Abstract
Recent developments in imaging, mapping, and ablation techniques have shown that the epicardial region of the heart is a key player in the occurrence of ventricular arrhythmic events in several cardiac diseases, such as Brugada syndrome, arrhythmogenic cardiomyopathy, or dilated cardiomyopathy. At the atrial level as well, the epicardial region has emerged as an important determinant of the substrate of atrial fibrillation, pointing to common underlying pathophysiological mechanisms. Alteration in the gradient of repolarization between myocardial layers favouring the occurrence of re-entry circuits has largely been described. The fibro-fatty infiltration of the subepicardium is another shared substrate between ventricular and atrial arrhythmias. Recent data have emphasized the role of the epicardial reactivation in the formation of this arrhythmogenic substrate. There are new evidences supporting this structural remodelling process to be regulated by the recruitment of epicardial progenitor cells that can differentiate into adipocytes or fibroblasts under various stimuli. In addition, immune-inflammatory processes can also contribute to fibrosis of the subepicardial layer. A better understanding of such ‘electrical fragility’ of the epicardial area will open perspectives for novel biomarkers and therapeutic strategies. In this review article, a pathophysiological scheme of epicardial-driven arrhythmias will be proposed.
Keywords: Cardiac arrhythmias, Atrial fibrillation, Arrhythmogenic cardiomyopathy, Epicardial progenitor cells, Fibro-fatty infiltration
Graphical Abstract
Graphical Abstract.
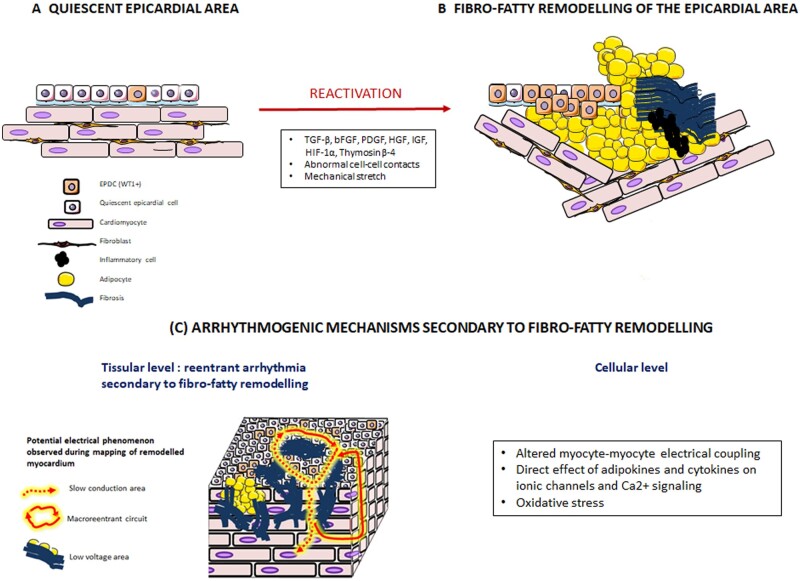
From the reactivation of the epicardium to the formation of an arrhythmogenic substrate. Transition from a quiescent epicardium (A) to epicardial reactivation followed by fibro-fatty infiltrations of subepicardial myocardial layers (B). Potential arrhythmogenic mechanisms (C) include (i) at the tissue level, conduction slowing or block and low-voltage area favouring formation of electrical reentry circuit within the myocardial wall and (ii) at the cellular level, altered myocyte coupling and adipokine- and cytokine-induced abnormal excitation contraction coupling and oxidative stress.
1. Introduction
Recent developments in imaging, mapping, and ablation techniques have shown that the epicardial region of the heart is a key player in the occurrence of ventricular arrhythmic events in several cardiac diseases, such as Brugada syndrome,1 arrhythmogenic cardiomyopathy (ACM),2 or dilated cardiomyopathy.3 At the atrial level as well, the epicardial region has emerged as an important determinant of the substrate of atrial fibrillation (AF), the most frequent cardiac arrhythmia in clinical practice. Taken together, these observations raise questions about whether distinct mechanisms underlie the ‘tissue fragility’ of the epicardial region and whether they could provide new therapeutic targets for cardiac arrhythmias. This article will describe specific features of the epicardial region that can contribute to activation of arrhythmogenic processes, it will review evidences for an epicardial origin of cardiac arrhythmias, and discuss the role of epicardial reactivation in the formation of the arrhythmogenic substrate.
2. Definitions and special features of the epicardial region
2.1 Anatomy and histology
The epicardial area is delineated by the epicardium, the outer mesothelial layer of the heart. The epicardium contains multipotent progenitors that can undergo epithelial-to-mesenchymal transition (EMT), migrate into the subepicardium and, during cardiac ontogeny, give rise to multipotent mesenchymal epicardium-derived cells (EPDCs).4 At that point, the EPDCs can differentiate into smooth muscle cells, coronary vessels,5 or myocardial fibroblasts6 and, less importantly, into coronary endothelial cells7 and cardiomyocytes.8 The epicardium has also important signalling functions and exchanges paracrine factors with the neighbouring myocardium. Quiescent in the healthy adult heart, the epicardium can be reactivated, becoming a source of myofibroblasts and of growth and angiogenic factors.9
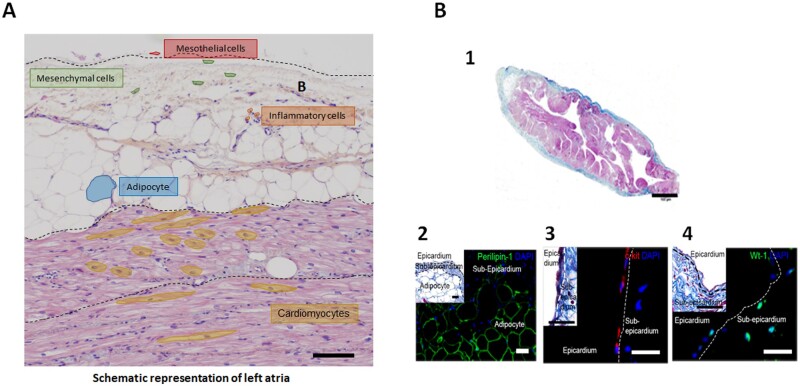
The subepicardium rapidly increases in volume from embryonic Day 6 to 11 during mesenchymal cell invasion. It is mainly composed of connective tissue, located between the epicardium and the myocardium with a predominance of collagen I and collagen III compared to the rest of the myocardium.10 It contains also mesenchymal cells, such as smooth muscle and Cajal-like cells together with lymphocytes, mast cells, macrophages, fibroblasts, nerves, and capillary (Figure 1). Myocardial trabeculations are present within the subepicardium, yet with a distinct spatial organization well visualized by clinical imaging techniques and characterized by a transmural orientation and a rotation of 120° on the axis of myocytes from epicardium to endocardium.12,13
Figure 1.
Histology and structural organization of the epicardial area. (A) Schematic representation of a cross section of left atrium showing the interaction between epicardium, adipose tissue, and myocardium with distinct orientations of fibres between layers (cross section vs longitudinal myocytes). (B) Masson’s trichrome staining of human atrial tissue (1) showing the epicardium in blue, myocardium in red and subepicardial adipose tissue in white (Scale bar, 100 µm). At high magnification, immunofluorescence staining of human atrial section reveals the presence of adipocytes expressing perilipin-1 (2) and progenitor cells expressing c-Kit (3) and WT-1 (4) in both epicardium and subepicardium (Scale bars, 10 µm), from Suffee et al.11
The adipose tissue localized between the myocardium and the visceral pericardium, referred to as the epicardial adipose tissue (EAT), can be considered as another component of the epicardial area. Firstly, EAT is histologically tightly associated with the epicardium and the neighbouring myocardium that it can infiltrate.14 Secondly, epicardial progenitors are the source of adipocytes that compose EAT.11 Finally, EAT differs from paracardiac adipose tissue located at the outer surface of the fibrous pericardium by its embryologic origin, its biological properties, and its vascularization from coronary arteries. However, the difficulty in distinguishing these two types of cardiac adipose tissue using clinical imaging techniques explains that they are often confused in studies. There is no barrier between EAT and the subepicardial myocardium such that peptides and adipokines can freely diffuse between the two tissues. For instance, EAT is an important source of free-fatty acids used for myocardial energetic metabolism; it releases twice as much fatty acid as pericardial depots and also protects the heart against toxic levels of fatty acids.14 EAT is a brown adipose tissue, expressing the uncoupling protein-1 located at the mitochondria inner membrane, a protein regulating heat production, permitting EAT to protect the heart against hypothermia.14 Moreover, EAT regulates myocardial oxidative stress by releasing adiponectine that inhibits nicotinamide adenine dinucleotide phosphate oxidase activity.15
2.2 Special electrical features of the epicardial region
The presence of distinct electrophysiological properties of the subepicardial myocardium also calls for the individualization of this cardiac region. A repolarization gradient exists between the subepicardial and subendocardial myocardium in the human heart as well as in the hearts of other animal species.16–18 This gradient prevents retrograde depolarization of subendocardial layers by operating as a secure lock against the occurrence of re-entry circuits.
This repolarization gradient is due to the distinct electrical properties of cardiomyoyctes between myocardial layers. Action potential (AP) of subepicardial cardiomyocytes is of shorter duration compared to other cardiomyocytes,19,20 having a more pronounced rate dependency indicated by the persistent suppression of the plateau phase with premature beats, i.e. AP duration shortening with decreasing S1S2 interval between S1 steady-state pacing and premature S2.16,18 Moreover, upon increased extracellular [K+]o, AP shortening is predominant in subepicardial layers resulting in tall positive T waves, whereas upon decreased [K+]o, epicardial AP lengthens and T waves flatten.19
At the cellular level, the repolarization gradient is generated by a regional difference of the fast component of the voltage-dependent outward current, Ito, of much higher density in subepicardial than subendocardial layers.18 This current governs the early repolarization phase18 of the AP, the notch, and tunes the duration of the plateau phase to heart rate.21 It is the functional expression of shaker voltage-gated potassium Kv 4.2 and Kv 4.3 channels depending on species.22,23 In human and large mammal hearts, the molecular basis for transmural difference of Ito is the expression gradient of gene encoding for Kv4.x channels and for KCHiP2, a β-subunit that chaperones ionic channels at the plasma membrane.24–29 The small density of delayed potassium currents, Iks and Ikr in M cells, too contributes to the electrical gradient.30
Other mechanisms underlying epicardial electrical heterogeneity are the left-handed helix to a right-handed31 arrangement of muscle fibres and the organization of the laminar structure that drives ionic current flow.32 Whereas in subendocardial and midwall myocardium, myocytes are grouped together by perimysial collagen into branching layers called myolaminae,33 in the subepicardium, perimysial collagen is present only as longitudinal cords.34
The fibre direction of the right ventricle (RV) appears to change suddenly at the level of the subepicardium35 which may make this area prone to conduction delay and block. Additionally, the embryologic origin of the subepicardium in the right ventricular outflow tract appears to differ from other sites which makes this area prone to conduction slowing in mice.36 Finally, fatty infiltration in the right ventricular subepicardium is more outspoken than at other sites even in normal subjects.37
3. Clinical evidence for epicardial-driven arrhythmias
3.1 Arrhythmogenic cardiomyopathy
Arrhythmogenic cardiomyopathy (ACM) classically occurs in young patients by 12-lead ECG QRS-T abnormalities and ventricular arrhythmias. Fibro-fatty replacement progresses from the epicardium to the endocardium and leads to areas of slow conduction that constitute the main substrate for re-entrant ventricular tachycardia (VT) in ACM.38 Other arrhythmia mechanisms, such as focal epicardial activities, have also been described in ACM patients.39 The epicardial origin of ventricular arrhythmias (VA) associated with ACM has been particularly highlighted by ablation procedures, showing the epicardial predominance of low-voltage areas and abnormal electrograms, such as fractioned or late potentials, when endocardium may be normal, especially in the earlier stages of the disease.2 Interestingly, endocardial unipolar low-voltage zones (<5.5 mV) were shown to predict epicardial substrate in patients with ACM, with respect to size and location.40,41 This predictive value of endocardial unipolar mapping was also confirmed in a recent study in which RV epicardial scar region significantly correlated with endocardial unipolar low-voltage zones (<5.5 mV).42
Non-invasive epicardial mapping with electrocardiographic imaging (ECGI) is also suitable for mapping electrophysiologic substrate on the epicardial surface. Andrews et al.43 performed ECGI and late gadolinium enhancement cardiac magnetic resonance in 20 ACM patients. Compared with controls, ACM patients had significantly longer ventricular activation duration and prolonged mean epicardial activation-recovery intervals. ECGI also showed varied epicardial activation breakthrough locations and regions of non-uniform conduction and fractionated electrograms that colocalized with late gadolinium enhancement scar.
Arrhythmic substrate cannot always be targeted by endocardial ablation, especially as layered and confined epicardial circuits of ventricular tachycardia were found in ACM patients.44 Given the high rate of VT recurrence after endocardial ablation,45 epicardial ablation was widely tested in ACM patients.46 Several studies have shown the superiority of a combined endo/epicardial approach to endocardial-only ablation.47 In a study comparing endocardial-alone ablation to endo-epicardial ablation, freedom from ventricular arrhythmias was observed at 3-year follow-up in 85% of the patients who benefitted from the combined approach and only 53% of the patients with endocardial-alone ablation.48 Berruezo et al.49 evaluated a combined endo-epicardial VT ablation approach associated with conducting channel elimination (scar dechannelling); freedom from VT recurrence was obtained in 90% of ACM patients.
3.2 Brugada syndrome
The other cardiac arrhythmia with a well-established epicardial substrate is Brugada syndrome (BrS). In a systematic review, Fernandes et al.50 showed that BrS patients undergoing both epicardial and endocardial mapping had exclusive epicardial substrate in 93% of cases. Simultaneous non-invasive epicardial and endocardial mapping further establishes the epicardial predominance of electrical abnormalities.51 Non-invasive epicardial mapping with ECGI was also conducted in 25 BrS patients and 6 patients with right bundle-branch block (RBBB) for comparison. Unlike patients with RBBB, BrS patients had delayed activation localized to the right ventricular outflow tract, and fractionation, or repolarization abnormalities on RVOT electrograms.52
Accordingly, targeting of epicardial rather than endocardial substrate appeared much more effective to prevent VT/VF in BrS. Brugada et al.53 performed right ventricular epicardial mapping of patients with BrS and identified low-voltage (<1.5 mV) and abnormal electrograms areas with abnormally prolonged fragmented epicardial potentials on the anterior right free wall and RVOT. Radiofrequency ablation of this area resulted in the suppression of the BrS ECG pattern and suppressed VT/VF inducibility. Data on 135 patients with BrS showed that elimination of abnormal epicardial electrograms led to a normalization of the BrS ECG pattern in all but two patients.1 In another study in which 28 symptomatic BrS patients underwent RV epicardial mapping, an anterior RVOT abnormal epicardial substrate was found in all patients.54 Abnormal electrograms covered the entire RVOT epicardium and extended to the RV body in more than half of the patients (53%). The chosen endpoint was to eliminate all abnormal electrograms detected during epicardial mapping after the administration of a sodium channel blocker. Only 3 of 28 patients had recurrent VF episodes and required reablation.54
3.3 Scar-related cardiomyopathy
Following the first description of the technique in 1996,55 transthoracic epicardial catheter ablation was mainly used to treat patients with Chagas disease55 or with VT related to inferior myocardial infarction.56 The technique and its indication have expanded over recent decades. In a multicentre study including all patients undergoing VT ablation, epicardial mapping and/or ablation was required in 17% of patients and 35% of those presented with non-ischaemic cardiomyopathy (NICM).3 Lateral subepicardial and anteroseptal intramural arrhythmogenic substrate are very common in NICM, related to presence of interstitial fibrosis that is usually more diffuse and patchy than in ICM.57 In a series of 22 patients with NICM who failed prior endocardial ablation and/or had ECG suggesting an epicardial origin, Cano et al.58 found larger low-voltage and dense scar areas within the epicardium compared to the endocardium. Almost half of the patients, showed an abnormal epicardial voltage map in contrast with a normal endocardial map. Despite the expansion of epicardial ablation, long-term outcomes after VT ablation in NICM remain poor when compared with ICM, possibly due to the diffuse and deep location of arrhythmic substrate. In the HELP-VT registry study, VT free-survival at 1-year follow-up was 40.5% in patients with NICM and 57% in patients with ICM, even though ablation procedures were performed with an epicardial access when necessary.59 These data reflect the highly complex substrate for VT in NICM and the further progress that remains to be made. Myocarditis-related ventricular arrhythmia is also likely to be related to an epicardial substrate, as suggested by Dello Russo et al.60 In this case series of 20 patients with drug refractory VT in the context of proven myocarditis, epicardial ablation was necessary for 30% of patients.60 These findings are consistent with contrast-enhanced cardiac magnetic resonance (CMR) imaging of subepicardial distribution of late enhancement in patients with active myocarditis.61
3.4 Atrial fibrillation and epicardial region
The thin atrial wall is a heterogeneous structure with electrical dissociation between endocardium and epicardium and transmural muscle fibres connecting those distinct layers62 that can constitute the substrate for re-entries. Indeed, high-density mapping of long-standing persistent atrial fibrillation (AF) performed during cardiac surgery63,64 has recorded epicardial-endocardial breakthroughs (EEB), a source of ‘focal’ fibrillation waves favouring AF persistence, that originate from the epicardial surface. Furthermore, during the progression from paroxysmal to persistent AF, secondary to fibro-fatty infiltration, endo-epi dissociation,65 and low-voltage areas have been shown to increase. After the restoration of sinus rhythm, EEB can still be recorded with epicardial-endocardial asynchrony and the muscular connections between the endo-epicardial layers clearly indicating the presence of an epicardial substrate.66 In this line, Pak et al.67 showed that epicardial catheter ablation with a pericardial approach was effective in patients with redo-AF ablation procedure at risk for PV stenosis. Another option is the hybrid AF ablation combining minimally invasive surgery and percutaneous electrophysiology study.68 In a meta-analysis of 563 patients, long-term success rate (sinus rhythm after a mean of 26 months) after hybrid ablation ranged from 60% to 87%.69
3.5 Value and limitations of the different epicardial mapping techniques
Contact electro-anatomical mapping systems have emerged as essential tools for precise mapping and ablation of cardiac arrhythmias. These magnetic and or impedance-based systems use dedicated mapping catheters that are introduced within the cardiac chamber of interest or the pericardial space for electrophysiological analysis. Once in place, they are able to determine the mechanism and delineate the site of origin of the arrhythmia with a precision of a few millimetres. As discussed above, ventricular endocardial unipolar low-voltage electrograms were shown to predict epicardial substrate location, notably in ACM40 and non-ischaemic left ventricular cardiomyopathy.41 Ventricular epicardial contact mapping using these technologies also clearly demonstrated the epicardial origin of numbers arrhythmias in BrS, ACM, and NICM.
The inability for catheters to reach the targeted site in order to efficiently suppress the arrhythmia is one of the limitations of this technology. This is particularly true for epicardial substrate for which an epicardial access is required. Due to past medical (pericarditis) or surgical (cardiac surgery) history, the virtual space between the two pericardium sheets could be inaccessible via a transcutaneous puncture. Closely located coronary arteries to the site of interest or surrounding subepicardial adipose tissue may also limit the ability to successfully suppress the arrhythmogenic substrate.
Finally, non-invasive epicardial mapping with ECGI is another effective option for mapping electrophysiologic substrate on the epicardial surface. Despite considerable recent development, validation of ECGI is still challenging70 as some important discrepancies have been found between ECGi and epicardial contact mapping which should theoretically produce identical maps.
4. Fibro-fatty infiltration and epicardial arrhythmias
Alteration of repolarization gradient and action potential heterogeneity between the subepicardial and subendocardial myocardium are well-established arrhythmogenic mechanisms. Brugada syndrome is an archetypal example with two main models, repolarization and depolarization,71,72 with a key role of the epicardium layer in both of them. The fibro-fatty infiltration of myocardial layer appears as another major shared pathophysiological mechanism between several epicardial-driven arrhythmias.
4.1 Adipose tissue infiltration at the ventricular level: the paradigm of ACM
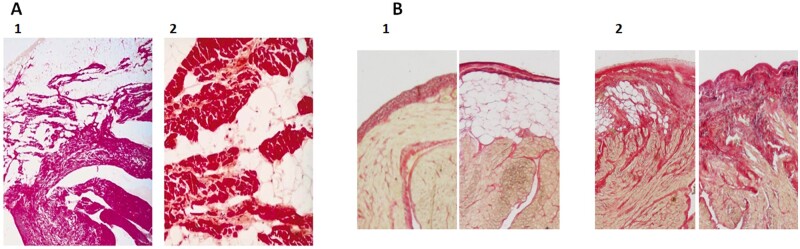
Both post-mortem studies and surgical ablation procedures have revealed the presence of adipose depots in the RV of ACM patients with a progressive replacement of myocardium by fibro-fatty infiltrates and the progressive loss of RV muscle fibres73 starting from the subepicardium and then progressively extending to the subendocardium, thereby become transmural (Figure 2).73,76 Clusters of mononuclear cells in the fatty infiltrates corresponding to immune cells have been observed in fibro-fatty infiltrates leading to the notion of lymphocytic myocarditis77 further supported by the recent observation of clinical myocarditis in patients with ACM.78
Figure 2.
Fibro-fatty infiltration of subepicardial layers of ventricle and atrial myocardium. (A) Haematoxylin–phloxin–safran staining of the right ventricle free wall of a patient with arrhythmogenic cardiomyopathy showing a large amount of adipose tissue occupying subepicardial layers (1, ×10) and isolated strands of myocardium bordered by or embedded in fibrous tissue (2, ×100), from Mallat et al.74 (B) Red sirius staining of a human right atrial section showing an example of non-fibrotic remodelled epicardium (1) either without subepicardial adipose tissue or with subepicardial adipose tissue and (2) fibrotic remodelled epicardium with various degree of fibro-fatty infiltration, from Haemers et al.75
Some degree of fibro-fatty remodelling has also been observed in the subepicardial layer of the right ventricle outflow track in BrS patients. In a post-mortem study, RVOT histological sections of BrS patients showed an increased epicardial surface collagen that was thicker than that in control hearts. This epicardial fibrosis infiltrated into the underlying epicardial myocardium, admixed with fat. BrS cases also had reduced Cx43 expression in the RVOT when compared with controls.79 Ohkubo et al.80 also performed endomyocardial biopsy in 25 patients with BrS. Moderate to severe fatty infiltration was observed in five patients and significant fibrosis infiltration in four. Noteworthy, right ventricular cardiomyopathy with fibro-fatty replacement was observed in patients with Brugada like-ST segment elevation.81 This histological feature together with the delayed activation of the RVOT points to a phenotypic overlap between ACM and BrS.82,83
4.2 Fibro-fatty infiltration of the atrial myocardium and the substrate of AF
Strikingly similar fibro-fatty remodelling of the subepicardium resembling the histology of the ACM heart has been described in the atria of patients suffering from AF (Figure 2).75,84 In healthy adult atria, the epicardium is mainly a cell monolayer with thin, extracellular matrix in contact with the myocardium or adipose tissue. However, with ageing or in patients with hypertension,85 mitral valve diseases,86 or AF, the epicardium can become thick and adipose tissue fibrotic, resulting in fibro-fatty infiltrates.75 In a model of persistent AF in sheep, the degree of fibrosis of the subepicardial adipose tissue follows the progression of AF from paroxysmal to permanent.75 As in ventricle, clusters of inflammatory cells can be observed at the epicardial site in the transition zone between adipocytes and fibrosis in both human and sheep atria. Immuno-histochemistry analysis of these cell clusters revealed a predominance of CD3+ T lymphocytes, with the vast majority of them CD8+ cytotoxic T cells, displaying functional cytotoxic activity through granzyme B against adipocytes.75 Indeed, such an immune component mediated by CD8+ cytotoxic T cells has been described for other visceral adipose tissues notably in obese patients and is considered as a major mechanism underlying the fibrosis of adipose tissue.87
4.3 Evidences for the arrhythmogenicity of fibro-fatty infiltration of myocardial layers
By comparing histology with electrical mapping of the right ventricular outflow tract, it has been possible to establish a relationship between arrhythmogenicity and the different distributions of subepicardial fibro-fatty infiltrations. For instance, 80 consecutive ACM patients in sinus rhythm were classified into three groups according to the type of fibro-fatty infiltration, referred to in this study as scar gradient (<10%: transmural, 10–20%: intermediate, >20%: horizontal).42 Patients with horizontal scars experienced significantly more syncope, sustained VT, and fatal VA when compared with patients with transmural and intermediate scar gradient, independently of the right ventricular volume. Horizontal extension of epicardial scars was an independent predictor of life-threatening VA, whereas patients with transmural scars had a greater number of clinical and EP induced PVCs that was not correlated with fatal VA.42 A heterogeneous distribution of scars between subepicardial and subendocardial layers was well described in ACM patients, with a predominance of scar area and late abnormal ventricular potentials within the subepicardium layer compared to the endocardium.2,44 Moreover, the dense fibrotic infiltrates, characteristic of ACM, may electrically isolate the epicardium from the endocardium. When studying the transmural right ventricular activation pattern, it was observed that the epicardium is activated with a major delay in ACM patients without direct transmural spread from the endocardium but with a laminar activation pattern from the border to the central scar favouring re-entry circuits.44
Pieroni et al.88 described the relationship between electroanatomic abnormalities and pathological substrate in BrS patients. Three-dimensional electroanatomic mapping-guided RVOT biopsies were performed in 20 patients and histopathological abnormalities including fibrosis were found in 15 of them and could provide an explanation for the areas of low voltage recorded in those patients.
In a goat model of AF, EEB incidence and degree of endo-epicardial dissociation increased with increasing AF substrate complexity.65 EED and breakthroughs also correlated with the degree of epicardial fibrosis89 as evidenced using a 3D computational model of human atria integrating MR images and histo-anatomical data. Slow electrical conduction was recorded in the region of the human right atrial myocardium infiltrated by fibro-fatty tissue and has been attributed to the deleterious effect of adipose tissue on normal myocyte–myocyte coupling.90
Several mechanisms can underlie the arrhythmogenicity of the fibro-fatty infiltration of subepicardial myocardium.91 First, by infiltrating subepicardial myocardial layers, adipocytes, and fibrosis disrupt normal myocyte–myocyte coupling leading to local conduction slowing/block and to the formation of myocardial area of low voltage.90 These altered conduction properties pave the way for re-entry circuits within this remodelled area (Graphical Abstract)
The adipose tissue can also secrete a myriad of cytokines and adipokines, such as leptine, and also small extracellular vesicles92 that can regulate directly the cardiac electrical properties93 or indirectly, for instance, by modulating the oxidative stress of the myocardium.94 Finally, the altered electrical properties caused by fibro-fatty infiltrates subepicardial myocardial layers could disturb the normal endo-epi repolarization gradient.
However, the difficulty to reproduce experimentally the precise composition of cardiac tissue with controlled texture of fibrosis and adipose tissues explains the lack of direct evidences for the arrhythmogenicity of fibro-fatty infiltration. To overcome this limitation, computational model of myocardial tissue has been used providing arguments for reduction in conduction velocity, enhanced spiral wave periodicity, and increased break-up with the degree of fibro-fatty infiltration.91
5. Epicardial reactivation and fibro-fatty infiltration of the subepicardium
Several explanations have been proposed for the apparent replacement of myocardium by adipose tissue notably in the context of ACM. This includes transdifferentiation of cardiomyocytes,95 proliferation and differentiation of cardiac progenitors,96 or a mesenchymal origin.97 For instance, in explanted ACM hearts, mesenchymal stromal cells were found to contribute to fibro-fatty infiltrates suggesting an epicardial EMT origin.97 The link between genetic defects underlying ACM and fibro-fatty remodelling of right ventricle myocardium is still actively investigated and the description of ‘the connexome’ had provided some clues. Connexome is a network of proteins localized at the intercalated disks (ID) that includes desmosome proteins, gap junction, and ionic channels. It plays a crucial role in the normal electrical and mechanical coupling between myocytes and also in the maintenance of differentiated myocardium through various signalling pathways that depend on the integrity of myocyte–myocyte contacts including Wnt/β-catenin or Hippo-Yap pathways. It is to be noted that the Hippo-Yap pathway was reported to be activated during ACM as the result of changes of the expression of several ID proteins.98
5.1 Epicardial reactivation and fibro-fatty infiltration of the atrial myocardium
The study of the origin of EAT and fibro-fatty infiltrates in the atria has provided strong evidence for the role played by the epicardium in this remodelling process (Graphical Abstract). Firstly, cells expressing markers of an epicardial origin, such as Wilm’s tumour (Wt-1) and Tbx18, have been detected in the fibro-fatty infiltrates of subepicardium of human atria. Secondly, using a genetic lineage tracing as Wt-1-CreERT2+/−RosatdT+/− mouse model, it has been possible to track EPDCs in situ and to show their migration into the subepicardium and their differentiation into adipocytes or fibroblasts during various atrial remodelling.11,99 Single-cell RNA-sequencing analysis revealed the heterogeneity of atrial epicardial cells with at least eight clusters.99 The analysis of inference trajectory of atrial EPDCs identifies, at least, two differentiation pathways, one towards adipocyte and another towards myofibroblast with an apparent progression from fat accumulation to fibrosis. Atrial natriuretic peptide (ANP) and angiotensin-II (Ang-II) have been shown to be important regulators of the epicardial reactivation and to control the signalling pathways that regulate differentiation lineages of atrial EPDCs (aEPDCs).11,99 They operate as a switch to induce differentiation of aEPDCs into fibroblasts or adipocytes, respectively. For instance, the atrial natriuretic peptide, at low concentration, activates cGMP-dependent protein kinase (PKG) that regulates the expression of transcription factors C/EBPα and PPARγ.11
These peptides are locally secreted explaining why areas with a thin epicardial layer co-exist with thick and fibrotic epicardial areas.99 This could contribute to focal structural reactivation of the epicardium and accumulation of fat or fibrosis. Finally, both Ang-II and ANP up-regulated their own receptors indicating a positive feedback of the agonists on their signalling pathways to secure a long-lasting response. Numerous factors can trigger epicardial reactivation and EMT, such as transforming growth factor-β, fibrobast growth factors, platelet derived growth factors, Notch, retinoic acid, or thymosin-beta-4 (Graphical Abstract).100,101
5.2 Are their evidences for reactivation of the epicardium at the ventricle level?
An epicardial reactivation has been also reported at the ventricle level in adult heart. For instance, following acute myocardial infarction an intense reactivation of the epicardium with recruitment of progenitor cells contributes to the scar formation and to the fibrotic remodelling of the ventricle myocardium. In this line, the inhibition of embryonic Wt1-Cre lineage mobilization is associated with the reduction in the number of epicardium-derived fibroblasts colonizing the post-myocardial injury scar.101
Epicardial progenitor cells are also a source of adipocytes that compose the fat depot of atrioventricular groove. Furthermore, depending on the activation PPARγ signalling pathway, epicardial progenitors can differentiate into adipocytes and colonize too the post-myocardial infarction scar.102 An epicardial reactivation due to local mechanical stretch is likely to explain fibro-fatty scars development in NICM patients and should be investigated.
5.3 Role of desmosome proteins in the differentiation of cardiac progenitors into adipocytes
Several evidences indicate a role of desmosomal proteins in fatty infiltration of the ventricular myocardium. First, desmosome proteins are present in fibro-adipogenic progenitors whereas some mutations of desmosomal proteins favour their differentiation into adipocytes.103 Second, in a population of non-excitable cardiac-resident cells, plakophiline has been shown to regulate intracellular lipid accumulation. Finally, epicardial progenitor cells derived from iPS cells, generated from skin fibroblasts obtained in patients suffering from ACM and harbouring Plakophilin-2 mutations, spontaneously differentiated into adipocytes. The activating enhancer binding protein 2 alpha (AP2α) could be the transcription factor involved in the adipogenic differentiation of EPDCs.104 Further studies using epicardial progenitor lineage models harbouring desmosomal protein gene mutations are necessary to establish firmly the role played by epicardial reactivation in the ventricle subepicardial remodelling during ACM.
Despite solid arguments for an epicardial origin of fibro-fatty infiltration of myocardium and several evidences for the arrhythmogenicity of such fibro-fatty infiltrates, a direct causal role of epicardial reactivation in the occurrence of cardiac arrhythmias remains to be established. Furthermore, there is no evidence for a reactivation of the epicardium during BrS.
6. Conclusion
Progress in the mapping of cardiac arrhythmias, notably ventricular tachycardia, has revealed the crucial role played by the epicardial region in the activation of arrhythmogenic mechanisms. This has led to important practical implications with the development of non-invasive and percutaneous catheter based epicardial mapping and ablation procedures. It has also given rise to intense research activity to better understand why the epicardial region can be a source of arrhythmias. It turns out that this region is characterized by distinct electrical and histological characteristics that under some circumstances can become a risk factor for arrhythmias. For instance, the epicardial origin of adipose tissue explains likely the propensity of the infiltration of subepicardial myocardial layers by fibro-fatty tissue as observed during several epicardial-driven arrhythmias and that has been shown to be potentially arrhythmogenic. In this line, the reactivation of the epicardium and the capacity of progenitor cells to differentiate into adipocytes or fibroblasts could be an early event in the pathophysiology of certain cardiac arrhythmias. Promising anti-arrhythmic strategies emerge from these novel pathological insights, one of them could target the accumulation of epicardial adipose tissue and its replacement by fibrosis. Progress will come also from the development of biological and imaging biomarkers that could detect the electrical fragility of the epicardial region.
Conflict of interest: F.A. is a consultant for and has received lecture fees from Boston Scientific, Medtronic, and Microport CRM. And the other authors have no conflict of interest to declare.
Funding
This work was supported by the French National Agency through the national program Investissements d’Avenir (Investments for the Future), grant ANR-10-IAHU-05 (to C.C., N.S., E.G., E.B., and S.N.H.) and through grant ANR-15-RHUS-0003 as well as the Fondation de La Recherche Medicale (Foundation of Medical Research) (to C.C., N.S., E.B., and S.N.H.). The project has received funding from European Union’s Horizon 2020 research and innovation programme under the Grant agreement number 965286; MAESTRIA.
Contributor Information
Corentin Chaumont, Cardiology Department, Rouen University Hospital, Rouen, France; FHU REMOD-VHF, UNIROUEN, INSERM U1096, F76000 Rouen, France.
Nadine Suffee, INSERM UMRS1166, ICAN—Institute of CardioMetabolism and Nutrition, Sorbonne University, Institute of Cardiology, Pitié-Salpêtrière Hospital, 91, boulevard de l’hôpital, 75013 Paris, France.
Estelle Gandjbakhch, INSERM UMRS1166, ICAN—Institute of CardioMetabolism and Nutrition, Sorbonne University, Institute of Cardiology, Pitié-Salpêtrière Hospital, 91, boulevard de l’hôpital, 75013 Paris, France.
Elise Balse, INSERM UMRS1166, ICAN—Institute of CardioMetabolism and Nutrition, Sorbonne University, Institute of Cardiology, Pitié-Salpêtrière Hospital, 91, boulevard de l’hôpital, 75013 Paris, France.
Frédéric Anselme, Cardiology Department, Rouen University Hospital, Rouen, France; FHU REMOD-VHF, UNIROUEN, INSERM U1096, F76000 Rouen, France.
Stéphane N Hatem, INSERM UMRS1166, ICAN—Institute of CardioMetabolism and Nutrition, Sorbonne University, Institute of Cardiology, Pitié-Salpêtrière Hospital, 91, boulevard de l’hôpital, 75013 Paris, France.
References
- 1. Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, Vitale R, Cuko A, Giannelli L, Calovic Z, Conti M, Pozzi P, Natalizia A, Crisà S, Borrelli V, Brugada R, Sarquella-Brugada G, Guazzi M, Frigiola A, Menicanti L, Santinelli V.. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053. [DOI] [PubMed] [Google Scholar]
- 2. Garcia FC, Bazan V, Zado ES, Ren J-F, Marchlinski FE.. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–375. [DOI] [PubMed] [Google Scholar]
- 3. Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG.. Epicardial ventricular tachycardia ablation: a multicenter safety study. J Am Coll Cardiol 2010;55:2366–2372. [DOI] [PubMed] [Google Scholar]
- 4. von GA, Pu WT.. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 2012;110:1628–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dettman RW, Denetclaw W, Ordahl CP, Bristow J.. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998;193:169–181. [DOI] [PubMed] [Google Scholar]
- 6. Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 1999;255:212–226. [DOI] [PubMed] [Google Scholar]
- 7. Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ.. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell 2012;22:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai C-L, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM.. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008;454:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou B, Honor LB, He H, Ma Q, Oh J-H, Butterfield C, Lin R-Z, Melero-Martin JM, Dolmatova E, Duffy HS, Gise AV, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT.. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 2011;121:1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tidball JG. Distribution of collagens and fibronectin in the subepicardium during avian cardiac development. Anat Embryol (Berl) 1992;185:155–162. [DOI] [PubMed] [Google Scholar]
- 11. Suffee N, Moore-Morris T, Farahmand P, Rücker-Martin C, Dilanian G, Fradet M, Sawaki D, Derumeaux G, LePrince P, Clément K, Dugail I, Puceat M, Hatem SN.. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc Natl Acad Sci U S A 2017;114:E771–E780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng W-YI, Wedeen VJ, Reese TG, Smith RN, Halpern EF.. Diffusion tensor MRI of myocardial fibers and sheets: correspondence with visible cut‐face texture. J Magn Reson Imaging 2003;17:31–42. [DOI] [PubMed] [Google Scholar]
- 13. Scollan DF, Holmes A, Winslow R, Forder J.. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol 1998;275:H2308–H2318. [DOI] [PubMed] [Google Scholar]
- 14. Iacobellis G, Corradi D, Sharma AM.. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536–543. [DOI] [PubMed] [Google Scholar]
- 15. Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM, Antoniades C.. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res 2016;118:842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Litovsky SH, Antzelevitch C.. Rate dependence of action potential duration and refractoriness in canine ventricular endocardium differs from that of epicardium: role of the transient outward current. J Am Coll Cardiol 1989;14:1053–1066. [DOI] [PubMed] [Google Scholar]
- 17. Ueda N, Zipes DP, Wu J.. Functional and transmural modulation of M cell behavior in canine ventricular wall. Am J Physiol-Heart Circ Physiol 2004;287:H2569–H2575. [DOI] [PubMed] [Google Scholar]
- 18. Litovsky SH, Antzelevitch C.. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res 1988;62:116–126. [DOI] [PubMed] [Google Scholar]
- 19. Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW.. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 1991;69:1427–1449. [DOI] [PubMed] [Google Scholar]
- 20. Sicouri S, Antzelevitch C.. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res 1991;68:1729–1741. [DOI] [PubMed] [Google Scholar]
- 21. Tseng GN, Hoffman BF.. Two components of transient outward current in canine ventricular myocytes. Circ Res 1989;64:633–647. [DOI] [PubMed] [Google Scholar]
- 22. Niwa N, Nerbonne JM.. Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation. J Mol Cell Cardiol 2010;48:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dixon Jane E, Shi Wenmei W, Hong-Sheng M, Christine Y, Hangang W, Randy S, Cohen Ira S, David M.. Role of the Kv4.3 K+ channel in ventricular muscle. Circ Res 1996;79:659–668. [DOI] [PubMed] [Google Scholar]
- 24. An WF, Bowlby MR, Betty M, Cao J, Ling H-P, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ.. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000;403:553–556. [DOI] [PubMed] [Google Scholar]
- 25. Rosati B, Pan Z, Lypen S, Wang H-S, Cohen I, Dixon JE, McKinnon D.. Regulation of KChIP2 potassium channel β subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol 2001;533:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM.. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res 2002;90:586–593. [DOI] [PubMed] [Google Scholar]
- 27. DescheNes I, DiSilvestre D, Juang GJ, Wu RC, An WF, Tomaselli GF.. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac I(to)? Circulation 2002;106:423–429. [DOI] [PubMed] [Google Scholar]
- 28. Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S.. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol 2004;561:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balse E, Steele DF, Abriel H, Coulombe A, Fedida D, Hatem SN.. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol Rev 2012;92:1317–1358. [DOI] [PubMed] [Google Scholar]
- 30. Liu DW, Antzelevitch C.. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res 1995;76:351–365. [DOI] [PubMed] [Google Scholar]
- 31. Sengupta PP, Korinek J, Belohlavek M, Narula J, Vannan MA, Jahangir A, Khandheria BK.. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol 2006;48:1988–2001. [DOI] [PubMed] [Google Scholar]
- 32. Hooks DA, Trew ML, Caldwell BJ, Sands GB, LeGrice IJ, Smaill BH.. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circ Res 2007;101:e103–e112. [DOI] [PubMed] [Google Scholar]
- 33. LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ.. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol 1995;269:H571–H582. [DOI] [PubMed] [Google Scholar]
- 34. Pope AJ, Sands GB, Smaill BH, LeGrice IJ.. Three-dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol 2008;295:H1243–H1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vetter FJ, Simons SB, Mironov S, Hyatt CJ, Pertsov AM.. Epicardial fiber organization in swine right ventricle and its impact on propagation. Circ Res 2005;96:244–251. [DOI] [PubMed] [Google Scholar]
- 36. Boukens BJ, Sylva M, de Gier-de Vries C, Remme CA, Bezzina CR, Christoffels VM, Coronel R.. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res 2013;113:137–141. [DOI] [PubMed] [Google Scholar]
- 37. Tansey DK, Aly Z, Sheppard MN.. Fat in the right ventricle of the normal heart. Histopathology 2005;46:98–104. [DOI] [PubMed] [Google Scholar]
- 38. Berruezo A, Acosta J, Fernández-Armenta J, Pedrote A, Barrera A, Arana-Rueda E, Bodegas AI, Anguera I, Tercedor L, Penela D, Andreu D, Perea RJ, Prat-González S, Mont L.. Safety, long-term outcomes and predictors of recurrence after first-line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic substrate distribution pattern. A prospective multicentre study. Europace 2017;19:607–616. [DOI] [PubMed] [Google Scholar]
- 39. Miljoen H, State S, Chillou CD, Magnin-Poull I, Dotto P, Andronache M, Abdelaal A, Aliot E.. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005;7:516–524. [DOI] [PubMed] [Google Scholar]
- 40. Polin GM, Haqqani H, Tzou W, Hutchinson MD, Garcia FC, Callans DJ, Zado ES, Marchlinski FE.. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2011;8:76–83. [DOI] [PubMed] [Google Scholar]
- 41. Hutchinson MD, Gerstenfeld EP, Desjardins B, Bala R, Riley MP, Garcia FC, Dixit S, Lin D, Tzou WS, Cooper JM, Verdino RJ, Callans DJ, Marchlinski FE.. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin C-Y, Lin Y-J, Li C-H, Chung F-P, Lo M-T, Lin C, Chang H-C, Chang S-L, Lo L-W, Hu Y-F, Chang Y-T, Lin C-H, Chen Y-Y, Walia R, Te ALD, Yamada S, Wu T-J, Chen S-A.. Heterogeneous distribution of substrates between the endocardium and epicardium promotes ventricular fibrillation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Europace 2018;20:501–511. [DOI] [PubMed] [Google Scholar]
- 43. Andrews CM, Srinivasan NT, Rosmini S, Bulluck H, Orini M, Jenkins S, Pantazis A, McKenna WJ, Moon JC, Lambiase PD, Rudy Y.. Electrical and structural substrate of arrhythmogenic right ventricular cardiomyopathy determined using noninvasive electrocardiographic imaging and late gadolinium magnetic resonance imaging. Circ Arrhythm Electrophysiol 2017;10:e005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haqqani HM, Tschabrunn CM, Betensky BP, Lavi N, Tzou WS, Zado ES, Marchlinski FE.. Layered activation of epicardial scar in arrhythmogenic right ventricular dysplasia. Circ Arrhythm Electrophysiol 2012;5:796–803. [DOI] [PubMed] [Google Scholar]
- 45. Wijnmaalen AP, Schalij MJ, Bootsma M, Kies P, Roos AD, Putter H, Bax JJ, Zeppenfeld K.. Patients with scar-related right ventricular tachycardia: determinants of long-term outcome. J Cardiovasc Electrophysiol 2009;20:1119–1127. [DOI] [PubMed] [Google Scholar]
- 46. Santangeli P, Zado ES, Supple GE, Haqqani HM, Garcia FC, Tschabrunn CM, Callans DJ, Lin D, Dixit S, Hutchinson MD, Riley MP, Marchlinski FE.. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8:1413–1421. [DOI] [PubMed] [Google Scholar]
- 47. Romero J, Cerrud-Rodriguez RC, Di Biase L, Diaz JC, Alviz I, Grupposo V, Cerna L, Avendano R, Tedrow U, Natale A, Tung R, Kumar S, Combined endocardial-epicardial versus endocardial catheter ablation alone for ventricular tachycardia in structural heart disease: a systematic review meta-analysis. JACC Clin Electrophysiol 2019;5:13–24. [DOI] [PubMed] [Google Scholar]
- 48. Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, Saenz LC, Vacca M, Verma A, Khaykin Y, Mohanty S, Burkhardt JD, Hongo R, Beheiry S, Dello Russo A, Casella M, Pelargonio G, Santarelli P, Sanchez J, Tondo C, Natale A.. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol 2011;4:478–485. [DOI] [PubMed] [Google Scholar]
- 49. Berruezo A, Fernández-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J.. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol 2012;5:111–121. [DOI] [PubMed] [Google Scholar]
- 50. Fernandes GC, Fernandes A, Cardoso R, Nasi G, Rivera M, Mitrani RD, Goldberger JJ.. Ablation strategies for the management of symptomatic Brugada syndrome: a systematic review. Heart Rhythm 2018;15:1140–1147. [DOI] [PubMed] [Google Scholar]
- 51. Rudic B, Chaykovskaya M, Tsyganov A, Kalinin V, Tülümen E, Papavassiliu T, Dösch C, Liebe V, Kuschyk J, Röger S, El‐Battrawy I, Akin I, Yakovleva M, Zaklyazminskaya E, Shestak A, Kim S, Chmelevsky M, Borggrefe M.. Simultaneous non‐invasive epicardial and endocardial mapping in patients with Brugada syndrome: new insights into arrhythmia mechanisms. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2016;5:e004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Junjie Z, Frédéric S, Kurt H, Thomas O, Maria S, Phillip C, Jennifer S, Daniel C, Mitchell F, Mélèze H, Michel H, Melvin S, Yoram R.. Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation 2015;131:1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brugada J, Pappone C, Berruezo A, Vicedomini G, Manguso F, Ciconte G, Giannelli L, Santinelli V.. Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol 2015;8:1373–1381. [DOI] [PubMed] [Google Scholar]
- 54. Nademanee K, Hocini M, Haïssaguerre M.. Epicardial substrate ablation for Brugada syndrome. Heart Rhythm 2017;14:457–461. [DOI] [PubMed] [Google Scholar]
- 55. Sosa E, Scanavacca M, d'Avila A, Pilleggi F.. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol 1996;7:531–536. [DOI] [PubMed] [Google Scholar]
- 56. Sosa E, Scanavacca M, Avila AD, Oliveira F, Ramires JAF.. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol 2000;35:1442–1449. [DOI] [PubMed] [Google Scholar]
- 57. Roberts WC, Siegel RJ, McManus BM.. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol 1987;60:1340–1355. [DOI] [PubMed] [Google Scholar]
- 58. Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE.. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009;54:799–808. [DOI] [PubMed] [Google Scholar]
- 59. Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C, Hindricks G, Arya A.. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy. Circulation 2014;129:728–736. [DOI] [PubMed] [Google Scholar]
- 60. Dello Russo A, Casella M, Pieroni M, Pelargonio G, Bartoletti S, Santangeli P, Zucchetti M, Innocenti E, Di Biase L, Carbucicchio C, Bellocci F, Fiorentini C, Natale A, Tondo C.. Drug-refractory ventricular tachycardias after myocarditis: endocardial and epicardial radiofrequency catheter ablation. Circ Arrhythm Electrophysiol 2012;5:492–498. [DOI] [PubMed] [Google Scholar]
- 61. De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A.. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 2006;47:1649–1654. [DOI] [PubMed] [Google Scholar]
- 62. Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL, Boineau JP.. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 1993;88:250–263. [DOI] [PubMed] [Google Scholar]
- 63. Groot ND, Houben RPM, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA.. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 2010;122:1674–1682. [DOI] [PubMed] [Google Scholar]
- 64. de GN, L van der D, Yaksh A, Lanters E, Teuwen C, Knops P, van de W, Bekkers J, Kik C, Bogers A, Allessie M.. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol 2016;9:e003648. [DOI] [PubMed] [Google Scholar]
- 65. Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule S, Allessie M, Schotten U.. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res 2011;89:816–824. [DOI] [PubMed] [Google Scholar]
- 66. Mouws EMJP, Lanters EAH, Teuwen Christophe P, van der Does Lisette JME, Kik C, Knops P, Bekkers JA, Bogers AJJC, de Groot NMS.. Epicardial breakthrough waves during sinus rhythm. Circ Arrhythm Electrophysiol 2017;10:e005145. [DOI] [PubMed] [Google Scholar]
- 67. Pak H-N, Hwang C, Lim HE, Kim JS, Kim Y-H.. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach for difficult cases. J Cardiovasc Electrophysiol 2007;18:917–923. [DOI] [PubMed] [Google Scholar]
- 68. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Esquivias GB, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 69. Vroomen M, Pison L.. Hybrid ablation for atrial fibrillation: a systematic review. J Interv Card Electrophysiol 2016;47:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cluitmans M, Brooks DH, MacLeod R, Dössel O, Guillem MS, Dam PV, Svehlikova J, He B, Sapp J, Wang L, Bear L.. Validation and opportunities of electrocardiographic imaging: from technical achievements to clinical applications. Front Physiol 2018;9:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilde AAM, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C.. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol 2010;49:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meregalli PG, Wilde AAM, Tan HL.. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res 2005;67:367–378. [DOI] [PubMed] [Google Scholar]
- 73. Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R.. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:784–804. [DOI] [PubMed] [Google Scholar]
- 74. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G.. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996;335:1190–1197. [DOI] [PubMed] [Google Scholar]
- 75. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, Claus P, LePrince P, Nicoletti A, Jalife J, Wolke C, Lendeckel U, Jaïs P, Willems R, Hatem SN.. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J 2017;38:53–61. [DOI] [PubMed] [Google Scholar]
- 76. Corrado D, Basso C, Thiene G.. Cardiomyopathy: arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart 2000;83:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burke AP, Farb A, Tashko G, Virmani R.. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium. Circulation 1998;97:1571–1580. [DOI] [PubMed] [Google Scholar]
- 78. Flavie A, Elodie S, Philippe C, Alban R, Amir Z, Alice M, Eloi M, Denjoy I, Alexis H, Véronique F, Estelle G.. Inherited cardiomyopathies revealed by clinically suspected myocarditis. Circ Genomic Precis Med 2020;13:e002744. [DOI] [PubMed] [Google Scholar]
- 79. Nademanee K, Raju H, Noronha SD, Papadakis M, Robinson L, Rothery S, Makita N, Kowase S, Boonmee N, Vitayakritsirikul V, Ratanarapee S, Sharma S, Wal AVD, Christiansen M, Tan HL, Wilde AA, Nogami A, Sheppard MN, Veerakul G, Behr ER.. Fibrosis, Connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol 2015;66:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ohkubo K, Watanabe I, Okumura Y, Takagi Y, Ashino S, Kofune M, Sugimura H, Nakai T, Kasamaki Y, Hirayama A, Morimoto S-I.. Right ventricular histological substrate and conduction delay in patients with Brugada syndrome. Int Heart J 2010;51:17–23. [DOI] [PubMed] [Google Scholar]
- 81. Corrado D, Nava A, Buja G, Martini B, Fasoli G, Oselladore L, Turrini P, Thiene G.. Familial cardiomyopathy underlies syndrome of right bundle branch block, ST segment elevation and sudden death. J Am Coll Cardiol 1996;27:443–448. [DOI] [PubMed] [Google Scholar]
- 82. Corrado D, Zorzi A, Cerrone M, Rigato I, Mongillo M, Bauce B, Delmar M.. Relationship between arrhythmogenic right ventricular cardiomyopathy and Brugada syndrome. Circ Arrhythm Electrophysiol 2016;9:e003631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Furushima H, Chinushi M, Okamura K, Iijima K, Komura S, Tanabe Y, Okada S, Izumi D, Aizawa Y.. Comparison of conduction delay in the right ventricular outflow tract between Brugada syndrome and right ventricular cardiomyopathy: investigation of signal average ECG in the precordial leads. Europace 2007;9:951–956. [DOI] [PubMed] [Google Scholar]
- 84. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim Y-H, Lip GYH, Ma C-S, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Wagoner DRV, Nattel S, Centurion DROA, Kuck K-H, Review coordinator: Alena Shantsila (UK) . EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm 2016;32:247–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Medi C, Kalman JM, Spence SJ, Teh AW, Lee G, Bader I, Kaye DM, Kistler PM.. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:1317–1324. [DOI] [PubMed] [Google Scholar]
- 86. John B, Stiles MK, Kuklik P, Chandy ST, Young GD, Mackenzie L, Szumowski L, Joseph G, Jose J, Worthley SG, Kalman JM, Sanders P.. Electrical remodelling of the left and right atria due to rheumatic mitral stenosis. Eur Heart J 2008;29:2234–2243. [DOI] [PubMed] [Google Scholar]
- 87. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R.. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920. [DOI] [PubMed] [Google Scholar]
- 88. Pieroni M, Notarstefano P, Oliva A, Campuzano O, Santangeli P, Coll M, Nesti M, Carnevali A, Fraticelli A, Iglesias A, Grassi S, Brugada R, Bolognese L.. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with Brugada syndrome. J Am Coll Cardiol 2018;72:2747–2757. [DOI] [PubMed] [Google Scholar]
- 89. Gharaviri A, Bidar E, Potse M, Zeemering S, Verheule S, Pezzuto S, Krause R, Maessen JG, Auricchio A, Schotten U.. Epicardial fibrosis explains increased endo–epicardial dissociation and epicardial breakthroughs in human atrial fibrillation. Front Physiol Frontiers 2020;11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, Lui E, Sim CB, Larobina M, O'Keefe M, Goldblatt J, Royse A, Lee G, Porrello ER, Watt MJ, Kistler PM, Sanders P, Delbridge LMD, Kalman JM.. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 2020;76:1197–1211. [DOI] [PubMed] [Google Scholar]
- 91. De Coster T, Claus P, Kazbanov IV, Haemers P, Willems R, Sipido KR, Panfilov AV.. Arrhythmogenicity of fibro-fatty infiltrations. Sci Rep 2018;8:2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gan L, Xie D, Liu J, Bond Lau W, Christopher TA, Lopez B, Zhang L, Gao E, Koch W, Ma X-L, Wang Y.. Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation 2020;141:968–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhao Y, Sun Q, Zeng Z, Li Q, Zhou S, Zhou M, Xue Y, Cheng X, Xia Y, Wang Q, Tu X.. Regulation of SCN3B/scn3b by Interleukin 2 (IL-2): IL-2 modulates SCN3B/scn3b transcript expression and increases sodium current in myocardial cells. BMC Cardiovasc Disord 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gómez-Hurtado N, Domínguez-Rodríguez A, Mateo P, Fernández-Velasco M, Val-Blasco A, Aizpún R, Sabourin J, Gómez AM, Benitah J-P, Delgado C.. Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse. J Physiol 2017;595:4227–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen H-SV.. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 2013;494:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lombardi R, Dong J, Rodriguez G, Bell A, Leung TK, Schwartz RJ, Willerson JT, Brugada R, Marian AJ.. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 2009;104:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sommariva E, Brambilla S, Carbucicchio C, Gambini E, Meraviglia V, Dello Russo A, Farina FM, Casella M, Catto V, Pontone G, Chiesa M, Stadiotti I, Cogliati E, Paolin A, Ouali Alami N, Preziuso C, Amati GD, Colombo GI, Rossini A, Capogrossi MC, Tondo C, Pompilio G.. Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur Heart J 2016;37:1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ.. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res 2014;114:454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Suffee N, Moore-Morris T, Jagla B, Mougenot N, Dilanian G, Berthet M, Proukhnitzky J, Le Prince P, Tregouet DA, Pucéat M, Hatem SN.. Reactivation of the epicardium at the origin of myocardial fibro-fatty infiltration during the atrial cardiomyopathy. Circ Res 2020;126:1330–1342. [DOI] [PubMed] [Google Scholar]
- 100. Blom JN, Feng Q.. Cardiac repair by epicardial EMT: current targets and a potential role for the primary cilium. Pharmacol Ther 2018;186:114–129. [DOI] [PubMed] [Google Scholar]
- 101. Quijada P, Trembley MA, Small EM.. The role of the epicardium during heart development and repair. Circ Res 2020;126:377–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR, Sucov HM.. Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc Natl Acad Sci U S A 2015;112:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lombardi R, Chen SN, Ruggiero A, Gurha P, Czernuszewicz GZ, Willerson JT, Marian AJ.. Cardiac fibro-adipocyte progenitors express desmosome proteins and preferentially differentiate to adipocytes upon deletion of the desmoplakin gene. Circ Res 2016;119:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kohela A, van Kampen S, Moens T, Monshouwer-Kloots J, Molenaar B, Wehrens M, Vink A, Chen Huei-Sheng V, van Rooij E.. Abstract 783: epicardial contribution to arrhythmogenic cardiomyopathy. Circ Res 2019;125:A783–A783. [Google Scholar]