Abstract
Background and Purpose:
A large proportion of ischemic stroke patients lack a definitive stroke etiology despite extensive diagnostic testing. Varicella-Zoster Virus (VZV) can directly invade blood vessels causing vasculitis and may be associated with cryptogenic stroke (CS).
Methods:
We conducted a retrospective cross-sectional study of CS patients tested for VZV. The following were considered evidence of VZV reactivation (VZV+): positive CSF VZV PCR, anti-VZV IgM in CSF, or anti-VZV IgG CSF/serum ratio of 1:10 or higher. We describe the cohort, report VZV+ proportion with 95% confidence intervals (CI) determined with the Wald method, and compare patient groups using standard statistical tests.
Results:
A total of 72 CS patients met full study inclusion criteria. Most of the patients were <65 years old, had few traditional vascular risk factors, and had multifocal infarcts. Mean age was 49 years (SD ±13) and 47% were women. A total of 14 patients (19.4%; CI: 11.4-30.8%) had evidence of CNS VZV reactivation. There was no difference in evaluated demographic or radiographic features between those with versus without evidence of VZV reactivation. History of ischemic stroke in the past year (11/14 vs 25/43, P<.05) and hypertension (13/14 vs 35/58 and P<.05) were associated with VZV+.
Conclusion:
We found a high proportion of CNS VZV reactivation in a cross-sectional cohort of CS patients selected for CSF testing. Testing for VZV might be reasonable in CS patients who are young, have multifocal infarcts, or had an ischemic stroke within the past year, but additional research is needed.
Keywords: ischemic stroke, vasculitis, cryptogenic stroke, varicella zoster virus
Introduction
Cryptogenic stroke (CS) accounts for approximately one third of ischemic strokes. 1 Despite ongoing research, the most cost-effective, accurate, and clinically useful diagnostic evaluation for CS patients is uncertain. 2 Many CS patients do not have atrial fibrillation after prolonged cardiac monitoring. 3 It remains unclear whether additional cardioembolic sources play a role in CS given that broadly inclusive trials of anticoagulation for secondary stroke prevention have not demonstrated benefit as compared to antiplatelet therapy. 3 Other stroke mechanisms such as dissection, non-stenotic atherosclerosis, and infectious vasculopathies are likely under-diagnosed stroke etiologies, particularly in young patients with CS.4-6
Infectious agents have long been implicated in development of atherosclerosis and in increased risk of stroke.7-9 Varicella Zoster Virus (VZV) is a well-established risk factor for stroke and a cause of cerebral vasculitis resulting in acute ischemic stroke.10-12 VZV, a member of the Herpesviridae family, is a globally prevalent human neurotropic virus. 13 Conditions that cause decline in cell-mediated immunity, such as aging or an immunosuppressed state, allow the virus to reactivate and travel peripherally in the neuroaxis to cause herpes zoster (shingles) as well as travel to the central nervous system to cause a variety of neurological complications.12,14 Reactivation of VZV can be difficult to detect clinically as a zoster rash is only noted in slightly more than half of confirmed VZV vasculitis patients. 15
We hypothesize that VZV reactivation in the central nervous system (CNS) might be found in a subgroup of CS patients suggesting a novel stroke etiology. In this study, we report the frequency of CNS VZV reactivation (VZV+) in a subset of CS patients who had VZV testing at our center and explore demographic, clinical, and radiographic features associated with VZV reactivation. To our knowledge, no prior studies have investigated whether or not VZV reactivation can be detected in CS.
Methods
Study Design
We conducted a retrospective cross-sectional cohort study of consecutive CS patients aged 18 years and above who underwent testing for VZV in cerebrospinal fluid (CSF) during their admission for acute ischemic stroke at 2 Montefiore Medical Center (MMC) sites, Montefiore Hospital and Jack D. Weiler Hospital, from 1/1/2016 to 1/1/2018. Both hospitals are located in the Bronx, NY. We initially identified all neurology patients who underwent CSF testing for VZV but only patients with acute ischemic stroke were included in the study. Selection of CS patients for VZV testing was done at the discretion of the treating neurologist, thus not every CS patient seen at MMC with CS had VZV testing. Patients selected for CSF VZV testing were typically young (age <65 years) with an unrevealing comprehensive diagnostic evaluation including at least 24 hours of cardiac rhythm monitoring.
To provide an estimate of the average rate of young (<65 year of age) CS patients seen at our center, we queried the MMC stroke registry from 10/1/2017 to 4/1/2018, which partially overlaps with our study time period. Stroke subtype in the registry was determined at the time of patient hospitalization by the treating neurologist and then confirmed retrospectively via chart review by board certified vascular neurologists (ALL and CCE) using TOAST criteria. 16
The MMC IRB granted a waiver of consent for our cohort study and had approved the MMC stroke registry for quality improvement.
Study Variables
Study variables were abstracted from the electronic medical records by a board-certified neurologist (EB) who was blinded to the results of VZV testing. Demographic variables included patient age, sex, and self-reported race/ethnicity. Clinical variables included history of hypertension, diabetes, hyperlipidemia, stroke/TIA, recent stroke/TIA defined as within 1 year of index admission, autoimmune disease, use of immunosuppressant or disease-modifying antirheumatic drugs (DMARDs), Human Immunodeficiency Virus (HIV), Acquired Immunodeficiency Syndrome (AIDS), and smoking status. We considered patients immunosuppressed if they had AIDS or were taking immunosuppressant medications or DMARDs. Non-contrast MRI brain imaging (diffusion weighted imaging, T1, and fluid attenuated inversion recovery [FLAIR]) was reviewed to determine infarct characteristics. Infarct location was further defined as borderzone, cortical or deep, involving gray or white matter or gray-white matter junction, anterior or posterior circulation, and according to vessel territory corresponding to the infarct location. 17 Infarct territory was further defined according to size of the vessel affected as large, small, or both. Vascular imaging was reviewed to identify large vessel stenoses >50% and severe stenosis was defined as >70% on any vascular imaging modality. Results of CSF testing for the presence of VZV reactivation as well as red blood cell count (RBC), white blood cell count (WBC), protein, and glucose were reported. Elevated WBC was defined as CSF WBC cell count of ≥5 per ul, elevated RBC was defined as CSF RBC cell count of ≥1 per ul, elevated protein was defined as CSF protein >42 mg/dl, and abnormal glucose was defined as >70 mg/dl or <40 mg/dl based on our institution’s results criteria.
VZV Testing
To identify patients with evidence of VZV reactivation in the CNS, enzyme-linked immunosorbent assay (ELISA) based laboratory test for the presence of anti-VZV IgG on paired CSF and serum samples was conducted by Viral Vaccine Preventable Disease Branch, Division of Viral Diseases, National Center for Immunization and Respiratory Disease at the Centers for Disease Control and Prevention in Atlanta, Georgia. ELISA assays were used to detect all antiviral IgG antibodies in serum and CSF. VZV IgG serology was performed as described. 18 Three additional ELISAs were performed to demonstrate VZV specificity: Herpes Select HSV-1 and HSV-2 type-specific IgG ELISAs (Focus Diagnostics, Cypress, CA), and Abnova HHV-6 ELISA (Fisher Scientific, Waltam, MA). All 3 were performed according to the manufacturer’s instructions. The presence of specific VZV antibody in CSF and serum at a ratio 1:1 to 1:10, from samples collected at the same time from the same patient was indicative of VZV-specific intrathecal antibody synthesis and marker of VZV reactivation (CSF adjusted optical density (OD)=.310 and serum adjusted OD =3.100). In addition, all 3 specificity controls needed to be negative in CSF, and at least 1 needed to be seropositive. This method of looking at CSF:serum optical density ratio of 1:10 or higher has been shown to correlate with other measures of VZV-associated neurologic disease and vasculopathies in multiple case report studies.19-25 This assay is distinguished from other systems for the measurement of specific intrathecal antibody by its reliance on 3 separate specificity controls, each of which targets a different herpesvirus that has been associated with neurologic diseases.
In our study, we categorized patients as VZV+ if they had either: (1) positive VZV PCR in CSF, (2) anti-VZV IgG CSF/serum ratio 1:10 or higher using ELISA assays as described above, or (3) the presence of anti VZV IgM in CSF ≥ 1:10 (Quest diagnostics, Nichols Institute).
Statistical Analyses
Standard descriptive statistics were used to describe the study cohort. For measures of VZV+ rate, point estimates and 95% confidence intervals (CIs) were reported using the modified Wald method. Categorical variables were compared between VZV+ and VZV− patients using chi-squared test or Fisher testing when the cell counts were <5. Means (SD) were reported for continuous variables and compared using Student’s t-test. All analyses were conducted using Stata/IC, version 16 (StataCorp, TX). Statistical significance was set at α = .05.
Results
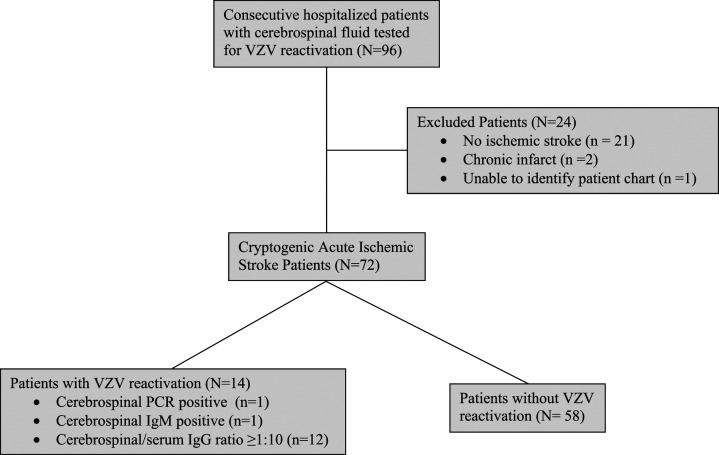
A total of 72 CS patients were included in the final study cohort. We initially identified all patients who underwent CSF testing for VZV but only patients with acute ischemic stroke were included in the final cohort. We excluded 23 patients who had CSF tested for VZV but did not have an acute ischemic stroke as well as 1 patient with an electronic medical record that could not be located (Figure 1). Of the 72 CS patients included in the final study cohort, 4 (5.6%) had intraparenchymal hemorrhages and 3 (4.2%) had subarachnoid hemorrhages associated with acute ischemic infarcts. Mean age was 49 years (SD ±13) and 34 (47%) were women (Table 1).
Figure 1.
Study flow diagram.
Table 1.
Demographics and clinical variables (N = 72).
| VZV+ (n = 14) | VZV−(n = 58) | P-Value | |
|---|---|---|---|
| Age (years) | 52.57 (10.14) | 48.05 (13.53) | 0.2 |
| Sex (female (N, %)) | 8 (57) | 26 (45) | 0.6 |
| Race & ethnicity | |||
| Hispanics n (%) | 7 (50) | 24 (41) | - |
| Non-Hispanics n (%) | 3 (21) | 21 (36) | - |
| Others, n (%) | 2 (14) | 10 (17) | - |
| Missing data, n (%) | 2 (14) | 3 (5) | - |
| Clinical history | |||
| Hypertension, n (%) | 13 (93) | 35 (60) | .02 |
| Hyperlipidemia, n (%) | 5 (36) | 20 (34) | .93 |
| Diabetes Mellitus, n (%) | 6 (43) | 26 (44) | .89 |
| Stroke/TIA, n (%) | 11 (79) | 31 (53) | .09 |
| - Stroke/TIA in 12 months, n (%) | 11 (79) | 25 (43) | .04 |
| Tobacco use, n (%) | 5 (36) | 17 (29) | .64 |
| HIV, n (%) | 0 | 2 (3) | 1.0 |
| Immunosuppressed, n (%) | 3 (21) | 5 (9) | .18 |
| Presenting Features | |||
| Rash | 1 (7) | 0 | .19 |
| Headache | 1 (.07) | 18 (31) | .09 |
| Treatment with IV Acyclovir | 11 (79%) | 8 (14) | <.001 |
A total of 14 (19.4% 95% CI: 11.4-30.8) patients were VZV+ (Figure 1). There were no differences in demographic factors between VZV+ and VZV− groups (Table 1). VZV+ patients were more likely to have history of hypertension (VZV+93% vs VZV-60%; P = .02; Table 1) and stroke/TIA within 12 months prior to index admission (79% vs 43%; P < .05). Most patients were not immunocompromised and did not have typical clinical signs of VZV reactivation; rash was only present in 1 VZV+ patient with thoracolumbar zoster.
Radiographic features, presented in Table 2, did not differ between VZV+ and VZV− patients, including infarction at the gray-white matter junction (16 patients, 22%), multifocal infarction (47 patients, 65%), and borderzone infarct location (15 patients, 22%). All study patients had noninvasive vessel imaging performed, either CT angiogram or MR angiogram, and 31 (43%) also underwent digital subtraction angiography. A total of 32 (44%) patients had evidence of significant vessel stenosis on imaging and the proportion did not differ between VZV+ and VZV− groups (Table 2). Two study patients (1 VZV+ and 1 VZV−) did not have any CSF studies performed aside from VZV testing. Among the 70 patients with routine CSF studies sent, there were no differences in elevated cell counts, elevated protein, or abnormal glucose values between VZV+ and VZV− groups (Table 3).
Table 2.
Radiographic variables (N = 72).
| VZV+ (n = 14) | VZV−(n = 58) | P-Value | |
|---|---|---|---|
| Infarct at gray-white junction, n (%) | 4 (29) | 12 (21) | .49 |
| Multifocal infarcts, n (%) | 10 (71) | 37 (64) | .52 |
| Border zone infarcts, n (%) | 2 (14) | 13 (22) | .72 |
| White matter disease, n (%) | 11 (79) | 51 (88) | .39 |
| Anterior circulation, n (%) | 9 (64) | 38 (66) | .76 |
| Vessel stenosis, n (%) | 6 (43) | 26 (45) | .89 |
Table 3.
Cerebrospinal fluid analysis (N = 70).
| VZV+ (n = 13) | VZV− (n = 57) | P-Value | |
|---|---|---|---|
| WBC ≥5, n (%) | 1 (8) | 4 (7) | 1.0 |
| RBC ≥1, n (%) | 10 (77) | 33 (58) | .3 |
| Protein >42 mg/dl, n (%) | 3 (23) | 32 (56) | .06 |
| Glucose >70 mg/dl or <40 mg/dl, n (%) a | 5 (38) | 29 (51) | .6 |
A single patient in the VZV- group had CSF glucose <40; the rest had glucose >70 mg/dl.
Eleven VZV+ patients received 2 weeks of treatment with intravenous acyclovir. In the VZV− group, 7 patients received above treatment prior to CSF results returned due to clinical suspicion for VZV reactivation (Table 1). Of the 3 VZV+ patients who did not receive treatment, 1 was made comfort care and 2 were lost to follow up.
The MMC registry identified 164 patients with confirmed CS admitted over a 6-month period. Of those CS patients, 60 (60/164; 36.6%) were <65 years of age and thus similar to our study cohort. The monthly rate of CS patients <65 years of age at our center in the registry data was 10 patients per month. The monthly rate of CS patients evaluated for VZV based in our primary study cohort was 3 patients per month. We therefore estimate that approximately 1/3 of young CS patients seen at our center had their CSF tested for evidence of VZV during the main study period.
Discussion
In a single center retrospective cross-sectional cohort study of CS patients who underwent CSF VZV testing, nearly 1 in 7 had evidence of VZV CNS reactivation. Our cohort consisted of mostly young patients with few traditional vascular risk factors, multifocal infarcts on imaging, the majority of whom were not immunocompromised and did not have other clinical signs of VZV reactivation including zoster rash. A history of stroke within 1 year prior to index event and hypertension were the only 2 clinical factors associated with being VZV+. There were no differences in pre-specified demographic or radiographic features between VZV+ and VZV-groups.
This is the first study that we are aware of to investigate the rate of CNS VZV reactivation in a subset of CS patients. Knowledge of clinical and radiographic features of VZV vasculopathy is limited and mainly based on case reports and a large case series of 30 virologically confirmed patients with CNS VZV reactivation.15,25-27 Among the 30 confirmed cases, VZV reactivation was associated with infarcts in both deep and cortical areas, particularly in gray-white matter junction. 15 Among VZV+ patients, we similarly found that nearly 1/3 had infarcts at gray-white matter junction. Our finding that nearly 70% of VZV+ patients had multifocal infarcts is also consistent with prior literature as multiple vessels are often involved in VZV vasculitis.15,28
The prior large case series of stroke patients with VZV CNS reactivation focused on stroke patients with recent zoster rash and found VZV vasculopathy in 63% of patients with rash. 15 In contrast, only 1 VZV+ patient in our cohort had preceding zoster rash. Additionally, few had significant CSF pleocytosis unlike prior work.15,29 Our findings are consistent with the fact that neither the presence of rash nor CSF pleocytosis is required to diagnose CNS VZV reactivation. 15
Interestingly, we found that CS patients who had an ischemic stroke/TIA within a year of index admission were more likely to be VZV+. While we lack detailed data on prior events for all patients in our study, including stroke etiology, it is possible that ischemic events in the past year might have also been associated with CNS VZV reactivation. Population-based studies have shown that stroke risk is highest short term (<1 year) after zoster as opposed to long-term (>1 year). 28 In children, VZV vasculopathy is usually a monophasic disease. 30 Data are limited regarding the temporal course of VZV vasculopathy in adults, but recurrent cerebrovascular events associated with VZV reactivation have been reported. 31 Both the risk of stroke after CNS VZV reactivation and the association between previous stroke within 1 year of CS in VZV+ patients requires further study since, to our knowledge, these relationships have not been previously explored in a systematic fashion.
Our study has several limitations. First, selection bias is possible and generalizability may be limited because we report results from a single institution where CSF testing for VZV in CS patients was not a protocolized or standardized practice; patient selection for CSF testing was at the discretion of the treating neurologist. We therefore do not know the prevalence of VZV reactivation among all CS patients hospitalized during the study period nor the clinical characteristics of CS patients who did not undergo CSF testing. Based on our stroke registry data, we estimate that approximately 1/3 of young CS patients seen at MMC underwent CSF evaluation during our primary study period. Second, the clinical accuracy of the VZV reactivation classification schema that we used has not been robustly established. Although the CDC uses a strong specificity control to look for anti-VZV antibody production in CSF, given the lack of rash and readily accessible tissue to analyze, it is difficult to define VZV reactivation in the CNS. Third, sample size is small with a total of 72 patients analyzed and only 14 with CNS VZV reactivation. We conducted several pre-specified tests for significant differences between the VZV+ and VZV− groups without adjustment or correction for multiplicity so the differences between groups that we found thus may be the result of multiple testing. 32 Fourth, we do not have radiographic data on presence of micro bleeds or areas of contrast enhancement in our cohort. Additional MRI sequences might have been helpful to better characterize the radiographic differences between groups, as prior work has shown that infarcts from VZV vasculitis may enhance.29,33 Finally, we did not use vessel wall imaging to evaluate for vasculitis which is a promising non-invasive method that can be used to clarify ischemic stroke mechanism. 34
Conclusion
We found a high proportion of CNS VZV reactivation in a cross-sectional cohort of CS patients who underwent CSF testing. Based on our findings, CS patients who are <65 year of age, have few traditional vascular risk factors, have multifocal infarcts, and a prior stroke within a year may be a reasonable subgroup of CS patients to undergo CSF testing for VZV reactivation. Future prospective studies to determine the prevalence of VZV reactivation in patients with CS and to identify additional factors associated with reactivation may be warranted.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Liberman Receives Research Support from National Institutes of Health Grant K23NS107643.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Diseases Control and Prevention.
Authors’ Note: The Study Was Performed in the Saul R. Korey Department of Neurology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA
Disclosures: Dr. Ekaterina Bakradze has nothing to disclose. Dr. Charles C. Esenwa has nothing to disclose. Dr. D. Scott Schmid has nothing to disclose. Dr. Kathryn F. Kirchoff-Torres has nothing to disclose. Dr. Daniel Antoniello has nothing to disclose. Dr. Peter C. Mabie has nothing to disclose. Dr. Daniel L. Labovitz has nothing to disclose. Ms. Congrong Miao has nothing to disclose. Dr. Ava L. Liberman has nothing to disclose.
ORCID iDs
Ekaterina Bakradze https://orcid.org/0000-0001-7534-7218
Daniel Antoniello https://orcid.org/0000-0002-4680-7948
References
- 1.Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14(9):903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saver JL. Cryptogenic stroke. N Engl J Med. 2016;375(11):e26. [DOI] [PubMed] [Google Scholar]
- 3.Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the approach to embolic stroke of undetermined source: a review. JAMA Neurol. 2019;76(7):855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Gialdini G, Lerario MP, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4(6):e002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedeltchev K, der Maur TA, Georgiadis D, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76(2):191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkind MS, Ramakrishnan P, Moon YP, et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol. 2010;67(1):33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34(10):2518-2532. [DOI] [PubMed] [Google Scholar]
- 9.Lian Y, Zhu Y, Tang F, Yang B, Duan R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: a systematic review and meta-analysis. PLoS One. 2017;12(2):e0171182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40(11):3443-3448. [DOI] [PubMed] [Google Scholar]
- 11.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74(10):792-797. [DOI] [PubMed] [Google Scholar]
- 12.Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27(3):356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol. 2011;6(3):341-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakradze E, Kirchoff KF, Antoniello D, et al. Varicella Zoster Virus Vasculitis and Adult Cerebrovascular Disease. Neurohospitalist. 2019;9(4):203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70(11):853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams HP, Jr., Woolson RF, Clarke WR, et al. Design of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Control Clin Trials. 1997;18(4):358-377. [DOI] [PubMed] [Google Scholar]
- 17.Mangla R, Kolar B, Almast J, Ekholm SE. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics. 2011;31(5):1201-1214. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MA, Kruszon-Moran D, Jumaan A, Schmid DS, McQuillan GM. Varicella seroprevalence in the U.S.: data from the National Health and Nutrition Examination Survey, 1999-2004. Public Health Rep. 2010;125(6):860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young-Barbee C, Hall DA, LoPresti JJ, Schmid DS, Gilden DH. Brown-Sequard syndrome after herpes zoster. Neurology. 2009;72(7):670-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haug A, Mahalingam R, Cohrs RJ, Schmid DS, Corboy JR, Gilden D. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system: Case report and review of the literature. J Neurol Sci. 2010;292(1-2):85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash in an immunocompetent woman. J Neurovirol. 2009;15(2):206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel MA, Russman AN, Feit H, et al. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology. 2013;80(2):220-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf J, Nagel MA, Mahalingam R, Cohrs RJ, Schmid DS, Gilden D. Chronic active varicella zoster virus infection. Neurology. 2012;79(8):828-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68(4):517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2 years’ duration. J Neurol Sci. 2012;323(1-2):245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CF, Hong CT, Lee WH, Wu D, Hu CJ, Chung CC. Disseminated cutaneous herpes zoster and multiple cerebral infarcts in an adult with diabetes mellitus. J Neurovirol. 2020;26(1):130-132. [DOI] [PubMed] [Google Scholar]
- 27.Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Engl J Med. 2002;347(19):1500-1503. [DOI] [PubMed] [Google Scholar]
- 28.Sreenivasan N, Basit S, Wohlfahrt J, et al. The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One. 2013;8(7):e69156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katchanov J, Siebert E, Klingebiel R, Endres M. Infectious vasculopathy of intracranial large- and medium-sized vessels in neurological intensive care unit: a clinico-radiological study. Neurocritical Care. 2010;12(3):369-374. [DOI] [PubMed] [Google Scholar]
- 30.Lanthier S, Armstrong D, Domi T, deVeber G. Post-varicella arteriopathy of childhood: natural history of vascular stenosis. Neurology. 2005;64(4):660-663. [DOI] [PubMed] [Google Scholar]
- 31.Liberman AL, Nagel MA, Hurley MC, Caprio FZ, Bernstein RA, Gilden D. Rapid development of 9 cerebral aneurysms in varicella-zoster virus vasculopathy. Neurology. 2014;82(23):2139-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest. 2011;140(1):16-18. [DOI] [PubMed] [Google Scholar]
- 33.Cheng-Ching E, Jones S, Hui FK, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci. 2015;351(1-2):168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]