Abstract
Context:
Only 55% of the athletes return to competitive sports after an anterior cruciate ligament (ACL) injury. Athletes younger than 25 years who return to sports have a second injury rate of 23%. There may be a mismatch between rehabilitation contents and the demands an athlete faces after returning to sports. Current return-to-sports (RTS) tests utilize closed and predictable motor skills; however, demands on the field are different. Neurocognitive functions are essential to manage dynamic sport situations and may fluctuate after peripheral injuries. Most RTS and rehabilitation paradigms appear to lack this aspect, which might be linked to increased risk of second injury.
Objective:
This systematic and scoping review aims to map existing evidence about neurocognitive and neurophysiological functions in athletes, which could be linked to ACL injury in an integrated fashion and bring an extensive perspective to assessment and rehabilitation approaches.
Data Sources:
PubMed and Cochrane databases were searched to identify relevant studies published between 2005 and 2020 using the keywords ACL, brain, cortical, neuroplasticity, cognitive, cognition, neurocognition, and athletes.
Study Selection:
Studies investigating either neurocognitive or neurophysiological functions in athletes and linking these to ACL injury regardless of their design and technique were included.
Study Design:
Systematic review.
Level of Evidence:
Level 3.
Data Extraction:
The demographic, temporal, neurological, and behavioral data revealing possible injury-related aspects were extracted and summarized.
Results:
A total of 16 studies were included in this review. Deficits in different neurocognitive domains and changes in neurophysiological functions could be a predisposing risk factor for, or a consequence caused by, ACL injuries.
Conclusion:
Clinicians should view ACL injuries not only as a musculoskeletal but also as a neural lesion with neurocognitive and neurophysiological aspects. Rehabilitation and RTS paradigms should consider these changes for assessment and interventions after injury.
Keywords: anterior cruciate ligament injury, cognition, athletes, neuronal plasticity, cortical activity
Most athletes who sustain an anterior cruciate ligament (ACL) injury will undergo an ACL reconstruction surgery (ACLR) with high expectations to return to preinjury levels of function. 17 The reality is 81% of the athletes return to sports (RTS) after ACLR, but only 55% to competitive sports. 4 Moreover, RTS paradigms are linked to an increased risk of a second ACL injury up to 23% in young athletes. 43 Currently, the RTS decision is taken at the “hypothetical end” of recovery without a gold standard to judge readiness to RTS with multifaceted perspectives after rehabilitation. 15
RTS protocols are primarily based on biomechanical and neuromuscular aspects, including range of motion, strength, and functional tests. 10 The latter represent closed motor skills defined as standardized movements in a predictable environment, for example a single-leg hop test. However, on return to the field after ACLR, the demands are vastly different. In open-skill sports (eg, football, tennis, etc), athletes are exposed to multiple stimuli such as the direction of the ball and moves of the opponent team, and athletes have to make decisions in this unpredictable, dynamically changing environment. This interpretation and the subsequent (subconscious) decision must be made quickly and be reevaluated within the dynamic demands on the field. 15 Indeed, any deficit or delay in sensory or attentional processing may contribute to an inability to correct potential errors in complex coordination, resulting in knee positions that increase the ACL injury risk. 37
Hence, the high incidence of second, noncontact ACL injuries may in part be explained by a higher neurocognitive load, as for example in soccer, when the player must allocate attentional resources to the location of opponents, the ball, and team mates. 3 Higher level neurocognitive functions, also referred to as executive functions, are important in tasks that demand concentration, adaptation, and control to override internal or external stimuli. 12 Core neurocognitive functions control complex, goal-directed thought and behavior, and involve multiple domains, such as inhibitory control, attention, working memory, and cognitive flexibility. 11 Deficits in reaction time and processing speed indicate a potential neurocognitive predisposition to ACL injury. 37 Athletes with lower levels of baseline neurocognitive performance demonstrate poor neuromuscular performance during landing relative to those with higher levels of baseline neurocognitive performance such that they may be at an elevated risk of injury. 23
Based on the aforementioned background, it appears that current RTS protocols after ACLR do not take this complexity into account. Specifically, there is a need to improve our understanding of how neurocognitive factors may influence second ACL injury risk. 34 Moreover, emerging evidence indicates that ACL injuries should also be considered as a neurophysiological lesion.22,30 A recent systematic review revealed cortical reorganization and summarized changes after ACL injuries. 32 Given the substantial evidence pointing at the role of the brain in sensorimotor processing, 16 it is fundamental to understand if these neuroplastic changes bridge with neurocognitive functions, which are essential to manage dynamic environments in sports. Such a linking exploration from both a neural and a behavioral perspective would allow for a broader understanding to optimize RTS tests and rehabilitation programs.
With this background, the primary aim of this systematic review is to summarize and map existing evidence about neurocognitive and neurophysiological functions in athletes who could contribute to or be affected by ACL injuries. The second aim is to present a framework integrating cognitive and neurophysiological principles to assessment and rehabilitation approaches after ACLR.
Methods
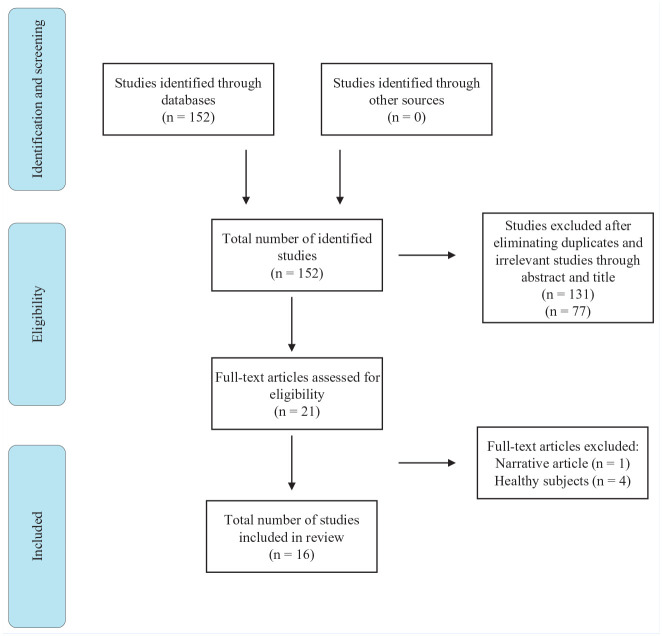
A systematic review methodology was used in accordance with PRISMA Extension for Scoping Reviews. 41 Two authors conducted the literature search in electronic databases PubMed and Cochrane. Studies focusing on injury-related neurocognitive and neurophysiological functions were searched using 2 different groups of keywords combined with Boolean operators. To find studies focusing on central nervous system (CNS) the keywords “brain (OR cortical OR neuroplasticity), ACL, and athletes,” and for those investigating neurocognitive functions the keywords “cognitive (OR neurocognitive OR cognition), ACL, and athletes” were used. Our detailed search strategy is presented in the PRISMA chart in Figure 1. The studies were selected in 2 groups: (1) those who investigated neurocognitive functions regardless of domains and tests and (2) those who investigated CNS regardless of techniques used in an ACL injury context. All experimental and observational studies with prospective and retrospective designs published in any year in English language were included. Reviews and conference abstracts were excluded. The main focus of this systematic review were athletes who have experienced an ACL injury with or without a primary ACLR with an autograft. Studies whose participants (1) had knee dislocation, (2) underwent a revision surgery, (3) were nonathletes, and (4) were older than 40 years were excluded (as middle-aged individuals with joint trauma may be more prone to knee osteoarthritis and this may influence the progression of rehabilitation). All studies that tested participants pre- or post-ACLR were included regardless of time elapsed. The relevant demographic, neurological, and behavioral data highlighting fluctuations in ACL-injured athletes were extracted from the studies by 1 reviewer and verified by a second reviewer.
Figure 1.
Flowchart of search strategy.
Results
Sixteen studies were included, exploring neurocognitive (n = 6) and neurophysiological functions (n = 10) related to injury. The detailed characteristics of studies in terms of subjects, demographics, designs, and techniques used are summarized in 2 groups and presented in Table 1 and the appendix (available in the online version of this article). A total of 295 subjects (118 women) with ACL injuries in the age range of 16 to 27 years were included in studies. Thirteen studies2,9,25,26,27,28,31,33,36,38,39,45,46 examined changes in athletes postinjury, whereas 3 of them13,14,22 focused on changes preceding injury with a prospective design. The time course between injury or surgery and examination varied between 2 weeks and 70 months. Except for 1, all studies compared ACL injured athletes with matched healthy controls.
Table 1.
Characteristics and findings of studies investigating neurophysiological changes related to ACL injury
| Study | Participants | Technique, Design | Time Course | Main Findings |
|---|---|---|---|---|
| Diekfuss et al (2018) 14 | ACL: n = 2, female, 16 ± 0 y Control: n = 8, female, 15.9 ± 0.8 y |
fMRI, case-control, prospective | Between testing and injury: 2 wk and 3.5 mo | Poorer connectivity between the left primary sensory cortex and right posterior lobe of cerebellum |
| Diekfuss et al (2019) 13 | ACL: n = 3, male, 16.33 ± 0.58 y Control: n = 12, male, 16.83 ± 0.39 y |
fMRI, case-control, prospective | Between testing and injury: 57, 67, and 243 d | Poorer connectivity between the left secondary somatosensory cortex and left supplementary motor area, left primary somatosensory cortex, and left primary motor cortex |
| Zarzycki et al (2018) 45 | ACL: n = 18, F:M = 10:8, 21.8 ± 3.3 y Control: n = 18, F:M = 10:8, 22.2 ± 2.5 y |
TMS, case-control | 2 weeks after ACLR | Corticospinal excitability is lower and intracortical facilitation is asymmetrical between 2 limbs in ACLR group |
| Zarzycki et al (2020) 46 | ACL: n = 18, F:M = 10:8, 21.6 ± 3.3 y Control: n = 18, F:M = 10:8, 22.3 ± 2.5 y |
TMS, case-control, longitudinal | 3 time points: (1) 2 wk after ACLR, (2) quiet knee, (iii) return to running | ICF is asymmetrical for the injured limb in ACL regardless of time point. Positive relationship between SICI and quadriceps strength at quite knee |
| Tang et al (2020) 39 | ACL: n = 20, F:M = 5:15, 24.1 ± 3.55 y Control: n = 20, F:M = 5:15, 22.3 ± 2.62 y |
TMS, case-control | Between testing and injury: 31 mo, between testing and ACLR: 27 mo | SICI was lower and ICF was higher in the injured limbs |
| Criss et al (2020) 9 | ACL: n = 15, F:M = 8:7, 20.9 ± 2.7 y Control: n = 15, F:M = 8:7, 22.5 ± 2.5 y |
fMRI, case-control | 43.3 ± 33.1 mo after surgery | Increased activity and connectivity in brain regions associated with visuospatial cognition and attention |
| Grooms et al (2015) 22 | ACL: n = 1, male, 25 y Control: n = 1, male, 26 y |
fMRI, case-control, prospective | 10 mo after initial, 26 d before secondary injury | Increased activity of motor planning, sensory, and visuomotor areas after the initial, before the second injury |
| Lepley et al (2020) 26 | ACL: n = 10, F:M = 6:4, 22.6 ± 1.9 y | MRI, TMS cross-sectional | 70.0 ± 23.6 mo after surgery | Reduced white matter volume and excitability in contralateral hemisphere |
| Lepley et al (2019) 25 | ACL: n = 11, F:M = 6:5, 22.6 ± 1.8 y Control: n = 11, F:M = 6:5, 23.2 ± 1.6 years |
fMRI, TMS, case-control | 69.4 ± 22.4 mo after surgery | Increased activation in frontal and cingulate cortex, increased active motor threshold and decreased motor-evoked potentials |
| Scheurer et al (2020) 33 | ACL: n = 16, F:M = 8:8, 20.4 ± 1.8 y Control: n = 16, F:M = 8:8, 21.0 ± 1.7 |
TMS, case-control | 33.9 ± 26.1 mo after surgery | Decreased corticospinal excitability and increased intracortical inhibition associate with reduced torque development |
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; F, female; fMRI, functional magnetic resonance imaging; ICF, intracortical facilitation; M, male; MRI, magnetic resonance imaging; SICI, short-interval intracortical inhibition; TMS, transcranial magnetic stimulation.
Six of the included studies assessed neurocognitive functions in athletes and 5 of them showed differences in different domains from 6 to 14 months after injury.2,28,31,36,38 The utilized tests and the main findings of these studies are summarized in the appendix (available online).
The remaining 10 studies13,14,22,25,26,33,38,39,45,46 investigated neurophysiological functions in athletes using either functional magnetic resonance imaging (fMRI) or transcranial magnetic stimulation (TMS). Seven of the studies25,26,33,38,39,45,46 underlined neurophysiological alterations 2 weeks to 70 months after injury, whereas the 3 prospective studies13,14,22 established these changes 2 to 34 weeks before injury in the athletic population. The main findings of these studies are summarized in Table 1.
Discussion
The findings of this systematic review underpin that athletes may present changes in different domains of neurocognitive functions and different features of CNS, such as decreased connectivity between brain regions and corticospinal excitability, before or predominantly after ACL injury in subacute and chronic phases. To our knowledge, this is the first systematic review to integrate neurocognitive and neurophysiological perspectives over ACL injuries in athletes. However, the paucity of research focusing on athletic population and the variability in methodology makes it difficult to quantify these findings statistically and systematically. The following sections discuss first the neurocognitive, subsequently the neurophysiological findings, and interpret them within a practical frame in terms of RTS and rehabilitation.
Reaction Time, Processing Speed, Memory, and Visual Performance
Six studies tested neurocognitive domains including reaction time, processing speed, visual processing, and memory, solely and in combination with an added motor task as a dual task paradigm.2,27,28,31,36,38 The study of Swanik et al 38 revealed deficits in reaction time, processing speed, and visual and verbal memory in ACL-injured athletes. Faster reaction time or processing speed are imperative in terms of being agile to unpredictable stimuli while maintaining neuromuscular control. 21 Similarly, the ability to keep the constantly changing environment in visual memory plays a fundamental role in feedforward mechanisms during motor planning. 35 These higher level neurocognitive functions are indeed correlated to athletic performance and their impairment may predispose athletes to second injury.18,23 However, Stone et al 36 found no differences in reaction time, but improved visuomotor scanning in ACL-injured participants compared with healthy controls. It is noteworthy to consider the nature of applied tests while interpreting these contradictory findings. Swanik et al 38 utilized ImPACT, in which the assessment of reaction time relies on a go/no-go paradigm, that is, response inhibition, which is indeed a discrete cognitive domain controlled by different neural mechanisms. 42 On the other hand, the task used by Stone et al 36 was only a reaction time task without having to inhibit the stimulus. This difference in tasks could have caused these conflicting findings regarding reaction time.
Concerning visual processing, it has been shown that ACL-injured athletes rely more on visual cues to compensate for postural control deficits.36,44 Although visuomotor performance correlates highly to visual memory, 21 the results of Swanik et al 38 contradict this fact and the findings of Stone et al, 36 pointing to an impaired visual memory performance in ACL-injured athletes. As a possible explanation, the trail making test with a “motor” component used by Stone et al 36 might be controlled by intertwined visual and motor mechanisms and reflect the “scanning” aspect of visual performance, rather than memory. As an additional remark, it is important to underline that the time elapsed after injury is not given in either of these studies and it is known that neural deficits might progress parallel to increased chronicity. 30
Added Cognitive Load
The influence of added cognitive load on postural control was investigated in 4 of the neurocognitive studies.2,27,28,31 Three of these studies2,28,31 demonstrated that either ACL-injured athletes sacrifice their cognitive performance to maintain sufficient postural control, or vice versa, postural stability declines with added cognitive load. Injured individuals demonstrate potentially maladaptive neuroplasticity in primary and premotor areas showing higher activation than healthy individuals during simple motor tasks. 30 Additionally, it was discussed that disrupted afference may be compensated through enhanced attentional processes in this population, when executing motor tasks. 6 These changes may therefore increase the processing demand for the primary task and constrain residual reserve for secondary tasks, as in the findings of these 3 studies.2,28,31 However, the findings of Lion et al 27 were contradictory, showing no differences between injured athletes and healthy controls when a cognitive load was added to a double-leg stance task. In this case, it could be questioned how challenging a double-leg stance task would be for recovered athletes. The difficulty level and complexity of a task are 2 important factors that determine to what extent compensatory mechanisms are recruited. More demanding motor tasks are affected to a greater extent when subjects are exposed to cognitive load. 8 Based on the sensory-reweighting hypothesis, the aforementioned compensatory attentional and visual processes in the injured population are explained by disrupted afferent information provided by articular mechanoreceptors. 5 When the cognitive reserve is exploited by secondary distracting tasks as in these studies, the attention is directed either to cognitive or motor task, which fluctuates the efficacy of the other, as also stated in capacity sharing model. 40
Changes in CNS
The remaining 10 studies reveal altered activity in different dimensions of CNS before and after injury, which could be in a “cause and effect” interaction with neurocognitive fluctuations discussed until here.9,13,14,21,25,26,33,39,45,46 The 2 fMRI studies with a prospective design suggest that neural changes, altered functional connectivity more specifically, might exist even before ACL injury.13,14 Diekfuss et al13,14 measured cortical activity via fMRI during resting state and suggested that athletes, who later on sustained an ACL injury, demonstrated decreased functional connectivity between pre- and primary sensorimotor areas and cerebellum, before injury. The highest level of hierarchical motor control in sports, which stands for producing strategies based on sensory information, includes areas of prefrontal and posterior parietal cortex. The primary sensory cortex within these areas play a fundamental role in terms of interacting with the prefrontal cortex and providing necessary sensory information to plan and initiate the movement. 7 Moreover, at the middle level of this hierarchical motor control, cerebellum interacts with the motor cortex to conceive “tactics.”7,29 In these 2 studies,13,14 the changes in connectivity between these crucial areas might cause reduced ability to produce congruent responses in complex sports situations. Similarly, the other prospective case report by Grooms et al 22 also demonstrated increased activity in sensori- and visuomotor areas during a unilateral knee flexion-extension task after the first and before the second ACL injury, which highlights that neuroplastic changes after an ACL injury may constitute a risk for second ACL injury. The retrospective fMRI study by Criss et al 9 reveals increased activity and connectivity in areas associated with visuospatial cognition and attention during hip and knee movement in ACL-injured participants and confirms that similar organizational changes exist years after injury. These CNS changes could constitute an organic basis for the aforementioned neurocognitive changes, which could be explained as compensatory mechanisms.
The TMS studies underlined injury-related changes in other features of CNS such as reduced corticospinal excitability and increased intracortical inhibition.25,26,33,39,45,46 Disrupted afferent input delivered from mechanoreceptors modulates motor cortex in time, which could lead to decreased corticospinal excitability. The increased inhibition on the other hand could be explained by theoretical models, which suggest that the human body might suppress motor tracts to prevent unwanted movements of an injured joint. 26 In addition, most of these studies have shown that these affected neurophysiological features reflect onto peripheral measures as reduced isometric quadriceps strength and torque development.33,46 Two studies emphasize that these excitability disruptions together with structural changes like reduced white matter volume persist even after years in the injured population.25,26
The Limitations of Studies and Their Practical Relevance in Sports
While translating these findings into practice, it is important to recognize the limitations. Dual-tasking paradigm has been assessed with added different cognitive tasks such as backward counting. Performing a discrete cognitive task on an unstable surface could represent a daily situation like playing with a smartphone in a moving bus, but in sports, the cognitive demand is a prerequisite to successfully achieve a motor goal and incorporating cognitive and motor tasks could automatize movements and induce a more efficient plasticity. 24 This notion is consolidated by studies that have shown that directing attention to an external focus while performing a motor task, that is, blending cognitive and motor aspects could enhance performance more than using an internal focus as in discrete motor and cognitive tasks. 19 Therefore, it would be questionable if all these tests are ecologically valid and reflect sport-relevant cognitive and motor incorporation, as on the field. Furthermore, the small sample size of some studies and covariates like differing timespan between scanning and injury, different tests and neurocognitive domains make it difficult to infer specific, quantifiable, and reliable results.
A Framework for Practical Applications
These findings reveal clearly that the athletic population demonstrates a loop of neurocognitive and CNS changes after ACL injury, even after returning to sports. However, most RTS and rehabilitation concepts lack this perspective and RTS decision is based on measures such as isokinetic muscle strength and closed motor skills. 1 Based on athletes’ high reinjury and low preinjury level rates, it may be speculated that they are not adequately prepared to meet the demands of field on return to participation after injury, whereas both the physical capacity and cognitive skills are modifiable through interventions. The latter may be underrepresented in rehabilitation as the focus is directed toward restoring range of motion, strength, power, and endurance. However, cognitive skills of an athlete in the context of complex sport situations play an important role to produce congruent strategies. The dynamic environment challenges cognitive reserve to keep movement “under attention” and simultaneously interpret multimodal stimuli, that is, pursue performance with high-risk biomechanics. When combined with compensatory neural changes, added cognitive load may pose a risk for second injury. While testing and rehabilitating athletes, increasing the complexity of functional environments with graded uncertainty may help restore both the physical and cognitive aspects of performance and prepare athletes for real-world sport situations. 20
Conclusion
The primary goal of this review was to scope evidence regarding neurocognitive and neurophysiological functions that could be related to ACL injury in athletes and to synthesize them into RTS and rehabilitation paradigms. The existing evidence shows that cognitive skills and CNS functions may be linked to an increased injury risk and diminish postinjury performance in athletes. Cortical reorganization may demand compensatory strategies and occupy cognitive reserve, which makes it difficult to manage dynamic environment in sports. RTS and rehabilitation concepts should consider this notion to prevent second injuries and to achieve an adequate competitive level in athletes.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_19417381211029265 for Neurocognitive and Neurophysiological Functions Related to ACL Injury: A Framework for Neurocognitive Approaches in Rehabilitation and Return-to-Sports Tests by Daghan Piskin, Anne Benjaminse, Panagiotis Dimitrakis and Alli Gokeler in Sports Health: A Multidisciplinary Approach
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42:601-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmadi P, Salehi R, Mehravar M, Goharpey S, Negahban H. Comparing the effects of external focus of attention and continuous cognitive task on postural control in anterior cruciate ligament reconstructed athletes. Neurosci Lett. 2020;715:134666. [DOI] [PubMed] [Google Scholar]
- 3. Almonroeder TG, Kernozek T, Cobb S, Slavens B, Wang J, Huddleston W. Divided attention during cutting influences lower extremity mechanics in female athletes. Sports Biomech. 2019;18:264-276. [DOI] [PubMed] [Google Scholar]
- 4. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament construction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48:1543-1552. [DOI] [PubMed] [Google Scholar]
- 5. Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J Neurophysiol. 2014;111:1852-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumeister J, Reinecke K, Liesen H, Weiss M. Cortical activity of skilled performance in a complex sports related motor task. Eur J Appl Physiol. 2008;104:625-631. [DOI] [PubMed] [Google Scholar]
- 7. Bear MF, Connors BW, Paradiso MA. Brain control of movement. In: Neuroscience: Exploring The Brain. Lippincott Williams & Wilkins; 2007:451-478. [Google Scholar]
- 8. Burcal CJ, Needle AR, Custer L, Rosen AB. The effects of cognitive loading on motor behavior in injured individuals: a systematic review. Sports Med. 2019;49:1233-1253. [DOI] [PubMed] [Google Scholar]
- 9. Criss CR, Onate JA, Grooms DR. Neural activity for hip-knee control in those with anterior cruciate ligament reconstruction: a task-based functional connectivity analysis. Neurosci Lett. 2020;730:134985. [DOI] [PubMed] [Google Scholar]
- 10. Davies GJ. Individualizing the return to sports after anterior cruciate ligament reconstruction. Oper Tech Orthop. 2017;27:70-78. [Google Scholar]
- 11. Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diamond A. The early development of executive functions. In: Bialystok A, Craik FIM, eds. Lifespan Cognition: Mechanisms of Change. Oxford University Press; 2006:70-95. [Google Scholar]
- 13. Diekfuss JA, Grooms DR, Nissen KS, et al. Alterations in knee sensorimotor brain functional connectivity contributes to ACL injury in male high-school football players: a prospective neuroimaging analysis. Braz J Phys Ther. 2019;24:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diekfuss JA, Grooms DR, Yuan W, et al. Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. J Sci Med Sport. 2018;22:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dingenen B, Gokeler A. Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: a critical step back to move forward. Sports Med. 2017;47:1487-1500. [DOI] [PubMed] [Google Scholar]
- 16. Edwards LL, King EM, Buetefisch CM, Borich MR. Putting the “sensory” into sensorimotor control: the role of sensorimotor integration in goal-directed hand movements after stroke. Front Integr Neurosci. 2019;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feucht MJ, Cotic M, Saier T, et al. Patient expectations of primary and revision anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24:201-207. [DOI] [PubMed] [Google Scholar]
- 18. Giesche F, Wilke J, Engeroff T, et al. Are biomechanical stability deficits during unplanned single-leg landings related to specific markers of cognitive function? J Sci Med Sport. 2020;23:82-88. [DOI] [PubMed] [Google Scholar]
- 19. Gokeler A, Benjaminse A, Welling W, Alferink M, Eppinga P, Otten B. The effects of attentional focus on jump performance and knee joint kinematics in patients after ACL reconstruction. Phys Ther Sport. 2015;16:114-120. [DOI] [PubMed] [Google Scholar]
- 20. Gokeler A, McKeon PO, Hoch MC. Shaping the functional task environment in sports injury rehabilitation: linking mind and body through situational training. Athl Train Sports Healthcare. 2020;12:283-292. [Google Scholar]
- 21. Grooms DR, Onate JA. Neuroscience application to noncontact anterior cruciate ligament injury prevention. Sports Health. 2016;8:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grooms DR, Page SJ, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: neuroimaging in musculoskeletal medicine. J Athl Train. 2015;50:1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herman DC, Barth JT. Drop-jump landing varies with baseline neurocognition: implications for anterior cruciate ligament injury risk and prevention. Am J Sports Med. 2016;44:2347-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herold F, Hamacher D, Schega L, Müller NG. Thinking while moving or moving while thinking—concepts of motor-cognitive training for cognitive performance enhancement. Front Aging Neurosci. 2018;10:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lepley AS, Grooms DR, Burland JP, Davi SM, Kinsella-Shaw JM, Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systematic differences in neural and morphological characteristics. Exp Brain Res. 2019;237:1267-1278. [DOI] [PubMed] [Google Scholar]
- 26. Lepley AS, Ly MT, Grooms DR, Kinsella-Shaw JM, Lepley LK. Corticospinal tract structure and excitability in patients with anterior cruciate ligament reconstruction: a DTI and TMS study. Neuroimage Clin. 2020;25:102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lion A, Gette P, Meyer C, Seil R, Theisen D. Effect of cognitive challenge on the postural control of patients with ACL reconstruction under visual and surface perturbations. Gait Posture. 2018;60:251-257. [DOI] [PubMed] [Google Scholar]
- 28. Mohammadi-Rad S, Salavati M, Ebrahimi-Takamjani I, et al. Dual-tasking effects on dynamic postural stability in athletes with and without anterior cruciate ligament reconstruction. J Sport Rehabil. 2016;25:324-329. [DOI] [PubMed] [Google Scholar]
- 29. Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247-259. [DOI] [PubMed] [Google Scholar]
- 30. Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47:1271-1288. [DOI] [PubMed] [Google Scholar]
- 31. Negahban H, Ahmadi P, Salehi R, Mehravar M, Goharpey S. Attentional demands of postural control during single leg stance in patients with anterior cruciate ligament reconstruction. Neurosci Lett. 2013;556:118-123. [DOI] [PubMed] [Google Scholar]
- 32. Neto T, Sayer T, Theisen D, Mierau A. Functional brain plasticity associated with ACL injury: a scoping review of current evidence. Neural Plast. 2019;2019:3480512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheurer SA, Sherman DA, Glaviano NR, Ingersoll CD, Norte GE. Corticomotor function is associated with quadriceps rate of torque development in individuals with ACL surgery. Exp Brain Res. 2020; 238:283-294. [DOI] [PubMed] [Google Scholar]
- 34. Schultz SJ, Schmitz RJ, Cameron KL, et al. ACL research retreat VIII summary statement: An update on injury risk identification and prevention across the anterior cruciate ligament injury continuum, March 14-16, 2019, Greensboro, NC. J Athl Train 2019;54:970-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith TQ, Mitroff SR. Stroboscopic training enhances anticipatory timing. Int J Exerc Sci. 2012;5:344-353. [PMC free article] [PubMed] [Google Scholar]
- 36. Stone AE, Roper JA, Herman DC, Hass CJ. Cognitive performance and locomotor adaptation in persons with anterior cruciate ligament reconstruction. Neurorehabil Neural Repair. 2018;32:568-577. [DOI] [PubMed] [Google Scholar]
- 37. Swanik CB. Brains and sprains: The brain’s role in noncontact anterior cruciate ligament injuries. J Athl Train. 2015;50:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35:943-948. [DOI] [PubMed] [Google Scholar]
- 39. Tang WT, Hsu MJ, Huang YM, Hsu YT, Chuang LL, Chang YJ. Low-intensity electrical stimulation to improve the neurological aspect of weakness in individuals with chronic anterior cruciate ligament lesion. Biomed Res Int. 2020;2020:7436274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tombu M, Jolicœur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3. [DOI] [PubMed] [Google Scholar]
- 41. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467-473. [DOI] [PubMed] [Google Scholar]
- 42. Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12:418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1861-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wikstrom EA, Song K, Pietrosimone BG, Blackburn JT, Padua DA. Visual utilization during postural control in anterior cruciate ligament-deficient and -reconstructed patients: systematic reviews and meta-analyses. Arch Phys Med Rehabil. 2017;98:2052-2065. [DOI] [PubMed] [Google Scholar]
- 45. Zarzycki R, Morton SM, Charalambous CC, Marmon A, Snyder-Mackler L. Corticospinal and intracortical excitability differ between athletes early after ACLR and matched controls. J Orthop Res. 2018;36:2941-2948. [DOI] [PubMed] [Google Scholar]
- 46. Zarzycki R, Morton SM, Charalambous CC, Pietrosimone B, Williams GN, Snyder-Mackler L. Athletes after anterior cruciate ligament reconstruction demonstrate asymmetric intracortical facilitation early after surgery. J Orthop Res. 2021;39:147-153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_19417381211029265 for Neurocognitive and Neurophysiological Functions Related to ACL Injury: A Framework for Neurocognitive Approaches in Rehabilitation and Return-to-Sports Tests by Daghan Piskin, Anne Benjaminse, Panagiotis Dimitrakis and Alli Gokeler in Sports Health: A Multidisciplinary Approach