Abstract
Venetoclax is a potent oral, highly selective small-molecule inhibitor of the antiapoptotic B-cell lymphoma 2 protein approved for chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma in treatment-naive patients (in combination with obinutuzumab) or for patients with relapsed/refractory CLL (in combination with rituximab). Venetoclax, in combination with azacitidine, decitabine, or low-dose cytarabine, is also approved in the United States for the treatment of newly diagnosed acute myeloid leukemia (AML) in adults who are ≥ 75 years or have comorbidities that preclude use of intensive induction chemotherapy. Clinical studies of patients with CLL or AML report both hematologic (e.g., neutropenia) and nonhematologic (e.g., gastrointestinal disorders and tumor lysis syndrome) adverse events associated with administration of venetoclax. It is therefore essential to provide information on the appropriate management of venetoclax-associated side effects. This article discusses the efficacy and safety of venetoclax administration and presents strategies specifically for the management of neutropenia and certain nonhematologic adverse events in patients receiving venetoclax for the treatment of AML and CLL.
Venetoclax (Venclexta) is a potent, selective, and orally bioavailable small-molecule inhibitor of B-cell lymphoma 2 (BCL-2), a protein that is overexpressed in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and acute myeloid leukemia (AML; Jia et al., 2008; Tzifi et al., 2012; Souers et al., 2013), independently of functional TP53 (Anderson et al., 2016). It was initially granted accelerated approval in the United States for the treatment of adult patients with CLL with 17p deletion (del[17p]) and received at least one prior line of therapy. This approval was expanded to all patients with relapsed/refractory (R/R) CLL on the basis of data from the MURANO trial (NCT02005471), a randomized, open-label, phase III study evaluating the efficacy of venetoclax in combination with rituximab in patients with R/R CLL (Seymour et al., 2018). In May 2019, venetoclax in combination with obinutuzumab received approval for the front-line treatment of adult patients (≥ 18 years) with CLL or SLL (AbbVie, Inc., 2021; Fischer et al., 2019). Venetoclax is also indicated in combination with azacitidine (AZA), decitabine (DAC), or low-dose cytarabine (LDAC) for the treatment of newly diagnosed AML in older patients (≥ 75 years) or in those with comorbidities precluding the use of intensive induction chemotherapy (DiNardo et al., 2020; Wei et al., 2020). This article reviews the efficacy and safety of venetoclax, focusing on the approach for the advanced practitioner to manage neutropenia and certain nonhematologic adverse events (AEs) in patients receiving venetoclax for CLL or AML.
CLINICAL STUDIES IN CHRONIC LYMPHOCYTIC LEUKEMIA
The efficacy of venetoclax monotherapy in patients with previously treated CLL/SLL and del(17p) was demonstrated in three single-arm, phase I to II studies (Coutre et al., 2018; Jones et al., 2018; Roberts et al., 2016; Stilgenbauer et al., 2016). On the basis of these studies, an open-label, randomized phase III trial (MURANO) assessed the efficacy of venetoclax in combination with rituximab (VEN+R, N = 194) for a 2-year fixed duration vs. that of bendamustine-rituximab (BR) for six cycles in patients with R/R CLL, regardless of del(17p) or TP53 status (Seymour et al., 2018). Demographics and baseline characteristics were well balanced in the two treatment groups, with a median age of 65 years and 73.8% males across both groups. Both arms were also balanced for high-risk disease features, including immunoglobulin heavy chain (IGHV) status and the presence of del(17p). In total, 246 of 360 (68%) patients who tested for IGHV mutational status had unmutated IGHV (VEN+R arm and BR arm: n = 123 each), while 92 of 342 (27%) assessed patients had del(17p) (VEN+R arm and BR arm: n = 46 each; Seymour et al., 2018). The independent review committee-assessed objective response rate (ORR) was 92.3% in the VEN+R arm and 72.3% in the BR arm, with complete remission (CR) achieved in 8.2% and 3.6% of patients in the VEN+R arm and BR arm, respectively (both p = .08; Seymour et al., 2018). The investigator-assessed ORR was 93.3% in the VEN+R arm and 67.7% in the BR arm, with CR or CR with incomplete hematologic recovery (CRi) achieved in 26.8% and 8.2% of patients, respectively. Different interpretations of residual adenopathy on computed tomography scans, specifically related to lesions measuring ≤ 30 mm, may explain the discordance in the investigator- and independent review committee-assessed CR or CRi rates. At 3 months after the last dose of rituximab, the minimal residual disease (MRD) negativity rate in the peripheral blood of patients who achieved partial response or better was 54% (104/194) in the VEN+R arm compared with 12% (23/195) in the BR arm by allele-specific oligonucleotide-PCR (AbbVie, Inc., 2021). Moreover, MRD status was predictive of progression-free survival (PFS) at the time of the combination-treatment response assessment visit. The most common AEs of any grade in the VEN+R cohort were neutropenia (61%), diarrhea (40%), upper respiratory tract infection (22%), and nausea (21%); and in the BR cohort, neutropenia (44%), nausea (34%), infusion-related reaction (34%), anemia (23%), thrombocytopenia (22%), and constipation and fatigue (20% each; Seymour et al., 2018).
After long-term follow-up (median: 59 months), the median PFS for VEN+R was 53.6 months (95% CI = 48.4–57.0) compared with 17.0 months (95% CI = 15.5–21.7) for BR, hazard ratio (HR), 0.19 (95% CI = 0.15–0.26; p < .0001; Kater et al., 2020). The average time to next therapy (TTNT) was longer for patients who received VEN+R compared with BR, with a median TTNT of 57.8 months (55.1–not estimable) vs. 23.9 months (20.7–29.5), respectively (HR, 0.26; 95% CI = 0.20–0.35). In total, 67 patients treated with VEN+R vs. 123 treated with BR received subsequent anti-CLL therapy following disease progression. The most common subsequent therapy was venetoclax on the VEN+R arm (47.8%) and Bruton tyrosine kinase inhibitor on the BR arm (58.5%; Harrup et al., 2020). A sustained overall survival (OS) rate at 5 years was also observed for VEN+R (82.1%) vs. BR (62.2%; HR, 0.40; 95% CI = 0.26–0.62; p < .0001; Kater et al., 2020).
A multinational, open-label, phase III trial of venetoclax in combination with obinutuzumab (VEN+G) vs. chlorambucil in combination with obinutuzumab (GClb) in patients with previously untreated CLL and coexisting medical conditions making them unfit (CLL14; NCT02242942) reported prolonged PFS with VEN+G in comparison with GClb (Fischer et al., 2019). The results from this study supported the regulatory approval of venetoclax in previously untreated CLL. A total of 432 patients (median age: 72 years) were randomized to each treatment group (n = 216, each). After a median follow-up of 28.1 months, PFS survival events had occurred in 30 patients in the VEN+G group compared with 77 patients in the GClb group (HR, 0.35; 95% CI = 0.23–0.53; p < .001). Response was achieved in 84.7% vs. 71.3% of patients treated with VEN+G or GClb, with CR rates of 49.5% vs. 23.1%, respectively (both p < .001). Three months after the completion of treatment, MRD negativity rate in the peripheral blood of patients who achieved CR was 87% (87/100) in patients treated with VEN+G and 62% (29/47) in patients treated with GClb. The most common AEs of any grade (≥ 15%) observed with VEN+G were neutropenia (58%), infusion-related reactions (45%), diarrhea (28%), thrombocytopenia (24%), pyrexia (23%), nausea (19%), anemia (17%), cough (16%), and fatigue (15%; Fischer et al., 2019). Four-year PFS estimate (median follow-up: 52.4 months) was 74.0% in the VEN+G arm compared with 35.4% in the GClb arm (HR, 0.33; 95% CI = 0.25–0.45; p < .0001; Al-Sawaf et al., 2021). The PFS benefit was also observed with VEN+G in patients with TP53 mutation/deletions and in patients with unmutated IGHV. With approximately 4 years of follow-up, data for OS remain immature with no difference in OS between the two arms (median OS: not reached in both arms; 4-year OS rate: VEN+G, 85.3%; GClb, 83.1% [HR, 0.85; 95% CI = 0.54–1.35; p = .49]).
CLINICAL STUDIES IN ACUTE MYELOID LEUKEMIA
Acute myeloid leukemia occurs more commonly in the elderly, with cure rates < 10%. This is likely due to an increase in adverse genomic features, limited therapeutic options due to functional status, and poor responses to induction therapy (Krug et al., 2011). BCL-2 overexpression has been implicated in survival of AML cells and treatment resistance (Mehta et al, 2013). On the basis of the limited therapeutic options for elderly patients and the known role of BCL-2 overexpression in AML, a phase Ib dose-escalation and -expansion study of venetoclax combined with hypomethylating agents (HMAs) DAC or AZA, in treatment-naive elderly patients with AML ineligible for standard induction therapy (N = 145), was initiated on October 6, 2014 (DiNardo et al., 2019). Patients had a median age of 74 years, and 49% had poor-risk cytogenetics [i.e.,–5,–7, abn(3q)]. The median time on study was 8.9 months, and 67% of patients achieved CR/CRi at all doses (venetoclax 400 mg-HMA: 73% CR/CRi; venetoclax 800 mg-HMA: 65% CR/CRi). Patients aged > 75 years and those with poor-risk cytogenetics had CR/CRi rates of 65% and 60%, respectively. The median duration of CR/CRi in all patients was 11.3 months, and median OS was 17.5 months. Early (30-day) mortality was 3%. Of note, in the 400-mg venetoclax cohort, the median OS had not been reached at the cutoff date of July 7, 2017. The most common treatment-emergent AEs of any grade (≥ 30%; 400-mg venetoclax cohort) in combination therapy with AZA/DAC were nausea (62%/55%), constipation (59%/45%), diarrhea (52%/42%), febrile neutropenia (38%/61%), fatigue (34%/39%), peripheral edema (34%/23%), thrombocytopenia (34%/19%), vomiting (31%/32%), anemia (31%/23%), decreased white blood cell (WBC) count (24%/42%), cough (21%/32%), and hypokalemia (17%/32%; DiNardo et al., 2019).
An international, open-label, phase Ib/II study assessed the safety and efficacy of venetoclax (600 mg) in combination with LDAC (VEN+LDAC) in previously untreated adults with AML who were ineligible for intensive chemotherapy (Wei et al., 2019). The use of HMAs for prior treatment of myelodysplastic syndrome was permitted. The median age was 74 years (N = 82) and 32% had poor-risk cytogenetics. The median treatment duration was 4.2 months. Early (30-day) mortality was 6%. CR/CRi was achieved in 54% of patients and the median time to first response was 1.4 months. The median OS for all patients was 10.1 months (95% CI = 5.7–14.2). Among patients achieving CR/CRi, the median duration of remission (DOR) was 8.1 months (95% CI = 5.3–14.9 months). CR/CRi was achieved in 62% of patients without prior HMA exposure, with a median DOR of 14.8 months (95% CI = 5.5 months–not reached), and median OS of 13.5 months (95% CI = 7.0–18.4 months). The most common treatment-emergent AEs of any grade (≥ 30%) were nausea (70%), diarrhea (49%), hypokalemia (48%), fatigue (43%), febrile neutropenia (43%), thrombocytopenia (38%), constipation (35%), decreased appetite (34%), decreased WBC count (34%), hypomagnesemia (33%), and vomiting (31%).
On the basis of the favorable safety profile and promising clinical activity reported in the phase Ib and phase I/Ib studies, the U.S. Food and Drug Administration (FDA) granted accelerated approval in November 2018 to venetoclax in combination with AZA, DAC, or LDAC for the treatment of newly diagnosed AML in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. Phase III confirmatory trials were designed to evaluate the efficacy and safety of VEN+AZA vs. placebo (Pbo)+AZA (VIALE-A) or VEN+LDAC vs. Pbo+LDAC (VIALE-C) in previously untreated patients with AML ineligible for intensive induction therapy (DiNardo et al., 2020; Wei et al., 2020). Overall survival was the primary endpoint for both studies in the United States.
For VIALE-A, patients (N = 431) had a median age of 76 years (range, 49–91) in both treatment groups (VEN+AZA, n = 286; Pbo+AZA, n = 145). At a median follow-up of 20.5 months, the median OS was 14.7 months (95% CI = 11.9–18.7) for patients treated with VEN+AZA vs. 9.6 months (95% CI = 7.4–12.7) for patients in the Pbo+AZA arm (HR, 0.66; 95% CI = 0.52–0.85; p < .001). The incidence of CR/CRi was higher in the VEN+AZA arm (66.4%) compared with the Pbo+AZA arm (28.3%); CR rates were 36.7% vs. 17.9% (p < .001), respectively. The most common reported hematologic AEs of grade ≥ 3 in the Ven+AZA and Pbo+AZA arms included thrombocytopenia (45% vs. 38%), neutropenia (42% vs. 28%), febrile neutropenia (42% vs. 19%), anemia (26% vs. 20%), and leukopenia (21% vs. 12%). Common gastrointestinal AEs of any grade in the Ven+AZA vs. Pbo+AZA arms included nausea (44% vs. 35%), constipation (43% vs. 39%), diarrhea (41% vs. 33%), and vomiting (30% vs. 23%). Infections of any grade occurred in 84% of patients in the VEN+AZA arm and 67% of patients in the Pbo+AZA arm; serious AEs occurred in 83% and 73%, respectively (DiNardo et al., 2020).
Patients enrolled in VIALE-C (N = 211) had a median age of 76 years (range, 36–93) in both treatment groups (VEN+LDAC, n = 143; Pbo+LDAC, n = 68). At a median follow-up of 12.0 months, the median OS was 7.2 months (95% CI = 5.6–10.1) vs. 4.1 months (95% CI = 3.1–8.8) for patients treated with VEN+LDAC or Pbo+LDAC, respectively (HR, 0.75; 95% CI = 0.52–1.07; p = .11). At an unplanned analysis with additional 6-month follow-up, the VEN+LDAC arm demonstrated a median OS of 8.4 months (95% CI = 5.9–10.1) compared with 4.1 months (95% CI = 3.1–8.1) for Pbo+LDAC (HR, 0.70; 95% CI = 0.50–0.99; nominal p = .04). The CR/CRi rates were 48% and 13% (p < .001) for VEN+LDAC compared with Pbo+LDAC, respectively; CR rates were 27% vs. 7% (p < .001). The most frequent grade ≥ 3 hematologic AEs for VEN+LDAC vs. Pbo+LDAC included neutropenia (46% vs. 16%), febrile neutropenia (32% vs. 29%), thrombocytopenia (45% vs. 37%), and anemia (25% vs. 22%). The most common non-hematologic AEs of any grade (≥ 25%) for VEN+LDAC vs. Pbo+LDAC included nausea (42% vs. 31%), hypokalemia (28% vs. 22%), diarrhea (28% vs. 16%), vomiting (25% vs. 13%), and constipation (18% vs. 31%). Serious AEs (any grade: VEN+LDAC, 66%; Pbo+LDAC, 62%) common to patients with AML included febrile neutropenia (16% vs. 18%), pneumonia (13% vs. 10%), and sepsis (6% in both arms); no other serious AEs were observed in ≥ 10% of patients in either arm (Wei et al., 2020).
The results from VIALE-A and VIALE-C supported the full FDA approval of venetoclax in combination with either an HMA (AZA/DAC) or LDAC in patients with previously untreated AML ineligible for intensive induction chemotherapy.
NEUTROPENIA
Proposed Mechanism
BCL-2 inhibition using venetoclax analogues and venetoclax was shown to cause neutropenia in rats and exacerbate taxane-induced neutropenia, as well as inhibit granulocyte colony formation in human bone marrow samples (Leverson et al., 2015). Furthermore, neutropenia was previously recorded in heavily pretreated patients with CLL or non-Hodgkin lymphoma in navitoclax trials (Wilson et al., 2010; Roberts et al., 2012) and may be a class effect of BCL-2–inhibiting drugs (Leverson et al., 2015). Indeed, it is likely that the potent and selective BCL-2 inhibition displayed by venetoclax, in particular its on-target inhibition of BCL-2 in neutrophil precursors, drives the incidence of neutropenia that has been observed (Lampson & Davids, 2017).
Neutropenia Incidence
In pooled single-arm trials of venetoclax monotherapy in patients with CLL or SLL (N = 352), 45% of patients had grade ≥ 3 neutropenia, with only 6% experiencing febrile neutropenia (Roberts et al., 2016; Stilgenbauer et al., 2016; Coutre et al., 2018; Jones et al., 2018). Dose interruptions and reductions due to AEs occurred in 36% and 13%, respectively, while only 9% required discontinuation (AbbVie, Inc., 2021).
When venetoclax was administered in combination with rituximab (VEN+R) or obinutuzumab (VEN+G), the rates of grade 3/4 neutropenia were 57.7% and 52.8%, respectively. Despite high rates of grade 3/4 neutropenia, rates of febrile neutropenia (3.6% VEN+R, 6% VEN+G) and grade 3/4 infections (17.5% VEN+R, 17.5% VEN+G) remained tolerable. Dose interruptions in both the VEN+R (43.3%) and VEN+G (41%) studies were mandated in patients with grade 3/4 neutropenia irrespective of infection. Dose reductions occurred in 12.4% and 13.0% of patients treated with VEN+R and VEN+G, respectively; discontinuation occurred in only 2.4% and 2.0% of patients, respectively. Grade ≥ 3 neutropenia was observed more commonly during the 6-month combination period of VEN+R (46%) and VEN+G (54%) and decreased significantly during the periods of venetoclax monotherapy (VEN+R, 11%; VEN+G, 23%). The median duration of grade 3/4 neutropenia in patients treated with VEN+G was 22 days (range, 2–363; AbbVie data on file; ABVRRTI68238) and 8 days (grade 3, range, 1–712; grade 4, range, 1–212) for patients treated with VEN+R (Seymour et al., 2018).
In patients with AML who received VEN+AZA or VEN+LDAC, the incidence of grade ≥ 3 neutropenia was 42% and 46%, respectively. Grade ≥ 3 febrile neutropenia was reported in 42% of patients treated with VEN+AZA and 32% of patients treated VEN+LDAC (DiNardo et al., 2019, 2020; Wei et al., 2020; Table 1). Dose reductions/interruptions for AEs occurred in 3%/72% of patients treated with VEN+AZA and 9%/63% of patients treated with VEN+LDAC, respectively. Discontinuation of venetoclax due to AEs was required in 24% and 25% of VEN+AZA- and VEN+LDAC-treated patients, respectively (AbbVie, Inc., 2021).
Table 1. Neutropenia and Febrile Neutropenia in AML Patients Treated With Venetoclax.
| Venetoclax + azacitidine (n = 283) | Venetoclax + LDAC (n = 142) | Venetoclax + decitabine (n = 31)a | |
|---|---|---|---|
| Grade ≥ 3 neutropenia | 42% | 46% | 42%b |
| Grade ≥ 3 febrile neutropenia | 42% | 32% | 61% |
Note.AML = acute myeloid leukemia; LDAC = low-dose cytarabine. Information from DiNardo et al. (2019, 2020); Wei et al. (2020).
Grade 3/4 events.
Decreased white blood cell count.
Neutropenia Management
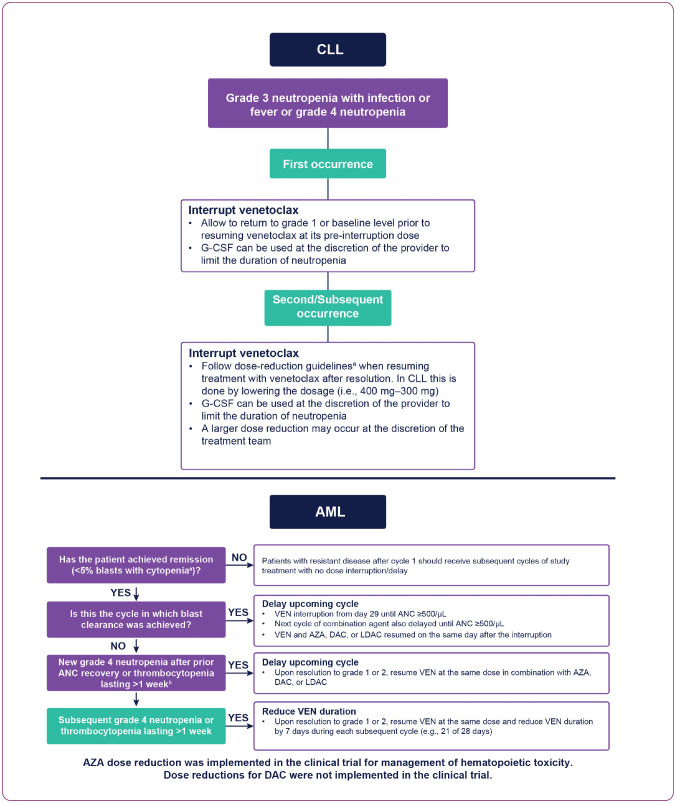
Optimal neutropenia management in CLL should include consideration of AE grade and occurrence frequency (Figure 1). In the case of grade 3 neutropenia with infection/fever or grade 4 neutropenia at first occurrence, venetoclax interruption is recommended until return to grade 1 or baseline level, followed by administration of venetoclax at its pre-interruption dose. For second/subsequent occurrences, venetoclax should be interrupted and subsequently resumed following dose-reduction guidelines (Figure 1 and Table 2). Additionally, growth factor support for substantial neutropenia should be provided according to institutional standards of care.
Figure 1.
Recommended venetoclax dose modifications or interruptions for neutropenia in CLL and AML. AML = acute myeloid leukemia; ANC = absolute neutrophil count; AZA = azacitidine; CLL = chronic lymphocytic leukemia; DAC = decitabine; G-CSF = granulocyte colony-stimulating factor; LDAC = low-dose cytarabine; VEN = venetoclax. aGrade 4 neutropenia with or without fever or infection, or grade 4 thrombocytopenia. bUnless due to underlying disease (e.g., relapse).
Table 2. Recommended Venetoclax Dose Modifications for Toxicities in CLL/SLL.
| Event | Occurrence | Action | |
|---|---|---|---|
| Tumor lysis syndrome | |||
| Blood chemistry changes or symptoms suggestive of TLS | Any | Withhold next day's dose; if resolved within 24–48 hr of last dose, resume at the same dose | |
| If resolved in 48 hr or more, resume at a reduced dose | |||
| For any clinical TLS events,a resume at a reduced dose following their resolution | |||
| Nonhematologic toxicities | |||
| Grade 3–4 | First occurrence | Interrupt venetoclax; resume at the same dose once the toxicity has resolved to grade 1 or baseline level. No dose modification is required | |
| ≥ Second occurrence | Interrupt venetoclax; when resuming treatment after resolution, use a reduced dose (for a dose at interruption of 400, 300, 200, 100, 50, or 20 mg use 300, 200, 100, 50, 20, or 10 mg, respectively) | ||
| Hematologic toxicities | |||
| All grade 4 (except lymphopenia) | First occurrence | Interrupt venetoclax; administer G-CSF to reduce infection risks associated with neutropenia, if clinically indicated. Once toxicity has resolved to grade 1 or baseline level, resume venetoclax at the same dose | |
| ≥ Second occurrence | Interrupt venetoclax; consider using G-CSF as clinically indicated; when resuming treatment after resolution, use a reduced dose (for a dose at interruption of 400, 300, 200, 100, 50, or 20 mg use 300, 200, 100, 50, 20, or 10 mg, respectively) | ||
Note. CLL = chronic lymphocytic leukemia; SLL = small lymphocytic lymphoma; G-CSF = granulocyte colony-stimulating factor; TLS = tumor lysis syndrome.
Clinical TLS was defined as laboratory TLS with clinical consequences such as acute renal failure, cardiac arrhythmias, or sudden death and/or seizures.
Granulocyte colony-stimulating factor (G-CSF) was used during clinical studies to help limit the duration of neutropenia. In previous monotherapy studies (N = 352), G-CSF use ranged from 24% to 39% (Coutre et al., 2018; Roberts et al., 2016; Stilgenbauer et al., 2016), while in venetoclax combination studies with venetoclax + obinutuzumab and venetoclax + rituximab, G-CSF was utilized in 43.5% and 47.9% of patients, respectively (Seymour et al., 2018; AbbVie data on file; ABVRRTI69512). Despite higher rates of neutropenia observed with venetoclax combinations with anti-CD20 antibodies, few patients required dose discontinuation due to neutropenia.
Neutropenia management was specifically defined in the study protocols for venetoclax combination studies with rituximab and obinutuzumab in patients with CLL. Both protocols mandated holding venetoclax for grade ≥ 3 neutropenia irrespective of fever/infection, which is not in line with current label recommendations. If an event occurred during the dose-combination phase, the obinutuzumab or rituximab doses were also held until resolution of the neutropenia per protocol specifications (rituximab: absolute neutrophil count [ANC] grade ≤ 2; obinutuzumab: ANC ≥ 1 × 109/L). G-CSF (or growth factors) for neutropenia was administered as indicated. For patients experiencing neutropenia requiring interruption during the combination phase of venetoclax + an anti-CD20, prophylactic G-CSF was initiated in subsequent combination cycles. For patients with prolonged neutropenia despite dose interruptions/reductions or those with new-onset cytopenias after previously stable blood counts, a bone marrow biopsy should be considered for disease assessment.
In AML, remission status by bone marrow assessment should be considered to guide the management of neutropenia (Figure 1). If neutropenia occurs prior to remission, treatment should not be interrupted in most cases (AbbVie, Inc., 2021). Patients should receive supportive care with blood products and prophylactic anti-infectives until remission or progression occurs. Once bone marrow blasts of < 5% are confirmed, the subsequent cycle of VEN+HMA or VEN+LDAC should be held until ANC ≥ 500/µL. G-CSF can be utilized when clinically indicated (Figure 1). When determining the timing of initial disease-response assessment, it is noteworthy that time to first response in AML was 1 month for VEN+AZA and VEN+LDAC-treated patients, and 1.9 months for VEN+DAC-treated patients. Therefore, patients with prolonged cytopenias during cycle 1 will benefit from early disease assessment to determine if the patient has persistent disease or CR/CRi (CRi/morphologic leukemia-free state), whereby VEN+HMA or VEN+LDAC dosing can be held to allow for count recovery.
For patients who are in remission with first occurrence of grade 4 neutropenia (ANC < 500/µL), with or without fever or infection, lasting > 7 days, the subsequent VEN+HMA or VEN+LDAC treatment cycle should be delayed and blood counts monitored. Supportive care, including G-CSF and antimicrobial prophylaxis, can be used if clinically indicated for neutropenia, and combination treatment resumed at its initial dose once the toxicity resolves to grade 1 to 2. For second/subsequent occurrences of grade 4 neutropenia, with or without fever or infection, lasting > 7 days, similar measures apply, with a reduction in venetoclax treatment duration by 7 days for each subsequent cycle, for example, 21 days of venetoclax in a 28-day cycle (see Figure 1). For patients who experience persistent/prolonged cytopenias or new-onset cytopenias while in remission, a bone marrow biopsy should be considered to assess ongoing response. In the VEN+HMA studies, AZA dose reductions could be considered after cycle 4 in patients whose recovery was > 21 days. AZA could be dose reduced by 50% if the bone marrow cellularity was between 15% to 50% and by 33% if the bone marrow cellularity was < 15%.
Recently, a post-hoc analysis of the frequency and management of cytopenia in patients from the VIALE-A study who achieved a best response of CR or CR with partial hematologic recovery (CRh) was reported (AbbVie data on file, ABVRRTI71565). In total, 66% (185/282) of VEN+AZA-treated patients and 23% (33/143) of Pbo+AZA-treated patients achieved a best response of CR/CRh. Blast clearance occurred early for patients treated with VEN+AZA who achieved CR/CRh, and a delay of the subsequent treatment cycle to allow for ANC recovery was common. A delay in the next treatment cycle after achieving blast clearance occurred in 74% and 67% of patients treated with VEN+AZA and Pbo+AZA, respectively, with a median duration per cycle delay post-blast clearance of 10 and 6.5 days. Among CR/CRh patients treated with VEN+AZA vs. Pbo+AZA, post-remission grade 4 cytopenia lasting ≥ 7 days was reported in 87% and 45% of patients, respectively. An increased proportion of CR/CRh patients in the VEN+AZA arm experienced post-remission cycle delays due to cytopenia compared with the Pbo+AZA arm (78% vs. 33%). For these patients, the median duration of cycle delays was 13 days in the VEN+AZA arm and 11 days in the Pbo+AZA arm. In addition, a higher percentage of CR/CRh patients had post-remission cycles with a reduction in venetoclax or placebo dosing days and/or cycle delays ≥ 7 days (total) due to cytopenia in the VEN+AZA arm (74%) than in the Pbo+AZA arm (27%). A post-remission cycle of ≤ 21 days after achieving CR/CRh occurred in 69% and 30% of patients treated with VEN+ AZA and Pbo+AZA, respectively, with a median time from remission to first ≤ 21-day cycle of 92 and 74 days, respectively.
NAUSEA/VOMITING
Gastrointestinal disorders are the prominent class of AEs frequently reported in patients with CLL and AML treated with venetoclax as a single agent or in combination studies. In pooled data (N = 352) from three single-arm trials of venetoclax monotherapy in patients with CLL, the rates of nausea and vomiting reported at any grade/grade ≥ 3 were 42%/1% and 16%/1%, respectively (Roberts et al., 2016; Stilgenbauer et al., 2016; Coutre et al., 2018; Jones et al., 2018; AbbVie, Inc., 2021). The rates of nausea and vomiting reported at any grade/grade ≥ 3 in patients with CLL receiving VEN+R were 21%/1% and 8%/(data not shown; Seymour et al., 2018); rates of 19%/0% and 10%/1% were reported for VEN+G (Fischer et al., 2019). It is important to note that the relative rate of grade 3/4 nausea and vomiting remained low. Patients with AML who received venetoclax in combination with AZA or LDAC reported any-grade/grade ≥ 3 nausea at rates of 44%/2% and 42%/1%, respectively; AEs of vomiting at any grade occurred at rates of 30%/2% and 25%/< 1%, respectively (DiNardo et al., 2020; Wei et al., 2020).
The National Comprehensive Cancer Network antiemetic guidelines classify venetoclax as low to minimal risk where antiemetics can be used as needed (National Comprehensive Cancer Network, 2021). Recommended oral agents should be provided daily on an as-needed basis (prn) prior to the start of therapy. These agents include metoclopramide (10–20 mg orally [po] and then every 6 hours prn), prochlorperazine (10 mg po and then every 6 hours prn; maximum 40 mg/day), and 5-hydroxytryptamine 3 antagonists (dolasetron [100 mg po daily prn], granisetron [1- to 2-mg total dose po daily prn], or ondansetron [8- to 16-mg total dose po daily prn]). Patients experiencing low/minimal emetogenicity should be escalated to the next higher level of antiemetic therapy for future cycles of anticancer therapy if nausea/vomiting is still experienced.
DIARRHEA
In patients with CLL, rates of diarrhea reported at any grade/grade ≥ 3 in pooled single-arm trials of venetoclax monotherapy (N = 352) were 43%/3% (Roberts et al., 2016; Stilgenbauer et al., 2016; Coutre et al., 2018; Jones et al., 2018; AbbVie, Inc., 2021), 40%/3% in combination with rituximab, and 28%/4% in combination with obinutuzumab (AbbVie, Inc., 2021). In patients with AML receiving venetoclax in combination with AZA or LDAC, any-grade/grade ≥ 3 diarrhea was reported in 41%/5% and 28%/3% (LDAC) of patients, respectively (DiNardo et al., 2020; Wei et al., 2020). Although prevalence was high, it is important to note that the relative rate of grade 3/4 diarrhea remained low. In pooled monotherapy data, the rate of grade 3 diarrhea was 3%, with only 1/352 patients having a serious AE related to diarrhea. Similarly, in patients treated with VEN+R and VEN+G, the rates of grade 3/4 diarrhea were 3% and 4%, respectively.
The study protocols for venetoclax combination studies with rituximab and obinutuzumab provide management guidelines for the treatment of nonhematologic events (Seymour et al., 2018; Fischer et al., 2019). In those trials, patients with grade 3 or 4 events were recommended to have venetoclax delayed for a maximum of 28 days; obinutuzumab and rituximab should be delayed if an event occurred during cycles 1 to 6. If an event was the first occurrence, previous doses of venetoclax and obinutuzumab or rituximab could be resumed if the event improved to grade ≤ 1 or baseline. For subsequent episodes, venetoclax should be restarted at one dose-level reduction (e.g., 400 mg reduced to 300 mg) upon improvement to grade ≤ 1 or baseline. Similar to grade 4 events, treatment with obinutuzumab or rituximab should also be delayed if the event occurred during cycles 1 to 6. After resolution, venetoclax and obinutuzumab or rituximab should be resumed at full dose. No dose reduction or delays are recommended for grade 1 events. Recommended dose-modification for patients with AML treated with venetoclax-based regimens who experience a grade 3 or 4 nonhematologic AE include interruption of venetoclax at any occurrence if the AE is not resolved with supportive care. Venetoclax can be resumed at the original dose upon resolution of AE to grade 1 or baseline level. No specific recommendations are provided for grade < 3 events (AbbVie, Inc., 2021). It remains important both in patients with CLL and AML to exclude other causes of diarrhea prior to considering the AE a result of the drug.
TUMOR LYSIS SYNDROME
CLL/AML Incidence of Tumor Lysis Syndrome
Tumor lysis syndrome (TLS), an infrequent but relevant AE, can be classified as laboratory or clinical (Howard et al., 2011). Laboratory TLS (LTLS) requires that two or more of the following abnormalities are met within 3 days before or 7 days after the initiation of chemotherapy: 25% decrease from baseline in serum calcium, and/or 25% increase from baseline in the serum values of uric acid, potassium, or phosphorus. Clinical TLS (CTLS) is defined as LTLS with one of the following abnormalities: creatinine > 1.5 × upper limit of normal, cardiac arrhythmia or sudden death, or seizure (Howard et al., 2011).
During the early development of venetoclax in CLL, CTLS events led to two deaths prior to adoption of the current 5-week ramp-up period with a lower dose: one death after an initial 50-mg venetoclax dose (Seymour et al., 2018), and one after a 1,200-mg dose (Roberts et al., 2016). To mitigate TLS risk, modifications including TLS risk-stratification, prophylaxis, monitoring, and starting with a lower initial dose (20 mg) were introduced to subsequent clinical protocols (Davids et al, 2018; Figure 2; Table 2). When the dose-mitigation strategies were followed, no clinical TLS events were recorded in subsequent monotherapy (Stilgenbauer et al., 2016; Coutre et al., 2018; Jones et al., 2018) or combination therapy (Fischer et al., 2019; Kater et al., 2018) studies.
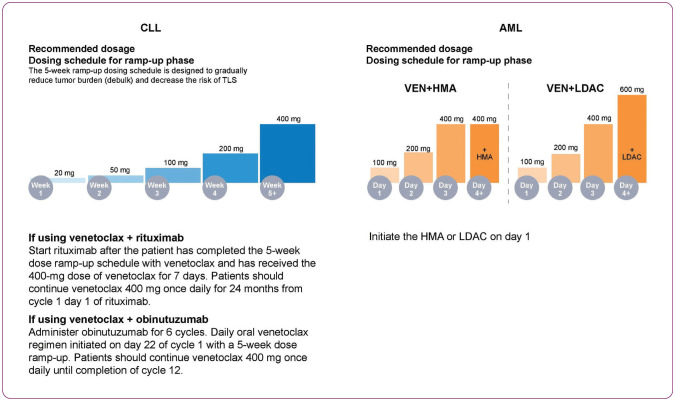
Figure 2.
Recommended venetoclax dosage ramp-up schedule for CLL (left) and AML (right). AML = acute myeloid leukemia; CLL = chronic lymphocytic leukemia; HMA = hypomethylating agent; LDAC = low-dose cytarabine; TLS = tumor lysis syndrome; VEN = venetoclax.
Currently, all venetoclax dosing regimens in CLL feature a 5-week ramp-up to a target dose of 400 mg. In the phase III MURANO trial, venetoclax was scaled up prior to the start of rituximab at the end of week 5. In CLL14, obinutuzumab was started prior to venetoclax, which allowed for absolute lymphocyte count (ALC) normalization in up to 98% of patients before beginning the venetoclax ramp-up (Figure 2). Following this ramp-up schedule, venetoclax was administered at a continued dose of 400 mg daily (AbbVie, Inc., 2021). Low rates of LTLS were reported in MURANO (3%; 6/194), with no incidence of CTLS. In CLL14, reported AEs of LTLS were 1% (3/212), with all events occurring during the obinutuzumab lead-in prior to initiation of venetoclax (Fischer et al., 2019). All LTLS events reported in combination studies resolved and did not lead to withdrawal from study. Subsequent studies have reported CTLS, including fatal events and renal failure requiring dialysis, in patients with high tumor burden when treated with venetoclax monotherapy (AbbVie, Inc., 2021).
Due to the more acute nature of AML compared with CLL, the venetoclax dose ramp-up is condensed over 3 to 4 days, depending on the backbone therapy used (Figure 2). Moreover, the venetoclax dose used depends on the combination agent, with the highest dose being 400 mg in combination with HMAs, and 600 mg when combined with LDAC (AbbVie, Inc., 2021). In the VIALE-C study, eight patients reported AEs of TLS, all within the VEN+LDAC treatment arm; seven were reported as grade ≥ 3 events. Out of the eight patients, two did not meet Howard criteria but were reported as TLS by the investigator due to kidney injury. CTLS was reported for four patients. Two of these four cases were reported as serious AEs of TLS and resulted in death (AbbVie data on file; ABVRRTI71500). Three events of TLS were reported during the dose ramp-up period in the VIALE-A study (DiNardo et al., 2020). All were within the VEN+AZA treatment arm and were considered LTLS. Patients had transient biochemical changes that resolved with uricosuric agents and calcium supplements. No interruptions in administration of study drug occurred.
Assessment of Tumor Lysis Syndrome Risk: CLL vs. AML
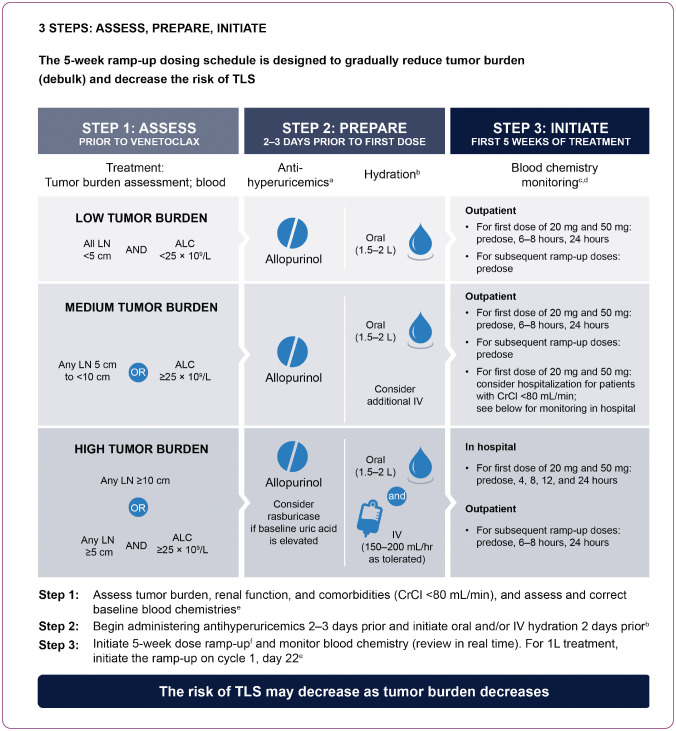
The risk of TLS in AML and CLL is based on multiple factors, including tumor burden and comorbidities. Recommendations for TLS prophylaxis and monitoring during venetoclax treatment are provided on the basis of tumor burden assessments from clinical trial data (AbbVie, Inc., 2021). Risk evaluation in CLL includes radiographic imaging of tumor burden assessments (on the basis of lymph node size, as detailed below), an accurate pretreatment ALC, serum blood chemistry, and an accurate creatinine clearance measurement. Patients with CLL are divided into three risk categories for TLS on the basis of tumor burden: low tumor burden (low lymph nodes [LN] < 5 cm and ALC < 25 × 109/L); medium tumor burden (LN 5 cm to < 10 cm or ALC ≥ 25 × 109/L); or high tumor burden (LN ≥ 10 cm or ALC ≥ 25 × 109/L and LN ≥ 5 cm). Creatinine clearance < 80 mL/min at screening was identified as a secondary risk factor (Figure 3). Splenomegaly should also be assessed, as its presence may also increase the risk of TLS (AbbVie Inc., 2021). Once tumor burden has been determined, prophylactic measures including adequate hydration and antihyperuricemic agents are administered to patients prior to the first dose of venetoclax. Prophylactic recommendation for low and medium risk is similar (oral hydration: 1.5–2 L/day; antihyperuricemics: allopurinol); intravenous (IV) fluids can also be considered for patients who cannot tolerate oral fluids (AbbVie, Inc., 2021).
Figure 3.
Initiating the 5-week venetoclax dose ramp-up in patients with chronic lymphocytic leukemia. 1L = first line; ALC = absolute lymphocyte count; CrCl = creatinine clearance; IV = intravenous; LN = lymph node; TLS = tumor lysis syndrome; VEN = venetoclax. aStart allopurinol or xanthine oxidase inhibitor 2–3 days prior to initiation of VEN. b1.5–2 L of water (6–8 glasses) should be consumed every day starting 2 days before the first dose and throughout the ramp-up phase, especially the first day of each dose increase. Administer intravenous hydration for any patient who cannot tolerate oral hydration. cReview in real time. dFor patients at risk of TLS, monitor blood chemistries at 6–8 hr and at 24 hr at each subsequent ramp-up dose. ePotassium, uric acid, phosphorous, calcium, and creatinine; correct any pre-existing abnormalities. fStarting at 20 mg and escalating weekly to 50 mg, 100 mg, 200 mg, and then 400 mg once daily clearance.
Hospitalization should be considered for patients with a high risk for TLS. Specifically, patients should receive oral and IV hydration (150–200 mL/hr IV fluids), antihyperuricemics, and rasburicase should be considered for hyperuricemia as per institutional guidelines. Laboratory monitoring (potassium, calcium, uric acid, creatinine, and phosphorus) for patients with high-risk CLL is performed predose, 4 hr, 8 hr, 12 hr, and 24 hr after the first doses of 20 mg and the first escalation to 50 mg as inpatients. Subsequent escalations to 100 mg, 200 mg, and 400 mg can be done in an outpatient setting, with lab monitoring predose, 6 to 8 hr, and 24 hr after escalation (Figure 3; AbbVie, Inc., 2021). Hospitalization should also be considered for those patients who are at medium risk for TLS and have creatinine clearance < 80 mL. For patients who are at medium risk for TLS, IV hydration can be considered in addition to oral hydration to mitigate risk. Antihyperuricemics and oral hydration (1.5–2 L/day) should be used in those at medium and low risk for TLS. Lab monitoring for TLS in the outpatient setting should be done at the predose, 6–8 hr, and 24-hr time frames with the 20-mg and first ramp-up to 50-mg doses (Figure 3; AbbVie, Inc., 2021).
Tumor lysis syndrome risk assessment in patients with AML is dependent on evaluation of WBC count, renal function, uric acid, potassium, calcium, phosphorus, and lactate dehydrogenase (Mato et al., 2006). All patients with AML should have WBC < 25,000/μL prior to initiation of venetoclax. Hydroxyurea or leukapheresis can be used for cytoreduction. Prior to first dose of venetoclax, all patients should be provided prophylactic measures including adequate hydration and antihyperuricemic agents (allopurinol, rasburicase, etc.), which continue during the ramp-up phase. Lab monitoring should occur predose and 6 to 8 hours at each dose-escalation level. Once the desired dose of venetoclax is achieved, a final lab evaluation should be performed at 24 hr post-dose (Figure 3; AbbVie, Inc., 2021). For patients with risk factors for TLS (e.g., circulating blasts, high burden of leukemia involvement in bone marrow, elevated pretreatment lactate dehydrogenase [LDH] levels, or reduced renal function), additional measures, including increased laboratory monitoring and reducing venetoclax starting dose should be considered (AbbVie, Inc., 2021).
Important Drug-Drug Interactions
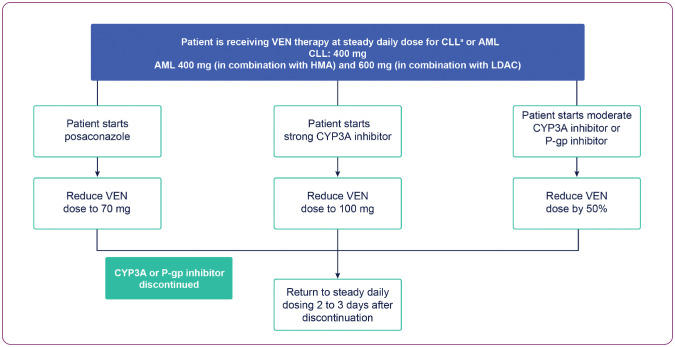
It is noteworthy that a key difference in the venetoclax dose ramp-up for patients with AML is that strong or moderate cytochrome P450, family 3, subfamily A inhibitors (CYP3Ai) are not contraindicated, given that many patients may require anti-infective prophylaxis due to profound neutropenia (Figure 4). However, the dose of venetoclax should be reduced to 100 mg. If moderate CYP3A or P-glycoprotein (P-gp) inhibitors are coadministered, the dose should be reduced by at least 50% (AbbVie, Inc., 2021).
Figure 4.
Recommended dose reductions for patients who are on concurrent CYP3A and P-gp inhibitor. AML = acute myeloid leukemia; CLL = chronic lymphocytic leukemia; CYP3A = cytochrome P450, family 3, subfamily A; HMA = hypomethylating agent; LDAC = low-dose cytarabine; P-gp = P-glycoprotein; VEN = venetoclax. aPosaconazole and strong CYP3A inhibitors are contraindicated during ramp-up.
Furthermore, administration of venetoclax with posaconazole requires a dose reduction to 70 mg, as evidenced by findings from a separate drug-drug interaction cohort (Agarwal et al., 2017). A recent clinical trial reported increases of 53% and 93% in venetoclax plasma concentrations and of 76% and 155% in exposure following 50-mg and 100-mg venetoclax coadministration with posaconazole, respectively (Agarwal et al., 2017).
Infection rates and efficacy outcomes were evaluated in the VIALE-A study in patients who received concomitant anti-infective prophylaxis with CYP3Ai (Jonas et al, 2020). Prophylactic antimicrobial CYP3Ai were administered to approximately 20% of patients. Within the first two cycles of therapy, concomitant anti-infective prophylaxis agents considered moderate/strong CYP3Ai were received by 14% (41/286)/8% (22/286) of patients in the VEN+AZA arm and 12% (18/145)/9% (13/145) of patients in the Pbo+AZA arm. The median duration of prophylactic CYP3Ai agent use was 12.5 days (range, 1–614) and 15 days (range, 1–731) in the VEN+AZA vs. Pbo+AZA arms, respectively. The rates of CR+CRi as a best response were similar with concomitant use of moderate (61%) or strong (64%) CYP3Ai with adjusted venetoclax dose vs. no use of CYP3Ai (67%). In the VEN+AZA arm, median OS was 15.2 months (95% CI = 11.2–20.8), 12.3 months (95% CI = 7.6–19.3), and 12.2 months (95% CI = 3.9–21.1) for patients receiving no, moderate, and strong CYP3Ai agents, respectively. The frequency of infections did not appear to decrease with CYP3Ai use. Invasive fungal infections occurred in 3%, 12%, and 9% with VEN+AZA and 0%, 0%, and 15% with Pbo+AZA in patients receiving no, moderate, and strong CYP3Ai agents, respectively.
DISCUSSION
Recent clinical trials support the activity of venetoclax in hematologic malignancies such as CLL/SLL and AML, and show that dose levels and certain toxicities differ across cancer types. The venetoclax ramp-up schedule varies according to disease, and concurrent use of CYP3A or P-gp inhibitors impacts the dosage employed (Figure 4). Hematologic and nonhematologic toxicities are common across malignancies and require regular monitoring.
As neutropenia remains the most common hematologic AE for patients receiving venetoclax, it is imperative that the treatment center has confidence in managing dose interruptions and reductions. Clinical reports on the management of AEs from large-scale phase III trials of combination targeted agents in CLL (Seymour et al., 2018; Fischer et al., 2019) and AML (VIALE-A [AbbVie, Inc., 2021], VIALE-C [AbbVie, Inc., 2021]) has shed light on these areas as the use of venetoclax in clinical practice is increasing worldwide. Specific attention should be paid to safety in these combinations, given that many targeted therapies use similar pathways of metabolism. Gastrointestinal disorders, including nausea, vomiting, and diarrhea, are prominent nonhematologic toxicities reported in patients treated with venetoclax. While prevalence of nonhematologic AEs in clinical studies of venetoclax treatment is high, it is important to note that the severity remains low and they are usually manageable. Tumor lysis syndrome, while less frequent, is an important AE that was observed in the early clinical studies of venetoclax prior to development of a comprehensive TLS mitigation strategy. Assessment of tumor burden, observance of TLS prevention recommendations, and adherence to dose ramp-up schedules can minimize risk (AbbVie, Inc., 2021). Advanced practitioners are essential in providing appropriate prophylaxis, patient counseling, and acknowledgment/treatment of common toxicities, thus enabling venetoclax to be administered safely at optimal dosages. Therefore, up-to-date reviews detailing common toxicities with venetoclax and feasible management options are of crucial relevance. Despite the incidence of these AEs discussed within, it is noteworthy that they can all be effectively managed, and their occurrence does not outweigh the efficacy benefit that has been observed in patients with CLL and AML.
Acknowledgment
AbbVie and the authors thank the patients, their families and caregivers, as well as the study investigators, research, and supporting staff. Medical writing support was provided by Mary L. Smith, PhD, Aptitude Health, Atlanta, GA, funded by AbbVie.
Footnotes
Venetoclax is being developed in a collaboration between AbbVie and Genentech. AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Mr. Waggoner is a former AbbVie employee and may own stock. Mr. Katsetos and Ms. Thomas are AbbVie employees and may own stock. Ms. Galinsky has served on advisory boards for Pfizer, Merus, and AbbVie. Ms. Fox has no conflicts of interest to disclose.
References
- AbbVie, Inc. (2021). Venclexta (venetoclax) package insert. https://www.rxabbvie.com/pdf/venclexta.pdf
- Agarwal, S. K., DiNardo, C. D., Potluri, J., Dunbar, M., Kantarjian, H. M., Humerickhouse, R. A.…Salem, A. H. (2017). Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: Evaluation of dose adjustments. Clinical Therapeutics, 39(2), 359–367. 10.1016/j.clinthera.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Al-Sawaf, O., Zhang, C., Lu, T., Liao, M. Z., Panchal, A., Robrecht S., & Fischer K. (2021). Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: Extended off-treatment follow-up from the randomized CLL14 study. Journal of Clinical Oncology, 39(36), 4049–4060. 10.1200/JCO.21.01181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M. A., Deng, J., Seymour, J. F., Tam, C., Kim, S. Y., Fein, J., …Roberts, A. W. (2016). The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood, 127(25), 3215–3224. 10.1182/blood-2016-01-688796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutre, S., Choi, M., Furman, R. R., Eradat, H., Heffner, L., Jones, J. A., …Davids, M. S. (2018). Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood, 131(15), 1704–1711. 10.1182/blood-2017-06-788133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids, M. S., Hallek, M., Wierda, W., Roberts, A. W., Stilgenbauer, S., Jones, J. A., …Seymour, J. F. (2018). Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clinical Cancer Research, 24(18), 4371–4379. 10.1158/1078-0432.CCR-17-3761 [DOI] [PubMed] [Google Scholar]
- DiNardo, C. D., Pratz, K., Pullarkat, V., Jonas, B. A., Arellano, M., Becker, P. S.…Letai, A. (2019). Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood, 133(1), 7–17. 10.1182/blood-2018-08-868752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo, C. D., Jonas, B. A., Pullarkat, V., Thirman, M. J., Garcia, J.S., Wei, A. H., & Pratz, K. W. (2020). Azacitidine and venetoclax in previously untreated acute myeloid leukemia. New England Journal of Medicine, 383(7), 617–629. 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- Fischer, K., Al-Sawaf, O., Bahlo, J., Fink, A. M., Tandon, M., Dixon, M., …Hallek, M. (2019). Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. New England Journal of Medicine, 380(23), 2225–2236. 10.1056/NEJMoa1815281 [DOI] [PubMed] [Google Scholar]
- Harrup, R. A., Owen, C., D’Rozario, J., Robak, T., Kater, A. P., Montillo, M., & Seymour, J. F. (2020). Efficacy of subsequent novel targeted therapies, including repeated venetoclax-rituximab (VenR), in patients (pts) with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) previously treated with fixed-duration Venr in the Murano study. Blood, 136(suppl 1), 44–45. 10.1182/blood-2020-137415 [DOI] [Google Scholar]
- Howard, S. C., Jones, D. P., & Pui, C. H. (2011). The tumor lysis syndrome. New England Journal of Medicine, 364(19), 1844–1854. 10.1056/NEJMra0904569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, F., Figueroa, S. D., Gallazzi, F., Balaji, B. S., Hannink, M., Lever, S. Z., …Lewis, M. R. (2008). Molecular imaging of bcl-2 expression in small lymphocytic lymphoma using 111In-labeled PNA-peptide conjugates. Journal of Nuclear Medicine, 49(3), 430–438. 10.2967/jnumed.107.045138 [DOI] [PubMed] [Google Scholar]
- Jonas, B. A., Dinardo, C. D., Fracchiolla, N., Pristupa, A., Ishizawa, K., Jin, J., & Pratz, K. W. (2020). CYP3A inhibitors and impact of these agents on outcomes in patients with acute myeloid leukemia treated with venetoclax plus azacitidine on the VIALE-A study. Blood, 136(suppl 1), 50–52. 10.1182/blood-2020-13485032430504 [DOI] [Google Scholar]
- Jones, J. A., Mato, A. R., Wierda, W. G., Davids, M. S., Choi, M., Cheson, B. D., …Byrd, J. C. (2018). Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: An interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncology, 19(1), 65–75. 10.1016/S1470-2045(17)30909-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater, A. P., Seymour, J. F., Hillmen, P., Eichhorst, B., Langerak, A. W., Owen, C., …Kipps, T. J. (2018). Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: Post-treatment follow-up of the MURANO phase III study. Journal of Clinical Oncology, 37(4), 269–277. 10.1200/JCO.18.01580 [DOI] [PubMed] [Google Scholar]
- Kater, A. P., Kipps, T. J., Eichhorst, B., Hillmen, P., D’Rozario, J., Owen, C., & Seymour, J. F. (2020). Five-year analysis of Murano study demonstrates enduring undetectable minimal residual disease (uMRD) in a subset of relapsed/refractory chronic lymphocytic leukemia (R/R CLL) patients (pts) following fixed-duration venetoclax-rituximab (VenR) therapy (Tx). Blood, 136(suppl 1), 19–21. 10.1182/blood-2020-136109 [DOI] [Google Scholar]
- Krug, U., Büchner, T., Berdel, W. E., & Müller-Tidow, C. (2011). The treatment of elderly patients with acute myeloid leukemia. Deutsches Ärzteblatt International, 108(51–52), 863–870. 10.3238/arztebl.2011.0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson, B. L., & Davids, M. S. (2017). The development and current use of BCL-2 inhibitors for the treatment of chronic lymphocytic leukemia. Current Hematologic Malignancy Reports, 12(1), 11–19. 10.1007/s11899-017-0359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson, J. D., Phillips, D. C., Mitten, M. J., Boghaert, E. R., Diaz, D., Tahir, S. K., …Souers, A. J. (2015). Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Science Translational Medicine, 7(279), 279ra40. 10.1126/scitranslmed.aaa4642 [DOI] [PubMed] [Google Scholar]
- Mato, A. R., Riccio, B. E., Qin, L., Heitjan, D. F., Carroll, M., Loren, A., …Luger, S. M. (2006). A predictive model for the detection of tumor lysis syndrome during AML induction therapy. Leukemia & Lymphoma, 47(5), 877–883. 10.1080/10428190500404662 [DOI] [PubMed] [Google Scholar]
- Mehta, S. V., Shukla, S. N, & Vora, H. H. (2013). Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: Its correlation with FLT3. Neoplasma, 60(6), 666–675. 10.4149/neo_2013_085 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2021). NCCN Clinical Practice Guidelines in Oncology: Antiemesis. V1.2022. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
- Roberts, A. W., Seymour, J. F., Brown, J. R., Wierda, W. G., Kipps, T. J., Khaw, S. L., …Humerickhouse, R. (2012). Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of Clinical Oncology, 30(5), 488–496. 10.1200/JCO.2011.34.7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A. W., Davids, M. S., Pagel, J. M., Kahl, B. S., Puvvada, S. D., Gerecitano, J. F., …Seymour, J. F. (2016). Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 374(4), 311–322. 10.1056/NEJMoa1513257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour, J. F., Kipps, T. J., Eichhorst, B., Hillmen, P., D’Rozario, J., Assouline, S., …Kater, A. P. (2018). Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. New England Journal of Medicine, 378(12), 1107–1120. 10.1056/NEJMoa1713976 [DOI] [PubMed] [Google Scholar]
- Souers, A. J., Leverson, J. D., Boghaert, E. R., Ackler, S. L., Catron, N. D., Chen, J., …Elmore, S. W. (2013). ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature Medicine, 19(2), 202–208. 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- Stilgenbauer, S., Eichhorst, B., Schetelig, J., Coutre, S., Seymour, J. F., Munir, T., …Wierda, W. G. (2016). Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncology, 17(6), 768–778. 10.1016/S1470-2045(16)30019-5 [DOI] [PubMed] [Google Scholar]
- Tzifi, F., Economopoulou, C., Gourgiotis, D., Ardavanis, A., Papageorgiou, S., & Scorilas, A. (2012). The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Advances in Hematology, 2012, 524308. 10.1155/2012/524308 [DOI] [PMC free article] [PubMed]
- Wei, A. H., Strickland, S. A., Jr., Hou, J. Z., Fiedler, W., Lin, T. L., Walter, R. B.,…Roboz, G .J. (2019). Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study. Journal of Clinical Oncology, 37(15), 1277–1284. 10.1200/JCO.18.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, A. H., Montesinos, P., Ivanov, V., DiNardo, C. D., Novak, J., Laribi, K., & Panayiotidis, P. (2020). Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase III randomized placebo-controlled trial. Blood, 135(24), 2137–2145. 10.1182/blood.2020004856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, W. H., O’Connor, O. A., Czuczman, M. S., LaCasce, A. S., Gerecitano, J. F., Leonard, J. P., …Humerickhouse, R. A. (2010). Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncology, 11(12), 1149–1159. 10.1016/S1470-2045(10)70261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]