Abstract
Context:
There are 3.8 million mild traumatic brain injuries (mTBIs) that occur each year in the United States. Many are left with prolonged life-altering neurocognitive deficits, including difficulties in attention, concentration, mental fatigue, and distractibility. With extensive data on the safety and efficacy of stimulant medications in treating attention deficit, concentration difficulties and distractibility seen with attention deficit disorder, it is not surprising that interest continues regarding the application of stimulant medications for the persistent neurocognitive deficits in some mTBIs.
Evidence Acquisition:
Studies were extracted from PubMed based on the topics of neurocognitive impairment, mTBI, stimulant use in mTBI, stimulants, and the association between attention deficit/hyperactivity disorder and mTBI. The search criteria included a date range of 1999 to 2020 in the English language.
Study Design:
Literature review.
Level of Evidence:
Level 4.
Results:
Currently, there is very limited literature, and no guidelines for evaluating the use of stimulant medication for the treatment of prolonged neurocognitive impairments due to mTBI. However, a limited number of studies have demonstrated efficacy and safety of stimulants in the treatment of neurocognitive sequelae of mTBI in the adult, pediatric, military, and athletic populations.
Conclusion:
There is limited evidence to suggest stimulant medication may be beneficial in patients with mTBI with persistent neurocognitive symtpoms. The decision to utilize stimulant medication for mTBI patients remains physician and patient preference dependent. Given the limited encouraging data currently available, physicians may consider stimulant medication in appropriate patients to facilitate the recovery of prolonged neurocognitive deficits, while remaining cognizant of potential adverse effects.
Keywords: neurocognitive impairment, mild traumatic brain injury (mTBI), attention deficit/hyperactivity disorder (ADHD), stimulant medication
Methods
In this narrative review, we aimed to summarize the relevant limited literature available on the use of stimulant medications for the treatment of prolonged neurocognitive impairments due to mild traumatic brain injury (mTBI). 55 Our search was selective in nature without a formal quality or risk of assessment of included studies. Chosen literature was extracted from a PubMed-based search on the topics of neurocognitive impairment, mTBI, stimulant use in mTBI, stimulants, and the association between attention deficit/hyperactivity disorder (ADHD) and mTBI. The search criteria included a date range of 1999 to 2020 in the English language. Content analysis of the reviewed studies was developed into a conceptual framework to provide clinicians basic guidance to consider stimulant medication in appropriate patients to facilitate the recovery of prolonged neurocognitive deficits, after an mTBI, while remaining cognizant of potential adverse effects26,72
Background of the Problem
In the United States, 100 to 300 per 100,000 people annually suffer traumatic brain injury (TBI), with most of these injuries being mild. 23 The majority of patients with mTBI recover quickly with no long-term effects11,23,41; however, a minority suffer persistent postconcussive symptoms (PPCS). 62 Results from a sample of 731 patients with mTBI showed that prevalence rates of PPCS ranged from 11.4% to 38.7% with most of these patients reporting neurocognitive dysfunction, including difficulties in attention, concentration, and distractibility (Table 1).29,71 Additionally, mTBI and ADHD have been increasingly associated, particularly in early childhood mTBI and the athletic population.1,51,57
Table 1.
Long-term sequelae of mild traumatic brain injury (mTBI) with prolonged postconcussive symptoms
| Long-Term mTBI Sequelae | Rates Among mTBI With Chronic Sequelae | Characteristics | Risk Factors for Chronic Impairments | Management |
|---|---|---|---|---|
| Chronic headache | Most prevalent reported symptom among patients at 47%-95%
a
18%-33% report headaches after 1 year b |
Mainly tension or migraine headaches; often temporal location a | Female sex vs male sex (74 vs 63%), individual history of headaches before injury (45% vs 19%) c | Pharmacotherapies tailored to type of headache, cognitive behavioral therapy, biofeedback, relaxation therapy d |
| Emotional disturbances | 18% report depression 1-year postinjury and is the most common report emotional disturbance e | Impairment of awareness, recognition, expression, and regulation of emotions f | Young age, previous mTBI, low education level, previous psychiatric diagnosis, alcohol use g | Pharmacotherapies aimed at depression such as SSRI/SNRIs, stimulants, tricyclic antidepressant; psychotherapy g |
| Cognitive dysfunction | Incidence of 15%, with newer literature suggesting this to be an underestimation h | Impairments of executive function, learning/memory, attention, processing speed, and language function h | Low education level and low preinjury IQ are correlated with higher rates of prolonged cognitive deficits i | First treatment: Cognitive rehabilitation, lifestyle modifications. Proposed use of NMDA antagonists, stimulants, and cholinergic augmentation j |
| Vestibular symptoms | Up to 10%-15% experience dizziness and vertigo at 1 year k | Dizziness, vertigo, disequilibrium | Blast exposure, previous mTBI l | Vestibular rehabilitation; short-term use of scopolamine, meclizine m |
IQ, intelligence quotient; NMDA, N-methyl-d-aspartate; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors.
Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther. 2014;22:36-44.
Lew HL, Lin PH, Fuh JL, Wang SJ, Clark DJ, Walker WC. Characteristics and treatment of headache after traumatic brain injury: a focused review. Am J Phys Med Rehabil. 2006;85:619-627. doi:10.1097/01.phm.0000223235.09931.c0
Hoffman JM, Lucas S, Dikmen S, et al. Natural history of headache after traumatic brain injury. J Neurotrauma. 2011;28:1719-1725.
Olesen J, Ramadan NM. Migraine mechanisms. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, eds. The Headaches. 3rd ed. Lippincott Williams & Wilkins; 2006:251-393.
Rao V, Bertrand M, Rosenberg P, et al. Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2010;22:100-104.
Neumann D. Treatments for emotional issues after traumatic brain injury. J Head Trauma Rehabil. 2017;32:283-285.
Schwarzbold M, Diaz A, Martins ET, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. 2008;4:797-816.
McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review [published correction appears in PLoS One. 2019;14:e0218423]. PLoS One. 2017;12:e0174847.
de Freitas Cardoso MG, Faleiro RM, de Paula JJ, et al. Cognitive impairment following acute mild traumatic brain injury. Front Neurol. 2019;10:198.
Wortzel HS, Arciniegas DB. Treatment of posttraumatic cognitive impairments. Curr Treat Options Neurol. 2012;14:493-508.
Skóra W, Stańczyk R, Pajor A, Jozefowicz-korczyńska M. Vestibular system dysfunction in patients after mild traumatic brain injury. Ann Agric Environ Med. 2018;25:665-668.
Akin FW, Murnane OD, Hall CD, Riska KM. Vestibular consequences of mild traumatic brain injury and blast exposure: a review. Brain Inj. 2017;31:1188-1194.
Franke LM, Walker WC, Cifu DX, Ochs AL, Lew HL. Sensorintegrative dysfunction underlying vestibular disorders after traumatic brain injury: a review.J Rehabil Res Dev. 2012;49:985-994.
Definition
The heterogeneity of definitions of mTBI, concussion, and TBI in the literature potentially compromises any standard review. 63 For the purposes of this review, we will be using the terminology of mTBI to agree with the World Health Organization Collaborating Centre Task Force on Mild Traumatic Brain Injury. 11 “mTBI is an acute brain injury resulting from mechanical energy to the head from external physical forces. Operational criteria for clinical identification include:
(1) One or more of the following: Confusion or disorientation, loss of consciousness for 30 minutes or less, post-traumatic amnesia for less than 24 hours, and/or other transient neurological abnormalities such as focal signs, seizure, and an intracranial lesion not requiring surgery.
(2) Glasgow Coma Scale score of 13-15 after 30 minutes post-injury or later upon presentation for healthcare”. 12
Given the array of symptoms reported, paucity of prospective studies and randomized control trials and conjunction with other psychiatric conditions, developing a consistent and specific definition of PPCS continues to be elusive. Additional conundrums include various terminology and lack of consistency regarding the length of time outside the normal course of recovery after mTBI. Within this article, PPCS will be defined as the persistence of concussion symptoms beyond 30 days from injury. Persistent symptoms are often a constellation of cognitive, physical, emotion, and sleep impairments. Postconcussional disorder is no longer included in the Diagnostic and Statistical Manual of Mental Disorders (DSM), as an alternative it is contained under “neurocognitive disorder due to traumatic brain injury.” 20 The World Health Organization’s International Classification of Diseases, 10th Revision (ICD-10), defines PCS as
A syndrome that occurs following head trauma (usually sufficiently severe to result in loss of consciousness) and includes a number of disparate symptoms such as headache, dizziness, fatigue, irritability, difficulty in concentration and performing mental tasks, impairment of memory, insomnia, and reduced tolerance to stress, emotional excitement, or alcohol. 79
The focus of this article is the utility of stimulant therapy in these patients with mTBI, who have persistent neurocognitive symptoms, either by patient symptom reporting, or objective neurocognitive findings.
Epidemiology
The Centers for Disease Control and Prevention estimates that 2.5 million patients present to emergency departments annually for mTBI. 22 Considering injuries that do not present to the emergency department or other medical care settings, the Centers for Disease Control and Prevention estimates up to 3.8 million mTBIs occur each year in the United States. 40 Of the 54 million Americans with disability, there are 5.3 million Americans (1 in 10) with disability related to TBI. 40 Sports-related concussion, a subset of mTBI, is estimated to account for 135,000 emergency department visits annually, with an overall incidence of 24 per 100,000. 12 Football accounts for nearly 75% of these injuries, but soccer accounts for nearly 50% of female injuries and women’s ice hockey presents the highest risk per exposure of mTBI (0.91 per 1000 exposures). 18
Prolonged Neurocognitive Sequealae
While the majority of patients with mTBI recover within 4 to 12 weeks, a significant minority have persistent symptoms leading to prolonged neurocognitive sequalae such as impaired school or work function and/or performance. 29 Children who sustained mTBIs are at risk of a range of neurocognitive and behavioral deficits such as impaired attention, concentration, memory, language, executive function skills, information processing, and problem solving.7,9,35,53,54 Studies vary in the frequency of PPCS in young athletes after mTBI from 1.5% to 15%.5,43,48,80 In a study of 1116 sports-related mTBI in young athletes through Vanderbilt Sports Concussion Center, 3.5% of concussions led to PPCS for 3 months or greater. 50 These patients with prolonged neurocognitive deficits post mTBI, and their hypothetical response to stimulant therapy, are the focus of this review (Figure 1).
Figure 1.
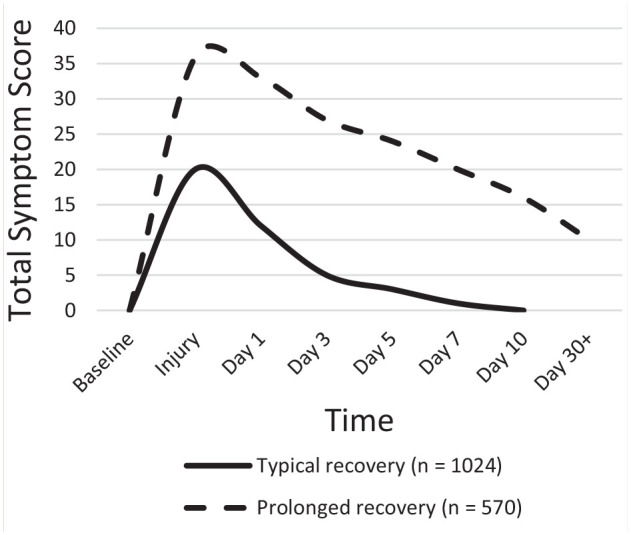
Typical and prolonged recovery patterns identified after sport-related concussions among high school and collegiate student-athletes. Adapted from Teel et al (2017),a McCrea et al (2013),b and McCrea et al (2003).c
aTeel EF, Marshall SW, Shankar V, McCrea M, Guskiewicz KM. Predicting recovery patterns after sport-related concussion. J Athl Train. 2017;52:288-298. doi:10.4085/1062-6050-52.1.12
bMcCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2013;19:22-33. doi:10.1017/S1355617712000872
cMcCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556-2563. doi:10.1001/jama.290.19.2556
Pathophysiology
The pathophysiology of mTBI is not completely understood and is outside the scope of this article. 4 Typically, in mTBI, the biochemical processes induced by mTBI are reversible, recovered fully within days to weeks. In more severe injury, or with a repetitive injury during the recovery process, biochemical changes can persist, possibly indicating irreversible cellular damage.4,38,64 The cognitive functions of memory and attention are not isolated to a single area of the brain. Rather, broad cerebral networks with significant overlap exist within brain regions. 3 These brain regions include prefrontal and parietal cortices, cingulate gyrus, basal ganglia, hippocampus and related mesial temporal structures, as well as regions of the brain stem and midbrain forming the reticular activating system. 3 The suspected benefit of stimulants lives in the augmentation of the cerebral dopaminergic and adrenergic systems located in these proposed brain regions and networks involved in cognitive function.
Pharmacology
Clinical Pharmacology
As mTBI often induces attention deficit, neurocognitive dysfunction, and executive function impairment similar to ADHD, some researchers have proposed that stimulant medications may be indicated in patients with mTBI.19,73 Methylphenidate and amphetamine are classified as sympathomimetic agonists. Both exhibit properties that stimulate the central nervous system and are readily bioavailable after oral ingestion. It is noted that methylphenidate has a more prominent effect on the central nervous system than does amphetamine, with more activity focused on the cognitive functions and less on motor and other systemic functions. Both agents have been used for the treatment of TBI.
In various studies, methylphenidate improved cognitive impairment and, along with cognitive rehabilitation, improved memory and overall executive functioning.28,47,56 Improvements in sustained and divided attention, reasoning ability, pattern recognition, and motor coordination have been demonstrated with methamphetamines. 17 However, with variations in study design and study populations, not all studies have shown sustained positive outcome. 56 On review of the literature, fewer studies have occurred with amphetamine as a treatment option for TBI than methylphenidate.
Efficacy of Stimulants IN mTBI
On extensive review of the literature, there are very few prospective, randomized, placebo-controlled studies assessing stimulant medication strictly in the mTBI population. Most studies include mTBI as well as moderate and/or severe TBI in their assessment of safety and efficacy of stimulant medications. 30 The only study found assessing only stimulants, specifically in mTBI, is the study by Mcallister et al. 45 This was a 12-week, double-blinded, randomized controlled multisite trial of galantamine or methylphenidate for persistent emotional and cognitive symptoms associated with posttraumatic stress disorder (PTSD) and/or mTBI. Participants were adults aged 18 to 55 years (n = 32), and were included if diagnosed with PTSD and/or mTBI by clinical interview and with demonstrated significant cognitive complaints (T score >60 on the Postmorbid Cognitive Scale of the Ruff Neurobehavioral Inventory). Treatment was well tolerated, and methylphenidate treatment was associated with clinically meaningful and statistically significant improvement compared with placebo on primary and secondary outcome measures (Ruff Neurobehavioral Inventory-Postmorbid Cognitive Scale, Rivermead Post Concussive Symptom Questionairre, and/or Posttraumatic Stress Disorder Checklist). 45
One other study restricted to mTBI assessing not stimulants, but the sympathomimetic amantadine by Reddy et al 61 assessed effect in a prospective, case-control design. The authors studied the sympathomimetic amantadine (100 mg twice a day) in 25 adolescent patients with sports-related mTBI. Medication was added, after an initial rest period, and patients were assessed by clinical examination, IMPACT computer-based neurocognitive battery, and symptom report. Authors found the medication well tolerated, and noted significantly greater improvements in symptoms, verbal memory, and reaction time performance in the amantadine group, than in matched controls. 61
For further guidance regarding safety and efficacy of stimulant medication in mTBI, one has to look to the still limited, and broader spectrum of TBI literature that includes mild, moderate, and severe TBI.
Adult Patients
In adults, limited studies of diverse populations, outcome measures, and treatment regimens have suggested at least modest stimulant efficacy in the treatment of neurocognitive sequelae of TBI. 35 In 2014, a 30-week double-blinded randomized controlled trial (RCT) of 33 adult post-TBI patients showed statistically significant improvement in levels of fatigue, cognitive function, and standardized depression scores with use of methylphenidate. 82 Patients were a broad TBI injury grouping, and they were randomized and analyzed for outcomes pre- and posttreatment with Mental Fatigue Scale, Choice Reaction Time, Compensatory Tracking Task, Mental Arithmetic Test, Digit Symbol Substitution Test, Mini-Mental State Examination (MMSE), Beck Depression Inventory (BDI), and Hamilton Rating Scale for Depression. In a RCT of patients with moderate to severe TBI, methylphenidate use improved reaction time and accuracy with sustained attention and improved reaction time in working memory. This study utilized the neurocognitive tests, two-back task and visual sustained attention task to assess accuracy, sustained attention, and working memory. The same study associated the improvement in reaction time with the magnitude of suppression of the left posterior superior parietal cortex and parieto-occipital junction on perfusion functional magnetic resonance imaging, correlating the efficacy of methylphenidate with the neuroanatomy in patients with TBI. 39
Lis-dexamphetamine showed benefits in the areas of attention, working memory, response speed and endurance, and executive functioning in patients with moderate to severe TBI with chronic attention deficits. 67 Another recent multicenter US study of at least mTBI analyzed the utility of combined cognitive rehabilitation and methylphenidate. Outcome measures included scores on neuropsychological measures and subjective rating scales, with results suggesting a statistically significant improvement with combination therapy as opposed to either form of monotherapy. Improvements were seen in attention, episodic and working memory, and executive functioning after TBI, most pronounced with stimulant plus metacognitive rehabilitation. 47
Special Populations: Pediatric
Studies of stimulant effect of high quality with standardized populations, outcome measures, and treatment protocols are limited in pediatric mTBI.54,76 Hornyak et al 27 conducted a chart review of 10 pediatric patients who had sustained TBI, which demonstrated improved cognitive function, behavior, and arousal by parent and teacher reports, evaluation by the rehabilitation team, and neuropsychometric testing results with the introduction of methylphenidate. Williams et al 76 found no significant differences in attention, memory, and processing speed measures of pediatric patients with TBI who received methylphenidate versus placebo during a 2-week testing period. 76 Mahalick et al 42 in a double-blinded, placebo-controlled trial with crossover design found significant positive treatment effects across all 6 measures of attention and concentration with methylphenidate compared with placebo, while no treatment effect was seen with placebo compared with baseline. Larger, randomized placebo-controlled studies are needed to better assess the use of stimulant medications in pediatric patients with TBI, particularly in the mTBI category of injury.
Special Populations: Preexisting ADHD
The management of mTBI in patients with preexisting ADHD is inconsistent due to limited research, therefore posing a challenge to clinicians. The complex and multifactorial relationship between ADHD and mTBI has led to an investigation of the association of learning disabilities, specifically ADHD and increased lifetime risk of mTBI. This speculation comes from described higher bodily injury rates in patients with ADHD compared with the general population. 33 A large cross-sectional, retrospective study by Iversion et al 33 examining lifetime concussion history in high school athletes found those with ADHD to have a greater lifetime history of concussion compared with high school athletes without ADHD. We have a poor understanding pertaining to the role of preinjury ADHD in postconcussive outcomes. Our current literature has found various mTBI recovery trajectories among patients with ADHD with selected studies showing worse postconcussive outcomes to have numerous limitations causing the reader to question the authors conclusions.15,32,44 Cook et al 15 developed the first prospective study examining whether having ADHD was associated with a worse postconcussive outcome and found that adolescents with ADHD did not take longer to recover compared with those without ADHD. Additionally, those with ADHD and prior concussions had no recovery differences compared with patients with ADHD and no prior concussion. 15 Even though preexisting ADHD in itself may not be a risk factor for prolonged recovery or worse outcomes after an mTBI continued management of ADHD with medications seems appropriate.
The effect of stimulant medications among patients with preexisting ADHD after a mTBI is limited. While multiple studies found no association with poorer recovery outcomes among patients with ADHD compared with those with ADHD, there is a paucity of data regarding medication status and longer recovery times among patients with preexisting ADHD. A large prospective study by Cook et al 16 is the first to examine concussion recovery among adolescents who were determined to have “treated” versus “untreated” ADHD. In this study, 623 adolescents aged 14 to 19 years were included, with only 14.4% self-reporting preexisting ADHD, and 5.9% of the sample population taking medications for ADHD. The authors concluded that recovery times did not differ based on student-reported medication use for the treatment of ADHD. This study had multiple limitations given the self-reported nature of the investigation, ambiguous criteria of ADHD diagnosis, confounding factors complicating the definition of recovery, unclear meaning of “treated” versus “untreated,” and small number of patients taking medications for the management of ADHD. There is an enormous need for more information to accurately determine the efficacy of continuing or altering medication among patients with preexisting ADHD as they recovery from an mTBI.
Special Populations: Pregnancy
Before prescribing stimulant medication to a post-mTBI reproductive-aged women, one should consider counseling and/or testing for pregnancy.21,49 Stimulant medication to improve cognition post mTBI at this time cannot be recommend as a safe option in the pregnant population.
Special Populations: Athletes
Although there is limited research available on the effects of stimulant medications in the athletic populations, team physicians should be aware of the potential ergogenic and adverse effects of stimulant medications in athletes and the controversy surrounding use.13,25,69,70 Both the American Academy of Pediatrics and the American Heart Association recommend against prescribing stimulant medications in athletes with preexisting cardiac abnormalities and underlying structural heart defects. 69 Team physicians should refer to the regulations governing prescription of stimulant medications to athletes established by various sport organizations such as the International Olympic Committee, World Anti-Drug Agency, and National Collegiate Athletic Association. 31
In all patients, a detailed history and physical examination should be performed before considering prescribing stimulant medications and should be continually monitored throughout use. Weighing the benefits and harms of this medication class to combat cognitive impairment after mTBI must be a mutual decision between the patient and physician. 59
Safety/Adverse Effects
Multiple studies have documented the safety of stimulants in a wide variety of patients, although minor side effects have been reported. Although further studies are needed, those that have assessed the incidence of side effects have generally confirmed that the exacerbation of symptoms is mild, dose dependent, and typically does not lead to medication discontinuation2,25,36,78 (Tables 2 and 3).12,14,18,24,25,34,52,60,66,74
Table 2.
Common/less common adverse effects of stimulant medications a
| Stimulant Adverse Effect | Suggested Intervention | Evidence |
|---|---|---|
| Appetite suppression | Strategies to improve nutrition; dose after meals; high fat intake may delay the onset and increase peak concentrations of some formulations Decrease dose Drug holiday |
(14, 15, 16) Common |
| Xerostomia | Educate on dental hygiene and hydration • Commercial salvia substitute • Saliva stimulation (sugar-free gum, lozenges) |
(20) Common |
| Insomnia | Educate on sleep hygiene, sleep diary Restrict or eliminate caffeine Change/reduce dose, timing, medication, omit or reduce the last dose of the day |
(13, 17, 20, 21) Varies among patients Inconsistent outcomes in children; sleep problems may be more related to attention deficit/hyperactivity disorder symptoms rather than medication |
| Irritability | Consider coexisting mental health conditions or medications Consider changing to extended-release form, a different stimulant medication |
(18, 21) |
| Elevations in heart rate Elevations in blood pressure |
Pretreatment checking and monitoring of pulse and blood pressure Children should be routinely evaluated |
(3, 4, 5) Less common Modest cardiovascular effects: 1. 3-10 beats per minute 2. 3-5 mm Hg |
| Headaches | Determine if symptoms develop: Acute phase (1-2 h after administration)—consider changing to extended-release form or reducing dose. Wean-off phase—consider adding a second dose or changing to extended-release form |
(20, 21) |
| Symptom rebound | Change to sustained-release stimulant | (20, 21) |
The numbers within parentheses refer to the numbers listed in footnote to Table 3.
Table 3.
Rare/theoretical adverse effects of stimulant medicationsa
| Stimulant Adverse Effect | Suggested Intervention | Evidence |
|---|---|---|
| Exacerbation of tics | Observe, change to different stimulant or medication class | (7) Children with tics may benefit from stimulant medication but high doses of dextroamphetamine should be avoided |
| Psychosis/mania | Discontinue medication, refer to mental health specialist | Rare (6) Slightly higher risk of new-onset psychosis with amphetamines than methylphenidate, but absolute risk remains low. |
| Depression/suicide-related events | Discontinue medication, refer to mental health specialist | (20, 21) Rare No causality has been established |
| Cardiovascular | Depends on severity of the adverse effect; consider decreasing dose, discontinue medication, refer to specialist | (1, 2, 20, 21) Stimulant therapy does not appear to increase the risk of sudden unexpected cardiac death or other serious cardiac complications Patients with known cardiac disease or concerns on physical examination should undergo further evaluation |
| Priapism | Discontinue medication | (8, 20,21) Rare Limited studies; uncertain correlation between stimulants and priapism |
| Growth Restriction | Regularly monitor growth in children Strategies to improve nutrition Drug-holiday |
(11, 12, 20, 21) Deceleration of linear growth may occur but appears to attenuate over time Adult height dose not appear to be affected |
| Seizures/epilepsy | Refer to specialist | Rare (9, 10, 20, 21) Low to medium doses of methylphenidate are safe and effective, even in children with active seizures |
aThe numbers in parentheses refer to the following reference list:
Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-1904.
FDA drug safety communication: safety review update of medications used to treat attention-deficit/hyperactivity disorder (ADHD) in children and young adults. Accessed November 7, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm277770.htm
Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50:978-990.
Kelly AS, Rudser KD, Dengel DR, et al. Cardiac autonomic dysfunction and arterial stiffness among children and adolescents with attention deficit hyperactivity disorder treated with stimulants. J Pediatr. 2014;165:755-759.
Hennissen L, Bakker MJ, Banaschewski T, et al. Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs. 2017;31:199-215.
Moran LV, Ongur D, Hsu J, Castro VM, Perlis RH, Schneeweiss S. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019;380:1128-1138.
Osland ST, Steeves TD, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2018;6:CD007990.
US Food and Drug Administration. FDA drug safety communication: FDA warns of rare risk of long-lasting erections in males taking methylphenidate ADHD medications and has approved label changes. Accessed June 25, 2021. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM378835.pdf
Santos K, Palmini A, Radziuk AL, et al. The impact of methylphenidate on seizure frequency and severity in children with attention-deficit-hyperactivity disorder and difficult-to-treat epilepsies. Dev Med Child Neurol. 2013;55:654-660.
Salpekar JA, Mishra G. Key issues in addressing the comorbidity of attention deficit hyperactivity disorder and pediatric epilepsy. Epilepsy Behav. 2014;37:310-315.
Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48:240-248. doi:10.1097/CHI.0b013e318197748f
Harstad EB, Weaver AL, Katusic SK, et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134:e935–e944.
Tirosh E, Sadeh A, Munvez R, Lavie P. Effects of methylphenidate on sleep in children with attention-deficient hyperactivity disorder. An activity monitor study. Am J Dis Child. 1993;147:1313-1315.
Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management, Wolraich M, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
Auiler JF, Liu K, Lynch JM, Gelotte CK. Effect of food on early drug exposure from extended-release stimulants: results from the Concerta, Adderall XR Food Evaluation (CAFE) Study. Curr Med Res Opin. 2002;18:311-316.
Leslie LK, Guevara JP. Attention-deficit/hyperactivity disorder. In: McInerny TK, ed. American Academy of Pediatrics Textbook of Pediatric Care. American Academy of Pediatrics; 2009:1201.
Corkum P, Davidson F, Macpherson M. A framework for the assessment and treatment of sleep problems in children with attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 2011;58:667-683.
Wender EH. Managing stimulant medication for attention-deficit/hyperactivity disorder [published correction appears in Pediatr Rev. 2001;22:292]. Pediatr Rev. 2001;22:183-190.
Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124:680-686.
Stevens JR, Wilens TE, Stern TA. Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges. Prim Care Companion CNS Disord. 2013;15:PCC.12f01472. doi:10.4088/PCC.12f01472
Graham J, Banaschewski T, Buitelaar J, et al. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20:17-37.
The use of stimulant medications for PPCS neurocognitive deficits should be used with caution or not considered in patients presenting with symptoms including somatic (headaches, loss of appetite), vestibular/visual (dizziness), and/or a head injury that was instigated by potential cardiogenic pathology (eg, syncopal episode). While the adverse effects of stimulant medications are rare, dizziness, headaches, and cardiac-related events have been reported. 25 Clinicians should perform a focused history and physical examination in addition to considering additional testing (eg, 12-lead electrocardiogram, echocardiogram, continuous heart monitoring, referral to specialist) in appropriate patients before beginning stimulant medications for the management of PPCS neurocognitive deficits.
Consideration of Mental Health Dysfunction
Since mood symptoms have been demonstrated in approximately half of patients reporting PPCS, clinicians should assess this possible comorbidity of mental health disorders before prescribing stimulant medication in mTBI patients. Silver et al 65 reported a higher likelihood of psychiatric disturbances including suicidal attempts among patients with a prior head injury compared with those without such a history. The development of a depressive syndrome after mTBI has been linked to preexisting mood disorders; nevertheless, clinicians must consider emotional and physiological distress due to financial burden, being held from sport/work, and/or other activities of daily living directly related to injury, and depressive symptoms before a concussion was associated with a greater number of reported symptoms postinjury. 81 Studies have shown that reported personal and family psychiatric disorders in addition to life stressors enhances the odds of developing PPCS after mTBI. 68 Depression has been linked to compromised sleep, slower reaction times, dysfunctional relationships, poor school/work performance, impaired post-mTBI testing outcomes and prolonged recovery.
Patients with diagnosed or undiagnosed ADHD can experience mTBI-like signs in the absence of head injury. Symptoms such as difficulty concentrating, poor academic/work performance, and mood dysregulation may be due to ADHD, inadequate ADHD treatment, or the presence of co-occurring anxiety and depression.15,16,44 Determining mTBI recovery, the definite cause of deficits, and appropriate course of management in such patients is challenging. It is recommended that clinicians use a broad diagnostic perspective and all available resources in these situations.
Boone 10 coined the term neurocognitive hypochondrosis, in which an individual reports cognitive impairment in the absence of any actual objective impairment.6,75 When using self-reported assessments of cognitive deficits, clinicians should be aware that self-reported inadequacies may be more related to physical and emotional factors rather than identifiable cognitive impairments. 58 Cognitive deficits after TBI can be inappropriately diagnosed as an organic pathology related to the injury rather than an overlaying mood dysfunction.
Neuropsychological evaluations typically include a clinical interview with detailed medical and psychological history, symptom review, self-reported measures, and a battery of cognitive testing (eg, assessment of attention, memory, processing speed, executive function). 58 Consideration of psychological comorbidity and its influence on persisting cognitive complaints is an essential aspect of neuropsychological assessments given the considerable overlap of symptomatology with anxiety, depression, and PTSD. 77 Clinicians should utilize resources such as extensive neuropsychological testing conducted by a neuropsychologist, particularly one who is well versed in TBIs, when there is a question of true neurocognitive impairment and whether this impairment is attributable to mTBI.
Stimulants not only assist improved concentration and focus but may also enhance vitality and even physical performance in some patients. While the enhancement of mood seen with stimulant medications should be reflected upon in greater detail outside of this manuscript, clinicians should consider this attribute when prescribing such medications for impaired neurocognitive symptoms post mTBI.
Genetic Considerations
Further research is needed before a change in practice is seen, but genetic predisposition seems to play a role in mTBI with regard to injury extent, recovery, preinjury cognition, and response to treatment with stimulant medications.37,46 The use of pharmacogenomics to optimize drug therapy and avoid adverse effects may be the future of TBI management. 8
Duration of Treatment
Currently, there exist limited data looking specifically at duration of stimulant therapy in neurocognitive deficits associated with mTBI, although studies have shown the potential for improvement in symptoms for up to 30 weeks. 82 General recommendations for the prescription of stimulant therapy is to initiate with the recommended starting dose, use a consistent titration schedule, establish a maximum dose, develop a method for assessing drug response, manage side effects, and schedule routine follow-up for long-term maintenance at least monthly dependent on side effects, compliance, and severity of symptoms. 24
Limitations
There is modest support in the literature for potential benefits of stimulant medications in post mild, moderate, and severe TBI patients with persistent neurocognitive deficits in the areas of attention, working memory, and executive function. This data is much more limited in the mTBI category alone. Furthermore, larger scale randomized, prospective, controlled clinical trials are needed in this area to more completely understand the role of stimulants in mTBI therapy. There are significant inconsistencies in the methodology of current, available RCTs regarding stimulant medication use in mTBI. Larger RCTs with standardized methodology, standardized definition of mTBI, and standardized outcome measures would greatly contribute to our understanding of the effect of stimulants in this patient population. 26 Future research should further investigate the use of combined cognitive rehabilitation and stimulant medication, given benefit seen from previous trials. 43 Further exploration of neuroimaging such as functional magnetic resonance imaging in conjunction with methylphenidate administration could also be beneficial in our understanding of the resulting neuroanatomical changes. 20 Laboratory and statistical genomic sequencing and pharmacogenomics methods will undoubtedly offer future value in the diagnosis and management of prolonged cognitive impairments after head trauma.
Given the extremely limited quantity of prospective, randomized controlled clinical trials for stimulants in mTBI, we did not pursue a formal systematic review structure for our manuscript. When a larger quantity of high quality, prospective literature is available in this domain, a formal systematic review may prove beneficial.
Clinical Recommendations
In appropriate patients, amantadine is a nonstimulant option for the improvement of verbal memory and reaction time for the treatment of persistent post-mTBI neurocognitive deficits.
Methylphenidate can be considered for the management of post-mTBI impaired reaction time, cognitive accuracy, episodic and working memory, attention, executive functioning.
The most pronounced benefit for the treatment of prolonged cognitive deficits post mTBI appears to be stimulant medication plus metacognitive rehabilitation.
Patients with preexisting ADHD taking a stimulant or nonstimulant medication who are experiencing persistent neurocognitive deficits related to mTBI should continue their current medication regimen. Discontinuation or adjustment of medication dose/frequency should be determined on an individual basis.
The use of stimulant medication to improve cognition post mTBI in the pregnant patient should be avoided.
All patients with preexisting cardiac abnormalities, underlying structural heart defects, or other contraindications should not be prescribed stimulant medications for the management of post-mTBI cognitive impairments.
Before prescribing stimulant medications in the athletic population, clinicians must be aware and complete appropriate protocols established by the athlete’s sport organization(s).
Although stimulants are well tolerated in a wide variety of patients, minor side effects have been reported; therefore, education and regular monitoring of adverse effects should be continued for the duration of treatment.
Clinicians must consider comorbid mental health disorders before prescribing stimulant medication in mTBI patients.
When there is a question regarding true neurocognitive impairment and whether this impairment is attributable to mTBI, clinicians should utilize resources such as extensive neuropsychological testing conducted by a neuropsychologist.
Conclusion
Stimulant medications in the treatment of persistent neurocognitive symptoms in patients with mTBI may be useful, but large randomized, controlled clinical trials with uniform patient populations and outcome measures are needed. Improvement in patients is most pronounced when combined with metacognitive rehabilitation. Improvements appear to be most substantiated in reaction time, attention, and working memory with stimulants in mTBI patients with persistent neurocognitive symptoms. Use remains dependent on patient and provider preference. Care must be exercised in stimulant utilization in various populations regarding comorbid mood disorders, potential for misuse, competitive restrictions on medications, potential side effects, and possible performance enhancing potential.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Adeyemo BO, Biederman J, Zafonte R, et al. Mild traumatic brain injury and ADHD: a systematic review of the literature and meta-analysis. J Atten Disord. 2014;18:576-584. [DOI] [PubMed] [Google Scholar]
- 2. Alban JP, Hopson MM, Ly V, Whyte J. Effect of methylphenidate on vital signs and adverse effects in adults with traumatic brain injury. Am J Phys Med Rehabil. 2004;83:131-167. [DOI] [PubMed] [Google Scholar]
- 3. Arciniegas DB, Wortzel HS, Frey KL. Rehabilitation and pharmacotherapy of cognitive impairments. In: Arciniegas DB, Anderson CA, Filley CM, eds. Behavioral Neurology and Neuropsychiatry. Cambridge University Press; 2013:511-542. [Google Scholar]
- 4. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury—an update. Phys Med Rehabil Clin N Am. 2016;27:373-393. [DOI] [PubMed] [Google Scholar]
- 5. Barlow M, Schlabach D, Peiffer J, Cook C. Differences in change scores and the predictive validity of three commonly used measures following concussion in the middle school and high school aged population. Int J Sports Phys Ther. 2011;6:150-157. [PMC free article] [PubMed] [Google Scholar]
- 6. Bass C, Wade DT. Malingering and factitious disorder. Pract Neurol. 2019;19:96-105. [DOI] [PubMed] [Google Scholar]
- 7. Bates G. Medication in the treatment of the behavioural sequelae of traumatic brain injury. Dev Med Child Neurol. 2006;48:697-701. [DOI] [PubMed] [Google Scholar]
- 8. Becquemont L. Pharmacogenomics of adverse drug reactions: practical applications and perspectives. Pharmacogenomics. 2009;10:961-969. [DOI] [PubMed] [Google Scholar]
- 9. Beers SR, Skold A, Dixon CE, Adelson PD. Neurobehavioral effects of amantadine after pediatric traumatic brain injury: a preliminary report. J Head Trauma Rehabil. 2005;20:450-463. [DOI] [PubMed] [Google Scholar]
- 10. Boone KB. Fixed belief in cognitive dysfunction despite normal neuropsychological scores: neurocognitive hypochondriasis? Clin Neuropsychol. 2009;23:1016-1036. [DOI] [PubMed] [Google Scholar]
- 11. Carroll LJ, Cassidy JD, Peloso PM, Garritty C, Giles-Smith L; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Systematic search and review procedures: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 suppl):11-14. [DOI] [PubMed] [Google Scholar]
- 12. Childress AC, Spencer T, Lopez F, et al. Efficacy and safety of dexmethylphenidate extended-release capsules administered once daily to children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:351-361. [DOI] [PubMed] [Google Scholar]
- 13. Clendenin AA, Businelle MS, Kelley ML. Screening ADHD problems in the sports behavior checklist: factor structure, convergent and divergent validity, and group differences. J Atten Disord. 2005;8:79-87. [DOI] [PubMed] [Google Scholar]
- 14. Conklin HM, Lawford J, Jasper BW, et al. Side effects of methylphenidate in childhood cancer survivors: a randomized placebo-controlled trial. Pediatrics. 2009;124:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook NE, Iaccarino MA, Karr JE, Iverson GL. Attention-deficit/hyperactivity disorder and outcome after concussion: a systematic review. J Dev Behav Pediatr. 2020;41:571-582. [DOI] [PubMed] [Google Scholar]
- 16. Cook NE, Iverson GL, Maxwell B, Zafonte R, Berkner PD. Adolescents with ADHD do not take longer to recover from concussion. Front Pediatr. 2021;8:606879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085-1099. [DOI] [PubMed] [Google Scholar]
- 18. Daly B, Kral MC, Brown RT, et al. Ameliorating attention problems in children with sickle cell disease: a pilot study of methylphenidate. J Dev Behav Pediatr. 2012;33:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daly BP, Brown RT. Scholarly literature review: management of neurocognitive late effects with stimulant medication. J Pediatr Psychol. 2007;32:1111-1126. [DOI] [PubMed] [Google Scholar]
- 20. Diagnostic and Statistical Manual of Mental Disorders . 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 21. Diav-Citrin O, Shechtman S, Halberstadt Y, et al. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31:540-545. [DOI] [PubMed] [Google Scholar]
- 22. Faul M, Xu L, Wald MM, Coronado V. Traumatic Brain Injury in the United States. National Center for Injury Prevention and Control, Centers for Disease Control; 2010. [Google Scholar]
- 23. Ferrari R, Rowe BH, Majumdar SR, et al. Simple educational intervention to improve the recovery from acute whiplash: results of a randomized, controlled trial. Acad Emerg Med. 2005;12:699-706. [DOI] [PubMed] [Google Scholar]
- 24. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S. [DOI] [PubMed] [Google Scholar]
- 25. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306:2673-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Wiley-Blackwell; 2008. [Google Scholar]
- 27. Hornyak JE, Nelson VS, Hurvitz EA. The use of methylphenidate in paediatric traumatic brain injury. Pediatr Rehabil. 1997;1:15-17. [DOI] [PubMed] [Google Scholar]
- 28. Huang CH, Huang CC, Sun CK, Lin GH, Hou WH. Methylphenidate on cognitive improvement in patients with traumatic brain injury: a meta-analysis [published correction appears in Curr Neuropharmacol. 2016;14:662]. Curr Neuropharmacol. 2016;14:272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62:535-541. [DOI] [PubMed] [Google Scholar]
- 30. Iaccarino MA, Philpotts LL, Zafonte R, Biederman J. Stimulant use in the management of mild traumatic brain injury: a qualitative literature review. J Atten Disord. 2020;24:309-317. [DOI] [PubMed] [Google Scholar]
- 31. International Olympic Committee. The International Olympic Committee anti-doping rules applicable to the Games of the XXX Olympiad, London. International Olympic Committee; 2012. Accessed June 25, 2021. https://stillmed.olympic.org/Documents/Games_London_2012/Anti-doping/IOC_Anti-Doping_Rules_London%20_2012-eng.pdf
- 32. Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51:941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iverson GL, Wojtowicz M, Brooks BL, et al. High school athletes with ADHD and learning difficulties have a greater lifetime concussion history. J Atten Disord. 2020;24:1095-1101. [DOI] [PubMed] [Google Scholar]
- 34. Jensen PS, Hinshaw SP, Swanson JM, et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22:60-73. [DOI] [PubMed] [Google Scholar]
- 35. Jin C, Schachar R. Methylphenidate treatment of attention-deficit/hyperactivity disorder secondary to traumatic brain injury: a critical appraisal of treatment studies. CNS Spectr. 2004;9:217-226. [DOI] [PubMed] [Google Scholar]
- 36. Johansson B, Wentzel AP, Andréll P, et al. Evaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and pain. Brain Inj. 2014;28:304-310. [DOI] [PubMed] [Google Scholar]
- 37. Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem Res. 2007;32:905-915. [DOI] [PubMed] [Google Scholar]
- 38. Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J Cereb Blood Flow Metab. 1992;12:12-24. [DOI] [PubMed] [Google Scholar]
- 39. Kim J, Whyte J, Patel S, et al. Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: a perfusion fMRI study. Psychopharmacology (Berl). 2012;222:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378. [DOI] [PubMed] [Google Scholar]
- 41. Lundin A, de Boussard C, Edman G, Borg J. Symptoms and disability until 3 months after mild TBI. Brain Inj. 2006;20:799-806. [DOI] [PubMed] [Google Scholar]
- 42. Mahalick DM, Carmel PW, Greenberg JP, et al. Psychopharmacologic treatment of acquired attention disorders in children with brain injury. Pediatr Neurosurg. 1998;29:121-126. [DOI] [PubMed] [Google Scholar]
- 43. Makdissi M, Cantu RC, Johnston KM, McCrory P, Meeuwisse WH. The difficult concussion patient: what is the best approach to investigation and management of persistent (>10 days) postconcussive symptoms? Br J Sports Med. 2013;47:308-313. [DOI] [PubMed] [Google Scholar]
- 44. Mautner K, Sussman WI, Axtman M, Al-Farsi Y, Al-Adawi S. Relationship of attention deficit hyperactivity disorder and postconcussion recovery in youth athletes. Clin J Sport Med. 2015;25:355-360. [DOI] [PubMed] [Google Scholar]
- 45. McAllister TW, Zafonte R, Jain S, et al. Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology. 2016;41:1191-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McAllister TW. Polymorphisms in genes modulating the dopamine system: do they influence outcome and response to medication after traumatic brain injury? J Head Trauma Rehabil. 2009;24:65-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDonald BC, Flashman LA, Arciniegas DB, et al. Methylphenidate and memory and attention adaptation training for persistent cognitive symptoms after traumatic brain injury: a randomized, placebo-controlled trial. Neuropsychopharmacology. 2017;42:1766-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meehan WP, 3rd, d’Hemecourt P, Comstock RD. High school concussions in the 2008-2009 academic year: mechanism, symptoms, and management. Am J Sports Med. 2010;38:2405-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e1-51.e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15:589-598. [DOI] [PubMed] [Google Scholar]
- 51. Narad ME, Kennelly M, Zhang N, et al. Secondary attention-deficit/hyperactivity disorder in children and adolescents 5 to 10 years after traumatic brain injury. JAMA Pediatr. 2018;172:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicholls E, Hildenbrand AK, Aggarwal R, McCarthy L, Daly B. The use of stimulant medication to treat neurocognitive deficits in patients with pediatric cancer, traumatic brain injury, and sickle cell disease: a review. Postgrad Med. 2012;124:78-90. [DOI] [PubMed] [Google Scholar]
- 53. Ornstein TJ, Levin HS, Chen S, et al. Performance monitoring in children following traumatic brain injury. J Child Psychol Psychiatry. 2009;50:506-513. [DOI] [PubMed] [Google Scholar]
- 54. Pangilinan PH, Giacoletti-Argento A, Shellhaas R, Hurvitz EA, Hornyak JE. Neuropharmacology in pediatric brain injury: a review. PM R. 2010;2:1127-1140. [DOI] [PubMed] [Google Scholar]
- 55. Paré G, Trudel MC, Jaana M, Kitsiou S. Synthesizing information systems knowledge: a typology of literature reviews. Inform Manage. 2015;52:183-199. [Google Scholar]
- 56. Ponsford J, Bayley M, Wiseman-Hakes C, et al. INCOG recommendations for management of cognition following traumatic brain injury, part II: attention and information processing speed. J Head Trauma Rehabil. 2014;29:321-337. [DOI] [PubMed] [Google Scholar]
- 57. Poysophon P, Rao AL. Neurocognitive deficits associated with ADHD in athletes: a systematic review. Sports Health. 2018;10:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prince C, Bruhns ME. Evaluation and treatment of mild traumatic brain injury: the role of neuropsychology. Brain Sci. 2017;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Putukian M, Kreher JB, Coppel DB, Glazer JL, McKeag DB, White RD. Attention deficit hyperactivity disorder and the athlete: an American Medical Society for Sports Medicine position statement. Clin J Sport Med. 2011;21:392-401. [DOI] [PubMed] [Google Scholar]
- 60. Rapport MD, Moffitt C. Attention deficit/hyperactivity disorder and methylphenidate. A review of height/weight, cardiovascular, and somatic complaint side effects. Clin Psychol Rev. 2002;22:1107-1131. [DOI] [PubMed] [Google Scholar]
- 61. Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil. 2013;28:260-265. [DOI] [PubMed] [Google Scholar]
- 62. Reitan RM, Wolfson D. The two faces of mild head injury. Arch Clin Neuropsychol. 1999;14:191-202. [PubMed] [Google Scholar]
- 63. Servadei F, Teasdale G, Merry G. Neurotraumatology Committee of the World Federation of Neurosurgical Societies. Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001;18:657-664. [DOI] [PubMed] [Google Scholar]
- 64. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 suppl 2):S359-S368. [DOI] [PubMed] [Google Scholar]
- 65. Silver JM, Kramer R, Greenwald S, Weissman M. The association between head injuries and psychiatric disorders: findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj. 2001;15:935-945. [DOI] [PubMed] [Google Scholar]
- 66. Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19:1802-1808. [DOI] [PubMed] [Google Scholar]
- 67. Tramontana MG, Cowan RL, Zald D, Prokop JW, Guillamondegui O. Traumatic brain injury–related attention deficits: treatment outcomes with lisdexamfetamine dimesylate (Vyvanse). Brain Inj. 2014;28:1461-1472. [DOI] [PubMed] [Google Scholar]
- 68. Trinh LN, Brown SM, Mulcahey MK. The influence of psychological factors on the incidence and severity of sports-related concussions: a systematic review. Am J Sports Med. 2020;48:1516-1525. [DOI] [PubMed] [Google Scholar]
- 69. US Department of Health and Human Resources, US Food and Drug Administration. FDA Drug Facts: stimulant ADHD medications—methylphenidate and amphetamines. Accessed May 25, 2012. http://www.drugabuse.gov/publications/drugfacts/stimulant-adhd-medications-methylphenidate-amphetamines
- 70. Veliz P, Boyd C, McCabe SE. Adolescent athletic participation and nonmedical Adderall use: an exploratory analysis of a performance-enhancing drug. J Stud Alcohol Drugs. 2013;74:714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Voormolen DC, Cnossen MC, Polinder S, von Steinbuechel N, Vos PE, Haagsma JA. Divergent classification methods of post-concussion syndrome after mild traumatic brain injury: prevalence rates, risk factors, and functional outcome.J Neurotrauma. 2018;35:1233-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Webster J, Watson RT. Analyzing the past to prepare for the future: writing a literature review. Manage Inform Syst Q. 2002;26:11. [Google Scholar]
- 73. Whyte J, Hart T, Vaccaro M, et al. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am J Phys Med Rehabil. 2004;83:401-420. [DOI] [PubMed] [Google Scholar]
- 74. Wilens TE, Boellner SW, López FA, et al. Varying the wear time of the methylphenidate transdermal system in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:700-708. [DOI] [PubMed] [Google Scholar]
- 75. Willer BS, Zivadinov R, Haider MN, Miecznikowski JC, Leddy JJ. A preliminary study of early-onset dementia of former professional football and hockey players. J Head Trauma Rehabil. 2018;33:E1-E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williams SE, Ris MD, Ayyangar R, Schefft BK, Berch D. Recovery in pediatric brain injury: is psychostimulant medication beneficial? J Head Trauma Rehabil. 1998;13:73-81. [DOI] [PubMed] [Google Scholar]
- 77. Williams WH, Potter S, Ryland H. Mild traumatic brain injury and Postconcussion Syndrome: a neuropsychological perspective. J Neurol Neurosurg Psychiatry. 2010;81:1116-1122. [DOI] [PubMed] [Google Scholar]
- 78. Willmott C, Ponsford J, Olver J, Ponsford M. Safety of methylphenidate following traumatic brain injury: impact on vital signs and side-effects during inpatient rehabilitation. J Rehabil Med. 2009;41:585-587. [DOI] [PubMed] [Google Scholar]
- 79. World Health Organization. International Statistical Classification of Diseases and Related Health Problems. World Health Organization; 2010. [Google Scholar]
- 80. Yeates KO, Taylor HG, Rusin J, et al. Premorbid child and family functioning as predictors of post-concussive symptoms in children with mild traumatic brain injuries. Int J Dev Neurosci. 2012;30:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yrondi A, Brauge D, LeMen J, et al. Depression and sports-related concussion: a systematic review. Presse Med. 2017;46:890-902. [DOI] [PubMed] [Google Scholar]
- 82. Zhang WT, Wang YF. Efficacy of methylphenidate for the treatment of mental sequelae after traumatic brain injury. Medicine (Baltimore). 2017;96:e6960. [DOI] [PMC free article] [PubMed] [Google Scholar]


