Abstract
A series of uniquely functionalized 2,3,-dihydro-1H-pyyrolo[3,4-b]quinolin-1-one derivatives were synthesized in one to two steps by utilizing a post-Ugi modification strategy and were evaluated for antileishmanial efficacy against visceral leishmaniasis (VL). Among the library compounds, compound 5m exhibited potential in vitro antileishmanial activity (CC50 = 65.11 μM, SI = 7.79, anti-amastigote IC50 = 8.36 μM). In vivo antileishmanial evaluation of 5m demonstrated 56.2% inhibition in liver and 61.1% inhibition in spleen parasite burden in infected Balb/c mice (12.5 mg kg−1, i.p.). In vitro pharmacokinetic study ascertained the stability of 5m in both simulated gastric fluid and simulated intestinal fluid. All the active compounds passed the PAINS filter and showed no toxicity in in silico predictions.
A series of uniquely functionalized 2,3,-dihydro-1H-pyyrolo[3,4-b]quinolin-1-one derivatives were synthesized in one to two steps by utilizing a post-Ugi modification strategy and were evaluated for antileishmanial efficacy against visceral leishmaniasis (VL).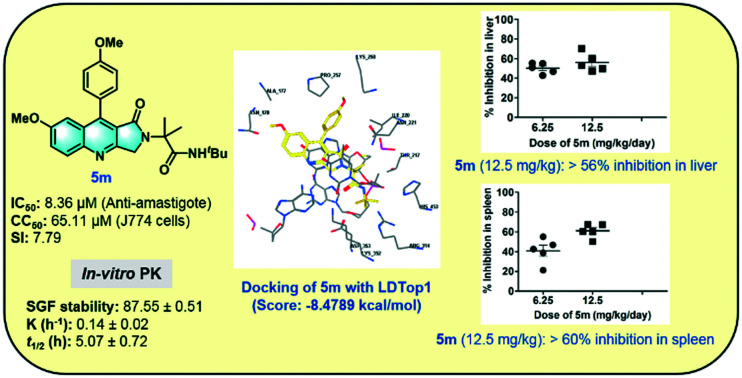
Introduction
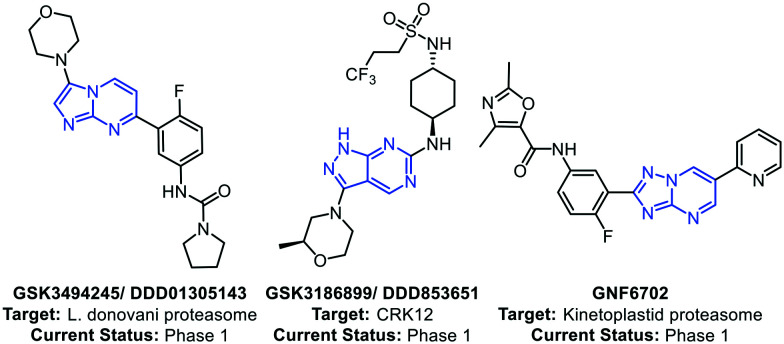
Leishmaniasis, a neglected tropical disease (NTD), is caused by a parasite of the genus Leishmania.1 The disease is endemic to India, Central Asia, East Africa, the Mediterranean and South America.2 The two most common forms of leishmaniasis are known as visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL), among which VL is prevalent in the Indian subcontinent.3 The common medicines for VL are sodium stibogluconate, amphotericin B, paromomycin, and miltefosine.4 Although the number of cases is in constant decline due to the implementation of the kala-azar elimination programme by the WHO, drug resistance has been a major concern.5 Also, there are speculations that the more resistant form of leishmaniasis may reoccur, causing major health concerns.6 Additionally, the existing drugs cause serious side effects and need to be used with caution.7 With the discovery of new drug targets, new drug candidates have been identified that are in various stages of clinical development. Wyllie et al. in 2019 reported a preclinical drug candidate GSK3494245/DDD01305143, which acts by inhibition of the chymotrypsin-like activity catalyzed by the β5 subunit of the L. donovani proteasome.8 The same group in 2018 reported a pyrazolopyrimidine-based antileishmanial compound GSK3186899/DDD853651 that inhibits the parasite cdc-2-related kinase 12 (CRK12).9 Khare et al. in 2016 reported a selective kinetoplastid proteasome inhibitor GNF6702 with excellent in vivo efficacy in mice models.10 GNF6702 does not inhibit the mammalian proteasome or growth of mammalian cells, and oral administration of GNF6702 in L. donovani-infected mice substantially reduced the liver parasite burden in comparison to miltefosine (Fig. 1).
Fig. 1. Representative antileishmanial agents under clinical development.
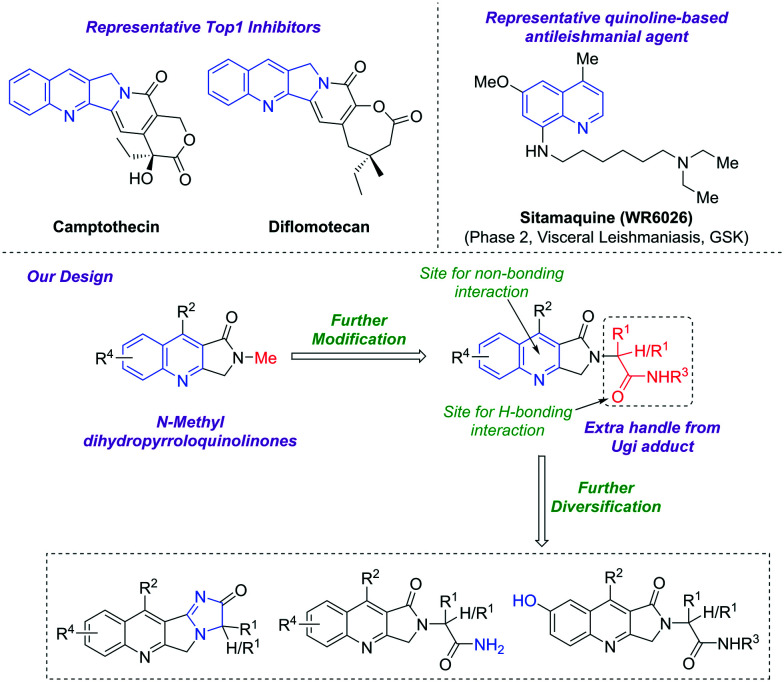
Over the years, DNA topoisomerases have emerged as an attractive target for antileishmanial drug discovery.11–14 Both forms of L. donovani topoisomerases, LDTop1 and LDTop2, have been reported as potential drug targets for leishmaniasis.15–17 The main rationale behind exploiting LdTop1 as a novel drug target is its unique bi-subunit heterodimeric structure18 that is completely distinct from the single-subunit structured human counterpart.19 Interestingly, camptothecin is a specific inhibitor of eukaryotic topoisomerase I and is cytotoxic to L. donovani parasite.20–23 However, the 8-aminoquinoline derivatives sitamaquine and tafenoquine have shown good efficacy against leishmaniasis.24,25 Sitamaquine has completed phase 2 trials for the treatment of visceral leishmaniasis (VL).26 Another 8-aminoquinoline analogue, WR242511, has also demonstrated potential in vitro antileishmanial activity against L. mexicana 227 (IC50 = 2.5 μM) (Fig. 2).27
Fig. 2. Quinoline-based antileishmanial agents and our designed prototypes.
Inspired by previous reports and in continuation of our ongoing efforts to identify medicinally important structural frameworks,28–36 a series of N-alkylated pyrroloquinolinone derivatives were designed and synthesized, and their antileishmanial efficacy was evaluated against visceral leishmaniasis (VL).
Results and discussion
Chemistry
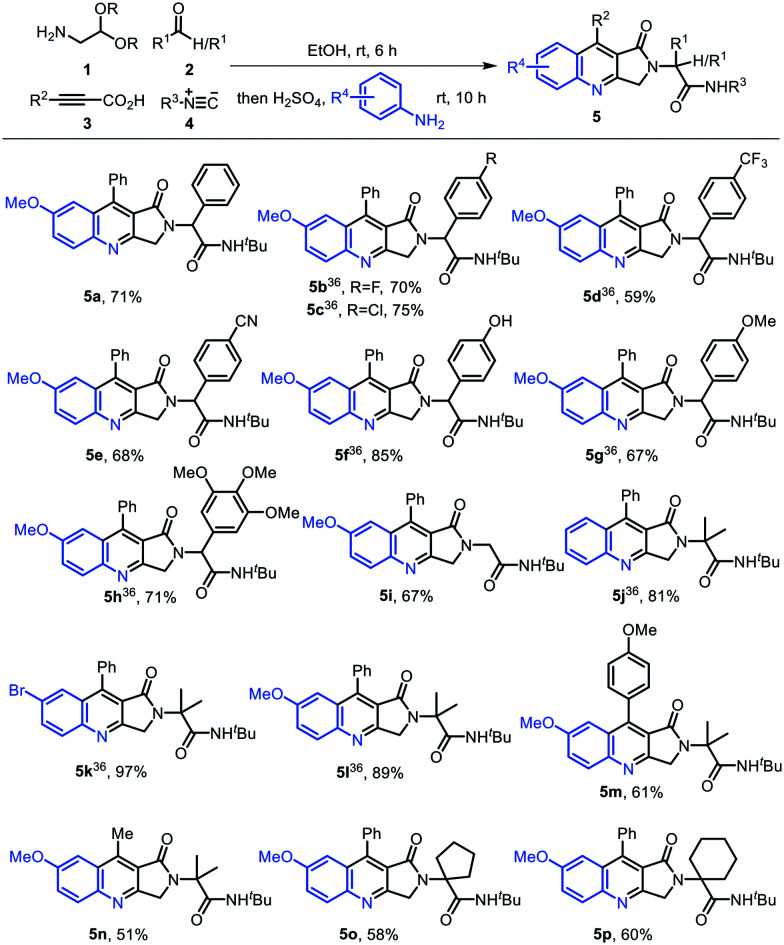
Using a post-Ugi modification strategy recently reported by our research group,36 structurally diverse dihydropyrroloquinolines 5a–5p were synthesized by performing Ugi condensation of aminoaldehyde acetal 1, aldehydes/ketones 2, propiolic acids 3, and isocyanides 4, followed by H2SO4-mediated deprotection and imino Diels–Alder reaction (Povarov reaction) with substituted anilines in ethanol (Scheme 1). The reaction proceeded smoothly with aldehydes bearing both electron-withdrawing and electron-donating groups, yielding the corresponding dihydropyrroloquinolines 5a–5h in 59–85% yields. With formaldehyde, dihydropyrroloquinoline 5i was obtained in 67% yield. Various ketones, viz. acetone (5j–5n), cyclopentanone (5o), and cyclohexanone (5p), were well tolerated in the reaction and afforded the compounds 5j–5p in good to excellent yields (51–97%) (Scheme 1).
Scheme 1. Synthesis of highly functionalized 2,3-dihydro-1H-pyrrolo[3,4-b]quinolin-1-ones.
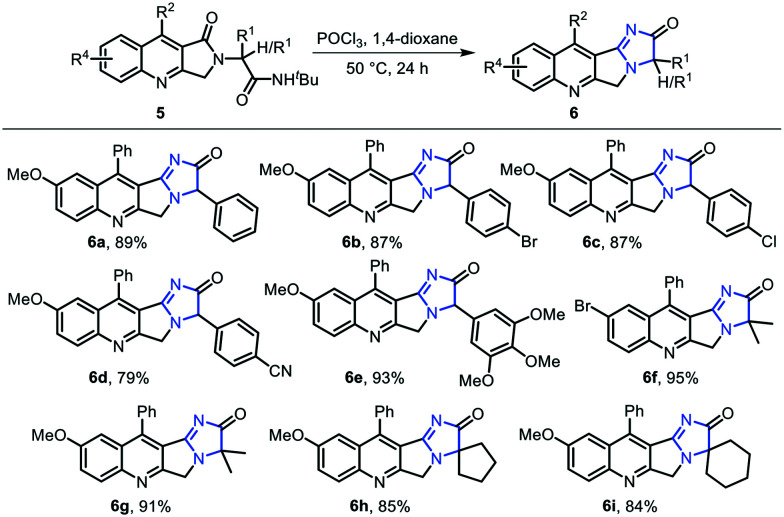
In order to construct imidazopyrroloquinolinones through intramolecular cyclization, dihydropyrroloquinolinone 5 was treated with phosphorus oxychloride in 1,4-dioxane at 50 °C, leading to the deprotection of the tert-butyl group and subsequent cyclization to afford 6a–6i in good to excellent yields (Scheme 2).
Scheme 2. Synthesis of imidazopyrroloquinolinones.
Aldehydes bearing electron-withdrawing and electron-donating groups on the phenyl ring yielded the corresponding imidazopyrroloquinolinones 6a–6e in 79–93% yields, while in the case of symmetric ketones like acetone (6f, 6g), cyclopentanone (6h), and cyclohexanone (6i), imidazopyrroloquinolinones 6f–6i were afforded in 84–95% yields.
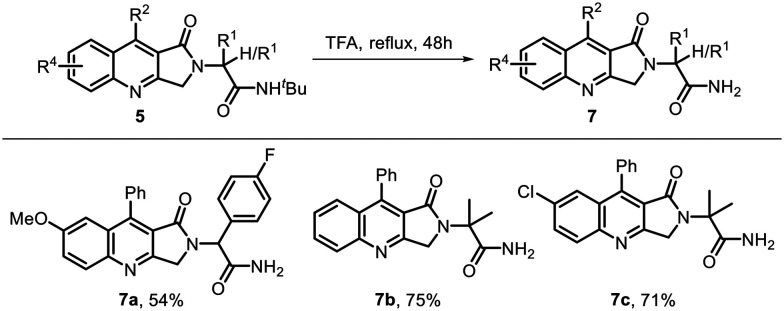
Free amide in a drug-like molecule helps to increase the polar character of the compound, enhances the water solubility, and also increases the hydrogen-bonding interactions with the target site. Thus, we introduced free amidic moieties in our molecule by deprotection of the tert-butyl group in dihydropyrroloquinolinones 5 using trifluoroacetic acid under reflux for 48 h. This led to the corresponding free amides 7a–7c in 54–75% yields (Scheme 3).
Scheme 3. Synthesis of amide.
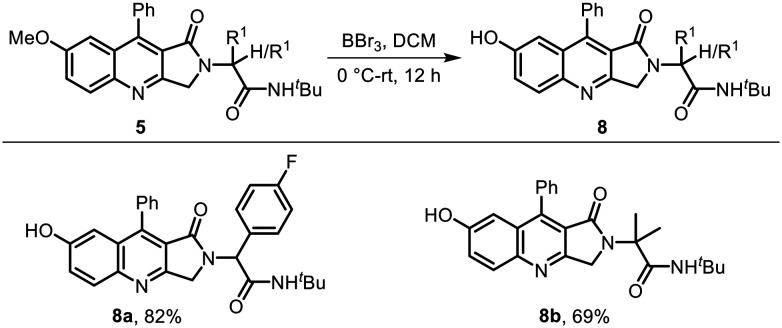
In another attempt to increase the polarity of the molecule and to enhance its binding interactions with the target site, we performed demethylation of dihydropyrroloquinolinone 5, which converted the OMe group into the OH group and afforded the corresponding hydroxy dihydropyrroloquinolines 8a and 8b in 56% and 82% yields, respectively (Scheme 4). Attempts to synthesize the hydroxyl group containing dihydropyrroloquinolines 8 in a single step from 4-hydroxyaniline using the method described in Table 1 failed, as the Povarov-type reaction did not take place and the Ugi adduct remained unreacted.
Scheme 4. Selective deprotection of the methoxy group.
IC50 values of compounds (>74% inhibition).
| Entry | Compound code | IC50 |
|---|---|---|
| 1 | 5c | 10.51 ± 0.85 |
| 2 | 5i | 8.39 ± 0.4 |
| 3 | 5k | 10.31 ± 0.44 |
| 4 | 5m | 8.36 ± 0.76 |
| 5 | 5n | 7.42 ± 0.77 |
| 6 | 5p | 5.35 ± 0.25 |
| 7 | 7a | 8.75 ± 1.26 |
| 8 | Miltefosine | 8.10 ± 0.32 |
Biological evaluations
In vitro antileishmanial activity
Evaluating the dose-dependent cytotoxicity of an active compound against host cells is a prerequisite to fully define its antileishmanial potency and safety index. Therefore, J774 host cell macrophages were treated with increasing concentrations of all the synthesized compounds and percentage inhibition was determined via MTT assay (Table S1, ESI†). The compounds displayed moderate to high toxicity at higher concentration, i.e. 50 and 100 μM (exhibited ≥30% loss in cell viability of macrophages). However, at lower concentrations of 25, 12.5, and 6.25 μM, the percentage loss in cell viability of host macrophages was evidently lower. The results obtained from this preliminary MTT-based cytotoxicity assay served as a guiding light for deducing the dosage of the compounds under study for subsequent assays.
Further, the in vitro antileishmanial potential of the compounds was evaluated at 25, 12.5, and 6.25 μM concentrations by serial two-fold dilution (Table S2, ESI†). The pyrroloquinolinone derivatives 5a–5p exhibited 40–85% inhibition of promastigotes at 25 μM. Amongst them, compounds 5a, 5d, 5k, 5m, 5n, and 5p showed more than 70% anti-promastigote inhibition. Notably, compound 5m was found to exhibit significant antileishmanial potency against L. donovani promastigotes with 85.42% inhibition at 25 μM. In contrast, imidazopyrroloquinolinone derivatives 6a–6i showed 39–52% inhibition of promastigotes at 25 μM. The free amide derivatives 7a–7c demonstrated 61–79% promastigote inhibition, while the hydroxy dihydropyrroloquinolinones 8a and 8b showed 72.74% and 77.16% inhibition of promastigotes at 25 μM, respectively. In parallel, anti-amastigote assays were conducted to gain perspective regarding the ability of the dihydropyrroloquinolinone derivatives to inhibit luciferase-expressing intra-macrophagic amastigotes, which are responsible for causing human VL. A similar trend was observed with respect to anti-promastigote activity. In coherence with the observations derived from anti-promastigote assays, compounds 5a–5p were found to exhibit 45–85% inhibition of amastigotes at 25 μM. Similar to the inhibition observed for promastigotes, compound 5m exhibited a noticeably higher percentage inhibition of luciferase activity against amastigote forms of the parasite, i.e. 85.5% at 25 μM. However, imidazopyrroloquinolinones 6a–6i exhibited a relatively lower anti-amastigote activity (31–59%) at 25 μM, as compared to the reference drug miltefosine, which demonstrated more than 90% inhibition against amastigote multiplication at the same dose. Compounds 7a–c showed 46–75% anti-amastigote activity, while compounds 8a and 8b demonstrated 69–71% inhibition against amastigotes at 25 μM. The results of all thirty compounds comprising their percentage inhibition values against promastigote and amastigote stages of L. donovani parasite at the three tested concentrations (25, 12.5, and 6.25 μM) are summarized in Table S2, ESI,† in comparison with the standard drug miltefosine. Since compounds 5c, 5i, 5k, 5m, 5n, 5p and 7a showed significant antileishmanial activity towards intracellular L. donovani amastigotes (>74% inhibition), we next performed a dose-dependent analysis to evaluate the 50% inhibitory concentration (IC50) of these compounds against the amastigote form of the parasite. The IC50 values of the active compounds tested are listed in Table 1.
Molecular docking
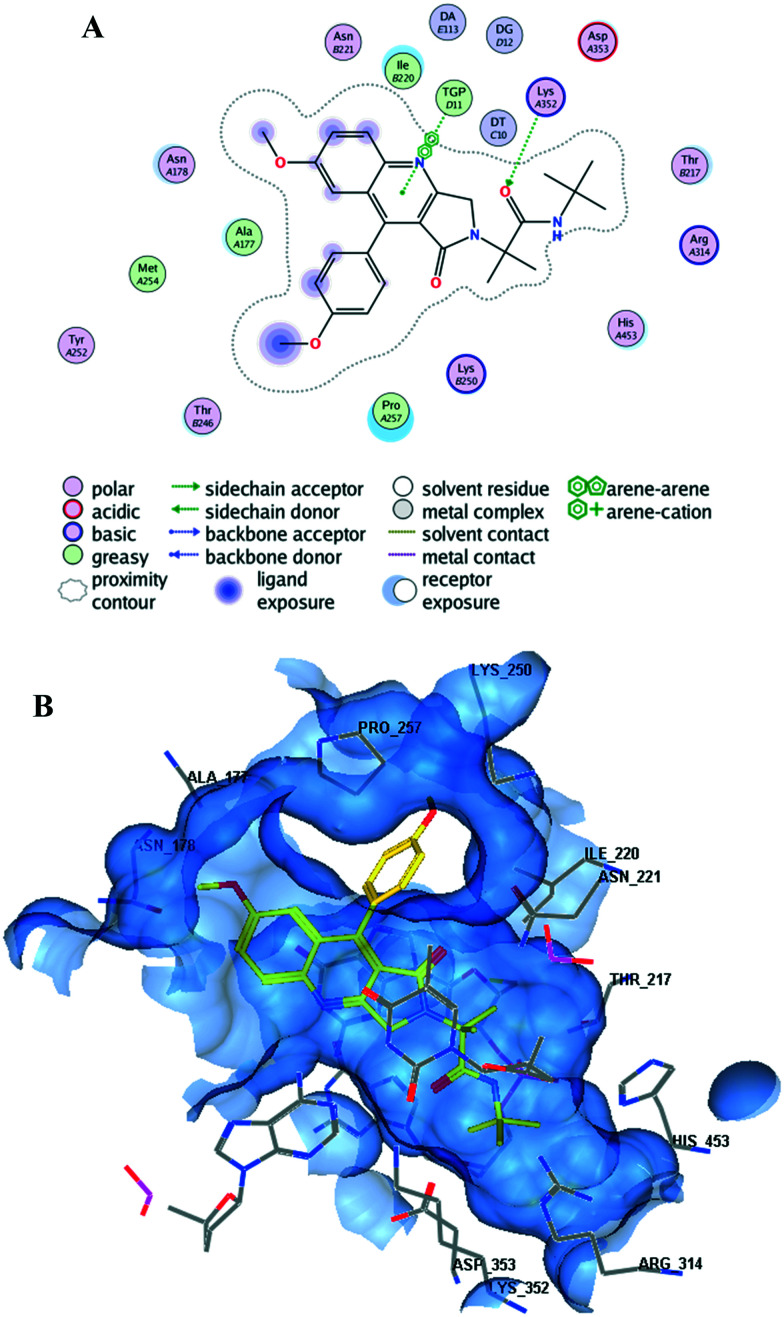
Leishmania donovani topoisomerase 1 (LDTop1) is an attractive therapeutic target for developing novel antileishmanial agents, and many of the quinoline derivatives act through the inhibition of LDTop1. Molecular docking was performed to study the binding mode of the active compounds at the LdTop1 active site (PDB ID 2B9S).38 The docking score of camptothecin at the LdTop1 binding site was found to be −7.7961 kcal mol−1, showing hydrogen bond interactions with residues Lys352, Asp353, and Arg314 and non-bonding interaction with residue TGP11 (docking protocol described in the ESI,† pages S24 and S25). Docking of compound 5m at the active site of LdTop1 (docking score −8.4789 kcal mol−1) exhibited intercalation at the DNA cleavage site, hydrogen bonding interaction with residue Lys352, and non-bonding interaction with residue TGP11 (Fig. 3A and B).
Fig. 3. (A) 2D and (B) 3D binding modes of compound 5m with LdTop1.
Among the other active compounds, 5n (docking score: −8.5766 kcal mol−1; H-bond interaction with Lys352 and non-bonding interactions with Tgp11 and DT10 residues), amide 7a (docking score: −8.3910 kcal mol−1; H-bond interactions with Asn221 and non-bonding interactions with DT10 and TGP11 residues) and hydroxy derivative 8a (docking score: −9.4846 kcal mol−1; H-bond interaction with Asn178 and non-bonding interactions with TGP11 and DC112 residues) also showed good binding at the LDTop1 active site.
Structure–activity relationship (SAR)
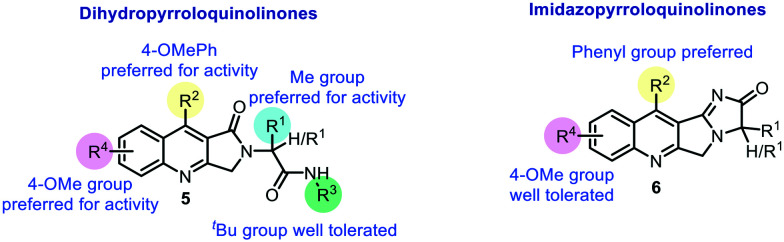
Based on the antileishmanial activity of compounds, their IC50 values, and the corresponding docking studies, a structure–activity relationship (SAR) analysis was performed (Fig. 4).
Fig. 4. A comparative SAR analysis.
Dihydropyrroloquinolinones and imidazopyrroloquinolinones are the two major classes of compounds described in this work, with diverse structural modifications affecting the antileishmanial activity of the compounds. Compounds 5c (IC50: 10.51 μM), 5i (IC50: 8.39 μM), 5k (IC50: 10.31 μM), 5m (IC50: 8.36 μM), 5n (IC50: 7.42 μM), 5p (IC50: 5.35 μM), and 7a (IC50: 8.75 μM) showed more than 70% inhibition against the intracellular amastigote form of L. donovani. Interestingly, the IC50 values showed good correlation with the docking scores. The docking score of 5m (−8.4789 kcal mol−1) was found to be higher than that of camptothecin (−7.7961 kcal mol−1), with H-bonded (Lys352) and non-bonded (TGP11) interactions. Compounds 5n (−8.5766 kcal mol−1) and 7a (−8.3910 kcal mol−1) also exhibited good binding at the LDTop1 active site. In contrast, imidazopyrroloquinolinones 6 showed low to moderate activity with less than 70% intracellular amastigote inhibition of L. donovani. Hence, the fused tetracyclic ring did not favour the antileishmanial potential of the compounds. Instead, the tricyclic dihydropyrroloquinolinones were found to be active, with R1 as methyl, R2 as phenyl/4-methoxyphenyl, R3 as tert-butyl, and R4 as 4-methoxy being the preferred structural requirements for antileishmanial activity.
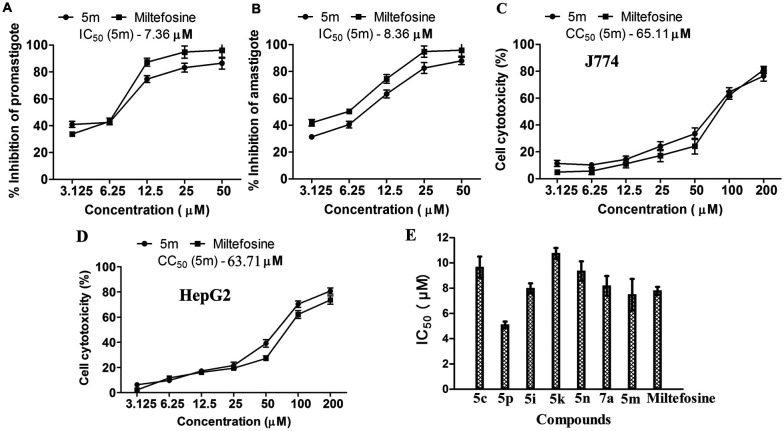
Amongst the screened compounds, compound 5m fulfilled our criteria of a promising lead compound owing to its ability to demonstrate >85% inhibition against both stages of the parasite. As a result, it was further selected for IC50 determination. The dose-dependent effect of compound 5m on parasite growth inhibition was evaluated upon treatment of L. donovani promastigotes and J774 macrophage-infected luciferase-expressing amastigotes with compound 5m. Results revealed a dose-dependent inhibition against promastigotes, with maximum killing of 85.42% and 86.5% observed at 25 μM and 50 μM, respectively, as determined via luciferase assay (Fig. 5A). Additionally, dose–response analysis against amastigote-infected host macrophages showed a similar trend of dose-dependent inhibition (Fig. 5B). The half-maximal inhibition concentration (IC50) of 5m was calculated to be 7.36 μM and 8.36 μM against promastigotes and intra-macrophagic amastigotes, respectively, at 72 h post-treatment, which is well comparable with that of the standard reference drug miltefosine (Fig. 5A and B). Calculated IC50 values for miltefosine against promastigotes and intracellular amastigotes following 72 h of treatment were 6.96 μM and 6.12 μM, respectively. The 50% cytotoxic concentration (CC50) of 5m was calculated to be 65.11 μM and 63.71 μM against host J774 macrophages and HepG2 cell line, respectively (Fig. 5C and D), as compared to the CC50 of miltefosine (72.35 μM and 78.62 μM against J774 macrophages and HepG2 cell line, respectively). The selectivity index (SI = CC50/IC50) of 5m was found to be 7.79 and 7.62 against J774 macrophages and HepG2 cell line, respectively, which is lower than that of the standard drug miltefosine (SI = 11.82 and 12.84 for J774 macrophages and HepG2 cell line, respectively).
Fig. 5. Determination of in vitro antileishmanial efficacy and cytotoxicity of 5m: (A) percent inhibition of L. donovani promastigotes was determined by MTT assay after treatment with various concentrations of 5m for 72 h. (B) J774 macrophages were seeded overnight followed by infection with luciferase-expressing L. donovani promastigotes (1 : 10) as described in experimental procedures followed by incubation with different doses of 5m (3.12–50 μM) for 72 h. Percent inhibition of amastigotes was also evaluated by luciferase assay. (C and D) J774 macrophages and HepG2 cells were treated with varying concentrations of 5m for 72 h and cell viability was checked by MTT assay. (E) The IC50 of active compounds was determined against intracellular amastigotes as described in the ESI† (pages S13–S20). Data are representative of three independent experiments done in replicates and the results shown are means ± SD for each time point.
Pharmacokinetic parameters of compound 5m
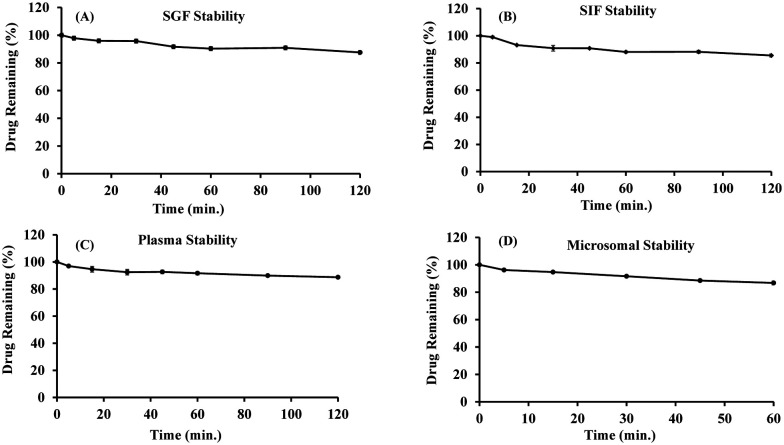
The stability of compound 5m at various physiological conditions was evaluated to assess the possibility of oral administration (Table 2 and Fig. 6). Compound 5m was found to be stable in both SGF and SIF under the established experimental conditions. The compound was found to be 87.55 ± 0.51% and 85.47 ± 1.12% intact, respectively, in SGF and SIF after 2 h of incubation (Fig. 6A and B). The stability result of compound 5m in mice plasma is illustrated in Fig. 6C and was found to be intact with 88.69 ± 0.59% remaining of drug after 2 h of incubation. It was evident from the results that compound 5m was stable in mice plasma. In mice liver microsomes (MLMs), in vitro microsomal stability test of the compound 5m was performed to examine the role of the liver in the clearance of the compound from the body, and the results are illustrated in Table 2 and Fig. 6. Compound 5m was found to be 86.77 ± 1.49% intact after 1 h of incubation in MLM. The half-life (t1/2) and the intrinsic clearance (CLint.) were found to be 5.07 ± 0.72 h and 4.61 ± 0.65 μL min−1 mg−1, respectively. Based on the preclinical stability studies, compound 5m was found to be stable under a different set of in vitro experimental conditions.
In vitro pharmacokinetic parameters of compound 5m.
| Parameters | 5m |
|---|---|
| SGF stability (% drug remaining, 2 h) | 87.55 ± 0.51 |
| SIF stability (% drug remaining, 2 h) | 85.47 ± 1.12 |
| Plasma stability (% drug remaining, 2 h) | 88.69 ± 0.59 |
| Microsomal stability (% drug remaining, 1 h) | 86.77 ± 1.49 |
| K (h−1) | 0.14 ± 0.02 |
| Half-life (h) | 5.07 ± 0.72 |
| Intrinsic clearance (μL min−1 mg−1) | 4.61 ± 0.65 |
Fig. 6. In vitro stability of compound 5m in simulated gastric fluids (A) and simulated intestinal fluids (B) up to 2 h. In vitro stability of compound 5m in mice plasma (C) and in mice liver microsomes (MLMs) (D).
Solubility and partition coefficient (log P) of 5m
The equilibrium solubility of compound 5m in triple distilled water (TDW), as determined by the shake flask method, was found to be 156.75 ± 22.62 μg mL−1, while the experimentally determined log P value for compound 5m was found to be 2.70 ± 0.19, which comply with Lipinski's rule for log P.37
Antileishmanial potential of 5m against a Balb/c mice model of VL
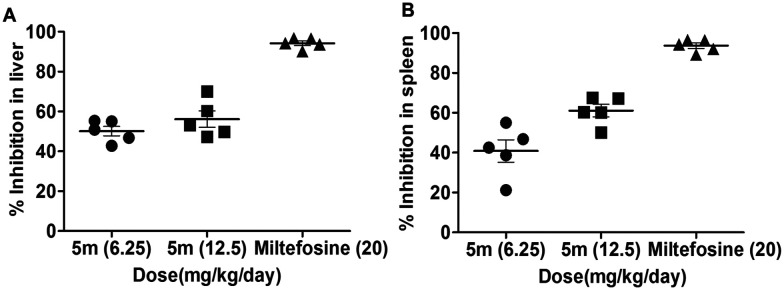
In vivo antileishmanial evaluation of compound 5m was conducted in a L. donovani-infected Balb/c mice model (Fig. 7). Following 15 days post-infection, Balb/c mice were given intraperitoneal administration of compound 5m for seven consecutive days at three doses: 6.25, 12.5, and 50 mg kg−1, respectively. One-week post-treatment, infected-treated and infected-untreated animals were sacrificed to assess the organ parasite burden. A moderate inhibition in both liver and spleen parasite burden was observed in infected Balb/c mice which were treated with a dose of 6.25 mg kg−1 (45.1% and 42.4%, respectively) and this was maximum at 12.5 mg kg−1 (56.2% and 61.1%, respectively) (Fig. 7A and B). Further increasing the dose of 5m to 50 mg kg−1 did not appreciably enhance its in vivo antileishmanial efficacy (57.8% and 63.4% inhibition of liver and spleen parasite burden, respectively, data not shown). The in vivo activity results clearly depict that compound 5m is moderately active against the in vivo experimental model of VL, as similar antileishmanial efficacy was observed at both lower and moderately higher doses (12.5 mg kg−1 and 50 mg kg−1, respectively), implying that 12.5 mg kg−1 is the optimal dose of the compound for demonstrating antileishmanial efficacy in the in vivo model of VL. Miltefosine was used as a standard reference drug (25 mg kg−1), which almost completely ameliorated organ parasite burden in infected Balb/c mice (97.4% and 98.9% inhibition of liver and spleen parasite burden, respectively).
Fig. 7. In vivo antileishmanial effect of 5m on organ parasite burden of infected Balb/c mice. 15 days infected Balb/c mice were treated with different doses of 5m as described in the experimental procedures. Mice of different experimental groups were sacrificed at one week post treatment for the determination of (A) hepatic and (B) splenic parasite loads by a stamp smear method. The mean percent inhibition (PI) ± SD was calculated by comparing the parasitic burden of 5m- or miltefosine-treated groups to infected animals treated with vehicle control.
5m induces mitochondrial programmed cell death of L. donovani promastigotes
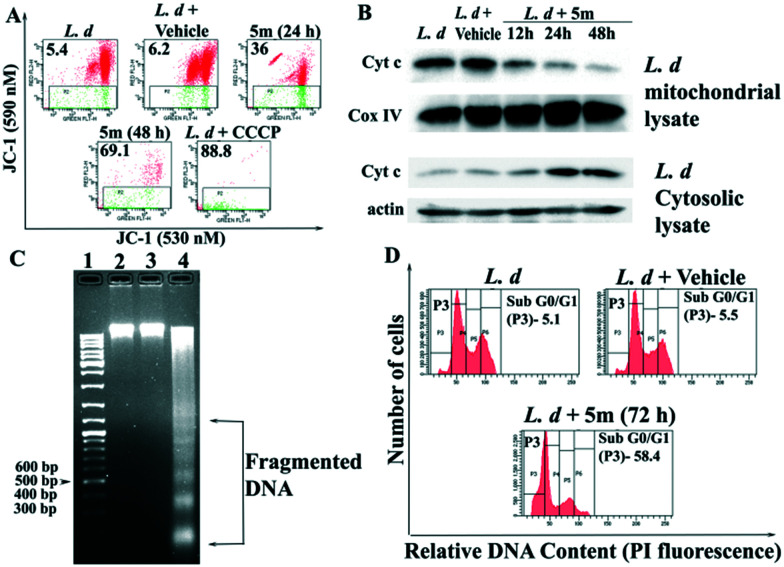
The apparent direct killing effect of 5m on L. donovani promastigotes further prompted us to evaluate its mechanism(s) of action, and to this end we next evaluated the biochemical parameters like mitochondrial membrane potential loss, cytochrome c release from damaged mitochondria, cell cycle arrest, and DNA damage in 5m-treated L. donovani promastigotes. In order to verify whether 5m induces apoptosis via mitochondrial dysfunction, we monitored the change in mitochondrial membrane potential via flow cytometry of 5m-treated L. donovani promastigotes. We observed that 5m exhibited a time-dependent increase in MMP loss (24 h and 48 h), as evidenced by the increasing percentage of cells in the green region (JC-1 extrudes into the cytoplasm from damaged mitochondria as monomers emitting green fluorescence, Fig. 8A). This loss in MMP was maximum at 48 h post-treatment (69.1%) as compared with the green fluorescence intensity of untreated parasites (5.4%) and vehicle-treated parasites (6.2%) (Fig. 8A). We next studied the downstream event following apoptotic stimulus-induced MMP loss, i.e. cytochrome c release in the cytosol of 5m-treated L. donovani promastigotes from mitochondria. Immunoblotting analysis showed that with increasing time, 5m increased cytochrome c abundance in the cytosolic fractions of L. donovani promastigotes at 24 h and 48 h with a concomitant decrease in the mitochondrial region (Fig. 8B). On the other hand, a higher cytochrome c abundance in the mitochondrial fractions of untreated and vehicle-treated parasites was indicative of functionally intact mitochondria (Fig. 8B).
Fig. 8. 5m-induced assessment of programmed cell death parameters in L. donovani promastigotes. (A) Log-phase LDd8 promastigotes were incubated with 50 μM 5m for 24 and 48 h or vehicle (0.1% DMSO, for 48 h). Mitochondrial membrane potential loss was evaluated by flow cytometry after staining with JC-1 dye (2 μM). CCCP-treated parasites were used as the positive control. (B) Immunoblotting analysis of cytochrome c in cytosolic and mitochondrial fractions of L. donovani promastigotes incubated with 50 μM 5m for 12–48 h or vehicle for 48 h. β-Actin was the loading control for cytosolic fractions, while COX IV was taken as the loading control for mitochondrial fractions. Results are representative of three independent experiments. (C) DNA fragmentation analysis by agarose gel electrophoresis of genomic DNA obtained from untreated (lane 2), vehicle (0.1% DMSO)-treated (lane 3) and 50 μM 5m-treated promastigotes for 72 h (lane 4). Lane 1 denotes molecular size marker (bp, base pairs). The results are representative of those from three independent experiments. (D) Representative histograms of cell cycle analysis of L. donovani promastigotes treated with 50 μM 5m or vehicle for 48 h. The changes in cell cycle progression of Leishmania promastigotes were analysed by flow cytometry after staining with propidium iodide. The percentage of parasites undergoing apoptosis-like cell death are denoted in the P3 region (sub-G0/G1 phase).
The triggering of apoptosis culminates with the activation of internucleosomal DNA digestion in leishmania parasites.39 Thus, in order to verify whether 5m treatment is met with genomic DNA cleavage resulting from internucleosomal DNA digestion, we checked for the presence of apoptotic DNA ladder formation in 5m-treated parasites post 72 h. Analysis of genomic DNA by agarose gel electrophoresis revealed a sharp, intact band for both control and vehicle-treated parasites, indicating the absence of DNA fragmentation. On the other hand, genomic DNA isolated from parasites incubated with 50 μM 5m for 72 h was visible in a ladder-like pattern, indicating breakdown of nuclear DNA into oligonucleosomal-sized fragments (in multiples of 200 base pairs, Fig. 8C).
The loss of genomic DNA integrity accompanied by DNA nicking and formation of oligonucleosomal structures eventually slows down parasite proliferation, causing cell cycle arrest.40 Thus, to check whether 5m-induced chromosomal damage correlates with perturbations in the cell cycle, we carried out flow cytometry analysis following PI staining of 5m-treated parasites at the maximum inhibitory concentration, i.e. 50 μM. As depicted in Fig. 8D, 5m induced an increase in the percentage of parasite population in the sub-G0/G1 phase of the cell cycle. This increase in the sub-G0 was indicative of cell cycle arrest and apoptosis as untreated and vehicle-treated parasites exhibited a normal cell cycle distribution with only 5.1% and 5.5% cells in the sub-G0/G1 region. On the other hand, 5m treatment of L. donovani promastigotes for 72 h induced a prominent increase of sub-G0/G1 population (58.4%), indicating inhibited entry into the S phase of the cell cycle and consequent cell cycle arrest.
Conclusions
In summary, a library of novel and functionalized pyrroloquinolinone derivatives were synthesized in good to excellent yields using atom-efficient and operationally simple post-Ugi functionalization. The antileishmanial activity of all the synthesized molecules was evaluated against luciferase-expressing L. donovani, wherein compound 5m was identified as a potential lead that showed >85% inhibition in both promastigote and amastigote stage at 25 μM concentration. The IC50 of compound 5m (7.36 μM and 8.36 μM) was found to be comparable with that of the standard drug miltefosine. Compound 5m could potentially reduce the parasite burden in Balb/c mice when treated with 12.5 mg kg−1 per day (i.p.). Molecular docking studies and in vitro/in vivo analysis against an experimental model of visceral leishmaniasis revealed that 5m could be acting through LDTop1 inhibition, thereby inducing cell cycle arrest and mitochondrial apoptosis of L. donovani promastigotes.
Experimental
General
All the reagents and solvents were used as received from commercial sources without further purification. All air- and moisture-sensitive reactions were conducted under an inert atmosphere of nitrogen or argon. Solvents like dichloromethane, dimethylformamide, and 1,4-dioxane were distilled before use. Reactions were monitored by thin-layer chromatography carried out on silica plates (silica gel 60 F254, Merck) using UV light, iodine, ninhydrin, and p-anisaldehyde for visualization. Column chromatography was carried out using silica gel (100–200 and 230–400 mesh) packed in glass columns. Technical grade ethyl acetate and petroleum ether used for column chromatography were distilled prior to use. 1H NMR and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 as solvent (300 and 75 MHz/400 and 100 MHz/500 and 125 MHz) at ambient temperature. 19F NMR spectra were recorded in CDCl3 as solvent at 283 MHz and 376 MHz, respectively. The coupling constant J is given in Hz. The chemical shifts (δ) are reported in ppm on scale downfield from TMS and using the residual solvent peak in CDCl3 (H: δ = 7.26 ppm and C: δ = 77.00 ppm) or TMS (δ = 0.00) as internal standard and signal patterns are indicated as follows: s = singlet, d = doublet, dd = doublet of doublet, ddd = doublet of doublet of doublet, dt = doublet of triplet, t = triplet, q = quartet, m = multiplet. High-resolution mass spectra (HRMS) were recorded on a Thermo Scientific Exactive ORBITRAP spectrometer using H2O/MeOH mixed with 0.1% formic acid as the mobile phase. Melting points were recorded on a Stuart SMP30 melting point apparatus and are uncorrected. HPLC analysis was performed using an Agilent 1260 Series HPLC instrument, solvent system (isocratic) acetonitrile (solvent A) and water (solvent B) 9 : 1, flow rate 0.8 mL min−1, run time 10 min, Agilent XDB-C18 column (mesh size 5.0 μm, diameter 4.6 × 250 mm), oven temperature 27 °C, UV detector (wavelength 220 nm and 254 nm). Compounds 5b, 5c, 5d, 5f, 5g, 5h, 5j, 5k, and 5l are reported in a previous publication from our research group.36
General procedure A for the synthesis of compounds 5a–5p
Compounds 5a–5p were prepared in accordance with a procedure previously reported by our research group.1 An equimolar mixture of amine (1.90 mmol, 1.0 equiv.), aldehyde (1.90 mmol, 1.0 equiv.), acid (1.90 mmol, 1.0 equiv.) and isocyanide (1.90 mmol, 1.0 equiv.) in EtOH was stirred at room temperature for 6 h. After consumption of all the substrates (based on TLC), amine (1.90 mmol, 1.0 equiv.) and concentrated H2SO4 (0.2 mL, 3.80 mmol, 1.0 equiv.) were added and the mixture was stirred for 10 h at rt. After completion of the reaction (based on TLC), the reaction mixture was diluted with dichloromethane and washed with saturated sodium bicarbonate solution. The aqueous layer was extracted with dichloromethane and the combined organic layers were dried over anhydrous sodium sulfate followed by evaporation of the solvent in vacuo. The crude product was purified by silica gel column chromatography to afford the desired product 5.
General procedure B for the synthesis of compounds 6a–6i
To a suspension of 5 (0.25 mmol, 1.0 equiv.) in 5.0 mL of anhydrous 1,4-dioxane was added 1.17 mL (12.5 mmol, 50.0 equiv.) of POCl3 and the reaction mixture was heated at 50 °C for 24 h. After completion of the reaction (based on TLC), the resulting suspension was poured into ice and the solution was made basic by the addition of 1 M NaOH solution. The mixture was extracted with three 10.0 mL portions of ethyl acetate. The combined organic phase was washed with two 5.0 mL portions of 1 M NaOH solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford the desired product 6.
General procedure C for the synthesis of compounds 7a–7c
To the required quantity of 5 (0.25 mmol), 3.0 mL of trifluoroacetic acid was added at room temperature and the resulting reaction mixture was refluxed in a preheated oil bath for 48 h. After completion of the reaction (based on TLC), the reaction mixture was quenched with sodium bicarbonate solution and the crude product was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo and the crude was purified by silica gel column chromatography to afford the desired amide 7.
General procedure D for the synthesis of compounds 8a and 8b
To a solution of 5 (0.25 mmol, 1.0 equiv.) in anhydrous DCM at 0 °C under nitrogen, BBr3 (0.07 mL, 0.75 mmol, 3.0 equiv.) was added dropwise. The resulting mixture was allowed to warm to room temperature and stirred overnight. After completion of the reaction (based on TLC), the reaction mixture was added slowly to a stirring solution of ice water, diluted with DCM and washed with saturated sodium bicarbonate solution. The organic layer collected was again washed with brine solution. The combined organic layers were dried over anhydrous sodium sulfate and concentrated in vacuo and the crude product was purified by silica gel column chromatography to afford the demethylated product 8.
5a (N-(tert-butyl)-2-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-phenylacetamide)
Light yellow solid (650.0 mg, 71%); mp: 279–281 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.02 (d, J = 9.2 Hz, 1H), 7.55–7.37 (m, 11H), 7.06 (d, J = 2.8 Hz, 1H), 6.09 (s, 1H), 5.72 (s, 1H), 5.08 (d, J = 17.1 Hz, 1H), 4.11 (d, J = 17.1 Hz, 1H), 3.73 (s, 3H), 1.30 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 168.6, 166.2, 158.9, 157.8, 146.2, 145.9, 135.2, 133.1, 130.4, 129.8, 129.1, 129.0, 128.7, 128.6, 128.0, 127.9, 123.5, 119.7, 105.0, 58.4, 55.4, 51.8, 48.9, 28.6; HRMS (ESI) m/z calcd for C30H30N3O3 [M + H]+: 480.2287, found: 480.2279; HPLC analysis: retention time = 5.166 min; peak area = 91.30% (220 nm), 96.61% (254 nm).
5e (N-(tert-butyl)-2-(4-cyanophenyl)-2-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)acetamide)
Yellow solid (650.0 mg, 68%); mp: 229–231 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 9.2 Hz, 1H), 7.69 (d, J = 8.4 Hz, 2H), 7.59–7.44 (m, 7H), 7.39 (d, J = 7.7 Hz, 1H), 7.05 (d, J = 2.8 Hz, 1H), 6.11 (s, 1H), 5.90 (s, 1H), 5.06 (d, J = 17.0 Hz, 1H), 4.15 (d, J = 17.0 Hz, 1H), 3.74 (s, 3H), 1.31 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 167.3, 166.6, 158.4, 157.9, 146.6, 146.2, 140.4, 132.9, 132.7, 130.5, 129.7, 128.8, 128.1, 128.0, 127.9, 123.9, 119.1, 118.2, 112.6, 105.0, 57.9, 55.4, 52.1, 48.8, 28.5; HRMS (ESI) m/z calcd for C31H29N4O3 [M + H]+: 505.2240, found: 505.2240; HPLC analysis: retention time = 4.371 min; peak area = 97.78% (220 nm), 99.36% (254 nm).
5i (N-(tert-butyl)-2-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)acetamide)
Light yellow solid (515.0 mg, 67%); mp: 201–203 °C; Rf = 0.3 (5% MeOH in DCM); 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 9.2 Hz, 1H), 7.59–7.53 (m, 3H), 7.49 (dd, J = 9.2, 2.8 Hz, 1H), 7.47–7.39 (m, 2H), 7.08 (d, J = 2.8 Hz, 1H), 5.77 (s, 1H), 4.69 (s, 2H), 4.16 (s, 2H), 3.75 (s, 3H), 1.32 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 167.1, 166.8, 158.1, 157.9, 146.5, 145.9, 132.8, 130.4, 129.6, 128.8, 128.2, 128.0, 123.9, 119.4, 105.1, 55.4, 51.9, 51.6, 47.4, 28.7; HRMS (ESI) m/z calcd for C24H26N3O3 [M + H]+: 404.1974, found: 404.1970; HPLC analysis: retention time = 3.687 min; peak area = 97.82% (220 nm), 98.59% (254 nm).
5m (N-(tert-butyl)-2-(7-methoxy-9-(4-methoxyphenyl)-1-oxo-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-methylpropanamide)
Brown solid (540.0 mg, 61%); mp: 229–231 °C; Rf = 0.3 (70% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.05 (d, J = 9.2 Hz, 1H), 7.46 (dd, J = 9.2, 2.8 Hz, 1H), 7.39–7.35 (m, 2H), 7.14 (d, J = 2.8 Hz, 1H), 7.08–7.04 (m, 2H), 6.25 (s, 1H), 4.65 (s, 2H), 3.91 (s, 3H), 3.77 (s, 3H), 1.70 (s, 6H), 1.32 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 172.8, 167.2, 159.9, 158.1, 157.9, 146.2, 146.1, 131.3, 130.4, 128.7, 124.8, 123.6, 120.6, 113.5, 105.2, 60.9, 55.5, 55.2, 51.2, 49.6, 28.6, 24.6; HRMS (ESI) m/z calcd for C27H32N3O4 [M + H]+: 462.2393, found: 462.2387; HPLC analysis: retention time = 4.238 min; peak area = 100% (220 nm), 100% (254 nm).
5n (N-(tert-butyl)-2-(7-methoxy-9-methyl-1-oxo-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-methylpropanamide)
Yellow sticky solid (360.0 mg, 51%); Rf = 0.3 (70% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 9.2 Hz, 1H), 7.46 (dd, J = 9.2, 2.8 Hz, 1H), 7.35 (d, J = 2.7 Hz, 1H), 6.15 (s, 1H), 4.59 (s, 2H), 3.98 (s, 3H), 3.07 (s, 3H), 1.72 (s, 6H), 1.37 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 172.8, 168.5, 157.7, 157.6, 145.0, 144.2, 130.8, 129.0, 123.4, 121.4, 102.6, 60.5, 55.5, 51.2, 49.3, 28.6, 24.5, 12.1; HRMS (ESI) m/z calcd for C21H28N3O3 [M + H]+: 370.2131, found: 370.2135; HPLC analysis: retention time = 3.581 min; peak area = 98.47% (220 nm), 95.19% (254 nm).
5o (N-(tert-butyl)-1-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)cyclopentane-1-carboxamide)
Light yellow solid (505.0 mg, 58%); mp: 182–184 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 9.2 Hz, 1H), 7.55 (dd, J = 5.1, 1.9 Hz, 3H), 7.47 (dd, J = 9.2, 2.8 Hz, 1H), 7.42–7.38 (m, 2H), 7.03 (d, J = 2.8 Hz, 1H), 6.85 (s, 1H), 4.64 (s, 2H), 3.74 (s, 3H), 2.55 (dd, J = 12.0, 5.5 Hz, 2H), 2.32–2.22 (m, 2H), 1.78–1.70 (m, 4H), 1.25 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 171.7, 167.7, 157.9, 157.9, 146.2, 146.0, 133.1, 130.4, 129.5, 128.8, 128.4, 128.0, 123.7, 120.6, 105.0, 71.4, 55.4, 51.0, 50.7, 35.1, 28.6, 22.7; HRMS (ESI) m/z calcd for C28H32N3O3 [M + H]+: 458.2444, found: 458.2433; HPLC analysis: retention time = 5.047 min; peak area = 99.00% (220 nm), 99.23% (254 nm).
5p (N-(tert-butyl)-1-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)cyclohexane-1-carboxamide)
Brown solid (539.0 mg, 60%); mp: 229–231 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 9.2 Hz, 1H), 7.58–7.51 (m, 3H), 7.47 (dd, J = 9.2, 2.8 Hz, 1H), 7.43–7.35 (m, 2H), 7.02 (d, J = 2.8 Hz, 1H), 6.67 (s, 1H), 4.64 (s, 2H), 3.74 (s, 3H), 2.43 (s, 2H), 2.24–2.10 (m, 2H), 1.59–1.32 (m, 6H), 1.26 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 171.7, 168.3, 157.9, 157.9, 146.1, 133.2, 130.4, 129.5, 128.8, 128.5, 127.9, 123.7, 120.8, 105.0, 64.9, 55.4, 51.1, 49.8, 32.7, 28.7, 25.3, 22.8; HRMS (ESI) m/z calcd for C29H34N3O3 [M + H]+: 472.2600, found: 472.2600; HPLC analysis: retention time = 4.554 min; peak area = 100% (220 nm), 100% (254 nm).
5q (2-(4-bromophenyl)-N-(tert-butyl)-2-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)acetamide)
Yellow sticky solid (605.0 mg, 57%); Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 9.2 Hz, 1H), 7.52 (ddd, J = 8.5, 6.8, 3.5 Hz, 4H), 7.45 (dd, J = 9.2, 2.8 Hz, 2H), 7.41–7.32 (m, 4H), 7.05 (d, J = 2.8 Hz, 1H), 6.09 (s, 1H), 5.95 (s, 1H), 5.10 (d, J = 17.1 Hz, 1H), 4.14 (d, J = 17.1 Hz, 1H), 3.74 (s, 3H), 1.28 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 168.1, 166.3, 158.7, 157.9, 146.5, 145.9, 134.1, 132.9, 132.2, 130.7, 130.4, 129.7, 128.7, 128.1, 128.0, 127.9, 123.7, 122.9, 119.5, 105.0, 57.7, 55.4, 51.9, 48.8, 28.5; HRMS (ESI) m/z calcd for C30H29BrN3O3 [M + H]+: 558.1392, found: 558.1386.
5r (N-(tert-butyl)-2-(7-chloro-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-methylpropanamide)
White sticky solid (520.0 mg, 63%); Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.11 (d, J = 8.9 Hz, 1H), 7.79–7.72 (m, 2H), 7.58–7.52 (m, 3H), 7.42–7.35 (m, 2H), 6.20 (s, 1H), 4.70 (s, 2H), 1.70 (s, 6H), 1.30 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 172.5, 166.4, 160.6, 148.2, 146.9, 132.8, 131.9, 130.7, 129.8, 129.2, 128.1, 126.2, 121.2, 60.9, 51.2, 49.9, 28.6, 24.6; HRMS (ESI) m/z calcd for C25H27ClN3O2 [M + H]+: 436.1792, found: 436.1786.
6a (9-methoxy-3,11-diphenyl-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
Brown solid (90.0 mg, 89%); mp: 189–191 °C; Rf = 0.7 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.05 (d, J = 9.2 Hz, 1H), 7.59 (dd, J = 5.9, 4.3 Hz, 3H), 7.54–7.49 (m, 3H), 7.48–7.41 (m, 5H), 7.08 (d, J = 2.8 Hz, 1H), 6.76 (s, 1H), 4.66 (d, J = 16.2 Hz, 1H), 4.24 (d, J = 16.2 Hz, 1H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.6, 158.2, 157.2, 147.3, 146.4, 132.6, 131.6, 130.5, 129.8, 129.7, 129.5, 129.4, 129.1, 128.3, 128.2, 128.1, 127.3, 124.3, 118.4, 115.7, 105.1, 55.5, 47.1, 46.1; HRMS (ESI) m/z calcd for C26H20N3O2 [M + H]+: 406.1556, found: 406.1540; HPLC analysis: retention time = 4.692 min; peak area = 96.49% (220 nm), 97.39% (254 nm).
6b (3-(4-bromophenyl)-9-methoxy-11-phenyl-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
Light yellow solid (105.0 mg, 87%); mp: 171–173 °C; Rf = 0.5 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 9.2 Hz, 1H), 7.63–7.56 (m, 5H), 7.52–7.43 (m, 3H), 7.40 (d, J = 8.2 Hz, 2H), 7.08 (d, J = 2.8 Hz, 1H), 6.71 (s, 1H), 4.66 (d, J = 16.0 Hz, 1H), 4.24 (d, J = 16.0 Hz, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.6, 158.2, 156.9, 147.5, 146.4, 132.7, 132.6, 130.7, 130.6, 129.7, 129.4, 129.1, 128.9, 128.2, 128.1, 124.5, 124.1, 118.2, 115.3, 105.1, 55.5, 46.9, 45.6; HRMS (ESI) m/z [M + H]+ calcd for C26H19BrN3O2 484.0661, found 484.0656; HPLC analysis: retention time = 5.519 min; peak area = 100% (220 nm), 100% (254 nm).
6c (3-(4-chlorophenyl)-9-methoxy-11-phenyl-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
White solid (96.0 mg, 87%); mp: 149–151 °C; Rf = 0.7 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 9.2 Hz, 1H), 7.63–7.56 (m, 3H), 7.52–7.40 (m, 7H), 7.08 (d, J = 2.7 Hz, 1H), 6.73 (s, 1H), 4.66 (d, J = 16.0 Hz, 1H), 4.24 (d, J = 16.0 Hz, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.6, 158.2, 156.9, 147.4, 146.4, 135.9, 132.6, 130.6, 130.2, 129.8, 129.7, 129.4, 129.1, 128.7, 128.3, 128.1, 124.4, 118.2, 115.3, 105.1, 55.5, 46.9, 45.5; HRMS (ESI) m/z calcd for C26H19ClN3O2 [M + H]+: 440.1166, found: 440.1166; HPLC analysis: retention time = 5.295 min; peak area = 100% (220 nm), 99.07% (254 nm).
6d (4-(9-methoxy-2-oxo-11-phenyl-2,5-dihydro-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-3-yl)benzonitrile)
White solid (85.0 mg, 79%); mp: 114–116 °C; Rf = 0.5 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 9.2 Hz, 1H), 7.79–7.72 (m, 2H), 7.66 (d, J = 8.2 Hz, 2H), 7.63–7.57 (m, 3H), 7.53–7.43 (m, 3H), 7.09 (d, J = 2.8 Hz, 1H), 6.81 (s, 1H), 4.70 (d, J = 15.9 Hz, 1H), 4.23 (d, J = 15.9 Hz, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.8, 158.3, 156.7, 147.6, 146.5, 136.7, 133.2, 132.5, 130.6, 129.7, 129.3, 129.2, 128.3, 128.2, 128.1, 124.6, 117.8, 117.6, 114.7, 113.9, 105.1, 55.5, 47.1, 45.8; HRMS (ESI) m/z calcd for C27H19N4O2 [M + H]+: 431.1508, found: 431.1495; HPLC analysis: retention time = 3.966 min; peak area = 99.17% (220 nm), 99.03% (254 nm).
6e (9-methoxy-11-phenyl-3-(3,4,5-trimethoxyphenyl)-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
Light yellow solid (115.0 mg, 93%); mp: 213–215 °C; Rf = 0.5 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 9.2 Hz, 1H), 7.64–7.57 (m, 3H), 7.53–7.48 (m, 2H), 7.47–7.42 (m, 1H), 7.08 (d, J = 2.8 Hz, 1H), 6.72 (s, 2H), 6.66 (s, 1H), 4.67 (d, J = 16.1 Hz, 1H), 4.30 (d, J = 16.1 Hz, 1H), 3.87 (s, 6H), 3.84 (s, 3H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.5, 158.2, 157.1, 153.9, 147.3, 146.4, 139.1, 132.6, 130.5, 129.8, 129.3, 129.1, 128.3, 128.1, 126.9, 124.4, 118.4, 115.8, 105.1, 104.7, 60.9, 56.4, 55.5, 47.1, 46.2; HRMS (ESI) m/z calcd for C29H26N3O5 [M + H]+: 496.1872, found: 496.1845; HPLC analysis: retention time = 4.306 min; peak area = 90.06% (220 nm), 98.26% (254 nm).
6f (9-bromo-3,3-dimethyl-11-phenyl-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
Brown solid (96.5 mg, 95%); mp: 242–244 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 9.0 Hz, 1H), 7.95 (d, J = 2.1 Hz, 1H), 7.90 (dd, J = 9.0, 2.1 Hz, 1H), 7.63–7.52 (m, 3H), 7.42 (dd, J = 6.5, 2.8 Hz, 2H), 4.70 (s, 2H), 1.94 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 165.4, 159.5, 148.6, 147.6, 134.9, 131.5, 130.8, 129.7, 129.6, 129.3, 128.5, 128.3, 121.3, 120.4, 120.1, 50.5, 49.4, 26.1; HRMS (ESI) m/z calcd for C21H17BrN3O [M + H]+: 406.0555, found: 406.0550; HPLC analysis: retention time = 21.262 min; peak area = 93.24% (254 nm).
6g (9-methoxy-3,3-dimethyl-11-phenyl-3H-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin-2(5H)-one)
Light yellow solid (81.5 mg, 91%); mp: 244–246 °C; Rf = 0.2 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 9.2 Hz, 1H), 7.60–7.42 (m, 6H), 7.06 (d, J = 2.7 Hz, 1H), 4.69 (s, 2H), 3.75 (s, 3H), 1.94 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 165.9, 158.1, 156.9, 146.8, 146.1, 132.6, 130.3, 129.6, 128.9, 128.5, 128.1, 124.2, 120.3, 119.9, 105.1, 55.4, 50.4, 49.2, 26.1; HRMS (ESI) m/z calcd for C22H20N3O2 [M + H]+: 358.1556, found: 358.1547; HPLC analysis: retention time = 3.766 min; peak area = 100% (220 nm), 99.45% (254 nm).
6h (9′-methoxy-11′-phenylspiro[cyclopentane-1,3′-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin]-2′(5′H)-one)
Brown solid (81.5 mg, 85%); mp: 200–202 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 9.2 Hz, 1H), 7.60–7.53 (m, 3H), 7.49 (dd, J = 9.2, 2.8 Hz, 1H), 7.47–7.40 (m, 2H), 7.06 (d, J = 2.8 Hz, 1H), 4.67 (s, 2H), 3.75 (s, 3H), 2.73–2.66 (m, 2H), 2.37 (ddd, J = 12.0, 6.1, 3.1 Hz, 2H), 1.99–1.87 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 166.7, 158.1, 157.2, 146.8, 146.3, 132.6, 130.4, 129.6, 128.9, 128.4, 128.1, 124.1, 120.1, 119.8, 105.1, 59.5, 55.4, 49.9, 37.9, 37.8, 22.4(3), 22.4(1); HRMS (ESI) m/z calcd for C24H22N3O2 [M + H]+: 384.1712, found: 384.1701; HPLC analysis: retention time = 4.213 min; peak area = 100% (220 nm), 100% (254 nm).
6i (9′-methoxy-11′-phenylspiro[cyclohexane-1,3′-imidazo[1′,2′:1,5]pyrrolo[3,4-b]quinolin]-2′(5′H)-one)
Light yellow solid (83.5 mg, 84%); mp: 226–228 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 9.2 Hz, 1H), 7.58–7.51 (m, 3H), 7.49 (dd, J = 9.2, 2.8 Hz, 1H), 7.46–7.41 (m, 2H), 7.06 (d, J = 2.8 Hz, 1H), 4.69 (s, 2H), 3.74 (s, 3H), 2.54 (d, J = 12.3 Hz, 2H), 2.07 (td, J = 12.8, 3.9 Hz, 2H), 1.98–1.60 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 166.1, 158.0, 157.2, 146.7, 146.2, 132.7, 130.4, 129.6, 128.9, 128.5, 128.1, 124.1, 120.1, 118.7, 105.1, 56.6, 55.4, 49.1, 33.8, 24.5, 22.9; HRMS (ESI) m/z calcd for C25H24N3O2 [M + H]+: 398.1869, found: 398.1863; HPLC analysis: retention time = 4.549 min; peak area = 91.55% (220 nm), 97.62% (254 nm).
7a (2-(4-fluorophenyl)-2-(7-methoxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)acetamide)
Yellow solid (59.5 mg, 54%); mp: 189–191 °C; Rf = 0.3 (5% MeOH in DCM); 1H NMR (300 MHz, CDCl3): δ 8.03 (d, J = 9.2 Hz, 1H), 7.45 (ddd, J = 8.7, 5.1, 2.4 Hz, 8H), 7.11–7.05 (m, 2H), 7.01 (d, J = 2.8 Hz, 1H), 6.32 (s, 1H), 6.22 (s, 1H), 5.35 (s, 1H), 5.03 (d, J = 17.0 Hz, 1H), 4.19 (d, J = 17.0 Hz, 1H), 3.73 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 171.1, 166.6, 162.9 (d, JC–F = 247.5 Hz), 158.6, 157.9, 146.3, 146.1, 133.2, 131.0, 130.9, 130.5, 130.4 (d, JC–F = 3.75 Hz), 129.7, 129.5, 128.7, 128.1, 128.0 (d, JC–F = 7.5 Hz), 123.8, 116.0 (d, JC–F = 21 Hz), 105.0, 56.8, 55.4, 48.8; 19F NMR (376 MHz, CDCl3): δ −112.3; HRMS (ESI) m/z calcd for C26H21FN3O3 [M + H]+: 442.1567, found: 442.1558; HPLC analysis: retention time = 3.623 min; peak area = 96.77% (220 nm), 90.33% (254 nm).
7b (2-methyl-2-(1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)propenamide)
White solid (65.0 mg, 75%); mp: 228–230 °C; Rf = 0.5 (10% MeOH in DCM); 1H NMR (400 MHz, CDCl3): δ 8.17 (d, J = 8.3 Hz, 1H), 7.85–7.78 (m, 2H), 7.56–7.49 (m, 4H), 7.42 (dd, J = 7.0, 2.6 Hz, 2H), 6.32 (s, 1H), 5.44 (s, 1H), 4.72 (s, 2H), 1.73 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 175.9, 166.9, 160.2, 149.8, 147.9, 132.6, 131.2, 129.9, 129.1, 128.8, 127.9, 127.6, 127.3, 126.7, 120.3, 59.8, 49.5, 24.5; HRMS (ESI) m/z calcd for C21H20N3O2 [M + H]+: 346.1556, found: 346.1552; HPLC analysis: retention time = 3.262 min; peak area = 98.87% (220 nm), 99.22% (254 nm).
7c (2-(7-chloro-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-methyl propanamide)
Light yellow solid (67.5 mg, 71%); mp: 279–281 °C; Rf = 0.5 (10% MeOH in DCM); 1H NMR (400 MHz, CDCl3): δ 8.13–8.08 (m, 1H), 7.77–7.72 (m, 2H), 7.58–7.52 (m, 3H), 7.43–7.38 (m, 2H), 6.28 (s, 1H), 5.41 (s, 1H), 4.71 (s, 2H), 1.73 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 175.8, 166.5, 160.5, 148.2, 147.1, 132.9, 132.1, 131.9, 130.7, 129.8, 129.2, 128.2, 126.2, 121.0, 59.9, 49.5, 24.5; HRMS (ESI) m/z calcd for C21H19ClN3O2 [M + H]+: 380.1166, found: 380.1157; HPLC analysis: retention time = 3.657 min; peak area = 99.82% (220 nm), 99.36% (254 nm).
8a (N-(tert-butyl)-2-(4-fluorophenyl)-2-(7-hydroxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)acetamide)
Light yellow solid (100.0 mg, 82%); mp: 307–309 °C; Rf = 0.3 (50% EtOAc in hexane); 1H NMR (300 MHz, DMSO-d6): δ 10.04 (s, 1H), 8.09 (s, 1H), 7.98 (d, J = 9.1 Hz, 1H), 7.55 (dd, J = 6.8, 3.5 Hz, 3H), 7.39 (ddd, J = 10.7, 7.6, 4.7 Hz, 5H), 7.25 (dt, J = 8.8, 2.3 Hz, 2H), 6.94 (d, J = 2.6 Hz, 1H), 6.01 (s, 1H), 4.88 (d, J = 17.1 Hz, 1H), 4.00 (d, J = 17.1 Hz, 1H), 1.27 (s, 9H); 13C NMR (75 MHz, DMSO-d6): δ 168.3, 165.1, 161.7 (d, JC–F = 243 Hz), 157.8, 155.8, 144.6, 144.4, 133.2, 132.6 (d, JC–F = 3 Hz), 130.5, 130.4, 130.3, 129.8 (d, JC–F = 10.5 Hz), 128.2, 127.9, 127.7, 123.6, 119.2, 115.6 (d, JC–F = 21 Hz), 107.5, 56.6, 50.6, 48.5, 28.3; 19F NMR (376 MHz, DMSO-d6): δ −114.1; HRMS (ESI) m/z calcd for C29H27FN3O3 [M + H]+: 484.2036, found: 484.2035; HPLC analysis: retention time = 3.486 min; peak area = 99.06% (220 nm), 96.73% (254 nm).
8b (N-(tert-butyl)-2-(7-hydroxy-1-oxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-2-methylpropanamide)
Light yellow solid (72.0 mg, 69%); mp: 271–273 °C; Rf = 0.4 (50% EtOAc in hexane); 1H NMR (400 MHz, DMSO-d6): δ 10.05 (s, 1H), 8.02 (d, J = 9.1 Hz, 1H), 7.54–7.50 (m, 3H), 7.41 (dd, J = 9.1, 2.7 Hz, 1H), 7.36 (dd, J = 6.6, 3.0 Hz, 2H), 6.98 (d, J = 2.6 Hz, 1H), 6.88 (s, 1H), 4.68 (s, 2H), 1.44 (s, 6H), 1.20 (s, 9H); 13C NMR (100 MHz, DMSO-d6): δ 172.5, 164.8, 158.5, 155.8, 144.3, 143.7, 133.3, 130.4, 129.8, 128.3, 127.8, 127.7, 123.4, 120.6, 107.5, 79.2, 59.3, 50.2, 48.2, 28.5, 23.6; HRMS (ESI) m/z calcd for C25H28N3O3 [M + H]+: 418.2131, found: 418.2142; HPLC analysis: retention time = 3.197 min; peak area = 98.92% (220 nm), 95.16% (254 nm).
Biological studies
General
L. donovani (MHOM/IN/80/Dd8), a WHO reference strain, was received in 1981 as a kind gift from Professor P. C. C. Garnham of Imperial College, London. J774 murine macrophages were obtained from the National Centre for Cell Sciences (Pune, India). Human liver carcinoma (HepG2) cells were obtained from the lab of Dr. Ruth S. Nussenzweig, New York University, Grossman School of Medicine. All animal procedures were performed in accordance with the guidelines laid out by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The animal experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) (registration no. 34/GO/ReBiBt-S/Re-L/99/CPCSEA) of the Council for Scientific and Industrial Research – Central Drug Research Institute (Lucknow, India).
Maintenance of L. donovani parasites and cell culture
Host macrophage J774 cell line was cultured in RPMI medium fortified with 10% fetal bovine serum (Gibco), in addition to streptomycin (100 μg mL−1) and penicillin (100 U mL−1). Cells were maintained in a humidified 5% CO2 atmosphere at 37 °C. L. donovani promastigotes, belonging to strain MHOM/IN/80/Dd8 expressing the luciferase gene,41 were maintained in M199 medium fortified with 15% FBS (Seralab) and antibiotic G418 (20 μg mL−1) at 24 °C.
Promastigote viability assay
For determination of the antileishmanial activity of dihydropyrroloquinolinone derivatives, exponentially growing Leishmania promastigotes expressing firefly luciferase were seeded onto 96-well plates enriched with 10% FBS at a count of 1 × 106 parasites per well and subsequently treated with the test compounds at three concentrations, 25, 12.5, and 6.25 μM. Control parasites were left untreated and incubated with medium only. Parasite-infected cells were also treated with the standard drug miltefosine, used as the positive control. Cells treated with 0.1% DMSO were the vehicle control. Following 72 h compound treatment, luciferin-enriched Steady-Glo reagent (Promega) was added along with PBS at a 1 : 1 ratio and plates were subjected to mild shaking for 2–3 minutes in order to allow the lysis of the parasite suspension. The viability of parasites in response to compound treatment was then evaluated by measuring luciferase activity using a luminometer (Berthold). The reading obtained expressed parasite multiplication in terms of relative luminescence units (RLUs), which was used to calculate percentage inhibition and IC50 values of compounds via non-linear regression analysis using the four-parameter Hill equation.
Intracellular amastigote inhibition assay
Host cell line J774 macrophages were seeded onto complete RPMI medium-enriched 96-well plates and 16-well chamber slides overnight at a count of 4 × 104 cells per 100 μl per well. Following overnight incubation at a humidified 5% CO2 atmosphere and 37 °C, cells were replaced with fresh medium and further infected with stationary-phase L. donovani promastigotes expressing luciferase at a ratio of 1 : 10 (macrophage : parasite). After a further 24 h of incubation, the medium from each well was discarded to remove non-internalized parasites and infected macrophages were treated with two-fold, serially diluted compounds at a range of concentrations (50–3.125 μM) for 72 h. After completion of the 72 h treatment, the medium was aspirated from individual wells, and PBS and luciferin-containing Steady Glo reagent (15 mg ml−1) were added in a 1 : 1 ratio to individual wells. The cells were then lysed by gentle shaking for 2–3 minutes and assessment of luciferase activity was carried out in a luminometer. The amastigote inhibition values of test compounds were calculated with relative luminescence units (RLUs) obtained through luminescence measurement. IC50 values of compound 5m were calculated by nonlinear regression analysis of dose–response curves using four-parameter Hill equations. The reference drug miltefosine was taken as the positive control for this assay.
Cell cytotoxicity assay using MTT reagent
J774 macrophages were adhered onto 96-well plates at a count of 1 × 105 cells per 100 μl per well and cultured in complete RPMI medium overnight. The following day, medium from individual wells was discarded to remove non-adherent macrophages and cells were treated with two-fold, serially diluted compounds at a range of concentrations (200–3.12 μM) for 72 h at 37 °C, 5% CO2. After completion of the 72 h treatment, cells in each of the wells were treated with 20 μL of MTT (5 mg mL−1) and further incubated in the dark for 3 h at 37 °C, 5% CO2. Shortly after, the medium was aspirated and the resultant purple-coloured formazan crystals were solubilized by adding 125 μL DMSO to the wells. The development of formazan dye was compared between living (compound-untreated) and compound-treated cells by measuring the absorbance at 570 nm using a microplate spectrophotometer plate reader (Biotek Powerwave XS2).
Infection and treatment in Balb/c mice
Balb/c mice were divided into five groups, with 5 animals per group. Balb/c mice of all groups were infected intravenously with stationary phase L. donovani promastigotes for establishment of Leishmania infection. Autopsy of two to three randomly chosen mice was carried out two weeks post-infection in order to perform infection checking. Different groups of infected animals were treated with either vehicle (5% DMSO) or different doses of 5m (6.25, 12.5, 50 mg kg−1) for 7 consecutive days through the intraperitoneal route. Standard drug miltefosine was administered orally at a dose of 20 mg kg−1 for 7 consecutive days to the infected animals. Different groups of animals were sacrificed after one week post treatment and liver and spleen samples were obtained from Balb/c mice of all groups. Thereafter, impression smears were made on glass slides, fixed with methanol and then stained with 30% Giemsa stain for 2.5 h. Assessment of amastigote count was carried out following visualization of Giemsa-stained spleen and liver tissue smears. The antileishmanial efficacy of the compound is expressed in terms of percentage inhibition (PI)42 using the following formula:PI = 100 − [ANAT × 100/(INAT × TIUC)]where PI is the percent inhibition of amastigote multiplication, ANAT is the actual number of amastigotes in treated animals, INAT is the initial number of amastigotes in treated animals and TIUC is the times increase of parasites in untreated control animals.
Measurement of mitochondrial membrane potential (ΔΨm) loss
Measurement of MMP was carried out in 5m-treated, vehicle (0.1% DMSO)-treated and control (untreated) LDd8 promastigotes following staining with JC-1 dye (MitoProbe JC-1 Assay Kit, Molecular Probes, USA) as described earlier.42 Briefly, 1 × 106 exponentially growing LDd8 promastigotes were incubated with compound 5m (50 μM) for 12–48 h or vehicle (0.1% DMSO for 48 h). Cells were then harvested, washed and resuspended in 1× PBS. Further, JC-1 dye (2 μM final concentration) was added to this cell suspension and the samples were then incubated at 37 °C in 5% CO2 for 30 min. In parallel, CCCP-treated parasites were processed similarly and taken as the positive control. The fluorescence intensity of all the samples was then measured using a FACSCalibur cytometer (Becton Dickinson, USA) at 530 nm (green fluorescence) and 590 nm (red fluorescence) and data were analysed using Cell Quest Pro software.
Detection of cytochrome c in cytosolic and mitochondrial fractions
Control (untreated), vehicle-treated and 5m-treated LDd8 promastigotes were harvested and washed with 1× PBS. Cell pellets were resuspended in cell fractionation buffer derived from an ApoAlert cell fractionation kit (Takara, Japan) and homogenized. Following separation of the cytosolic and mitochondrial fractions in accordance with the manufacturer's instructions, 50 μg protein of each fraction was separated on 10% SDS-PAGE and immunoblotted with polyclonal cytochrome c antibody. The same blots were further reprobed with cytochrome c oxidase subunit IV (COX IV) antibody and actin for the assessment of relative abundance of loading controls in both mitochondrial and cytosolic fractions, respectively. HRP-conjugated secondary antibody was used and visualization of protein bands was done by chemiluminescence reaction.
Evaluation of DNA ladder formation
Genomic DNA from exponentially growing LDd8 promastigotes treated with 50 μM compound 5m or vehicle (0.1% DMSO) for 72 h was isolated following the manufacturer's instructions using an apoptotic DNA ladder kit (Roche). Samples were electrophoresed on 1.5% agarose gel at 75 V for 2 h. Bands were further visualized under UV-illumination following staining with ethidium bromide.
Cell cycle analysis
Flow cytometry analysis of DNA content was conducted to evaluate cell cycle perturbations in untreated, vehicle-treated or 5m-treated parasites as described previously43 with slight modifications. Briefly, after treatment, parasites were harvested, washed twice with 1× PBS and fixed in chilled methanol at 4 °C for 30 minutes. The fixed cells were washed again and treated with 500 μl of PBS containing 100 μg ml−1 RNase A and 1 μg ml−1 propidium iodide. Samples were incubated in the dark for 45 minutes and quantitation of apoptotic-like cells was evaluated as the percentage of cells in the sub-G0/G1 region in cell cycle analysis,40,44 using a fluorescence-activated cell sorting (FACSCalibur) flow cytometer equipped with a 488 nm Ar laser.
Evaluation of DNA ladder formation
Genomic DNA from log-phase growing LDd8 promastigotes treated or untreated with 50 μM compound 5m following 72 h of incubation was isolated according to the manufacturer's instructions using an apoptotic DNA ladder kit (Roche). Genomic DNA from untreated, 0.1% DMSO (vehicle)-treated and 5m-treated parasites were electrophoresed on 1.2% agarose gel at 75 V for 2 h. Bands were further visualized and photographed under UV illumination following staining with ethidium bromide.
Pharmacokinetic studies
UPLC conditions for in vitro PK study
On a Shimadzu UPLC system fitted with a photodiode array detector, compound 5m was analyzed. The mobile phase composition of acetonitrile : formic acid (0.1%) on a C18 column (100 × 4.6 mm, 5.0 μm) was used for chromatographic separation of the compound. The mobile phase ratio for 5m was optimized as 60 : 40 (v/v). The flow rate for elution was set as 0.8 mL min−1, while the complete run time for sample analysis was set for 10 min. The sample was analyzed at 254 nm wavelength.
In vitro SGF and SIF stability
USP specifications were followed for the preparation of SGF and SIF.45 Stability studies of compound 5m in SGF and SIF were performed at 10 μg mL−1 in duplicate. SGF was prepared in 100 mL of triple-distilled water by dissolving NaCl (234.0 mg) and KCl (37.2 mg) and the pH was adjusted to 1.2 with concentrated hydrochloric acid. In 100 mL triple-distilled water, SIF was prepared by dissolving 680.0 mg of KH2PO4 and 90.0 mg of NaOH and the pH was adjusted to 6.8 with orthophosphoric acid. SGF and SIF (990 μL each) were taken in two separate glass test tubes and pre-incubated for 10–15 min at 37 ± 2 °C in a shaking water bath. Afterward, an aliquot of compound 5m was spiked in the pre-incubated SGF and SIF and immediately subjected to incubation. 100 μL of the incubation mixture was withdrawn at 0, 5, 10, 15, 30, 45, 60, 90, and 120 min. Each sample was quenched with 200 μL of acetonitrile 1 : 2 (% v/v), vortexed, and centrifuged at 10 000 rpm. The supernatant was then analyzed by UPLC.
In vitro plasma stability
The plasma stability study of compound 5m was done at 10 μg mL−1 in triplicate. Blank mice plasma was taken in test tubes and pre-incubated in a shaking water bath for 10 min at 37 ± 0.2 °C. Afterward, an aliquot of compound 5m was spiked to the pre-incubated plasma and immediately subjected to incubation. Plasma samples were withdrawn at predetermined time points, i.e. 0, 5, 10, 15, 30, 45, 60, 90, and 120 min. The samples were precipitated by adding acetonitrile (200 μL) at a ratio of 1 : 2 (v/v) at each time interval, followed by vortexing and centrifugation at 10 000 rpm for 10 min. Supernatants were separated and subjected to UPLC analysis.
In vitro microsomal stability
The microsomal stability test of 5m was performed in mice liver microsomes (MLMs) as specified previously with slight modifications.46 Briefly, incubation buffer (Tris buffer (50 mM, pH 7.4) and MgCl2) was mixed with microsomal protein of mice (MLM) and incubated in a shaking water bath for 5 min at 37 ± 0.2 °C. Afterward, an aliquot of compound 5m was added from the stock solution to each glass tube. The reaction was initiated by the addition of NADPH. Each sample (50 μL) was collected at pre-defined time points, 0, 5, 15, 30, 45, 60, 90, and 120 min, and immediately quenched with ice-cold acetonitrile (100 μL) to stop the reaction. Samples were vortexed and centrifuged and the supernatant (20 μL) was directly analyzed by UPLC. Testosterone was used as a positive control. Experiments were performed in triplicate.
Data analysis
The percentage of compound remaining intact after incubation at different time intervals was calculated using eqn (1) while the in vitro microsomal half-life (t1/2) was determined by plotting the natural logarithm of percentage compound remaining versus time, and the slope of the curve was represented as depletion rate constant K (min−1). The half-life of metabolic stability was estimated using the first-order eqn (2). The in vitro intrinsic clearance (CLint) was calculated using eqn (3).
| % Drug remaining = (Concentration of drug after incubation)/(Concentration of drug before incubation) × 100 | 1 |
| Half-life (t1/2) = (0.693)/K | 2 |
| Intrinsic clearance CLint = K × (Volume of incubation (μL))/(Amount of protein incubated (mg)) | 3 |
where K represents the depletion rate constant and was calculated from the slope of linear regression from the natural log percentage of the substrate remaining versus incubation time.
Solubility studies and log P calculation
Solubility estimation
An excess quantity of compound 5m (∼10 mg) was added to glass vials containing 2.0 mL triple-distilled water and the vials were shaken in a water bath at 37 ± 2 °C for 24 h to attain equilibrium. After incubation, samples were filtered through a 0.22 μm filter and centrifuged at 13 000g for 20 min; the supernatant was collected, diluted with ACN at a 1 : 5 ratio and further centrifuged to remove precipitated salts. The concentration of 5m was analysed using UPLC against a standard calibration curve prepared in corresponding equal amounts of ACN and TDW. All assays were carried out in triplicate.47
Partition coefficient (log P) determination
The shake flask method was experimentally used for determination of log P of compound 5m with slight modification.32,48 Initially, filtered n-octanol and triple-distilled water were mixed with each other for saturation up to 24 h. Then both the phases were separated out. A weighed quantity of drug was mixed with the n-octanol-saturated water in a flask and shaken for 30 min; afterwards, n-octanol was added and the mixture was agitated on a swing bed for 24 h. The samples were allowed to stand for layering. Both water and n-octanol fractions were collected separately in different vials. Each sample was diluted appropriately with ACN and analysed by UPLC. The experiment was performed in triplicate. Log P was calculated by taking the logarithm of the ratio of the peak area of compound 5m in n-octanol to the corresponding peak area in TDW. Consequently, log P values were based on analysis of both water and octanol fractions. The equilibrium solubility of 5m in TDW was determined by the shake flask method and was found to be 156.75 ± 22.62 μg mL−1, while the experimental log P value for compound 5m was found to be 2.70 ± 0.19, which comply with Lipinski's rule for log P.37
Statistical analysis
Data analysis was performed using Student's t-test. Results were expressed as mean values with standard deviations. Graphical analysis and representation were done using GraphPad Prism version 7.0 and statistical significance was taken at p < 0.05.
Molecular docking studies
The crystal structure of LdTop1 (PDB ID 2B9S)38 was downloaded from the RCSB Protein Data Bank for performing docking. There are large protein subunits (27–456 residues) and small subunits (221–262) with a 22 bp DNA covalent complex in the crystal structure. The MOE Structure Preparation Module was used to model and fix the biomolecular structure 2B9S for any missing data followed by Protonate3D and water molecules were removed. In addition, we used the method defined by Roy et al. in which the 22 bp double-stranded DNA is replaced by the 22 bp double-stranded DNA present in the human Top1–DNA complex (PDB ID 1K4T) by fitting the two molecules on the atoms of the backbone.49 The replacement was performed because the presence of a cleaved site in the 22 bp double-stranded DNA in the 1K4T X-ray structure containing the topotecan molecule enables the compound 5m to investigate the ability to interact with the cleavable complex of LdTOP1LS DNA. The MOE energy minimization module with MMFF94x force field was used for whole system energy minimization to remove any clashes that may have occurred during substitution of the cleaved site in the 22 bp double-stranded DNA present in the 1K4T. Using the MOE docking protocol, 5 Å grids were generated around the topotecan ligand transferred during substitution. Docking experiments were carried out using the MOE software's docking module with the default parameters (in the Method option, placement is set to Triangle Matcher using the London dG score for 30 poses, whereas in the Refinement option, rigid receptor was used with the GBVI/WSA dG score followed by 5 poses). The docking scores and bonding/non-bonding interactions of selected compounds with LDTOP1 (PDB 2BPS) are shown in Table S3.†
Screening against PAINS
To evaluate a library of the synthesized compounds against PAINS, all tested compounds were screened against the PAINS filter using the online tool, https://www.cbligand.org/PAINS/.50 All the tested compounds passed the PAINS filter.
Author contributions
AS and AG contributed equally to this work. AG designed and synthesized all the compounds. AS, AR, MD and SK performed the biological studies. VD and SPS performed docking studies. SKS and MR performed pharmacokinetic studies. SK supervised biological studies and MW supervised pharmacokinetic studies. AKS conceptualized the work, designed the experiments and supervised the whole project. The manuscript was written through the contributions of all the authors. All the authors have given approval to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
Financial support from SERB, New Delhi (Grant No. CRG/2018/001897 [GAP0320], Grant No. CRG/2020/002932 [GAP0371]), and DBT, New Delhi (Grant No. BT/PR32490/MED/29/1457/2019) is acknowledged. A. G. thanks ICMR; A. S., V. D., S. P. S., A. R., and M. R. thank UGC; M. D. and S. K. S. thank CSIR for providing research fellowships. The transgenic L. donovani promastigotes were originally procured from Dr. Neena Goyal, Chief Scientist, Division of Biochemistry and Structural Biology, CSIR-CDRI, Lucknow, India. The authors acknowledge the Division of Sophisticated Analytical Instrument Facility and Research, CSIR-CDRI, for providing analytical support. This manuscript bears CDRI Communication Number 10400.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00078d
References
- Tamiru H. F. Mashalla Y. J. Mohammed R. Tshweneagae G. T. BMC Infect. Dis. 2019;19:1–10. doi: 10.1186/s12879-019-4506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.who.int/news-room/fact-sheets/detail/leishmaniasis, Accessed on August 14, 2021
- Thakur L. Singh K. K. Shanker V. Negi A. Jain A. Matlashewski G. Jain M. PLoS Neglected Trop. Dis. 2018;12:1–17. doi: 10.1371/journal.pntd.0006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F. Bilbe G. Blesson S. Goyal V. Monnerat S. Mowbray C. Ouattara G. M. Pécoul B. Rijal S. Rode J. Solomos A. Strub-Wourgaft N. Wasunna M. Wells S. Zijlstra E. E. Arana B. Alvar J. Clin. Microbiol. Rev. 2018;31:1–30. doi: 10.1128/CMR.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Independent Assessment of Kala-Azar Elimination Programme India, 2020
- Sundar S. Singh O. P. Chakravarty J. Expert Rev. Anti-infect. Ther. 2018;16:805–812. doi: 10.1080/14787210.2018.1532790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. Frasca K. Scherrer S. Henao-Martínez A. F. Newman S. Ramanan P. Suarez J. A. Curr. Trop. Med. Rep. 2021;8:121–132. doi: 10.1007/s40475-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Brand S. Thomas M. Rycker M. D. Chung C.-W. Pena I. Bingham R. P. Bueren-Calabuig J. A. Cantizani J. Cebrian D. Craggs P. D. Ferguson L. Goswami P. Hobrath J. Howe J. Jeacock L. Ko E.-J. Korczynska J. MacLean L. Manthri S. Martinez M. S. Mata-Cantero L. Moniz S. Nühs A. Osuna-Cabello M. Pinto E. Riley J. Robinson S. Rowland P. Simeons F. R. C. Shishikura Y. Spinks D. Stojanovski L. Thomas J. Thompson S. Gaza E. V. Wall R. J. Zuccotto F. Horn D. Ferguson M. A. J. Fairlamb A. H. Fiandor J. M. Martin J. Gray D. W. Miles T. J. Gilbert I. H. Read K. D. Marco M. Wyatt P. G. Proc. Natl. Acad. Sci. U. S. A. 2019;116:9318–9323. doi: 10.1073/pnas.1820175116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Thomas M. Patterson S. Crouch S. Rycker M. D. Lowe R. Gresham S. Urbaniak M. D. Otto T. D. Stojanovski L. Simeons F. R. C. Manthri S. MacLean L. M. Zuccotto F. Homeyer N. Pflaumer H. Boesche M. Sastry L. Connolly P. Albrecht S. Berriman M. Drewes G. Gray D. W. Ghidelli-Disse S. Dixon S. Fiandor J. M. Wyatt P. G. Ferguson M. A. J. Fairlamb A. H. Miles T. J. Read K. D. Gilbert I. H. Nature. 2018;560:192–197. doi: 10.1038/s41586-018-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S. Nagle A. S. Biggart A. Lai Y. H. Liang F. Davis L. C. Barnes S. W. Mathison C. J. N. Myburgh E. Gao M.-Y. Gillespie J. R. Liu X. Tan J. L. Stinson M. Rivera I. C. Ballard J. Yeh V. Groessl T. Federe G. Koh H. X. Y. Venable J. D. Bursulaya B. Shapiro M. Mishra P. K. Spraggon G. Brock A. Mottram J. C. Buckner F. S. Rao S. P. S. Wen B. G. Walker J. R. Tuntland T. Molteni V. Glynne R. J. Supek F. Nature. 2016;537:229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R. P. Shapiro T. A. Mini-Rev. Med. Chem. 2003;3:597–608. doi: 10.2174/1389557033487863. [DOI] [PubMed] [Google Scholar]
- Nitiss J. L. Biochim. Biophys. Acta. 1998;1400:63–81. doi: 10.1016/S0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Pommier Y. Pourquier P. Fan Y. Strumberg D. Biochim. Biophys. Acta. 1998;1400:83–105. doi: 10.1016/S0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P. Majumder H. K. Bhattacharya S. J. Med. Chem. 2007;50:2536–2540. doi: 10.1021/jm0610604. [DOI] [PubMed] [Google Scholar]
- Mamidala R. Majumdar P. Jha K. K. Bathula C. Agarwal R. Chary M. T. Majumder H. K. Munshi P. Sen S. Sci. Rep. 2016;6:1–12. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. R. Godinho J. L. P. Vinayagam J. Zuma A. A. Silva S. T. D. M. Jaisankar P. Rodrigues J. C. F. Souza W. D. Majumder H. K. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-30405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. R. Kumar A. Godinho J. L. P. Silva S. T. D. M. Zuma A. A. Saha S. Kumari N. Rodrigues J. C. F. Sundar S. Dujardin J.-C. Roy S. Souza W. D. Mukhopadhyay S. Majumder H. K. Biochem. Pharmacol. 2017;138:19–30. doi: 10.1016/j.bcp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Das B. B. Sen N. Ganguly A. Majumder H. K. FEBS Lett. 2004;565:81–88. doi: 10.1016/j.febslet.2004.03.078. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Bodley A. L. Shapiro T. A. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3726–3730. doi: 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. Nat. Rev. Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- Lavergne O. Lesueur-Ginot L. Rodas F. P. Kasprzyk P. G. Pommier J. Demarquay D. Prévost G. Ulibarri G. Rolland A. Schiano-Liberatore A.-M. Harnett J. Pons D. Camara J. Bigg D. C. H. J. Med. Chem. 1998;41:5410–5419. doi: 10.1021/jm980400l. [DOI] [PubMed] [Google Scholar]
- Pommier Y. Cushman M. Mol. Cancer Ther. 2009;8:1008–1014. doi: 10.1158/1535-7163.MCT-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K. A. Loughlin W. A. Young D. J. Mini-Rev. Med. Chem. 2013;13:730–743. doi: 10.2174/1389557511313050010. [DOI] [PubMed] [Google Scholar]
- Loiseau P. M. Cojean S. Schrével J. Parasite. 2011;18:115–119. doi: 10.1051/parasite/2011182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha T. K. Sundar S. Thakur C. P. Felton J. M. Sabin A. J. Horton J. Am. J. Trop. Med. Hyg. 2005;73:1005–1011. doi: 10.4269/ajtmh.2005.73.1005. [DOI] [PubMed] [Google Scholar]
- Liu H. Nolan L. Acta Hortic. 1996;426:221–226. doi: 10.17660/ActaHortic.1996.426.26. [DOI] [Google Scholar]
- Ghoshal A. Yadav A. Srivastava A. K. J. Org. Chem. 2020;85:14890–14904. doi: 10.1021/acs.joc.0c01539. [DOI] [PubMed] [Google Scholar]
- Singh S. P. Tripathi S. Yadav A. Kant R. Srivastava H. K. Srivastava A. K. Chem. Commun. 2020;56:12789–12792. doi: 10.1039/D0CC04415F. [DOI] [PubMed] [Google Scholar]
- Tripathi S. Ambule M. D. Srivastava A. K. J. Org. Chem. 2020;85:6910–6923. doi: 10.1021/acs.joc.0c00108. [DOI] [PubMed] [Google Scholar]
- Ghoshal A. Ambule M. D. Sravanthi R. Taneja M. Srivastava A. K. New J. Chem. 2019;43:14459–14474. doi: 10.1039/C9NJ03533H. [DOI] [Google Scholar]
- Ghoshal A. Kumar A. Yugandhar D. Sona C. Kuriakose S. Nagesh K. Rashid M. Singh S. K. Wahajuddin M. Yadav P. N. Srivastava A. K. Eur. J. Med. Chem. 2018;152:148–159. doi: 10.1016/j.ejmech.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Ramanivas T. Parameshwar M. Gayatri G. Nanubolu J. B. Srivastava A. K. Eur. J. Org. Chem. 2017;2017:2245–2257. doi: 10.1002/ejoc.201700031. [DOI] [Google Scholar]
- Yugandhar D. Kuriakose S. Nanubolu J. B. Srivastava A. K. Org. Lett. 2016;18:1040–1043. doi: 10.1021/acs.orglett.6b00164. [DOI] [PubMed] [Google Scholar]
- Yugandhar D. Srivastava A. K. ACS Comb. Sci. 2015;17:474–481. doi: 10.1021/acscombsci.5b00065. [DOI] [PubMed] [Google Scholar]
- Ghoshal A. Yugandhar D. Nanubolu J. B. Srivastava A. K. ACS Comb. Sci. 2017;19:600–608. doi: 10.1021/acscombsci.7b00095. [DOI] [PubMed] [Google Scholar]
- Rothwell J. A. Day A. J. Morgan M. R. J. Agric. Food Chem. 2005;53:4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- Davies D. R. Mushtaq A. Interthal H. Champoux J. J. Hol W. G. J. J. Mol. Biol. 2006;357:1202–1210. doi: 10.1016/j.jmb.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Zangger H. Mottram J. C. Fasel N. Cell Death Differ. 2002;9:1126–1139. doi: 10.1038/sj.cdd.4401071. [DOI] [PubMed] [Google Scholar]
- Sen R. Bandyopadhyay S. Dutta A. Mandal G. Ganguly S. Saha P. Chatterjee M. J. Med. Microbiol. 2007;56:1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- Ashutosh Gupta S. Ramesh Sundar S. Goyal N. Antimicrob. Agents Chemother. 2005;49:3776–3783. doi: 10.1128/AAC.49.9.3776-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay A. Chandrakar P. Gupta S. Parmar N. Singh S. K. Rashid M. Kushwaha P. Wahajuddin M. Sashidhara K. V. Kar S. J. Med. Chem. 2019;62:5655–5671. doi: 10.1021/acs.jmedchem.9b00628. [DOI] [PubMed] [Google Scholar]
- Antwi C. A. Amisigo C. M. Adjimani J. P. Gwira T. M. PLoS Neglected Trop. Dis. 2019;13:e0007206. doi: 10.1371/journal.pntd.0007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J. Singh N. Singh B. Dube A. Tripathi R. Singh P. Singh N. Exp. Parasitol. 2010;125:310–314. doi: 10.1016/j.exppara.2010.02.011. [DOI] [PubMed] [Google Scholar]
- XXIV U. P., The United States of Pharmacopoeia Convention, Rockville, Maryland, 2000, p. 185 [Google Scholar]
- Chandrakar P. Gunaganti N. Parmar N. Kumar A. Singh S. K. Rashid M. Wahajuddin M. Mitra K. Narender T. Kar S. Eur. J. Med. Chem. 2019;182:1–14. doi: 10.1016/j.ejmech.2019.111632. [DOI] [PubMed] [Google Scholar]
- Singh S. K. Rashid M. Bhalala K. Malik Y. Chaturvedi S. Raju K. S. R. Sultana N. Mitra K. Gayen J. R. Wahajuddin M. J. Drug Delivery Sci. Technol. 2021;61:102254. doi: 10.1016/j.jddst.2020.102254. [DOI] [Google Scholar]
- Paschke A. Neitzel P. L. Walther W. Schüürmann G. J. Chem. Eng. Data. 2004;49:1639–1642. doi: 10.1021/je049947x. [DOI] [Google Scholar]
- Roy A. Chowdhury S. Sengupta S. Mandal M. Jaisankar P. D'Annessa I. Desideri A. Majumder H. K. PLoS One. 2011;6:e28493. doi: 10.1371/journal.pone.0028493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J. B. Holloway G. A. J. Med. Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.