Abstract
Background
In hepatocellular carcinoma (HCC), pulmonary metastasis (PM) after hepatectomy is associated with poor clinical outcomes. The crucial phases of tumour cell proliferation, angiogenesis, and metastasis all entail platelet activation. In HCC, platelet distribution width (PDW) suggests platelet size changes and predicts a worse prognosis. The aim of this study was to assess the association between PDW and PMs in HCC patients receiving hepatectomy.
Material/methods
From January 2013 to December 2015, a cohort of patients who underwent hepatectomy for HCC at the Harbin Medical University Cancer Hospital in China were retrospectively evaluated. The relationship between PDW levels and clinical and demographic parameters was examined. To investigate the relationships between predicted factors and PM, a competing risk model was used. From January 2016 to December 2018, a validation cohort of 109 patients from the First Affiliated Hospital of Harbin Medical University was studied independently.
Results
In the primary cohort, 19 out of 214 patients had postoperative PMs. In HCC patients with PM, PDW levels were lower than in those without PM. There was a significant difference in the cumulative incidence of 2-year PM between the high-PDW and low-PDW groups after controlling for competing risk events (death prior to the development of PM) (p < 0.001). In addition, PDW was also found to be an independent predictor for PM in a multivariable competing risk analysis. The results were externally validated in another cohort.
Conclusions
In HCC, preoperative PDW is significantly associated with PM. PDW could be a biomarker for post-operative PM in HCC patients.
Keywords: Hepatocellular carcinoma, Pulmonary metastases, Platelet distribution width, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of malignancy-related death [1, 2]. Curative hepatectomy remains the most common treatment for HCC patients. However, there is a substantial risk of recurrence after curative hepatectomy. Despite the fact that intrahepatic recurrence is more common, extrahepatic metastases (EHMs) represent 14.0% to 25.5% of all recurrences, After curative hepatectomy, pulmonary metastasis (PM) accounts for roughly half of all EHMs [3, 4].
The presence of PM after hepatectomy indicates a poor prognosis. Despite advances in PM therapy, strategies for accurately predicting the incidence of PM following curative hepatectomy remain inadequate [4]. Identifying high-risk patients for PM before surgery is helpful in early detection and early intervention. Thus, investigation of novel biomarkers for PM is urgently needed.
Recently, abundant evidence shows that platelet activation is involved in tumor proliferation, angiogenesis, and metastasis [5]. Through direct signal transduction with hepatocytes and liver parenchymal cells, platelets have been demonstrated to increase HCC cell proliferation and infiltration, as well as liver regeneration. Moreover, antiplatelet therapy has also been demonstrated to reduce liver damage and improve patient outcomes [6]. Platelet distribution width (PDW) reveals variations in platelet size and is considered a hallmark of platelet morphology [7, 8]. At present, PDW has been proven to be critical in the prediction of liver metastasis in colorectal cancer and distant metastasis in gastric cancer [9]. PDW levels were also found to predict poor survival in HCC in our previous study [10]. However, no study has investigated the association between PDW and PM following hepatectomy for HCC.
The aim of this study was to assess the association between PDW and PMs in HCC patients receiving hepatectomy.
Materials and methods
Patients
The clinical data of 214 patients with histologically diagnosed HCC at the Harbin Medical University Cancer Hospital in China were reviewed retrospectively from January 2013 to December 2015. All of the patients were subjected to radical surgical resection. They exhibited no signs of substantial portal vein/hepatic vein invasion and had not received any adjuvant therapy prior to surgery. This study excluded participants with other malignancies, haematological illness, infectious disease, and cardiovascular disease. The subjects who had treatment with anticoagulants, statins, or acetylic salicylic acid were also excluded. Information from another independent cohort of patients who underwent hepatectomy for HCC at the First Affiliated Hospital of Harbin Medical University, from January 2016 to December 2018, was retrospectively collected. Two hospitals' ethics committees gave their approval for the study. An informed consent form was signed by all participants.
Data collection
The following demographic and clinicopathological information were collected: age, sex, body mass index (BMI), hepatitis B virus surface antigen (HBsAg), antibodies to hepatitis C virus (anti-HCV), the presence of liver cirrhosis, Child–Pugh’s grade, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GGT), albumin, total bilirubin, creatinine, alphafetoprotein (AFP), international normalized ratio (INR), tumor size, tumor number, capsule, tumor differentiation, macrovascular invasion, model of end-stage liver disease score (MELD score), fibrosis-4 index (FIB-4), aspartate aminotransferase to platelet ratio index (APRI), platelet-albumin-bilirubin (PALBI), and albumin-bilirubin (ALBI) score. White blood cell count (WBC), platelet count, PDW, mean platelet volume (MPV), and haemoglobin were directly obtained by an automated hematological analyzer. PDW had a normal range of 11–17%.
Follow-up
After hepatectomy, patients were followed up every three months. At each appointment, a routine abdomen and chest computed tomography (CT) was conducted.
Liver function and serum AFP were measured. When tumor recurrence or metastasis is suspected, abdominal contrast-enhanced CT and/or magnetic resonance imaging (MRI) are performed every 6 months or earlier. The following criteria were used to diagnose PM: (a) A dynamic chest CT scan revealed freshly emerging lesions, particularly many round nodules around the lungs; and (b) AFP levels were increased. The CT findings were confirmed by at least two independent radiologists. Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1) was used to define the response. A bronchial perfusate examination and sputum cytological test were used to differentiate other pulmonary lesions. All of the patients were tracked for up to two years. Patients who were diagnosed with PM less than a month after hepatectomy were excluded.
Statistical analysis
Statistical analyses were completed using SPSS software (version 26.0), and R software (version 4.1.2). Categorical and continuous variables were analyzed using Chi-squared test and Student’s t-test, respectively. Receiver operating characteristic (ROC) curve was constructed to define the optimal cut-off value of PDW using MedCalc software (version 15.0). Among these survival outcomes, PM was the interest event, while death was considered a competing risk. Survival analyses were performed using univariable and multivariable competing risk models. Variables were included in the multivariable competing risk analysis if the P value on univariable competing risk analysis was < 0.10. The cumulative incidence of PM was estimated using the cumulative incidence function (CIF) curves and intergroup comparison was analyzed using the Gray’s test. The results were presented as subdistribution hazard ratios (sHR) with 95% CI. P < 0.05 was regarded as significant.
Results
The clinicopathological characteristics of HCC patients in the derivation set and validation set are summarized in Table 1. There were 214 patients (mean age, 52.6 ± 9.2 years; range, 26.0 to 74.0 years) in the derivation cohort, including 162 males (75.7%) and 52 females (24.3%). However, no statistically significant differences were detected between the derivation and validation cohorts with regard to age, sex, hepatitis C, vascular invasion, tumor number, MELD score, APRI score, FIB-4 score, AFP levels, platelet count, and ALT levels.
Table 1.
Baseline characteristics of patients with HCC
| Variables | Derivation set | Validation set | P-value |
|---|---|---|---|
| N | 214 | 109 | |
| Age (years) | 52.6 ± 9.2 | 52.7 ± 10.1 | 0.966 |
| BMI (kg/m2) | 24.2 ± 3.8 | 22.0 ± 2.6 | < 0.001 |
| Sex (male, %) | 162 (75.7) | 82 (75.2) | 0.926 |
| HBsAg (%) | 190 (88.8) | 65 (59.6) | < 0.001 |
| Hepatitis C (%) | 11 (5.1) | 2 (1.8) | 0.153 |
| Cirrhosis (%) | 196 (91.6) | 41 (37.6) | < 0.001 |
| Child Pugh score | < 0.001 | ||
| A | 205 (95.8) | 85 (78.0) | |
| B | 9 (4.2) | 24 (22.0) | |
| Vascular invasion | 0.631 | ||
| No | 186 (86.9) | 24 (22.0) | |
| Yes | 28 (13.1) | 85 (78.0) | |
| Tumor number | 0.631 | ||
| Single | 183 (85.5) | 91 (83.5) | |
| Multiple | 31 (14.5) | 18 (16.5) | |
| Tumor differentiation | < 0.001 | ||
| Poor | 38 (17.8) | 50 (45.9) | |
| Moderate/well | 176 (82.2) | 59 (54.1) | |
| Capsule | 0.001 | ||
| Complete | 183 (85.5) | 77 (70.6) | |
| Incomplete | 31 (14.5) | 32 (29.4) | |
| Tumor size (cm) | 4.9 ± 2.9 | 6.3 ± 4.2 | 0.002 |
| MELD score | -1.84 (-3.58 to 0.02) | 1.57 (-4.20 to 0.90) | 0.451 |
| FIB-4 | 1.9 (1.4–3.2) | 1.9 (1.1–3.2) | 0.417 |
| APRI | 0.6 (0.4–0.9) | 0.7 (0.4–1.2) | 0.439 |
| PALBI | -3.3 (-3.6 to -3.1) | -4.8 (-5.2 to -4.2) | < 0.001 |
| ALBI | -2.6 (-2.9 to -2.3) | -2.0 (-2.4 to -1.7) | < 0.001 |
| AFP (ng/mL) | 14.8 (4.1–227.5) | 23.7 (4.7–471.2) | 0.390 |
| WBC (× 109/L) | 5.84 ± 2.10 | 8.39 ± 3.98 | < 0.001 |
| Haemoglobin (g/L) | 142.0 ± 17.3 | 122.2 ± 23.7 | < 0.001 |
| Platelet count (× 109/L) | 161.5 ± 63.2 | 177.9 ± 90.6 | 0.093 |
| MPV (fL) | 10.6 ± 1.4 | 11.1 ± 1.1 | < 0.001 |
| PDW (%) | 15.2 ± 2.4 | 13.2 ± 2.2 | < 0.001 |
| INR | 1.06 ± 0.09 | 1.21 ± 0.26 | < 0.001 |
| Albumin (g/L) | 39.5 ± 4.6 | 34.3 ± 5.8 | < 0.001 |
| Creatinine (μmol/L) | 76.8 ± 15.0 | 60.6 ± 15.5 | < 0.001 |
| AST (U/L) | 35 (28–47) | 51 (31–88) | < 0.001 |
| ALT (U/L) | 39 (28–52) | 39 (26–60) | 0.589 |
| γ-GGT (U/L) | 49 (32–84) | 78 (44–135) | < 0.001 |
| Total bilirubin (μmol/L) | 13.9 (10.4–18.7) | 18.2 (14.1–25.6) | < 0.001 |
AFP Alphafetoprotein, ALBI Albumin-bilirubin, APRI Aspartate aminotransferase to platelet count ratio index, AST Aspartate aminotransferase, ALT Alanine aminotransferase, BMI Body mass index, FIB-4 Fibrosis-4 index, γ-GGT γ-glutamyl transferase, INR International normalized ratio, MPV Mean platelet volume, MELD score model of end-stage liver disease score, PDW, Platelet distribution width, PALBI Platelet-albumin-bilirubin, WBC White blood cell
Table 2 displays the characteristics of HCC patients stratified by PM status. In the derivation cohort, over a median follow-up period of 27.0 (range 4.0–82.0) months, 19 (8.88%) patients had PM events. Moreover, statistical significance was found in vascular invasion, capsule, tumor size, AFP, and PDW levels between the PM and non-PM groups (Table 2). Other clinical parameters were not in correlation with PM. In the validation set, the median follow-up time was 25.0 (range 4.0–46.0) months. Cirrhosis, Child Pugh score, tumor size, MELD score, PALBI, AFP, haemoglobin, platelet count, and PDW levels between the two groups had a significant difference.
Table 2.
The characteristics of HCC patients stratified by PM status
| Variables | Without PM | With PM | P-value |
|---|---|---|---|
| Development set | |||
| N | 195 | 19 | |
| Age (years) | 52.6 ± 9.2 | 53.0 ± 9.1 | 0.855 |
| BMI (kg/m2) | 24.2 ± 3.8 | 24.4 ± 3.5 | 0.780 |
| Sex (male, %) | 151 (77.4) | 11 (57.9) | 0.058 |
| HBsAg (%) | 173 (88.7) | 17 (89.5) | 0.921 |
| Hepatitis C (%) | 10 (5.1) | 1 (5.3) | 0.980 |
| Cirrhosis (%) | 179 (91.8) | 17 (89.5) | 0.728 |
| Child Pugh score | 0.810 | ||
| A | 187 (95.9) | 18 (94.7) | |
| B | 8 (4.1) | 1 (5.3) | |
| Vascular invasion | < 0.001 | ||
| No | 176 (90.3) | 10 (52.6) | |
| Yes | 19 (9.7) | 9 (47.4) | |
| Tumor number | 0.060 | ||
| Single | 164 (84.1) | 19 (100.0) | |
| Multiple | 31 (15.9) | 0 (0.0) | |
| Tumor differentiation | 0.099 | ||
| Poor | 32 (16.4) | 6 (31.6) | |
| Moderate/well | 163 (83.6) | 13 (68.4) | |
| Capsule | 0.004 | ||
| Complete | 171 (87.7) | 12 (63.2) | |
| Incomplete | 24 (12.3) | 7 (36.8) | |
| Tumor size (cm) | 4.7 ± 2.8 | 7.5 ± 3.5 | < 0.001 |
| MELD score | -1.8 (-3.6 to -0.0) | -2.0 (-2.9 to 1.2) | 0.422 |
| FIB-4 | 1.9 (1.3–3.1) | 2.3 (1.6–3.3) | 0.327 |
| APRI | 0.6 (0.4–0.9) | 0.7 (0.4–0.9) | 0.714 |
| PALBI | -3.3 (-3.6 to -3.0) | -3.3 (-3.7 to -3.2) | 0.353 |
| ALBI | -2.6 (-2.9 to -2.3) | -2.7 (-3.0 to -2.4) | 0.201 |
| AFP (ng/mL) | 12.3 (3.9–174.3) | 231.0 (22.6–11,894.3) | 0.001 |
| WBC (× 109/L) | 5.82 ± 1.92 | 6.06 ± 3.48 | 0.634 |
| Haemoglobin (g/L) | 142.5 ± 16.3 | 137.4 ± 25.7 | 0.404 |
| Platelet count (× 109/L) | 160.3 ± 64.3 | 174.0 ± 50.5 | 0.367 |
| MPV (fL) | 10.7 ± 1.5 | 10.2 ± 1.1 | 0.175 |
| PDW (%) | 15.4 ± 2.3 | 13.3 ± 2.3 | < 0.001 |
| INR | 1.05 ± 0.08 | 1.08 ± 0.17 | 0.255 |
| Albumin (g/L) | 39.4 ± 4.6 | 40.9 ± 4.7 | 0.158 |
| AST (U/L) | 35 (28–47) | 38 (34–56) | 0.137 |
| ALT (U/L) | 40 (28–53) | 31 (26–50) | 0.250 |
| γ-GGT (U/L) | 49 (32–84) | 48 (34–76) | 0.927 |
| Total bilirubin (μmol/L) | 13.9 (10.4–18.7) | 14.6 (10.4–21.2) | 0.423 |
| Creatinine (μmol/L) | 76.8 ± 15.1 | 76.6 ± 14.2 | 0.967 |
| Validation set | |||
| N | 98 | 11 | |
| Age (years) | 53.2 ± 9.8 | 47.9 ± 11.9 | 0.099 |
| BMI (kg/m2) | 22.1 ± 2.6 | 21.0 ± 2.3 | 0.193 |
| Sex (male, %) | 76 (77.6) | 6 (54.5) | 0.094 |
| HBsAg (%) | 60 (61.2) | 5 (45.5) | 0.312 |
| Hepatitis C (%) | 2 (2.0) | 0 (0) | 0.632 |
| Cirrhosis (%) | 40 (40.8) | 1 (9.1) | 0.039 |
| Child Pugh score | 0.048 | ||
| A | 79 (80.6) | 6 (54.5) | |
| B | 19 (19.4) | 5 (45.5) | |
| Vascular invasion | 0.063 | ||
| No | 24 (24.5) | 0 (0) | |
| Yes | 74 (75.5) | 11 (100.0) | |
| Tumor number | 0.311 | ||
| Single | 83 (84.7) | 8 (72.7) | |
| Multiple | 15 (15.3) | 3 (27.3) | |
| Tumor differentiation | 0.212 | ||
| Poor | 43 (43.9) | 7 (63.6) | |
| Moderate/well | 55 (56.1) | 4 (36.4) | |
| Capsule | 0.591 | ||
| Complete | 70 (71.4) | 7 (63.6) | |
| Incomplete | 28 (28.6) | 4 (36.4) | |
| Tumor size (cm) | 5.6 ± 3.5 | 13.0 ± 3.4 | < 0.001 |
| MELD score | -1.4 (-4.0 to -1.3) | -4.3 (-6.3 to-1.6) | 0.041 |
| FIB-4 | 2.0 (1.3–3.3) | 0.9 (0.4–3.1) | 0.103 |
| APRI | 0.7 (0.4–1.2) | 0.4 (0.2–1.2) | 0.300 |
| PALBI | -4.8 (-5.2 to -4.1) | -5.3 (-5.8 to -4.7) | 0.050 |
| ALBI | -2.0 (-2.4 to -1.7) | -2.0 (-2.5 to -1.8) | 0.904 |
| AFP (ng/mL) | 16.2 (4.5–416.4) | 415.7 (94.4–1000.0) | 0.001 |
| WBC (× 109/L) | 8.45 ± 4.08 | 7.80 ± 2.98 | 0.609 |
| Haemoglobin (g/L) | 123.9 ± 22.3 | 107.0 ± 31.3 | 0.024 |
| Platelet count (× 109/L) | 171.8 ± 87.0 | 232.3 ± 108.3 | 0.035 |
| MPV (fL) | 11.2 ± 1.1 | 10.7 ± 0.5 | 0.134 |
| PDW (%) | 13.4 ± 2.1 | 11.2 ± 1.1 | < 0.001 |
| INR | 1.21 ± 0.27 | 1.17 ± 0.11 | 0.613 |
| Albumin (g/L) | 34.4 ± 5.9 | 33.6 ± 5.9 | 0.639 |
| Creatinine (μmol/L) | 60.8 ± 13.9 | 59.7 ± 27.0 | 0.902 |
| AST (U/L) | 38 (27–57) | 56 (24–73) | 0.794 |
| ALT (U/L) | 52 (31–87) | 50 (26–203) | 0.665 |
| γ-GGT (U/L) | 78 (42–134) | 77 (65–252) | 0.289 |
| Total bilirubin (μmol/L) | 18.9 (14.6–26.0) | 14.0 (10.4–25.4) | 0.155 |
Abbreviations: see to Table 1
The optimal cut-off value of PDW was determined as 14.1% with an area under the curve (AUC) value of 0.732 (0.667–0.790) using ROC curve in the derivation cohort (Fig. 1). The HCC patients were classified into two parts based on the cut-off value (low-PDW (≤ 14.1%) and high-PDW (> 14.1%)). Among all patients, 57 patients (26.6%) had PDW ≤ 14.1 and 157 patients (73.4%) had PDW > 14.1. Over a median follow-up of 27.0 months, 7 patients in high-PDW group and 12 patients in low-PDW group had PM events. In the validation cohort, the HCC patients were classified into two parts by the same cut-off value of PDW. During the follow-up of 25.0 months, 1 patient in high-PDW group and 10 patients in low-PDW group developed PM.
Fig. 1.
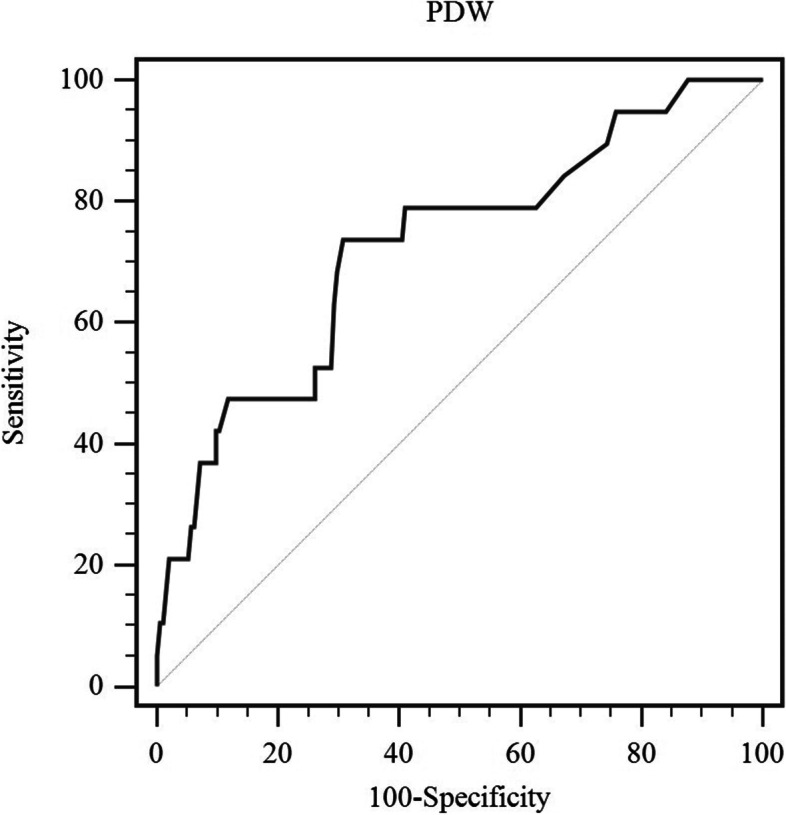
ROC curve to determine an optimal cut-off value of PDW
Death was treated as an event competing with PM. Table 3 shows the results of the competing-risk analysis. In the univariable analysis, sex, vascular invasion, capsule, PDW (continuous variable) and tumor size (continuous variable) were significant prognostic factors for PM in the derivation cohort (p < 0.05). All these factors were included in the multivariable model. The multivariable competing-risk analysis revealed that PDW (sHR = 0.850, 95%CI [0.736–0.983]) and tumor size (sHR = 1.240, 95%CI [1.045–1.481]) were the independent predictive factors for PM. The same results were externally validated in another cohort.
Table 3.
The predictors of PM in HCC patients
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| sHR (95% CI) | P-value | sHR (95% CI) | P-value | |
| Development set | ||||
| Sex (male vs female) | 0.405(0.164–0.997) | 0.049 | 0.443(0.170–1.153) | 0.095 |
| Vascular invasion (Yes vs No) | 5.360(2.200–13.100) | < 0.001 | 2.610(0.898–7.596) | 0.078 |
| Tumor number (Multiple vs Single) | 0.712(0.209–2.430) | 0.590 | ||
| Tumor differentiation (Poor vs Moderate/well) | 1.310(0.498–3.450) | 0.580 | ||
| Capsule (Incomplete vs Complete) | 3.110(1.230–7.850) | 0.017 | 1.490(0.534–4.181) | 0.440 |
| Tumor size (cm) | 1.330(1.200–1.460) | < 0.001 | 1.240(1.045–1.481) | 0.014 |
| AFP (ng/mL) | 1.000(1.000–1.000) | 0.090 | ||
| PDW (%) | 0.798(0.706–0.901) | < 0.001 | 0.850(0.736–0.983) | 0.028 |
| Validation set | ||||
| Cirrhosis (Yes vs No) | 0.089(0.011–0.703) | 0.022 | 0.376(0.028–4.970) | 0.460 |
| Child Pugh score (B vs A) | 6.270 (1.970–19.900) | 0.001 | 2.587(0.378–17.682) | 0.330 |
| Tumor size (cm) | 1.530(1.350–1.730) | < 0.001 | 1.940(1.291–2.915) | 0.001 |
| MELD score | 0.944(0.686–1.300) | 0.720 | ||
| PALBI | 0.400(0.146–1.100) | 0.070 | ||
| AFP (ng/mL) | 1.000(1.000–1.000) | 0.630 | ||
| Haemoglobin (g/L) | 0.972(0.961–0.983) | < 0.001 | 1.046(0.988–1.107) | 0.120 |
| Platelet count (× 109/L) | 1.010(1.000–1.010) | 0.016 | 1.001(0.990–1.011) | 0.920 |
| PDW (%) | 0.764(0.645–0.904) | 0.001 | 0.523(0.304–0.898) | 0.019 |
sHR Subdistribution hazard ratio, CI Confidence interval Abbreviations: see to Table 1
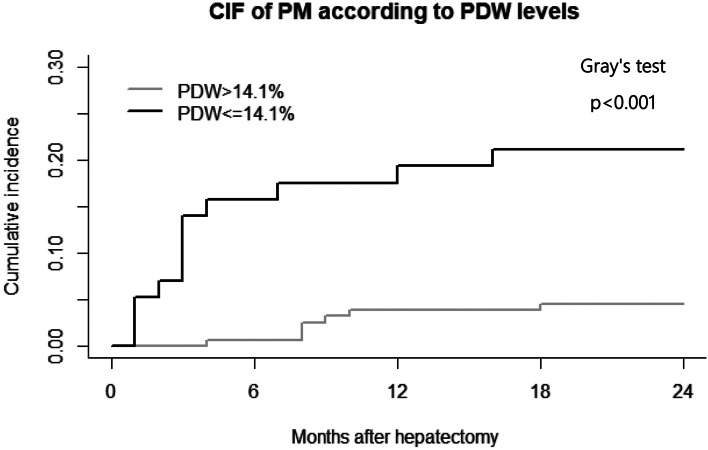
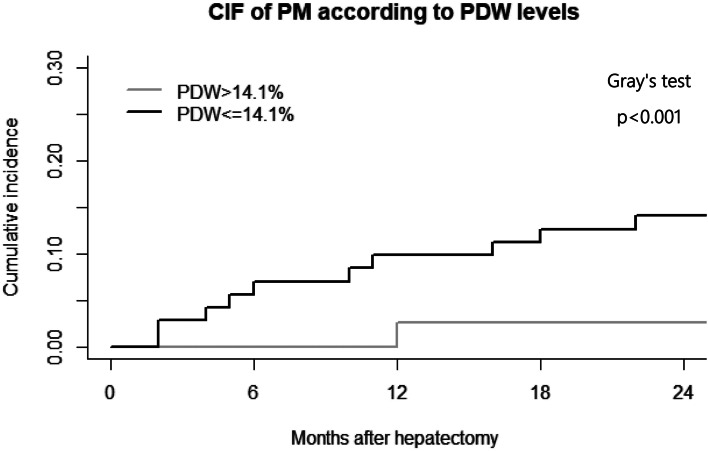
In the derivation cohort, Fig. 2 showed the cumulative incidence of PMs in the high-PDW and low-PDW groups. PM was the interest event, while death was considered a competing risk. After controlling for competing risk event, there was a significant difference in the incidence of PM between the high-PDW and low-PDW groups (p < 0.001). We found that individuals with low PDW levels tended to develop PM more than individuals with high PDW levels, with 2-year cumulative incidence of 21.0% and 4.5%, respectively. In the validation cohort, the cumulative incidence of 2-year PM in HCC patients was 21.3% in the high-PDW group and 1.6% in the low-PDW group (p < 0.001) (Fig. 3).
Fig. 2.
Timing of development of pulmonary metastases in development set
Fig. 3.
Timing of development of pulmonary metastases in validation set
Discussion
This study observed that in HCC patients with PM, PDW levels were lower than in those without PM. In addition, multivariable analysis found that PDW was the independent predictor of PM after HCC resection. And an external validation cohort came to the same conclusion.
Platelets are traditionally considered the principal cells active in thrombosis and hemostasis. Extensive research has demonstrated that platelets make a substantial contribution to cancer growth and dissemination. Platelet activation is caused by tumor cells interacting with platelets, which promotes tumor development and metastasis [11]. Although the functions of platelets in tumor metastasis have been widely studied in other malignancies, the exact effects of platelets on HCC metastasis are unknown [12–14]. Compared with HCC patients without metastases, the patients with extrahepatic metastases had a higher platelet count. Moreover, platelet count is a valuable diagnostic for predicting extrahepatic metastasis in patients with early-stage HCC receiving curative therapy [15]. In a metastatic HCC mouse model, pharmacological inhibition of platelet activation prevents platelets from adhering to tumor cells and reduces metastasis [16]. Krüppel-like factor 6 (KLF6), a tumor suppressive gene, inhibits tumor growth and invasion in HCC. Previous studies revealed that platelet release downregulates KLF6 expression in vivo and in vitro in HCC cells [17]. In addition, platelet extracts may also be able to counteract sorafenib or regorafenib-mediated inhibitory effects in HCC cells [18]. According to our findings, platelet activation has a crucial role in HCC. Furthermore, our data supports the use of antiplatelet treatment in patients with HCC who have undergone hepatectomy.
The mechanisms behind the link between decreased PDW and PMs are still unknown. PDW is an early biomarker of platelet activation and indicates the average change in platelet volume. In megakaryocyte development and thrombopoiesis, platelet volume is determined. The failure of heterogenic megakaryocytic maturation is reflected in the decline in PDW levels [19]. In addition, numerous clinical studies revealed a strong link between PDW and the prognosis of various cancers such as breast cancer, colon cancer, ovarian cancer, and non-small cell lung cancer [20–23]. Meanwhile, several reports have also confirmed that PDW is an independent predictor of poor clinical outcome in HCC [10, 24, 25]. Interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), and macrophage colony-stimulating factor (M-CSF) have all been found to influence megakaryocytic maturation, platelet production, and platelet volume [26]. Furthermore, tumor-derived G-CSF creates a pre-metastatic environment in distant organs, and anti-G-CSF or anti-M-CSF antibodies have been shown to significantly prevent PMs [27]. Furthermore, the presence of thrombocytopenia in HCC patients with cirrhosis indicates that the disease is in an advanced stage. Thrombocytopenia before treatment could be a low-cost and practical predictor of postoperative recurrence in HCC patients [28]. This also partly explains why PDW levels in HCC patients with PM were lower than those without PM.
In the present study, there are several limitations that deserve mention. Firstly, it was a small-sized study with a retrospective nature. Secondly, the mechanisms of PDW involved in PM were not explored and further research is needed. Lastly, participants only included Chinese people, so a larger study is needed to extrapolate our findings to other ethnic groups.
In brief, preoperative PDW may predict PM in HCC patients. Further studies are warranted.
Acknowledgements
Not applicable.
Abbreviations
- AFP
Alphafetoprotein
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- APRI
Aspartate aminotransferase to platelet count ratio index
- BMI
Body mass index
- CIF
Cumulative incidence function
- CT
Computerized tomography
- CI
Confidence interval
- EHMs
Extrahepatic metastases
- FIB-4
Fibrosis-4 index
- γ-GGT
γ-Glutamyl transferase
- G-CSF
Granulocytes colony-stimulating factor
- HCC
Hepatocellular carcinoma
- IL-6
Interleukin-6
- INR
International normalized ratio
- IQR
25%, 75% Interquartile range
- KLF6
Krüppel-like factor 6
- MPV
Mean platelet volume
- M-CSF
Macrophage colony-stimulating factor
- MELD score
Model of end-stage liver disease score
- PALBI
Platelet-albumin-bilirubin
- ALBI
Albumin-bilirubin
- PM
Pulmonary metastasis
- PDW
Platelet distribution width
- RECIST
Response evaluation criteria in solid tumors
- ROC
Receiver operating characteristic
- sHR
Subdistribution hazard ratios
- SD
Standard deviation
- WBC
White blood cell
Authors' contributions
XZ and RTW conceived and designed the study; WW, and MLZ collected the data; WJH, ZYL, WW and GYW analyzed the data; WJH, GYW and ZYL wrote and edited the manuscript; WJH, GYW, ZYL, MLZ, RTW and XZ revised the manuscript; RTW and XZ participated in the quality control of the study and manuscript review. All authors read and approved the final manuscript.
Funding
The study was funded by the Harbin Medical University Cancer Hospital (JJZD2017-05). The funding body had no role in the design of the study, collection, analysis, interpretation of data, and writing of the manuscript. Harbin Medical University Cancer Hospital,JJZD2017-05,Rui-tao Wang
Availability of data and material
The data used in the study can be obtained from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Harbin Medical University Cancer Hospital and the First Affiliated Hospital of Harbin Medical University Institutional Review Boards. All patients involved in the study gave written consent for this study.
Consent for publication
Not Applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-juan Huang, Guang-yu Wang and Zeng-yao Liu contributed equally to this work.
Contributor Information
Xin Zhang, Email: Dr_xinzhang@outlook.com.
Rui-tao Wang, Email: ruitaowang@126.com.
References
- 1.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223-38. [DOI] [PMC free article] [PubMed]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-49. [DOI] [PubMed]
- 3.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 4.Schutte K, Schinner R, Fabritius MP, Moller M, Kuhl C, Iezzi R, Ocal O, Pech M, Peynircioglu B, Seidensticker M, et al. Impact of Extrahepatic Metastases on Overall Survival in Patients with Advanced Liver Dominant Hepatocellular Carcinoma: A Subanalysis of the SORAMIC Trial. Liver Cancer. 2020;9(6):771–786. doi: 10.1159/000510798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlovic N, Rani B, Gerwins P, Heindryckx F. Platelets as Key Factors in Hepatocellular Carcinoma. Cancers (Basel). 2019;11(7):1022. [DOI] [PMC free article] [PubMed]
- 7.Kaito K, Otsubo H, Usui N, Yoshida M, Tanno J, Kurihara E, Matsumoto K, Hirata R, Domitsu K, Kobayashi M. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128(5):698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunaldi M, Erdem D, Goksu S, Gunduz S, Okuturlar Y, Tiken E, Aksoy H, Yildirim M. Platelet Distribution Width as a Predictor of Metastasis in Gastric Cancer Patients. J Gastrointest Cancer. 2017;48(4):341–346. doi: 10.1007/s12029-016-9886-5. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Huang XY, Li N, Cui MM, Wang RT. Platelet Indices in Colorectal Cancer Patients with Synchronous Liver Metastases. Gastroenterol Res Pract. 2019;2019:6397513. doi: 10.1155/2019/6397513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue CX, Liu YX, Yun ZY, Li N, Zhao CJ, Wang RT. Decreased platelet distribution width predicts a worse prognosis in patients undergoing surgical resection for hepatocellular carcinoma. Cancer Biomark. 2019;26(3):361–366. doi: 10.3233/CBM-190474. [DOI] [PubMed] [Google Scholar]
- 11.Gresele P, Momi S, Malvestiti M, Sebastiano M. Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev. 2017;36(2):331–355. doi: 10.1007/s10555-017-9679-8. [DOI] [PubMed] [Google Scholar]
- 12.Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8(1):310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D, Remer K, Nurden P, Nurden AT, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135(14):1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 14.Plantureux L, Mege D, Crescence L, Carminita E, Robert S, Cointe S, Brouilly N, Ezzedine W, Dignat-George F, Dubois C, et al. The Interaction of Platelets with Colorectal Cancer Cells Inhibits Tumor Growth but Promotes Metastasis. Cancer Res. 2020;80(2):291–303. doi: 10.1158/0008-5472.CAN-19-1181. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Lin YJ, Lin CC, Yen CL, Shen CH, Chang CJ, Hsieh SY. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35(10):2327–2336. doi: 10.1111/liv.12817. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang M, Xin G, Wei Z, Li S, Xing Z, Ji C, Du J, Niu H, Huang W. Dihydrodiosgenin inhibits endothelial cell-derived factor VIII and platelet-mediated hepatocellular carcinoma metastasis. Cancer Manag Res. 2019;11:4871–4882. doi: 10.2147/CMAR.S202225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen PH, Wang DY, Zhang JK, Wang ZH, Pan J, Shi XY, Yang H, Zhang SJ, Guo WZ. Kruppel-like factor 6 suppresses growth and invasion of hepatocellular carcinoma cells in vitro and in vivo. Int J Immunopathol Pharmacol. 2016;29(4):666–675. doi: 10.1177/0394632016655171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Alessandro R, Refolo MG, Lippolis C, Giannuzzi G, Carella N, Messa C, Cavallini A, Carr BI. Antagonism of sorafenib and regorafenib actions by platelet factors in hepatocellular carcinoma cell lines. BMC Cancer. 2014;14:351. doi: 10.1186/1471-2407-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon K, Kim M, Lee J, Lee JS, Kim HS, Kang HJ, Lee YK. Immature platelet fraction: A useful marker for identifying the cause of thrombocytopenia and predicting platelet recovery. Medicine (Baltimore) 2020;99(7):e19096. doi: 10.1097/MD.0000000000019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Cui MM, Huang YX, Fu S, Zhang X, Guo H, Wang RT. Preoperative platelet distribution width predicts breast cancer survival. Cancer Biomark. 2018;23(2):205–211. doi: 10.3233/CBM-181267. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Zhang H, Qi Q, Zhang B, Yue D, Wang C. The preoperative platelet distribution width: A predictive factor of the prognosis in patients with non-small cell lung cancer. Thorac Cancer. 2020;11(4):918–927. doi: 10.1111/1759-7714.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin L, Li JY, Huang WJ, Zhang ML, Wang RT, Shen W. Higher platelet distribution width is associated with unfavorable prognosis in ovarian cancer. Cancer Biomark. 2020;28(3):365–370. doi: 10.3233/CBM-191190. [DOI] [PubMed] [Google Scholar]
- 23.Sakin A, Sahin S, Sakin A, Karatas F, Sengul Samanci N, Yasar N, Arici S, Demir C, Geredeli C, Dikker O, et al. Mean platelet volume and platelet distribution width correlates with prognosis of early colon cancer. J BUON. 2020;25(1):227–239. [PubMed] [Google Scholar]
- 24.Guo F, Zhu X, Qin X. Platelet Distribution Width in Hepatocellular Carcinoma. Med Sci Monit. 2018;24:2518–2523. doi: 10.12659/MSM.909474. [DOI] [PubMed] [Google Scholar]
- 25.Zuo X, Kong W, Feng L, Zhang H, Meng X, Chen W. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2019;24(3):307–313. doi: 10.3233/CBM-182076. [DOI] [PubMed] [Google Scholar]
- 26.Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92(2):345–344. doi: 10.1182/blood.V92.2.345. [DOI] [PubMed] [Google Scholar]
- 27.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang Q, Qu K, Bi JB, Liu SS, Zhang JY, Song SD, Lin T, Xu XS, Wan Y, Tai MH, et al. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J Gastroenterol. 2015;21(25):7895–7906. doi: 10.3748/wjg.v21.i25.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the study can be obtained from the corresponding author upon request.