Abstract
Background and Purpose
The purpose is to determine the impact of an academic neurohospitalist service on clinical outcomes.
Methods
We performed a retrospective, quasi-experimental study of patients discharged from the general neurology service before (August 2010–July 2014) and after implementation of a full-time neurohospitalist service (August 2016–July 2018) compared to a control group of stroke patients. Primary outcomes were length of stay and 30-day readmission. Using the difference-in-difference approach, the impact of introducing a neurohospitalist service compared to controls was assessed with adjustment of patients’ characteristics. Secondary outcomes included mortality, in-hospital complications, and cost.
Results
There were 2706 neurology admissions (1648 general; 1058 stroke) over the study period. The neurohospitalist service was associated with a trend in reduced 30-day readmissions (ratio of ORs: .52 [.27, .98], P = .088), while length of stay was not incrementally changed in the difference-in-difference model (-.3 [-.7, .1], P = .18). However, descriptive results demonstrated a significant reduction in mean adjusted LOS of .7 days (4.5 to 3.8 days, P < .001) and a trend toward reduced readmissions (8.9% to 7.6%, P = .42) in the post-neurohospitalist cohort despite a significant increase in patient complexity, shift to higher acuity diagnoses, more emergent admissions, and near quadrupling of observation status patients. Mortality and in-hospital complications remained low, patient satisfaction was stable, and cost was not incrementally changed in the post-neurohospitalist cohort.
Conclusions
Implementation of a neurohospitalist service at an academic medical center is feasible and associated with a significant increase in patient complexity and acuity and a trend toward reduced readmissions.
Keywords: Neurohospitalist, outcomes, quality, safety, length of stay
Introduction
The hospitalist model of inpatient care brought a fundamental shift in the practice of acute care medicine and has been followed by the emergence of specialty hospitalists, including neurohospitalists. Neurohospitalists comprise a rapidly growing specialty area of neurology. Since its inception in 2010, the Neurohospitalist Section of the American Academy of Neurology has grown to over 1000 members who practice in community and academic settings. 1 As of 2014, 38% of neurology departments nationally employed neurohospitalists and 32% planned to hire more. 2
Neurohospitalists practice in diverse settings, including hospital group practice, academic medical centers, telehealth, and locum tenens. The services staffed by neurohospitalists also vary, with some serving primary functions in acute stroke care, including neuro-interventional cases, and critical care consultation. Other programs have adopted consultative or co-management models of care in collaboration with internal medicine hospitalist services. 3 At our institution, in addition to staffing the general neurology consult service, neurohospitalists serve as the primary admitting attending for the general neurology service.
Although neurohospitalist programs have been adopted rapidly in recent years, the last major study to evaluate the outcomes of program implementation at an academic medical center was published a decade ago. 4 In this study, we sought to determine the impact of a neurohospitalist service on clinical outcomes.
Methods
Study Design
We conducted a retrospective, quasi-experimental study with a difference-in-difference (DID) design comparing changes in clinical outcomes associated with implementation of a neurohospitalist model to controls on a separate stroke service. We chose the stroke service as a control group because of a shared geographic unit and similarities in patient population and team structure to the general neurology service.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Stanford University institutional review board (Protocol 48647)
Background and Setting
The pre-neurohospitalist general neurology inpatient service was staffed by a rotating pool of 24–26 faculty, each attending for 1–3 weeks per year. This role involved serving as the primary attending for the general neurology inpatient ward service and concurrently staffing the general neurology consult service. The inpatient general neurology attending would also continue to staff some of their outpatient clinics and depending on subspecialty, the inpatient stroke, and/or epilepsy services.
At our institution, stroke patients are admitted to a separate stroke service, elective admissions for electroencephalography are admitted to the epilepsy monitoring service, and there is a separate neurocritical service that cares for all neurology patients in the intensive care unit (ICU). There was no change to this division of services during the study period.
Intervention
On August 1, 2014, Stanford piloted a neurohospitalist model for the general neurology ward and consult services. By August 1, 2016, the inpatient general neurology service had fully reorganized to a teaching service staffed by 3–4 neurohospitalist attendings 51 weeks per year. Each neurohospitalist had completed either a neurohospitalist or stroke fellowship and was on service a minimum of 8 weeks each year. While on service, the neurohospitalist attending’s only clinical responsibilities were the inpatient ward service and general neurology consult service. After conducting morning rounds on both services, the on-service neurohospitalist was present throughout the afternoon to attend family meetings, supervise procedures, staff new admissions and consultations, and attend afternoon case management rounds.
Study Population
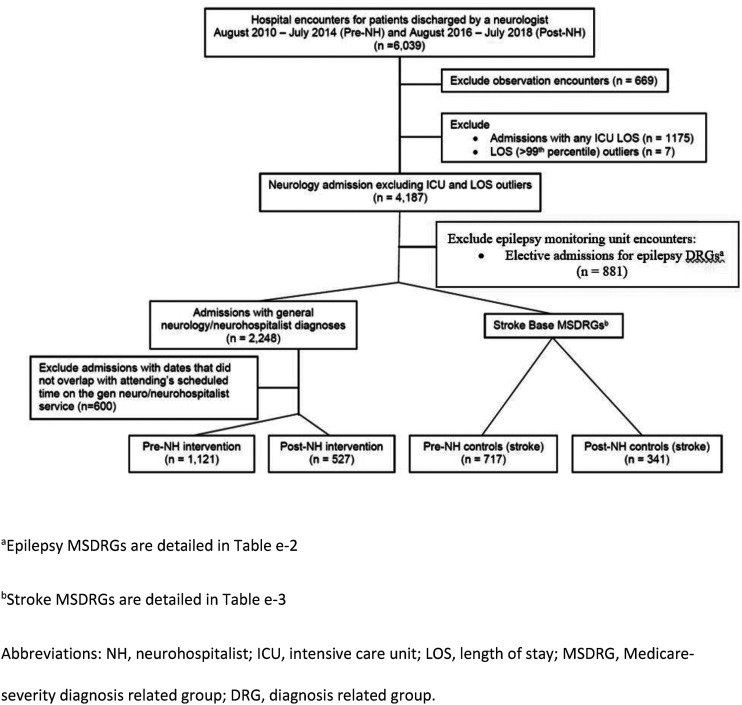
We used our institutional finance database to identify neurology patients by “attending physician” (Figure 1). Observation status patients were excluded from analysis; since they are considered outpatients by most payors and with the shorted length of stay, there is less opportunity for the neurohospitalist model to impact their overall care. This method is similar to other studies.4–7 Patients in either group with any ICU days were excluded since the clinical decision-making in our “closed” ICU may impact the outcomes of interest and our intervention was unlikely to affect patients’ care while they were in the ICU. Length of stay (LOS) outliers (> 99th percentile from the mean within each Medicare-Severity Diagnosis-Related Group (MSDRG)) were excluded to avoid a skewed distribution.4,8–10 Because attendings subspecializing in epilepsy would occasionally concurrently round on both the general neurology and epilepsy services in the pre-neurohospitalist period, we excluded patients with epilepsy diagnosis-related groups (DRGs) and admission type “elective” (Supplementary Table e-2). Stroke controls were identified based on “attending physician” name matching one of our known stroke division faculty and stroke-specific DRGs (Supplementary Table e-3). Of the remaining encounters, “attending physician” and hospitalization dates were matched to the rounding schedules and only encounters where there was overlap in dates for the assigned attending were included. “Attending physician” refers to the attending who discharged the patient. We validated the inclusion and exclusion criteria in two hundred random encounters and found 99% accuracy (198/200) in identifying the correct service.
Figure 1:
Study flow diagram.
The pre-neurohospitalist (pre-NH) period was defined as patients discharged from August 2010 to July 2014. The post-neurohospitalist (post-NH) period included discharges from August 2016 to July 2018. We excluded the August 2014–July 2016 transition period, during which there were 1–2 neurohospitalists and a combination of neurohospitalist and traditional neurology attending coverage.
Demographic information including age, sex, insurance carrier, primary DRG, medical comorbidities as defined by ICD9/ICD10 codes, Charlson Comorbidity Index, case mix index (CMI), severity of illness indicator (3M™ All Patients Refined Diagnosis Related Groups; APR-DRG), admission source, and discharge disposition was collected from the hospital finance database.11-14
Outcomes
The primary outcomes were length of stay and 30-day readmission. Secondary clinical and cost outcomes included: in-hospital mortality, incidence of any in-hospital complication, direct cost, lab cost, pharmacy cost, imaging cost, imaging use, and whether there were two or more medical consultants during the inpatient stay. 5
Clinical and cost outcomes were obtained from the hospital billing database with the exception of readmissions which were obtained from hospital quality reporting. LOS was measured in days. All unplanned readmissions within 30 days to any service at our institution were included. 15 In-hospital complications were selected a priori based on frequency in the hospitalized neurology population.16,17 We prioritized complications for which incidence might be directly influenced by the primary neurology service attending. ICD-9/ICD-10 codes were used to identify complications. 5 Conditions that were present on admission were excluded. Total direct costs attributed to the encounter were included and were adjusted for inflation (Consumer Price Index for medical costs) and reported in 2018 dollars. 18 Direct costs related to lab, pharmacy, and imaging associated with each patient were also included and imaging use (proportion of encounters with any imaging) was calculated. Patient satisfaction was an additional secondary outcome. Patient satisfaction data was obtained from Hospital Consumer Assessment of Healthcare Services (H-CAHPS) and Press Ganey survey responses. We looked topbox scores (percent of patients who rated 5 on a 5-point Likert scale) for overall likelihood to recommend the hospital and the 8 physician-specific subdomains (see Supplementary Table e-5).
Statistical Analysis
Descriptive statistics were compared for patients’ demographic and clinical characteristics by pre-NH neurology service, post-NH neurology service, pre-NH stroke service, and post-NH stroke service. The unit of analysis was the hospital encounter and multiple encounters for the same patient were included. For non-normal distributed continuous variables, median and interquartile range (IQR) were reported. To compare the difference between pre-NH and post-NH group for either neurology service or stroke service, standardized mean difference (SMD), which is the mean difference between the two groups divided by the pooled standard deviation, was reported for each variable. For categorical variables, means of each group were the proportions of patients who had the event whereas pooled standardized deviations of the two groups were calculated assuming binomial distributions. The higher the SMD, the greater the magnitude of difference between the two groups. Cohen offered the following guidelines for interpreting the magnitude of the SMD: small, SMD = .2; medium, SMD = .5; and large, SMD = .8.19,20
In the DID analysis, generalized estimating equation (GEE) models were used to estimate the impact of neurohospitalist program implementation on the study outcomes. A GEE model was fit for each outcome, adjusting for patients' age, sex, primary insurance, Charlson Comorbidity Index and quarter of discharge. With the inclusion of a clustering variable of the primary attendings, we accounted for intra-provider correlations in our standard error estimates. For binary outcomes, we applied logit link with binomial distribution. LOS, however, was assumed to follow gamma distribution so that our estimates were robust to skewed distribution with outliers. Specifically, for each study outcome, we fit a GEE regression model on an indicator for the study period (post-NH vs pre-NH), an indicator for service group (general neurology service as the intervention group vs stroke service as the control) and interaction of the 2, adjusting for patients’ characteristics and clustering within provider. DID estimators for our study are interpreted as the difference in the changes of the outcomes from pre-NH period to post-NH period for the general neurology service compared to the stroke service control. A check of the parallel trend assumption across all our primary outcomes demonstrated the stroke cohort to be a suitable control (Supplementary Figure e1a-e). We adjusted familywise type I error for multiple comparisons on the primary outcomes using Benjamini’s method that controls for false discovery rate (FDR). 21 Adjusted P < .05 was interpreted as statistically significant. We performed exploratory analysis to obtain absolute estimates of length of stays accounting for the potential difference in patients’ clinical and demographic characteristics by time periods and service groups. Specifically, we reported predicted population margins and the corresponding 95% confidence intervals from the DID model. 22
We performed an additional sensitivity analysis to determine whether inclusion of observation status patients would affect our primary outcomes. Because observation stays are not associated with a DRG, we were not able to distinguish whether individual patients cared for by attendings who rotated on multiple services were in the intervention, control or excluded (EMU) group as rigorously as our primary analysis. Patients discharged by a stroke attending were all included in the intervention cohort, patients cared for by an epilepsy attending were excluded and only those patients cared for by other neurology attendings were included in the intervention cohort.
All analyses were performed using R statistical programming languages, version 3.4.3. 23
Results
Study Cohort and Patient Characteristics
A total of 2706 neurology service admissions (1648 general; 1058 stroke) occurred during the study period. Table 1 presents characteristics of included patients in the intervention and control groups. In both the intervention and stroke control post-NH groups, as compared to the pre-NH period, patients were significantly more likely to have a managed medical insurance (Medicaid/MediCal or Medicare), higher Charlson Comorbidity Index, higher CMI, higher APR-DRG severity of illness, and were less likely to be discharged home. As compared to pre-intervention, patients in the post-NH intervention cohort were significantly older (median [IQR]: 50 years [34, 66] vs 56 [38, 69], SMD = .18, P = .001), have an admission source of “emergency” and less likely to be “clinic/direct admit” (emergency 62.4% vs 75.9% and clinic/direct admit 20.1% vs 6.6%, SMD=.42, P < .001).
Table 1.
Patient characteristics before and after neurohospitalist intervention.
| Intervention (neurohospitalist) | Control (stroke) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre (n = 1121) | Post (n = 527) | SMD a | P-value | Pre (n = 717) | Post (n = 341) | SMD a | P-value | |
| Age (%), y | ||||||||
| Median [IQR] | 50 [34, 66] | 56 [38, 69] | .18 | .001 | 75 [62, 84] | 74 [60, 85] | .05 | .52 |
| Male, % | 492 (43.9) | 262 (49.7) | .12 | .03 | 366 (51.0) | 165 (48.4) | .05 | .46 |
| Primary insurance (%) | .31 | <.001 | .28 | <.001 | ||||
| Medicare | 363 (32.4) | 218 (41.4) | 467 (65.1) | 213 (62.5) | ||||
| Medicaid/Medi-Cal | 167 (14.9) | 84 (15.9) | 58 (8.1) | 52 (15.2) | ||||
| Private | 525 (46.8) | 219 (41.6) | 163 (22.7) | 72 (21.1) | ||||
| Other | 66 (5.9) | 6 (1.1) | 29 (4.0) | 4 (1.2) | ||||
| Charlson Comorbidity Index | .38 | <.001 | .79 | <.001 | ||||
| 0 (none) | 668 (59.6) | 227 (43.1) | 4 (.6) | 5 (1.5) | ||||
| 1-2 (mild) | 330 (29.4) | 185 (35.1) | 478 (66.7) | 103 (30.2) | ||||
| 3-4 (moderate) | 80 (7.1) | 69 (13.1) | 171 (23.8) | 165 (48.4) | ||||
| >=5 (severe) | 43 (3.8) | 46 (8.7) | 64 (8.9) | 68 (19.9) | ||||
| Median [IQR] | 0 [0,1] | 1 [0, 2] | .35 | <.001 | 2 [1, 3] | 3 [2, 4] | .66 | <.001 |
| Top DRG b (%) | .42 | <.001 | .86 | <.001 | ||||
| Seizures | 188 (16.8) | 96 (18.2) | ||||||
| Degenerative disorders | 80 (7.1) | 60 (11.4) | ||||||
| Nonbacterial CNS infections excluding viral meningitis | 46 (4.1) | 52 (9.9) | ||||||
| Nervous system neoplasms | 81 (7.2) | 41 (7.8) | ||||||
| MS or Cerebellar ataxia | 75 (6.7) | 27 (5.1) | ||||||
| Other diseases of nervous system | 52 (4.6) | 27 (5.1) | ||||||
| Bacterial Tb of nervous system | 49 (4.4) | 25 (4.7) | ||||||
| Neurologic eye disease | 37 (3.3) | 24 (4.6) | ||||||
| Cranial or Peripheral nerve | 47 (4.2) | 20 (3.8) | ||||||
| Headache | 115 (10.3) | 17 (3.2) | ||||||
| ICH or stroke | 639 (89.1) | 269 (78.9) | ||||||
| Acute stroke with thrombolytic | 8 (1.1) | 32 (9.4) | ||||||
| TIA | 40 (5.6) | 10 (2.9) | ||||||
| Case Mix Index (CMI) | ||||||||
| Mean (sd) | 1.1 (.6) | 1.4 (.9) | .43 | <.001 | 1.1 (.4) | 1.4 (.5) | .60 | <.001 |
| APR DRG (%) | .46 | <.001 | .58 | <.001 | ||||
| Extreme | 34 (3.0) | 46 (8.7) | 22 (3.1) | 40 (11.7) | ||||
| Major | 270 (24.1) | 199 (37.8) | 191 (26.6) | 128 (37.5) | ||||
| Moderate | 510 (45.5) | 202 (38.3) | 379 (52.9) | 159 (46.6) | ||||
| Minor | 302 (26.9) | 78 (14.8) | 119 (16.6) | 14 (4.1) | ||||
| Missing | 5 (.4) | 2 (.4) | 6 (.8) | 0 (.0) | ||||
| Weight, mean (sd) | .9 (.7) | 1.0 (.8) | .21 | <.001 | 1.0 (.4) | 1.2 (.6) | .46 | <.001 |
| Admission source (%) | .42 | <.001 | .24 | .006 | ||||
| Hospital transfers | 193 (17.2) | 92 (17.5) | 105 (14.6) | 73 (21.4) | ||||
| Emergency/trauma | 700 (62.4) | 400 (75.9) | 592 (82.6) | 288 (78.0) | ||||
| Clinic/direct admit | 225 (20.1) | 35 (6.6) | 18 (2.5) | 2 (.6) | ||||
| Other | 3 (.3) | 0 (.0) | 2 (.3) | 0 (.0) | ||||
| Discharge disposition (%) | .37 | <.001 | .43 | <.001 | ||||
| Home (routine/with care) | 889 (79.3) | 337 (63.9) | 471 (65.7) | 162 (47.5) | ||||
| Other acute care facility/hospital | 97 (8.7) | 88 (16.7) | 74 (10.3) | 77 (22.6) | ||||
| Skilled nursing facility (SNF) | 93 (8.3) | 79 (15.6) | 116 (16.2) | 71(20.8) | ||||
| Hospice | 30 (2.7) | 20 (3.8) | 31 (4.3) | 22 (6.5) | ||||
| In-hospital death | 4 (.4) | 2 (.4) | 17 (2.4) | 7 (2.1) | ||||
SMD: standardized mean difference; IQR: interquartile range; DRG: diagnosis-related group, CNS: central nervous system; MS: multiple sclerosis; Tb: tuberculosis; ICH: intracranial hemorrhage; APR-DRG: 3M™ All Patients Refined Diagnosis-Related Groups; SNF: skilled nursing facility.
aThe higher the SMD, the greater the magnitude of difference between the two groups. Cohen offered the following guidelines for interpreting the magnitude of the SMD: small, SMD = .2; medium, SMD = .5; and large, SMD = .8.19,20
bRepresentative diagnoses include (see Table e-1): Degenerative disorders—myasthenia gravis, amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s dementia, and dementia with Lewy bodies; nonbacterial CNS infections—viral encephalitis, fungal meningitis, acute disseminated encephalomyelitis, transverse myelitis, and autoimmune encephalitis; bacterial Tb of the nervous system—Guillain-Barre syndrome and intracranial abscess
There was a significant shift in DRG (P < .001) with notably fewer headache and more degenerative disorders and nonbacterial central nervous system (CNS) infections excluding viral meningitis (headache pre 10.3% to post 3.2%, CNS infection pre 4.1% to post 9.9%, and degenerative disease pre 7.1% to post 11.4%) (Table 2 and Supplementary Table e-1). There was no significant change in patient age pre-NH vs post-NH period in the stroke controls, but there was a shift to more transfers (14.6% to 21.4%). On the stroke service, there was a shift in DRG toward more stroke with thrombolytics (pre 1.1% to post 9.4%) and fewer TIAs (TIA pre 5.6% to post 2.9%, SMD .86, P < .001).
Table 2.
Clinical outcomes of neurohospitalist intervention and controls.
| Intervention (neurohospitalist) | Control (stroke) | DID estimator | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre (n = 1121) | Post (n = 527) | P-value | Pre (n = 717) | Post (n = 341) | P-value | Est. | 95% CI | P-value a | ||
| Lower | Upper | |||||||||
| Length of stay, median (IQR) | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.5) | .02 | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | .11 | -.30 b | -.74 | .13 | .18 |
| 30-day readmissions (%) | 100 (8.9) | 40 (7.6) | .05 | 36 (5.0) | 27 (7.9) | .12 | .52 c | .27 | .98 | .09 |
DID: difference-in-difference; SMD: standardized mean difference; IQR: interquartile range; Est: estimator; NH: neurohospitalist; GEE: generalized estimating equation; OR: odds ratio.
aP-values were adjusted for family-wise type I error using Benjamini’s method that controls for false discovery rate. Adjusted P-value<.05 was considered as statistically significant. 21
bDifference-in-difference in days (Ref: Pre-NH, stroke control), where DID estimator < 0 is a positive impact of the intervention, with reduction of LOS from pre period to post period for NH service comparing to stroke service.
cRatio of OR (Ref: Pre-NH, stroke control), where DID estimator < 1 is a positive impact of the intervention. GEE logistic model was fit for each binary outcome, adjusting for patients' age, gender, primary insurance, CMI, Charlson Comorbidity Index and quarter of discharge. Standard Error estimates for confidence intervals were adjusted for intra-provider correlations by including a clustering variable of primary attendings into the GEE model.
Primary Clinical Outcomes
The neurohospitalist service was associated with a reduction in 30-day readmissions compared to stroke controls (ratio of ORs: .52 [.27, .98], P = .088) although not statistically significant after adjustment for multiple comparisons. Length of stay was not incrementally changed in the DID model (-.3 [-.7, .1], P = .176) (Table 2).
In secondary descriptive analysis, risk-adjusted mean length of stay was significantly shorter following implementation of the neurohospitalist service (4.5 pre to 3.8 days post, P < .001) but not in stroke controls (3.1 pre to 2.7 days post, P = .14) (Table 3). Notably, during the study period percentage of observation status patients nearly quadrupled, from 8.3% of all discharges (102/1223) pre-NH to 30.4% (230/757) in the post-NH intervention group. Because our stroke group was defined by inpatient DRG, we were not able to measure change in observation status in the controls. During the same period, the proportion of observation status patients increased slightly from 20% to 25% across the hospital.
Table 3.
Predicted marginal mean length of stay in neurohospitalist and control groups
| Predicted marginal mean LOS | P-value | ||
|---|---|---|---|
| LOS in days a , [95% CI] | Pre | Post | |
| Intervention (neurohospitalist) | 4.5 [4.3, 4.8] | 3.8 [3.6, 4.1] | <.001 |
| Control (stroke) | 3.1 [2.8, 3.3] | 2.7 [2.4, 3.0] | .18 |
LOS: length of stay
aAdjusted for patients’ characteristics included age, sex, primary insurance, case mix index (CMI), Charlson Comorbidity Index, and quarter of discharge .
Secondary Outcomes
Additional Clinical Outcomes: In the DID analysis, mortality and in-hospital complication rate showed a trend toward fewer complications, but ultimately no significant incremental impact of the neurohospitalist intervention (Table 4). Among the pre-specified complications, there was a significant decrease in delirium (DID estimator .14, 95% CI: .02–.7. P = .027) although absolute number of cases remained low across all groups (<50) (Supplementary Table e-4). Mortality remained < 1% for the neurohospitalist service. The rate of any in-hospital complication was significantly higher in the post-NH period compared to pre-NH period for both neurohospitalist and control groups but absolute rates remained low (<4% for each complication) (Supplementary Table e-4).
Table 4.
Secondary clinical, cost & utilization outcomes—difference-in-difference analysis
| DID estimator | |||||
|---|---|---|---|---|---|
| Estimator a | 95% CI | P-value | FDR-adjusted P-value | ||
| Clinical | Lower | Upper | |||
| In-hospital death (%) b | .80 | .08 | 7.82 | .85 | 1.00 |
| In-hospital complication (%) b POA not yes | |||||
| Any | .42 | .16 | 1.10 | .08 | .24 |
| Sepsis | 1.47 | .10 | 21.53 | .78 | 1.00 |
| Pneumonia | .74 | .03 | 19.84 | .86 | 1.00 |
| UTI c | - | - | - | - | |
| Delirium | .14 | .02 | .79 | .03 | .12 |
| AKI | .72 | .11 | 4.77 | .73 | 1.00 |
| Thrombosis c (DVT/PE) | - | - | - | - | |
| Cost and utilization | |||||
| >=2 consultants b | .77 | .46 | 1.28 | .31 | .74 |
| Total direct cost d | .97 | .86 | 1.10 | .68 | 1.00 |
| Lab cost d | 1.39 | 1.15 | 1.69 | <.001 | .012 |
| Imaging cost d | 1.07 | .92 | 1.24 | .38 | .76 |
| Imaging use e | .12 | .03 | .52 | .004 | .024 |
| Rx/IV therapy cost d | 1.00 | .69 | 1.44 | .99 | 1.00 |
DID: difference-in-difference; POA: present on admission; UTI: urinary tract infection; AKI: acute kidney injury; DVT: deep venous thrombosis; PE: pulmonary embolism; Rx/IV: medication and/or intravenous therapy; NH: neurohospitalist; GEE: generalized estimating equation.
aRatio of OR (Ref: Pre-NH, stroke control)
bIf ratio < 1, intervention significantly reduced complication rate compared to controls.
cModel did not converge due to low event rate
dIf ratio < 1, intervention significantly reduced cost compared to controls. Main log gamma model.
eIf ratio < 1, intervention resulted in significantly lower odds of having any imaging during hospital encounter compared to controls. Hurdle model.
Cost & Resource Use. Total direct cost showed no significant incremental impact of the neurohospitalist service as compared to controls, although lab costs were increased and imaging use was less as compared to stroke (DID estimator 1.39, 95% CI: 1.15–1.69, P = .0009 for labs; estimator .12, 95% CI: .03–.52, P = .0043 for imaging utilization). Direct cost per case significantly increased in both cohorts compared to the pre-NH period (bootstrapped mean increase of $3827 in intervention, 95% CI ($2,859, $4921); bootstrapped mean increase $3262 in control, ($2,066, $4454), both P < .001). In the neurohospitalist cohort, lab and imaging costs increased (lab increased $130, imaging increase $394, both P < .001) while imaging increased and pharmacy decreased in controls (imaging increase $821, P < .001; pharmacy decrease -$73, P = .006). Proportion of encounters with at least 2 medical consultants showed no change in the neurohospitalist group despite an increase in patient complexity but a significant increase in stroke controls (intervention: pre 14.0%, post 16.3%, P = .25; control: pre 9.1%, post 14.4%, P = .01).
Patient Satisfaction. Response rates for patient satisfaction surveys were low: intervention pre-NH (11.5%), intervention post-NH (8.9%), control pre-NH (10%), control post-NH (3.2%). There was no significant change in overall patient satisfaction in either cohort as compared to the pre-NH period (Supplementary Table e-5).
Sensitivity Analysis
Using our revised exclusion criteria to include observation and inpatient status patients, distinguished by whether they were cared for by a stroke attending or general neurology attending regardless of diagnosis, we had 1121 patients in the pre-intervention cohort, 527 in the post-intervention cohort, 717 in the pre-control and 341 in the post-control (SupplementaryTable e-6). In this model, the difference-in-difference analysis showed no significant impact of the neurohospitalist intervention on 30-day readmissions (DID estimator: 1.34, 95% CI: .55–3.31, P = .52). Adjusted mean LOS in the intervention cohort was significantly reduced from 4.3 days to 3.5 days (P < .001), while there was only a trend in reduction in the stroke cohort (3.3 days to 2.8 days, P = .06) (Supplementary Table e-7).
Discussion
In this retrospective study, implementation of a neurohospitalist program was associated with meaningful shifts in patient characteristics and clinical outcomes. General neurology patients in the post-NH period were sicker than those in the pre-NH group, as evidenced by a significant shift in discharge diagnoses from headache to CNS infections and neoplasms, significantly increased emergency admissions, and concordant shifts in Charlson Comorbidity Index, CMI, APR-DRG, and discharge disposition. Despite this substantial shift in the patient population cared for by the newly created neurohospitalist service, hospital complications and inpatient mortality rates remained low, 30-day readmission rate was stable, and mean adjusted LOS decreased by .7 days. Using a quasi-experimental design with a DID analytic approach, implementation of the neurohospitalist program was associated with a marginally significant reduction in 30-day readmission rate compared to the stroke service control group.
Only one other study evaluating outcomes associated with the implementation of a neurohospitalist program has been published. 4 Pre-post analyses in our study replicate several findings from that earlier one, including reduced mean adjusted LOS, stable mortality rates and stable patient satisfaction. The patient population cared for by neurohospitalists in our study was older (median age 56 vs 51) and more severely ill (extreme or major severity 46.4% vs 28.3%) than in the earlier study yet the current population had a slightly shorter mean unadjusted LOS (4.2 days vs 4.6).
Numerous studies evaluating the impact of medicine and pediatric hospitalist programs on clinical and educational outcomes have been published since the launch of the hospitalist movement.7,24-27 Relatively few have included a control group in their analyses.28-31 In studies of hospitalist program implementation without a control group, contemporaneous interventions at the level of local practice (e.g., changes in emergency room admission process; moving into a new hospital building with more beds) or national policy (e.g., redefinition of observation status; pay for performance initiatives; and changes in resident duty hour limits) may positively or negatively confound the results.32-35 In the current study, it is possible that contemporaneous interventions drove outcomes in which both the general neurology and stroke services shifted in the same direction.
The DID analysis suggested a potential reduction in 30 day readmissions that could be attributed to the neurohospitalist intervention. The 30-day readmission rate for general neurology patients decreased from 8.9% to 7.6%, while the 30-day readmission rate on the stroke service increased from 5.0% to 7.9%. The modest reduction in readmission rate associated with the implementation of the neurohospitalist program is consistent with the neurohospitalist, medicine hospitalist, and pediatric hospitalist literature.4,7,25-27 Drivers of the increased readmission rate in the stroke control group are less clear, but may in part reflect the shift in payor mix to Medicaid in the post-NH period. 36
Even though the neurohospitalist intervention was associated with a significant reduction in adjusted LOS and stroke LOS showed a nonsignificant decreased trend, we were not able to demonstrate a significant incremental impact on LOS in the DID analysis. A larger sample size may have resulted in a statistically significant difference between intervention and controls. The substantial shift in observation status patients (from 8% to 30% of all discharges) unique to the neurohospitalist intervention cohort likely limited our ability to detect a more substantial change in LOS compared to stroke controls. We speculate this shift might in part relate to neurohospitalists more effectively transitioning patients to outpatient care as soon as clinically appropriate (such as outpatient-based infusions for multiple sclerosis flares or intractable headache) leading to fewer patients requiring subsequent conversion to inpatient admission. Additionally, and perhaps most importantly, several new practices started by the neurohospitalist program were deliberately spread to the stroke service, including a documentation improvement initiative, afternoon case management rounds, and a cost-conscious rounding checklist. This may have reduced the likelihood of detecting statistically significant improvement associated with the intervention in the DID analyses.
We note the significantly increased rate of in-hospital complications in the post-NH period in both the neurohospitalist and stroke cohorts. We speculate this may relate to changes in documentation and coding processes rather than a change in clinical outcomes. We also suspect that these outcomes are significantly undercoded here and therefore our ability to detect any significant effect of the intervention is limited. For example, incidence of delirium on a neurology unit has previously been reported to be 27–32% and studies have demonstrated that delirium is significantly undercoded with ICD9/10 codes having only 13–20% sensitivity.37-39 Further study, incorporating chart abstraction, is needed to better understand whether neurohospitalist models impact in-hospital complications and other clinical outcomes.
While overall costs were not significantly different between intervention and control groups, we note the neurohospitalist intervention was associated with significantly lower imaging use and significantly higher lab costs. We might speculate that neurohospitalist care may have led to fewer unnecessary neuroimaging studies given the shift away from outpatient subspecialists seeing patients outside their typical scope of practice. Alternatively, neurohospitalist care may have led to fewer non-neuroimaging studies such as chest x-rays in patients without clinically concerning findings. We did not have granular data to be able to determine whether the decrease in imaging use was due to a reduction in x-rays, CTs, MRIs, or other. Similarly, we did not have line item data to be able to determine what drove the increase in lab costs following the intervention, whether this was driven by higher utilization of a handful of more expensive tests (e.g. autoantibody panels in encephalitis and ganglioside antibodies in neuropathy) or a global increase in lab testing. These are both interesting questions for further study.
We acknowledge that while the controlled design minimizes most confounders, the impact of clinical practice changes such as the rise in interventional stroke cases may have impacted the composition and outcomes of our control group. 40 We also recognize that our findings may not be generalizable to other settings where neurohospitalists also see stroke cases or staff non-teaching services. We were only able to capture 30-day readmissions to our center which is an additional limitation. Finally, the outcomes presented in this study may not fully capture the impact of neurohospitalist program implementation. Other outcomes might have been informative, including volume and characteristics of inpatient consultations, effectiveness of transitions from inpatient to outpatient care, connectedness of the inpatient neurology interdisciplinary team. Educational ratings from neurology residents and medical students will be described in a separate manuscript.
Conclusions
Hospital systems may expect reduced length of stay and 30-day readmission rate and an improved capacity to care for more severely ill patients when moving from a traditional model to a neurohospitalist program.
Supplemental Material
Supplemental Material, sj-pdf-1-nho-10.1177_19418744221083182 for Outcomes of a Neurohospitalist Program at an Academic Medical Center by Carl A. Gold, Brian J. Scott, Yingjie Weng, Eric Bernier and Kathryn A. Kvam in The Neurohospitalist
Acknowledgments
The authors thank Drs. Frank Longo, Yuen So, Greg Albers, Neil Schwartz, Nirali Vora, and Alison Kerr for their contributions to the development of the Neurohospitalist Program. The authors thank Drs. Neera Ahuja and Nidhi Rohatgi for their input on the conceptualization of this study. The authors acknowledge the contributions to data acquisition and data analysis of Roslind Wiley, Noelle Wang, Hannah Wetmore, and Christina Sabathia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Note: Statistical Analysis: performed by Yingjie Weng, MHS, Quantitative Sciences Unit, Stanford University, Stanford, CA.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Carl A. Gold, MD, MS https://orcid.org/0000-0002-4868-4152
Kathryn A. Kvam, MD https://orcid.org/0000-0002-2532-8315
References
- 1.AAN Neurohospitalist Section Synapse Community Website. https://synapse.aan.com/communities/community-home?CommunityKey=e980e436-eaa3-49d6-b69f-0b8baa945418&_ga=2.112205508.1392577039.1598590931-1828190297.1586208821. Accessed December 1, 2020.
- 2.Probasco JC, George BP, Dorsey ER, Venkatesan A. Neurohospitalists: perceived need and training requirements in academic neurology. The Neurohospitalist. 2014;4(1):9-17. doi: 10.1177/1941874413495880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene JG. Collaborative comanagement between neurohospitalists and internal medicine hospitalists decreases provider costs and enhances satisfaction with neurology care at an academic medical center. The Neurohospitalist. 2018;8(2):74-81. doi: 10.1177/1941874417735173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas VC, Scott BJ, Berg G, Freeman WD, Josephson SA. Effect of a neurohospitalist service on outcomes at an academic medical center. Neurology. 2012;79(10):988-994. doi: 10.1212/WNL.0b013e31826846cb. [DOI] [PubMed] [Google Scholar]
- 5.Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes. Annals of Surgery. 2016;264(2):275-282. doi: 10.1097/SLA.0000000000001629. [DOI] [PubMed] [Google Scholar]
- 6.Rifkin WD, Holmboe E, Scherer H, Sierra H. Comparison of hospitalists and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics. Journal of General Internal Medicine. 2004;19(11):1127-1132. doi: 10.1111/j.1525-1497.2004.1930415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic Proceedings. 2009;84(3):248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wachter RM, Katz P, Showstack J, Bindman AB, Goldman L. Reorganizing an Academic Medical Service. JAMA. 1998;279(19):1560-1565. doi: 10.1001/jama.279.19.1560. [DOI] [PubMed] [Google Scholar]
- 9.Cots F, Mercadé L, Castells X, Salvador X. Relationship between hospital structural level and length of stay outliers. Health Policy. 2004;68(2):159-168. doi: 10.1016/j.healthpol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Freitas A, Silva-Costa T, Lopes F, et al. Factors influencing hospital high length of stay outliers. BMC Health Services Research. 2012;12(1):265. doi: 10.1186/1472-6963-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasey JO. Icd: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes. R package. version 4.0.6. https://CRAN.R-project.org/package=icd. [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 13.OSHPD Patient Discharge Data, 1996-current year, FY Final Rule, Table 5, CHHS Open Data Portal. https://data.chhs.ca.gov/dataset/case-mix-index. Accessed October 19, 2020.
- 14.3MTM All Patient Refined Diagnosis Related Groups Methodology Overview. https://apps.3mhis.com/docs/Groupers/All_Patient_Refined_DRG/Methodology_overview_GRP041/grp041_aprdrg_meth_overview.pdf. Accessed October 19, 2020.
- 15.Yale New Haven Health Services Corporation - Center for Outcomes Research & Evaluation . All-Cause Hospital Wide Measure Updates and Specifications Report. 2018. https://www.qualitynet.org/inpatient/measures/readmission/resources#tab3 https://www.qualitynet.org/inpatient/measures/readmission/resources#tab3. Accessed November 12, 2020. Version 7.0.
- 16.Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-Hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214-3218. doi: 10.1161/STROKEAHA.110.610881. [DOI] [PubMed] [Google Scholar]
- 17.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223-1229. [DOI] [PubMed] [Google Scholar]
- 18.Measuring Price Change in the CPI: Medical Care. https://www.bls.gov/cpi/factsheets/medical-care.htm. Accessed August 28, 2020
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences /. 2nd ed. L. Erlbaum Associates; 1988. [Google Scholar]
- 20.Yoshida K. Create “Table 1” to Describe Baseline Characteristics. R package version 0.11.1. http://CRAN.R-project.org/package=tableone. http://CRAN.R-project.org/package=tableone. Accessed October 19, 2020.
- 21.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001;29(4):1165-1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 22.Lenthemmeans RV. Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.3. https://cran.r-project.org/web/packages/emmeans/emmeans.pdf https://cran.r-project.org/web/packages/emmeans/emmeans.pdf. Accessed October 7, 2021.
- 23.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R-project.org/. Accessed August 28, 2020. [Google Scholar]
- 24.The Emerging Role of “Hospitalists” in the American Health Care System | NEJM. https://www.nejm.org/doi/full/10.1056/NEJM199608153350713. Accessed August 28, 2020 [DOI] [PubMed]
- 25.White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Medicine. 2011;9(1):1-22. doi: 10.1186/1741-7015-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim SA, Elmaraezy A, Pamarthy A, Thongprayoon C, Cheungpasitporn W, Palabindala V. Impact of hospitalists on the efficiency of inpatient care and patient satisfaction: a systematic review and meta-analysis. Journal of Community Hospital Internal Medicine Perspectives. 2019;9(2):121-134. doi: 10.1080/20009666.2019.1591901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. doi: 10.1542/peds.2005-0609. [DOI] [PubMed] [Google Scholar]
- 28.Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. New England Journal of Medicine. 2016;375(11):1009-1011. doi: 10.1056/NEJMp1607958. [DOI] [PubMed] [Google Scholar]
- 29.Halasyamani LK, Valenstein PN, Friedlander MP, Cowen ME. A comparison of two hospitalist models with traditional care in a community teaching hospital. The American Journal of Medicine. 2005;118(5):536-543. doi: 10.1016/j.amjmed.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of Hospital Resource Use and Outcomes Among Hospitalists, Primary Care Physicians, and Other Generalists. JAMA Internal Medicine. 2017;177(12):1781-1787. doi: 10.1001/jamainternmed.2017.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of Care by Hospitalists, General Internists, and Family Physicians. New England Journal of Medicine. 2007;357(25):2589-2600. doi: 10.1056/NEJMsa067735. [DOI] [PubMed] [Google Scholar]
- 32.Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. Journal of General Internal Medicine. 2004;19(3):266-268. doi: 10.1111/j.1525-1497.2004.30431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright B, Martin GP, Ahmed A, Banerjee J, Mason S, Roland D. How the Availability of Observation Status Affects Emergency Physician Decisionmaking. Annals of Emergency Medicine. 2018;72(4):401-409. doi: 10.1016/j.annemergmed.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. New England Journal of Medicine. 2012;366(17):1606-1615. doi: 10.1056/NEJMsa1112351. [DOI] [PubMed] [Google Scholar]
- 35.Silber JH, Bellini LM, Shea JA, et al. Patient Safety Outcomes under Flexible and Standard Resident Duty-Hour Rules. New England Journal of Medicine. 2019;380(10):905-914. doi: 10.1056/NEJMoa1810642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regenstein M, Andres E. Reducing hospital readmissions among medicaid patients. Quality Management in Health Care. 2014;23(1):20-42. doi: 10.1097/QMH.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 37.Brown EG, Josephson SA, Anderson N, Reid M, Lee M, Douglas VC. Predicting inpatient delirium: The AWOL delirium risk-stratification score in clinical practice. Geriatric Nursing. 2017;38(6):567-572. doi: 10.1016/j.gerinurse.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 38.von Hofen-Hohloch J, Awissus C, Fischer MM, Michalski D, Rumpf J-J, Classen J. Delirium Screening in Neurocritical Care and Stroke Unit Patients: A Pilot Study on the Influence of Neurological Deficits on CAM-ICU and ICDSC Outcome. Neurocritical Care. 2020;33(3):708-717. doi: 10.1007/s12028-020-00938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiology and Drug Safety. 2017;26(8):945-953. doi: 10.1002/pds.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tate WJ, Polding LC, Kemp S, et al. Thrombectomy results in reduced hospital stay, more home-time, and more favorable living situations in DEFUSE 3. Stroke. 2019;50(9):2578-2581. doi: 10.1161/STROKEAHA.119.025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-nho-10.1177_19418744221083182 for Outcomes of a Neurohospitalist Program at an Academic Medical Center by Carl A. Gold, Brian J. Scott, Yingjie Weng, Eric Bernier and Kathryn A. Kvam in The Neurohospitalist