Abstract
Aims
Hydrogen sulfide (H2S) is a potent signalling molecule that activates diverse cardioprotective pathways by post-translational modification (persulfidation) of cysteine residues in upstream protein targets. Heart failure patients with reduced ejection fraction (HFrEF) exhibit low levels of H2S. Sulfide:quinone oxidoreductase (SQOR) catalyses the first irreversible step in the metabolism of H2S and plays a key role in regulating H2S-mediated signalling. Here, the aim of this study was to discover a first-in-class inhibitor of human SQOR and evaluate its cardioprotective effect in an animal model of HFrEF.
Methods and results
We identified a potent inhibitor of human SQOR (STI1, IC50 = 29 nM) by high-throughput screening of a small-molecule library, followed by focused medicinal chemistry optimization and structure-based design. STI1 is a competitive inhibitor that binds with high selectivity to the coenzyme Q-binding pocket in SQOR. STI1 exhibited very low cytotoxicity and attenuated the hypertrophic response of neonatal rat ventricular cardiomyocytes and H9c2 cells induced by neurohormonal stressors. A mouse HFrEF model was produced by transverse aortic constriction (TAC). Treatment of TAC mice with STI1 mitigated the development of cardiomegaly, pulmonary congestion, dilatation of the left ventricle, and cardiac fibrosis and decreased the pressure gradient across the aortic constriction. Moreover, STI1 dramatically improved survival, preserved cardiac function, and prevented the progression to HFrEF by impeding the transition from compensated to decompensated left ventricle hypertrophy.
Conclusion
We demonstrate that the coenzyme Q-binding pocket in human SQOR is a druggable target and establish proof of concept for the potential of SQOR inhibitors to provide a novel therapeutic approach for the treatment of HFrEF.
Keywords: Heart failure, Cardiac disease, Hydrogen sulfide signalling, Sulfide:quinone oxidoreductase, Cardioprotection
Graphical Abstract
Graphical Abstract.
1. Introduction
Heart failure is a highly lethal cardiovascular syndrome currently affecting 6.5 million Americans with a projected patient population of over 8 million by 2030. Although improvements in Guideline Determined Medical Therapy have reduced morbidity and mortality, the 5-year survival rate in treated heart failure patients remains low at ≈50%. Approximately half of heart failure patients are characterized by reduced ejection fraction1 (HFrEF). Current standard-of-care pharmacologic therapy for symptomatic (NYHA Class II–IV) HFrEF patients primarily aims to decrease the workload of the heart by reducing blood pressure, blood volume, and/or heart rate. There is a compelling need for new drugs that reduce the major risk factors for myocardial injury and mitigate the pathological remodelling induced by injury that results in HFrEF.
The gasotransmitter hydrogen sulfide (H2S) is a widely recognized cardioprotective signalling molecule that hinders the development of atherosclerosis and hypertension, major risk factors for HFrEF,2–6 and mitigates pathological remodelling by activating diverse cardioprotective pathways (e.g. antihypertrophic, antioxidant, antiapoptotic, antifibrotic, proangiogenic, anti-inflammatory).7–17 Importantly, patients with HFrEF exhibit a pronounced decrease in the plasma concentration of H2S,18–20 a finding replicated in rodent models of HFrEF.8–10,21 Moreover, administration of exogenous H2S in animal models of HFrEF protects against functional remodelling of the left ventricle, induced by myocardial infarction or pressure overload.7–13
Reduced plasma levels of H2S constitute an apparent biomarker for decreased H2S-mediated cardioprotective signalling in tissues of patients and animal models of HFrEF. Studies over the past decade have established that H2S signalling is mediated by protein persulfidation, a reversible post-translational modification of reactive cysteine residues to cysteine persulfide (CysSSH) that alters protein structure and/or function.22 Cardiac remodelling and progression to HFrEF are strongly associated with oxidative stress, which leads to oxidation of cysteine residues in proteins.23,24 Importantly, protein persulfidation by reaction of H2S with oxidized cysteines, such as cysteine sulfenic acid (CysSOH + H2S → CysSSH + H2O), constitutes a major pathway for H2S signalling in response to oxidative stress.25,26
H2S-mediated signalling is regulated by small differences between the rapid rate of mitochondrial consumption of H2S and the rapid rate of H2S biosynthesis.27 Sulfide:quinone oxidoreductase (SQOR) catalyses the first irreversible step in the mitochondrial oxidation of H2S28,29 and thus sits at the optimal pharmacological intervention point in this pathway. SQOR is a monotopic inner mitochondrial membrane protein that uses coenzyme Q (CoQ) as electron acceptor and is expressed throughout the cardiovascular system. Our recently solved X-ray structure of human SQOR reveals a large, predominantly hydrophobic CoQ-binding pocket likely to bind drug-like molecules.30 SQOR plays a key role in regulating H2S-mediated signalling, as inferred from the large increase in tissue H2S levels when the mitochondrial metabolism of H2S is blocked by a genetic deficiency of SQOR.31
Collectively, these data led us to hypothesize that small-molecule inhibitors of SQOR constitute a potential novel class of drugs for the treatment of HFrEF that slow the mitochondrial metabolism of H2S to activate cardioprotective signalling in patients via persulfidation of pivotal target proteins. The purpose of this study was to (i) identify a first-in-class inhibitor of human SQOR and (ii) evaluate its cardioprotective effect in an animal model of HFrEF.
Here, we describe our discovery of a potent and highly selective small-molecule inhibitor, SQOR-targeted inhibitor 1 (STI1), that binds to the CoQ-binding pocket in human SQOR. A proof of concept study, conducted in a mouse model of heart failure induced by pressure overload, provides compelling evidence for the ability of STI1 to protect against pathological remodelling of the left ventricle and the progression to HFrEF.
2. Methods
2.1 High-throughput screening of a small-molecule library
High-throughput screening (HTS) was performed using recombinant human SQOR28 and a 2,6-dichlorophenolindophenol (DCIP) endpoint assay developed in this study, as described in Supplementary material online, Methods. Briefly, this assay employs a water-soluble coenzyme Q derivative (CoQ1) and sulfite as electron and sulfane sulfur (S0) acceptor, respectively, in the SQOR-catalysed oxidation of H2S to S0. Reaction mixtures containing enzyme, substrates (H2S, sulfite, CoQ1), and potential inhibitors are incubated in 96-well plates. The reactions are quenched after 60 s by the addition of formaldehyde to denature SQOR and N-ethylmaleimide to consume unreacted H2S, a step that prevents the non-enzymic reduction of DCIP by H2S. DCIP is added after a 10 min incubation. Reduced CoQ1, produced in the SQOR reaction, reduces DCIP in a non-enzymatic reaction that abolishes the intense blue colour of the oxidized dye at 600 nm. The absorbance at 600 nm is read 2 min after DCIP addition.
The DCIP endpoint assay was used to screen 41 000 compounds from a library provided by the Baruch S. Blumberg Institute (Doylestown, PA). This library was assembled by cherry-picking compounds from four commercial collections: Chemical Diversity Inc. (San Diego, CA); Asinex Inc. (Moscow, Russia); Chembridge Inc. (DIVERSet Library) (San Diego, CA); Maybridge Inc. (HitFinderTM sub-library) (Cornwall, UK). The library compounds exhibit an average molecular weight of ∼350 Da, a mean cLogP of 3.4, and a mean topological polar surface area molecular (tPSA) of 74.
Compounds that exhibited percentage inhibition values above the μ + 3 σ threshold were classified as hits. Hit confirmation was conducted by performing dose-response measurements, as described in Supplementary material online, Methods.
2.2 Inhibitor mode of action studies
Steady-state kinetic assays to determine the mode of inhibitor action were conducted as described in Supplementary material online, Methods.
2.3 Selectivity assays
The selectivity of STI1 was determined by assessing the effect of the compound on the catalytic activity of complex I, complex II, complex III, electron-transfer flavoprotein:ubiquinone oxidoreductase, and dihydroorotate dehydrogenase, as described in Supplementary material online, Methods.
2.4 Model of the SQOR•STI1 complex
Docking of STI1 to ligand-free SQOR (PDB: ID 6M06) was performed with GLIDE (Schrödinger Suite 2019-3; Schrödinger, LLC), as described in Supplementary material online, Methods.
2.5 Animals
Animal care and experimental protocols were approved by the Institutional Animal Care & Use Committee of Drexel University. All studies complied with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.6 Cell-based hypertrophy and cytotoxicity assays
Hypertrophy and cytotoxicity assays were conducted with neonatal rat ventricular cardiomyocytes (NRVMs) and H9c2 cells, as described in Supplementary material online, Methods. Sprague–Dawley rat pups were placed indirectly on ice to cool before decapitation euthanasia. Following euthanasia, NRVMs were isolated from the hearts of 1 to 2-day-old pups, as described in Supplementary material online, Methods. Sprague–Dawley rat dams were euthanized by gradual displacement of room air to carbon dioxide (CO2) at a rate of ∼20% CO2 per minute.
2.7 Transverse aortic constriction protocol
Male C57BL/6J CD-1 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in a 12:12 light-dark facility with food and water ad libitum. Mice (22–25 g body weight) were anaesthetized with 2–3% isoflurane gas (in 100% oxygen, 1.0 L/min). The surgical plane of anaesthesia was assessed by pedal reflex (firm toe pinch), and the adequacy of anaesthesia was monitored every 10 min by examining respiratory rate and pattern. Prior to surgery, the animals were administered with 0.05 mg/kg buprenorphine. The mice were orally intubated by direct visualization of the intubation cannula (1.0 mm OD, Harvard Apparatus, 73–2737) inside the trachea. The lung ventilation was started immediately in the pressure-controlled ventilation mode using Kent Scientific TOPO Dual Mode Rodent Ventilator (100% oxygen flow at 0.6–0.8 L/min; isoflurane 1.5%; respiration rate of 120–130 b.p.m., peak inspiration pressure 12–15 cm H2O). After intubation, the transverse aortic constriction (TAC) procedure was performed by tying the aortic arch between the brachiocephalic trunk (innominate artery) and the left common carotid artery, as described in Supplementary material online, Methods. Following surgical closure of the chest in layers, the anaesthesia was stopped, and the animals switched to inhale 100% oxygen for several minutes and then were extubated and removed from the ventilator to recover under a warming heat lamp, followed by post-operative care. Animals were administered with 10 mg/kg of meloxicam and carefully monitored for post-surgical complications. Those that displayed abnormalities or complications were removed from the study. Mice subjected to TAC surgery were randomly assigned to receive STI1 or vehicle. The animals were euthanized 12 weeks post-surgery by carbon dioxide (CO2) overdose with a displacement rate of 50% chamber volume per minute, followed by exsanguination. Hearts and lungs were harvested, weighed, and tibia lengths were measured.
2.8 Preparation of stock solutions and administration of STI1
Solutions of STI1 were prepared and administered as described in Supplementary material online, Methods.
2.9 Echocardiography
Transthoracic echocardiography was used to non-invasively quantify cardiac structural and functional changes during the progression to heart failure in the TAC mouse model. Measurements were conducted in animals under anaesthesia with 2–3% isoflurane gas mixed with 100% oxygen flowing at 1.0 L/min using a Vevo® 2100 Imaging System (VisualSonics, Canada) with a high-frequency (18–38 MHz) probe (MS400). The heart and respiration rates were continuously monitored, with the target heart rate of 430–480 b.p.m. Echocardiography using colour Doppler was performed 1 week after surgery to confirm the constriction. The aortic constriction was further validated by conducting colour Doppler measurements 12 weeks after surgery. Echocardiography using pulsed-wave Doppler was performed 12 weeks after surgery to determine the pressure gradient across the aortic constriction. Blood velocity peaks (V) were measured, and a peak pressure gradient was calculated using the modified Bernoulli equation (Pressure gradient = 4 × V2).32,33 Baseline echocardiography evaluations were performed 1–2 days prior to surgery. For these studies, parasternal long- and short-axis images were obtained in 2D B-mode and M-mode. Additional images were obtained at regular intervals (2, 4, 6, 8, 10, and 12 weeks) after surgery. To evaluate cardiac structure and function, the echocardiographic data were analysed in a blinded manner to determine left ventricle (LV) end-diastolic diameter, LV end-systolic diameter, LV end-diastolic volume, LV end-systolic volume, LV ejection fraction, LV fractional shortening, and LV end-diastolic anterior wall thickness (see Supplementary material online, Table S2).
2.10 Cardiac histology
Cardiac histology was conducted as described in Supplementary material online, Methods.
2.11 Statistical analysis
All data are expressed as means ± SEM, except as noted in Supplementary material online, Figure S12B. Statistical analyses were performed using GraphPAD Prism 8.4 (GraphPAD Software, LLC). Survival curves were analysed by the use of the Kaplan–Meier log-rank (Mantel–Haenszel approach) test. Statistical significance for comparison between three or more means was evaluated using one-way ANOVA with the Tukey–Kramer post hoc test. For echocardiographic data, obtained 1–2 days prior to (baseline) and at 2-week intervals after surgery, statistical significance was evaluated using two-way repeated ANOVA with the Bonferroni post hoc test. For all analyses, P < 0.05 was considered statistically significant. Post hoc power analysis was performed using G*Power (Version 3.1.9.7) on outcome measures observed with sham, TAC + STI1, and TAC + vehicle mice at 12 weeks post-surgery. We confirmed that for our observed effect size with a significance level of α = 0.05, that a sample size of four per group was sufficient to produce moderately robust results (power = 0.76).
3. Results
3.1 Identification of drug-like SQOR inhibitors by screening a small-molecule library
No specific inhibitors of human SQOR were known at the start of this project. Studies to identify an inhibitor suitable for in vivo efficacy testing were initiated by screening a small-molecule library. The catalytic activity of human SQOR can be measured by monitoring the reduction of a water-soluble derivative of coenzyme Q (CoQ1) in the UV region.28 However, this continuous spectrophotometric assay is unsuitable for HTS because most drug-like small-molecules absorb in the UV region. To overcome this limitation, we developed a two-step endpoint assay that is based on the ability of reduced CoQ1, produced in the SQOR reaction, to non-enzymatically reduce a blue dye (DCIP, 2,6-dichlorophenolindophenol) in a second step that abolishes the intense blue colour of the oxidized dye (Supplementary material online, Figure S1A). The DCIP endpoint assay meets the performance criteria required for HTS (Z-factor = 0.78)34 (Supplementary material online, Figure S1B).
The Baruch S. Blumberg Institute library was assembled by cherry-picking small-molecules from commercial collections for diversity, solubility, and drug-like qualities, while filtering out compounds with undesired features.35 We used the DCIP endpoint assay to screen ∼41 000 compounds from this library at one concentration (10 μM). A scatter plot of the observed percentage inhibition data highlights numerous apparent potent inhibitors (Figure 1A), but, as anticipated, most compounds were inactive. A histogram plot of the data exhibits an expected normal distribution with a mean close to zero (Supplementary material online, Figure S1C). A threshold for the selection of hits was specified as a percentage inhibition value above μ + 3σ (μ, mean; σ, standard deviation), as indicated by the upper dashed line in Figure 1A. By applying this criterion, we identified 677 compounds as hits, corresponding to a hit rate of 1.7%. Samples of the primary hits were retrieved from the mother plates and retested at eight concentrations ranging from 2.4 nM to 15 μM. Out of 677 retested compounds, 646 exhibited a sigmoidal dose-response curve expected for true positives, as illustrated by results obtained for several confirmed hits (Figure 1B). The impressive hit confirmation rate (95%) provides evidence in support of the quality of the library and the HTS assay.
Figure 1.
Discovery of potent small-molecule inhibitors of human SQOR. (A) Inhibition of SQOR observed during a high-throughput screen of 41 000 compounds at a single concentration (10 μM) from the Baruch S. Blumberg Institute small-molecule library using the DCIP endpoint assay (see Supplementary material online, Figure S1A), conducted in singlicate. Data points are colored according to the commercial source of the tested compound: Asinex, red; Chembridge DIVERSet Library, blue; Chemical Diversity, magenta; Maybridge HitFinderTM sub-library, green. Black dashed lines are shown at μ ± 3σ. (B) Hit validation IC50 curves for the class A inhibitors are shown in C. Each data point is an average of two replicate assays. (C) Structures and properties of class A/A’ SQOR inhibitors. (D–G) Class A/A’ inhibitors and CoQ compete for the same binding site in SQOR. A continuous spectrophotometric assay was used to monitor the reduction of CoQ1 at the concentrations of STI1, HTS12441, RH00520, and HTS12442 indicated in panels D–G, respectively. Each data point is an average of two replicate assays. The solid lines in each panel were obtained by fitting for an equation for competitive inhibition (Eq. (1), see Supplementary material online, Methods) to the data (R2 = 0.9882, 0.9907, 0.9894, and 0.9751, respectively). (H) View of the SQOR CoQ-binding pocket with bound substrate (decylubiquinone, DCQ)30 or STI1. The surface of the CoQ-binding cavity is shown in white. Carbons in amino acid side chains, DCQ, and STI1 are colored white, green, and yellow, respectively. The model of the SQOR complex with STI1 was produced using GLIDE (Schrödinger). A hydrogen bond between the 2-pyridyl ring in STI1 and W435:NE1 is indicated by the dashed yellow line.
3.2 Discovery of a potent series (class A/A’) of SQOR inhibitors
Typically, 3–5 structural classes from a successful screen emerge as suitable candidates for launching a hit-to-lead optimization campaign.36 Our confirmed HTS hits included >500 compounds that inhibit SQOR with IC50 <20 μM. We narrowed down the number of compounds by using a StarDrop 6.1 (Optibrium Ltd., Cambridge, UK) profile developed based on properties exhibited by successful, orally administered non-CNS drugs,37 which we amended to include an SQOR-specific inhibition criterion (Supplementary material online, Figure S2). Additionally, we performed in silico screening of confirmed hits by using StarDrop P450 metabolism and Derek Nexus knowledge-based prediction models38,39 to evaluate metabolic stability and toxicity, respectively. We selected three chemical classes of compounds based on the combined results of these analyses, with the highest priority assigned to 2,4-diphenylpyridines (class A/A’) (Figure 1C). The three top class A/A’ HTS hits (HTS12411, RH00520, and HTS07545) are potent SQOR inhibitors (IC50 ≤ 30 nM) but exhibit poor aqueous solubility and cLogP values (cLogP > 5) considerably above the desired range (1–3) (Figure 1B and C, Supplementary material online, Figure S2). As we will report elsewhere, structure-activity relationship and structure-based design studies resulted in the identification of the cell-permeable class A’ derivative, STI1, that retains the high potency observed for the top class A/A’ HTS hits (IC50 = 29.3 nM) with improved polarity (cLogP = 3.0), topological polar surface area (tPSA = 84), and aqueous solubility (Figure 1C).
3.3 Class A/A’ SQOR inhibitors block substrate access to the CoQ-binding site
Our recently solved X-ray structure of human SQOR reveals the presence of two active sites: (i) a small-hydrophilic H2S-oxidizing active site; (ii) a large, predominantly hydrophobic CoQ-binding pocket, which appeared more likely to bind drug-like molecules.30 To evaluate this hypothesis, we investigated the mechanism of inhibitor action to determine whether the inhibitors and CoQ compete for the same binding site. Accordingly, we conducted steady-state kinetic studies with STI1 and three class A HTS hits by monitoring SQOR activity at various concentrations of inhibitor and CoQ1 in the presence of saturating concentrations of other substrates (sulfite, sulfide). The inhibition kinetics observed with STI1, HTS12441, RH00520, and HTS12442 are described best by an equation defining competitive inhibition, as illustrated by the Lineweaver–Burk plot fits shown in Figure 1D–G. It is worth noting that the IC50 values of these inhibitors are two-fold larger than values determined for the dissociation constant of the corresponding E•I complexes (Ki) in steady-state kinetic studies (Supplementary material online, Table S1). The observed relationship, IC50 = 2 Ki, is a characteristic feature of competitive inhibitors.40 The results indicate that class A/A’ inhibitors interfere with SQOR catalysis by blocking substrate access to the CoQ-binding site and are consistent with the proposal that the inhibitors also bind to the CoQ-binding pocket.
3.4 Model of the SQOR•STI1 complex
The CoQ-binding pocket in human SQOR is lined with mostly hydrophobic residues (Figure 1H) and accessed via an entrance on the enzyme’s membrane-binding surface (Supplementary material online, Figure S3).30 The polar quinone ring of decylubiquinone (DCQ) (Figure 1H, green carbons) is bound deep within the CoQ-binding pocket. The non-polar decyl substituent of DCQ extends from the quinone ring through a tunnel leading to an opening on the surface of the protein; the terminal carbon of the decyl tail is exposed on the protein surface (Figure 1H, Supplementary material online, Figure S3).
A model of the SQOR•STI1 complex was produced using GLIDE (Schrödinger) with flexible ligand/rigid protein docking to ligand-free SQOR (PDB: ID 6M06). A similar model for the preferred docking pose was obtained using the GOLD Suite of Programs (CCDC Software Ltd.). As predicted, docking studies indicate that STI1 binds within the CoQ-binding pocket (Figure 1H, yellow carbons), occupying a binding site very similar to that observed for DCQ. Thus, the ring nitrogen of the 2-pyridyl substituent in STI1 superimposes with the O2 carbonyl oxygen in DCQ, and both atoms are hydrogen-bonded to Trp435:NE1. The amino moiety of the para-aminophenyl substituent in STI1 points towards the entrance of the pocket and is exposed on the protein surface, as observed for terminal carbon of the decyl tail in DCQ. Other class A/A’ inhibitors also bind in the CoQ-binding pocket, as judged by results obtained in docking studies that we will report elsewhere.
3.5 STI1 exhibits high selectivity for SQOR
The known mammalian CoQ-dependent reactions are catalysed by metabolically essential respiratory complexes or monotopic integral membrane proteins located in the inner mitochondrial membrane (Figure 2A). Selectivity studies were conducted to determine whether STI compounds will be safe, with little or no off-target toxicity. Except as indicated, the selectivity of STI1 was evaluated at a concentration of 10.5 μM, more than 350-fold higher than the IC50 value observed with SQOR (IC50 = 29.3 nM). Solubility constraints precluded studies at higher concentrations. The catalytic activity of each of the respiratory complexes (I, II, and III) was measured using mouse muscle homogenate preparations in assays (Figure 2B–D) that are conducted in the presence or absence of a target-specific inhibitor (rotenone, malonate, and antimycin A, respectively) to evaluate the contribution due to nonspecific substrate oxidation.41 The addition of 10.5 μM STI1 resulted in a 21.6% decrease in the activity observed for reduced nicotinamide adenine dinucleotide (NADH) oxidation by complex I (Figure 2B). Succinate oxidation by complex II was unaffected by the addition of STI1 (Figure 2C). Similarly, STI1 did not affect the initial rate of decylubiquinol oxidation by complex III (Figure 2D). The oxidation of dihydroorotate by dihydroorotate dehydrogenase (DHODH) was measured using recombinant human DHODH in an assay similar to that previously described.42 Enzyme activity was nearly completely inhibited (98.2%) in the presence of 100 nM brequinar, a DHODH-specific inhibitor (IC50 = 10.3 nM),42 whereas the addition of 10.5 μM STI1 resulted in a 22.4% decrease in DHODH activity (Figure 2E). Electron transferring flavoprotein:ubiquinone oxidoreductase (ETF:QO) activity was measured by using a coupled succinate-ETF reductase assay43 (Supplementary material online, Figure S4) that is conducted using heart submitochondrial particles and recombinant porcine ETF. In this assay, ETF:QO catalyses a rate-limiting reduction of ETF that is measured by monitoring the decrease in the fluorescence of oxidized ETF. The addition of 1.0 μM STI1 did not affect ETF:QO activity (Figure 2F); the weak intrinsic fluorescence of STI1 precluded studies at higher concentrations.
Figure 2.
STI1 exhibits a high degree of selectivity for the CoQ-binding site in SQOR. (A) The known mammalian CoQ-dependent reactions include sulfide:quinone oxidoreductase (SQOR), complexes I, II, and III, dihydroorotate dehydrogenase (DHODH), and electron transferring flavoprotein:ubiquinone oxidoreductase (ETF:QO). (B–D) Representative traces of the activity of complex I, complex II, and complex III observed in assays with mitochondrial membrane preparations conducted in the absence of inhibitor or in the presence of STI1 or a complex-specific inhibitor: rotenone, malonate, and antimycin A (AMA), respectively. Complex I activity was measured by monitoring the oxidation of NADH at 340 nm. Succinate oxidation by complex II with decylubiquinone (DCQ) as electron acceptor generates decylubiquinol (DCQH2), which reduces DCIP; the activity of complex II was assessed by monitoring DCIP reduction at 600 nm. The oxidation of decylubiquinol by complex III was assessed by monitoring the rate of cytochrome C reduction at 550 nm. (E) Averages of DHODH activity (n = 2) observed with recombinant enzyme in the absence of inhibitor or in the presence of STI1 or brequinar (solid lines). Dihydroorotate oxidation by DHODH with DCQ as electron acceptor produces DCQH2; DHODH activity was measured by monitoring the DCQH2-dependent reduction of DCIP at 600 nm. The dotted red lines were generated by linear regression analysis of data: r2 = 0.9956 (no inhibitor), 0.9945 (10.5 μM STI1), 0.9325 (100 nM brequinar). (F) ETF:QO activity was measured by monitoring the decrease in the fluorescence of oxidized ETF in a coupled succinate-ETF reductase assay43that was conducted using mouse heart submitochondrial particles and recombinant porcine ETF. The black and red circles show averages (n = 3) of relative fluorescence (RF) intensity ( ± SEM), measured at 3 s intervals, in the absence and presence of 1.0 μM STI1, respectively. The black and red lines were generated by linear regression analysis of the data: r2 = 0.9871 (no inhibitor), 0.9837 (1.0 μM STI1). (G) The selectivity profile of STI1 was constructed using estimated IC50 values for complexes I–III, DHODH, and ETF:QO, as described in the text.
The selectivity index (SI) of an inhibitor is determined by comparing the IC50 value observed for an off-target reaction with that observed for the target reaction (SI = IC50 off-target/IC50 target). IC50 values for STI1 inhibition of complex I and DHODH were estimated based on the modest inhibition observed at 10.5 μM STI1 (∼460 μM and ∼300 μM, respectively). Lower limits for the corresponding IC50 values for STI1 inhibition of complex II or complex III (IC50 > 1000 μM) or ETF: QO (IC50 > 100 μM) were estimated based on the absence of inhibition at the highest STI1 concentration that could be tested. The estimated off-target IC50 values are at least three orders of magnitude larger than the IC50 value observed with SQOR. The results indicate that STI1 is a highly selective inhibitor of SQOR (SI ≥ 1000) (Figure 2G). For comparison, we used an IC50 value determined for the off-target reaction of brequinar with SQOR (IC50 = 3.6 μM) (Supplementary material online, Figure S5) to calculate an SI value for the DHODH-specific inhibitor. The value obtained for brequinar (SI = 350) is at least three-fold smaller than the SI values estimated for our SQOR-specific inhibitor.
3.6 Characterization of the in vitro cardioprotective and in vivo pharmacokinetic properties of STI1
Before in vivo testing in a mouse heart failure model, STI1 was subjected to extensive in vitro evaluation. Firstly, we found that STI1 exhibits very low cytotoxicity in H9c2 cells, a rat ventricular cardiomyoblast cell line (CC50 = 56 ± 10 μM) and in neonatal rat ventricular cardiomyocytes (NRVMs) (CC50 = 26 ± 4 μM) (Supplementary material online, Figure S6). The results are consistent with expectations based on our biochemical studies, which show that STI1 is a potent and highly selective inhibitor of SQOR.
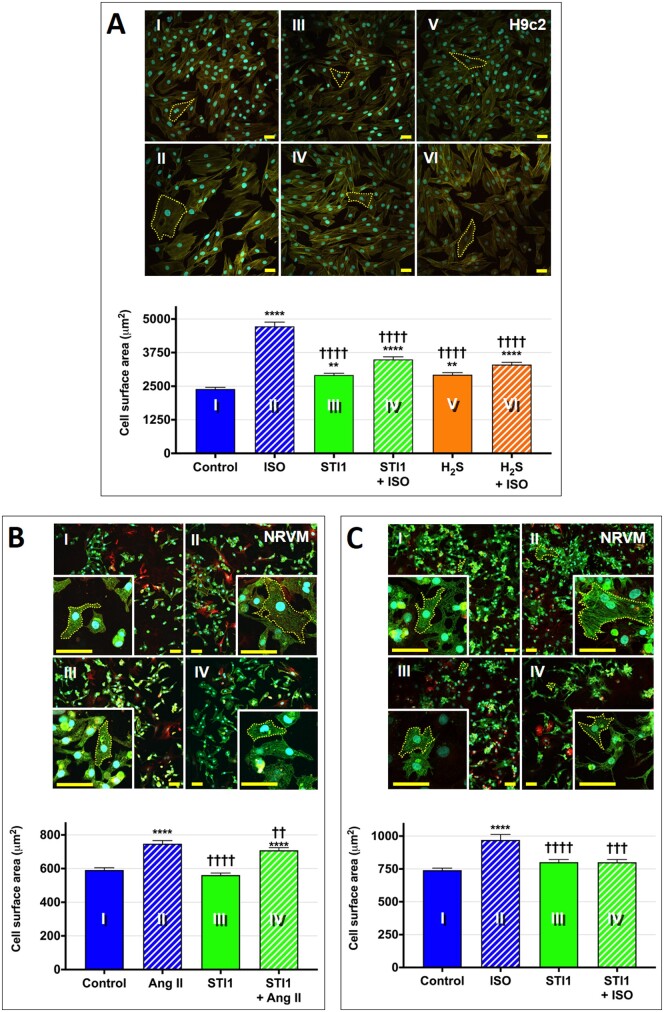
Abnormal enlargement of the heart is characterized at the cellular level by an increase in cardiomyocyte size and may be induced by chronic elevation of stress hormones (epinephrine, norepinephrine) and activation of the renin–angiotensin–aldosterone system (RAAS).44 The in vivo response can be simulated in vitro by treating cells with agonists that induce cardiac hypertrophy in animal models, such as isoproterenol, an analogue of epinephrine and β-adrenergic receptor agonist, or angiotensin II (Ang II), a key mediator of the RAAS.45–47 To investigate the cardioprotective effect of SQOR inhibition, we assessed the ability of STI1 to inhibit hypertrophic growth of NRVMs and H9c2 cells induced by various agonists. We found that STI1 significantly mitigated the two-fold increase in the cell surface area of H9c2 cells induced by isoproterenol, similar to the anti-hypertrophic effect observed with a 10-fold higher concentration of H2S (Figure 3A). Further, we found that STI1 significantly attenuated or completely blocked the 1.3-fold increase in the size of NRVMs induced by treatment with Ang II (Figure 3B) or isoproterenol (Figure 3C), respectively. The results demonstrate that inhibition of SQOR with STI1 significantly attenuates the in vitro hypertrophic response induced by neurohormonal stressors.
Figure 3.
SQOR inhibition with STI1 attenuates hypertrophic growth in vitro. (A) Representative confocal images of H9c2 cells treated with ±STI1 (10.5 μM) or ±H2S (100 μM) and ±isoproterenol (ISO) (100 μM, 24 h; then 50 μM, 24 h) with quantification of cell area (n = 300 cells/condition). Cells were stained using fluorescent reagents to visualize actin fibres (green), cell membranes (red), and nuclei (blue); scale bar = 50 μm. One-way ANOVA with the Tukey–Kramer post hoc test, **P < 0.01 or ****P < 0.0001 vs. control (I); ††††P < 0.0001 vs. ISO (II). (B) Representative confocal images of neonatal rat ventricular cardiomyocytes (NRVMs) treated with ±STI1 (10.5 μM) and ± angiotensin II (Ang II) (200 nM, 24 h) with quantification of cell area (n = 300–550 cells/condition). Cells were stained using fluorescent reagents to visualize α-actinin (green), cell membranes (red), and nuclei (blue); scale bar = 50 μm. One-way ANOVA with the Tukey–Kramer post hoc test, ****P < 0.0001 vs. control (I); ††P < 0.01 or ††††P < 0.0001 vs. Ang II (II). (C) Representative confocal images of NRVMs treated with ±STI1 (10.5 μM) and ± ISO (100 nM, 24 h) with cell area quantification (n = 250–300 cells/condition). Cells were stained as in panel B; scale bar = 50 μm. One-way ANOVA with the Tukey–Kramer post hoc test, ****P < 0.0001 vs. control (I); †††P < 0.001 or ††††P < 0.0001 vs. ISO (II).
Mouse plasma pharmacokinetics studies demonstrate that STI1 exhibits an acceptable t1/2 value (1.13 h) and good apparent exposure, as judged by the observed area under the curve value (1082 ng·h/mL) (Supplementary material online, Figure S7). Importantly, the plasma concentration of STI1 (measured 5 min after IP administration at 10 mg/kg) did not exceed the concentration (10.5 μM) tested in in vitro cytotoxicity studies.
A control study was conducted to determine whether STI1 might affect the expression of SQOR or cystathionine γ-lyase (CSE), the enzyme that provides the major source of H2S in the mammalian cardiovascular system.2 We found that daily IP administration of STI1 for 15 days at 10 mg/kg did not change the cardiac expression of the gene for SQOR (sqrdl) or the gene for CSE (Cth), as determined by reverse transcription-polymerase chain reaction (RT-PCR) analysis (Supplementary material online, Figure S8).
3.7 STI1 improves survival and mitigates cardiomegaly and pulmonary congestion in a heart failure model
The demonstrated in vitro safety and potency of STI1 and the observed pharmacokinetic properties provided a strong rationale to focus on the second objective of this study: explore for the first time the cardioprotective efficacy of a small-molecule inhibitor of H2S metabolism in an animal model of heart failure. Aortic stenosis or elevated blood pressure can trigger an initially compensatory enlargement of the heart to sustain cardiac output but ultimately results in pressure overload-induced heart failure with reduced ejection fraction (HFrEF). TAC, which mimics human aortic stenosis, is a highly regarded mouse model for pressure-induced myocardial enlargement and HFrEF.48–50 We selected the TAC mouse model for a proof of concept study with STI1. Daily IP dosing with the SQOR inhibitor was initiated 24 h after surgery at the same dosage (10 mg/kg) tested in pharmacokinetic studies (Figure 4A). STI1-treated TAC mice exhibited 100% survival at 12 weeks after surgery, as observed for sham-operated mice. In contrast, only a 67% survival was observed for animals receiving vehicle after TAC surgery, although the difference was not statistically significant (Figure 4B).
Figure 4.
Inhibition of SQOR with STI1 improves survival and mitigates cardiomegaly and pulmonary oedema in vivo. (A) Experimental protocol for the TAC mouse study to evaluate the in vivo efficacy of STI1. (B) Kaplan–Meier survival curves for 84 days following surgery in sham-operated mice (n = 8), TAC mice that received STI1 (n = 5), and TAC mice that received vehicle (n = 4 at t > 35 days post-surgery). (C) Representative photos of freshly excised whole hearts. (D) Ratio of heart weight to tibia length (HW:TL) observed for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals. One-way ANOVA with the Tukey–Kramer post hoc test, ††P < 0.01 vs. sham, *P < 0.05 vs. TAC + STI1. (E) Representative short-axis end-diastolic B-mode images obtained at 12 weeks post-surgery. Scale bar, 2.25 mm. (F) Ratio of lung weight to tibia length (LW:TL) observed for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals. The dotted red line indicates the 95% confidence interval for sham mice.
Visual inspection of hearts harvested at 12 weeks after TAC surgery showed that the organs from vehicle-treated mice were considerably enlarged in comparison with those from animals that received STI1 after surgery (Figure 4C). The gross morphology of the hearts was substantiated by the observed ratios of heart weight to tibia length which demonstrate that STI1 mitigated the statistically significant two-fold cardiac enlargement observed with animals receiving vehicle after surgery in comparison with the smaller increase (1.4-fold) observed with STI1-treated animals, which was not statistically significant (Figure 4D). Further evidence that STI1 treatment mitigated pathological cardiomegaly is provided by representative 2D end-diastolic images obtained at 12 weeks after surgery (Figure 4E).
Based on the observed ability of STI1 to mitigate cardiac enlargement, we anticipated that treatment with STI1 would also attenuate TAC-induced cardiac expression of a hypertrophic marker gene, such as brain natriuretic protein (BNP; Nppb). However, RT-PCR analysis revealed that TAC animals receiving vehicle or STI1 exhibited a similar increase in BNP expression (3.7- and 3.3-fold, respectively) as compared with sham animals (Supplementary material online, Figure S9).
Pulmonary oedema, the most severe cardinal manifestation of heart failure, can be detected based on the ratio of lung weight to tibia length. TAC mice exhibiting a ratio above the 95% confidence interval observed for sham-operated animals were classified as having heart failure, similar to a metric used in a prior study.33 On this basis, 75% of vehicle-treated TAC mice had developed heart failure and exhibited ratios that were 1.6-fold larger than sham-operated mice. In contrast, STI1-treated TAC mice exhibited ratios that were, on average, only 1.1-fold higher than sham mice, and just 20% developed heart failure (Figure 4F).
3.8 STI1 impedes the transition to decompensated left ventricle hypertrophy and preserves cardiac function
Dilatation of the left ventricle is a distinctive feature observed as the left ventricle transitions from compensatory enlargement to decompensated hypertrophy and HFrEF.51 A time-dependent evaluation of left ventricle dilatation was conducted via B-mode and M-mode echocardiography at baseline and 2-week intervals following surgery (Supplementary material online, Figure S10). Vehicle-treated TAC mice exhibited a progressive increase in the diameter (Figure 5A and B) and the volume (Figure 5C and D) of the left ventricle in end-diastole and end-systole; these changes were prevented by daily treatment with STI1 and not observed with sham-operated mice. The anti-dilatative effect of STI1 is highlighted in plots showing the quantification of the total change in the volume of the left ventricle in end-diastole and end-systole (ΔV), from baseline to 12 weeks after TAC or sham surgery, (Figure 5E and F). The TAC-induced ventricular dilation in vehicle-treated mice was accompanied by an increase in left ventricular wall thickness (Supplementary material online, Figure S11). A subsequent gradual thinning of the left ventricle wall, expected for the transition to decompensated hypertrophy, was not observed within the 12-week time frame of this study. STI1 delayed the onset of wall thickening but did not significantly reduce the extent of thickening observed by 4 weeks after surgery (Supplementary material online, Figure S11).
Figure 5.
STI1 prevents adverse cardiac remodeling and heart failure after transverse aortic constriction. Parasternal long-axis echocardiographic images were obtained in two-dimensional B-mode and M-mode 1–2 days prior to surgery (B = baseline) and then at 2-week intervals after surgery for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals. (A) Left ventricular end-diastolic diameter (LVDdiastole) (mm). (B) Left ventricular end-systolic diameter (LVDsystole) (mm); (C) Left ventricular volume during diastole [LVVdiastole (µL)]; (D) Left ventricular volume during systole [LVVsysstole (µL)]. (E–F) Quantification of the total change from baseline to 12 weeks post-surgery in LVVdiastole (ΔLVVdiastole) and LVVsysstole (ΔLVVsysstole), respectively; (G) Left ventricular ejection fraction (EF) (%). (H) Left ventricular fractional shortening (FS) (%). (I and J) Quantification of the total change from baseline to 12 weeks post-surgery in EF (ΔEF) and FS (ΔFS), respectively. Each data point in panels A–D and G and H is an average of measurements obtained for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals. Two-way ANOVA-Bonferroni post hoc test (A–D, G, H) or one-way ANOVA with the Tukey–Kramer post hoc test (E, F, I, J), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs. sham. †P < 0.05, ††P < 0.01, and †††P < 0.001 vs. TAC + STI1.
Vehicle-treated TAC mice exhibited a rapid initial loss of systolic function at 2 weeks, followed by a slower progressive decline from 2 to 12 weeks after TAC, as judged by the longitudinal evaluation of ejection fraction and fractional shortening. In contrast, no loss of systolic function was observed with STI1-treated TAC mice for 4 weeks following surgery, after which a gradual decline was observed (Figure 5G and H). Moreover, the total decline in ejection fraction and fractional shortening at 12 weeks following TAC was significantly greater in vehicle-treated TAC mice than in STI1-treated animals (Figure 5I and J).
3.9 STI1 attenuates pressure-induced development of cardiac fibrosis and cardiomyocyte hypertrophy
Pressure overload-induced cardiac fibrosis contributes to the dysfunction of the left ventricle observed in patients with high blood pressure or aortic stenosis and the TAC mouse HFrEF model.52,53 STI1 blocked the TAC-induced three-fold increase in interstitial fibrosis observed in mice receiving vehicle after TAC surgery (Figure 6A and B). Treatment with STI1 slightly, but significantly, blunted the increase in the size of left ventricular cardiomyocytes compared with vehicle-treated TAC mice, as estimated based on the cross-sectional cell area measured in short-axis sections (Figure 6C).
Figure 6.
Inhibition of SQOR with STI1 attenuates fibrosis and left ventricle cardiomyocyte hypertrophy after transverse aortic constriction. (A) Representative images of Masson trichrome-stained hearts were captured at 12.5× magnification; scale bar = 2000 μm. (B) Representative confocal images of left ventricle sections were stained with Masson’s trichrome to reveal the deposition of interstitial collagen (blue). Images were captured at 160× magnification; scale bar = 50 μm. Collagen fraction (fibrosis area, expressed as % of left ventricle area) for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals was quantified using Fiji Image J software. One-way ANOVA with the Tukey–Kramer post hoc test, *P < 0.05 vs. sham + vehicle. (C) Representative confocal images of left ventricle sections stained with CF®594 WGA conjugate. Scale bar = 50 μm. Cardiomyocyte cross-sectional area was quantified using Fiji Image J software. We measured 500 cells per heart section from each animal. Averages of 2000–4000 measurements for each group of animals are plotted in the bar graph. One-way ANOVA with the Tukey-Kramer post hoc test, ****P < 0.0001 vs. sham + vehicle; ††††P < 0.0001 vs. TAC + STI1.
3.10 STI1 mitigates the pressure gradient across the aortic constriction created by TAC
Mice subjected to TAC surgery were randomly assigned to receive STI1 or vehicle. The average size of the constriction was determined for each animal using colour Doppler recordings and Vevo® 2100 Imaging System software (Supplementary material online, Figure S12). The results indicate that the physical constriction (mean ± SD mm) was of the same degree in TAC mice that received STI1 (0.4095 ± 0.0026 mm) or vehicle (0.4112 ± 0.0058 mm) (Supplementary material online, Figure S12B). Furthermore, the measured physical constriction is in excellent agreement with a value expected (0.413 mm) based on the outer diameter of the 27-gauge needle used to create the constriction. However, echocardiography studies using pulsed-wave Doppler at 12 weeks after surgery revealed that the peak pressure gradient in TAC animals receiving vehicle was significantly greater (1.4-fold) than observed with STI1-treated TAC mice (Figure 7). Differences in the peak pressure gradient observed with sham and the two groups of TAC animals exhibit a modest linear correlation (r2 ∼ 0.4, P < 0.005) with differences in the extent of cardiac enlargement or loss of systolic function, as measured by the increase in the HW/TL ratio or the decrease in EF, respectively (Supplementary material online, Figure S13A and B). By contrast, a strong correlation (r2 = 0.83, P < 0.0001) is observed between the increase in the HW/TL ratio and the decrease in EF (Supplementary material online, Figure S13C). The results indicate that reduction in the TAC-induced pressure gradient can account for some of the cardioprotective effects observed with STI1.
Figure 7.

Inhibition of SQOR with STI1 mitigates the pressure gradient across the aortic constriction. Peak pressure gradients at 12 weeks post-surgery were calculated for sham (n = 8), TAC + STI1 (n = 5), and TAC + vehicle (n = 4) animals from Doppler velocities using the modified Bernoulli equation. One-way ANOVA with the Tukey–Kramer post hoc test, ††††P < 0.0001 vs. sham, *P < 0.05 vs. TAC + STI1.
4. Discussion
Here, we provide proof of concept for an innovative approach for the treatment of heart failure that involves a postulated novel mechanism of action, namely stimulation of H2S-mediated cardioprotective signalling by inhibition of SQOR, the enzyme that catalyses the first irreversible step in the mitochondrial oxidation of H2S. We demonstrate that SQOR is a druggable target, based on our discovery of a potent first-in-class inhibitor, STI1, that binds competitively and selectively to the CoQ-binding pocket in the enzyme. Our results are consistent with the observed selectivity of drugs that target CoQ-binding sites in mitochondrial complex III and DHODH.54–57 We present compelling evidence for the in vivo efficacy of STI1 in the TAC mouse model of heart failure, which mimics human aortic stenosis. We show that STI1 mitigates pressure-induced cardiomegaly, pulmonary congestion, and dilatation of the left ventricle. We also demonstrate that STI1 preserves cardiac function and prevents the progression to HFrEF by impeding the transition from compensated to decompensated left ventricle hypertrophy. This work establishes SQOR as a promising target for pharmacologic intervention in the treatment of pressure-induced HFrEF. We predict that STI1 and related SQOR inhibitors will exhibit similar efficacy in the treatment of MI-induced heart failure, a pathology also found to be strongly associated with impaired H2S-mediated cardioprotective signalling in patients and animal models.18–21
Cardiac remodelling and the progression to HFrEF are strongly associated with oxidative stress, which leads to the oxidation of cysteine residues to cysteine sulfenic acid.23,24 We propose that the cardioprotection observed with STI1 is triggered by protein persulfidation via reaction of H2S with cysteine sulfenic acid (Supplementary material online, Figure S14A). An overview of potential protein persulfidation targets and downstream cardioprotective signalling pathways is shown in Supplementary material online, Figure S14B. For example, the ability of STI1 to attenuate pressure-induced cardiomegaly may involve persulfidation of specificity protein 1 (SP-1), which is known to suppress expression and activity of Krüppel-like factor 5 to prevent myocardial hypertrophy.14 Similarly, the ability of STI1 to mitigate the pressure gradient across the aortic constriction may involve persulfidation of ATP-sensitive potassium (KATP) channels in vascular smooth muscle cells, which relaxes blood vessels and lowers blood pressure.2,58
With the discovery of the biological significance of H2S-induced cardioprotective signalling, the therapeutic potential of H2S donors has attracted keen interest.59,60 Our strategy of inhibiting H2S metabolism circumvents potential problems associated with H2S donors, including uncontrolled patterns of H2S release, unknown by-product profiles, and potentially toxic side effects of the donor compounds and/or their decomposition by-products. Additionally, SQOR inhibitors are predicted to preferentially increase cardioprotective signalling in mitochondria, an important site of H2S-mediated protection against myocardial ischemia/reperfusion injury.61 Moreover, SQOR inhibitors will have maximal efficacy in organs where the enzyme is highly expressed, such as the heart, whereas H2S donors will have maximal impact in organs with low levels of SQOR expression, such as the brain.62,63
We recognize that the current study has several limitations. Our results are consistent with the hypothesis that pharmacological inhibition of SQOR activates cardioprotective pathways via H2S-mediated protein persulfidation. Future studies using a mass spectrometry-based approach for proteome-wide identification of persulfidation sites64 are needed to document changes in the persulfidome of TAC mice produced by treatment with STI1. Notably, this approach was recently used to identify more than 800 persulfidated proteins that are produced in an adaptive response to endoplasmic reticulum stress.26 The ability of STI1 to reduce the pressure gradient across the aortic constriction suggests that STI1 may be acting, at least in part, as a vasodilator, to reduce afterload and oxygen demand. Studies to determine the effect of STI1 on blood pressure and cardiac haemodynamics are needed to provide additional insight into the mechanism of STI1 action. Studies involving delayed administration of STI1 following TAC surgery are also needed to determine whether STI1 can reverse existing and/or attenuate further ventricular remodelling. Translation of our study into the clinic will require studies to confirm the cardioprotective efficacy of SQOR-targeted inhibitors in MI-induced heart failure, especially in large animal models of HFrEF.
In conclusion, this proof of concept study identifies SQOR-inhibiting drugs as promising agents with the potential to provide first-in-class therapy for the treatment of heart failure patients with reduced ejection fraction. Due to the well-established protective properties of H2S-induced signalling in renal physiology and pathology,65 this novel class of heart failure therapeutics may also address the large unmet need of therapies for ∼50% of heart failure patients that have concurrent chronic renal disease.66
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institute of General Medical Sciences at the National Institutes of Health [GM107389 to M.S.J.]; the National Heart, Lung, and Blood Institute at the National Institutes of Health [R41 HL134435 to M.S.J.]; and Coulter-Drexel Translational Research Partnership Awards (to M.S.J.).
Data availability
The data underlying this article are available in the article and its Supplementary material online.
Translational perspective.
In Heart failure patients with reduced ejection fraction (HFrEF), there is a compelling need for new drugs that mitigate the pathological remodelling induced by injury and improve patient survival. This study identifies SQOR-inhibiting drugs as a promising first-in-class therapy for HFrEF patients. Due to the well-established protective properties of H2S-induced signalling in renal physiology and pathology, this novel class of heart failure therapeutics may also address the large unmet need of therapies for ∼50% of heart failure patients that have concurrent chronic renal disease.
Supplementary Material
Acknowledgements
The authors express their thanks to R. Roy for technical guidance on the TAC procedure and echocardiology, A. Cuconati and J. Kulp III for assembling and providing samples of compounds from the Baruch S. Blumberg Institute’s small-molecule library, E. S. Goetzman for a generous gift of recombinant porcine ETF, and S. Cocklin and A. B. Reitz for helpful discussions.
Contributor Information
Michael R Jackson, Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
Kristie D Cox, Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
Simon D P Baugh, Fox Chase Chemical Diversity Center, Inc., Doylestown, PA 18902, USA.
Luke Wakeen, Department of Surgery, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
Adel A Rashad, Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
Patrick Y S Lam, Fox Chase Chemical Diversity Center, Inc., Doylestown, PA 18902, USA.
Boris Polyak, Department of Surgery, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
Marilyn Schuman Jorns, Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R.. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 2008;322:587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan H, Du J, Tang C.. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 2004;313:22–27. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J.. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2009;29:173–179. [DOI] [PubMed] [Google Scholar]
- 5. Mani S, Li HZ, Untereiner A, Wu LY, Yang GD, Austin RC, Dickhout JG, Lhotak S, Meng QH, Wang R.. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013;127:2523–2534. [DOI] [PubMed] [Google Scholar]
- 6. Cheung SH, Kwok WK, To KF, Lau JYW.. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One 2014;9:e113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC.. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci 2012;8:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW.. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 2013;6:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ.. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013;127:1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Givvimani S, Munjal C, Gargoum R, Sen U, Tyagi N, Vacek JC, Tyagi SC.. Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure. J Appl Physiol 2011;110:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ.. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 2009;105:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan L, Liu X, Shen Y, Wang N, Xu J, Wu D, Xiong Q, Deng H, Huang G, Zhu YC.. Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int J Cardiol 2013;168:3770–3778. [DOI] [PubMed] [Google Scholar]
- 13. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow C, Lefer DJ.. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 2007;104:15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng G, Xiao Y, Ma Y, Tang X, Xie L, Liu J, Gu Y, Yu Y, Park C, Xian M, Wang X, Ferro A, Wang R, Moore PK, Zhang Z, Wang H, Han Y, Ji Y.. Hydrogen sulfide regulates Krüppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J Am Heart Assoc 2016;5:e004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang M, Kobayashi A, Yamamoto M, Kensler TW, Talalay P.. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 2004;101:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altaany Z, Ju YJ, Yang GD, Wang R.. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 2014;7:ra87. [DOI] [PubMed] [Google Scholar]
- 17. Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH.. Hydrogen sulfide-linked sulfhydration of NF-kB mediates its antiapoptotic actions. Mol Cell 2012;45:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovacic D, Glavnik N, Marinsek M, Zagozen P, Rovan K, Goslar T, Mars T, Podbregar M.. Total plasma sulfide in congestive heart failure. J Card Fail 2012;18:541–548. [DOI] [PubMed] [Google Scholar]
- 19. Jiang HL, Wu HC, Li ZL, Geng B, Tang CS.. Changes of the new gaseous transmitter H2S in patients with coronary heart disease. J First Mil Med Univ 2005;25:951–954. [PubMed] [Google Scholar]
- 20. Polhemus DJ, Calvert JW, Butler J, Lefer DJ.. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica (Cairo) 2014;2014:768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Wang Q, Guo W, Zhu YZ.. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep 2011;31:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filipovic MR, Zivanovic J, Alvarez B, Banerjee R.. Chemical biology of H2S signaling through persulfidation. Chem Rev 2018;118:377–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA.. Oxidant stress from nitric oxide synthase–3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 2005;115:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsutsui H, Kinugawa S, Matsushima S.. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 2011;301:H2181–H2190. [DOI] [PubMed] [Google Scholar]
- 25. Cuevasanta E, Lange M, Bonanata J, Coitiño EL, Ferrer-Sueta G, Filipovic MR, Alvarez B.. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J Biol Chem 2015;290:26866–26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao X, Krokowski D, Guan B, Bederman I, Majumder M, Parisien M, Diatchenko L, Kabil O, Willard B, Banerjee R, Wang B, Bebek G, Evans CR, Fox PL, Gerson SL, Hoppel CL, Liu M, Arvan P, Hatzoglou M.. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife 2015;4:e10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vitvitsky V, Kabil O, Banerjee R.. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Redox Signal 2012;17:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson MR, Melideo SL, Jorns MS.. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 2012;51:6804–6815. [DOI] [PubMed] [Google Scholar]
- 29. Augustyn KDC, Jackson MR, Jorns MS.. Use of tissue metabolite analysis and enzyme kinetics to discriminate between alternate pathways for hydrogen sulfide metabolism. Biochemistry 2017;56:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson MR, Loll PJ, Jorns MS.. X-ray structure of human sulfide:quinone oxidoreductase: insights into the mechanism of mitochondrial hydrogen sulfide oxidation. Structure 2019;27:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friederich MW, Elias AF, Kuster A, Laugwitz L, Larson AA, Landry AP, Ellwood-Digel L, Mirsky DM, Dimmock D, Haven J, Jiang H, MacLean KN, Styren K, Schoof J, Goujon L, Lefrancois T, Friederich M, Coughlin CR, Banerjee R, Haack TB, Van Hove JLK.. Pathogenic variants in SQOR encoding sulfide:quinone oxidoreductase are a potentially treatable cause of Leigh disease. J Inherit Metab Dis 2020;43:1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Guo X, Chen Y, Yin H, Li J, Doan J, Liu Q.. Assessment of cardiac morphological and functional changes in mouse model of transverse aortic constriction by echocardiographic imaging. J Vis Exp 2016;112:e54101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammed SF, Storlie JR, Oehler EA, Bowen LA, Korinek J, Lam CSP, Simari RD, Burnett JC Jr, Redfield MM.. Variable phenotype in murine transverse aortic constriction. Cardiovasc Pathol 2012;21:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang JH, Chung TD, Oldenburg KR.. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J Biomol Screen 1999;4:67–73. [DOI] [PubMed] [Google Scholar]
- 35. Pouliot M, Jeanmart S.. Pan assay interference compounds (PAINS) and other promiscuous compounds in antifungal research: miniperspective. J Med Chem 2016;59:497–503. [DOI] [PubMed] [Google Scholar]
- 36. Wunberg T, Hendrix M, Hillisch A, Lobell M, Meier H, Schmeck C, Wild H, Hinzen B.. Improving the hit-to-lead process: data-driven assessment of drug-like and lead-like screening hits. Drug Discov Today 2006;11:175–180. [DOI] [PubMed] [Google Scholar]
- 37. Segall MD. Multi-parameter optimization: identifying high quality compounds with a balance of properties. Curr Pharm Des 2012;18:1292–1310. [DOI] [PubMed] [Google Scholar]
- 38. Tyzack JD, Hunt PA, Segall MD.. Predicting regioselectivity and lability of cytochrome P450 metabolism using quantum mechanical simulations. J Chem Inf Model 2016;56:2180–2193. [DOI] [PubMed] [Google Scholar]
- 39. Marchant CA, Briggs KA, Long A.. In silico tools for sharing data and knowledge on toxicity and metabolism: Derek for windows, meteor, and vitic. Toxicol Mech Methods 2008;18:177–187. [DOI] [PubMed] [Google Scholar]
- 40. Macarron R, Hertzberg RP.. Design and implementation of high throughput screening assays. In: Janzen WP (ed.). High Throughput Screening: Methods and Protocols. Totowa, NJ: Human Press Inc.; 2002. p1–29. [DOI] [PubMed] [Google Scholar]
- 41. Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C.. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 2012;7:1235–1246. [DOI] [PubMed] [Google Scholar]
- 42. Knecht W, Henseling J, Löffler M.. Kinetics of inhibition of human and rat dihydroorotate dehydrogenase by atovaquone, lawsone derivatives, brequinar sodium and polyporic acid. Chem Biol Interact 2000;124:61–76. [DOI] [PubMed] [Google Scholar]
- 43. Frerman FE. Reaction of electron-transfer flavoprotein ubiquinone oxidoreductase with the mitochondrial respiratory chain. Biochim Biophys Acta 1987;893:161–169. [DOI] [PubMed] [Google Scholar]
- 44. Heineke J, Molkentin JD.. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- 45. Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD.. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc Natl Acad Sci USA 2002;99:4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frank D, Kuhn C, van Eickels M, Gehring D, Hanselmann C, Lippl S, Will R, Katus HA, Frey N.. Calsarcin-1 protects against angiotensin-II-induced cardiac hypertrophy. Circulation 2007;116:2587–2596. [DOI] [PubMed] [Google Scholar]
- 47. Watkins SJ, Borthwick GM, Arthur HM.. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 2011;47:125–131. [DOI] [PubMed] [Google Scholar]
- 48. deAlmeida AC, van Oort RJ, Wehrens XHT.. Transverse aortic constriction in mice. J Vis Exp 2010;38:e1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stansfield WE, Rojas M, Corn D, Willis M, Patterson C, Smyth SS, Selzman CH.. Characterization of a model to independently study regression of ventricular hypertrophy. J Surg Res 2007;142:387–393. [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, Javan H, Li L, Szucsik A, Zhang R, Deng Y, Selzman CH.. A modified murine model for the study of reverse cardiac remodelling. Exp Clin Cardiol 2013;18:e115. [PMC free article] [PubMed] [Google Scholar]
- 51. van Berlo JH, Maillet M, Molkentin JD.. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 2013;123:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kong P, Christia P, Frangogiannis NG.. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG.. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 2009;131:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Capper MJ, O'Neill PM, Fisher N, Strange RW, Moss D, Ward SA, Berry NG, Lawrenson AS, Hasnain SS, Biagini GA, Antonyuk SV.. Antimalarial 4(1H)-pyridones bind to the Qi site of cytochrome bc1. Proc Natl Acad Sci USA 2015;112:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nilsen A, LaCrue AN, White KL, Forquer IP, Cross RM, Marfurt J, Mather MW, Delves MJ, Shackleford DM, Saenz FE, Morrisey JM, Steuten J, Mutka T, Li Y, Wirjanata G, Ryan E, Duffy S, Kelly JX, Sebayang BF, Zeeman A-M, Noviyanti R, Sinden RE, Kocken CHM, Price RN, Avery VM, Angulo-Barturen I, Jiménez-Díaz MB, Ferrer S, Herreros E, Sanz LM, Gamo F-J, Bathurst I, Burrows JN, Siegl P, Guy RK, Winter RW, Vaidya AB, Charman SA, Kyle DE, Manetsch R, Riscoe MK.. Quinolone-3-diarylethers: a new class of antimalarial drug. Sci Transl Med 2013;5:177ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, du Pre S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M.. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA 2016;113:12809–12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vyas VK, Ghate M.. Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors. Mini Rev Med Chem 2011;11:1039–1055. [DOI] [PubMed] [Google Scholar]
- 58. Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH.. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 2011;109:1259–I268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Powell CR, Dillon KM, Matson JB.. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol 2018;149:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK.. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137). Circulation 2008;117:2351–2360. [DOI] [PubMed] [Google Scholar]
- 61. Calvert JW, Coetzee WA, Lefer DJ.. Novel insights into hydrogen sulfide-mediated cytoprotection. Antioxid Redox Signal 2010;12:1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F.. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta Bioenerg 2010;1797:1500–1511. [DOI] [PubMed] [Google Scholar]
- 63. Ackermann M, Kubitza M, Maier K, Brawanski A, Hauska G, Pina AL.. The vertebrate homolog of sulfide-quinone reductase is expressed in mitochondria of neuronal tissues. Neuroscience 2011;199:1–12. [DOI] [PubMed] [Google Scholar]
- 64. Longen S, Richter F, Kohler Y, Wittig I, Beck KF, Pfeilschifter J.. Quantitative persulfide site identification (qPerS-SID) reveals protein targets of H2S releasing donors in mammalian cells. Sci Rep 2016;6:29808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Feliers D, Lee HJ, Kasinath BS.. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal 2016;25:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S.. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 2016;12:610–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and its Supplementary material online.
Translational perspective.
In Heart failure patients with reduced ejection fraction (HFrEF), there is a compelling need for new drugs that mitigate the pathological remodelling induced by injury and improve patient survival. This study identifies SQOR-inhibiting drugs as a promising first-in-class therapy for HFrEF patients. Due to the well-established protective properties of H2S-induced signalling in renal physiology and pathology, this novel class of heart failure therapeutics may also address the large unmet need of therapies for ∼50% of heart failure patients that have concurrent chronic renal disease.