Abstract
In oncology practices across the United States, biosimilars—highly similar versions of licensed, innovator (reference) biological medicines—are currently emerging as more affordable therapeutic options. Only after a rigorous product development program, during which a proposed biosimilar is analyzed and compared with its reference biologic to demonstrate comparable clinical efficacy, safety, and tolerability, is biosimilarity supported and licensure granted by the US Food and Drug Administration. Coincidentally, many advanced practitioners (APs) are finding themselves at the forefront of introducing monoclonal antibody (mAb) biosimilars in their oncology practice. Advanced practitioners are often tasked with building the confidence of colleagues and patients who may be unfamiliar with biosimilars, skeptical about integrating them, or have yet to consider mAb biosimilars as a viable and more sustainable cancer treatment option. With this responsibility comes a number of challenges that require APs to become knowledgeable about biosimilars and approaches to their implementation. This review aims to highlight the practical implications of streamlining the integration of biosimilar therapies in an oncology practice.
Biologics derived from living organisms comprise half of the pharmacologic market share in oncology (Konstantinidou et al., 2020). Manufacturing exact copies of any biologic is impossible, given their inherent variability (Congressional Research Service Report, 2019). Biosimilars are highly similar versions of already licensed biologics (referred to as the reference, originator, or innovator products). Biosimilars can only be approved after regulatory exclusivity for the originator expires and the data submitted to regulatory authorities are deemed sufficiently robust to support high similarity to the reference product (FDA, 2009). The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have rigorous science-based regulatory frameworks and approval pathways in place for biosimilars. All US-licensed and European (EU)-approved biosimilars are required to demonstrate no clinically meaningful differences in safety, purity/pharmaceutical quality, and potency with respect to the reference product (European Medicines Agency, 2019; FDA, 2015). Biosimilars are an attractive therapeutic option because of their potential to contribute to the alleviation of affordability and accessibility issues associated with originator biologics, which can largely benefit underserved patient populations (Biosimilars Council, 2017; Lyman et al., 2018; Transparency Market Research, 2016; Vulto & Jaquez, 2017; Yuan et al., 2018). Accordingly, biosimilars are emerging as a practical reality across health-care systems under growing economic pressure (Kobberling, 2017). Streamlining the integration of biosimilars into clinical practice necessitates a deliberate and coordinated effort that advanced practitioners (APs)—including pharmacists—can undertake to support this evolution in treatment (Zlatkus et al., 2020).
By virtue of the patient-centered and holistic approach to health care adopted by APs, they possess the relevant competencies to play an important role in the education of physician colleagues, oncology professionals, patients, and their caregivers about biosimilars (Mayden et al., 2015; Walsh et al., 2014). Extending general education about biosimilars to practice support staff and administrative staff can also help to promote one voice among all patient-facing personnel (Manolis et al., 2016; Rathore et al., 2019; Renton et al., 2019). Healthcare-system pharmacists are ideally situated to take the lead in multidisciplinary efforts to evaluate biosimilars for institutional use via Pharmacy and Therapeutics (P&T) committee reviews and formulary management, and, when necessary, on a case-by-case basis (American Society of Health-System Pharmacists, 2013; Cuellar et al., 2019; Dolan, 2018; Hobbs & Crawford, 2019; Ventola, 2015; Zlatkus et al., 2020). Pharmacists often initiate the integration of biosimilars into clinical practice based on their roles in managed- and acute-care settings (Hobbs & Crawford, 2019). Clinical oncology pharmacists also play an important role in educating patients and prescribers about the biosimilars that are available on the health-plan formulary, and explaining why these treatments can be suitable for patients (Heaton & Skiermont, 2019; Lanton, 2019; Smerker, 2016). Collectively, the efforts of APs can advance the type of clinical management and informed decision-making that facilitates effective communications with patients and/or their caregivers, to empower and reassure them about biosimilars (Armuzzi et al., 2019; Heaton & Skiermont, 2019). This review shines a light on practical communication and integration strategies that can enable APs to increase the likelihood that informed and appropriate considerations are given to biosimilars as treatment options in the oncology practice setting.
THE BASICS MATTER
When discussing biosimilars compared with reference/originator monoclonal antibody (mAb) biologics as therapeutic options with oncology patients, the ability of APs to exude confidence will depend heavily upon having a bona fide understanding of the key concepts that underpin the rationale for considering biosimilars. Suboptimal knowledge among health-care providers concerning the potential of biosimilars, or preconceived notions about their benefit–risk profile, can limit the treatment options patients may be asked to consider (Lyman et al., 2018), or undermine patient confidence should they discern uncertainty on the part of the AP. These are important considerations in oncology and supportive-care settings where barriers to the integration of biosimilars can impact affordability, access, and the optimization of patient care (Weise, 2019).
In a survey of 77 oncology clinicians, including physicians and APs, 74% revealed that they could not satisfactorily define a biosimilar (Cook et al., 2019); of these, 40% (who considered themselves “moderately familiar” with biosimilars) and 34% (who claimed they were “somewhat familiar”) erroneously equated a biosimilar with a generic drug (Cook et al., 2019). Discrepancies between declared and actual knowledge about biosimilars are noteworthy and should be resolved to encourage confident communications about biosimilars that do not involve guesswork (Leonard et al., 2019). As has been well-documented previously, there is an important need for the ongoing education of APs with respect to all aspects of biosimilars pertinent to their use in clinical practice (Mayden et al., 2015). Such an approach can enhance the level of self-confidence necessary to recommend and prescribe biosimilars, manage patients receiving them, and educate others (Cuellar et al., 2019; Lyman et al., 2018; Mayden et al., 2015; Wolff-Holz et al., 2018). For a basic understanding of biosimilars, a number of “need to know” principles are foundational to the regulatory pathway for biosimilars and considered important by medical societies and personnel (Table 1). Here we describe some key biosimilar concepts in more depth for the benefit of APs interacting as part of a multidisciplinary oncology team, tasked with caring for patients to optimize the management of their health care.
Table 1. Biosimilarity Concepts and Terms.
| Topic | Brief explanation | |
|---|---|---|
| What is a biosimilar? |
|
|
| What makes biosimilars different from generic drugs? | Biosimilars are:
|
Generics are:
|
| What differences are permitted between approved biological products such as biosimilars? |
|
|
| How are biosimilars developed? |
|
|
| How does the development of biosimilars proceed? | A comprehensive stepwise development program is implemented.
|
|
| What is the approval process for biosimilars? |
|
|
| Why are there fewer clinical studies for a biosimilar compared with a reference product? |
|
|
| Why is the cost of biosimilars lower? |
|
|
| Treatment changes | ||
| Substitution |
|
|
| Switching |
|
|
| Extrapolation |
|
|
| Interchangeability |
|
|
| Immunogenicity | ||
| What are ADAs? |
|
|
| What are NAbs? |
|
|
Note. ADAs = anti-drug antibodies; AP = advanced practitioner; FDA = US Food and Drug Administration; NAbs = neutralizing antibodies; RP = reference product. Information from American Society of Clinical Oncology (2015); Blevins (2019); Chiu & Gilliland (2016); Christl (2018); Christl & Lim (2018); Cuellar et al. (2019); Curigliano et al. (2016); Demler (2018); FDA (2015, 2017b, 2019b, 2020b); Gabay (2017); Grampp & Ramanan (2015); Isaacs et al. (2017); Kim et al. (2020); Krishna & Nadler (2016); Lyman et al. (2018); Markus et al. (2017); Mayden et al. (2015); McClellan et al. (2019); McMahon Publishing (2013); NIH National Cancer Institute (2019); Pittman et al. (2016, 2019); Schellekens et al. (2016); Stanton (2017); Stebbing et al. (2020); Thill et al. (2019); Rumore et al. (2016); Webster et al. (2019); Welch (2017); van Brummelen et al. (2016).
BIOSIMILARS ARE NOT GENERIC
Understanding the difference between generic drugs and biosimilars is pivotal to being able to explain biosimilars to colleagues and patients alike. Generic drugs are small-molecule agents, synthesized chemically to be exact copies of brand-name medications in every respect, including structurally (McMahon Publishing, 2013; Thill et al., 2019). Conversely, biosimilars—like all biologics—are large-molecule drugs originating from living cells and complex biotechnologic processes (McMahon Publishing, 2013; Thill et al., 2019; Table 1). There is an expectation that minor batch-to-batch and production-lot differences may occur with all biologics, including reference and biosimilar products (Ebbers et al., 2020; Isaacs et al., 2017; Schiestl et al., 2011). Although the primary amino acid sequence of the products must be identical, other minor differences are acceptable, providing they occur in inactive components of the product (FDA, 2015). These minor differences must be demonstrated not to be clinically meaningful; that is, they cannot impact clinical efficacy, safety, or immunogenicity (FDA, 2015). Rigorous and thorough evaluations by manufacturers and the FDA ensure these variations are monitored and controlled to stay within stringent analytical margins (FDA, 2017a). One can think of acceptable variations in terms of a lock-and-key concept (Webster et al., 2019). Even if the bow of one key—the part we hold when turning the key in the lock—is slightly different to the bow of another key, both keys will still open the lock when the part of each key that performs the important unlocking function is identical (Figure 1).
Figure 1.

Biosimilars and their reference products must function in a clinically equivalent manner. Concept adapted from Webster et al. (2019).
A FAVORABLE RISK–BENEFIT PROFILE
The biosimilar approval process is rigorous but differs from that for novel biologics (Figure 2). The biosimilar pathway relies on extensive comparative analytical testing to demonstrate high similarity in structure and biological function to the reference product, which has already been extensively studied in humans and has a well-understood clinical profile (Janjigian et al., 2018; Yuan et al., 2018). After these characteristics, which are known to impact safety and efficacy, have been thoroughly compared and shown to be highly similar, an abbreviated comparative clinical program can be implemented to answer any unresolved questions arising from preclinical development (Janjigian et al., 2018). This program typically comprises a clinical pharmacology study, including pharmacokinetic (PK) and pharmacodynamic (PD) evaluations—the latter when valid biomarkers are identified—and is conducted in a sensitive population (Li et al., 2020). Lastly, a comparative clinical study is designed to confirm biosimilarity to the reference biologic, rather than establish de novo clinical efficacy and safety of the proposed biosimilar. This confirmatory study is conducted in a sensitive indication; in other words, one in which potential differences are likely to be observed, should they exist (Schiestl & Krendyukov, 2017).
Figure 2.
A stepwise approach is adopted to amass the science-based evidence required for the regulatory approval of biosimilars. PD = pharmacodynamic; PK = pharmacokinetic; US = United States. Information from Christl & Lim (2018); McClellan et al. (2019); Stebbing et al. (2020); Yuan et al. (2018).
The selected primary study endpoints need to be suitably sensitive to measure any differences in potential product-related activity, as opposed to the long-term patient benefit between a reference product and its biosimilar (Thill, 2019). Meaningful, relatively shorter-term surrogate endpoints, such as pathologic complete response and overall response rates, are considered more appropriate than survival endpoints (e.g., progression-free survival or overall survival) typically used to support the initial licensure of reference products (Thill, 2019). A determination of clinical equivalence is contingent upon the confidence interval for the primary endpoint(s) being entirely contained within an equivalence margin based on historical clinical performance data of the reference product in randomized controlled trials. The construction of prespecified equivalence margins represents what regulators agree reflects differences that are not clinically meaningful (Rugo et al., 2016; Stebbing et al., 2020; Webster et al., 2019). Safety, including immunogenicity, is also evaluated (Stebbing et al., 2020). No single pivotal study demonstrates biosimilarity (Christl & Lim, 2018). Evidence from each stage of the biosimilar development program, or what is called the “totality-of-the-evidence,” is used to evaluate biosimilarity (Rugo et al., 2016; Figure 2). In effect, the regulatory pathway ensures that, once biosimilars are FDA-approved, they are “just as safe and effective as their reference products” (FDA, 2020a). Nevertheless, confusion can ensue among prescribers who do not fully appreciate the rigorous development process involved and incorrectly presume “highly similar” could mean “riskier” than a reference product; therefore, a misplaced reluctance to consider such a treatment option for patients can prevail (Bhatt, 2018; Reinke, 2019; Weise, 2019).
BIOSIMILARS: NOT TOO UNFAMILIAR
The first EMA-approved biosimilar (in 2006) was a biosimilar to the recombinant human growth-hormone reference medicine, somatropin (Lopez-Siguero et al., 2017). The following year, the EMA approved several biosimilars of epoetin for chemotherapy-related anemia and chronic kidney failure (GaBI Online, 2020). In 2015, Zarxio (filgrastim-sndz, Sandoz), a biosimilar to Neupogen (filgrastim, Amgen) became the first US-approved biosimilar for use in supportive care, followed in 2018 by Nivestym (filgrastim-aafi, Pfizer; Biehn & Nell, 2022). Other colony-stimulating factor biosimilars subsequently approved in the US include the following biosimilars to Neulasta (pegfilgrastim, Amgen): Udenyca (pegfilgrastim-cbqv, Coherus), Fulphila (pegfilgrastim-jmbd, Mylan), Ziextenzo (pegfilgrastim-bmez, Sandoz), and Nyvepria (pegfilgrastim-apgf, Pfizer), as well as Retacrit (epoetin alfa-epbx, Pfizer/Vifor), a biosimilar to Epogen/Procrit (epoetin alfa, Amgen; Biehn & Nell, 2022). To date, there has been high similarity between biosimilars and their respective reference products, and rarely are biosimilars not approved after the acceptance of a marketing application for safety concerns (Webster et al., 2019). No rejections or withdrawals of proposed biosimilars have occurred following analytical and human PK studies that have shown high similarity between a proposed biosimilar and its reference product owing to subsequent clinical inequivalence between compared products (Webster et al., 2019). Additionally, analytical and clinical pharmacology studies and results have so far never been refuted by clinical confirmatory studies, which has inspired proposals to adopt even more flexible or truncated regulatory pathways in the future, in which clinical trials may not always be necessary (Schiestl et al., 2020; Webster et al., 2019).
The introduction of biosimilars of relatively higher molecular weight, i.e., more complex therapeutic mAbs, for use in oncology is more recent (Thill et al., 2019). In 2017, Truxima (rituximab-abbs, Celltrion), a rituximab biosimilar, became the first ever mAb biosimilar approved in Europe in an oncology indication, and its administration continues without new issues emerging, according to prescribing physicians (Leslie, 2019; Trollope et al., 2017). The first trastuzumab (Herceptin, Genentech) biosimilar (Ontruzant, trastuzumab-dttb, Samsung Bioepis) was launched in Europe in 2018 (DiGrande, 2018). In the US, the first mAb biosimilar was launched in mid-2019 (Figure 3). It should be noted that the potential to consider waiving confirmatory efficacy trials is typically restricted to proposed biosimilars with appropriate biomarkers that have already been well described; this does not include the majority of therapeutic mAb biosimilars for cancer, which are comprehensively evaluated (Schiestl et al., 2020). Overall, biosimilar medication use in Europe has spanned > 700 million patient-days over a > 10-year period with safety monitoring, which should provide some reassurance to prescribers (van den Hoven, 2017). Most APs are familiar with oncology reference biologics, and biosimilars—by their meticulous design and rigorous evaluation—are not expected to be clinically meaningfully different from them, so their integration into practice should be reasonably straightforward.
Figure 3.
Existing and pipeline oncology monoclonal antibody biosimilars. FDA = Food and Drug Administration; mAb = monoclonal antibody; US = United States. Information from Biehn & Nell (2022)a aThis approval and development timeline is current to July 6, 2020.
Although the emergence of mAb biosimilars creates new opportunities for APs to make a meaningful difference to patients’ lives, new challenges also arise (Campen, 2017). For example, confusion appears to prevail about the need for a patient who transitions from the reference product to a corresponding biosimilar to re-consent. Some APs consider that because a biosimilar is not identical, it merits “new” drug-administration status, given that the original consent form only contains the international nonproprietary name (INN) and brand name of the reference biologic (Oncology Nursing Society, 2019). In an era in which biosimilars are continuing to emerge as a potential treatment option, a proactive approach would be to ensure the original consent form for any biologic is all-inclusive and states the INN followed by “products” (e.g., trastuzumab products). All biosimilars of that reference product are then seamlessly covered, smoothing therapy transitions for patients and circumventing extra work involved in obtaining re-consent.
KNOW YOUR PATIENTS
The ability of APs to initiate biosimilar treatment, or transition a patient to a biosimilar from an oncology reference product, relies on an intuitive balance between knowledge and real-world application. The capacity to grasp the level of each patient's awareness and/or concerns, detect knowledge deficits, and be able to address them in an efficient and practical manner exemplifies an AP's ability to bridge any gaps between the art and science of their profession (Wolff-Holz et al., 2018).
Knowledge gaps affect patient attitudes about biosimilars as a treatment option for their disease (Jacobs et al., 2016). In 2014, up to 70% of the general population in the United States (US) had never heard of biosimilars (Jacobs et al., 2016). At that time, patient awareness about biosimilars resulted in more positive perceptions (by up to 20% compared with patients lacking awareness) in terms of the efficacy, safety, accessibility, and affordability of biosimilars (Jacobs et al., 2016; Figure 4).
Figure 4.
Patients in the US who were already aware of biosimilars viewed many aspects about them more positively than those who were unaware of biosimilars. An independent survey company conducted a 10- to 20-minute survey consisting of up to 56 closed-ended as well as open-ended questions. Among the 1,241 US respondents, 312 commented from an oncology perspective, comprising 188 patients diagnosed with cancer in the past 2 years, 89 patients diagnosed with cancer who had participated in patient support groups, and 35 respondents who were caregivers. The remaining 679 and 250 respondents had inflammatory disease and were from the general population, respectively. Perceptions were assessed among 610 respondents who were unaware of biosimilars and 270 respondents who were aware. US = United States. Information from Jacobs et al. (2016).
It could be overwhelming for patients who first find out about biosimilars when diagnosed with cancer. Coping with their disease can intensify feelings of vulnerability at a time when personal goals and priorities may be changing (Canadian Breast Cancer Network, 2017; Frey et al., 2020). In contrast, patients on chronic therapy can be caught off guard by a recommendation to switch to a biosimilar, particularly when their current reference biologic appears to be safe and effective (Manolis et al., 2016). Knowledge gaps and misconceptions about biosimilars, or indeed about any drug, can heighten a patient's concerns about what it means for their quality of care. This highlights the importance of educating patients early on and in practical ways that will empower them, promote improved communications with APs, and allow for informed decision-making.
Monetary constraints can lead to “financial toxicity” (i.e., problems related to the cost of medical care) and add to the burden of cancer patients receiving treatment (Oubre, 2019). Common reasons for financial distress are an uninsured status, high medical copayments or high drug and treatment copayments, loss of income, loss of coverage tied to work benefits, the need for medical leave or early retirement, or the uncertainty about how to navigate insurance coverage issues or safety-net resources (Vorobiof et al., 2020; Rotter et al., 2019; Warsame et al., 2019). Given that financial hardship is associated with reductions in quality of life, adherence to treatment, and adverse psychological consequences, finances can become a source of harm to patients (Rotter et al., 2019).
Based on a study of recorded conversations during routine oncology visits of patients with solid tumors at three different oncology clinics in the US, 28% (151/529) of the conversations about treatment in general addressed cost, and lasted, on average, less than 2 minutes (Warsame et al., 2019). Cost discussions centered on insurance processes/eligibility, employment insecurities, costs related to drugs that impact medical decision making, and to proactively avert the potential for behaviors that could lead to medication self-rationing and the like. Clinicians mostly engaged in conversations about costs for the purpose of contingency planning, to alleviate out-of-pocket and treatment-related costs. Referrals to social services occurred less than 4% (6/151) of the time and patients were more often encouraged to contact their insurance provider before filling prescriptions to determine copays and assess how they might be affected financially. Patients and caregivers were most interested in discussing insurance approvals, anticipated coverage denials, and the writing of appeals to mitigate treatment delays. It was suggested that multiple stakeholders, such as financial navigators, could be involved in these conversations to better facilitate patient navigation of financial issues. Clinicians, including nurse practitioners, chose to verbally acknowledge 60% of cost conversations and took action in response to them 25% of the time. In another study reporting 677 outpatient visits to an oncology clinic, 147 (22%) conversations about cost were monitored and reported. Initiators of the cost conversations were physicians (59% of the time), patients (37%), caregivers or companions (3%), and nurses (1%; Hunter et al., 2017). Demonstrating a willingness to at least address or discuss the cost of treating cancer is considered an important element of high-quality health care (Hunter et al., 2017). Some practices prefer to employ financial counselors or utilize the expertise of coders and payer experts to help patients navigate their benefits (McGraill, 2019; Shah, 2019). Financial toxicity was reduced from 48% to 30% among surveyed patients receiving cancer immunotherapy after pretreatment financial counseling from their healthcare provider (Vorobiof et al., 2020). Examples of brief conversations, including switching a patient's drug and other scenarios, demonstrate possible strategies for approaching cost conversations that could be adapted when discussing biosimilars (Hunter et al., 2017).
In contrast, although biosimilars typically offer a more affordable version of the same reference product, patient skepticism can be triggered by the lower cost of biosimilars compared with reference products that are typically more expensive (Renton et al., 2019). Irrational thoughts about potentially receiving an inferior product, and the negative expectations stemming from a patient's misconceptions that their medication will harm rather than improve their health (nocebo effects), can pose clinical challenges (Janjigian et al., 2018; Spanou et al., 2019). Failing to address the potential for cost-related concerns early on could, for some patients, foster an anxiety-driven internal monologue that reduces their receptivity to the education that follows.
IMPLEMENTING STRATEGIES FOR THE INTEGRATION OF BIOSIMILARS
Advanced practitioners and treating physicians are considered primary sources of patient education (Lyman et al., 2018), which means they can influence perceptions about biosimilars and influence the type of informed decision-making that can empower patients (Armuzzi et al., 2019; Janjigian et al., 2018; Renton et al., 2019). However, time is at a premium in any clinic or hospital (Smith & Zsohar, 2013), so anticipating a patient's need to have, at minimum, a general awareness of biosimilars can save time and avoid later disruption if initiating or switching to a biosimilar is recommended. Indeed, it is preferable for APs to broach the topic of biosimilars in person with patients, and ensure they have all the relevant information, rather than have an unknown insurance company representative notifying them by letter or telephone about a non-medical or formulary-driven change (Dolan, 2018).
Ideally, education about biosimilars is best delivered in the same way as other oncology therapeutics. As with all patient-education endeavors, judicious alignment within a multidisciplinary team among physicians, nurses, pharmacy colleagues, support staff, and other stakeholders (e.g., financial advocates) improves the likelihood that the patient will receive consistent information, guidance, recommendations, and education (Armuzzi et al., 2019; Welch, 2017). Standardizing patient education has been associated with increased patient satisfaction and improved competency in terms of self-care (Gee, 2016). In addition, structured communications can enhance acceptance of, and adherence to, treatment for patients prescribed biosimilars (Armuzzi et al., 2019).
In an era of unprecedented accessibility and exposure to vast amounts of information, patients can easily become overwhelmed and be tempted to “switch off” when faced with the complexity of care and surplus information (Canadian Breast Cancer Network, 2017). Simply asking a patient whether they are familiar with biosimilars and, if so, having them explain what they know can be an effective starting point. Listening to their explanation will provide an insight into their understanding (or lack thereof) of biosimilars, making it an opportune time for APs to supply patients with more information about the biosimilar(s) they will be offered. Explaining details of the biosimilar approval process to patients is probably unnecessary. What matters, first and foremost, is telling patients that the biosimilar recommended for them is an FDA-approved medication and is clinically equivalent to the best available treatment option for them (Canadian Breast Cancer Network, 2017; National Comprehensive Cancer Network (NCCN), 2019; Welch, 2018). Second, it may be beneficial for patients to know that there are potential cost savings associated with biosimilars use, both in terms of their out-of-pocket costs compared with being prescribed reference drugs and due to health-care system efficiencies (e.g., when no preauthorizations are required) that can help to ensure long-term drug availability and treatment when necessary (Canadian Breast Cancer Network, 2017; Pakizegee et al., 2020). Emphasizing that shared decision-making is important to the success of the treatment plan as a whole, and answering any remaining questions is good way to conclude the conversation.
Each discussion should be tailored to the patient's specific circumstances, disease, and how much they want to know (Askren, 2013; Jacobs et al., 2016; Smith & Zsohar, 2013). The extent of these discussions will depend upon the stage of the patient's journey. Education will be particularly important when switching patients from a reference product to a biosimilar; this is because the patient will presume they are already receiving the optimum medication, so will need reassurance as to why a change is recommended (Armuzzi et al., 2019; De Munter & Crombez, 2018; Zack, 2018). The rationale for a switch to a biosimilar could take more time to explain than when a patient initiates biological therapy with a biosimilar and feels confident about their prescriber's decision to treat them with the best available option (Hobbs & Crawford, 2019). Reinforcing any face-to-face education with materials the patient can take home and review together with their family and/or caregiver can be of added benefit.
Patient education is not a one-time event: it is ongoing and will need to be incorporated into the patient's plan of care, along with other required education that is likely ongoing with their sometimes complex infusion-therapy regimens (Smith & Zsohar, 2013). An infusion center is an ideal venue where oncology nurses, educated about biosimilars by APs, could dedicate some face-to-face time with the patient (Gee, 2016). Teaching techniques and communication styles will need to be adapted to each patient's individual learning preferences for maximal effect, reinforced by periodically evaluating each patient's comprehension (i.e., by asking questions that require explanations rather than “Yes/No” answers, or by demonstration; Smith & Zsohar, 2013). Although it may not be needed for some patients who are comfortable with technical explanations, for others who are not and ask for more details about how biosimilars are manufactured, an AP can opt to explain the concept in everyday terms, which may facilitate an understanding of the process (Welch, 2018). For example, the FDA has likened the manufacturing of a biosimilar to the approach taken when brewing beer that is intended to be just like someone else's beer (Welch, 2017). A sample of that person's beer would be required in order to reverse-engineer the recipe and figure out how it was made (Wehrwein, 2020). Just like a biosimilar is not an exact copy of a biologic drug, a batch of beer made following a comparable recipe can never be truly identical to a similarly prepared batch of beer. However, when the formulation and brewing process is set up to be so highly similar in nature, it becomes possible to make a version of the original beer that is just as good because it has no meaningful differences in terms of its quality characteristics. Likewise, a biosimilar will only receive FDA approval when it is manufactured in a safe and tightly controlled manner and all tests show that it is highly similar to its reference product with no clinically meaningful differences in terms of its safety, purity, and potency (FDA, 2015).
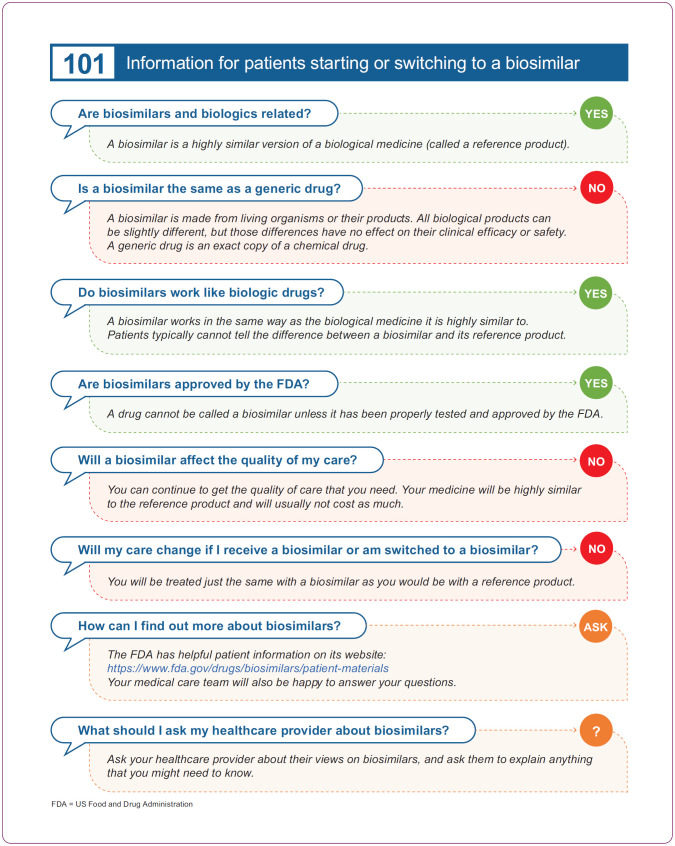
To move a patient forward in their treatment plan, any indecision based on misconceptions about the safety or efficacy of a biosimilar will need to be resolved (Campen, 2017). Patient advocacy groups also provide excellent patient support; group members generally have a positive attitude about biosimilars (Jacobs et al., 2016). All resources warrant a critical review by APs to evaluate their suitability before recommending them to patients. A summary of some potential patient resources is shown in Table 2. In addition, Figure 5 depicts a simple patient handout for use or adaption; it contains some rudimentary facts about biosimilars to create initial patient awareness.
Table 2. Potential Resources to Consider for Patient Use.
| Organization | Details of materials | Link |
|---|---|---|
| US Food and Drug Administration (FDA) | Regularly updated patient materials about the basics of biosimilars, FAQs, and PDF infographics. | https://www.fda.gov/drugs/biosimilars/patient-materials |
| Canadian Agency for Drugs and Technologies in Health (CADTH) | A concise tool about biosimilar basics for patients prescribed a biosimilar. The CADTH is an independent, not-for-profit organization. Patients can subscribe to a CADTH newsletter and follow CADTH on Twitter. | https://cadth.ca/sites/default/files/pdf/biosimilar_drugs_patient_en.pdf |
| American Pharmacists Association (APhA) | Biologics and biosimilar drug products: pharmacist guide to patients’ frequently asked questions. | https://www.pharmacist.com/Advocacy/Issues/Biosimilars/Biologics-and-Biosimilar-Drug-Products-Pharmacist-Guide-to-Patients-Frequently-Asked-Questions |
| Cancer Support Community | A website with patient information, a quick patient guide, and videos about why biosimilars are important. Includes a Cancer Support Community locater. | https://www.cancersupportcommunity.org/what-biosimilar |
| Susan G. Komen | Patient materials related to basic facts about biosimilars: includes various fact sheets, a video, and podcasts. | https://blog.komen.org/?s=biosimilar |
| American Cancer Society (ACS) Cancer Action Network | Public policy resources that provide patient information and videos on understanding biosimilars, and FAQs. | https://www.fightcancer.org/policy-resources/understanding-biologic-and-biosimilar-drugs |
| Cancer Care Ontario (CCO) | Biosimilar information that patients need to know (more specific to Canada) with an online format, video, and PDF handout with space for patients to make notes about questions they may have. | https://www.cancercareontario.ca/en/cancer-treatments/biologic-biosimilar-therapy/biosimilars |
| European Society for Medical Oncology (ESMO) Biosimilars Portal: Patient Resources | Predominantly EU-focused materials that address patient concerns regarding biosimilars. The materials include infographics, a video (in 8 languages), and questions and answers about biosimilars (in 23 languages). A link is also provided to US FDA patient materials. | https://www.esmo.org/policy/biosimilarsportal/patient-resource |
| Explanation of Benefits (EOB) information | A website that patients can use to help them decipher their explanation of benefits. | https://www.verywellhealth.com/understanding-your-eob-1738641 |
Note. EU = European Union; FAQs = frequently asked questions.
Figure 5.
An example of a basic patient handout that could be used or adapted for patients starting or switching to a biosimilar.
Important information about biosimilars should be presented to patients in clear and easy-to-understand terms, free of medical jargon and appropriate for their health literacy (American Society of Clinical Oncology, 2008; Campen, 2017; Canadian Breast Cancer Network, 2017; Janjigian et al., 2018). Materials could include handouts, brochures about device handling and proper drug-storage conditions, visual aids, instructional videos, video vignettes of patient experiences, and information about the most common adverse drug reactions and how to manage them (Armuzzi et al., 2019; Bestvina and Polite, 2017). Links to reputable online resources developed by professional organizations and plain-language summaries of peer-reviewed research articles may benefit patients who express a need for more information or direct clinical evidence (Lyman et al., 2018; Pushparajah et al., 2018; Wolff-Holz et al., 2018). This approach will help avoid random internet searches about oncology medications that could provide misleading or incorrect information and advice (Gee, 2016). As knowledgeable and trusted prescribers, the views of APs will be important to their patients. So, if APs advise their patients that they would have no reservations about receiving a biosimilar or prescribing biosimilars for their own family members, this can provide some welcome personal context and insight.
COLLABORATIVE EFFORTS
The P&T committee members will be adept at determining the availability of patient-education materials from manufacturers of biosimilars (Cuellar et al., 2019). Given that some biosimilars can be extraordinarily expensive to develop, and on rare occasions can exceed the cost of a reference product for which there is copay assistance, pharmacists can generally inform about whether the pharmaceutical company of a marketed biosimilar or an associated patient advocacy organization offers a Patient Assistance Program to improve drug affordability (Cuellar et al., 2019; Welch, 2017). Pharmacists will also be aware of any insurance coverage discrepancies in indications covered between the reference product and the label of a given biosimilar (i.e., FDA-approved indications of the reference product not covered by the biosimilar label because they are still under patent and have been “carved out” for regulatory or legal reasons not related to clinical performance). These resulting “skinny” labels for biosimilars, which omit patented uses of the reference product, may not include all the recommended indications of the reference product and biosimilars that are covered in the NCCN guidelines. The NCCN Steering Committee voted unanimously in May 2020 to revise all of its guidelines to indicate that an FDA-approved biosimilar is an “appropriate substitution” for a brand-name biologic (NCCN, 2020; Wehrwein, 2020). NCCN panels for some specific cancers had previously approved this, but the policy in May 2020 applied to guidelines for all cancers. Payers may also make decisions that differ from the NCCN guidelines.
Pharmacists play a key role in remaining alert to issues of practical importance, such as any differences in excipients, devices, or other subtle product nuances that could necessitate consideration and/or counseling (Lutz, 2015). They monitor post-approval biosimilar surveillance and raise any concerns with the potential to cause dispensing or treatment errors in hospitals, clinics, or among patients. Although not a problem limited to biosimilars, a pegfilgrastim biosimilar product was introduced with packaging very similar in appearance to that of an already well-established osteoporosis drug, resulting in reports of potential look-alike mix-ups and prompting strategies to eradicate the incidence of confusion between products (Institute for Safe Medication Practices, 2019). Ensuring accurate product traceability of any adverse events (AEs), determining supply-chain reliability, and keeping abreast of information pertinent to drug delivery are in the pharmacists’ bailiwick. For example, a switch to a biosimilar could also involve a new presentation of a patient's usual treatment (e.g., prefilled syringe co-packaged with an on-body auto-injector for the pegfilgrastim reference product [Neulasta Onpro] compared with a pegfilgrastim biosimilar requiring manual administration via a prefilled syringe; Cuellar et al., 2019). Pharmacists are also integral to implementing technologic protocols related to biosimilar extrapolation, switching, substitution, and interchangeability, and ensuring that electronic prescriptions are recorded in patients’ electronic medical records for use in treatment regimens (Cuellar et al., 2019).
Conversations between oncology APs and physicians will remain central to the successful integration of biosimilars. Pharmacists are relied upon to comprehend PK/PD and clinical study data for biosimilars while remaining mindful of risk–benefit profiles and cost-containment objectives (Jarrett & Dingermann, 2015). Pharmacists also take into consideration a variety of stakeholder preferences, and ensure prescribing APs have access to the data supporting treatment decisions (Jarrett & Dingermann, 2015). Beyond a treating provider wanting to discuss with pharmacy the proper procedures for ordering a biosimilar, one of the most important conversations concerns a biosimilar being added to a formulary or that needs to be substituted based on a third-party's requirement. Treatment providers know the details underlying each patient's unique medical history (contraindications, polypharmacy, etc.). Moreover, providers will want to avoid being caught unaware when a patient has a biosimilar substituted by their pharmacy and wants to know more about the biosimilar in question. In some US states, the provider must be notified before the pharmacist substitutes a biosimilar for a reference product. In cases where the reference product is preferred, writing on a prescription “dispense as written or DAW” or “brand medically necessary” will be essential to prevent any state-legislated pharmacy substitutions from occurring in the future (Cauchi, 2019; Smeeding et al., 2019). In the US, legislation for the regulation of biosimilar substitution is state-specific (Cauchi, 2019). It is the responsibility of APs to stay apprised of the laws enacted within their state, the notification rules, and record retention requirements related to biosimilars (Lutz, 2015). Advanced practitioners can access an array of website links to sites with more in-depth information of this nature (Table 3).
Table 3. Resources for Health-Care Providers.
| Organization | Description of resources | Reference/Link |
|---|---|---|
| US Food and Drug Administration (FDA) product information | A repository of FDA-licensed biosimilar products with links to prescribing information. | https://www.fda.gov/drugs/biosimilars/biosimilar-product-information |
| US FDA Biosimilar Guidances | Biosimilars guidance drafted for industry. | https://www.fda.gov/vaccines-blood-biologics/general-biologics-guidances/biosimilars-guidances |
| US FDA | A premier source of regularly updated information on biosimilar topics relevant to the education of health-care professionals. Also available are healthcare-provider outreach materials such as webinars, videos, fact sheets, FDA staff presentations and articles, infographics, and a stakeholder toolkit with links to social media posts (i.e., Twitter, Facebook, and LinkedIn) among other tools, including patient materials. | https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars |
| US FDA | All the FDA Guidance documents concerning their interpretation of policy on regulatory issues relevant to biologics, which includes biosimilars. Covered are the design, production, labeling, promotion, manufacturing, and testing of regulated products, as well as inspection and enforcement policies, among other aspects. | https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances |
| US FDA Center for Drug Evaluation and Research | The “Purple Book” lists all available US FDA-licensed biological products showing exclusivity expiry dates and biosimilarity or interchangeability evaluations to date. | https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-products-reference-product-exclusivity-and-biosimilarity-or |
| National Comprehensive Cancer Network (NCCN) | A platform (with free registration) that provides the NCCN guidelines by cancer site and includes a reference to FDA-licensed biosimilars within the context of the current clinical practice guidelines in oncology for: breast cancers, cervical cancers, central nervous system cancers, rectal cancers, non-small cell lung cancers, B-cell lymphoma, kidney cancers, chronic lymphocytic leukemia/small lymphocytic lymphoma. | https://www.nccn.org/guidelines/category_1 |
| National Conference of State Legislatures (NCSL) | State laws and legislation related to biologic medications and substitution of biosimilars (enacted state laws, state-by-state). | http://www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx |
| Congress.Gov | Search this website to learn about and track US legislative bills related to biosimilars that have been introduced into Congress. A search term of “biosimilar” will yield many results that can subsequently be refined according to need. | http://www.congress.gov |
| MedWatch | The FDA safety information and adverse event-reporting program. | https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program |
| American Society of Clinical Oncology (ASCO) CancerLinQ database | A network designed to improve patient care via the transfer of a practice's electronic health record into the secure CancerLinQ platform. Large amounts of real-world data are de-identified, sorted, and harmonized to provide quality metrics in real-time, which can help inform clinicians’ care decisions. | https://www.cancerlinq.org/ |
| Biosimilars Council | A website with a multitude of materials, videos, blogs, news, and other resources. | https://biosimilarscouncil.org/resources/ |
| Biosimilars Forum | A website hosting evidence-based information to inform and support public policies pertaining to biosimilars, with links to downloadable educational resources, the latest news on biosimilars, and surveys of interest. | http://www.biosimilarsforum.org/ |
| European Medicines Agency (EMA) | European guidelines relevant to biosimilars, specific biosimilar products, and other guidelines relevant for biosimilars. | https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-biosimilar |
| The Center for Biosimilars | News, resources by specialty (including oncology), multimedia (insights, interviews, peer exchange and podcasts), upcoming conferences, approved biosimilars, etc. | https://www.centerforbiosimilars.com/about |
| European Specialist Nurses Organisation (ESNO) | An EU information and communication guide for nurses addressing efficient switching from biological medicines to biosimilars. It includes FAQs and sample answers that, although they will differ by country/region/hospital, could be adapted to fit specific circumstances. | https://www.esno.org/publications.html#header1-3f |
| Cancer Connect | Information about biosimilar medications for the treatment of cancer and topline information covering interchangeability, extrapolation, and the cost of biosimilars. | https://news.cancerconnect.com/treatment-care/biosimilar-drugs-and-cancer-treatment-what-you-need-to-know |
| Oncology Nursing Society (ONS): An oncology nursing overview of biosimilars | A nursing overview of biosimilars with an embedded article (requires ONS membership). | https://voice.ons.org/news-and-views/an-oncology-nursing-overview-of-biosimilars |
| National Institutes of Health – National Cancer Institute | News and events information (a blog) on the emergence of biosimilars for the treatment of cancer. Explanations are provided for various concepts related to biological products, extrapolation, interchangeability, and the European experience with biosimilars. | https://www.cancer.gov/news-events/cancer-currents-blog/2018/biosimilars-cancer-treatment |
| Position statements | ||
| American Society of Clinical Oncology (ASCO) | Lyman, GH, et al. ASCO statement: biosimilars in oncology. J Clin Oncol. 2018;36:1260–5. | https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2017.77.4893 |
| National Comprehensive Cancer Network (NCCN) Biosimilars Work Group discussions and Policy Summit | NCCN Biosimilars White Paper: Regulatory, Scientific, and Patient Safety Perspectives. 2011 | https://jnccn.org/view/journals/jnccn/9/Suppl_4/article-pS-1.xml |
| Academy of Managed Care Pharmacy (AMCP) | Pharmacy-focused professional organization that supports the provision of “positive health-care outcomes through quality, accessible, and affordable pharmaceutical care.” A position statement and other policy and advocacy resources related to biosimilars can be accessed from the AMCP site. | https://www.amcp.org/policy-advocacy/policy-resource-center/where-we-stand-position-statements |
| International Coalition of Medicines Regulatory Authorities (ICMRA) | ICMRA statement about confidence in biosimilar products (for health-care professionals). | https://www.icmra.info/drupal/sites/default/files/2019-07/ICMRA_statement_about_confidence_in_biosimilar_product_HCP.PDF |
| Community Oncology Alliance (COA) | Position statement. | https://communityoncology.org/wp-content/uploads/2019/04/Biosimilars-positiion-statement-.pdf |
| Biosimilar Medicines (EU) | A summary of EU position statements on physician-led switching for biosimilar medicines (Sept 2019). | https://www.medicinesforeurope.com/wp-content/uploads/2017/03/M-Biosimilars-Overview-of-positions-on-physician-led-switching.pdf |
| ISOPP International Society of Oncology Pharmacy Practitioners | Resources relevant to oncology pharmacists. | https://www.medicinesforeurope.com/wp-content/uploads/2017/03/M-Biosimilars-Overview-of-positions-on-physician-led-switching.pdf |
| European Society for Medical Oncology (ESMO) | The ESMO position paper on biosimilars in oncology: enhancing the provision of accurate education and information. 2017. Biosimilars: A position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. 2016. | https://www.esmoopen.com/article/S2059-7029(20)32437-6/fulltext https://www.esmoopen.com/article/S2059-7029(20)32501-1/fulltext |
Note. EU = European Union; FAQs = frequently asked questions. Information from National Conference of State Legislatures, (2019); Pittman et al. (2019).
PHARMACOVIGILANCE
As with all new therapies, post-approval monitoring of the safety of biosimilars is paramount. Safety monitoring contributes additional real-world evidence. To capture any rare AEs that coincide with exposure to biosimilars, or events that differ from those characterized for the reference product, routine documentation is advised. Similar to the procedure with reference products, AEs may not be reported in small study populations or within biosimilar study timeframes when immunogenicity is delayed. Detection of AEs can be complicated by real-world therapy combinations or polypharmacy, beyond what occurs in more homogeneous clinical trial populations, and/or could be the result of extrapolating for off-label use of reference products and biosimilars alike (Baldo et al., 2018; Grampp & Ramanan, 2015; Thill et al., 2019; Webster et al., 2019; Zelenetz et al., 2011). Post-marketing safety registries and integrated health-information systems (such as the American Society of Clinical Oncology's CancerLinQ database and MedWatch) can also add to the information available about the safety and effectiveness of biosimilars (Lyman et al., 2018). Ongoing safety monitoring is required to establish and build confidence in the long-term safety and effectiveness of biosimilars (Emmanouilides et al., 2016).
Two safety-monitoring surveillance systems provide opportunities within pharmacy and medical settings to contribute to biosimilar pharmacovigilance (Grampp & Ramanan, 2015). These are (1) spontaneous reporting emanating from voluntary reports from physicians, APs, other healthcare providers, and patients; and (2) active surveillance, using retrospective analyses of medical records, data in drug and disease registries, and prescription survey data. To ensure AEs can be properly traced to the correct biologic in pharmacovigilance systems, pharmacists, prescribers, and nurses recording patient-reported safety issues in hospital and physician office settings need to ensure sufficient product identifiers are used. In the US, biosimilars and their reference product, and any related biologic product, share the same core name (FDA, 2019a). The core or nonproprietary name of each biosimilar is distinguished by a unique hyphenated suffix of four lowercase letters that are devoid of meaning; together, the core and suffix make up the “proper name” of each biosimilar (FDA, 2017b). In some cases, suffixes have exceeded the character limit of electronic health displays, making it difficult to determine whether a reference product or a biosimilar had been prescribed (Pittman et al., 2019). Therefore, additional identifiers such as the manufacturer, the 10-digit, 3-segment National Drug Code number, the lot and batch numbers of a biosimilar, and the billing code could increase the likelihood of accurate attribution of product-related AE reports under such circumstance (FDA, 2017b). Medical staff should also use barcode scanning to verify any medication before it is dispensed or administered to improve traceability and avoid any inadvertent product mix-ups (Grampp & Ramanan, 2015; Institute for Safe Medication Practices, 2019).
LOOKING AHEAD
Globally, biosimilars are recognized as playing an important role in the treatment paradigm for oncology (Cazap et al., 2018). This is evidenced by the authorization of oncology mAb biosimilars to well-established and widely used reference biologic products considered “life-saving, life-changing, and budget-breaking” (Jarrett & Dingermann, 2015). Advanced practitioners have pivotal roles to play in the acceptance and integration of biosimilars as relatively more affordable and sustainable biologic treatment options, with the potential to improve patients’ access to targeted therapy. The knowledge, attitude, and ability of APs to communicate and reassure patients and their colleagues about biosimilars will be influential. However, the integration of biosimilars into clinical practice will also need to be achieved against the backdrop of challenging external factors, including the need for prompt regulatory guidance (finalizing interchangeability guidance has taken several years), uncertainties about product launch timing following FDA approval (biosimilars to trastuzumab and bevacizumab [Avastin, Genentech] reference products were launched up to 2 years after FDA licensure), complex federal reimbursement and insurance issues, and misinformation campaigns promoting doubt about biosimilars (Cohen & McCabe, 2020; Cottler et al., 2017; Mehr & Brook, 2019; Wechsler, 2020). Key issues to consider in terms of streamlining reimbursement challenges will be addressing appropriate therapy for insurance approval, ensuring the provision of correct Patient Assistance Programs, and providing transparent communications to enable adaption and adoption of these therapies into practice to ensure patient care and prevent denials when payers have their own formulary.
CONCLUSIONS
Based on the potential for improvements in the affordability of, and access to, biologics afforded by biosimilars, in addition to their comparable safety and efficacy to reference biologics, biosimilars warrant fair consideration by APs as a value-based treatment option for patients with cancer.
The confidence APs have in the rigor of the regulatory pathway for the development, manufacture, scientific review, authorization, and monitoring of biosimilars will be central to their comfort level when it comes to prescribing biosimilars and their management in patients who receive them. Patients with cancer receiving curative and/or supportive-care therapies can benefit from upfront and continued education about biosimilars. Patients’ acceptance of biosimilars as a safe and effective treatment will largely depend on how confidently the rationale for prescribing them is communicated, how streamlined the education they receive on biosimilars is, and ultimately how well they perceive their needs for receiving quality care are being met.
Acknowledgment
Medical writing support was provided by Sue Reinwald, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Dr. Mayden has received payment for service on speakers bureaus from Pfizer, Amgen, and Puma outside the submitted work. Dr. McBride has received payment from Pfizer and Amgen for consultancy work, and payment for lectures including service on speakers bureaus from Pfizer and Coherus, all outside the submitted work. Dr. Kelton and Dr. Ryan are full-time employees of and hold stock or options in Pfizer Inc.
References
- American Society of Clinical Oncology. (2015). FDA's biosimilar product approval process: A closer look. https://ascopost.com/issues/april-25-2015/fda-s-biosimilar-product-approval-process-a-closer-look/?utm_source=TrendMD&utm_medium=cpc&utm_campaign=The_ASCO_Post_TrendMD_0
- American Society of Health-System Pharmacists. (2013). A health-system pharmacist’ guide to biosimilars: Regulatory, scientific, and practical considerations (Continuing Education Study Guide). https://www.ashpadvantagemedia.com/downloads/biosimcentral_guidelines.pdf
- Armuzzi, A., Avedano, L., Greveson, K., & Kang, T. (2019). Nurses are critical in aiding patients transitioning to biosimilars in inflammatory bowel disease: Education and communication strategies. Journal of Crohn's and Colitis, 13(2), 259–266. 10.1093/ecco-jcc/jjy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askren, H. (2013). Patient binders: Creating a tool that helps patients and nurses. Oncology Nurse Advisor, 4, 33–39. [Google Scholar]
- Baldo, P., Fornasier, G., Ciolfi, L., Sartor, I., & Francescon, S. (2018). Pharmacovigilance in oncology. International Journal of Clinical Pharmacy, 40(4), 832–841. 10.1007/s11096-018-0706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, P. S., Griffiths, E. A., Alwan, L. M., Bachiashvili, K., Brown, A., Cool, R., & Perez, L. E. (2020). NCCN Guidelines Insights: Hematopoietic Growth Factors, Version 1.2020. Journal of the National Comprehensive Cancer Network, 18(1), 12–22. 10.6004/jnccn.2020.0002 [DOI] [PubMed] [Google Scholar]
- Bestvina, C. M., & Polite, B. N. (2017). Implementation of advance care planning in oncology: A review of the literature. Journal of Oncology Practice, 13(10), 657–662. 10.1200/JOP.2017.021246 [DOI] [PubMed] [Google Scholar]
- Bhatt, V. (2018). Current market and regulatory landscape of biosimilars. American Journal of Managed Care, 24(21 Suppl), S451–S456. [PubMed] [Google Scholar]
- Biehn, B., & Nell, C. (2022). U.S. Biosimilar report. https://www.amerisourcebergen.com/-/media/assets/amerisourcebergen/biosimilars-page/sgs-biosimilars-usmarketlandscape-041422.pdf?la=en&hash=DC34E27B09855CFD4F85BFE61F878CF2EFA6127B
- Council Biosimilars. (2017). Biosimilars in the United States: Providing more patients greater access to lifesaving medicines. http://biosimilarscouncil.org/wp-content/uploads/2019/03/Biosimilars-Council-Patient-Access-Study.pdf
- Campen, C. J. (2017). Integrating biosimilars into oncology practice: Implications for the advanced practitioner. Journal of the Advanced Practitioner in Oncology, 8(7), 688–699. [PMC free article] [PubMed] [Google Scholar]
- Canadian Breast Cancer Network. (2017). Breast cancer & biosimilars. Recommendations on use, implementation and patient communications. https://cbcn.ca/web/default/files/public/Reports/CBCN%20Biosimilars%20Whitepaper%20-%202019%20Final.pdf.
- Cazap, E., Jacobs I., McBride, A., Popovian, R., & Sikora, K. (2018). Global acceptance of biosimilars: Importance of regulatory consistency, education, and trust. The Oncologist, 23(10), 1188–1198. 10.1634/theoncologist.2017-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, M. L., & Gilliland, G. L. (2016). Engineering antibody therapeutics. Current Opinion in Structural Biology, 38, 163–173. 10.1016/j.sbi.2016.07.012 [DOI] [PubMed] [Google Scholar]
- Christl, L. (2018). FDA's overview of the regulatory guidance for the development and approval of biosimilar products in the US. https://www.fda.gov/files/drugs/published/FDA%E2%80%99s-Overview-of-the-Regulatory-Guidance-for-the-Development-and-Approval-of-Biosimilar-Products-in-the-US.pdf
- Christl, L., & Lim, S. (2018). Biosimilar and interchangeable products in the United States: Scientific concepts, clinical use, and practical considerations. https://www.fda.gov/media/122832/download [Google Scholar]
- Cohen, H. P., & McCabe, D. (2020). The importance of countering biosimilar disparagement and misinformation. BioDrugs, 34(4), 407–414. 10.1007/s40259-020-00433-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communication: What do patients want and need? (2008). Journal of Oncology Practice, 4(5), 249–253. 10.1200/JOP.0856501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congressional Research Service Report. (2019). Biologics and biosimilars: Background and key issues. https://fas.org/sgp/crs/misc/R44620.pdf.
- Cook, J. W., McGrath, M. K., Dixon, M. D., Switchenko, J. M., Harvey, R. D., & Pentz, R. D. (2019). Academic oncology clinicians’ understanding of biosimilars and information needed before prescribing. Therapeutic Advances in Medical Oncology, 11, 1758835918818335. 10.1177/1758835918818335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler, M., Whitehill, J., & Siedor, A. (2017). The 2018 biosimilar litigation landscape: A primer. https://www.biopharmadive.com/news/the-2018-biosimilar-litigation-landscape-a-primer/512982/ [Google Scholar]
- Cuellar, S., McBride, A., & Medina, P. (2019). Pharmacist perspectives and considerations for implementation of therapeutic oncology biosimilars in practice. American Journal of Health-System Pharmacy, 76(21), 1725–1738. 10.1093/ajhp/zxz190 [DOI] [PubMed] [Google Scholar]
- Curigliano, G., O’Connor, D. P., Rosenberg, J. A., & Jacobs, I. (2016). Biosimilars: Extrapolation for oncology. Critical Reviews in Oncology/Hematology, 104, 131–137. 10.1016/j.critrevonc.2016.06.002 [DOI] [PubMed] [Google Scholar]
- De Munter, J., & Crombez, P. (2018). Cancer nurse perspective on the emerging field of biosimilars in cancer. Annals of Oncology, 29(suppl 8), viii696. 10.1093/annonc/mdy277.006 [DOI] [Google Scholar]
- Demler, T. (2018). Navigating the generic regulatory landscape. https://www.uspharmacist.com/article/navigating-the-generic-regulatory-landscape
- DiGrande, S. (2018). Celltrion's trastuzumab biosimilar launches in Europe. https://www.centerforbiosimilars.com/news/celltrions-trastuzumab-biosimilar-launches-in-europe.
- Dolan, C. (2018). Opportunities and challenges in biosimilar uptake in oncology. American Journal of Managed Care, 24(11 Suppl), S237–s243. [PubMed] [Google Scholar]
- Ebbers, H. C., Fehrmann B., Ottosen, M., Hvorslev, N., Hoier, P., Hwang, J. W., & Rezk, M. F. (2020). Batch-to-batch consistency of sb4 and sb2, etanercept and infliximab biosimilars. BioDrugs, 34(2), 225–233. 10.1007/s40259-019-00402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilides, C. E., Karampola, M. I., & Beredima, M. (2016). Biosimilars: Hope and concern. Journal of Oncology Pharmacy Practice, 22(4), 618–624. 10.1177/1078155215603232 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. (2019). Biosimilar medicines: Overview. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview.
- Frey, M. K., Ellis, A., Shyne, S., Kahn, R., Chapman-Davis, E., & Blank, S. V. (2020). Bridging the gap: A priorities assessment tool to support shared decision making, maximize appointment time, and increase patient satisfaction in women with ovarian cancer. JCO Oncology Practice, 16(2), e148–e154. 10.1200/jop.19.00455 [DOI] [PubMed] [Google Scholar]
- Gabay, M. (2017). Biosimilar substitution laws. Hospital Pharmacy, 52(8), 544–545. 10.1177/0018578717726995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GaBi Online. (2017). Integrating biosimilars into clinical practice. http://www.gabionline.net/Biosimilars/Research/Integrating-biosimilars-into-clinical-practice
- GaBI Online. (2020). Biosimilars approved in Europe. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
- Gee, N. R. (2016). Quality improvement: An update for outpatient oncology education [Masters Projects and Capstones. 324]. https://repository.usfca.edu/capstone/324 [Google Scholar]
- Grampp, G., & Ramanan, S. (2015). The diversity of biosimilar design and development: Implications for policies and stakeholders. BioDrugs, 29(6), 365–372. 10.1007/s40259-015-0147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, A., & Skiermont, K. (2019). Role of specialty pharmacy regarding biosimilars. https://www.pharmacytimes.com/view/role-of-specialty-pharmacy-regarding-biosimilars [Google Scholar]
- Hobbs, A. L., & Crawford, J. P. (2019). Biosimilars and implications for pharmacy practice: Ready or not, here they come! Pharmacy Practice, 17(3), 1659. 10.18549/PharmPract.2019.3.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, W. G., Zafar, S. Y., Hesson, A., Davis, J. K., Kirby, C., Barnett, J. A., & Ubel, P. A. (2017). Discussing health care expenses in the oncology clinic: analysis of cost conversations in outpatient encounters. Journal of Oncology Practice, 13(11), e944–e956. 10.1200/JOP.2017.022855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Safe Medication Practices. (2019). Prolia-Udenyca look-alike update. https://www.ismp.org/resources/prolia-udenyca-look-alike-update
- Isaacs, J., Goncalves, J., Strohal, R., Castañeda-Hernández, G., Azevedo, V., Dörner, T., & McInnes, I. (2017). The biosimilar approval process: How different is it? Considerations in Medicine, 1, 3–6. 10.1136/conmed-2017-100003 [DOI] [Google Scholar]
- Jacobs, I., Singh, E., Sewell, K. L., Al-Sabbagh, A., & Shane, L. G. (2016). Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Preference and Adherence, 10, 937–948. 10.2147/ppa.s104891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjigian, Y. Y., Bissig, M., Curigliano, G., Coppola, J., & Latymer, M. (2018). Talking to patients about biosimilars. Future Oncology, 14(23), 2403–2414. 10.2217/fon-2018-0044 [DOI] [PubMed] [Google Scholar]
- Jarrett, S., & Dingermann, T. (2015) Biosimilars are here: A hospital pharmacist's guide to educating health care professionals on biosimilars. Hospital Pharmacy, 50(10), 884–893. 10.1310/hpj5010-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Alten, R., Avedano, L., Dignass, A., Gomollón, F., Greveson, K., & Jeong, J. H. (2020). The future of biosimilars: Maximizing benefits across immune-mediated inflammatory diseases. Drugs, 80(2), 99–113. 10.1007/s40265-020-01256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobberling, J. (2017). Economic pressure in hospitals. Deutsches Ärzteblatt International, 114(47), 795–796. 10.3238/arztebl.2017.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou, S., Papaspiliou, A., & Kokkotou, E. (2020). Current and future roles of biosimilars in oncology practice. Oncology Letters, 19(1), 45–51. 10.3892/ol.2019.11105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, M., & Nadler, S. G. (2016). Immunogenicity to biotherapeutics - the role of anti-drug immune complexes. Frontiers in Immunology, 7, 21. 10.3389/fimmu.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanton, R. (2019). The specialty pharmacist's role in educating patients about biosimilars and biologics. https://www.pharmacytimes.com/news/the-specialty-pharmacists-role-in-educating-patients-about-biosimilars-and-biologics
- Leonard, E., Wascovich, M., Oskouei, S., Gurz, P., & Carpenter, D. (2019). Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. Journal of Managed Care & Specialty Pharmacy, 25(1), 102–112. 10.18553/jmcp.2019.25.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, M. (2019). News in brief: Truxima gets high marks from physicians. Cancer Discovery, 9(2), OF2. 10.1158/2159-8290.CD-NB2018-170 [DOI] [PubMed] [Google Scholar]
- Li, J., Florian, J., Campbell, E., Schrieber, S. J., Bai, J. P. F., Weaver, J. L., & Strauss D. G. (2020) Advancing biosimilar development using pharmacodynamic biomarkers in clinical pharmacology studies. Clinical Pharmacology and Therapeutics, 107(1), 40–42. 10.1002/cpt.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Siguero, J. P., Pfaffle, R., Chanson, P., Szalecki, M., Hobel, N., & Zabransky, M. (2017). Ten years’ clinical experience with biosimilar human growth hormone: A review of efficacy data. Drug Design, Development and Therapy, 2017(11), 1489–1495. 10.2147/DDDT.S130320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz, R. (2015). Biosimilar substitution: A primer for pharmacists. https://www.pharmacytimes.com/news/biosimilar-substitution-a-primer-for-pharmacists
- Lyman, G. H., Zon, R., Harvey, R. D., & Schilsky, R. L. (2018). Rationale, opportunities, and reality of biosimilar medications. New England Journal of Medicine, 378(21), 2036–2044. 10.1056/NEJMhle1800125 [DOI] [PubMed] [Google Scholar]
- Manolis, C. H., Rajasenan, K., Harwin, W., McClelland, S., Lopes, M., & Farnum, C. (2016). Biosimilars: Opportunities to promote optimization through payer and provider collaboration. Journal of Managed Care & Specialty Pharmacy, 22(9 Suppl), S3–9. 10.18553/jmcp.2016.22.9-a.s3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus, R., Liu, J., Ramchandani, M., Landa, D., Born, T., & Kaur, P. (2017). Developing the totality of evidence for biosimilars: Regulatory considerations and building confidence for the healthcare community. BioDrugs, 31(3), 175–187. 10.1007/s40259-017-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayden, K. D., Larson, P., Geiger, D., & Watson, H. (2015). Biosimilars in the United States: Considerations for oncology advanced practitioners. Journal of the Advanced Practitioner in Oncology, 6(2), 108–116. https://dx.doi.org/10.6004%2Fjadpro.2015.6.2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan, J. E., Conlon, H. D., Bolt, M. W., Kalfayan, V., Palaparthy, R., Rehman, M. I., & Kirchhoff, C. F. (2019). The ‘totality-of-the-evidence’ approach in the development of PF-06438179/GP1111, an infliximab biosimilar, and in support of its use in all indications of the reference product. Therapeutic Advances in Gastroenterology, 12, 1756284819852535. 10.1177/1756284819852535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraill, S. (2019). Leveraging advanced practice providers for team-based care. https://patientengagementhit.com/news/leveraging-advanced-practice-practitioners-for-team-based-care
- McMahon Publishing. (2013). Special Report. Understanding key differences between biosimilars and small molecule generics. Pharmacy Practice News, 40, 1–8. [Google Scholar]
- Mehr, S. R., & Brook, R. A. (2019). Biosimilars in the USA: Will new efforts to spur approvals and access spur uptake and cost savings? Pharmaceutical Medicine, 33(1), 1–8. 10.1007/s40290-018-00262-z [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2020). NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. V2.2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed]
- National Comprehensive Cancer Network. (2019). Framework for Resource Stratification. https://www.nccn.org/framework/default.aspx.
- Cauchi, R. (2019). State laws and legislation related to biologic medications and substitution of biosimilars. http://www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx
- NIH National Cancer Institute. (2019). Immunotherapy to treat cancer. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet.
- Oncology Nursing Society. (2019). ONS Member Community topic thread: Biosimilars and re-consent. https://communities.ons.org/communities/community-home/digestviewer/viewthread?MessageKey=bcb49689-77ad-4f20-b041-23e015a2003b&CommunityKey=0b54ba80-79aa-446b-80ae-5434d67c4adf&tab=digestviewer#bm46c4552f-ea22-46d2-a878-2007d7f80482
- Oubre, K. (2019). Empower physicians to fight financial toxicity with biosimilars. Evidence-Based Oncology, 25(12). https://www.ajmc.com/view/empower-physicians-to-fight-financial-toxicity-with-biosimilars [PubMed] [Google Scholar]
- Pakizegee, M., Stefanacci, R., Ornstein, C., & Hardesty, J. (2020). Oncolytic biosimilars: An opportunity to reduce oncology treatment costs. Journal of Clinical Pathways, 6(3), 28–30. 10.25270/jcp.2020.4.00003 [DOI] [Google Scholar]
- Pittman, W. L., Wer, C., & Glode, A. E. (2019). Review of biosimilars and their potential use in oncology treatment and supportive care in the United States. Journal of Hematology Oncology Pharmacy, 9(3), 133–141. [Google Scholar]
- Primeau, A. S. B. (2019). Biosimilar therapies for cancer. https://www.cancertherapyadvisor.com/home/tools/fact-sheets/biosimilar-therapies-for-cancer-treatment-patient-fact-sheet/.
- Pushparajah, D. S., Manning, E., Michels, E., & Arnaudeau-Begard, C. (2018). Value of developing plain language summaries of scientific and clinical articles: A survey of patients and physicians. Therapeutic Innovation & Regulatory Science, 52(4), 474–481. 10.1177/2168479017738723 [DOI] [PubMed] [Google Scholar]
- Rathore, A. S., Vulto, A. G., Stevenson, J. G., & Shah, R. (2019). Challenges with successful commercialization of biosimilars. BioPharm International, 32(5), 22–31. [Google Scholar]
- Reinke, T. (2019). Why biosimilars can never be identical to originators-and why they don't need to be. Managed Care, 28(1), 10–11. [PubMed] [Google Scholar]
- Renton, W. D., Leveret, H., Guly, C., Smee, H., Leveret, J., & Ramanan, A. V. (2019). Same but different? A thematic analysis on adalimumab biosimilar switching among patients with juvenile idiopathic arthritis. Pediatric Rheumatology Online Journal, 17(1), 67. 10.1186/s12969-019-0366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter, J., Spencer, J. C., & Wheeler, S. B. (2019). Financial toxicity in advanced and metastatic cancer: overburdened and underprepared. Journal of Oncology Practice, 15(4), e300–e307. 10.1200/JOP.18.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo, H. S., Linton, K. M., Cervi, P., Rosenberg, J. A., & Jacobs, I. (2016). A clinician's guide to biosimilars in oncology. Cancer Treatment Reviews, 46, 73–79. 10.1016/j.ctrv.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Rumore, M. M., Cobb, E., Sullivan, M., & Wittman, D. (2016). Biosimilars: Opportunities and challenges for nurse practitioners. Journal for Nurse Practitioners, 12(3), 181–191. 10.1016/j.nurpra.2015.08.027 [DOI] [Google Scholar]
- Schellekens, H., Smolen, J. S., Dicato, M., & Rifkin, R. M. (2016). Safety and efficacy of biosimilars in oncology. Lancet Oncology, 17(11), e502–e509. 10.1016/S1470-2045(16)30374-6 [DOI] [PubMed] [Google Scholar]
- Schiestl, M., & Krendyukov, A. (2017). The ESMO position paper on biosimilars in oncology: Enhancing the provision of accurate education and information. ESMO Open, 2(3), e000245. 10.1136/esmoopen-2017-000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, M., Stangler, T., Torella, C., Čepeljnik, T., Toll, H., & Grau R. (2011). Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nature Biotechnology, 29(4), 310–312. 10.1038/nbt.1839 [DOI] [PubMed] [Google Scholar]
- Schiestl, M., Ranganna, G., Watson, K., Jung, B., Roth, K., Capsius, B., & Marechal-Jamil, J. (2020). The path towards a tailored clinical biosimilar development. BioDrugs, 34, 297–306. 10.1007/s40259-020-00422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, R. (2019). Communicating with patients about the value of biosimilars. https://www.centerforbiosimilars.com/interviews/robin-shah-communicating-with-patients-about-the-value-of-biosimilars-?utm_medium=email&utm_campaign=CFBSS%20Biosimilars%20enews%20-%2011-13-19&utm_content=CFBSS%20Biosimilars%20enews%20-%2011-13-19+CID_23532e5e1ccb697dfcae219d537a937b&utm_source=CM%20BioSim&utm_term=Robin%20Shah%20Communicating%20With%20Patients%20About%20the%20Value%20of%20Biosimilars
- Smeeding, J., Malone, D. C., Ramchandani, M., Stolshek, B., Green, L., & Schneider, P. (2019). Biosimilars: Considerations for payers. Pharmacy and Therapeutics, 44(2), 54–63. [PMC free article] [PubMed] [Google Scholar]
- Smerker, J. (2016). The role of biosimilars in the growth of specialty pharmacy. https://www.pharmacytimes.com/news/the-role-of-biosimilars-in-the-growth-of-specialty-pharmacy
- Smith, J. A., & Zsohar, H. (2013). Patient-education tips for new nurses. Nursing, 43(10), 1–3. 10.1097/01.NURSE.0000434224.51627.8a [DOI] [PubMed] [Google Scholar]
- Spanou, I., Mavridis, T., & Mitsikostas, D. D. (2019). Nocebo in biosimilars and generics in neurology: A systematic review. Frontiers in Pharmacology, 10, 809. 10.3389/fphar.2019.00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, D. (2017). Biosimilar interchangeability: Do you know your switching form your substitution? https://www.biopharma-reporter.com/Article/2017/03/21/Biosimilr-switching-interchangeability-and-substitution-the-EU-view
- Stebbing, J., Mainwaring, P. N., Curigliano, G., Pegram, M., Latymer, M., Bair, A. H., & Rugo, H. S. (2020). Understanding the role of comparative clinical studies in the development of oncology biosimilars. Journal of Clinical Oncology, 38(10), 1070–1080. 10.1200/JCO.19.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill, M. (2019). Biosimilar trastuzumab in clinical trials: Differences or not? Breast Care (Basel), 14(1), 17–22. 10.1159/000496503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill, M., Thatcher, N., Hanes, V., & Lyman, G. H. (2019). Biosimilars: What the oncologist should know. Future Oncology, 15(10), 1147–1165. 10.2217/fon-2018-0728 [DOI] [PubMed] [Google Scholar]
- Transparency Market Research. (2016). According to new research study, oncology biosimilars market is expected to witness substantial growth over the forecast period of 2016 – 2024. https://www.biosimilardevelopment.com/doc/according-to-new-research-study-oncology-biosimilars-market-0001
- Trollope, R., Johnson, S., & Ireland, H. (2017). Launching biosimilar rituximab: An industry opinion on biosimilar uptake in Europe. Immunotherapy, 9(7), 527–529. 10.2217/imt-2017-0054 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. (2009). Assay development for immunogenicty testing of therapeutic proteins. Guidance for industry. http://www.nihs.go.jp/dbcb/immunogenicity/FDA_2009_Assay%20Development%20for%20Immunogenicity%20Testing%20of%20Therapeutic%20Proteins_Draft.pdf
- US Food and Drug Administration. (2015). Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry https://www.fda.gov/media/82647/download
- US Food and Drug Administration. (2017a). Biosimilar and interchangeable biologics: more treatment choices. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- US Food and Drug Administration. (2017b). Nonproprietary naming of biological products. Guidance for industry. https://www.fda.gov/media/93218/download
- US Food and Drug Administration. (2017c). Biosimilar development, review, and approval. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval
- US Food and Drug Administration. (2019a). Nonproprietary naming of biological products: update. Guidance for industry. Draft guidance. https://www.fda.gov/media/121316/download
- US Food and Drug Administration. (2019b). Considerations in demonstrating interchangeability with a reference product. Guidance for industry. https://www.fda.gov/media/124907/download
- US Food and Drug Administration. (2020a). Biosimilar basics for patients. https://www.fda.gov/drugs/biosimilars/patient-materials
- US Food and Drug Administration. (2020b). Prescribing interchangeable products. https://www.fda.gov/media/108107/download
- van Brummelen, E. M., Ros, W., Wolbink, G., Beijnen, J. H., & Schellens, J. H. (2016). Antidrug antibody formation in oncology: Clinical relevance and challenges. The Oncologist, 21(10), 1260–1268. 10.1634/theoncologist.2016-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoven, A. (2017). Biosimilar medicines clinical use: An experience based EU-perspective. https://www.medicinesforeurope.com/docs/20170713%20-%20Biosimilar%20Medicines%20Group,%20EU%20experience-AVH-US%20FDA%20Adcom.pdf
- Ventola, C. L. (2015). Evaluation of biosimilars for formulary inclusion: Factors for consideration by P&T committees. P T, 40(10), 680–689. [PMC free article] [PubMed] [Google Scholar]
- Vorobiof, D. A., Hasid, L., Litvin, A., Deutsch, I., & Malki, E. (2020). Real-world evidence data (RWED) of financial toxicity (FT) in patients (pts)receiving cancer immunotherapy treatments. Journal of Clinical Oncology, 38(5), 90. https://ascopubs.org/doi/10.1200/JCO.2020.38.5_suppl.90 [Google Scholar]
- Vulto, A. G., & Jaquez, O. A. (2017). The process defines the product: What really matters in biosimilar design and production? Rheumatology (Oxford, England), 56(suppl_4), iv14–iv29. 10.1093/rheumatology/kex278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, A., Moore, A., Barber, A., & Opsteen, J. (2014). Educational role of nurse practitioners in a family practice centre: Perspectives of learners and nurses. Canadian Family Physician [Médecin de famille canadien], 60(6), e316, e318–321. [PMC free article] [PubMed] [Google Scholar]
- Warsame, R., Kennedy, C. C., Kumbamu, A., Branda, M., Fernandez, C., Kimball, B., & Tilburt J. C. (2019). Conversations about financial issues in routine oncology practices: A multicenter study. Journal of Oncology Practice, 15(8), e690–e703. 10.1200/jop.18.00618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, C. J., Wong, A. C., & Woollett, G. R. (2019). An efficient development paradigm for biosimilars. BioDrugs, 33(6), 603–611. 10.1007/s40259-019-00371-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, J. (2020). Feds target biosimilar anticompetitive practices. Pharm Exec, 40(3). http://www.pharmexec.com/feds-target-biosimilar-anticompetitive-practices [Google Scholar]
- Wehrwein, P. (2020). Biosimilars for cancer an ‘emerging success’ says NCCN's Koh. https://www.managedhealthcareexecutive.com/view/biosimilars-for-cancer-an-emerging-success-says-nccn-s-koh
- Weise, M. (2019). From bioequivalence to biosimilars: How much do regulators dare? ZEFQ, 140, 58[en]62. 10.1016/j.zefq.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Welch, A. R. (2017). How biosimilar companies can meet patients’ education needs. https://www.biosimilardevelopment.com/doc/how-biosimilar-companies-can-meet-patients-education-needs-0001.
- Welch, A. R. (2018). The importance of humanizing biosimilars. https://www.biosimilardevelopment.com/doc/the-importance-of-humanizing-biosimilars-0001
- Wolff-Holz, E., Garcia Burgos, J., Giuliani, R., Befrits, G., de Munter, J., Avedano, L., & Tabernero, J. (2018). Preparing for the incoming wave of biosimilars in oncology. ESMO Open, 3(6), e000420. 10.1136/esmoopen-2018-000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J., Xu, W. W., & Poon, H. F. (2018). Analytical approach for biosimilar development: Special focus on monoclonal antibody biosimilars. International Journal of Biopharmaceutical Sciences, 1(2), 1–4. 10.31021/ijbs.20181109 [DOI] [Google Scholar]
- Zack, E. (2018). Nursing roles: Clinical implications regarding trends, administration, and education for biosimilars in oncology practice. Clinical Journal of Oncology Nursing, 22(5), 21–26. 10.1188/18.CJON.S1.21-26 [DOI] [PubMed] [Google Scholar]
- Zelenetz, A. D., Ahmed, I., Braud, E. L., Cross, J. D., Davenport-Ennis, N., Dickinson, B. D., & Hoffman J. M. (2011). NCCN biosimilars white paper: regulatory, scientific, and patient safety perspectives. Journal of the National Comprehensive Cancer Network, 9(suppl 4), S1–22. 10.6004/jnccn.2011.0136. [DOI] [PubMed] [Google Scholar]
- Zlatkus A., Bixby T., & Goyal, K. (2020). Considerations for the US health-system pharmacist in a world of biosimilars. Drugs in Context, 9, 2019-12-1. 10.7573/dic.2019-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]