuss, PubMed, Web of Science, and Scopus. The following search was carried out: (beetroot OR nitrate) (Title) AND beetroot (Abstract) AND (muscle damage OR oxidative stress OR recovery OR exercise OR muscle pain OR antioxidant OR inflammation OR soreness (Title)) AND sports (All Fields). The search was limited to English language and journal articles. The detailed search strategy can be found in Appendix 1 (available in the online version of this article).
Data Extraction
Two of the 4 authors independently did the literature search and the data extraction. All discrepancies were resolved through a consensus meeting. After applying inclusion and exclusion criteria, the following data were extracted from each study: first author name, year of publication, the intervention and placebo group characteristics, dosage of supplements, supplementation duration, exercise protocol to induce muscle damage and the effects of supplementation on functional measures, muscle soreness, and markers of muscle damage, inflammation, and oxidative stress.
Methodological Quality Assessment
The methodological quality of the articles was assessed with the PEDro scale, which is based on the Delphi list developed by Verhagen et al 50 and is a reliable and objective tool that helps identify which studies are likely to be externally valid (criterion 1), internally valid (criteria 2-9), and could have sufficient statistical information to make their results interpretable (criteria 10 and 11). 1 Points are only awarded when a criterion is clearly satisfied, and criterion 1, which relates to external validity, is not used to calculate the PEDro score.
A score of 9 to 10 on the PEDro scale was considered to be “high quality,” scores of 5 to 8 were considered to be “moderate quality,” and studies that scored less than 5 were considered to be “low quality.” 45 Two researchers assessed the manuscripts independently; if there was a difference in the criteria, it was resolved through discussion until a consensus was reached.
Results
Study Selection
Leaving aside the systematic reviews and meta-analysis, the literature search provided a total of 212 articles identified through the combined descriptors. After examination by titles, 177 articles were excluded because they were neither conducted in humans nor did they study recovery. After elimination of duplicates, 15 articles were selected for abstract screening and 5 of them were also excluded for not being conducted in humans not studying recovery.
Finally, 1 study was excluded because it was a 2-part study, and the first part was published as a separate study and included in this systematic review. Therefore, the total number of studies finally included was 9.8,11-16,30,37 There was a high level of agreement between reviewers at the title and abstract screening stages (95.5%; 169 of 177 articles) and a perfect agreement in the full-text screening stage. A summary of the search process can be seen in Figure 1.
Figure 1.
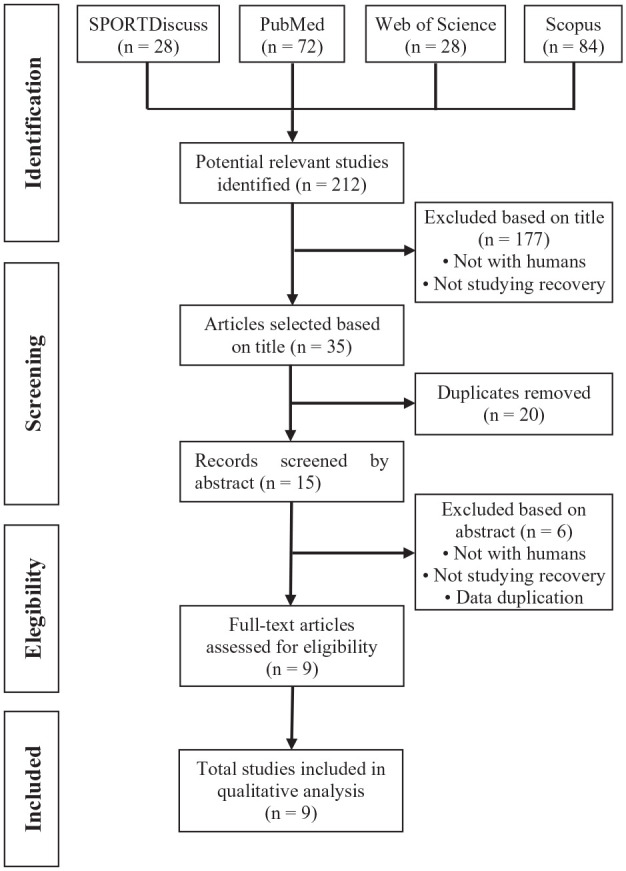
Flowchart for identification and selection of eligible studies for the systematic review.
Study Characteristics
The characteristics of the included studies are summarized in Table 1. All selected studies implemented a placebo-controlled experimental design. Four studies had a crossover design,8,16,30,37 3 studies used 2 experimental arms (placebo and beetroot supplementation),11,13,14 and 2 studies used 3 experimental arms with at least 1 of them with beetroot supplementation.12,15
Table 1.
Characteristics of the included studies
| Study | Participants | Groups | Age, y | Phenolic Content/Nitrate Content/Betanin Content | Supplementation Period | Exercise Protocol to Induce Muscle Damage |
|---|---|---|---|---|---|---|
| Clifford et al 12 | Recreationally active males | 10 (BR HD) 10 (BR LD) 10 (PLA) |
22 ± 6 21 ± 3 21 ± 3 |
HD: ~803.44 mg pc ~500 mg nit ~388 mg betanin LD: ~401.72 mg pc ~250 mg nit ~194 mg betanin |
3 days (exercise on day 0) | 100 Drop jumps (60 cm) |
| Clifford et al 13 | Male collegiate team-sport players | 10 (BR) 10 (PLA) |
23 ± 3 21 ± 2 |
810 mg pc 286 mg nit 388 mg betanin |
4 days (exercise on days 0 and 3) | Repeated sprint test (20 maximal-effort 30-m sprints) |
| Montenegro et al 37 | Male and female competitive triathletes | 22 (BR) 22 (PLA) |
38.0 ± 11.3 COD |
— nit pc: NP 24 mg betanin |
6 days (exercise on day 7) | 40 min submaximal cycling + 10 km running TT |
| Clifford et al 11 | Male and female marathon runners | 17 (BR) 17 (PLA) |
42 ± 10 39 ± 12 |
1200 mg pc 630 mg nit 582 mg betanin |
2 + 1/3 days (exercise on day 0) | Marathon race |
| Clifford et al 15 | Recreationally active males | 10 (SN) 10 (BR) 10 (PLA) |
21.7 ± 2.8 22.6 ± 2.8 21.0 ± 1.4 |
SN: — pc ~420 mg nit — betanin BR: ~810 mg pc ~420 mg nit ~388 mg betanin |
3 days (exercise on day 0) | 100 Drop jumps (60 cm) |
| Carriker et al 8 | Well-trained male cyclists | 9 (BR) 9 (PLA) |
29 ± 7 COD |
pc: NP 793.6 mg nit betanin: NP |
One single dose 2.5 h before exercise | Incremental cycling test at 25%, 40%, 50%, 60%, and 70% wattages of the normobaric VO2max in hypobaric chamber |
| Clifford et al 14 | Male and female marathon runners | 7 (BR) 7 (PLA) |
45 ± 7 45 ± 10 |
803.5 mg pc 501.2 mg nit 389 mg betanin |
5 + 1/2 days (exercise on day 5) | Marathon race |
| Daab et al 16 | Semiprofessional male soccer players | 13 (BR) 13 (PLA) |
22.12 ± 0.56 COD |
115.5 mg pc 250 mg nit 48.9 mg betanin |
7 days (exercise on day 4) | Loughborough intermittent shuttle test |
| Kozłowska et al 30 | Elite fencers (men) | 20 (BR) 20 (PLA) |
27.2 ± 5.4 (men) 22.6 ± 5.3 (women) COD |
38.34 mg pc nit: NP 371.83 mg betanin |
4 weeks | Normal training |
BR, beetroot; COD, crossover design; HD, high dose; LD, low dose; nit, nitrate; NP, not provided; pc, phenolic content; PLA, placebo group; SN, sodium nitrate; TT, time trial.
All participants were active people: team-sport players (n = 2), recreationally active males (n = 2), marathon runners (n = 2), triathletes (n = 1), well-trained cyclists (n = 1), and elite fencers (n = 1). The sample size ranged from 7 to 22 participants in each group. Four studies11,13,15,37 carried out an a priori statistical power analysis and used adequate sample sizes based on those estimations. The other 5 studies8,12,14,16,30 did not perform a power analysis, but only 1 of them 14 concluded that the number of participants was too low to detect differences in their primary outcome measure.
One study 37 used beetroot concentrate capsules and the rest8,11-16,30 used beetroot juice. Beetroot dosage varied widely across the included studies, and the phenolic content, nitrate content, and betanin content of the dosages of most studies are summarized in Table 1. Only 1 study 30 did not use a special protocol to induce muscle damage, and markers of muscle damage, inflammation, and oxidative stress were measured before and after a period of normal training.
Functional Measures and Muscle Soreness
Six studies11-13,15,16,37 measured 1 or more of the following functional variables: maximal isometric voluntary contraction (MIVC) of the knee extensors, countermovement jump (CMJ), reactive strength index (RSI), squat jump, 5-km time trial (TT), and 20-m sprint performance (SP). Four of them12,13,16,37 found that, after beetroot juice supplementation, there was a significant improvement in any of the variables measured at some point after exercise or throughout the whole recovery period (Table 2). The same studies also measured muscle soreness, and 4 of them12,13,15,16 found significantly lower values in beetroot group (BRG), 2 of them during the whole recovery period while the other 2 studies found reduced muscle soreness at the beginning or at the end of the recovery period (Table 2).
Table 2.
Variables measured and summary of findings of the included studies
| Study | Functional Measures and Muscle Soreness | Biochemical Markers of Muscle Damage, Inflammation, and Oxidative Stress | Significant Differences in BR Group (vs PLA/SN Group) |
|---|---|---|---|
| Clifford et al 12 | Muscle soreness of lower limb, MIVC of knee extensors, CMJ. Measurements: baseline, post and 24 h, 48 h, and 72 h post | CK; TNF-α, IL-1β, IL-6, IL-8. Measurements: baseline, post and 2 h, 24 h, 48 h, 72 h post |
>CMJ 48 h and 72 h post in HD <Muscle soreness 24 h, 48 h, and 72 h post in HD and LD |
| Clifford et al 13 | Muscle soreness of the lower limb, MIVC of knee extensors, CMJ, RSI. Measurements: baseline, post and 24 h, 48 h, 72 h post RST1; post and 24 post RST2 (96 h post RST1) | CK; CRP; PC, LOOH, AFR Measurements: baseline, post and 2.5 h, 24 h, 48 h, 72 h post RST1, post and 2.5 h, 24 h post RST2 (96 h post RST1) |
>recovery of CMJ from 24 h post RST1 >RSI throughout the trial <Muscle soreness 96 h post RST1 |
| Montenegro et al 37 | Whole body muscle soreness. Measurements: baseline, post, and 24 h post. 5 km TT. Measurements: 24 h post |
CK, LDH Measurements made baseline, post and 24 h post |
<5 km TT 24 h post <CK post |
| Clifford et al 11 | Muscle soreness of lower limb, MIVC of knee extensors, CMJ. Measurements: baseline, post and 24 h, 48 h post | CK, AST; TNF-α, IL-1ra, -2, -4, -6, -8, -10, IFN-γ, CRP Measurements: baseline, post and 48 h post |
No significant differences between groups |
| Clifford et al 15 | Muscle soreness of lower limb, MIVC of knee extensors, CMJ. Measurements: baseline, post and 24 h, 48 h, 72 h post | CK; CRP Measurements: baseline, post and 2,5 h, 24 h, 48 h, 72 h post |
<Muscle soreness in BR group vs SN and PLA in the 72-h postexercise period |
| Carriker et al 8 | — | Cat (baseline and post) 8-IsoP (baseline and 1 h post) |
No significant differences between groups |
| Clifford et al 14 | — | mtDNA damage; ROS production Measurements: baseline, pre, post, and 24 h post |
No significant differences between groups |
| Daab et al 16 | Muscle soreness of quadriceps and hamstrings, MIVC of knee extensors, SJ, CMJ, 20-m SP. Measurements: baseline, post and 24 h, 48 h, 72 h post | CK, LDH; CRP Measurements: baseline, post and 24 h, 48 h, and 72 h post |
>CMJ 24 h, 48 h, and 72 h post. >MIVC post, 24 h and 48 h. <SP 48 h post <Muscle soreness post and 24 h post |
| Kozłowska et al 30 | — | CK, LDH; IL-6, CP; AOPP, MDA, GPx-1, GPx-3, 8-oxodG Measurements: pre and post (4 weeks after) |
>Gpx-1 post >MDA post |
8-IsoP, 8-isoprostane; 8-oxodG, 8-oxo-deoxyguanosine; AFR, ascorbyl free radical; AOPP, advanced oxidation protein products; AST, aspartate transaminase; BR, beetroot; Cat, catalase; CK, creatine kinase; CMJ, countermovement jump; CP, ceruloplasmin; CRP, C-reactive protein; GPx, glutathione peroxidase; HD, high dose; IFN-γ, interferon-γ; IL, interleukin; LD, low dose; LDH, lactate dehydrogenase; LOOH, lipid hydroperoxides; MDA, malondialdehyde; MIVC, maximal isometric voluntary contraction; mtDNA, mitochondrial DNA; PC, protein carbonyls; PLA, placebo; post, postexercise; pre, pre-exercise; ROS, reactive oxygen species; RSI, reactive strength index; RST, repeated sprint test; SJ, squat jump; SN, sodium nitrate; SP, sprint performance; TNF-α, tumor necrosis factor alpha.
Muscle Damage
All but 1 of the studies 8 analyzed serum concentration of any of the following markers of muscle damage: creatine kinase (CK), lactate dehydrogenase, aspartate transaminase, and mithocondrial DNA (mtDNA) damage. Only 1 of them 37 found significant differences between groups in serum CK levels after exercise (Table 2).
Inflammation and Oxidative Stress
Six studies11-13,15,16,30 measured 1 or more of the following markers of inflammation: C-reactive protein (CRP); tumor necrosis factor alpha; interleukin-1β, -1ra, -2, -4, -6, -8, -10; IFN-γ; and ceruloplasmin, but none of them reported significant differences between groups in any of the inflammatory markers assessed.
With regard to oxidative stress, 4 studies8,13,14,30 analyzed 1 or more of the following markers: protein carbonyls, lipid hydroperoxides, ascorbyl free radical, catalase, 8-isoprostane, ROS production, advanced oxidation protein products, malondialdehyde (MDA), glutathione peroxidase 1 (GPx-1), glutathione peroxidase 3 (GPx-3), and 8-oxo-deoxyguanosine, but only 1 of them 30 found significant differences between groups after exercise in 2 of the markers measured: Gpx-1 and MDA.
The complete summary of findings for markers of inflammation and oxidative stress can be seen in Table 2.
With regard to study characteristics, the level of agreement between reviewers was almost perfect. Unfortunately, the same level of agreement regarding functional measures, muscle soreness, markers of muscle damage, and markers of inflammation and oxidative stress was not possible given the vast amount of information extracted from the articles. Nonetheless, all the differences were resolved after a consensus meeting.
Methodological Quality Assessment
The scores of all studies ranged from 5 to 9, with a mean PEDro score of 7.33 ± 1.05. Only 1 study was deemed high quality while the rest were considered to be of moderate quality. No study was excluded because of its low quality. Table 3 details the results of the criteria evaluated. All studies failed to blind all assessors who measured at least 1 key outcome (item 7) and only 1 study carried out a concealed allocation (item 3). 37 Both reviewers agreed on the evaluation of most items (92.9%) and after additional discussion all the differences were solved.
Table 3.
Methodological quality of the included studies assessed with the PEDro scale
| Items | Clifford et al 12 | Clifford et al 13 | Montenegro et al 37 | Clifford et al 11 | Clifford et al 15 | Carriker et al 8 | Clifford et al 14 | Daab et al 16 | Kozłowska et al 30 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Eligibility criteria were specified | + | + | + | + | + | + | + | + | + |
| 2. Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received) | − | + | + | − | + | − | − | + | − |
| 3. Allocation was concealed | − | − | + | − | − | − | − | − | − |
| 4. The groups were similar at baseline regarding the most important prognostic indicators | + | + | + | + | + | + | + | + | + |
| 5. There was blinding of all subjects | + | + | + | + | + | + | + | + | − |
| 6. There was blinding of all therapists who administered the therapy | + | + | + | + | + | + | + | + | − |
| 7. There was blinding of all assessors who measured at least 1 key outcome | − | − | − | − | − | − | − | − | − |
| 8. Measures of at least 1 key outcome were obtained from more than 85% of the subjects initially allocated to groups | + | + | + | + | + | + | + | + | + |
| 9. All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least 1 key outcome was analyzed by “intention to treat” | + | + | + | + | + | + | + | + | + |
| 10. The results of between-group statistical comparisons are reported for at least 1 key outcome | + | + | + | + | + | + | + | + | + |
| 11. The study provides both point measures and measures of variability for at least 1 key outcome | + | + | + | + | + | + | + | + | + |
| Total score | 7 | 8 | 9 | 7 | 8 | 7 | 7 | 8 | 5 |
Discussion
Beetroot contains high amounts of polyphenols, betalains, and nitrates, 18 and these are considered good anti-inflammatory and antioxidant compounds. Therefore, the purpose of this systematic review was to summarize the effects of beetroot supplementation on oxidative stress, inflammation, and recovery after EIMD. Our discussion will be carried out considering only the total phenolic content, the nitrate content, and the betanin content of the supplementations.
Effects of Beetroot Supplementation on Functional Measures and Muscle Soreness
Four of the studies that measured functional variables and muscle soreness11-13,15 had a supplementation period of only 3 to 4 days after exercise, and they showed contradictory results. Clifford et al 12 found a better recovery of CMJ height after exercise in high-dose BRG, and Clifford et al 13 also found a better recovery of CMJ height and RSI in BRG after exercise. None of those studies reported a better recovery of MIVC of the knee extensors in BRG, maybe because beetroot supplementation enhances recovery of dynamic but not static muscle function, 13 because CMJ and RSI are more valid tests for monitoring functional recovery than MIVC of the knee extensors 22 or simply because MIVC of the knee extensors was less affected by the exercise than the other functional variables.
However, Clifford et al 11 did not find a better recovery after exercise in CMJ height or MIVC of the knee extensors. The differences in exercise protocols and the different training levels of participants are potential explanations. Another possible explanation could be the differences in the magnitude of muscle damage induced. It is reasonable to assume that the experienced marathon runners in this study were sufficiently well protected from symptoms of muscle damage and, therefore, beetroot supplementation conferred no benefits for recovery. 11
Nonetheless, Howatson et al 25 reported an enhanced recovery of MICV of the knee extensors after a marathon race in marathon runners who consumed tart cherry supplementation 5 days before the marathon and 3 days after. Apart from the obvious differences in the supplements used, this result may suggest that a more long-term dosage strategy (including a loading phase) could have obtained similar recovery benefits. 11 Likewise, Clifford et al 15 did not find a better recovery after exercise neither in CMJ height nor in MIVC of the knee extensors and the explanation for these disparate results remains unclear because the subjects, the supplementation dosages, and the protocol to induce muscle damage were exactly the same used by Clifford et al 12
Of the 4 studies with a short supplementation period, only Clifford et al 11 did not show significantly lower levels of muscle soreness after exercise in BRG. A potential explanation is the way muscle soreness was measured because Clifford et al 11 used a visual analogue scale (VAS) while in the other 3 studies, soreness was assessed as pressure pain threshold with a handheld algometer applied on the muscle belly at 3 premarked sites, which is probably more accurate than a VAS. However, as with CMJ or MIVC of the knee extensors, the authors state that this may be due to differences in exercise protocol or participants or even to the magnitude of the muscle damage, markedly lower in this study, which made beetroot supplementation confer no benefits for recovery. 11
Two studies carried out a longer supplementation period.16,37 Montenegro et al 37 used a nitrate depleted supplementation with low amount of betanins, but the supplementation had a loading phase of 6 days pre-exercise and BRG had a lower 5 km TT 24 hours postexercise. However, they did not find a significant better recovery of muscle soreness in BRG, probably because the amount of betanins of the dosages was enough for enhancing recovery of 5-km TT but too low for enhancing recovery of muscle soreness. The supplementation used by Daab et al 16 was higher in nitrates and betanins and lasted for 7 days, 3 of them before exercise, and they reported better recovery of CMJ, MIVC of the knee extensors, SP, and muscle soreness after exercise in BRG.
Therefore, even with some contradictory results, it appears that beetroot supplementation is an effective strategy for recovery of functional measures and muscle soreness, especially if supplementation is administered some days before muscle damage is induced and for at least 72 hours after, for a total period of more than 5 to 6 days. These results are similar to those found by Rojano et al 45 in their review of supplementation with tart cherry or pomegranate and to those reported by Fernández-Lázaro et al 19 in their review of supplementation with curcumin.
Effects of Beetroot Supplementation on Markers of Muscle Damage
In all but 1 study, 14 markers of muscle damage demonstrated main effects for time, being significantly higher after exercise than baseline, which means that the protocol used to induce muscle damage was effective. Only Clifford et al 14 indicated no changes in mtDNA damage after exercise, but they concluded that the protocol they used was not sufficient to induce a large efflux of mtDNA into the circulation.
From the rest of the studies, only Montenegro et al 37 reported significant differences between groups in serum CK levels after exercise, showing BRG lower values. Most of those studies11-13,15,16 suggested that beetroot supplementation did not seem to be beneficial for reducing markers of muscle damage. Same contradictory results have been obtained with other functional foods such as tart cherry or pomegranate with some studies reporting beneficial effects5,33 and others not.3,24 It has to be said that circulating muscle specific proteins are probably best served as a marker that tissue damage has occurred rather than to assess its magnitude, 21 which can explain those inconclusive results.
Effects of Beetroot Supplementation on Markers of Inflammation and Oxidative Stress
None of the studies that measured any marker of inflammation11-13,15,16,30 reported significant differences between BRG and placebo groups. However, it has to be mentioned that 3 studies did not even find a significant elevation in serum levels of inflammatory markers after exercise.13,15,30 Clifford et al13,15 measured only CRP and they found that serum concentration of CRP remained close to baseline values throughout the trials, but maybe those studies were underpowered to detect small changes in CRP marker because they had 10 subjects per group while Clifford et al 11 had groups of 17 subjects and showed that CRP was significantly elevated postexercise. It is also possible that differences between these 3 studies were because of the different reliability of the methods used to assess CRP levels because CV was <2% for Clifford et al13,15 and <5% for Clifford et al 11
With regard to oxidative stress, only Kozłowska et al 30 found significant differences between groups in Gpx-1 and MDA, with higher values in BRG. The authors explained these unexpected results by the fact that they carried out a crossover design with a first stage of 4 weeks with all participants without supplementation and a second stage of another 4 weeks with all participants with supplementation. And, in the second stage, after some weeks of training, their participants engaged in a relatively high-intensity training, especially the last days of the stage just before blood sampling. The larger oxidative stress was the result of this high-intensity training and could be the result of improved physical performance due to the beetroot supplementation. 30 The rest of the studies8,13,14 found no evidence that beetroot supplementation attenuated oxidative stress, probably because of the fact that the benefits are limited to non-well-trained individuals who have not suffered physiologic adaptations resulting from training.9,28
Although the findings of the studies included in this systematic review do not support the use of beetroot supplementation to attenuate inflammation or oxidative stress after EIMD, most studies lack of a supplement loading phase in the days before exercise. As Clifford et al 11 concluded, if supplements had been consumed for some days before exercise, group differences might have been apparent. It is true that Daab et al 16 had a supplementation period of 7 days with 3 days before exercise, but the amount of polyphenols, nitrates, and betanins was probably very low to have any beneficial effects on reducing markers of inflammation and oxidative stress.
Limitations
The main limitations of this review are the small number of studies included, the small sample sizes of some studies, leading to a low statistical power, and the differences in the supplementation dosages and the duration of the interventions. Additionally, the markers of muscle damage, inflammation and oxidative stress were measured using different methods, the exact amount of polyphenols, betalains, and nitrates was not always provided, and some studies did not analyze the supplements before the interventions and used the results reported from previous analysis, regardless of inter-batch differences. Last, some of the studies used a crossover design, which may have attenuated the response of the body to intense exercise because of the repeated bout effect.
Conclusion
Despite the important differences in the amount of polyphenols, nitrates, and betanins used in the included studies, the evidence indicates that short-term beetroot supplementation has the potential to accelerate recovery of functional measures and muscle soreness. However, although the recovery of those variables might point to a potential blunting of the secondary damage response, improvements in markers of muscle damage, inflammation, and oxidative stress have not been reported.
Further research is needed to clarify whether a more long-term beetroot supplementation consumed for some days before exercise and some days after might be a good strategy to enhance recovery of markers of muscle damage, inflammation, and oxidative stress, as it has been already demonstrated with other functional foods. Positive effects are more likely with enough amounts of polyphenols, nitrates and betanins, but more research is also needed to shed some light on this matter and determine the minimum dose to achieve a positive impact.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_19417381211036412 for Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review by Daniel Rojano-Ortega, José Peña Amaro, Antonio J. Berral- Aguilar and Francisco J. Berral-de la Rosa in Sports Health: A Multidisciplinary Approach
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Ammar A, Bailey SJ, Chtourou H, et al. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: a systematic review. Br J Nutr. 2018;120:1201-1216. [DOI] [PubMed] [Google Scholar]
- 2. Aoi W, Naito Y, Takanami Y, et al. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med. 2004;37:480-487. [DOI] [PubMed] [Google Scholar]
- 3. Bell PG, Stevenson E, Davison GW, Howatson G. The effects of Montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients. 2016;8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245-263. [DOI] [PubMed] [Google Scholar]
- 5. Botwell JL, Sumners DP, Dyer A, Fos P, Mileva KN. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc. 2011;43:1544-1551. [DOI] [PubMed] [Google Scholar]
- 6. Buchwald-Werner S, Naka I, Wilhelm M, Schütz E, Schoen C, Reule C. Effects of lemon verbena extract (Recoverben®) supplementation on muscle strength and recovery after exhaustive exercise: a randomized, placebo-controlled trial. J Int Soc Sports Nutr. 2018;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byrne C, Twist C, Estom R. Neuromuscular function after exercise-induced muscle damage. theoretical and applied implications. Sports Med. 2004;34:49-69. [DOI] [PubMed] [Google Scholar]
- 8. Carriker CR, Rombach P, Stevens BM, Vaughan RA, Gibson AL. Acute dietary nitrate supplementation does not attenuate oxidative stress or the hemodynamic response during submaximal exercise in hypobaric hypoxia. Appl Physiol Nutr Metab. 2018;43:1268-1274. [DOI] [PubMed] [Google Scholar]
- 9. Carriker CR, Vaughan RA, VanDusseldorp TA, et al. Nitrate-containing beetroot juice reduces oxygen consumption during submaximal exercise in low but not high aerobically fit male runners. J Exerc Nutrition Biochem. 2016;20:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chazaud B. Inflammation during skeletal muscle regeneration and tissue remodeling: application to exercise-induced muscle damage management. Immunol Cell Biol. 2016;94:140-145. [DOI] [PubMed] [Google Scholar]
- 11. Clifford T, Allerton DM, Brown MA, et al. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metab. 2017:42:263-270. [DOI] [PubMed] [Google Scholar]
- 12. Clifford T, Bell O, West DJ, Howatson G, Stevenson EJ. The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur J Appl Physiol. 2016;116:353-362. [DOI] [PubMed] [Google Scholar]
- 13. Clifford T, Berntzen B, Davison GW, West DJ, Howatson G, Stevenson EJ. Effects of beetroot juice on recovery of muscle function and performance between bouts of repeated sprint exercise. Nutrients. 2016;8:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clifford T, Bowman A, Capper T, et al. A pilot study investigating reactive oxygen species production in capillary blood after a marathon and the influence of an antioxidant-rich beetroot juice. Appl Physiol Nutr Metab. 2018;43:303-306. [DOI] [PubMed] [Google Scholar]
- 15. Clifford T, Howatson G, West DJ, Stevenson EJ. Beetroot juice is more beneficial than sodium nitrate for attenuating muscle pain after strenuous eccentric-bias exercise. Appl Physiol Nutr Metab. 2017;42:1185-1191. [DOI] [PubMed] [Google Scholar]
- 16. Daab W, Bouzid MA, Lajri M, Bouchiba M, Saafi MA, Rebai H. Chronic beetroot juice supplementation accelerates recovery kinetics following simulated match play in soccer players. J Am Coll Nutr. 2020;3:1-9. [DOI] [PubMed] [Google Scholar]
- 17. Drobnic F, Riera J, Appendino G, et al. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): a randomised, placebo-controlled trial. J Int Soc Sports Nutr. 2014;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esatbeyoglu T, Wagner AE, Motafakkerazad R, Nakajima Y, Matsugo S, Rimbach G. Free radical scavenging and antioxidant activity of betanin: electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem Toxicol. 2014;73:119-126. [DOI] [PubMed] [Google Scholar]
- 19. Fernández-Lázaro D, Mielgo-Ayuso J, Seco J, Córdova A, Caballero A, Fernandez-Lazaro CI. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: a systematic review. Nutrients. 2020;12:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filippin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157-163. [DOI] [PubMed] [Google Scholar]
- 21. Friden J, Lieber RL. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand J Med Sci Sports. 2001;11:126-127. [DOI] [PubMed] [Google Scholar]
- 22. Gathercole RJ, Sporer BC, Stellingwerf T, Sleivert GG. Comparison of the capacity of different jump and sprint field tests to detect neuromuscular fatigue.J Strength Cond Res. 2015;29:2522-2531. [DOI] [PubMed] [Google Scholar]
- 23. Georgiev VG, Weber J, Kneschke EM, Denev PN, Bley T, Pavlov AI. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Food Hum Nutr. 2010;65:105-111. [DOI] [PubMed] [Google Scholar]
- 24. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1-10. [DOI] [PubMed] [Google Scholar]
- 25. Howatson G, McHugh MP, Hill JA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. 2010;20:843-852. [DOI] [PubMed] [Google Scholar]
- 26. Howatson G, van Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38:483-503. [DOI] [PubMed] [Google Scholar]
- 27. Jackson MJ, Vasilaki A, McArdle A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic Biol Med. 2016;98:13-17. [DOI] [PubMed] [Google Scholar]
- 28. Jonvik KL, Nyakayiru J, van Loon LJC, Verdijk LB. Can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol (1985). 2015;119:759-761. [DOI] [PubMed] [Google Scholar]
- 29. Kaminski HJ, Andrade FH. Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscul Disord. 2001;11:517-524. [DOI] [PubMed] [Google Scholar]
- 30. Kozłowska L, Mizera O, Gromadzinska J, et al. Changes in oxidative stress, inflammation, and muscle damage markers following diet and beetroot juice supplementation in elite fencers. Antioxidants. 2020;9:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamb KL, Ranchordas MK, Johnson E, Denning J, Downing F, Lynn A. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients. 2019;11:E1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larsen FJ, Schiffer TA, Ekblom B, et al. Dietary nitrate reduces resting metabolic rate: a randomized, crossover study in humans. Am J Clin Nutr. 2014;99:843-850. [DOI] [PubMed] [Google Scholar]
- 33. Levers K, Dalton R, Galvan E, et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J Int Soc Sports Nutr. 2015;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:65-94. [DOI] [PubMed] [Google Scholar]
- 35. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395-400. [DOI] [PubMed] [Google Scholar]
- 36. Mason BC, Lavallee ME. Emerging supplements in sports. Sports Health. 2012;4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montenegro CF, Kwong DA, Minow ZA, Davis BA, Lozada CJ, Casazza GA. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl Physiol Nutr Metab. 2017;42:166-172. [DOI] [PubMed] [Google Scholar]
- 38. Moreno B, Soto K, González D. El consumo de nitrato y su potencial efecto benéfico sobre la salud cardiovascular. Rev Chil Nutr. 2015;42:199-205. [Google Scholar]
- 39. Nakhostin B, Nasirvand A, Bolboli L. Influence of curcumin supplementation on exercise-induced oxidative stress. Asian J Sports Med. 2017;8:e35776. [Google Scholar]
- 40. Owens DJ, Twist C, Cobley JN, Howatson G, Close GL. Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? Eur J Sport Sci. 2019;19:71-85. [DOI] [PubMed] [Google Scholar]
- 41. Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1:941-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radak Z, Pucsok J, Mecseki S, Csont T, Ferdinandy P. Muscle soreness induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med. 1999;26:1059-1063. [DOI] [PubMed] [Google Scholar]
- 44. Reid MB. Redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol. 2001;90:724-731. [DOI] [PubMed] [Google Scholar]
- 45. Rojano D, Molina A, Moya H, Berral FJ. Tart cherry and pomegranate supplementations enhance recovery from exercise-induced muscle damage: a systematic review. Biol Sport. 2021;38:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rojas-Valverde D, Montoya-Rodríguez J, Azofeifa-Mora C, Sánchez-Urena B. Effectiveness of beetroot juice derived nitrates supplementation on fatigue resistance during repeated-sprints: a systematic review. Crit Rev Food Sci Nutr. 2020;25:1-12. [DOI] [PubMed] [Google Scholar]
- 47. Stamle JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209-237. [DOI] [PubMed] [Google Scholar]
- 48. Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345-R353. [DOI] [PubMed] [Google Scholar]
- 49. Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37:284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235-1241. [DOI] [PubMed] [Google Scholar]
- 51. Whitfield J, Ludzki A, Heigenhauser GJF, et al. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J Physiol. 2016;594:421-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wruss J, Waldenberger G, Huemer S, et al. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J Food Compos Anal. 2015;42:46-55. [Google Scholar]
- 53. Zamani H, de Joode MEJR, Hossein IJ, et al. The benefits and risks of beetroot juice consumption: a systematic review. Crit Rev Food Sci Nutr. 2021;61:788-804. [DOI] [PubMed] [Google Scholar]
- 54. Zembron-Lacny A, Orysiak J, Kalina K, Morawin B, Pokrywka A. The role of nitric oxide in skeletal muscle regeneration. Trends Sport Sci. 2013;4:173-179. [Google Scholar]
- 55. Zielinska-Przyjemska M, Olejnik A, Dobrowolska-Zachwieja A, Grajek W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother Res. 2009;23:49-55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_19417381211036412 for Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review by Daniel Rojano-Ortega, José Peña Amaro, Antonio J. Berral- Aguilar and Francisco J. Berral-de la Rosa in Sports Health: A Multidisciplinary Approach


