Abstract
Background
We implemented a multi-disciplinary process improvement intervention at our Comprehensive Stroke Center with speech/language pathologists to expedite oral medication delivery in stroke patients. Following a failed nursing dysphagia screen, trained neurology physicians screened dysphagia further to approve use of oral medications. We analyzed the safety and efficacy of this intervention.
Methods
We analyzed retrospectively collected data for hospital course, timing of first screen, first oral medication use, and complications (e.g., aspiration pneumonia) in consecutive ischemic stroke patients (9/2019-07/2021). Patients were included if they passed a dysphagia assessment by physicians (Ph), nurses (RN), or speech/language pathologists (SLP). Arrival-to-dysphagia screen and arrival-to-antithrombotic were assessed using restricted mean survival time (RMST).
Results
Of the 789 included patients, 673 were passed by RN, 104 by SLP, and 12 by Ph. Compared to patients passed by SLP, those passed by Ph were younger and had less severe deficits (P < .01 for both). Patients were screened more quickly by Ph than RN or SLP (median 38 vs 182 vs 1330-min post-arrival, P = .0001; 299-min RMST difference vs RN [95%CI 22-575, P = .03]; 470-min RMST difference vs SLP [95%CI 175-765, P = .002]). This translated to faster oral antithrombotic use for Ph-passed patients (138-min RMST difference vs RN [95%CI 59-216]; 332-min RMST difference vs SLP [95%CI 253-411]). No patients passed by Ph experienced aspiration pneumonia (0%).
Conclusions
We safely conducted a physician-driven dysphagia screening paradigm which led to faster oral antithrombotic delivery without signal of patient harm. Physician availability to complete dysphagia screens in acute stroke patients was a limitation.
Keywords: Dysphagia, Stroke, Process Improvement
Introduction
Following acute cerebral infarction, there is a transient increase in platelet activation, inflammation, and clot formation, which results in a heightened risk of early recurrence that attenuates with time. 1 Early administration of oral antithrombotic therapy in acute ischemic stroke has been shown to reduce stroke recurrence and mortality in randomized clinical trials, 2 but the beneficial effect of antithrombotic therapy dwindles the longer that treatment is delayed.1,2
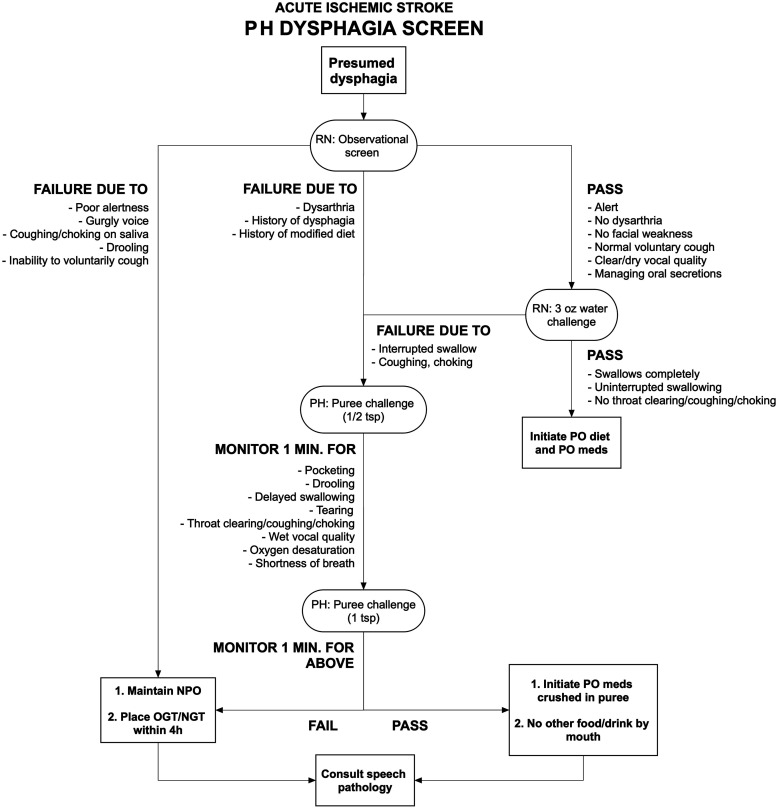
Oral antithrombotic therapy in acute stroke is frequently delayed due to symptomatic dysphagia, which occurs in up to 55% of patients.3,4 Starting March 2020, the neurology department at Cooper University Hospital implemented a prospective process improvement (PI) intervention to expedite oral medication delivery for patients with acute ischemic stroke. With the support and approval of the speech and language pathology (SLP) team, neurology residents and attending physicians were trained in dysphagia screening to assess patients swallowing function. In the previous paradigm, nurses would conduct an initial screening for dysphagia in all acute stroke patients. Among other circumstances, any patient with prior history of dysphagia, facial asymmetry, or speech disturbance would fail the screen. At this point, the patient would not be permitted anything by mouth until approval by an SLP. With the PI intervention, a trained physician could perform further screening and approve the use of oral medications if certain criteria were met (Figure 1).
Figure 1.
Dysphagia screen pathway. Notes: PH, denotes physician; RN, registered nurse; PO, per os; NPO, nil per os; OGT/NGT, orogastric or nasogastric tube.
We sought to examine the impact of this PI protocol on acute stroke patient outcomes. We hypothesized that physician dysphagia screenings would occur more quickly than assessments performed by SLP and could expedite oral medication administration without increasing the risk of adverse events.
Methods
Data will be made available to any qualified investigator upon reasonable request.
Process Improvement Initiative
Beginning in March 2020, we implemented a multi-disciplinary PI intervention to safely expedite oral medication delivery, with the support of and education by SLP. The intervention was investigator-initiated by the study PI (JS) in collaboration with institutional support from emergency department and inpatient nursing staff, SLP staff, neurology resident physicians and neurohospitalist staff. In preparation for the PI intervention, all neurology resident and attending neurohospitalists underwent formal training for the screening of dysphagia by SLP, and were given privileges to permit oral medication delivery until a formal SLP evaluation could be performed. The training involved a 1-h interactive training session between SLP staff and physicians, which involved a didactic session on swallowing mechanics followed by a case-based review to ensure content retention. Furthermore, all physicians were provided with a laminated, pocket-sized flowchart to keep with them as a reference when needed. As part of this PI intervention, physicians could permit a patient to receive oral medications (no other foods or liquids) until the patient would be ultimately cleared by SLP. A pathway in which resident or attending physicians could approve a patient for oral medications was designed using a modified Delphi consensus with representation from SLP and neurohospitalist team members in the months prior to the PI intervention. The final pathway was agreed upon by all stakeholders and is summarized in Figure 1. Physicians trained in the dysphagia screening could perform dysphagia screens as a part of their initial urgent evaluation or later following medical stabilization. While physicians were encouraged to perform dysphagia screens following failed nursing assessments, physicians were allowed to perform dysphagia screens for any patient who would be anticipated to fail a nursing assessment on the basis of having a history of dysphagia, facial asymmetry, or significant speech disturbance.
Study Design and Participants
We conducted a retrospective analysis of a prospective observational cohort of all consecutive patients 18 years of age or older admitted to a Comprehensive Stroke Center with acute ischemic stroke from 9/23/2019 to 7/16/2021. As per our institutional protocol, patients with an acute stroke are screened by a registered nurse (RN) for dysphagia, and if patients are deemed to be at high risk of aspiration (for reasons indicated in Figure 1), these patients are recommended for formal evaluation by SLP before any oral medications or nutrition can be delivered. While all patients with suspected stroke were potentially eligible for this PI intervention, patients were eligible for this formal analysis if their time of initial dysphagia assessment (by nursing staff or physician) was clearly documented and if the patient experienced an out-of-hospital stroke. In-hospital stroke patients were excluded from the planned analyses as their time-to-antithrombotic and time-to-dysphagia screen is limited by a clear “start” time. In-hospital stroke patients, which represent a minority of patients, often have an unclear symptom onset (or last known well time), therefore any estimate of time-to-event may be confounded.
Data Collection
We retrospectively collected demographic information, including age, sex, race, pre-morbid disability according to the modified Rankin Scale (mRS), pertinent past medical history, National Institutes of Health Stroke Scale (NIHSS) at presentation, and NIHSS subscores. All patients underwent dysphagia screening by nursing staff. During the initial medical evaluation and if time was permissible, resident or attending physicians who received appropriate training with speech pathology, would perform a dysphagia screen. The date/time of arrival, all dysphagia screen and assessment times, and time of first oral antithrombotic medication were captured. Hospital course, including aspiration pneumonia events, use of an orogastric tube, and severe disability or death by 90 days (mRS 5-6) were also captured. Aspiration pneumonia was defined clinically on the basis of a serum leukocytosis and radiographic evidence of airspace disease on chest x-ray, with or without fever. Missing data were not imputed.
Statistical Analyses
Descriptive statistics were used to compare patient groups stratified by the provider who approved the patient for oral medications following a dysphagia screen (nurse vs physician) or assessment with SLP). Continuous variables are reported as medians with interquartile range and compared using the Kruskal-Wallis equality of populations rank test. Categorical variables are reported as proportions and compared using the χ2 test, or Fisher’s exact test when contingency table cell counts were less than 5, as appropriate.
Differences in time-to-event were compared between the three groups using a cox proportional hazards model, however despite multivariable adjustment, all models violated the proportional hazards assumption. Therefore, between-group differences in time-to-event were assessed using restricted mean survival time (RMST) as previously described. 5 Time-to-event analyses were performed for two separate events: (1) arrival-to-dysphagia screen and (2) arrival-to-antithrombotic. To meet model specifications, the models were truncated at a reasonable minimum of the largest time point of the event between groups. For example, 511 min was the shortest interval of arrival-to-dysphagia screen among each group (physician group), so arrival-to-dysphagia was truncated at 480 min (τ = 480), or 8 h. The minimum of the largest interval for arrival-to-antithrombotics was also observed in the physician group at 2225 min, so arrival-to-antithrombotic was truncated at 1440 min (τ = 1440), or 24 h. This tau was also chosen as it represents the time window recommended by the American Heart Association/American Stroke Association for patients who do not receive intravenous thrombolysis. 6 (A subgroup analysis for time-to-antithrombotic was also performed, after excluding patients who were treated with thrombolysis, in order to account for expected delays among thrombolyzed patients [τ = 900].) Absolute differences (with 95% confidence intervals and corresponding P-values) are reported as average minutes of delay for a given patient, using the physician group as a referent. For analyses that were adjusted for baseline NIHSS, a ratio is also provided which describes the proportion of patients who failed to reach the time point (τ) among the nurse or SLP group when compared to the referent physician group. Survival curves were constructed using Kaplan-Meier estimates, following exclusion of outliers (n = 15 patients who were screened >10, 000 min after arrival, 1.4%).
All tests were performed at the two-sided level, with an alpha set at .05. Analyses were performed using STATA 15.0 (College Station, TX). These results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. This study was approved by the local institutional review board with waiver of informed consent.
Results
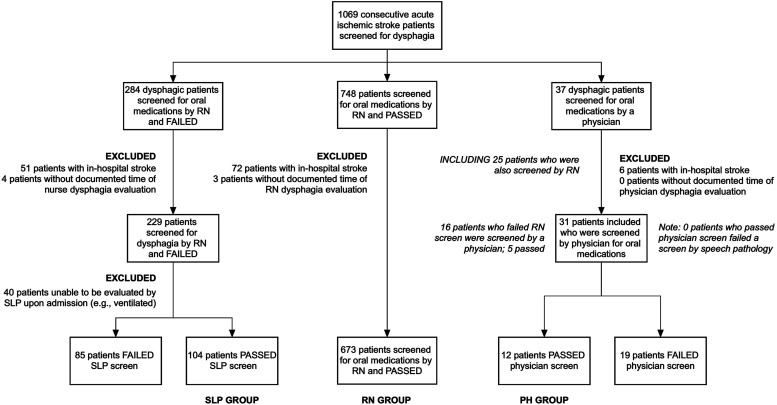
Of the 1069 consecutive patients with documented dysphagia screen times evaluated during the study period, 673 were passed following nurse screening (63.0%), 104 were ultimately passed by SLP following failure of nurse screening (9.7%), and 12 (1.1%) were ultimately passed by a physician (Figure 2). Compared to patients who failed a dysphagia screen by SLP and nurse/physician screen (and were therefore excluded), those who ultimately passed the dysphagia screen were older (median 75y [IQR 67-83] vs 67y [IQR 58-76], P < .01) and had more severe deficits (median NIHSS 17 [IQR 11-23] vs 3 [IQR 1-7], P < .01), with more severe deficits attributed to level of consciousness, facial weakness, and dysarthria using NIHSS subscores. Other key differences between included and excluded patients are summarized in Supplemental Table 1. There were several key differences between patients screened by physicians vs those who were not screened by physicians, notably that patients screened by physicians had more severe strokes (median NIHSS 18 [IQR 4-25] vs 4 [IQR 1-10], P < .01) with more frequent proximal large vessel occlusions (52% vs 16%, P < .01; Supplemental Table 2).
Figure 2.
Inclusion flowchart. Notes: RN, denotes registered nurse; SLP, speech and language pathologist; and PH, physician.
The median age of the cohort was 67 (IQR 58-76) with a median NIHSS of 3 (IQR 1-7), of whom 345 (48%) were female. One-hundred four (13%) had an occlusion of the intracranial internal carotid, proximal middle cerebral, or basilar arteries, of whom 80 (79%) underwent endovascular thrombectomy. Compared to patients who required further dysphagia evaluation and were eventually cleared for oral intake by SLP, those passed by physicians were younger and had milder cumulative neurologic deficits (based on total NIHSS) as well as fewer deficits associated with dysphagia (based on NIHSS subscores) at presentation (Table 1). The majority of patients received aspirin as the first antiplatelet agent (99.2%), with proportionally more patients passed by SLP receiving the first dose of aspirin rectally (28%SLP vs 4%RN vs 8%PH, P < .01). Compared to patients who received oral aspirin, those who received their first aspirin dose rectally had shorter arrival-to-antithrombotic times (median 508 min [IQR 264-1632] vs 994 min [IQR 410-1754], P < .01). When patients who were passed by physicians were compared against any patient who was given aspirin per rectum as the first antithrombotic, the arrival-to-antithrombotic time was non-significantly shorter for patients screened by physicians (median 335 min [IQR 80-705] vs 517 min [IQR 264-1632], P = .07).
Table 1.
Demographics.
| Passed SLP evaluation (n = 104) | Passed RN screen (n = 673) | Passed physician screen (n = 12) | P-value | |
|---|---|---|---|---|
| Age, median y (IQR) | 70 (63-78) | 67 (58-76) | 63 (55-72) | .04 |
| Female, no. (%) | 56 (54%) | 284 (42%) | 5 (42%) | .08 |
| mRS prior to admission, median (IQR) | 1 (0-2) | 0 (0-2) | 0 (0-1) | .04 |
| Race, no. (%) | .49 | |||
| Caucasian | 60 (58%) | 351/672 (52%) | 9 (75%) | |
| Black | 30 (29%) | 194 (29%) | 3 (25%) | |
| Asian | 2 (2%) | 12 (2%) | 0 (0%) | |
| Other | 12 (12%) | 115 (17%) | 0 (0%) | |
| Hispanic, no. (%) | 10/100 (10%) | 90/643 (14%) | 0 (0%) | .26 |
| Past medical history, no. (%) | ||||
| Hypertension | 90 (87%) | 566/671 (84%) | 11 (92%) | .68 |
| Dyslipidemia | 59 (57%) | 434/672 (65%) | 10 (83%) | .12 |
| Diabetes | 37 (36%) | 273/672 (41%) | 6 (50%) | .48 |
| Prior stroke | 27 (26%) | 187/672 (28%) | 1 (8%) | .35 |
| Atrial fibrillation | 24 (23%) | 116/671 (17%) | 1 (8%) | .25 |
| Coronary artery disease | 22 (21%) | 152/672 (23%) | 1 (8%) | .61 |
| Congestive heart failure | 19/103 (18%) | 98/672 (15%) | 2 (17%) | .53 |
| Baseline NIHSS, median (IQR) | 11 (5-18) | 3 (1-6) | 3 (2-5) | <.01 |
| Baseline NIHSS subscores, no. (%) | ||||
| ≥2 points for level of alertness | 40 (38%) | 83 (12%) | 4 (33%) | <.01 |
| ≥2 points for facial weakness | 79 (76%) | 241 (36%) | 6 (5%) | <.01 |
| ≥1 point for dysarthria a | 79 (76%) | 241 (36%) | 6 (5%) | <.01 |
| Anterior circulation only infarction, no. (%) | 73/102 (72%) | 396/630 (63%) | 10/11 (90%) | .05 |
| ASPECTS b , median (IQR) | 9 (8-10) | 9 (9-10) | 10 (8-10) | .04 |
| Occlusion of ICA, M1, or basilar, no. (%) | 30 (29%) | 71 (11%) | 3 (25%) | <.01 |
| Thrombolysis, no. (%) | 24 (23%) | 53 (8%) | 2 (17%) | <.01 |
| Administration of first antiplatelet, no. (%) | ||||
| Aspirin | 101 (97%) | 670 (99.6%) | 12 (100%) | .046 |
| Rectally administered for first dose? | 28/101 (28%) | 24/670 (4%) | 1/12 (8%) | <.01 |
| Non-aspirin | 3 (3%) | 3 (.5%) | 0 (0%) | .046 |
SLP denotes: speech and language pathology; RN: registered nurse; IQR: interquartile range; mRS: Modified Rankin Score; NIHSS: NIH Stroke Scale/Score; ASPECTS: Alberta Stroke Program Early CT Score; and ICA: internal carotid artery.
aNote: 105/789 (13%) patients had ≥1 point for dysarthria and ≥2 points for facial weakness, 221/789 (28%) had dysarthria without facial weakness, and 22/789 (3%) had facial weakness without dysarthria.
bASPECTS provided for patients with anterior circulation only infarctions.
Regarding safety, rates of aspiration pneumonia were no different between patient groups although patients passed by SLP had numerically higher rates of pneumonia (P = .11; Table 2). No aspiration pneumonia occurred among patients passed by physicians. All 16 patients diagnosed with aspiration pneumonia demonstrated radiographic evidence of airspace disease on chest x-ray. Of these 16 patients, 14 had serum leukocytosis (>11.00 × 103/uL), while the remaining 2 had low-normal leukocyte counts due to immune-deficient status. Of these 16 patients, 9 manifested with fever (>100.4 °F). Length of hospital stay was longer for patients who ultimately passed a dysphagia evaluation by SLP when compared to other providers (P < .01). However, there was no significant difference in length of stay between patients who passed RN or physician dysphagia screens (pRank-Sum = .20).
Table 2.
Primary and safety outcomes of the dysphagia screening.
| Passed SLP evaluation (n = 104) | Passed RN screen (n = 673) | Passed physician screen (n = 12) | P-value | |
|---|---|---|---|---|
| Primary efficacy outcome | ||||
| Time to first antithrombotic, median min. (IQR) | 1409 (459-2540) | 774 (316-1359) | 335 (80-705) | <.01 |
| Secondary efficacy and safety outcomes | ||||
| Time to dysphagia screen, median min. (IQR) | 1330 (1009-2414) | 182 (65-503) | 38 (21-61) | <.01 |
| Placement of orogastric tube on admission, no. (%) | 32/104 (31%) | 30 (4%) | 0 (0%) | <.01 |
| Aspiration pneumonia, no. (%) | 5 (5%) | 11 (2%) | 0 (0%) | .11 |
| Length of stay, median d (IQR) | 2 (1-6) | 3 (2-6) | 6 (3-10) | <.01 |
| Death or severe disability by 90 days (mRS 5-6), no. (%) | 19/75 (25%) | 83/474 (18%) | 0/6 (0%) | .18 |
SLP denotes: speech and language pathology; RN: registered nurse; IQR: interquartile range; and mRS: Modified Rankin Scale.
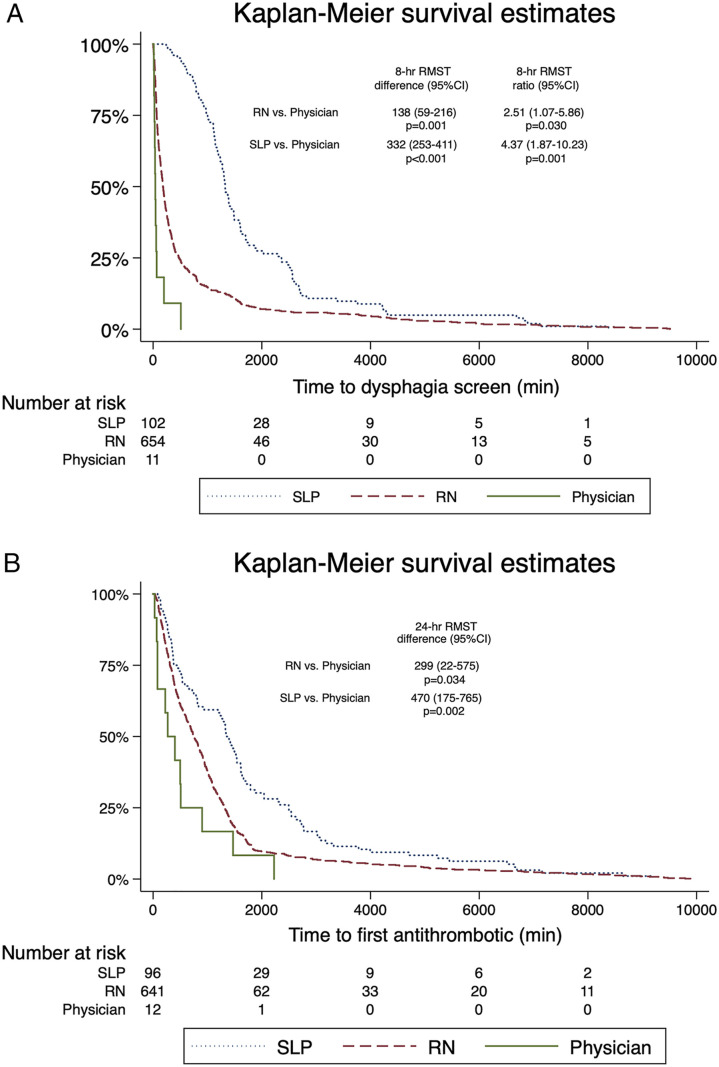
In the time-to-event analysis, patients screened and passed by physicians were generally screened and treated with an oral antithrombotic more quickly than patients screened by nurses or assessed by SLP. Compared to patients passed by physicians, the 8-h RMST for arrival-to-dysphagia screen was 138 min (95%CI 59-216) slower for patients passed by nurses, and 332 min (95%CI 253-411) slower for patients passed by SLP, and these differences remained significant after adjustment for stroke severity (P < .01 for both; Figure 3A). Compared to patients passed by physicians, the 24-h RMST for arrival-to-antithrombotic among all included patients was 299 min (95%CI 22-575) slower for patients passed by nurses (P = .03), and 470 min (95%CI 175-765) slower for patients passed by SLP (P < .01; Figure 3B). In these models, baseline NIHSS remained independently associated with longer delays to dysphagia screen, with an average delay of 6 min (95%CI 4-7) for every NIHSS point (P < .01), and longer delays to first antithrombotic, with an average delay of 7 minutes (95%CI 0-14) for every NIHSS point (P = .04).
Figure 3.
Time-to-dysphagia screen and antithrombotic administration. A Survival estimates for time-to-dysphagia screen. RMST results and P-values are provided for adjusted comparisons, accounting for baseline stroke severity. B Survival estimates for time-to-antithrombotic. RMST results and P-values are unadjusted as stroke severity was not independently associated with treatment delay (P = .97). RMST denotes restricted mean survival time and indicates an absolute average difference (in minutes, with 95% confidence intervals) in time-to-event for a patient evaluated by one provider vs another, as well as the relative difference in ratio form in time-to-event. For example, a patient who was passed by a nurse for dysphagia screen was evaluated on average 138 min later, or 251% more slowly, than a patient passed by a physician (P = .001). SLP denotes speech and language pathology, RN registered nurse. Note that Kaplain-Meier curves are truncated at 10 000 minutes to exclude extreme outliers (1.4% of population), as described in the Methods.
In the subgroup of patients who did not receive intravenous thrombolysis (n = 710, 90%), there remained differences in time-to-antithrombotic between groups. Given the shorter time-to-antithrombotic among non-recipients of thrombolysis (maximum delay 902 min for patients screened by physicians), the truncation time was set at 900 minutes (τ = 900), or 15 h. Therefore, the 15-h RMST for arrival-to-antithrombotic was 298 min (95%CI 107-488) slower for patients screened by nurses (P < .01), and 349 minutes (95%CI 149-550) slower for patients passed by SLP (P < .01). In this model, NIHSS was excluded as it was not associated with treatment times (P = .98).
Discussion
As a result of this PI intervention, stroke patients who underwent dysphagia screening (and were passed) by physicians were evaluated significantly more quickly than patients passed by nursing or SLP staff. The time from arrival to physician dysphagia screen was reduced by a magnitude of hours when compared to nurses or speech and language pathologists. And while stroke severity was greater for patients who ultimately required SLP evaluation, the difference in time to evaluation remained significant between groups regardless of stroke severity.
Earlier dysphagia screening performed by physicians during the inpatient course paralleled faster oral antithrombotic delivery. Although a higher proportion of patients who ultimately required dysphagia clearance by SLP were given their first antithrombotic rectally (28%), and this was associated with a significant reduction in time to treatment, patients ultimately requiring clearance by SLP were treated significantly more slowly when compared to those screened by physicians. Furthermore, rectal administration of aspirin was generally (although non-significantly) slower than antithrombotic administration following physician clearance. This is likely the consequence of physician encouragement and oversight of antiplatelet administration when acute stroke is suspected.
When considering all patients, antithrombotic therapy was delayed by approximately 5 h for patients who were ultimately passed by nurses when compared to physicians, and delayed by 8 h for patients passed by SLP. These estimates were calculated using restricted mean survival time that was truncated at 24 h, and indicate that for stroke patients who were screened for dysphagia over 24 h, the average patient would experience a 5-h delay to dysphagia assessment by nursing staff, and 8-h delay among SLP, when compared to physicians. A 24-h threshold for dysphagia screening is a reasonable goal for all-comers with acute ischemic stroke, and it is the time window recommended by the American Heart Association/American Stroke Association for non-thrombolyzed stroke patients. Therefore it was an acceptable tau for our primary outcome measure. 6
As expected, treatment with intravenous thrombolysis was associated with delays in oral antithrombotic administration. For this reason, we conducted a subgroup analysis limited to patients who were not treated with thrombolysis. In these patients, physician screening continued to be strongly associated with earlier clearance for oral medication delivery.
Importantly, this study population was restricted to patients who ultimately passed any dysphagia screening or evaluation for oral medication and/or nutrition. Some patients who were ultimately passed by SLP dysphagia evaluation may have failed an initial screening, and only later were advanced following recovery of swallow function. The more severe symptoms at presentation (as captured by the NIHSS) and longer length of stay observed in patients passed by SLP likely reflect how some patients in this arm recovered their ability to swallow with time.7,8 Approximately one-third of patients passed by SLP required an orogastric tube after admission. By contrast, patients passed by nurse or physician screens were able to tolerate oral medications upon hospital arrival, none of whom required an orogastric tube. This introduces an important bias in our results (immortal time bias), in which some SLP patients were physically unable to pass the initial dysphagia screen. Because they could not pass the dysphagia screen within an initial window, it is the effect of this dysphagia recovery period rather than delay in SLP evaluation which contributed to delays in dysphagia evaluation for this group. Therefore, we would emphasize differences in time to screen and time to treatment between physician and nurse arms in order to escape the immortal time bias. Other than slight differences with respect to age, NIHSS subscore items, and presence of an intracranial occlusion (which was more common in the physician arm), the physician and nursing patient groups are better matched. Thus, differences in screening and treatment time between these groups are more likely to reflect changes that were made possible by this PI initiative.
Expediting the screening process using this simple intervention was not associated with any appreciable patient harm. Although the findings of this PI project are limited by the small sample of patients who passed a physician screen, there were no aspiration pneumonia events in this arm. If anything, cases of aspiration pneumonia were numerically higher for patients who passed SLP evaluation, but this difference did not achieve statistical significance. The higher rate of pneumonia in the SLP arm likely speaks to the more severe neurologic symptoms, and likely more dysphagia at the time of presentation, among patients who required SLP evaluation. In addition to pneumonia, we also examined incidences of death or severe disability by 90 days, and this was no different between groups. As a whole, our findings illustrate the promising potential outcomes of incorporating resident physician dysphagia screening into the stroke assessment workflow.
Our study may be limited by its single center nature, however it was implemented with multi-disciplinary input and planning. Other centers may be able to adapt our methods to meet their needs based on institutional and team goals. Although physician-administered dysphagia screens have previously been described in the literature,9,10 none include a three-tiered RN, physician, and SLP system. At our center, the NP dysphagia screen is meant to judiciously sort out patients at risk for complications in oral intake, such as clinically significant aspiration pneumonia. 11 These initial screens incorporate a wider breadth of fail criteria, including history of dysphagia or history of modified diet. Meanwhile, the goal of SLP evaluation is to comprehensively determine the extent of dysphagia and swallowing/speech capacity of the patient. SLP assessments delineate patient oropharyngeal capacity, and recommendations in route of therapeutic courses. In light of this framework, physician screenings were adapted to retain patients who failed the NP screen criteria but could reasonably tolerate oral antithrombotic therapy while awaiting further SLP evaluation. As a result, physician screens effectively bridge the RN screen and SLP evaluation. This intermediary screen provides a rapid dysphagia characterization that can be used to guide time-sensitive medical decisions.
We also found that physician screening could be integrated into a routine stroke assessment for many patients. The convenience of adapting the physician dysphagia screen comes from performing it as part of the initial stroke assessment by the neurology team. The physician screen functions as an elaboration on the NP screen, in addition to the dysphagia assessment needed for NIHSS.
Limitations
These results reflect the observations of a single-center PI intervention. The non-randomized nature may have contributed to a selection bias in which physicians were more or less likely to screen patients with a high probability of passing (or failing), therefore leading to earlier screening completion times. However, this was not conducted as a randomized clinical trial, but rather a PI intervention with a pragmatic design. Patients screened by physicians had more severe deficits with more frequent proximal large vessel occlusions, which likely reflects our institutional practice of administering loading doses of oral P2Y12 inhibitors (clopidogrel, ticagrelor) in patients with high grade atherosclerotic stenosis with or without occlusion. This is often done when emergent stenting is planned, however procedural data regarding occlusions and endovascular treatment were not captured as part of this study. While the findings may not be generalizable to many centers, the protocol we implemented is simple and can be quickly and easily translated to other institutions. Physician and non-physician providers can be educated regarding a rapid dysphagia screening which can be completed during the course of an urgent evaluation. In this study, in order to confirm an independent relationship between the provider type and time to clearance for oral medication, we calculated restricted mean survival times with adjustment for measurable confounders. RMST has attracted recent attention as an alternative to cox proportional hazards modeling as it is easier to interpret and explain to patients. Compared to cox modeling, which estimates relative differences over time, RMST provides an absolute magnitude of the exposure effect and can provide relative differences after adjustment for measurable confounders (RMST ratio). Furthermore, and perhaps more importantly, RMST is advantageous for circumstances in which events do not occur at a constant rate–and would therefore violate the proportional hazards assumption required in a cox regression.12,13 One important disadvantage of RMST is the truncation time (τ) which is effectively the time after which data are censored for statistical testing. For example, the time to dysphagia screen can only be statistically compared between provider groups based on the reasonable minimum at which the greatest time-to-event occurred (at approximately 511 minutes). Therefore, significant absolute and relative differences in time-to-event could only be compared up to 511 minutes, or 8 and one-half hours. We do not find this a significant limitation of the analysis because we believe 8 hours to be a reasonable goal for performing dysphagia screens by any provider (physician, nurse, speech pathologist) in most clinical circumstances.
The small number of patients screened (and passed) by physicians is also a limitation. While all our physicians were trained in dysphagia screens, the evaluation of acute ischemic stroke patients is time-sensitive. It is likely impractical for many physicians to screen patients for dysphagia while also completing a medical history, conducting a comprehensive neurologic assessment, and making emergent medical decisions regarding acute stroke treatment. There were significant differences in patients who underwent physician screening compared to those who did not, with patients screened by physicians generally having more severe deficits. It is possible that the PI intervention was not more consistently implemented due to insufficient investment, or prioritization of other acute care responsibilities by physician stakeholders for all-comers with stroke. These, and other unmeasured confounders are likely to account for the low overall proportion of patients screened by physicians. Now that these results confirm more rapid medication administration, and show no signal of patient harm, we are eager to extend this dysphagia paradigm to other acute stroke patients.
In addition to the faster time-to-antithrombotic intervals among stroke patients screened by a physician, there are other theoretical advantages to this PI initiative. In particular, when there may be shortages in nursing staff or the emergency department may be at (or above) capacity (such as the COVID-19 pandemic), physicians may be helpful in off-loading nursing staff of this screening assessment in order to proceed with next steps in medical care. This PI initiative was planned in advance of the COVID-19 pandemic, and was adopted the month in which the first case of the novel human coronavirus was diagnosed in our state. However, patient data in this analysis was collected for more than 1 year after the first wave, therefore we believe that our local emergency department services and staff to have adjusted to limitations in staffing, contact precautions, and other collateral effects of the pandemic. In a separate investigation, we have shown that an unrelated PI intervention (switching from alteplase to tenecteplase) during the pandemic led to faster treatment times without any incidental improvements (or delays) in time to thrombectomy or time to first antithrombotic among patients for whom our neurology service was consulted. 14
Conclusions
In this single-center, multi-disciplinary PI intervention, we successfully implemented a physician-driven dysphagia screen which was associated with faster dysphagia screens and earlier antithrombotic delivery. While there was a small number of patients who ultimately passed physician screening, there was no signal of harm with the intervention. This simple protocol may be adapted at centers with more stringent nursing dysphagia screening protocols in which physicians or advanced practice providers may be trained on fundamental swallow screens. Additional implications of this protocol include its use in prospective clinical trials in which ultra-early administration of oral medications need to be given to patients with acute stroke and mild dysphagia.
Supplemental Material
Supplemental Material for Resident-Driven Dysphagia Screening Protocol for Expedited Antithrombotic Delivery in Acute Ischemic Stroke by Linda Zhang, Scott Kamen, Jennifer Niles, Jessica Goss, Mark E. Heslin, Nicholas Vigilante, Lauren Thau, Christopher Edwards, Kyle R. Marden, Jesse M. Thon, Terri Yeager, and James E. Siegler in The Neurohospitalist
Acknowledgments
We would like to thank Dr Erika Mejia for her statistical support.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Linda Zhang https://orcid.org/0000-0001-6843-9587
Nicholas Vigilante https://orcid.org/0000-0003-0540-4816
Jesse M. Thon https://orcid.org/0000-0002-3255-760X
Terri Yeager https://orcid.org/0000-0003-4217-4984
James E. Siegler https://orcid.org/0000-0003-0287-3967
References
- 1.Rothwell PM, Algra A, Chen Z, Diener H, Norrving B, Mehta ZD. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet (British edition). 2016;388(10042):365-375. doi: 10.1016/S0140-6736(16)30468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston S, Elm J, Easton J, et al. Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke: A secondary analysis from the POINT randomized trial. Circulation (New York, N.Y.). 2019;140(8):658-664. doi: 10.1161/CIRCULATIONAHA.119.040713. [DOI] [PubMed] [Google Scholar]
- 3.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 4.Smithard DG, O'Neill PA, England RE, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997;12(4):188-193. doi: 10.1007/PL00009535. [DOI] [PubMed] [Google Scholar]
- 5.Royston P, Parmar MKB. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13(1):152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers W, Rabinstein A, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 7.Okubo PCMI, Fábio SRC, Domenis DR, Takayanagui OM. Using the National Institute of Health Stroke Scale to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012;33(6):501-507. doi: 10.1159/000336240. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M, Liesirova K, Broeg-Morvay A, et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PloS one. 2016;11(2):e0148424. doi: 10.1371/journal.pone.0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonios N, Carnaby-Mann G, Crary M, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: The modified mann assessment of swallowing ability. J Stroke Cerebrovasc Dis. 2010;19(1):49-57. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Turner-Lawrence DE, Peebles M, Price MF, Singh SJ, Asimos AW. A feasibility study of the sensitivity of emergency physician dysphagia screening in acute stroke patients. Ann Emerg Med. 2009;54(3):344-348. doi: 10.1016/j.annemergmed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Feng M, Lin Y, Chang Y, et al. The mortality and the risk of aspiration pneumonia related with dysphagia in stroke patients. J Stroke Cerebrovasc Dis. 2019;28(5):1381-1387. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Uno H, Wei L. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179-1180. doi: 10.1001/jamacardio.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloecker DE, Davies MJ, Khunti K, Zaccardi F. Uses and limitations of the restricted mean survival time: Illustrative examples from cardiovascular outcomes and mortality trials in Type 2 diabetes. Ann Internal Med. 2020;172(8):541-552. doi: 10.7326/M19-3286. [DOI] [PubMed] [Google Scholar]
- 14.Hall J, Thon JM, Heslin M, et al. Tenecteplase improves door‐to‐needle time in real‐world acute stroke treatment. Stroke: Vasc Intervent Neurol. 2021;1(1):e000102. doi: 10.1161/SVIN.121.000102. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Resident-Driven Dysphagia Screening Protocol for Expedited Antithrombotic Delivery in Acute Ischemic Stroke by Linda Zhang, Scott Kamen, Jennifer Niles, Jessica Goss, Mark E. Heslin, Nicholas Vigilante, Lauren Thau, Christopher Edwards, Kyle R. Marden, Jesse M. Thon, Terri Yeager, and James E. Siegler in The Neurohospitalist