Abstract
Mitogen-activated protein kinases (MAPKs) govern various cellular programs and crucial intermediate pathways in signaling. Microtubule affinity-regulating kinase 4 (MARK4) is a part of the kinase family recognized for actively phosphorylating neural microtubule-associated proteins (MAPs) like MAP2, MAP4 and most importantly, tau. The Ser/Thr kinase MARK4 overexpression is associated with various life-threatening conditions such as neurodegenerative disorders, diabetic neuropathy, and cancer. Functionally, MARK4 is correlated with many important signaling cascades and transcription factors contributing to neurodegeneration and cancer onset and progression. Serotonin is a key molecule associated with regulating mood, stress, and various behavioral aspects. Low serotonin levels promote the progression of neurological and psychotic disorders, which is also a consequence of tau accumulation. MARK4 being a major contributor to phosphorylating tau, leading to its accumulation, and contributing to tauopathy, is targeted for inhibition by serotonin. The study deals with the inhibition of MARK4 by serotonin using combined computational and experimental studies. The results presented in this paper provide strong evidence for the direct physical binding of serotonin to recombinant MARK4 and subsequent inhibition of its kinase activity. In addition, we have performed molecular docking, followed by 100 ns MD simulations of MARK4 in the presence of serotonin, to estimate the stability of the protein–ligand complex. Since MARK4 is a potential drug target and can be exploited for drug design and discovery for cancer and neurodegenerative disorders, the results presented here are of interest and may be further exploited for Alzheimer's disease (AD) and other neurodegenerative diseases.
Mitogen-activated protein kinases (MAPKs) govern various cellular programs and crucial intermediate pathways in signaling.
Introduction
Alzheimer's disease (AD), late-life depression, anxiety, and mood disorders such as OCD (obsessive–compulsive disorder) are the current common disorders of public health. AD is characterized by an irreversible degradation of neurons, sluggishly spreading over the brain, leading to significant complications such as memory loss, a decline in cognitive abilities, and dementia. The major hallmarks of the disease are the formation of neurofibrillary tangles (NFTs) made up of hyperphosphorylated tau and the formation of amyloid plaques and insoluble β-amyloid (Aβ). Aβ peptides are generated from APP's amyloidogenic degradation by the action of β and γ-secretases. The underlying cause of the disease is largely unknown, but many factors are known to contribute to the disease. Many signaling pathways such as MAPK and related kinases are known to aid the progression of the disease by phosphorylating specific serine residues of tau.1,2 Similarly, imbalances in the molecules responsible for neurotransmission also play a significant role in disease onset and progression. One such neurotransmitter is serotonin (5-hydroxytryptamine; 5-HT), which modulates the signal transduction in the CNS.3
The neurotransmitter functions as a major link between neuronal signaling and immune response. It makes up well-established neuro-immune networking for communication in the body. l-Tryptophan, an essential amino acid, is the precursor for serotonin. It is synthesized in two steps, aided by multiple enzymes such as monoamine decarboxylase and 5-hydroxytryptophan decarboxylase (TPH).4 TPH exists in two isoforms, TPH1 and 2. Expression of both the isoforms is varied, as one is specific to the CNS while the other is present in the periphery and not restricted only to the brain. The other sources of serotonin are mast cells and the brain.5 The brain-generated serotonin performs various neurological functions, such as regulating mood, appetite, sleep and libido, by using serotonergic cells.6 The peripheral serotonin also plays major roles such as regulation of angiogenesis,7 bone density management8 and modulation of other pathways.9 The role of serotonin is diverse; it acts as a growth factor, governing the cell cycle10 and inflammation. Serotonin secreted in the blood is taken up by the platelets and mast cells by the action of serotonin transporter (SERT) molecules. Intracellular serotonin in the blood has a standard range of 0.7–2.5 μM. However, at higher densities, it is stored in granules.11 After the function of serotonin is fulfilled, it is degraded with the aid of the enzyme monoamine oxidase, which produces melatonin and other by-products.12
Several studies found an association between measures related to serotonin and mood in the normal range. Lower platelet serotonin2 receptor function was associated with a lower mood in one study,13 whereas a better mood was associated with higher blood serotonin levels in another.14 Serotonin is stored in small vesicles within the nerve terminal. When the serotonergic neuron is excited, the molecule is released in the synaptic cleft and binds to serotonin receptors located on the post synaptic neuron. The action of serotonin is terminated by its removal from the synaptic space via proteins known as serotonin-transporters. Low serotonin levels are associated with various conditions, including depression, anxiety, and adverse effects, and neurodegenerative disorders such as AD.15 Current treatments of the disease aim to increase the level of serotonin and are termed as “selective serotonin reuptake inhibitors” (SSRIs). Serotonin in the brain also interacts with many other signalling pathways modulating various important functions. The role of serotonin in AD is still not very clear.16
Protein kinases are major regulators in signaling pathways; their altered expression is associated with various ailments such as cancer, neurodegenerative disorders and several other metabolic disorders.17 As they are involved in many major pathways, kinases are targeted for inhibition by various therapeutic molecules.18 MARK4 belongs to the Ser/Thr family of kinases and is targeted for inhibition by various drugs and other molecules.1,19,20 MARKs came into recognition due to their potential for phosphorylate specific serine residues of tau protein.21 Overexpression of the protein is associated with cancer onset, progression and metastasis.22 MARK4 has crucial roles in the proliferation of breast cancer via hippo signaling and regulating miR-515-5p involved in breast cancer onset and metastasis.23 MARK4 is an essential target in many signaling pathways and for inhibition to cure cancer and other neurodegenerative diseases.24 Phosphorylated tau has many consequences, such as neurofibrillary deposits and the formation of amyloid precursor protein (APP). Further, transfection of retinal ganglion cells with tau inhibits mitochondria's axonal transport leading to axon starvation and being highly vulnerable to stress. Tau deposition is further linked with stress-related psychotic conditions such as obsessive–compulsive disorder.25,26
Serotonin is an essential molecule in modulating various signaling pathways leading to blockage of AD progressions and psychotic conditions. Low levels of serotonin associated with AD and other neurological and behavioral syndromes are modulated and targeted to increase by various mechanisms.27 In the current study, we have targeted MARK4, the tauopathy promoter for inhibition by serotonin. The present study evaluates the binding of serotonin with MARK4 employing molecular docking, fluorescence quenching and isothermal titration calorimetric studies. An MD simulation approach was used to understand the serotonin's binding and inhibition mechanism and to delineate the dynamics of the MARK4–serotonin complex. Additionally, kinase assay revealed significant inhibition of MARK4 by serotonin.
Materials and methods
Materials
Serotonin (cat number H9523) was purchased from Sigma Aldrich (USA). BIOMOL® Green was obtained from Enzo Life Sciences, Inc. Ni-NTA resin was purchased from Qiagen. All the buffers were prepared with double distilled water and filtered before use.
Molecular docking
The crystal structure of MARK4 was downloaded from the Protein Data Bank (PDB ID 5ES1).28 It is a decent high-resolution structure (2.80 Å) with no mutations and missing residues. It was then ported to Molecular Operating Environment MOE2020 (ref. 29) for structure preparation and optimization of hydrogen bonds. The X-ray crystal structure was stripped of all non-protein atoms and then prepared at physiological pH. The Protonate3D30 tool was utilized to add hydrogen atoms, and all tautomeric states were analyzed to optimize the hydrogen bonds. Histidine tautomers were checked for the correct protonation states, and Asn and Gln residues were also manually checked, and flips were done if needed. The resulting structure was then used as a setup for the docking experiment in MOE. The ligand molecule (serotonin) was downloaded from PubChem in mol2 format. The ligand is shown in Fig. S1, and Table S1† depicts the critical descriptors of serotonin obtained from the MOE platform. The induced fit for the receptor was chosen in the setting tab to allow protein flexibility, and the conventional ATP binding site was used as the site centroid for docking. The top pose was cross-checked with our in-house high-performing InstaDock31 tool, and the RMSDs were <1 Å showing a great level of accuracy of these programs.
System preparation prior to the MD setup
The top docked pose was considered for initializing the MD simulations. To obtain the parameters of the ligand serotonin, we performed the geometry optimization of the fragment using the B3LYP/6-31G(d) method in the Gaussian 16 program.32 We calculated the electrostatic potential (ESP) charges using the Merz–Kollman radii.33 The ligand was then parametrized using an antechamber in AmberTools, and ligand frcmod and library files were generated. In tleap, the ff19SB forcefield34 was used for protein atom parameterization and the gaff2 (ref. 35) forcefield for the ligand atoms. The complex was then solvated in a 12 Å box of TIP3P36 water. Sodium and chloride ions were added to maintain the electroneutrality of the system. This entire protocol was done using tleap in AmberTools2020.37
MD simulation
The complex was then subjected to MD simulations using the GPU accelerated AMBER20.38 SHAKE39 was turned on, and a time step of 2 fs was used. Our previous publications inspired all the minimization, heating, equilibration stages, and final production.46 The exact same MD protocol was followed in simulating apo-MARK4 kinase without any ligands present. This serves as our control simulation.
Fluorescence binding assay
To validate in silico observations, we performed a fluorescence binding assay that reveals the actual binding of the ligand to the protein. Fluorescence spectroscopy is used to find the binding parameters for a protein–ligand system as per previously published reports.40 MARK4 was excited at 280 nm with emission recorded in the range of 300–400 nm; the response was set to medium with the excitation and emission slit width set at 10 nm. Fluorescence quenching data were fitted in the modified Stern–Volmer equation and analyzed as earlier studies.19,41
Isothermal titration calorimetry
ITC is amongst the most sensitive techniques used to study protein–ligand interactions.42,43 ITC was performed on a VP-ITC microcalorimeter (GE, MicroCal, USA) at 25 °C. MARK4 was degassed before it was loaded into the main sample cell. Degassing is carried out to remove bubbles that might interfere with the binding process. The syringe was filled with 200 μM serotonin with its speed set at 307 rpm. A programmed titration was set up in which the first injection was a false one with a volume of 2 μL followed by 10 μL injections of serotonin into MARK4, spacing set at 280 s. The final figure was plotted with the aid of Origin 8.0 software attached to the instrument.
Kinase inhibition assay
This assay is a malachite green reagent-based microtiter plate assay that checks the ATPase activity of the enzyme in the absence and presence of varying ligand concentrations. MARK4 (2 μM) was incubated with varying serotonin concentrations (0–15 μM) for 1 hour at 25 °C. ATP (200 μM) was freshly prepared, and MgCl2 (10 mM) was added to the reaction mixture that was incubated for 30 min at 25 °C. The reaction was terminated with the BIOMOL® reagent, and the green color that was obtained upon the addition of the BIOMOL® reagent was read at 620 nm on an ELISA reader.
Results and discussion
Molecular docking
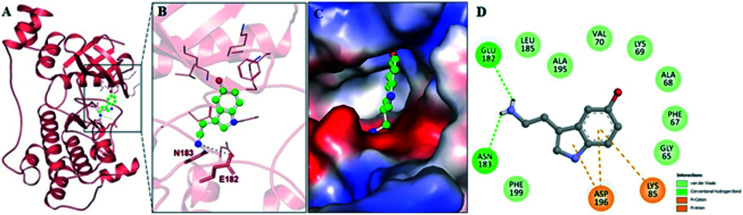
Molecular docking of serotonin with MARK4 was carried out to delineate different interactions governing the MARK4–serotonin complex formation. We investigated the selected docked pose of MARK4–serotonin using PyMOL and Discovery Studio Visualizer. Fig. 1A shows serotonin in the binding pocket of MARK4 with hydrogen bonds shown by the black dotted lines. Moreover, Discovery Studio Visualizer further sheds light on the type of interactions along with the participating residues. Fig. 1B shows the binding of serotonin to MARK4, where critical interaction residues are labeled. Fig. 1C shows the electrostatic potential surface of the serotonin in the active pocket of MARK4. The critical residues of the binding pocket of MARK4 are found to interact with serotonin, highlighting the importance of this binding (Fig. 1D).
Fig. 1. (A) Serotonin in the binding pocket of MARK4. (B) Magnified view of the MARK4–serotonin interaction drawn in PyMoL using the top docked pose obtained through InstaDock. (C) Charged surface view of serotonin occupying the binding pocket of MARK4. (D) 2D diagram of serotonin binding to MARK4 with critical interacting residues labeled.
Structural changes and analyses post MD
The binding of small-molecule ligands to proteins can cause significant conformational fluctuations, and MD simulations are vital to probe these phenomena atomistically.
Root mean square deviation (RMSD) is a conventional parameter for investigating residue-based fluctuations in proteins. We calculated the RMSD of the apoprotein and serotonin bound protein to compare these dynamics. The results are shown in Fig. 2A. The RMSD of serotonin was also computed to analyse its movement during the 100 ns production run (Fig. 2B). The RMSD plots reveal the significant stability of the protein backbone during the simulation, and an average RMSD of <2 Å from the initial solvated complex. This reinforces that serotonin binding to MARK4 slightly stabilizes the protein as evident from the red RMSD trajectory of the bound protein compared to the apo form. The radius of gyration (Rg) is an important marker for detecting compactness in a protein tertiary structure; it is the RMS distance of the set of atoms from their collective center of mass. The radius of gyration (Rg) of the apo and bound MARK4 kinase is depicted in Fig. 3A.
Fig. 2. (A) RMSD plots of backbone atoms (red is serotonin bound protein; black is apo protein). (B) RMSD plot of the ligand during the 100 ns run.
Fig. 3. (A) Radius of gyration plotted during the 100 ns MD run of apo (black) and serotonin bound MARK4 (red). (B) SASA plots of the protein backbone atoms as a function of the number of frames in the production runs.
The solvent accessible surface area (SASA) is directly related to the stability of proteins, and it has been used for exploring various conformational microstates in biomolecules. The SASA relates to the surface area of the protein that is exposed to solvent molecules, and it is utilized routinely to analyze artefacts in MD simulations of proteins. The SASA should be relatively stable and should not have huge deviations during the run (Fig. 3B). The SASA plots of the apo and bound MARK4 are almost similar, proving the stability of the MD simulations.
The intramolecular hydrogen bonds were also monitored to evaluate the structural stability of the protein. The hydrogen bonds (backbone) formed during serotonin binding and apoprotein do not show huge deviations, and they appear to be consistent (Fig. 4A). The ligand hydrogen bonds were also calculated during the simulation, and there are 6–7 main hydrogen bonding interactions that appear consistently throughout the 100 ns run. Asp-147, Asn-134, Glu-133, and Glu-90 are the main hydrogen bond acceptors with donor hydrogens from serotonin. The hydrogen bonds between serotonin and MARK4 are plotted in Fig. 4B. The key hydrogen bonding residues interacting with serotonin are ASP-147, ASN-134, GLU-133, GLU-90 and ILE-13.
Fig. 4. (A) Backbone hydrogen bonds (both apo and bound Mark4) plotted as a function of the number of snapshots. (B) Intermolecular hydrogen bonds monitored between the protein and serotonin.
Free energy analyses
For computational chemists worldwide, the calculation of the binding affinity of a molecule to a target protein is of common interest. Using MD simulations, we can focus on ensemble-based methods essential to obtain statistically significant estimates of free energies. The free energy of binding is very challenging to compute computationally. For this study, the molecular mechanics-generalized Born surface area (MMGBSA) technique and linear interaction energy (LIE) methods were employed. The MMGBSA/PBSA methods have evolved in the last 3 decades to minimize the computational time needed to perform these exhaustive calculations albeit with few approximations. The LIE method is also quite accurate in estimating the net electrostatics and van der Waals interaction of a ligand binding to the protein, scaled using adjustable parameters α and β.
The electrostatic and van der Waals interaction energies were computed for the apoprotein and the serotonin-bound forms. The net differences in electrostatic and van der Waals components (serotonin bound-Apo form) are plotted in Fig. 5. The net ΔGbinding was computed as −19.6 kcal mol−1. We used AmberTools to post-process the trajectory to calculate the MMGBSA and PBSA energy estimates. We needed to provide the solvated serotonin-bound-MARK4 complex, dry complex (no water), protein, and ligand topology files to the MMPBSA.py script and the 100 ns trajectory data. It took approximately 12 hours to compute the energies using 12 cores. The data are shown in Table 1. The MMGBSA overestimates the binding affinity value as compared to the LIE and Poisson–Boltzmann estimates. The free energy values estimated are −19.6 kcal mol−1 (LIE), −32.81 kcal mol−1 (MMGBSA) and −15.78 kcal mol−1 (MMPBSA). The MMPBSA method is the most computationally exhaustive and rigorous as it compares quite well with the LIE estimate.
Fig. 5. LIE energies are plotted as a function of the number of snapshots.
MMGBSA free energy estimate for serotonin binding.
| Energy factors | Average | Standard deviation | Std error of mean |
|---|---|---|---|
| VDWAALS | −28.3725 | 3.65 | 0.10 |
| EEL | −73.55 | 28.24 | 0.81 |
| EGB | 72.95 | 23.61 | 0.68 |
| ESURF | −3.82 | 0.21 | 0.006 |
| ΔGgas | −101.9 | 27.60 | 0.80 |
| ΔGsolv | 69.12 | 23.60 | 0.68 |
| ΔG (total) | −32.80 | 6.05 | 0.17 |
Fluorescence binding
Our in silico results confirmed that serotonin binds into the active pocket of MARK4, forming a stable complex. The next aim was to check the actual binding of serotonin to MARK4 with the aid of fluorescence spectroscopy. Fluorescence spectroscopy is usually deployed to ascertain the actual binding affinity of a ligand to the protein. It gives an estimate of the strength of the binding by giving an estimate of the different binding parameters of the complex. Fig. 6A shows the fluorescence emission spectra of the native MARK4 in the absence and presence of varying serotonin concentrations (0–1.5 μM). A decrease in the fluorescence intensity of MARK4 is evident with a corresponding increase in the serotonin concentration, referred to as fluorescence quenching. We fitted this quenching data into the modified Stern–Volmer equation to estimate the binding constant (K) and the number of binding sites (n) (Fig. 6B) of the complex giving an overview of the strength of binding between the ligand and protein. Serotonin binds to MARK4 with a good affinity; the binding constant was 0.3 × 106 M−1. The binding constant obtained is comparable to other reported MARK4 inhibitors,40,44,45 implicating serotonin as a potent MARK4 inhibitor adding up to the existing repertoire of kinase inhibitors that play a vital role in the disease therapeutics. In present times, the discovery of kinase inhibitors is a domain attracting researchers across the globe because kinases have critical roles in cancer and neurodegenerative disease progression.
Fig. 6. (A) Fluorescence emission spectra of MARK4 in the absence and presence of varying serotonin concentrations (0–1.5 μM). (B) Modified Stern–Volmer plot of the MARK4–serotonin complex.
Isothermal titration calorimetry
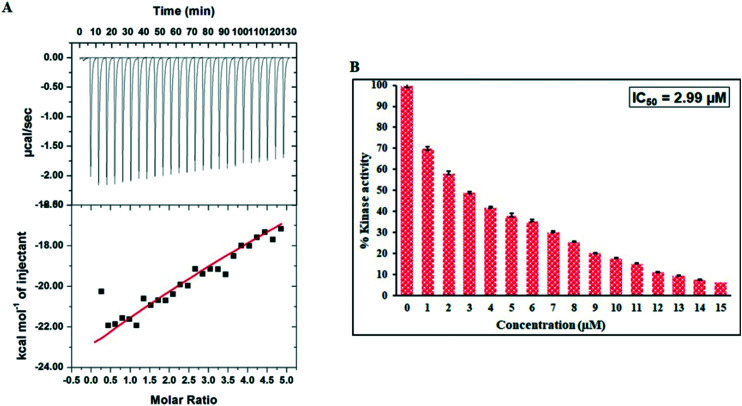
Once the actual binding is confirmed from the fluorescence binding assay, ITC was performed to delineate the thermodynamic parameters of the MARK4–serotonin complex. Fig. 7A shows a characteristic ITC isotherm obtained when serotonin was titrated with MARK4. In the upper panel, the raw data with negative heat pulses indicate exothermic binding. Once the dilution heats of both the compound and the protein are subtracted, the binding curves are obtained as shown in the lower panel. This reveals the degree of heat produced corresponding to each injection as a quantity of the molar ratio of serotonin to that of MARK4. We obtained the fitting from one site model, and the thermodynamic parameters obtained for the MARK4–serotonin complex are presented in Table 2. The ITC results further corroborate our fluorescence and in silico observations and affirm serotonin's strong binding to MARK4.
Fig. 7. (A) ITC measurements for the titration of serotonin with MARK4 at 25 °C. (B) Kinase assay of MARK4 with varying serotonin concentrations (0–15 μM).
Thermodynamic parameters of the MARK4–serotonin complex obtained from ITC.
| K a (association constant) M−1 | ΔH (enthalpy change) cal mol−1 | ΔS (cal mol−1 deg−1) |
|---|---|---|
| K a1 = 148 ± 1.71 × 104 | ΔH1 = −8.82 × 104 ± 1.53 × 106 | ΔS1 = −286 |
Kinase assay
All the above-mentioned assays confirmed the strong binding of serotonin with MARK4. The next aim was to assess the effect of serotonin binding on the kinase activity of MARK4 using a kinase assay. Kinase assay was carried out in the absence and presence of varying serotonin concentrations (0–15 μM). The kinase activity of MARK4 alone was taken as 100% for reference (Fig. 7B). It was found that when the concentration of serotonin was increased gradually, there was a corresponding decrease in the activity of MARK4. Thus, it can be concluded that serotonin binds to the active pocket of MARK4 and inhibits its kinase activity, thereby inhibiting the function of MARK4. This inhibition is implicated in various diseases, namely a variety of cancers and Alzheimer's disease, wherein MARK4 is overexpressed. Thus, it is concluded from the enzyme inhibition assay that serotonin binds to MARK4, inhibiting its kinase activity. IC50 is an operational parameter that can be defined as the concentration of the inhibitor (ligand) required for attaining 50% inhibition (binding saturation) of the enzyme (receptor). IC50 was found to be 2.99 μM which is much higher than those of the other reported MARK4 inhibitors,40,45 implicating serotonin's potency as a MARK4 inhibitor.
Conclusion
Studies focusing on kinase inhibitors are of interest in present times due to immense importance of these kinases in various pathways. Overexpression of MARK4 is implicated in various cancer types and neurodegenerative diseases, namely AD. Studies are being carried out to find potent inhibitors of MARK4 that can aid in disease therapeutics. The present study establishes serotonin, a neurotransmitter, as an inhibitor of MARK4. We have performed fluorescence binding study, isothermal titration calorimetry, molecular docking and MD simulation studies for estimating the binding affinity and inhibiting potential of serotonin for MARK4. MD simulation studies suggested that serotonin forms a stable complex with MARK4 without inducing any significant conformational changes in the protein structure. This work opens a new domain for the inhibition of MARK4 by a neurotransmitter. This study opens new avenues for medicinal chemists working in the field of cancer and AD therapeutics by establishing serotonin as a potent MARK4 inhibitor. Thus, targeting MARK4 with serotonin may serve as a breakthrough in managing the clinical manifestations of neurodegenerative and other MARK4 directed diseases.
Data availability statement
All the data have been provided in the manuscript.
Author contributions
All the authors have read and agreed to publish the current version of the manuscript. Conceptualization: AS, DDG, DKY and MIH; data curation: AS, DDG, FAA, MSK, WAA; formal analysis, AS, DDG, DKY, SAA, FAA; funding acquisition: DDG and MIH; investigation, MSK, AS, DDG, DKY and MSK; methodology: AS, FAA, MSK, WAA; project administration: DKY and MIH; resources: AS, DDG and MSK; software: DDG and AS; supervision: MIH, FAA and DKY; validation: AS and DDG; visualization, DDG, AS and MIH; writing – original draft: AS, DDG and MSK; writing – review and editing, AS and MIH.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
MSK acknowledges the generous support from the Research Supporting Project (RSP-2021/352) by King Saud University, Riyadh, Kingdom of Saudi Arabia.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00053a
References
- Anwar S. Shamsi A. Shahbaaz M. Queen A. Khan P. Hasan G. M. Islam A. Alajmi M. F. Hussain A. Ahmad F. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H. Gustafsson E. Svensson A. Nilsson M. Berg M. Sunnemark D. von Euler G. Acta Neuropathol. Commun. 2014;2:1–15. doi: 10.1186/2051-5960-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeysen S. Bockaert J. Giannoni P. ACS Chem. Neurosci. 2015;6:940–943. doi: 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- Walther D. J. Peter J.-U. Bashammakh S. Hortnagl H. Voits M. Fink H. Bader M. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Arreola R. Becerril-Villanueva E. Cruz-Fuentes C. Velasco-Velázquez M. A. Garcés-Alvarez M. E. Hurtado-Alvarado G. Quintero-Fabian S. Pavón L. J. Immunol. Res. 2015;2015:354957. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J. Anderson G. M. Cook Jr E. H. Eur. J. Pharmacol. 2000;410:165–181. doi: 10.1016/S0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- Zamani A. Qu Z. FEBS Lett. 2012;586:2360–2365. doi: 10.1016/j.febslet.2012.05.047. [DOI] [PubMed] [Google Scholar]
- Yadav V. K. Balaji S. Suresh P. S. Liu X. S. Lu X. Li Z. Guo X. E. Mann J. J. Balapure A. K. Gershon M. D. Nat. Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. M. Young R. L. Leong L. Rogers G. B. Spencer N. J. Jessup C. F. Keating D. J. Endocrinology. 2017;158:1049–1063. doi: 10.1210/en.2016-1839. [DOI] [PubMed] [Google Scholar]
- Nebigil C. G. Launay J.-M. Hickel P. Tournois C. Maroteaux L. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr N. Bode C. Duerschmied D. Front. Cardiovasc. Med. 2017;4:48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S. Lal G. Theranostics. 2021;11:5296. doi: 10.7150/thno.55986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson A. Heuchert J. Psychol. Rep. 2000;87:707–716. doi: 10.2466/PR0.87.7.707-716. [DOI] [PubMed] [Google Scholar]
- Williams E. Stewart-Knox B. Helander A. McConville C. Bradbury I. Rowland I. Biol. Psychol. 2006;71:171–174. doi: 10.1016/j.biopsycho.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lacasse J. R. Leo J. PLoS Med. 2005;2:e392. doi: 10.1371/journal.pmed.0020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldenhuys W. J. Van der Schyf C. J. CNS Drugs. 2011;25:765–781. doi: 10.2165/11590190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Shchemelinin I. Sefc L. Necas E. Folia Biol. 2006;52:81. [PubMed] [Google Scholar]
- Noble M. E. Endicott J. A. Johnson L. N. Science. 2004;303:1800–1805. doi: 10.1126/science.1095920. [DOI] [PubMed] [Google Scholar]
- Anwar S. Mohammad T. Shamsi A. Queen A. Parveen S. Luqman S. Hasan G. M. Alamry K. A. Azum N. Asiri A. M. Biomedicines. 2020;8:119. doi: 10.3390/biomedicines8050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S. Shamsi A. Mohammad T. Islam A. Hassan M. I. Biochim. Biophys. Acta, Rev. Cancer. 2021:188568. doi: 10.1016/j.bbcan.2021.188568. [DOI] [PubMed] [Google Scholar]
- Cho J. H. Johnson G. V. J. Neurochem. 2004;88:349–358. doi: 10.1111/j.1471-4159.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- Marx A. Nugoor C. Panneerselvam S. Mandelkow E. FASEB J. 2010;24:1637–1648. doi: 10.1096/fj.09-148064. [DOI] [PubMed] [Google Scholar]
- Pardo O. E. Castellano L. Munro C. E. Hu Y. Mauri F. Krell J. Lara R. Pinho F. G. Choudhury T. Frampton A. E. EMBO Rep. 2016;17:570–584. doi: 10.15252/embr.201540970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz F. Khan F. I. Mohammad T. Khan P. Manzoor S. Hasan G. M. Lobb K. A. Luqman S. Islam A. Ahmad F. Int. J. Biol. Macromol. 2018;107:2580–2589. doi: 10.1016/j.ijbiomac.2017.10.143. [DOI] [PubMed] [Google Scholar]
- De Oliveira G. N. M. Kummer A. Salgado J. V. Portela E. J. Sousa-Pereira S. R. David A. S. Teixeira A. L. Seizure. 2010;19:479–484. doi: 10.1016/j.seizure.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Machado R. A. Benjumea-Cuartas V. Berruecos J. F. Z. Agudelo-Flóres P. M. Salazar-Peláez L. M. Epilepsy Behav. 2019;96:192–199. doi: 10.1016/j.yebeh.2019.04.052. [DOI] [PubMed] [Google Scholar]
- Meltzer C. C. Smith G. DeKosky S. T. Pollock B. G. Mathis C. A. Moore R. Y. Kupfer D. J. Reynolds C. F. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Sack J. S. Gao M. Kiefer S. E. Myers, Jr. J. E. Newitt J. A. Wu S. Yan C. Acta Crystallogr., Sect. F: Struct. Biol. Commun. 2016;72:129–134. doi: 10.1107/S2053230X15024747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molecular Operating Environment (MOE), 2020.09 Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2022 [Google Scholar]
- Labute P. Proteins. 2009;75:187–205. doi: 10.1002/prot.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad T. Mathur Y. Hassan M. I. Briefings Bioinf. 2020;22(4) doi: 10.1093/bib/bbaa279. [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Petersson G. A., Nakatsuji H., Li X., Caricato M., Marenich A. V., Bloino J., Janesko B. G., Gomperts R., Mennucci B., Hratchian H. P., Ortiz J. V., Izmaylov A. F., Sonnenberg J. L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V. G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery, Jr. J. A., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J. J., Brothers E. N., Kudin K. N., Staroverov V. N., Keith T. A., Kobayashi R., Normand J., Raghavachari K., Rendell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Millam J. M., Klene M., Adamo C., Cammi R., Ochterski J. W., Martin R. L., Morokuma K., Farkas O., Foresman J. B. and Fox D. J., Gaussian 16, Gaussian, Inc., Wallingford CT, 2016 [Google Scholar]
- Chandra Singh U. Kollman P. A. J. Comput. Chem. 1984;5:129–145. doi: 10.1002/jcc.540050204. [DOI] [Google Scholar]
- Tian C. Kasavajhala K. Belfon K. A. A. Raguette L. Huang H. Migues A. N. Bickel J. Wang Y. Pincay J. Wu Q. Simmerling C. J. Chem. Theory Comput. 2020;16:528–552. doi: 10.1021/acs.jctc.9b00591. [DOI] [PubMed] [Google Scholar]
- Salomon-Ferrer R. Götz A. W. Poole D. Le Grand S. Walker R. C. J. Chem. Theory Comput. 2013;9:3878–3888. doi: 10.1021/ct400314y. [DOI] [PubMed] [Google Scholar]
- Mark P. Nilsson L. J. Phys. Chem. A. 2001;105:9954–9960. doi: 10.1021/jp003020w. [DOI] [Google Scholar]
- Case D. A., Belfon K., Ben-Shalom I. Y., Brozell S. R., Cerutti D. S., Cheatham I. T. E., Cruzeiro V. W. D., Darden T. A., Duke R. E., Giambasu G., Gilson M. K., Gohlke H., Goetz A. W., Harris R., Izadi S., Izmailov S. A., Kasavajhala K., Kovalenko A., Krasny R., Kurtzman T., Lee T. S., LeGrand S., Li P., Lin C., Liu J., Luchko T., Luo R., Man V., Merz K. M., Miao Y., Mikhailovskii O., Monard G., Nguyen H., Onufriev A., Pan F., Pantano S., Qi R., Roe D. R., Roitberg A., Sagui C., Schott-Verdugo S., Shen J., Simmerling C. L., Skrynnikov N. R., Smith J., Swails J., Walker R. C., Wang J., Wilson L., Wolf R. M., Wu X., Xiong Y., Xue Y., York D. M. and Kollman P. A., AMBER2020, 2020
- Götz A. W. Williamson M. J. Xu D. Poole D. Le Grand S. Walker R. C. J. Chem. Theory Comput. 2012;8:1542–1555. doi: 10.1021/ct200909j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckaert J.-P. Ciccotti G. Berendsen H. J. C. J. Comput. Phys. 1977;23:327–341. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- Shamsi A. Anwar S. Mohammad T. Alajmi M. F. Hussain A. Rehman M. Hasan G. M. Islam A. Hassan M. Biomolecules. 2020;10:789. doi: 10.3390/biom10050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi A. Ahmed A. Khan M. S. Al Shahwan M. Husain F. M. Bano B. J. Mol. Liq. 2020;311:113348. doi: 10.1016/j.molliq.2020.113348. [DOI] [Google Scholar]
- Yousuf M. Shamsi A. Khan P. Shahbaaz M. AlAjmi M. F. Hussain A. Hassan G. M. Islam A. Rizwanul Haque Q. M. Hassan M. Int. J. Mol. Sci. 2020;21:3526. doi: 10.3390/ijms21103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi A. Mohammad T. Anwar S. Alajmi M. F. Hussain A. Hassan M. I. Ahmad F. Islam A. Int. J. Biol. Macromol. 2020;148:533–542. doi: 10.1016/j.ijbiomac.2020.01.134. [DOI] [PubMed] [Google Scholar]
- Anwar S. Khan S. Shamsi A. Anjum F. Shafie A. Islam A. Ahmad F. Hassan M. I. J. Cell. Biochem. 2021;122:1445–1459. doi: 10.1002/jcb.30022. [DOI] [PubMed] [Google Scholar]
- Anwar S. Khan S. Anjum F. Shamsi A. Khan P. Fatima H. Shafie A. Islam A. Hassan M. I. J. Cell. Biochem. 2021;123:359–374. doi: 10.1002/jcb.30176. [DOI] [PubMed] [Google Scholar]
- Anwar S. J. Mol. Liq. 2022;355 doi: 10.1016/j.molliq.2022.118928. doi: 10.1016/j.molliq.2022.118928. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data have been provided in the manuscript.