Abstract
We present the case of a 72-year-old female with multifocal strokes found to have multiple, mobile intracardiac masses. We discuss the differential diagnosis and evaluation of intracardiac masses, and the challenges in management of the ultimately diagnosed etiology of stroke in this patient.
Keywords: cerebrovascular accident, cardioembolism, cardiac tumor, vascular neurology, fibroelastoma
A 72- year-old woman presented to neurology clinic because a brain MRI (ordered for mild tinnitus) revealed an incidental, punctate subacute right frontal stroke ( Figures 1A and 1B ). Her neurologic examination was normal. Past medical history included carotid endarterectomy for asymptomatic severe right internal carotid artery stenosis 9 years prior, hyperlipidemia, and a fifty-pack-year smoking history. Her medications included daily atorvastatin 40 mg, aspirin 325 mg, and clopidogrel 75 mg, the latter initiated by her primary care doctor after discovery of the incidental stroke.
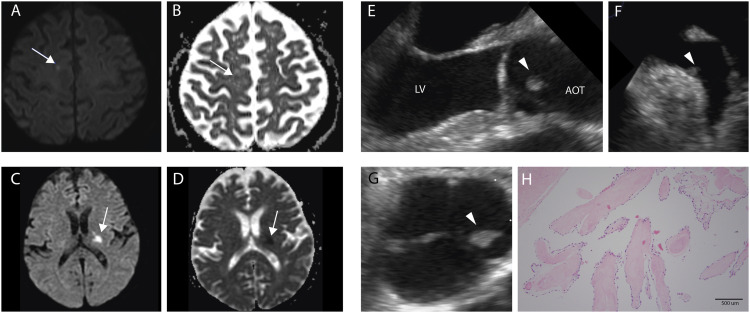
Figure 1.
A-B. Initial stroke in the right parasagittal convexity on MRI. Punctate hyperintensity on diffusion weighted imaging (A, white arrow) with apparent diffusion coefficient (B, black arrow) correlate; C-D. Second stroke in the left posterior limb of the internal capsule on MRI. Hyperintensity on diffusion weighted imaging (C, white arrow) with apparent diffusion coefficient (D, white arrow) correlate; E. Three chamber view on transesophageal echocardiogram showing fibroelastoma (arrowhead) on the left coronary cusp of aortic valve facing towards the aortic outflow tract; F. View of left atrial appendage on transesophageal echocardiogram showing the second visualized fibroelastoma (arrowhead) near the ostium of the left atrial appendage; G. Parasternal short axis view at level of aortic valve on transesophageal echocardiogram showing another view of fibroelastoma (arrowhead) on the left coronary cusp; H. Hematoxylin and eosin stain of fibroelastoma from left coronary cusp. Histology shows a single outer layer of endothelium surrounding a thin layer of mucopolysaccharide-rich matrix. The tumor core is made of largely acellular, avascular elastic fibers. 10x view on microscopy. Abbreviations: LV is left ventricle. AOT is aortic outflow tract.
The cortical-based location of her stroke suggests an embolic etiology. She requires evaluation for an embolic source to determine optimal secondary prevention. Vascular imaging of the head and neck (e.g. CTA, MRA, or Doppler ultrasound) should be performed with attention to the right internal carotid artery (ICA) for significant restenosis or high-risk plaque features (e.g. plaque ulceration, thrombus). Evaluation for cardiac sources of embolus should include transthoracic echocardiogram (TTE) and prolonged cardiac monitoring for occult arrhythmias (e.g. atrial fibrillation). Blood lipid profile, diabetes screening, and blood pressure monitoring should be performed to look for modifiable vascular risk factors. Smoking cessation counseling should be offered.
The patient’s blood pressure was normal. Hemoglobin A1c (6.1%) and low-density lipoprotein level (64 mg/dL) were normal. MRA showed < 50% stenosis of the cervical ICAs without high-risk plaque features and moderate focal stenosis of the right posterior cerebral artery without other vascular abnormalities. 14-day cardiac monitor was normal. TTE was scheduled, but prior to this, the patient awoke with right-sided weakness and dysarthria and presented to an outside hospital.
Brain MRI demonstrated acute stroke in the posterior limb of the left internal capsule ( Figures 1C and 1D ). This second stroke occurred 3 weeks after her clinic visit (and 3 months from initial MRI). Repeat stroke labs and vessel imaging were unremarkable. No atrial fibrillation was detected during her hospitalization.
This patient had two strokes in a 3-month period, the first embolic-appearing, the second lacunar, based on the pure motor syndrome, small size (∼10 mm), and subcortical location. Lacunar infarcts are most commonly caused by vascular risk factor-associated lipohyalinosis of small cerebral arteries resulting in lumen obliteration and subsequent stroke, 1 but may in some cases be due to other mechanisms such as cardio-embolism. 2 Our patient’s first stroke appeared embolic and her second stroke occurred on dual antiplatelet therapy, with controlled vascular risk factors, and without significant ICA pathology. Therefore, a cardioembolic source was considered.
Transesophageal echocardiography (TEE) performed at the outside hospital showed normal left ventricular chamber size, ejection fraction, and wall motion without patent foramen ovale, left ventricular thrombus, or significant aortic arch atherosclerosis.
Two pedunculated, mobile sub-centimeter echo-densities were observed: one on the left coronary cusp of the aortic valve and the other on the inferobasal aspect of the left atrial appendage. The masses had a homogenous speckled pattern with areas of echo-lucency and peripheral stippling.
The differential diagnosis for an intracardiac valvular mass includes infectious endocarditis, non-infectious thrombotic endocarditis (e.g. Libman-Sacks in systemic lupus erythematosus (SLE); malignancy), and intracardiac tumor. Evaluation should include at least two sets of blood cultures, serum testing for hypercoagulability, screen for SLE, and full-body imaging for occult malignancy.
Cardiac tumors are a rare cause of stroke but carry a high risk of embolization from either tumor embolism or thrombus generated on the tumor surface.3,4 Cardiac tumors are divided into primary and secondary tumors, with secondary tumors from metastasis being 20- to 40-fold more common. 5 Most primary cardiac tumors are benign; myxoma is the most common (over 50% of cases). 5 Myxomas are typically found in the left atrium with the highest incidence in women 30-60 years old. 3 Myxomas may present with systemic signs (e.g. weight loss, fever) that can mimic bacteremia. 5 The second and third most common adult primary cardiac tumors are lipomas and papillary fibroelastomas, respectively; both increase in incidence with age. 5 Fibroelastomas represent 75% of valvular tumors, 5 occurring most frequently on the aortic and mitral valves. 6 Malignant cardiac tumors (e.g. sarcomas and lymphomas) represent only 25% of primary cardiac tumors, 5 but may also cause stroke. 7
TTE is the first line diagnostic imaging modality for cardiac tumors due to its wide availability, non-invasiveness, ease of performance, and high rate of detection of tumors (sensitivity 90%; specificity 95%). 5 TEE usually is performed subsequently to improve visualization of valvular lesions in the left atrium. Cardiac CT can provide information on tumor vascularity (via contrast enhancement), calcification, and presence of fat. 8 Cardiac MRI (CMR) offers high spatial resolution, avoids ionizing radiation, and may be used for surgical planning; limitations include long acquisition time and limited availability compared to ultrasonography.8,9 Specific characteristics on CMR sequences can also predict the tumor type and likelihood of malignancy with high diagnostic accuracy. 9
Based on characteristic echocardiographic findings, our patient’s masses are most likely papillary fibroelastomas, as they are small (<1.5 centimeters), mobile, pedunculated, valvular in location, and have a homogenous speckled pattern with peripheral stippling.10,11 Papillary fibroelastomas should be differentiated from Lambl’s excrescence. Lambl’s excresence are thin, filiform strands that can form along valve closure contacts, are thought to arise due to valvular shearing forces that result in tissue overgrowth, and can lead to embolization in rare cases. 12 Their morphology and location at valve closure contacts distinguishes them echocardiographically from fibroelastomas, which are generally pedunculated and found on the mid portion of the valve, as in our patient. Approximately 80% of fibroelastomas are valvular (aortic more common than mitral), but usually do not cause valvular dysfunction. 10 90% of fibroelastomas occur in isolation, but multiple masses are possible and smaller tumors (<.2 centimeters) may not be visualized. 11
This patient’s blood cultures and hypercoagulable testing were negative, including lupus anticoagulant screen, anticardiolipin antibody, and beta-2-glycoprotein 1 antibody. She was up to date on her cancer screening. Based on the patient’s age, valvular location of the masses, and characteristic echocardiographic features, fibroelastomas were considered most likely.
Fibroelastomas carry a high risk for systemic embolization. 11 Stroke or transient ischemic attack (TIA) is one of the most common initial presentations of this tumor. 6 In one study, 23/26 (88%) of patients with fibroelastoma had symptoms attributed to cerebral or systemic embolization over an 11-month period. 11 Tumor location influences embolic risk; while the most commonly involved valve is the aortic valve, stroke appears more common with mitral valve tumors. 6 It is unclear which imaging characteristics of fibroelastomas predict higher risk for embolization, although mobility may be associated with higher risk.6,13
Since fibroelastomas are rare, there are limited data on optimal management. Based on small studies, surgery appears to reduce stroke recurrence rates (from 6% at 1 year and 13% at 5 years without surgery to 0-4% at 1 year and 5-11% at 5 years with surgery).6,13 Current guidelines recommend surgical removal of fibroelastomas that are symptomatic, larger than one centimeter in diameter, or mobile.11,14 Valve-sparing surgery is achieved in approximately 81% of resections; some patients require valve repair or replacement. 6 A full resection can be curative, as the risk of tumor recurrence after successful resection is low. 6 Therefore, if mobile, even asymptomatic fibroelastomas found incidentally are recommended to be removed as primary prophylaxis in good surgical candidates. 13
In symptomatic patients who are not good surgical candidates, an antithrombotic may be considered to reduce embolization risk.6,13 There are no randomized controlled trials comparing the efficacy of various antithrombotic therapies in patients with fibroelastoma. 2 In one small study of 121 patients with suspected fibroelastoma (based on TTE) and prior neurological event (TIA, stroke, or both), who did not undergo surgical resection, rates of recurrent stroke over 5 years were 14% on warfarin, 13% on aspirin, and 9% on clopidogrel (alone or with aspirin); comparison between antithrombotic agents was not performed due to small sample size. 13 The optimal antithrombotic regimen for patients with fibroelastoma therefore remains uncertain.
Since our patient’s fibroelastoma was symptomatic and mobile, she was referred for surgery. As our patient’s second stroke occurred while on dual antiplatelet therapy, her antithrombotic regimen was changed to apixaban and aspirin while awaiting surgery.
She underwent valve-sparing surgery and was found to have four fibroelastomas intraoperatively (two of which were not visualized on TEE), all of which were successfully removed. Tumor pathology revealed a core of connective tissue that was surrounded by a layer of endocardial cells ( Figure 1H ). No mural thrombus was visualized.
On gross pathology, papillary fibroelastomas have arm-like projections (“papillary fronds”) emanating from a central stalk. Histology shows a single outer layer of endothelium surrounding an intermediate, thin layer of a mucopolysaccharide-rich matrix. The tumor core is comprised of largely acellular, avascular elastic fibers. 15 Thrombus may be visualized along the surface, although not in all cases. 6 Our patient’s pathology confirmed fibroelastoma.
The patient was treated with IV heparin during the periprocedural period, and then transitioned to apixaban and aspirin on discharge.
Although surgery reduces the risk of stroke recurrence, post-operatively patients may continue to have elevated stroke risk due to tumor recurrence and thrombogenicity of operated cardiac tissue. 13 Most patients are therefore maintained on antithrombotics post-operatively. The optimal antithrombotic regimen and course remains unclear.6,13 Therefore, antithrombotic choice should involve shared decision-making among the neurologist, cardiologist, cardiac surgeon, and patient, and should take into consideration other co-morbid conditions (e.g. atrial fibrillation).
Fibroelastoma recurrence is uncommon. Interval surveillance imaging can be considered to look for cardiac tumor recurrence, but the ideal imaging modality and frequency interval is unclear.4,6
In a study of 98 patients with mean follow-up of 5.4 years (range 1-17), 12 (12.2%) had echocardiographic recurrence, with mean time to recurrence 6.7 years post-operatively. 4 In four patients, recurrent fibroelastomas were visualized only on TEE, but not on TTE. 4 Based on available evidence, it appears reasonable to observe patients with surveillance imaging after excision. Since post-surgical changes may be challenging to distinguish from recurrence soon after surgery, the authors recommend TTE at 1 year and surveillance TEE at 5 years. 4
With physical therapy, the patient made a good recovery from her stroke and surgery with a modified Rankin Scale of 1. She uses a cane outside the home. She has quit smoking. She continues on apixaban, aspirin, and a statin for secondary stroke prevention and has not had recurrent symptoms. Surveillance TTE obtained 8 months after surgery showed no recurrence of fibroelastoma. She will undergo annual TTE, with plan for TEE at 5 years.
Discussion
This case of a common condition (stroke) caused by an uncommon pathology (fibroelastoma) holds several important lessons.
First, not all lacunar-appearing infarcts should be attributed to small vessel disease pathology. In patients with well-controlled vascular risk factors on maximal medical management, embolic etiology should be considered.
Second, although primary cardiac tumors are rare—identified on only 0.02% of autopsies 5 –they should be considered as a potential etiology of cardioembolic stroke. While myxomas are the most common primary cardiac tumor, fibroelastomas should be considered in patients found to have a valvular mass. Due to advances in echocardiography, fibroelastomas are increasingly recognized, identified on approximately 0.1% of TTEs. 13 Despite increasing sensitivity, however, 33% of fibroelastomas are missed on TTE and later detected on TEE. 13 TEE should therefore be considered if initial TTE is negative and the cause of embolism is undetermined.
Finally, while data for management of fibroelastoma is limited, surgical resection should be considered in large, mobile, and symptomatic tumors to reduce stroke recurrence.6,11,13,14 Long-term antithrombotic therapy and surveillance imaging is recommended, although further research is necessary to determine optimal management strategies for patients with fibroelastoma.
Footnotes
Author Contributions: Alexis T. Roy, M.D. drafted the initial manuscript, revised the manuscript, and was involved in the clinical care of the patient. Galina Gheihman, M.D. drafted the initial manuscript and revised the manuscript. Aaron L. Berkowitz, M.D., Ph.D. revised the manuscript and was involved in the clinical care of the patient.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Alexis T. Roy https://orcid.org/0000-0001-7406-307X
Galina Gheihman https://orcid.org/0000-0003-1599-3271
References
- 1.Fisher CM. Lacures: small, deep cerebral infarcts. Neurology. 1965;15:774-784. [DOI] [PubMed] [Google Scholar]
- 2.Jung DK, Devuyst G, Maeder P, Bogousslavsky J. Atrial fibrillation with small subcortical infarcts. J Neurol Neurosurg Psychiatry. 2001;70(3):344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernatchez J, Gaudreault V, Vincent G, et al. Left atrial myxoma presenting as an embolic shower: a case report and review of literature. Ann Vasc Surg. 2018;53:266.e13-266.e20. [DOI] [PubMed] [Google Scholar]
- 4.Sorour AA, Kurmann RD, El-Am EA, et al. Recurrence of pathologically proven papillary fibroelastoma. Ann Thorac Surg. 2021;S0003-4975(21):00862-00866. [DOI] [PubMed] [Google Scholar]
- 5.Paraskevaidis IA, Michalakeas CA, Papadopoulos CH, Anastasiou-Nana M. Cardiac tumors. ISRN Oncol. 2011;2011:208929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gowda RM, Khan IA, Nair CK, et al. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146(3):404-410. [DOI] [PubMed] [Google Scholar]
- 7.Park CK, Cho YA, Kim M, et al. Malignant lymphoma arising in cardiac myxoma, presenting with peripheral arterial emboli. Cardiovasc Pathol. 2018;32:26-29. [DOI] [PubMed] [Google Scholar]
- 8.Tyebally S, Chen D, Bhattacharyya S, et al. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2(2):293-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268(1):26-43. [DOI] [PubMed] [Google Scholar]
- 10.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784- 790. [DOI] [PubMed] [Google Scholar]
- 11.Sun JP, Asher CR, Yang XS, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103:2687-2693. [DOI] [PubMed] [Google Scholar]
- 12.Kondamareddy D, Kerndt CC, Masood W. Lambls excrescences. [Updated 2021 Nov 26]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 13.Tamin SS, Maleszewski JJ, Scott CG, et al. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. J Am Coll Cardiol. 2015;65:2420-2429. [DOI] [PubMed] [Google Scholar]
- 14.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devanabanda AR, Lee LS. Papillary fibroelastoma. 2021 Aug 11. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]