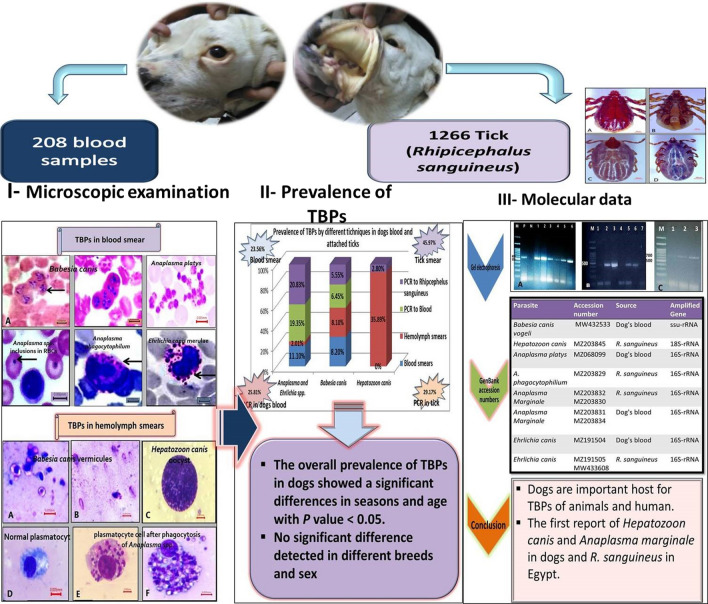
Abstract
Background
The incidence or recurrence of tick-borne diseases (TBDs) in animals and humans is increasing rapidly worldwide, but there is insufficient information about TBDs infecting dogs in Egypt. Thus, the present study was conducted to screen and genetically identify tick-borne pathogens (TBPs) in dogs and associated ticks by microscopic examination and polymerase chain reaction (PCR).
Methods
In Cairo and Giza governorates, 208 blood samples were collected from dogs of different breeds, ages, and sex. In addition, 1266 dog-associated ticks were collected (546 ticks were used to prepare hemolymph smears, and 720 ticks were kept in 70% ethanol until PCR analysis). PCR was applied to 124 dog blood samples and 144 tick pools prepared from 720 ticks.
Results
All ticks collected from dogs were Rhipicephalus sanguineus (s.l.). Microscopic examination revealed that TBP prevalence among dogs was 23.56% (49/208), including Anaplasma and Ehrlichia with 11.1% (23/208) and Babesia canis with 8.2% (17/208). Hepatozoon canis was not detected in blood smears. Co-infections with two pathogens were visible in 4.33% (9/208) of examined dogs. The prevalence of TBPs in hemolymph smears was 45.97% (251/546) including 35.89% (196/546) for H. canis, 8.1% (44/546) for B. canis, and 2.01% (11/546) for Anaplasmataceae (A. phagocytophilum, A. marginale, A. platys, and E. canis). The overall molecular prevalence rate of TBPs was 25.81% and 29.17% in the blood of examined dogs and in ticks, respectively. The molecular prevalence of Anaplasmataceae family, Babesia canis, and H. canis in dog blood samples was 19.35%, 6.45%, and 0.0%, respectively, while in ticks, it was 20.83%, 5.55%, and 2.8%, respectively. A sequential analysis identified six different species of TBPs, namely B. canis vogeli, Hepatozoon canis, A. phagocytophilum, A. marginale, A. platys, and E. canis. The obtained sequences were submitted to GenBank and assigned accession numbers.
Conclusions
The present study detected a wide range of TBPs (B. canis, H. canis, A. platys, A. phagocytophilum, A. marginale, and E. canis) that are considered a threat to domestic animals and humans in Egypt. Hepatozoon canis and A. marginale were reported in dogs and associated ticks for the first time in Egypt.
Graphical Abstract
Keywords: Anaplasma, Babesia, Ehrlichia, Egypt, Hepatozoon, PCR, Rhipicephalus sanguineus (s.l.), Tick-borne diseases
Highlights
Traditional and molecular techniques were used to screen domestic dogs and attached ticks for TBPs.
A wide range of TBPs in dogs and attached ticks was detected in Egypt.
Some accidental pathogens, such as A. marginale from other hosts, were identified in dogs.
Some canine isolates from Anaplasmataceae family have high homology to those isolated from humans.
Background
Ticks, as blood-feeding ectoparasites, directly damage vertebrate hosts during blood sucking and indirectly by transmitting many pathogens such as bacteria, viruses, and protozoa [1–4]. Rhipicephalus sanguineus (s.l.) is a widespread tick responsible for transmitting many pathogens, such as Babesia canis vogeli, Ehrlichia canis, and Anaplasma platys to dogs. Furthermore, many authors recorded fatal human cases due to Ehrlichia and Anaplasma spp. infection [5, 6]. Tick-borne pathogens (TBPs) are the leading cause of morbidity and mortality in pet dogs and a major public health concern. To date, three protozoa (Babesia, Theileria, and Hepatozoon) and five bacterial genera (Anaplasma, Ehrlichia, Rickettsia, Coxiella, and Bartonella) are recorded in dogs globally [7]. Dogs subclinically infected with TBPs act as reservoirs of these diseases for their owners and contact animals [8, 9]. Signs of TBDs are usually diffuse and overlapping, particularly in mixed infections; therefore, accurate diagnostic methods are required for a correct treatment and control strategy [10]. TBP diagnoses are usually based on microscopic examination of blood smears. Although these morphology-based methods save time and money, they are less sensitive and need highly skilled investigators. Thus, molecular techniques confirm the identity of canine blood parasites [11]. The molecular methods have higher sensitivities and specificities in detecting TBPs in peripheral blood and tick tissues than traditional techniques [12, 13]. Additionally, molecular technique result analysis helped better understand new species that infect dogs. For example, some authors genetically divided dog piroplasms into three groups: true Babesia, B. microti, and B. conradae group [14]. As pet dog ownership has increased recently in Egypt and constitutes a potential TBD reservoir, human infections will increase. Therefore, the current study was conducted to detect and genetically identify TBPs in dogs (household and kenneled) and associated ticks. A combination of screening microscopic examination and molecular techniques [polymerase chain reaction (PCR) and sequencing] were applied to address these issues.
Methods
Sample collection
Blood samples were collected from 144 tick-infested dogs and 64 previously infested dogs (both sexes, different ages, and breeds) from government and private clinics and shelters in Cairo and Giza governorates (n = 208) based on sample size calculation using Open EPI free software https://www.openepi.com/SampleSize/SSPropor.htm and a confidence level of 95%. Ticks were collected directly from animals, kept in well-ventilated jars, and transported to the laboratory. From 1266 engorged ticks, 546 were used to prepare hemolymph smears, and 720 ticks were stored in 70% alcohol at − 20 °C till DNA extraction. Morphological identification of ticks was performed microscopically using identification keys [15]. Blood samples were collected from the cephalic vein into 5-ml EDTA tubes and then divided into two parts; one part was stored at − 20 °C till DNA extraction. The other part was used to prepare thin blood smears according to the method described by [16]. Smears were fixed by absolute methyl alcohol and stained with 10% Giemsa solution. The stained slides were examined with an oil immersion lens (1000 ×). The TBP stages in smears were measured according to the method described by [17].
Hemolymph smear preparation
Five hundred forty-six ticks were washed twice with 70% ethanol and once with phosphate-buffered saline before preparing hemolymph smears, as [18] described.
DNA extraction
One hundred twenty-four blood samples were analyzed by PCR based on using the Open EPI free software https://www.openepi.com/SampleSize/SSPropor.htm.htm and a confidence level of 95%. This study used 720 ticks to prepare 144 tick pools by crushing 5 ticks collected from each dog as a pool before DNA extraction. DNA extraction from tick and blood samples used blood and tissue extraction kits (G-spin total DNA extraction kit from Intron Biotechnology-Korea) following the kit manufacturer's instructions.
PCR reactions were performed in a total volume of 50 μl using 25 µl of Cosmo PCR red master mix (Willowfort Co.,UK) with 20 pmol of each primer (Table 1) and 5 µl of extracted DNA, completed with sterile nuclease-free water. Amplification was performed using a programmable conventional thermocycler (Biometra, Göttingen, Germany) according to the thermal profile (Table 2).
Table 1.
Primers sequences used in the current study
| Target organism | Primer sequence | Amplified gene/ length (bp) | Reference |
|---|---|---|---|
| Babesia spp. |
5’-GTCTTGTAATTGGAATGATGG-3’ 5’-CCAAAGACTTTGATTTCTCTC-3’ |
ssu-rRNA/560 | [12] |
| Hepatozoon canis |
5’-ATACATGAGCAAAATCTCAAC-3’ 5’- CTTATTATTCCATGCTGCAG-3’ |
18S-rRNA/666 | [19] |
| Anaplasmataceae |
5’-TTTATCGCTATTAGATGAGCCTATG-3’ 5’-CTCTACACTAGGAATTCCGCTAT -3’ |
16S-rRNA/450 | [20] |
Table 2.
Thermal profile used for PCR procedures
| Parasite Step | Babesia | Cycle no. | Hepatozoon canis | Cycle NO | Anaplasmataceae | Cycle no. | |||
|---|---|---|---|---|---|---|---|---|---|
| Temp | Time | Temp | Time | Temp | Time | ||||
| Initial denaturation | 94 °C | 5 min | 1 | 95 °C | 5 min | 1 | 95 °C | 5 min | 1 |
| Denaturation | 94 °C | 30 Sec | 35 | 95 °C | 30 Sec | 35 | 95 °C | 30 Sec | 40 |
| Annealing | 49 °C | 30 Sec | 51 °C | 30 Sec | 55 °C | 30 Sec | |||
| Extension | 72 °C | 1 min | 72 °C | 1.5 min | 72 °C | 30 Sec | |||
| Final extension | 72 °C | 10 min | 1 | 72 °C | 10 min | 1 | 72 °C | 10 min | 1 |
The amplified products were electrophoresed on 1.5% agarose gels in 1× Tris Acetate EDTA (TAE). The agarose gel was stained with ethidium bromide and visualized by UV transillumination. The amplified fragment's size was compared to a 100-bp DNA molecular weight marker (Genedirex 100-bp DNA ladder H3 RTU). For each assay, the genomic DNA of known blood parasites was used as a positive control while nuclease-free water was used as a negative control [21].
DNA sequencing and phylogenetic analysis
Positive PCR products of Babesia and Hepatozoon canis and Anaplasma species were excised from the gel and then purified using a QIAquick purification kit (Qiagen, Germany) according to the manufacturer’s protocol. Purified products were subjected to one-direction Sanger sequencing in the ABI 3500 Genetic Analyzer (Applied Biosystems, USA) using the Big Dye Terminator V3.1 kit (Applied Biosystems). After that, the PCR products’ nucleotide sequences were analyzed for similarity with those available in the GenBank using the BLAST server on the NCBI website. Obtained sequences were compared to sequences recorded in Egypt and some selected sequences isolated from dogs, ticks, and other animals worldwide. The analysis was done using the Clustal W, BioEdit software (ver. 7.0.9). A maximum likelihood method and Tamura-Nei model were constructed for the phylogenetic tree using Mega 6.06 software, and bootstrap analysis was obtained with 1000 replicates. A similarity matrix was carried out using the DNASTAR program (Lasergene, version 8.0). The genetic distance values of species variations were analyzed with the Meg-Align project of DNSTAR software [22].
Nucleotide sequence accession numbers
A partial sequence of ssu-rRNA of Babesia canis vogeli gene, 18S-rRNA of Hepatozoon canis, and a partial sequence of 16S-rRNA of A. platys, n. phagocytophilum, A. marginale, and E. canis from the dog blood samples and R. sanguineus (s.l.) were submitted to GenBank, which assigned them the following accession numbers of pathogens and sequence length (B. canis vogeli: MW432533 (534 bp), H. canis:MZ203845 (600 bp), A. platys: MZ068099 (427 bp), A. phagocytophilum: MZ203829 (233 bp), A. marginale: MZ203830 (251 bp), MZ203832 (261 bp), MZ203831 (271 bp), MZ203834 (260 bp), E. canis: MW433608 (421 bp), MZ191505 (310 bp), and MZ191504 (350 bp).
Statistical analysis
Data obtained from the blood smears were analyzed using SPSS. Pearson chi-square (χ2) tested the effect of different seasons, age, sex, and dog breeds on the prevalence of TBPs by blood smears. P-values < 0.05 were considered statistically significant [23].
Results
Tick identification
All ticks collected from dogs in the present study were morphologically identified as R. sanguineus (s.l.). They are small ticks that have an elongated body with reddish-brown coloration. They have hexagonal basis capituli with pointed lateral angles and short palps and eyes; 11 festoons are present, but they are usually inornate. Males have a triangular adenal plate, and females have a wide U-shaped genital opening. The tail of the spiracular plate in male and female ticks is narrower than the festoon (Fig. 1).
Fig. 1.
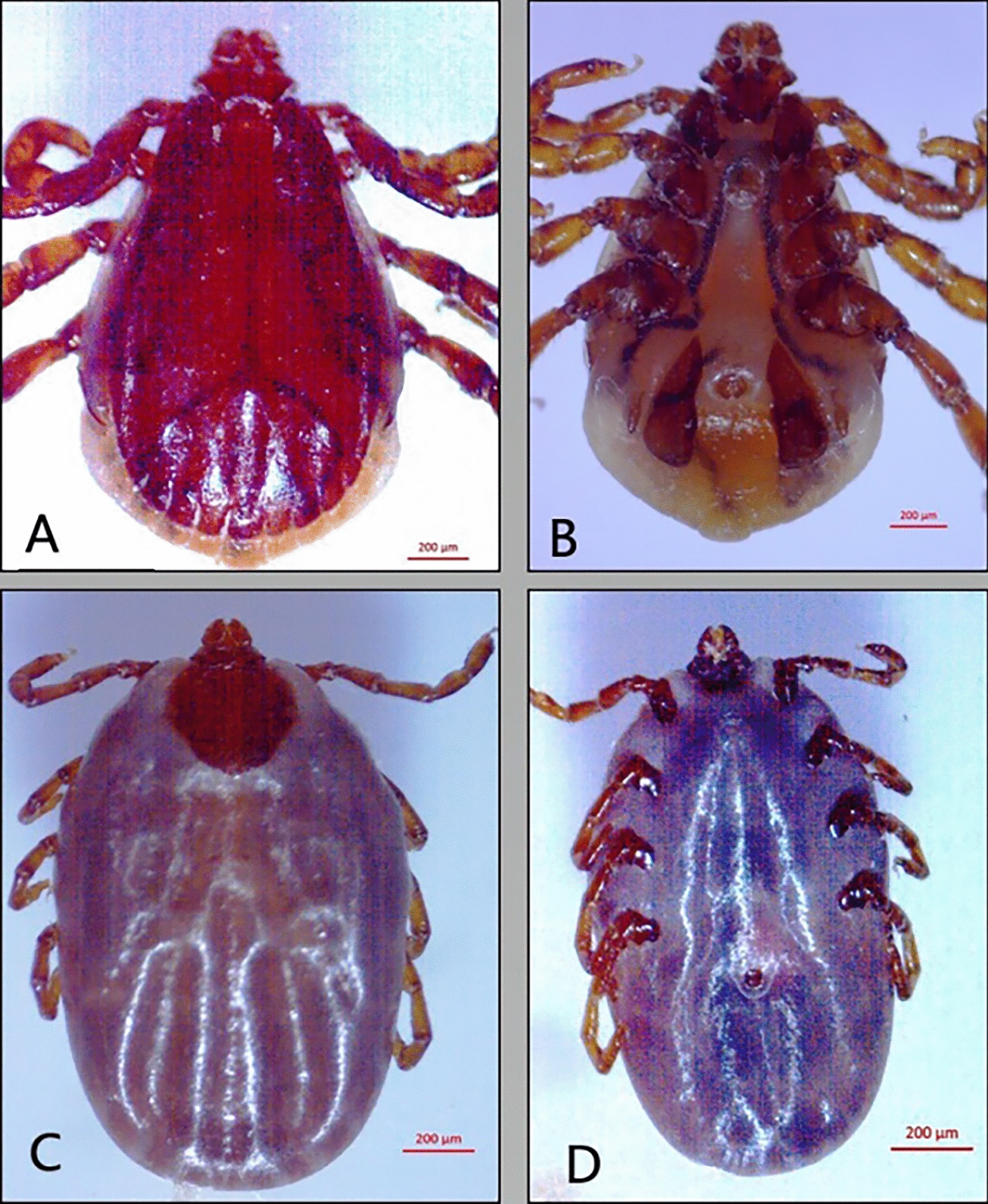
Male and female R. sanguineus (s.l.): (A) Male dorsal view, (B) Male ventral view, (C) female dorsal view, (D) female ventral view (bare = 200 µm)
Microscopic finding
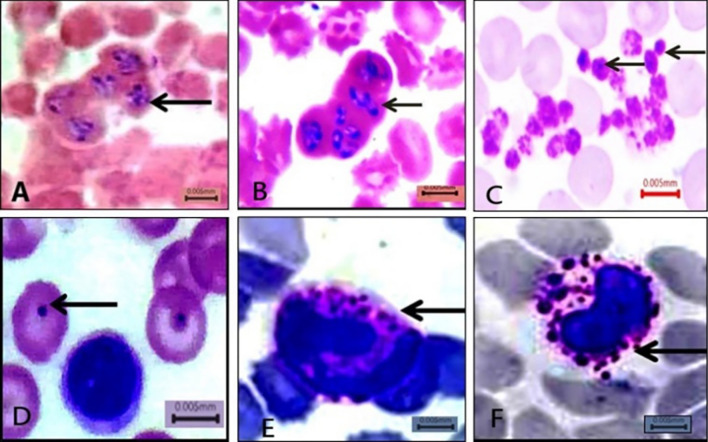
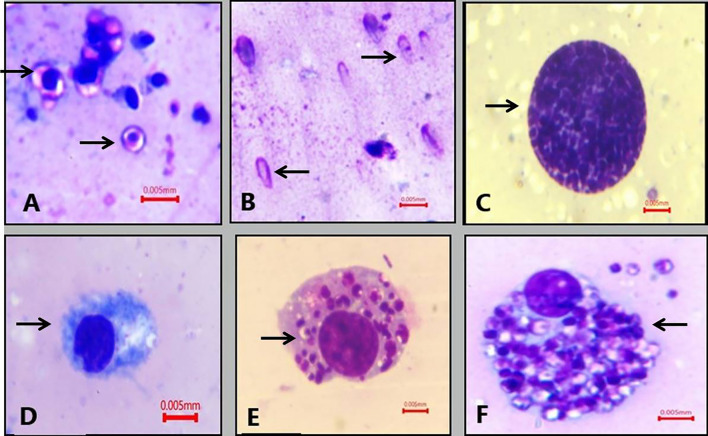
Giemsa-stained thin blood smear illustrated the presence of B. canis merozoites. They appeared polymorphic in shape, either typically large pear-shaped or oval. More than one piroplasm was detected in a single RBC, and the infected RBCs appeared clumped together, with sizes ranging from 3.9 to 4.8 µm. Regarding the Anaplasmataceae family in blood smears, Anaplasma inclusions inside RBCs appeared as dark-stained dots surrounded by pale areas in the cytoplasm. Furthermore, in infected neutrophils and monocytes, A. phagocytophilum and Ehrlichia canis multiplied organisms (morulae) appeared intracytoplasmic. Anaplasma platys appeared as basophilic inclusions in blood platelets. On the other hand, Hepatozoon canis gamont was not detected in peripheral blood smears (Fig. 2). Hemolymph smears of R. sanguineus (s.l.) examination revealed that Babesia spp. vermicules take many shapes, including round, amoeboid (3–4.8 µm), and club shaped with rounded and pointed ends, sized 6– 8 µm. Mature club-shaped B. canis vermicules appeared with an empty cytoplasm. Additionally, H. canis oocysts were described in hemolymph smears as ball-like structures packed with the dividing sporocysts; their size ranged from 150 to 200 µm. The light microscope could not detect sporozoites inside the oocyst. Anaplasma and Ehrlichia spp. were detected in hemolymph smears through some alterations in hemocytes. The plasmatocytes showed aggregates of them filling their cytoplasm (Fig. 3).
Fig. 2.
Giemsa-stained dog blood smears showing (A and B) B. canis, C Anaplasma platys inclusion inside thrombocytes, D Anaplasma inclusion inside RBCs. E A. phagocytophilum morulae inside neutrophil, F E. canis morulae inside monocyte (X1000 bare = 5 µm)
Fig. 3.
Giemsa-stained hemolymph smears of R. sanguineus (s.l.) showed A: spherical and amoeboid form of Babesia canis. B: Mature club-shaped vermicules of B. canis. C: Hepatozoon canis mature oocysts. D: Non-infected plasmatocyte cell. E: Plasmatocyte cell after phagocytosis of Anaplasma spp. F: Plasmatocyte eliminate Anaplasma spp. by nodulation (1000×, bare = 5 µm)
Epidemiological data
The prevalence of TBPs among dogs was 23.56% (49/208), including Anaplasma and Ehrlichia with a prevalence of 11.1% (23/208), Babesia canis 8.2% (17/208), and mixed infections with two pathogens observed in 4.33% (9/208) of examined dog blood smears. However, Hepatozoon canis was undetected in blood smears (Table 3). Furthermore, TBP prevalence in hemolymph smears was 45.97% (251/546), including 35.89% (196/546) for H. canis, 8.1% (44/546) for B. canis, and 2.01% (11/546) for Anaplasmataceae.
Table 3.
Prevalence of tick-borne pathogen infections related to some risk factors (season, age, sex, and breed) by microscopic examination of blood smears
| Risk factor | Groups | Total examined dogs | Babesia canis | Anaplasmataceae | Mixed infection | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infected dogs | % | Infected dogs | % | Infected dogs | % | Infected dogs | % | |||
| Season | Winter | 62 | 3 | 4.84 | 4 | 6.45 | 1 | 1.61 | 8 | 12.9 |
| Spring | 43 | 3 | 6.98 | 6 | 13.95 | 3 | 6.98 | 12 | 27.9 | |
| Summer | 47 | 6 | 12.77 | 0 | 21.28a | 5 | 10.64 | 21 | 44.68a | |
| Autumn | 56 | 5 | 8.93 | 3 | 5.36 | 0 | 0 | 8 | 14. 29 | |
| Age/year | < year | 66 | 2 | 3.03 | 3 | 4.55 | 2 | 3.03 | 7 | 10.61 |
| 1–5 years | 98 | 11 | 11.22 | 16 | 16.33a | 6 | 6.12 | 33 | 33.67a | |
| > 5 years | 44 | 4 | 9.1 | 4 | 9.1 | 1 | 2.27 | 9 | 20.45 | |
| Sex | Male | 135 | 10 | 7.41 | 14 | 10.37 | 6 | 4.44 | 30 | 22.22 |
| Female | 73 | 7 | 9.59 | 9 | 12.33 | 3 | 4.11 | 19 | 26.02 | |
| Breeds | Long hair | 111 | 8 | 7.21 | 10 | 9.01 | 6 | 5.41 | 24 | 21.62 |
| Short hair | 97 | 9 | 9.28 | 13 | 13.4 | 3 | 3.1 | 31 | 25.77 | |
| Total | 208 | 17 | 8.2 | 23 | 11.1 | 9 | 4.33 | 49 | 23.56 | |
aSignificant differences at p < 0.05, %: percentage of infection
Notably, clinical signs, including anorexia, depression, fever, lethargy, bleeding disorders, and jaundice or paleness of external mucosal membranes, were recorded in 31/49 infected animals, and the other 18 cases were healthy. Additionally, all infected animals were tick-infested (Fig. 4).
Fig. 4.
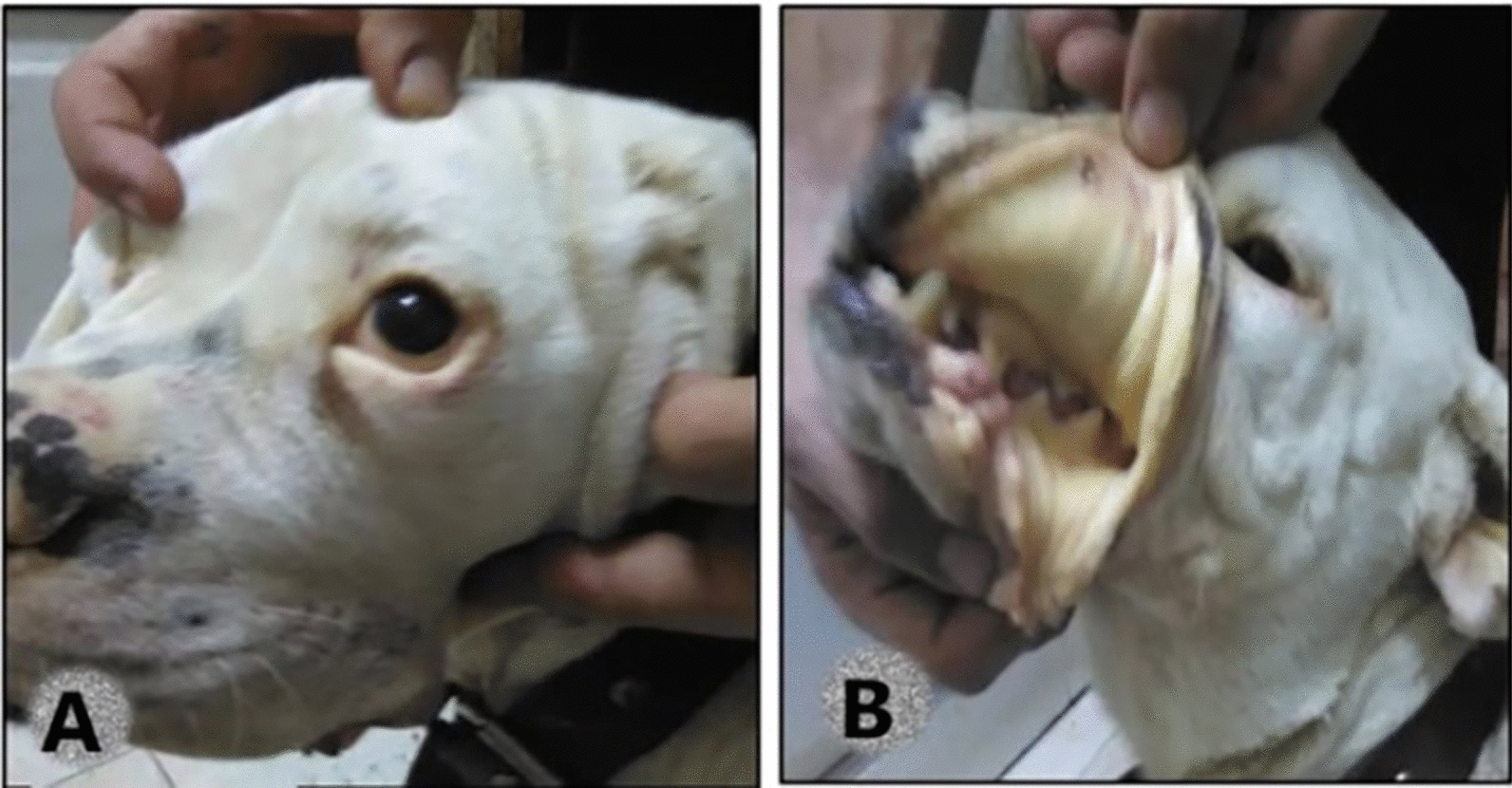
Signs of TBP infection in dogs (A): jaundice in sclera of eye (B): icteric mucosal membrane
Regarding risk factor analysis from data obtained by microscopic examination, the chi-square (χ2) test revealed significant differences in season and age on the overall prevalence of TBPs in dogs, particularly for the Anaplasmataceae family (P-value < 0.05). At the same time, there was no significant difference between different breeds and sexes. Moreover, the B. canis infection rate was not significantly affected by variation in season, breeds, age, and sex (P-value > 0.05).
Molecular findings
All blood samples that were positive on microscopic examination were also positive on PCR, and four samples from the negative samples on microscopic examination gave positive results by PCR. After PCR, the total prevalence rate of TBPs was 25.81% of the examined dog blood and 29.17% of the examined ticks. Anaplasmataceae family recorded the highest prevalence rate (19.35% and 20.83%) followed by Babesia canis (6.45% and 5.55%) in dog blood samples and ticks, respectively, while H. canis recorded the lowest prevalence rate (0% and 2.8%) in blood and ticks (Table 4). PCR amplified a monomorphic DNA fragment of 560-bp size of ssu-rRNA gene in case of B. canis and 670 bp of 18SrRNA gene in case of H. canis. The PCR product size of the Anaplasmataceae family was 450 bp of the 16SrRNA gene.
Table 4.
Prevalence of tick-borne pathogen infection in dog serum samples and associated ticks by traditional and molecular techniques
| Parasite | Total examined samples | Babesia canis | Hepatozoon canis | Anaplasmataceae | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Infected samples | % | Infected samples | % | Infected samples | % | Infected samples | % | |
| Blood | |||||||||
| Blood smear | 208 | 17 | 8.17 | 0 | 0 | 23 | 11.1 | 40 | 19.23 |
| PCR | 124 | 8 | 6.45 | 0 | 0 | 24 | 19.35 | 32 | 25.81 |
| Tick | |||||||||
| Hemolymph smear | 546 | 44 | 8.1 | 196 | 35.89 | 11 | 2.01 | 55 | 10.07 |
| PCR | 144 | 8 | 5.55 | 4 | 2.8 | 30 | 20.83 | 42 | 29.17 |
Sequencing and phylogenetic analysis data
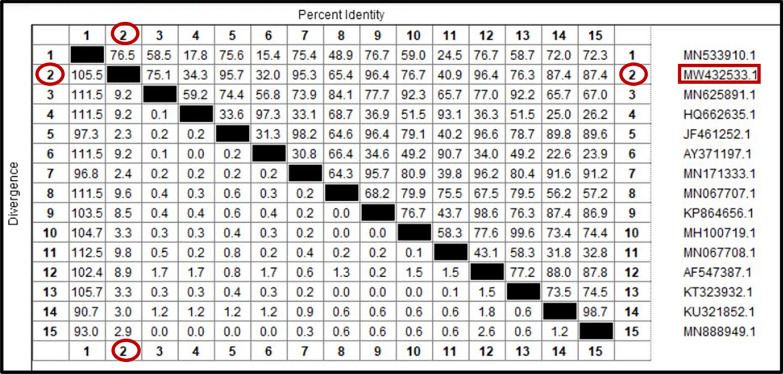
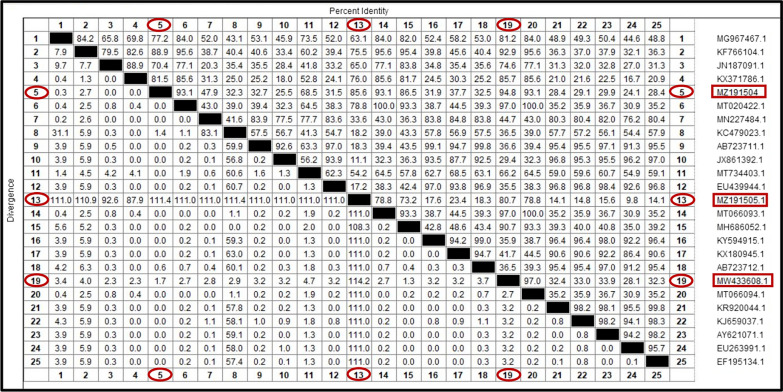
Sequence analysis identified six different species of tick-borne pathogens, namely B. canis vogeli, H. canis, A. platys, A. phagocytophilum, A. marginale, and E. canis. The data were accessed on GenBank. The phylogenetic analysis based on the ssu-rRNA sequences of B. canis vogeli isolated from dogs’ blood (MW432533) showed 96.4% similarity with B. canis vogeli from Thailand and South Africa (KP864656 and AF547387), respectively. However, the lowest genetic diversities between our isolate and other isolates on GenBank were 2.3% and 2.4%, with B. canis vogeli isolated from dogs in Germany and China (JF461252 and MN171333), respectively (Figs. 5, 6).
Fig. 5.
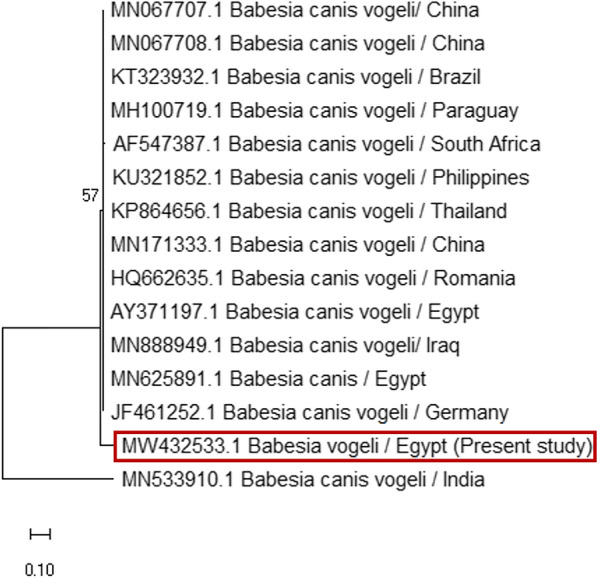
Phylogenetic relationships based on small subunit ribosomal RNA (ssu-rRNA) sequences of Babesia canis vogeli. The tree was constructed and analyzed using a maximum likelihood method and Tamura-Nei model, and numbers above internal nodes indicate the percentages of 1000 bootstrap replicates that supported the branch
Fig. 6.
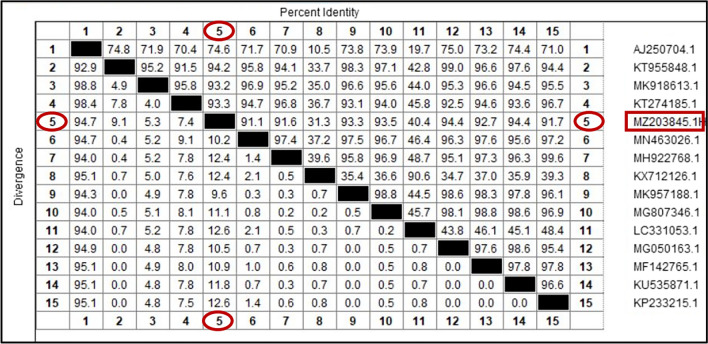
Similarity (percent identity) and genetic divergence of small subunit ribosomal RNA (ssu-rRNA) sequences of Babesia canis vogeli isolated from dog serum samples in Egypt (representing number, 2) compared with the most similar reference sequences (GenBank)
The phylogenetic analysis of the current H. canis isolated from R. sanguineus (s.l.) (MZ203845) revealed a relatively high homology (94.4%) with H. canis isolated from Pakistan and India (KU535871 and MG050163). The lowest divergence ratios (9.1% and 9.6%) were detected with H. canis isolated from dog and red fox in Pakistan and Iraq (KT955848 and MK957188), respectively (Figs. 7, 8).
Fig. 7.

Phylogenetic relationships based on 18S ribosomal RNA (18S-rRNA) sequences of Hepatozoon canis. The tree was constructed and analyzed using a maximum likelihood method and Tamura-Nei model, and numbers above internal nodes indicate the percentages of 1000 bootstrap replicates that supported the branch
Fig. 8.
Similarity (percent identity) and genetic divergence of 18S ribosomal RNA (18S-rRNA) sequences of Hepatozoon canis isolated from Rhipicephalus sanguineus (s.l.) ticks collected from dogs in Egypt (representing number, 5) compared with the most similar reference sequences (GenBank)
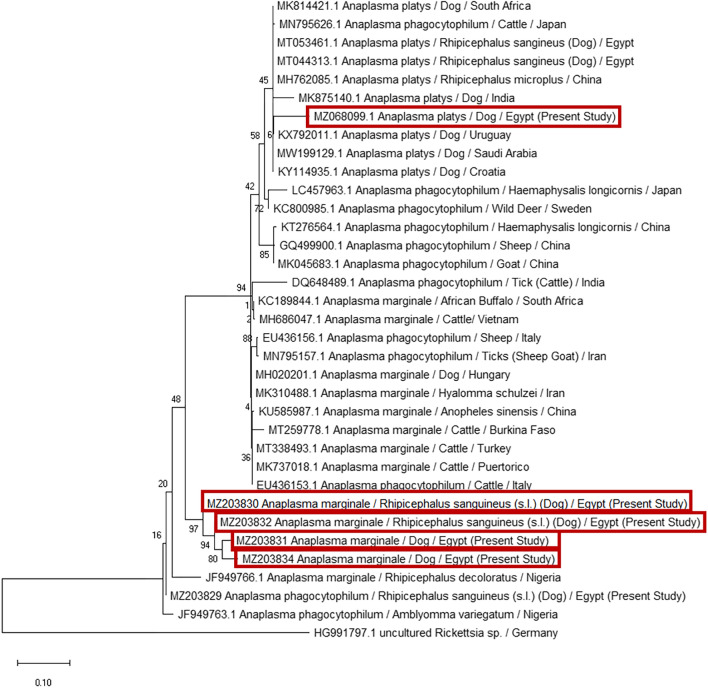
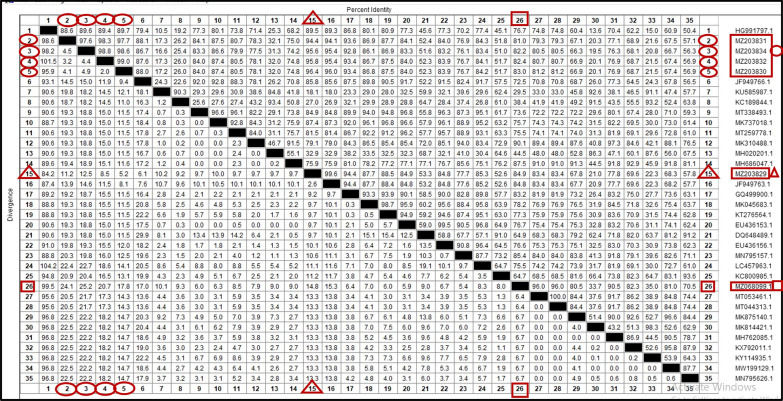
The present study identified three different subspecies of Anaplasma (A. platys, A. phagocytophilum, and A. marginale) in dog blood and ticks. Anaplasma platys (MZ068099) was detected in dog, showing the highest percentage identity (96%) and the lowest divergence ratio (6.4%) with A. platys isolated from R. sanguineus (s.l.) in Egypt (MT053461). Additionally, A. phagocytophilium isolated from R. sanguineus (s.l.) showed high homology (99.6%) and low divergence ratio (2.6%) with A. phagocytophilum identified from Amblyomma variegatum collected from cattle in Nigeria (JF949763). Furthermore, the current study identified four sequences as An. marginale in dog and R. sanguineus (s.l.). When these sequences aligned, the highest genetic homology was obtained between A. marginale isolated from R. sanguineus (s.l.) (MZ203830 and MZ203832) with a similarity percentage (99%) and divergence ratio (2%), while the highest similarity between our An. marginale isolates and other isolates accessed on the GenBank was 91.24%, with A. marginale identified from Rhipicephalus decoloratus in Nigeria (JF949766) (Figs. 9, 10).
Fig. 9.
Phylogenetic relationships based on 16S ribosomal RNA (16S-rRNA) sequences of Anaplasmataceae. The trees were constructed and analyzed using a maximum likelihood method and Tamura-Nei model, and numbers above internal nodes indicate the percentages of 1000 bootstrap replicates that supported the branch
Fig. 10.
Similarity (percent identity) and genetic divergence of 16S ribosomal RNA (16S-rRNA) sequences of A. platys, A. phagocytophilum, and A. marginale isolated from dogs and Rhipicephalus sanguineus (s.l.) ticks in Egypt (representing number, 2–5, 15, 26) compared to the most similar reference sequences (GenBank). The 16S rRNA of A. marginale sequenced in this study is marked with an oval shape and represented as numbers 2–5, A. phagocytophilum is marked with a triangular shape and represented as number 15, and A. platys is marked with square shape and represented as number 26
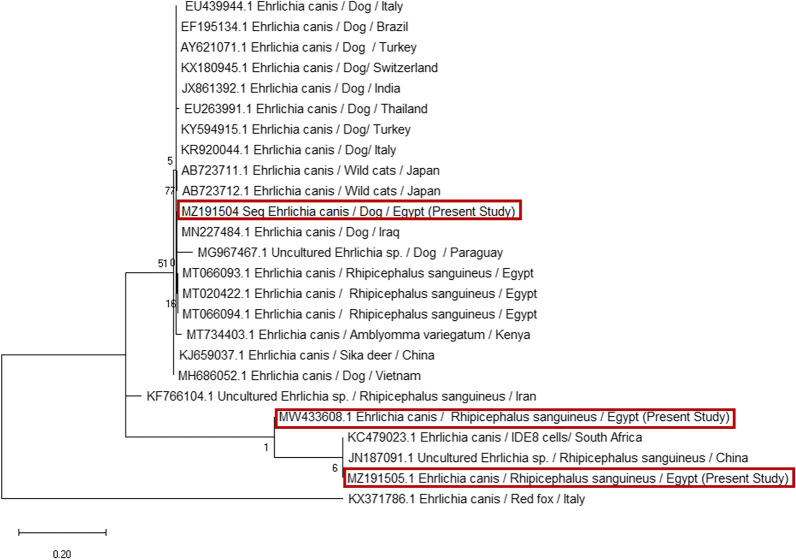
In the current phylogenetic analysis of E. canis isolates based on the 16S-rRNA sequences, three sequences of E. canis were isolated from dog and R. sanguineus (s.l.). After these sequences were aligned, a relatively high similarity (97%) with a very low genetic divergence ratio (2.7%) was recorded between E. canis isolated from R. sanguineus (s.l.) in Egypt (MW433608 and MT066094), respectively. Moreover, the current E. canis isolated from ticks (MZ191505) showed complete homology (100%) with E. canis isolated from R. sanguineus (s.l.) collected from dogs in Egypt (MT020422) (Figs. 11, 12).
Fig. 11.
Phylogenetic relationships based on 16S ribosomal RNA (16S-rRNA) sequences of Ehrlichia canis. The trees were constructed and analyzed using a maximum likelihood method and Tamura-Nei model, and numbers above internal nodes indicate the percentages of 1000 bootstrap replicates that supported the branch
Fig. 12.
Similarity (percent identity) and genetic divergence of 16S ribosomal RNA (16S rRNA) sequences of Ehrlichia canis isolated from dogs and Rhipicephalus sanguineus (s.l.) ticks in Egypt (representing number 5, 13, 19) compared with the most similar reference sequences (GenBank). The 16S rRNA of Ehrlichia canis sequenced in this study is marked and represented as numbers 5, 13, 19
Notably, the current isolates of A. platys isolated from dogs (MZ068099) had high similarity of 99.7% with A. platys isolated from humans in Mexico (MK386768).
Discussion
The present study revealed that all TBP-infected cases were infested by Rhipicephalus sanguineus only. This observation agreed with previous reports in Egypt [24–26]. Clinical manifestations of TBDs mainly recorded in 63.27% (31/49) of infected animals, particularly B. canis infection, were detected in diseased dogs with fever, emaciation jaundice, and red urine. This was unlike what was observed by [27] who reported that canine babesiosis in Egypt usually takes the chronic form. The reason for our finding might be the weak immune status of infected dogs or the presence of mixed infections that increase disease expression.
Concerning the morphological description of the stages of B. canis, H. canis, Anaplasma spp., and E. canis in dog blood and tick hemolymph, they had the same morphology as reported previously [28–32]. The overall prevalence of TBPs by microscopic examination of blood smears in the present study was 23.56%. Among the positive cases, there was a higher prevalence of Anaplasmataceae family (Anaplasma spp. and E. canis) infection (11.1%) than Babesia (8.2%). These findings contrast previous records of [33] in India, who observed Babesia spp. infection in 88% of the positive cases with a higher prevalence than other blood parasites such as E. canis and H. canis. For B. canis the prevalence is nearly the same as that of previous studies [34, 35]; 6.7% and 7.47% prevalences were reported from naturally infected dogs from Tunisia and India, respectively. However, this is lower than what was detected by other findings [24, 26] reporting Babesia infection in 76.92% and 12.0% of examined dogs from different governorates in Egypt. Anaplasma spp. and E. canis prevalences were lower than that recorded in Malaysia (13.6%) [32] and higher than (4%) in Israel [36]. The current low prevalence might be because Babesia parasites are difficult to detect in blood smears except during the acute phase; thus, the negative results do not exclude the possibility of Babesia infection. Also, E. canis is difficult to detect by blood smear examination because of low parasitemia even during the acute stage of the disease [36]. This discrepancy in prevalence ratios could be attributable to the difference in sampling design and geographical variations.
Co-infections with two pathogens were observed microscopically in 9/208 (4.33%) of examined dogs. This result coincides with those of with many authors [37–39] who reported co-infection of Ehrlichia/Anaplasma with other pathogens in Brazil, Costa Rica, and the Philippines, respectively. This finding might be attributed to thrombocytopenia, usually found in dog cases infected with Anaplasma platys or Ehrlichia canis [40–42]. Another explanation for this finding is that vectors of these pathogens (R. sanguineus) (s.l.) can carry several pathogens simultaneously, which might lead to co-infection [43].
Regarding the prevalence rates of TBPs in hemolymph smears of R. sanguineus, H. canis was the most prevalent pathogen (35.89%), followed by Babesia canis (8.1%) and finally the Anaplasmataceae family (2.01%). Hepatozoon prevalence was lower than that recorded by [31] in Israel, who found that 85% of R. sanguineus (s.l.) that had artificially fed on a Hepatozoon infected dog were infected with H. canis. On the other hand, the current B. canis prevalence was much lower than that detected by [44] in Nigeria, who found 64.5% of hemolymph smears from five Hyalomma spp. detached from cattle. In addition, [45] reported a higher prevalence of Anaplasma phagocytophilium (51%) in hemolymph smears prepared from Hyalomma spp. collected from horses in Iraq. These variations in prevalence ratios might be due to differences in vector and vertebrate hosts. However, H. canis stages in hemolymph smears represented 35.89% not detected in blood smears. This finding is consistent with [46] who detected H. americanum oocysts in A. maculatum ticks collected from a dog that did not have any H. americanum stages in its blood or tissues. This result might be because oral intake of ticks is the main route of transmission of Hepatozoon spp. to dogs and not tick biting. Therefore, the possibility of dog infection from infected ticks is minimum. Another explanation is the long period elapsed (28 days) from oral tick intake until gamont appeared in the peripheral circulation. The infected adult ticks were still uninfective to dogs 40 days post molting [31, 47].
Concerning the epidemiological results, the overall seasonal prevalence of TBPs was most prevalent in warm seasons (summer 44.68% and spring 27.9%) compared to winters (12.9%). This result follows [33] in Chennai city, India, who found that blood parasite infections in dogs were more prevalent during the monsoon (windy) season. The probable reason behind this observation may be affected by the seasonal activity of the vector [R. sanguineus (s.l.)] in Egypt, which is maximum in summer. At the same time, complete disappearance occurs in the winter months, December and January [48]. Also, statistical analysis of risk factors detected a significant effect of age and no significant difference in different breeds and sex. This finding contrasts with [49] in Turkey, who found that the TBP infection rate was higher in adults than in young dogs. On the other hand, [33], in India no significant differences were found between different age groups, breeds, and sexes in the prevalence of canine vector-borne pathogens.
The effect of risk factors on B. canis infection rate showed no significant effects of age, sex, and breed. This finding parallels [27] and disagrees with [24, 50], who reported that canine babesiosis in Egypt infection increased by age; Malino and German Shepherds were more susceptible to infection. Furthermore, [51–53] in the US and Hungary recorded that some dog breeds such as Pit bull terriers, German shepherds, and heavy-coated Komondors were more susceptible to Babesia infection. In the Anaplasmataceae family, summer season and age significantly affected the infection rate, but no significant difference was recorded for sex and breeds. This result coincides with [8, 54, 55] in Egypt, Germany, and Morocco, while [56] reported that Anaplasma spp. and Ehrlichia spp. were more prevalent in female dogs and German shepherds in Egypt.
The total molecular prevalence of TBPs in dog blood was 25.81% including 6.45% for Babesia canis and 19.35% for the Anaplasmataceae family. The total prevalence was higher than [48, 57] 23.4% and 5.4% in Thailand and Turkey, respectively, and lower than [10, 58, 59] 72%, 45%, and 52% in Nigeria, Romania, and India, respectively. Regarding the present study, B. canis prevalence was nearly similar to (6%) [60] in Brazil and higher (0.6%, 1.9%, and 0.26%) than [61–63] in Nigeria, Palestine, and India, respectively. On the other hand, our finding was lower than [10, 50] 10.8% and 29.17% in dogs from Egypt and Romania. Concerning Anaplasma and Ehrlichia, the molecular prevalence was the same as that reported by [61], who recorded Ehrlichia canis in 12.7% and A. platys in 6.6% of examined dogs from Nigeria. While it was higher than what was detected by [49], E. canis was 4.9% and A. platys 0.5% in Turkey. Different prevalence worldwide might be due to variations in climatic conditions.
The overall molecular prevalence of TBPs in R. sanguineus (s.l.) was 29.17%, including 20.83% for the Anaplasmataceae family, followed by 5.55% for Babesia canis and 2.8% for H. canis. The highest prevalence of B. canis vogeli (8.7%) was recorded by [60] in R. sanguineus (s.l.) collected from dogs in Brazil. On the other hand, a lower prevalence (0.5%) was detected by [62] in R. sanguineus (s.l.) collected from dogs in Palestine. In Anaplasma and Ehrlichia spp. infection rates, our findings are higher than [64, 65] who detected A. phagocytophilum, A. platys, and E. canis in R. sanguineus (s.l.) ticks collected from dogs in Egypt with ratios of 13.7%, 1.32%, and 1.98%, respectively, while higher prevalence rates for Anaplasma and Ehrlichia spp. of 21.1% and 45.5% were reported by [61, 66] in Nigeria and Iran, respectively. The present low ratio of Babesia spp. and H. canis in infected ticks might be caused by many PCR inhibitors in the extracted DNA from ticks.
Regarding the sequencing and phylogenetic analysis, the present study genetically identified six tick-borne pathogens in dog blood samples and R. sanguineus (s.l.), including B. canis vogeli, H. canis, A. platys, A. phagocytophilum, A. marginale, and E. canis. The current study found that B. canis vogeli was the only species responsible for canine babesiosis in Egypt. This finding agreed with [24, 27, 50]. Moreover, A. marginale and A. phagocytophilum identified in the current study from tick [R. sanguineus (s.l.)] showed relatively high homology (96.4%) with each other. This result is consistent with the observation of [67] in Spain, who mentioned that A. marginale and A. phagocytophilum shared similarities at a molecular level. Anaplasma phagocytophilum detected in R. sanguineus (s.l.) shared high similarity (99.6%) with A. phagocytophilum detected in Amblyomma variegatum attached to cattle in Nigeria [68]. Notably, this is the first report of DNA of A. marginale in dogs and their associated R. sanguineus (s.l.) ticks in Egypt. Anaplasma marginale was also detected in dogs from Hungary and registered in the GenBank under accession number (MH020201) in an unpublished report. This finding supported that some TBPs accidentally parasitized other hosts, as reported by [69].
Ehrlichia canis showed complete homology (100%) with E. canis isolates from R. sanguineus (s.l.) collected from dogs in Egypt [65]. Furthermore, the current isolates of A. platys isolated from dog serum samples and ticks showed high similarity (99.7%) with A. platys isolated from humans in Mexico. This observation is alarming because of the zoonotic potentiality of these species, similar to a previous observation in Egypt [65].
Conclusion
The present study detected a wide range of TBPs (B. canis, H. canis, A. platys, A. phagocytophilum, A. marginale, and E. canis) that indicates the emergence of tick-borne diseases in domestic animals and humans in Egypt. However, these diseases might be misdiagnosed because of the lack of public health and veterinary resources, increasing the possibility of human infection. Anaplasma marginale is a non-canine tick-borne pathogen identified in dogs as an accidental host. One of the most important findings is the high genetic similarity percentage between the detected E. canis and A. platys isolates with other species isolated from humans in Egypt and other countries. This observation is alarming because of the zoonotic potentiality of these species. To the best of our knowledge, this is the first time that H. canis and A. marginale were detected in dogs and associated ticks in Egypt.
Acknowledgements
The authors are grateful to Dr. Hend Abdullah (Researcher in National Research Centre, Egypt) and Dr. Eman Ahmed El-Kelesh (Chief Researcher-Animal Health-Research Institute) for providing us with positive DNA controls required for PCR.
Author contributions
MMF, responsible for the project design and supervised all work. HMO supervised all work and drafted the manuscript. MA, interpreted the PCR and sequencing results, phylogenetic analysis and drafted the manuscript, SGG identified ticks and protozoal stages in blood and ticks, NEM drafted the manuscript, and AAH responsible for samples and data collection and performed microscopic and PCR procedures. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
The datasets generated or analyzed during the current study are available in the Gene Bank repository, accsession numbers: MZ191504, MZ068099, MZ203831, MZ203834, MW432533, MW433608, MZ203829, MZ203832, MZ203845 and MZ203830.
Declarations
Ethics approval and consent to participate
The study complied with the guidelines of the Research Ethical Committee (REC) for experimental and clinical studies at the Animal Health-Research Institute, Egypt (Research Ethical Committee, REC) with serial number 165711. We obtained oral consent from the owners of the animals for ticks and blood samples collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 2.Otranto D, Dantas-Torres F, Breitschwerdt EB. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. 2009;25:157–163. doi: 10.1016/j.pt.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Ghoneim NH, Abdel-Moein KA, Zaher HM, Abuowarda MM. Investigation of Ixodidae ticks infesting camels at slaughterhouse and its potential role in transmitting Coxiella burnetii in Egypt. Small Rum Res. 2020;20:106173. doi: 10.1016/j.smallrumres.2020.106173. [DOI] [Google Scholar]
- 4.Elhelw R, Elhariri M, Hamza D, Abuowarda M, Ismael E, Farag H. Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet Res. 2021;17:1–9. doi: 10.1186/s12917-020-02733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaffner W, Standaert SM. Ehrlichiosis- in pursuit of an emerging infection. N Engl J Med. 1996;334:262–263. doi: 10.1056/NEJM199601253340410. [DOI] [PubMed] [Google Scholar]
- 6.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerging Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng W, Liu M, Moumouni PFA, Liu X, Efstratiou A, Liu Z, Liu Y, Tao H, Guo H, Wang G, Gao Y, Li Z, Ringo AE, Jirapattharasate C, Chen H, Xuan X. First molecular detection of tick-borne pathogens in dogs from Jiangxi. China J Vet Med Sci. 2017;79:248–254. doi: 10.1292/jvms.16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatat SE, Khallaayoune K, Errafyk N, Van Gool F, Duchateau L, Daminet S, Kachani M, El Amri H, Azrib R, Sahibi H. Detection of Anaplasma spp. and Ehrlichia spp. antibodies, and Dirofilaria immitis antigens in dogs from seven locations of Morocco. Vet Parasitol. 2017;239:86–89. doi: 10.1016/j.vetpar.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Salim B, Alanazi AD, Omori R, Alyousif MS, Alanazi O, Katakura K, Nakao R. Potential role of dogs as sentinels and reservoirs for piroplasms infecting equine and cattle in Riyadh City. Saudi Arabia Acta Tropica. 2019;193:78–83. doi: 10.1016/j.actatropica.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Andersson MO, Tolf C, Tamba P, Stefanache M, Waldenström J, Dobler G, Dobler LC. Canine tick-borne diseases in pet dogs from Romania. Parasit Vectors. 2017;10:155–161. doi: 10.1186/s13071-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe Part I. Epizootiological Aspects Vet Parasitol. 2003;113:189–201. doi: 10.1016/S0304-4017(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 12.Beck R, Vojta L, Mrljak V, Marinculic A, Beck A, Zˇivicnˇjak T, Caccio SM. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int J Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Irwin PJ. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2010;40:1141–1156. doi: 10.1016/j.cvsm.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe-part ii. phylogenetic analysis and evolutionary history. Vet Parasitol. 2003;114:173–194. doi: 10.1016/S0304-4017(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 15.Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, Pegram RG, Preston PM. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Reports, 42 Comiston Drive, Edinburgh EH10 5QR, Scotland, U.K. 2003.
- 16.Ministry of Agricultural, Fisheries and Food. 1981. Manual of veterinary investigation laboratory techniques, 2nd ed. HMSO (Technical Bulletin Part 4 Haematology).
- 17.Otify YZ. Movable computer ruler (MCR): A new method for measuring the size of Toxoplasma gondii cysts, tachyzoits and other selected parasites. Exp Parasitol. 2012;130:1–5. doi: 10.1016/j.exppara.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Patton TG, Dietrich G, Brandt K, Dolan MC, Piesman J, Gilmore RD. Saliva, salivary gland, and hemolymph collection from Ixodes scapularis ticks. J Vis Exp. 2012;60:e3894. doi: 10.3791/3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inokuma H, Ohno K, Yamamoto S. Serosurvey of Ehrlichia canis and Hepatozoon canis infection in dogs in Yamaguchi Prefecture. Japan J Vet Med Sci. 1999;61:1153–1155. doi: 10.1292/jvms.61.1153. [DOI] [PubMed] [Google Scholar]
- 20.Munderloh UG, Madigan JE, Stephen DJ, Goodman JL, Hayes SF, Barlough JE, Nelson CM, Kurtti TJ. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J Clin Microbiol. 1996;34:664–670. doi: 10.1128/jcm.34.3.664-670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 22.Abuowarda M, AbuBakr HO, Ismael E, Shaalan M, Mohamed MA, Aljuaydi SH. Epidemiological and genetic characteristics of asymptomatic canine leishmaniasis and implications for human Leishmania infections in Egypt. Zoonoses Public Health. 2021;68:413–430. doi: 10.1111/zph.12824. [DOI] [PubMed] [Google Scholar]
- 23.Shedecor GW, Cochran WG. Staistical methods. 8nd ed. Iowa State Univ Press. 1989
- 24.Salem NY, Farag HS. Clinical, Hematologic, and molecular findings in naturally occurring Babesia canis vogeli in Egyptian Dogs. Vet Med Int Article ID. 2014 doi: 10.1155/2014/270345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abuowarda MM, Haleem MA, Elsayed M, Farag H, Magdy S. Bio-pesticide control of the brown dog tick (Rhipicephalus sanguineus) in Egypt by using two entomopathogenic fungi (Beauveria bassiana and Metarhizium anisopliae) Int J Vet Sci. 2020;9:175–181. [Google Scholar]
- 26.El-Neshwy WM, Hassanen EAA, Morsi AM, Anter RGA. Clinical, morphological and molecular characterization of canine babesiosis and its compatible tick vector in naturally infected dogs. Egypt Zag Vet J. 2020;48:242–253. doi: 10.21608/zvjz.2020.24982.1103. [DOI] [Google Scholar]
- 27.Abdel-Rhman AA, Hegazy YM, Al-Gaabary MH. Canine babesiosis in an endemic area of the Middle East; Causative Agent Identification, Prevalence Estimation and Risk Factors Determination. Alex J Vet Sci. 2015;47:104–112. [Google Scholar]
- 28.Riek RF. The cycle of Babesia bigemina (Smith and Kilborne, 1893) in the tick vector Boophilus microplus (Canestrini) Aust J Agric Res. 1964;15:802–821. doi: 10.1071/AR9640802. [DOI] [Google Scholar]
- 29.Levine ND. Veterinary Protozoology. Ames, IA: Iowa State University Press; 1985. [Google Scholar]
- 30.Soulsby EJL. Helminths, arthopods and protozoa of domesticated animals. 7th ed. Bailliere Tindall. 1st Anne's road, Eastbourne, East Sussex BN 21 3UN. 1986.
- 31.Baneth G, Samish M, Shkap V. Life cycle of Hepatozoon canis (apicomplexa: adeleorina: hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris) J Parasitol. 2007;93:283–299. doi: 10.1645/GE-494R.1. [DOI] [PubMed] [Google Scholar]
- 32.Jamnah O, Chandrawathani P, Premaalatha B, Zaini CM, Abdul Muizz SIS, Tharshini J, Naheed MH. Blood protozoa findings in pet dogs Screened in ipoh. Malaysia MJVR. 2016;7:9–14. [Google Scholar]
- 33.Kumar KS, Vairamuthu S, Kathiresan D. prevalence of haemoprotozoans in canines in Chennai City. Tamilnadu J Vet Anim Sci. 2009;5:104–108. [Google Scholar]
- 34.Ghirbi YM, Bouattour A. Detection and molecular characterization of Babesia canis vogeli from naturally infected dogs and Rhipicephalus sanguineus ticks in Tunisia. Vet Parasitol. 2008;152:1–7. doi: 10.1016/j.vetpar.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Singh H, Singh NK, Singh ND, Rath SS. Canine babesiosis in northwestern India: molecular detection and assessment of risk factors. Biomed Res Int. 2014;2014:741785. doi: 10.1155/2014/741785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waner T. Haematopathological changes in dogs infected with Ehrlichia canis. Israel J Vet Med. 2008;63:19–22. [Google Scholar]
- 37.Santos F, Coppede JS, Pereira AL, Oliveira LP, Roberto PG, Benedetti RB, Marins M. Molecular evaluation of the incidence of Ehrlichia canis Anaplasma platys and Babesia spp. in dogs from RibeirãoPreto. Brazil. Vet. J. 2009;179:145–148. doi: 10.1016/j.tvjl.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Rojas A, Rojas D, Montenegro V, Gutiérrez R, Yasur-Landau D, Baneth G. Vector-borne pathogens in dogs from Costa Rica first molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet Parasitol. 2014;199:121–128. doi: 10.1016/j.vetpar.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Ybañez RHD, Ybañez AP, Arnado LLA, Belarmino LMP, Malingin KGF, Cabilete PBC, Amores ZRO, Talle MG, Liu M, Xuan X. Detection of Ehrlichia, Anaplasma, and Babesia spp. in dogs of Cebu. Philippines Vet World. 2018;11:14–19. doi: 10.14202/vetworld.2018.14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyindo M, Huxsoll DL, Ristic M, Kakoma I, Brown JL, Carson CA, Stephenson EH. Cell-mediated and humeral immune responses of German shepherd dogs and beagles to experimental infection with Ehrlichia canis. Am J Vet Res. 1980;41:250–254. [PubMed] [Google Scholar]
- 41.Macieira DB, Messick JB, Cerqueira AMF, Freire IMA, Linhares GFC, Almeida NKO, Almosny NRP. Prevalence of Ehrlichia canis infection in thrombocytopenic dogs from Rio de Janeiro. Brazil Vet Clin Pathol. 2005;34:44–48. doi: 10.1111/j.1939-165X.2005.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 42.Gaunt SD, Beall MJ, Stillman BA, Lorentzen L, Diniz PPVP, Chandrashekar R, Breitschwerdt EB. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasites Vectors. 2010;3:33. doi: 10.1186/1756-3305-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegab AA, Fahmy MM, Omar HM, Abuowarda MM, Gattas SG. Investigation of tick-borne pathogens within naturally infected brown dog tick (Ixodidae: Rhipicephalus Sanguineus) in Egypt by light and electron microscopy. Int J Vet Sci. 2020;9:476–482. doi: 10.37422/IJVS/20.064. [DOI] [Google Scholar]
- 44.Dipeolu OO, Amoo A. The presence of kinetes of a Babesia species in the hemolymph smears of engorged Hyalomma ticks in Nigeria. Vet Parasitol. 1984;17:41. doi: 10.1016/0304-4017(84)90063-3. [DOI] [PubMed] [Google Scholar]
- 45.Albadrani BA, Al-Iraqi OM. First detection of equine anaplasmosis and hemoplasmosis of horses in Mosul city. Iraq. Adv Anim Vet Sci. 2019;7:106–111. doi: 10.17582/journal.aavs/2019/7.2.106.11. [DOI] [Google Scholar]
- 46.Vincent-Johnson NA, Macintire DK, Lindsay DS, Lenz SD, Baneth G, Shkap V, Blagburn BL. A new hepatozoon species from dogs: description of the causative agent of canine hepatozoonosis in North America. J Parasitol. 1997;83:1165–1172. doi: 10.2307/3284379. [DOI] [PubMed] [Google Scholar]
- 47.Smith TG. The genus Hepatozoon (Apicomplexa: Adeleina) J Parasitol. 1996;82:565–585. doi: 10.2307/3283781. [DOI] [PubMed] [Google Scholar]
- 48.Abbas AA, Abd El-Baky SM, Ahmed NH, Abd El-Mohsen A, Awaad ES. Seasonal abundance of hard ticks (ixodidae) in two localities (Giza and Esmaieliya governorates) of Egypt. Sci J Fac Sci Minufia Univ. 2006;20:65–87. [Google Scholar]
- 49.Aktas M, Özübek S, Altay K, Sayinipek ND, Balkaya İ, Utuk AE, Kırbas A, Şimsek S, Dumanl N. Molecular detection of tick-borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasit Vectors. 2015;8:157. doi: 10.1186/s13071-015-0763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atta HSF. Epidemiological, diagnostic and therapeutical studies on canine babesiosis. M.V.Sc. Vet.Thesis Fac.Vet. Med. Cairo Univ. Egypt. 2012
- 51.Birkenheuer AJ, Correa MT, Levy MG, Breitschwerd EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) J Am Vet Med Assoc. 2005;227:942–947. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- 52.Birkenheuer AJ, Levy MG, Stebbins M, et al. Serosurvey of anti-Babesia antibodies in stray dogs and American Pit Bull Terriers and American Staffordshire Terriers from North Carolina. J Am Anim Hosp Assoc. 2003;2003:551–557. doi: 10.5326/0390551. [DOI] [PubMed] [Google Scholar]
- 53.Hornok S, Edelhofer R, Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasitol Res. 2006;99:638–642. doi: 10.1007/s00436-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 54.Botros B, Elmolla M, Salib A, Calamaio C, Dasch G, Arthur R. Canine ehrlichiosis in Egypt sero-epidemiological survey. Onderstepoort J Vet Res. 1995;1995:41–43. [PubMed] [Google Scholar]
- 55.Jensen J, Simon D, Escobar HM, Soller JT, Bullerdiek J, Beelitz P, Pfister K, Nolte I. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health. 2007;54:94–101. doi: 10.1111/j.1863-2378.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- 56.Selim A, Alanazi AD, Sazmand A, Otranto D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasites Vectors. 2021;14:175. doi: 10.1186/s13071-021-04670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laummaunwai P, Sriraj P, Aukkanimart R, Boonmars T, Wonkchalee N, Boonjaraspinyo S, Sangmaneedet S, Mityodwong T, Potchimplee P, Khianman P, Maleewong W. Molecular detection and treatment of tick-borne pathogens in domestic dogs in Khon Kaen, northeastern Thailand. Southeast Asian. J Trop Med Public Health. 2014;45:1166. [PubMed] [Google Scholar]
- 58.Adamu M, Troskie M, Oshadu DO, Malatji DP, Penzhorn BL, Matjila PT. Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State. Nigeria Parasites Vectors. 2014;2014:119. doi: 10.1186/1756-3305-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarma K, Nachum-Biala Y, Kumar M, Baneth G. Molecular investigation of vector-borne parasitic infections in dogs in Northeast India. Parasites Vectors. 2019;12:122. doi: 10.1186/s13071-019-3389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Araujo AC, Silveira JAG, Azevedo SS, Nieri-Bastos FA, Ribeiro MFB, Labruna MB, Horta MC. Babesia canis vogeli infection in dogs and ticks in the semiarid region of Pernambuco. Brazil Pesq Vet Bras. 2015;35:456–461. doi: 10.1590/S0100-736X2015000500012. [DOI] [Google Scholar]
- 61.Kamani J, Baneth G, Mumcuoglu KY, Waziri NE, Eyal O, Guthmann Y, Harrus S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl Trop Dis. 2013;7:e2108. doi: 10.1371/journal.pntd.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azmi K, Al-Jawabreh A, Nasereddin A, Abdelkader A, Zaid T, Ereqat S, Sawalha SS, Baneth G, Abdeen Z. Detection and molecular identification of Hepatozoon canis and Babesia vogeli from domestic dogs in Palestine. Parasitology. 2016 doi: 10.1017/S0031182016002201. [DOI] [PubMed] [Google Scholar]
- 63.Singla LD, Sumbria D, Mandhotra A, Bal MS, Kaur P. Critical analysis of vector-borne infections in dogs: Babesia vogeli, Babesia gibsoni, Ehrlichia canis and Hepatozoon canis in Punjab. India Acta Parasitologica. 2016;61:697–706. doi: 10.1515/ap-2016-0098. [DOI] [PubMed] [Google Scholar]
- 64.Ghafar MW, Amer SA. Prevalence and first molecular characterization of Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, in Rhipicephalus sanguineus ticks attached to dogs from Egypt. Cairo University: J Adv Res. 2012;3:189–194. [Google Scholar]
- 65.Nasr A, El Hariri M, Ghafar MW. Detection of Anaplasma platys and Ehrlichia canis in Rhipicephalus sanguineus ticks attached to dogs from Egypt; a public health concern. VMJ-G. 2020;66:1–9. [Google Scholar]
- 66.Bekloo AJ, Bakhshi H, Soufizadeh A, Sedaghat MM, Bekloo RJ, Ramzgouyan MR, Chegeni AH, Faghihi F, Telmadarraiy Z. Ticks circulate Anaplasma, Ehrlichia, Babesia and Theileria parasites in North of Iran. Vet Parasitol. 2017;248:21–24. doi: 10.1016/j.vetpar.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 67.De La Fuente J, Naranjo V, RuizFons F, Höfle U, Fernández De Mera IG, Villanúa D, Almazán C, Torina A, Caracappa S, Kocan KM, Gortázar C. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A phagocytophilum in Central Spain. Vector-borne Zoonotic Dis. 2005;5:390–401. doi: 10.1089/vbz.2005.5.390. [DOI] [PubMed] [Google Scholar]
- 68.Ogoa NI, Fernández de Mera IG, Galindo RC, Okubanjoc OO, Inuwac HM, Agbedec RIS, Torina A, Alongi A, Vicente J, Gortázar C, De la Fuente J. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet Parasitol. 2012;187:572–577. doi: 10.1016/j.vetpar.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 69.Criado-Fornelio A, Gónzalez-del-R’ıo MA, Buling-Sarana A, Barba-Carretero JC. The expanding universe of piroplasms. Vet Parasitol. 2004;2004:337–343. doi: 10.1016/j.vetpar.2003.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available in the Gene Bank repository, accsession numbers: MZ191504, MZ068099, MZ203831, MZ203834, MW432533, MW433608, MZ203829, MZ203832, MZ203845 and MZ203830.