Abstract
Although both protein tyrosine phosphatases and kinases are constitutively active in healthy human red blood cells (RBCs), the preponderance of phosphatase activities maintains the membrane proteins in a predominantly unphosphorylated state. We report here that unlike healthy RBCs, proteins in sickle cells are heavily tyrosine phosphorylated, raising the question regarding the mechanism underpinning this tyrosine phosphorylation. Upon investigating possible causes, we observe that protein tyrosine phosphatase 1B (PTP1B), the major erythrocyte tyrosine phosphatase, is largely digested to a lower molecular weight fragment in sickle cells. We further find that the resulting truncated form of PTP1B is significantly less active than its intact counterpart, probably accounting for the intense tyrosine phosphorylation of Band 3 in sickle erythrocytes. Because this tyrosine phosphorylation of Band 3 promotes erythrocyte membrane weakening that causes release of both membrane vesicles and cell free hemoglobin that in turn initiates vaso-occlusive events, we conclude that cleavage of PTP1B could contribute to the symptoms of sickle cell disease. We further posit that methods to inhibit proteolysis of PTP1B could mitigate symptoms of the disease.
Keywords: Band 3 tyrosine phosphorylation, calpain proteolysis, cleavage of PTP1B, phosphatase activity, PTP1B, sickle cells
1 |. INTRODUCTION
Previous studies have suggested that the elevated tyrosine phosphorylation of erythrocyte membrane protein Band 3 in sickle cells may contribute to the symptoms of sickle cell disease (SCD).1 In this reasoning, heightened tyrosine phosphorylation of Band 3 has been shown to promote dissociation of the spectrin-actin cortical cytoskeleton from the erythrocyte membrane.2,3 This disjunction of the cytoskeleton from the membrane has in turn been found to cause membrane weakening that permits the localized rupture and fragmentation of the membrane upon exposure to the shear stresses in the microvasculature. The consequent release of hemoglobin and heme have long been established to both consume nitric oxide4,5 and activate the vascular endothelium,6 inducing vasoconstriction and promoting expression of adhesion receptors, respectively.6–8 Moreover, the concomitantly discharged erythrocyte-derived membrane microparticles have been demonstrated to initiate intravascular thrombosis that can independently impede blood flow and contribute to the cumulative vaso-occlusive events that cause SCD.9–11
In an attempt to identify the underlying mechanism responsible for the elevated Band 3 tyrosine phosphorylation in SCD, we have examined the two obvious alternatives, namely that tyrosine phosphorylation of Band 3 is either elevated or its dephosphorylation is inhibited in sickle cells.12,13 We report here that the major erythrocyte membrane tyrosine phosphatase, protein tyrosine phosphatase 1B (PTP1B),14 is primarily present at a lower molecular weight in sickle cells than normal red blood cells (RBCs). We further demonstrate that this lower molecular weight species derives from proteolysis of PTP1B and that cleavage of PTP1B leads to its inhibition. Upon obtaining additional data that PTP1B constitutes the major tyrosine phosphatase that dephosphorylates Band 3 and that the disappearance of intact PTP1B in sickle cells correlates with an increase in Band 3 tyrosine phosphorylation in the same sickle cell samples, we conclude that elevated tyrosine phosphorylation of Band 3 likely contributes to development of the symptoms of SCD.1 We further speculate that a highly selective inhibitor of PTP1B cleavage could conceivably constitute a treatment for the disease.
2 |. METHODS
2.1 |. Sickle cell blood samples
Blood samples from 20 homozygous (SS) SCD patients (age range 3–20 years) were enrolled at Cincinnati Children’s Hospital (CCHM) following informed and written consent. Sixteen of these patients were being treated with hydroxyurea, while four (where indicated in figures) received regular transfusions. Following blood collection at CCHM, sickle cell samples along with healthy controls (total n = 8) were shipped overnight to Purdue University for analysis.
2.2 |. Analysis of phosphorylation of erythrocyte membrane proteins from sickle cells
Blood samples from sickle cell patients were obtained after enrollment, following informed written consent, on a protocol approved from Cincinnati Children’s Hospital Medical Center IRB. Following blood collection, samples were shipped overnight to Purdue University. Patient samples were subjected to centrifugation at 800 rcf for 10 min at room temperature and the pelleted erythrocytes were washed 3× with phosphate-buffered saline containing 5 mM glucose (PBS-G). Erythrocyte membranes were then prepared as described previously1 and separated by SDS polyacrylamide gel electrophoresis in preparation for analysis by Western blotting. Blotted nitrocellulose membranes were incubated with the desired primary and secondary antibodies labeled with near infrared dyes detectable at 800 and 700 nm wavelengths and then scanned using a LI-COR Odyssey infrared imager. Protein densitometry was conducted using Image J.
2.3 |. Evaluation of the relative contributions of the three major erythrocyte tyrosine phosphatases to the dephosphorylation of Band 3
To obtain an initial indication of which tyrosine phosphatase might be most responsible for dephosphorylating Band 3, washed healthy human erythrocytes suspended at 20% hematocrit in PBS-G were incubated with one or more inhibitors with established specificity for SHP-1 (Axon Medchem #Axon 2723) or SHP-2 (Axon Medchem #Axon 2633), nonspecific PTP1B inhibitors (PTP Inhibitors V and XVIII; Calbiochem CAS 314291-83-3, and CAS 1229246-07-4, respectively), or a specific PTP1B inhibitor (Cayman Chemical #15782) at 37°C for 30 min with 35 rpm shaking. Erythrocyte ghosts were then prepared, and Band 3 tyrosine phosphorylation was quantitated as summarized above. The phosphatase whose inhibition led to the greatest increase in endogenous Band 3 tyrosine phosphorylation was considered a candidate for the phosphatase responsible for Band 3 tyrosine dephosphorylation in vivo.
2.4 |. Comparison of the protein tyrosine phosphatase activities of PTP1B and SHP2 on phosphorylated recombinant Band 3
To assess the contribution of PTP1B and SHP2 to the dephosphorylation of Band 3, untagged cytoplasmic domain of Band 3 (cdb3, 1–379) was expressed in Escherichia coli and purified using anion exchange chromatography. Purified Band 3 was then tyrosine phosphorylated by incubation with Syk tyrosine kinase (SignalChem, S52–10G) and ATP. Phosphorylated Band 3 was then incubated with equal activity units of either full length PTP1B or SHP2. Reactions were quenched by addition of Laemmli buffer and boiling at 95°C for 5 min. Samples were then immunoblotted using anti-phosphotyrosine (Cell Signaling, Clone PY100) and anti-Band 3 (Sigma, Clone BIII-136, #B9277) antibodies and then imaged using a Licor infrared scanner.
2.5 |. Comparison of the protein tyrosine phosphatase activities of intact and cleaved PTP1B
The enzymatic activities of intact and cleaved PTP1B were analyzed using p-nitrophenyl phosphate phosphatase assay (ScienCell™ #8108). Quantitation of the activities of intact and cleaved PTP1B in dephosphorylating p-nitrophenyl phosphate15 was performed using a colorimetric assay based on the manufacturer’s instructions.
3 |. RESULTS
Because most tyrosine kinases have restricted substrate specificities whereas the majority of tyrosine phosphatases do not,12,16–18 one strategy for distinguishing whether the elevated tyrosine phosphorylation of Band 3 seen in sickle cells might have arisen from an overactive tyrosine kinase or underactive tyrosine phosphatase might be to examine whether the elevated tyrosine phosphorylation is restricted to just a few proteins or observed in many proteins. To perform this test on sickle cells, we immunoblotted the membrane proteins from sickle and healthy erythrocytes with anti-phosphotyrosine antibodies and examined the diversity of tyrosine phosphorylated proteins in the immunoblot. As shown in Figure 1, elevated tyrosine phosphorylation of healthy erythrocyte membrane proteins was primarily restricted to three or four polypeptides, whereas analogous phosphorylation in sickle cells was distributed across almost all membrane proteins, including Band 3. These data would suggest that the elevated tyrosine phosphorylation seen in sickle cells might derive from inadequate phosphatase activity19 rather than excessive kinase activity.
FIGURE 1.
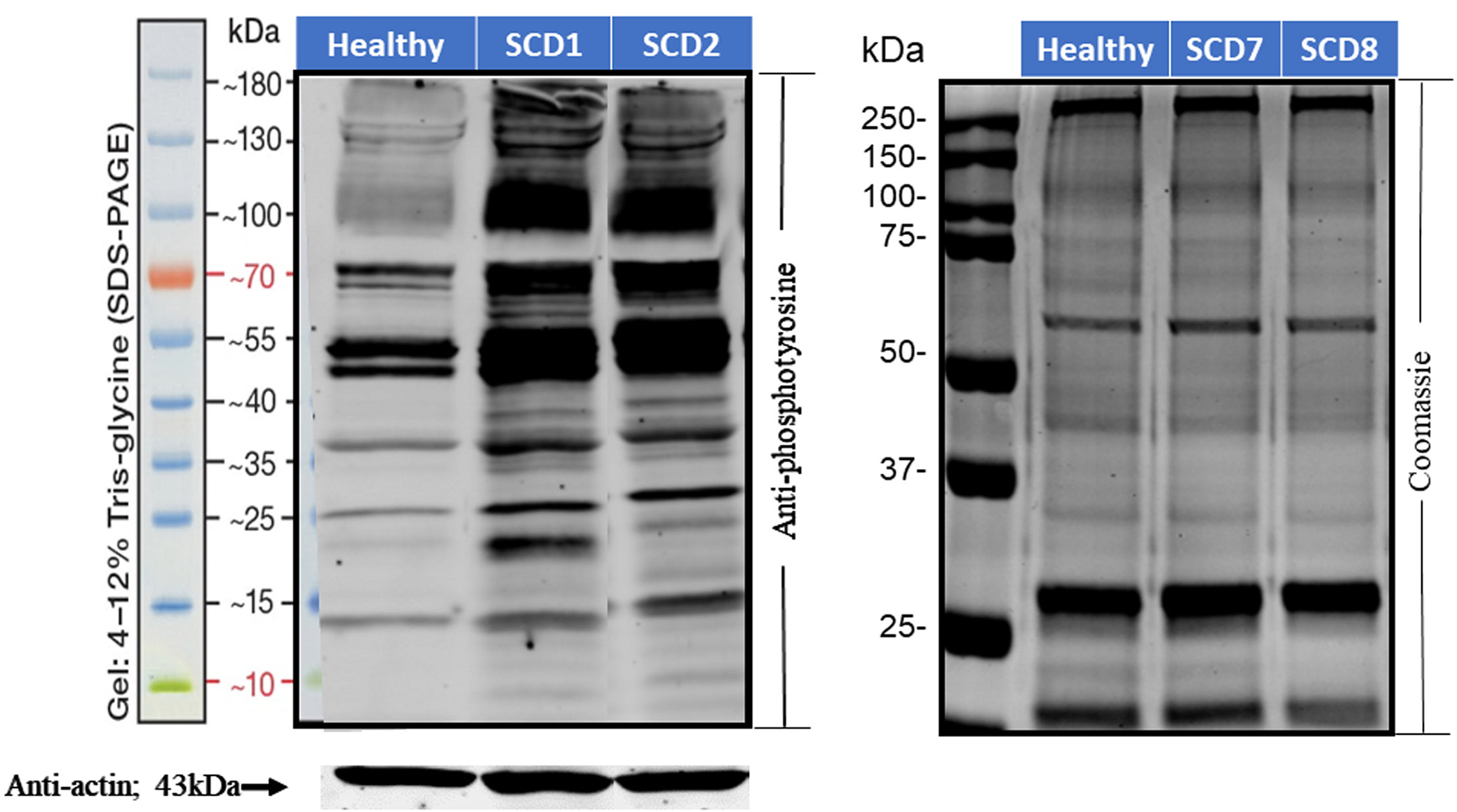
Tyrosine phosphorylation of erythrocyte membrane proteins in healthy individuals and sickle cell disease patients. Erythrocyte membrane proteins from healthy volunteers and sickle cell patients were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed with anti-phosphotyrosine antibodies (left panel). Anti-actin immunostaining was used as a loading control. Coomassie staining was used to evaluate the integrity of the major membrane proteins (right panel).
Next, to explore which erythrocyte tyrosine phosphatase might be most responsible for dephosphorylating Band 3, we treated healthy RBCs with different tyrosine phosphatase inhibitors and looked for changes in Band 3 tyrosine phosphorylation. As seen in Figure 2, only inhibition of PTP1B significantly enhanced the steady state of Band 3 tyrosine phosphorylation, whereas inhibition of the two other major erythrocyte tyrosine phosphatases (i.e. SHP-120 and SHP-221) had little effect on Band 3 tyrosine phosphorylation. Inspection of all major RBC membrane proteins revealed that only inhibition of PTP1B significantly enhanced tyrosine phosphorylation of almost all other membrane proteins (Figures S1–S4). To further investigate the contribution of the erythrocyte tyrosine phosphatases on the dephosphorylation of Band 3, the cdb3 was expressed and phosphorylated by the tyrosine kinase, Syk. This phosphorylated Band 3 cytoplasmic domain (cdb3-PO4) was incubated with the major erythrocyte tyrosine phosphatases, PTP1B and SHP2. Although both tyrosine phosphatases were able to dephosphorylate phosphorylated cdb3 to some extent, PTP1B was much more efficient at dephosphorylating Band 3, indicating that PTP1B may have a greater ability to dephosphorylate Syk phosphorylated Band 3 than SHP2 (Figure 3). Taken together, these data suggest that PTP1B is the major contributor to maintaining Band 3 in an unphosphorylated state and that a deficiency in PTP1B activity might specifically be responsible for the elevated steady state of Band 3 tyrosine phosphorylation in sickle cells.
FIGURE 2.
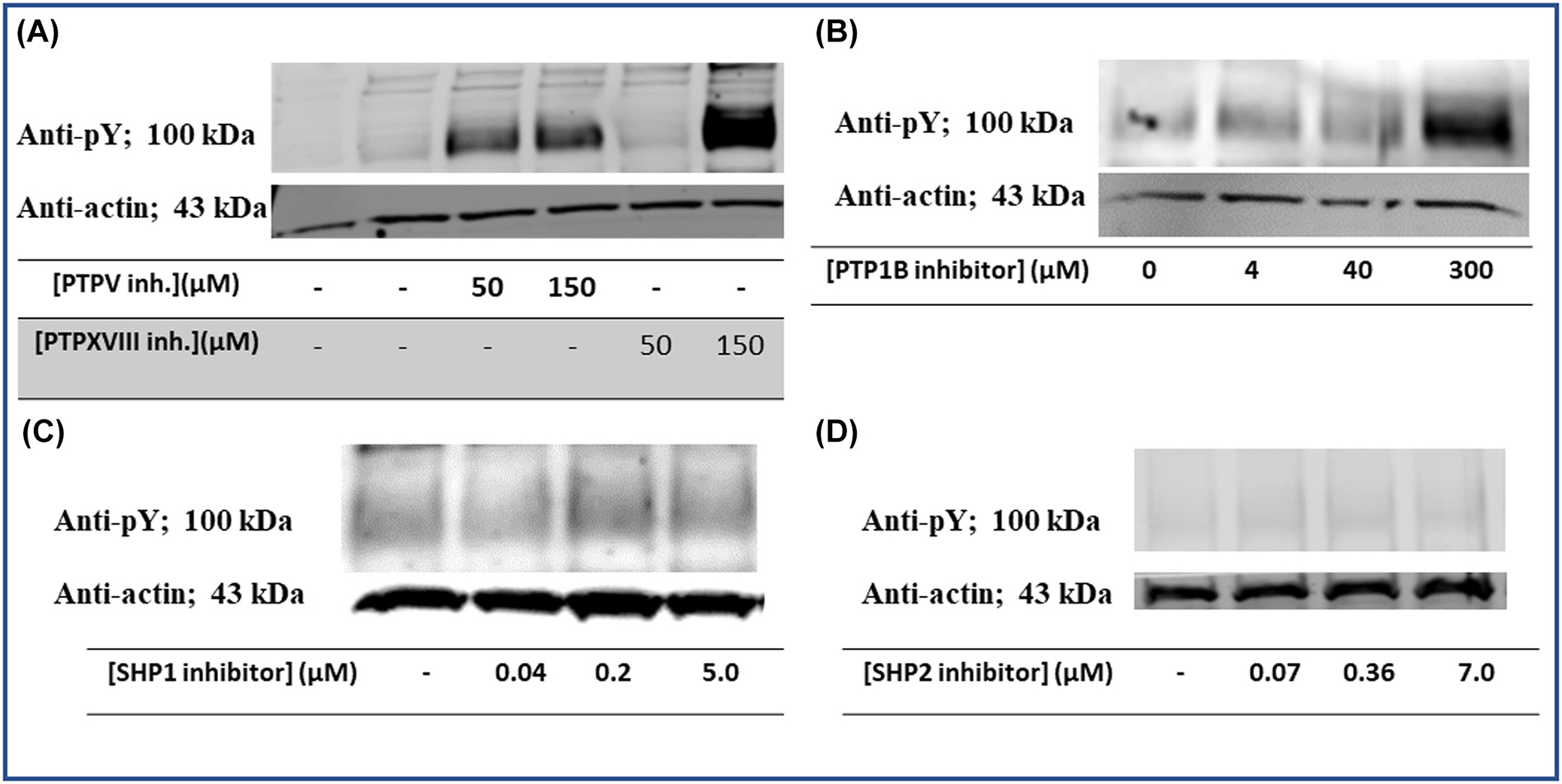
Effect of different tyrosine phosphatase inhibitors on tyrosine phosphorylation of Band 3. Erythrocytes from healthy donors were treated with different protein tyrosine phosphatase inhibitors, after which the extent of Band 3 tyrosine phosphorylation was determined by Western blotting. The phosphatase inhibitors examined were: (A) PTPV (Calbiochem CAS #314291-83-3) and PTPXVIII (CAS #1229246-07-4) that have moderate specificity for protein tyrosine phosphatase 1B (PTP1B), (B) PTP1B inhibitor (Cayman #15782) that has high specificity for PTP1B, (C) SHP-1 inhibitor (Axon Medchem #2723), and (D) SHP-2 inhibitor (Axon Medchem #2633).
FIGURE 3.

Comparison of the abilities of tyrosine phosphatases protein tyrosine phosphatase 1B (PTP1B) and SHP2 to dephosphorylate Syk phosphorylated Band 3 cytoplasmic domain. Equal units of activity of full length PTP1B and SHP2 enzymes were incubated with tyrosine phosphorylated Band 3 (1–379). Cbd3, cytoplasmic domain of band 3; cdb3-PO4, phosphorylated cdb3.
Because intracellular tyrosine phosphatases are generally not allosterically regulated, their activities are more commonly controlled by changes in their rates of synthesis or degradation.14,22,23 To determine whether elevated tyrosine phosphorylation in sickle cells might derive from accelerated degradation of PTP1B, we separated membrane proteins from sickle and healthy erythrocytes by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed the derived immunoblots with anti-phosphotyrosine (Santa Cruz Biotechnology SC-70200) and anti-PTP1B antibodies (Sigma Chemical Co. #MABS197). As shown in the anti-PTP1B blot of Figure 4(A) (lower panel), PTP1B exists as a single polypeptide of the expected 50 kDa molecular weight in healthy erythrocytes, but as two polypeptides of molecular weights 50 and 42 kDa in sickle cells. Importantly, both molecular weight species have been previously described in other cell types, including platelets,24–26 endothelial cells,27 and senescent erythrocytes,14 where the 42 kDa band has been ascribed to a degradation product of intact PTP1B.14,22,24,27
FIGURE 4.
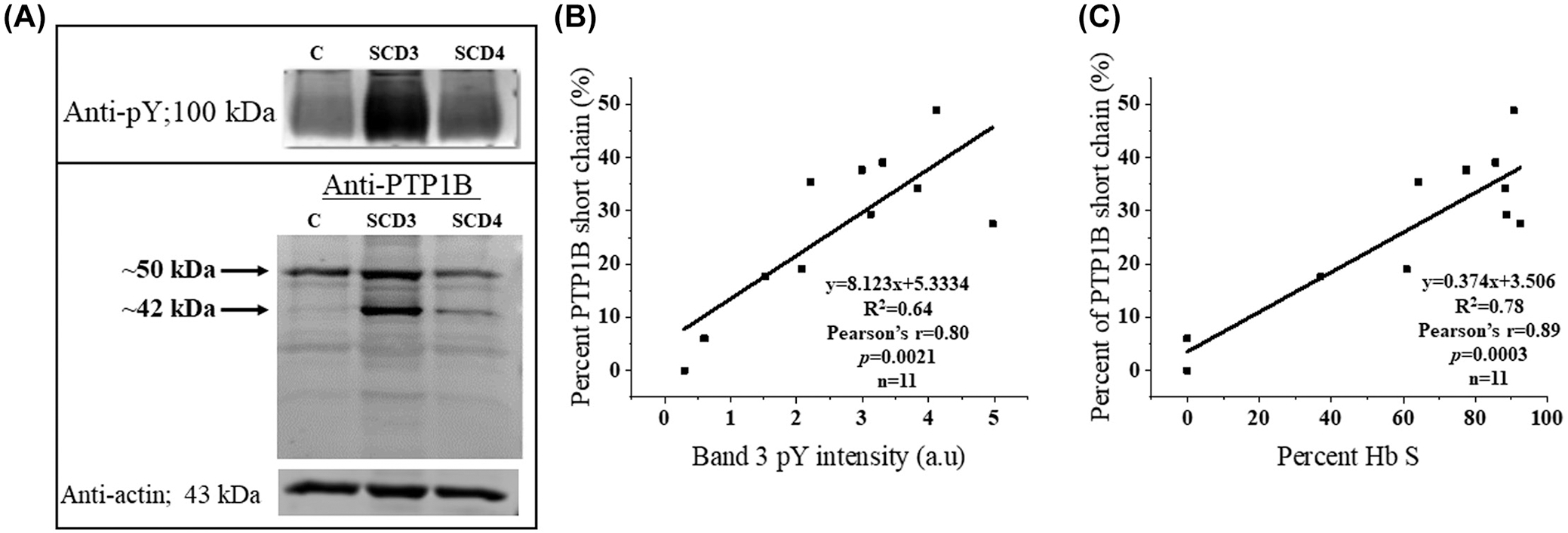
(A) Assessment of protein tyrosine phosphatase 1B (PTP1B) cleavage in healthy and sickle erythrocytes by anti-PTP1B immunoblotting. (B) Examination of the correlation between PTP1B cleavage and Band 3 tyrosine phosphorylation in each blood sample. (C) Examination of the correlation between PTP1B cleavage and percent HbS in each blood sample. Blood from healthy control (C), and sickle cell patients treated with hydroxyurea (SCD3) or repeated transfusions (SCD4) was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunostained with mouse anti-phosphotyrosine (A, top panel) or anti-PTP1B antibody (A, bottom panel) antibodies. Actin content was used as a loading control (A, bottom panel).
Although truncated 42 kDa PTP1B has been shown to localize to different intracellular organelles than intact PTP1B in other cell types, the impact of PTP1B cleavage on its phosphatase activity has remained controversial.14,22,24,28 Therefore, to determine whether the observed proteolysis of PTP1B might affect its ability to dephosphorylate Band 3, we examined the relationship between the fraction of cleaved PTP1B (ie [42 kDa]/[50 kDa + 42 kDa]) and the level of Band 3 tyrosine phosphorylation in each sickle cell sample. As shown in panel B, a statistically significant (p = .0021) positive correlation between the two variables emerged (Pearson’s r = .80), suggesting that cleavage of PTP1B might be mechanistically related to elevated Band 3 tyrosine phosphorylation. The fact that cleavage of PTP1B also displayed a significant (p = .0003) correlation with the percent Hb S in each patient’s blood sample (panel C; Pearson’s r = .89) further supported the hypothesis that PTP1B proteolysis might be involved in the heightened Band 3 tyrosine phosphorylation seen in sickle cells.1
The question next arose whether the shorter form of PTP1B might exhibit reduced catalytic activity relative to full length PTP1B. To address this question, the abilities of identical molar concentrations of short and full length PTP1B to dephosphorylate the classical tyrosine phosphatase substrate, p-nitrophenyl phosphate,15 were quantitated. As shown in Figure 5, the catalytic activity of full-length PTP1B was >3 times greater than that of the truncated PTP1B, suggesting that the elevated protein tyrosine phosphorylation in sickle cells might indeed derive from cleavage of PTP1B to a less active form.
FIGURE 5.
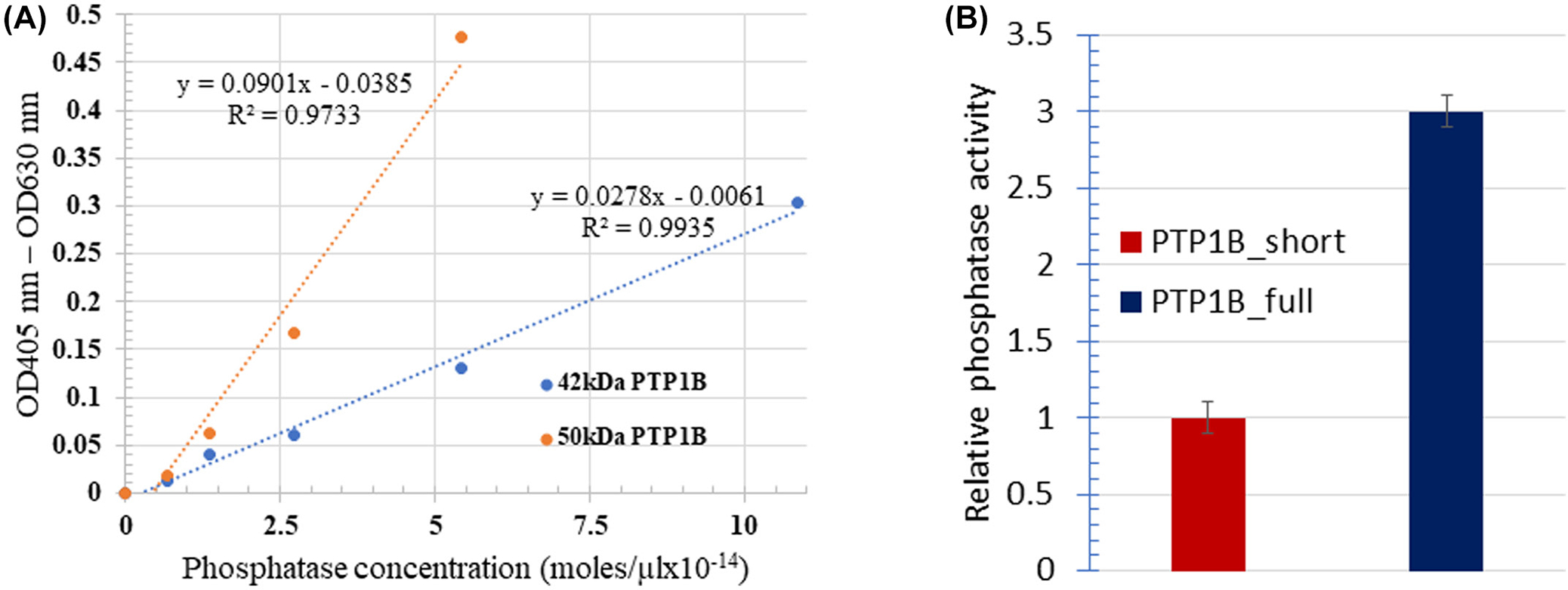
Impact of protein tyrosine phosphatase 1B (PTP1B) cleavage on its phosphatase activity. Catalytic activities of the 42 kDa short and 50 kDa full length PTP1B enzymes were analyzed using 4-nitrophenyl phosphate as a substrate (A), yielding the results shown in panel (B).
Finally, because the reticulocyte concentration in sickle blood samples is usually much higher than that in healthy patient samples, a question has been raised whether the higher reticulocyte count in the sickle samples might somehow be related to the accelerated cleavage of PTP1B. To explore this question, we examined the relative levels of reticulocytes in sickle and healthy blood samples and determined the cleavage of PTP1B in the same samples. As shown in Figure 6, blood samples with higher reticulocyte numbers indeed exhibited larger percentages of cleaved PTP1B. However, whether a causative link exists between reticulocyte numbers and PTP1B proteolysis cannot be ascertained from these data and will have to wait on further data.
FIGURE 6.
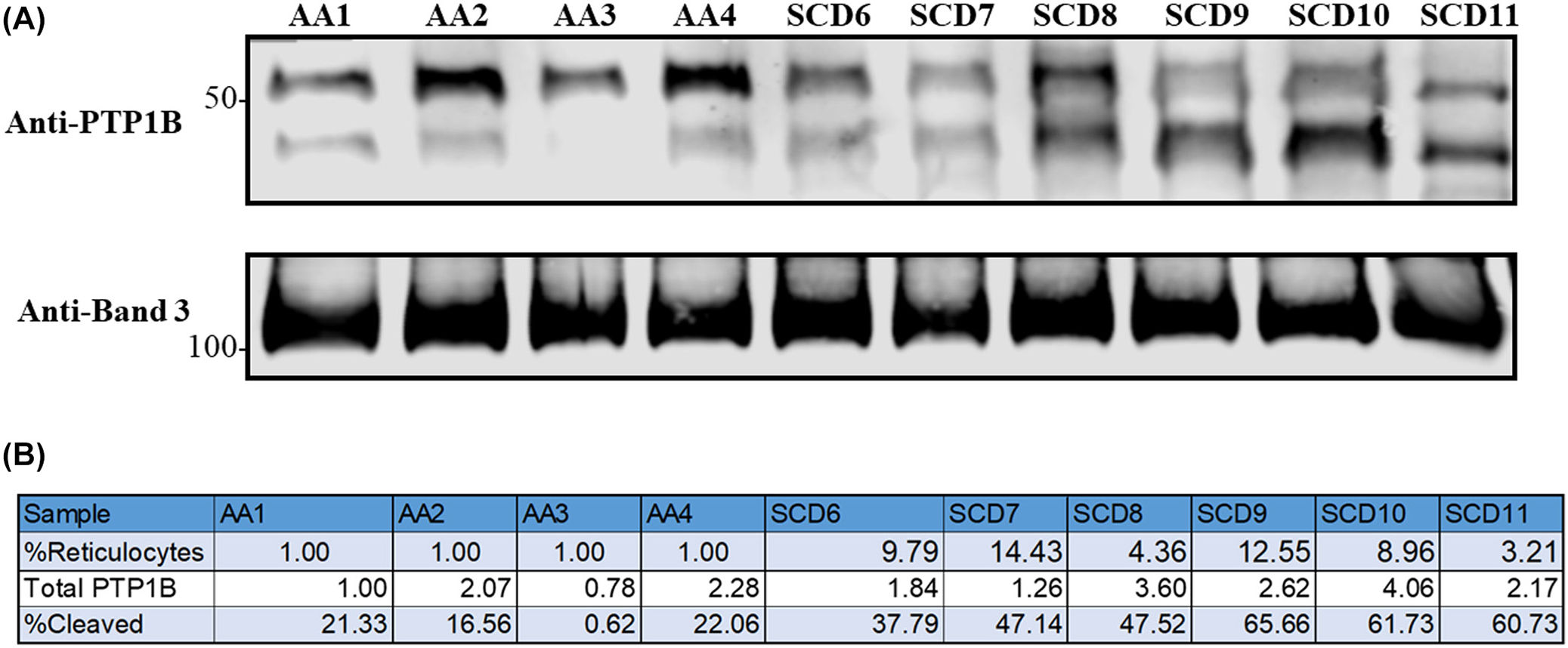
Correlation between reticulocyte content and protein tyrosine phosphatase 1B (PTP1B) cleavage in whole blood samples from healthy donors and sickle cell patients. (A) Immunoblot assessing the content of cleaved and intact PTP1B in healthy volunteer and sickle cell patient blood samples. (B) Total amount of the sum of intact plus cleaved PTP1B relative to the amount measured in sample AA1. Also shown is the percent of total PTP1B in each sample that is cleaved.
4 |. DISCUSSION
Elevated tyrosine phosphorylation of membrane proteins in sickle cells has been reported by several previous laboratories. Merciris et al29 and Siciliano et al30 have observed that deoxygenation of sickle cells induces a general increase in membrane protein tyrosine phosphorylation, while Terra et al31 have further noted that this elevated tyrosine phosphorylation is especially concentrated on Band 3. While the Merciris study did speculate that an imbalance between PTK and PTP activities might cause a potential miscue that could be relevant to red cell survival,1,13,14,19 none of these previous publications offered a mechanism to explain how elevated tyrosine phosphorylation might be linked to pathology of SCD.
In our previous studies, we have shown that tyrosine phosphorylation of Band 3 induces dissociation of the spectrin-actin cytoskeleton from Band 3, resulting in destabilization and fragmentation of the erythrocyte membrane.2,3 We have further shown that this membrane fragmentation can contribute to the vaso-occlusive events that characterize SCD by (1) generating membrane-derived microparticles1 that activate the coagulation pathway,11,32,33 (2) discharging cell-free hemoglobin that consumes the nitric oxide required for vaso-dilation,5,34,35 and (3) releasing unbound heme that stimulates the Toll-like receptors 4 on the vascular endothelium, thereby promoting their upregulation of adhesion receptors.6,36,37 However, despite these advances, the important question still remained regarding the molecular cause of the elevated Band 3 tyrosine phosphorylation in sickle cells. In this paper we have demonstrated that cleavage of PTP1B, the major enzyme that dephosphorylates Band 3,14 contributes prominently to the elevated phosphorylation of Band 3 in sickle cells. This observation suggests that design of a specific inhibitor that would prevent cleavage of PTP1B could constitute a therapy for SCD.
Ciana et al14 demonstrated several years ago that PTP1B is similarly degraded toward the end of a healthy erythrocyte’s life span, and that the degradation was accompanied by an increase in Band 3 tyrosine phosphorylation.13,14,38 The authors further hypothesized that PTP1B cleavage might suppress the association of PTP1B with Band 3, since PTP1P co-immunoprecipitated with Band 3 more readily from young RBCs than old RBCs.13,14,38 The data presented herein support these findings and suggest that the loss of plasma membrane that occurs as healthy RBCs age39 may derive from a similar membrane destabilization that leads to sickle cell hemolysis and microvesiculation.1,40,41
Although the cause of PTP1B cleavage was not investigated here, cleavage of PTP1B has been shown by others to be catalyzed by calpain,14,26,27 which in turn must be activated by Ca2+ influx.27 Calcium levels are normally maintained at very low levels in healthy RBCs by a calcium ATPase,42–44 but due to a cation leak, can be very high in sickle erythrocytes.45–47 The binding of calpain to the protein, calpastatin, has also been shown to regulate calpain by inhibiting proteolytic activity and preventing the localization of calpain to the cell membrane, where it can also be activated.48 Joiner et al demonstrated that a calpain inhibitor (BDA-410) could significantly improve the morphology of sickle cells from SAD mice while simultaneously reducing RBC density and elevating cytoplasmic potassium.49 While the Joiner lab focused primarily on the role of BDA-410 in preventing sickle cell dehydration, it should not go unnoticed that BDA-410 also reduced membrane distortion and fragmentation. In our view, this maintenance of normal membrane stability and morphology could have mechanistically derived from its prevention of PTP1B cleavage and the associated elevation of Band 3 tyrosine phosphorylation. Given that improvement in sickle cell hydration combined with an enhancement of erythrocyte membrane stability could synergistically suppress symptoms of SCD, it would be interesting to explore the administration of a calpain 1 inhibitor for the treatment of SCD. Alicapistat, a calpain 1 and 2 inhibitor, has in fact recently been examined for treatment of Alzheimer’s disease with no obvious adverse effects.50 Alternatively, inhibitors of the erythrocyte tyrosine kinase responsible for Band 3 tyrosine phosphorylation might also be investigated for treatment of SCD.1
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by funds from the NIH (GM24417-40) (P.S.L.).
Abbreviations:
- Cdb3
cytoplasmic domain of Band 3
- Cdb3-PO4
tyrosine phosphorylated cytoplasmic domain of Band 3
- IRB
Institutional Review Board
- PBS-G
phosphate-buffered saline containing 5 mM glucose
- PTP1B
protein tyrosine phosphatase 1 B
- RBCs
red blood cells
- SCD
sickle cell disease
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- SHP-2
Src homology region 2 domain-containing phosphatase 2
Footnotes
DISCLOSURES
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the methods.
REFERENCES
- 1.Noomuna P, Risinger M, Zhou S, et al. Inhibition of Band 3 tyrosine phosphorylation: a new mechanism for treatment of sickle cell disease. Br J Haematol. 2020;190(4):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferru E, Giger K, Pantaleo A, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117(22):5998–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puchulu-Campanella E, Turrini FM, Li Y-H, Low PS. Global transformation of erythrocyte properties via engagement of an SH2-like sequence in band 3. Proc Natl Acad Sci. 2016;113(48):13732–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parise LV, Telen MJ. Erythrocyte adhesion in sickle cell disease. Curr Hematol Rep. 2003;2(2):102–108. [PubMed] [Google Scholar]
- 8.Kucukal E, Ilich A, Key NS, Little JA, Gurkan UA. Red blood cell adhesion to heme-activated endothelial cells reflects clinical phenotype in sickle cell disease. Am J Hematol. 2018;93(8):1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camus SM, Gausserès B, Bonnin P, et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood. 2012;120(25):5050–5058. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Meijden PEJ, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HMH. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10(7):1355–1362. [DOI] [PubMed] [Google Scholar]
- 11.Gerotziafas G, Van Dreden P, Chaari M, et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb Haemost. 2012;107(6):1044–1052. [DOI] [PubMed] [Google Scholar]
- 12.Hafen E Kinases and phosphatases--a marriage is consummated. Science. 1998;280(5367):1212–1213. [DOI] [PubMed] [Google Scholar]
- 13.Minetti G, Ciana A, Balduini C. Differential sorting of tyrosine kinases and phosphotyrosine phosphatases acting on band 3 during vesiculation of human erythrocytes. Biochem J. 2004;377(Pt 2):489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciana A, Minetti G, Balduini C. Phosphotyrosine phosphatases acting on band 3 in human erythrocytes of different age: PTP1B processing during cell ageing. Bioelectrochemistry. 2004;62(2):169–173. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz U Protein tyrosine phosphatase assays. Curr Protoc Immunol. 2011;11:11.7. 10.1002/0471142735.im1107s93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danforth WH, Helmreich E, Cori CF. The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci USA. 1962;48(7):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–846. [DOI] [PubMed] [Google Scholar]
- 18.Keyse SM. An emerging family of dual specificity MAP kinase phosphatases. Biochim Biophys Acta. 1995;1265(2–3):152–160. [DOI] [PubMed] [Google Scholar]
- 19.George A, Pushkaran S, Konstantinidis DG, et al. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121(11):2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragadin M, Ion-Popa F, Clari G, Bordin L. SHP-1 tyrosine phosphatase in human erythrocytes. Ann N Y Acad Sci. 2007;1095:193–203. [DOI] [PubMed] [Google Scholar]
- 21.Bordin L, Brunati AM, Donella-Deana A, Baggio B, Toninello A, Clari G. Band 3 is an anchor protein and a target for SHP-2 tyrosine phosphatase in human erythrocytes. Blood. 2002;100(1):276–282. [DOI] [PubMed] [Google Scholar]
- 22.Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68(3):545–560. [DOI] [PubMed] [Google Scholar]
- 23.Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PIH. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295(5560):1708–1711. [DOI] [PubMed] [Google Scholar]
- 24.Frangioni JV, Oda A, Smith M, Salzman EW, Neel BG. Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J. 1993;12(12):4843–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati P, Markova B, Göttlicher M, Böhmer F-D, Herrlich PA. UVA inactivates protein tyrosine phosphatases by calpain-mediated degradation. EMBO Rep. 2004;5(8):812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol Cell Biol. 2007;27(17):6038–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li Q, Youn JY, Cai H. Protein phosphotyrosine phosphatase 1B (PTP1B) in calpain-dependent feedback regulation of vascular endothelial growth factor receptor (VEGFR2) in endothelial cells: implications in VEGF-dependent angiogenesis and diabetic wound healing. J Biol Chem. 2017;292(2):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picha KM, Patel SS, Mandiyan S, Koehn J, Wennogle LP. The role of the C-terminal domain of protein tyrosine phosphatase-1B in phosphatase activity and substrate binding. J Biol Chem. 2007;282(5):2911–2917. [DOI] [PubMed] [Google Scholar]
- 29.Merciris P, Hardy-Dessources M-D, Giraud F. Deoxygenation of sickle cells stimulates Syk tyrosine kinase and inhibits a membrane tyrosine phosphatase. Blood. 2001;98(10): 3121–3127. [DOI] [PubMed] [Google Scholar]
- 30.Siciliano A, Turrini F, Bertoldi M, et al. Deoxygenation affects tyrosine phosphoproteome of red cell membrane from patients with sickle cell disease. Blood Cells Mol Dis. 2010;44(4):233–242. [DOI] [PubMed] [Google Scholar]
- 31.Terra HT, Saad MJ, Carvalho CR, Vicentin DL, Costa FF, Saad ST. Increased tyrosine phosphorylation of band 3 in hemoglobinopathies. Am J Hematol. 1998;58(3):224–230. [DOI] [PubMed] [Google Scholar]
- 32.Whelihan MF, Lim MY, Mooberry MJ, et al. Thrombin generation and cell-dependent hypercoagulability in sickle cell disease. J Thromb Haemost. 2016;14(10):1941–1952. [DOI] [PubMed] [Google Scholar]
- 33.Atmis A, Sasmaz I. Microparticle profile during painful crisis and steady state period in sickle cell anemia. Blood. 2013;122(21):4681. [Google Scholar]
- 34.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. [DOI] [PubMed] [Google Scholar]
- 36.Kaul DK, Nagel RL, Chen D, Tsai HM. Sickle erythrocyte-endothelial interactions in microcirculation: the role of von Willebrand factor and implications for vasoocclusion. Blood. 1993;81(9):2429–2438. [PubMed] [Google Scholar]
- 37.Wautier J-L, Wautier M-P. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin Hemorheol Microcirc. 2013;53(1–2):11–21. [DOI] [PubMed] [Google Scholar]
- 38.Dumaswala UJ, Greenwalt TJ. Human erythrocytes shed exocytic vesicles in vivo. Transfusion. 1984;24(6):490–492. [DOI] [PubMed] [Google Scholar]
- 39.Fibach E The redox balance and membrane shedding in RBC production, maturation, and senescence. Front Physiol. 2021;12:604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Advani R, Sorenson S, Shinar E, Lande W, Rachmilewitz E, Schrier SL. Characterization and comparison of the red blood cell membrane damage in severe human alpha- and betathalassemia. Blood. 1992;79(4):1058–1063. [PubMed] [Google Scholar]
- 41.Alaarg A, Schiffelers RM, van Solinge WW, van Wijk R. Red blood cell vesiculation in hereditary hemolytic anemia. Front Physiol. 2013;4:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiffert T, Bookchin RM, Lew VL. Calcium homeostasis in normal and abnormal human red cells. In: Bernhardt I, Ellory JC (Eds.). Red Cell Membrane Transport in Health and Disease. Springer; Berlin Heidelberg; 2003:373–405. [Google Scholar]
- 43.Scharff O Regulation of (Ca2+, Mg2+)-ATPase in human erythrocytes dependent on calcium and calmodulin. Acta Biol Med Ger. 1981;40(4–5):457–463. [PubMed] [Google Scholar]
- 44.Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in red blood cells—a perilous balance. Int J Mol Sci. 2013;14(5):9848–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellory JC, Robinson HC, Browning JA, Stewart GW, Gehl KA, Gibson JS. Abnormal permeability pathways in human red blood cells. Blood Cells Mol Dis. 2007;39(1):1–6. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y-L, Rees DC, Gibson JS, Ellory JC. The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors. J Physiol. 2012;590(9):2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Browning JA, Staines HM, Robinson HC, Powell T, Ellory JC, Gibson JS. The effect of deoxygenation on whole-cell conductance of red blood cells from healthy individuals and patients with sickle cell disease. Blood. 2007;109(6): 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawasaki H, Emori Y, Suzuki K. Calpastatin has two distinct sites for interaction with calpain-effect of calpastatin fragments on the binding of calpain to membranes. Arch Biochem Biophys. 1993;305(2):467–472. [DOI] [PubMed] [Google Scholar]
- 49.De Franceschi L, Franco RS, Bertoldi M, et al. Pharmacological inhibition of calpain-1 prevents red cell dehydration and reduces Gardos channel activity in a mouse model of sickle cell disease. FASEB J. 2013;27(2):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lon H-K, Mendonca N, Goss S, et al. Pharmacokinetics, safety, tolerability, and pharmacodynamics of alicapistat, a selective inhibitor of human calpains 1 and 2 for the treatment of alzheimer disease: an overview of phase 1 studies. Clin Pharmacol Drug Dev. 2019;8(3):290–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the methods.


