Abstract
The nematode C. elegans has a contingent of five sod genes, one of the largest among aerobic organism. Earlier studies revealed each of the five sod genes is capable of making perfectly active SOD proteins in heterologous expression systems therefore none appears to be a pseudogene. Yet deletion of the entire contingent of sod genes fails to impose any effect on the survival of C. elegans except these animals appear more sensitive to extraneously applied oxidative stress condition. We asked how many of the five sod genes are actually making active SOD enzymes in C. elegans through usage of in-gel SOD activity analysis and by using KCN as a selective inhibitor against Cu-ZnSOD enzyme(s). Here we provide evidence that out of the five SOD proteins only the mitochondrial SOD is active in the water soluble fraction of C. elegans extracts albeit at an apparently much lower activity than the multiple active SODs in D. melanogaster and E. coli. We had no opportunity to test the activity of Sod-4a isoform which is possibly a membrane bound form of SOD. Mutant analysis further confirmed that among the two mitochondrial SOD proteins, SOD-2 is the only naturally active SOD in C. elegans.
Keywords: SOD, C. elegans, Free Radicals, Superoxide, Oxidative Stress, Mitochondria
1. Introduction
Aerobic organisms utilize Superoxide Dismutase (SOD) enzymes to scavenge and catabolize partially reduced oxygen molecules (superoxide radicals; O2−) into hydrogen peroxide (Fridovitch, 1995). Superoxides are generated in the electron transport chain from aerobic respiration. In different aerobes, SOD enzymes entrap superoxide radicals with the help of a variety of redox active transition metals such as Cu, Mn, Fe or Ni. Despite all SODs performing the single task of scavenging superoxide radicals, the activity of specific SOD enzymes are noticeably limited to either the mitochondrial matrix, cytoplasm, inter-membrane space, or to the extracellular environment (Weisiger and Fridovich 1973; Missirilis et al., 2003; Muller et al., 2004). Loss-of-function studies in different species have established the variable requirement of the SOD protection system because the spectrum of defects appearing in SOD mutants are wide ranging. In general, lack of mitochondrial SOD in vertebrates cause neonatal lethality accompanied by signs of mitochondrial damage, cardiomyopathy and neuropathology (Lebovitch et al., 1996; Li et al., 1995). Early lethality is also evident in the Drosophila mitochondrial SOD (SOD2) loss of function condition (Kirby et al., 2002; Duttaroy et al., 2003) with evidence of neuropathology (Paul et, al., 2007), loss of mitochondrial aconitase activity and reduced ATP production in the muscle (Godenschwege et al., 2009).
On the other hand, loss of the Cu-ZnSOD enzyme (SOD1) causes more subtle effects particularly in higher animals. For example, mice lacking Cu-ZnSOD activity exhibit no effect on longevity although a multitude of other damages are reported (Valentine et al., 2005; Culotta et al., 2006). Drosophila Cu-ZnSod null mutants survive albeit with a shorter adult life span (Phillips et al., 1989). Finally, bacterial and yeast mutants of sod1 and sod2 are hypersensitive to hyperbaric Oxygen concentration, and exhibit numerous biochemical defects in these mutants (Culotta, 2000).
The nematode C. elegans is a free-living, soil dwelling aerobe traditionally used as a workhorse in studies related to aging, cell death, and developmental biology (Corsi et al., 2015). Homology analysis established five sod genes in C. elegans, possibly one of the largest contingents of SOD isoforms among aerobic organism (Landis and Tower, 2005; Worm Base). C. elegans sod-1, sod-2 and sod-4 represent the traditional cytoplasmic, mitochondrial and extracellular forms of SODs respectively, while sod-3 and sod-5 are recognized as additional and minor mitochondrial and cytoplasmic SODs (Doonan et al., 2008). sod-1 and sod-2 mRNAs represent the bulk of the sod RNAs (~76% and 18% respectively) within the total SOD mRNA populations whereas the remaining three sod mRNAs make up only between 5–0.5% of the total (Doonan et al., 2008). Hence in C. elegans all sod genes are expressed but differentially.
Are the extra copies of sod purposefully acquired to gain additional protection against some pro-oxidative environment or are most of these sod genes evolutionary relics? Mutational analyses have already provided a striking answer as a C. elegans strain containing a quintuple deletions of all sod genes survives as long as the wild type control worms (Van Raamsdonk and Hekimi, 2012). Additionally, no tissue damage or loss of mitochondrial proteins was evident in this quintuple sod mutant due to the lack of a complete SOD protection system. The only noticeable change seems to be these animals are hypersensitive to different kinds of oxidants and exhibit reduced fertility (Van Raamsdonk et al., 2009). However, C. elegans sod genes are not evolutionary relics because sod-2 and sod-3 genes and sod1 and sod-5 genes can make perfectly active SOD proteins in heterologous systems like bacteria and yeast (Hunter et al., 1997; Jensen and Cullota, 2005). This suggests therefore that no coding sequence deficiencies are expected in these genes like one might observe in a pseudogene. Additionally, SOD enzyme activity assays support that C. elegans do contain active SOD enzyme(s) (Doonan et al., 2008; Ramsdonk and Hekimi, 2012) however which one or how many of the five SOD proteins are naturally active remains an open question.
Mutational analyses have begun to provide some intriguing answers to the requirement of SOD enzymes in C. elegans. C. elegans null mutants for either sod-1 or sod-2 exhibit a reduced brood phenotype (Sakamoto and Idahi, 2017). For the sod-2 mutant this was shown to be due to a sperm defect, while the sod-1 mutant fertility defect is hypothesized to be due to defects in oogenesis (Sakamoto and Idahi, 2017). The sod-2 null sperm defect can be rescued by treatment with exogenous hydrogen peroxide, suggesting that it is a lack of active SOD enzymatic activity in the sperm upon mutation of sod-2 that causes the defect.
With the help of in-gel SOD activity assays of the soluble protein extract we demonstrate that only the mitochondrial SOD protein maintains enzymatic activity C. elegans and of the two mitochondrial SODs, SOD-2 remains naturally active in C. elegans.
2. Materials and Methods
C. elegans strains and culture conditions:
The following strains were used in this work: wild type N2; CB1370 [daf-2(e1370)III], FX776 [sod-1(tm776)II]; VC433 [sod-3(gk235)X]; RB1072 [sod-2(ok1030)I]; MQ1766 [sod-2(ok1030)I; sod-5(tm1146) sod-1(tm783) II; sod-4(gk101)III; sod-3(tm760)X]. All strains were grown and maintained under standard conditions at 20°C.
Protein extraction
C. elegans protein lysates were prepared by washing the appropriately staged worms off MYOB plates grown at 20°C with 1X PBS and collected in 1.5 ml microcentrifuge tube. Worms were pelleted with gentle centrifugation, homogenized in 1 X PBS plus 10ul/ml Halt protease and phosphatase inhibitor (single use cocktail, Thermo Fisher Scientific Cat. number 78442) on ice for 10 mins followed by centrifugation at 16000 G at 4°C for 15mins. Supernatant was aspirated carefully avoiding the floating lipid layer. Bradford assay was performed to measure the protein concentration. Similar technique is used for extracting Drosophila (Canton-S) and E. coli proteins.
In gel SOD activity assay:
For in-gel SOD activity assay total soluble protein extracts were loaded into a gel (4–20% Criterion Precast Gel, Biorad Cat #3450032) and run at 4° C. Once completed gels were soaked in 40 ml phosphate buffer containing 80 mg of NBT (2.43 mM), 170 μl of TEMED (28 mM) and 8 μl of stock riboflavin-5’-phosphate (0.14 M [53 mg ml−1] in 50 mM phosphate buffer, pH 7.8) for 20 minutes under dark. Following incubation, the gels were washed in dH2O three times and put under fluorescent light for 2 hours. During this time the gels turned purple and achromatic band begins to appear. In principal, SOD will compete with NBT for O •−. In the absence of SOD, NBT will react with O •− and form a blue precipitate throughout the gel except in areas where SOD enzyme(s) is active which appears as clear bands (achromatic) because it outcompetes NBT for the O2•− in those areas (Weydert and Cullen, 2010). For KCN treatment 10 mM KCN is added to C. elegans protein extracts and incubated in room temperature for 30 mins prior to loading.
2. Results and Discussion
To answer how many and which particular SOD protein is active we performed in situ SOD gel analysis using preparations of native protein extracts from C. elegans and compared this with Drosophila and E. coli extracts. Starting with Drosophila, two active SOD enzymes are identified (Figure 1A) with the top band being the mitochondrial SOD (MnSOD or SOD2) and the lower band representing the cytoplasmic SOD (Cu-ZnSOD or SOD1) (Phillips et al., 1989; Duttaroy et al., 2003). As expected, the E. coli extract shows three forms of bacterial SOD enzymes MnSOD, Fe-SOD, and the Cu-ZnSOD (Figure 1A). On the other hand, wild type C. elegans (N2) extract shows only a single SOD enzyme band indicating that it carries no more than one active form of a SOD enzyme (Figure 1A).
FIGURE 1:
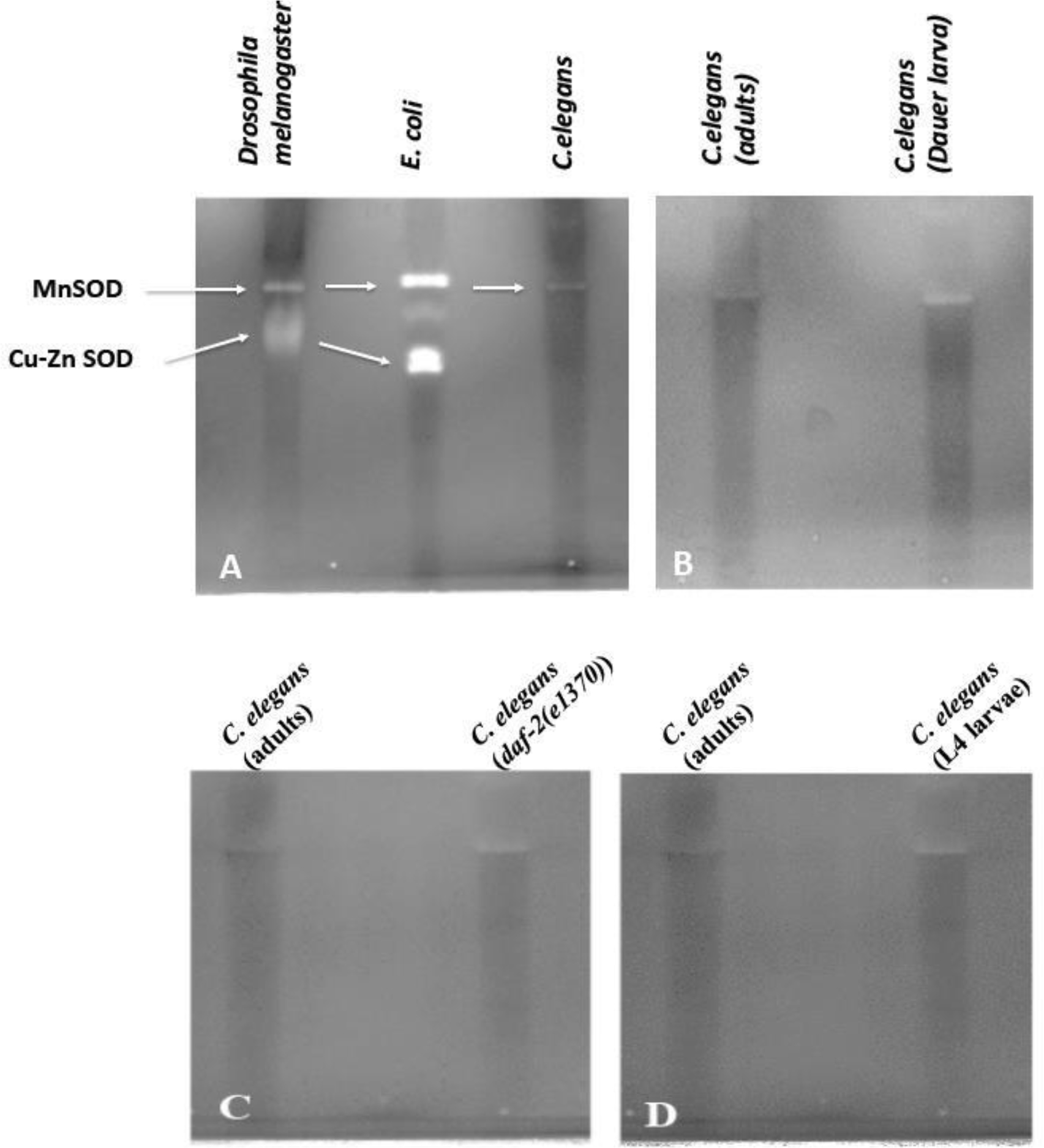
In situ SOD enzyme profiles in three different species. (A) Comparison of SOD activity profiles in D. melanogaster, E. coli and C. elegans. C. elegans has no more than one active SOD enzyme. (B, C, and D) The dauer larval stage, daf-2 mutant and L4larvae all carry one active form of SOD.
Two things are notable with respect to the single active C. elegans SOD enzyme. First it lines up perfectly with the MnSOD/SOD2 isoforms of Drosophila and E. coli, suggesting that this SOD activity is from either of the two C. elegans MnSODs- SOD-2 or SOD-3. Second, the C. elegans SOD activity appears as a much less intense band in comparison to the activity observed in Drosophila and E. coli despite loading equal amounts of protein extracts per lane (60 μg/lane). This leads us to hypothesize that either C. elegans makes much less SOD enzyme or that the enzyme itself is less active in this organism compared to the Drosophila and E. coli SODs. Further research should resolve if C. elegans SOD is either expressed at a lesser amount or if it is less active relative to other organisms.
The previous result led us to ask why is there only one naturally active SOD enzyme present in the C. elegans system when there are five sod genes? As none of the five sod genes in C. elegans are pseudogenes since each is capable of making perfectly active SOD protein in a heterologous system we had anticipated multiple active SODs in our in-gel activity assay (Hunter et al., 1997; Jensen and Cullota, 2005). We tested two possibilities such as some of the SOD enzymes might be made and active during specific developmental stages of the worm (Jenese and Cullota, 2005), or second, that some SODs are active only under high stress condition. C. elegans dauer arrested larvae, a stage adopoted to survive in high stress environment show no more than one active SOD enzyme (Figure 1B). Similarly, no additional SOD enzymes appear active in the L4 larval stage protein extracts compared to adult extracts (Figure 1D) nullifying the possibility of activation of certain SOD enzymes during the L4 stage of development (Figure 1D). As we have not tested the other worm developmental stages, we cannot comment for each of those. It has previously been reported that daf-2 mutants exhibit overproduction of sod mRNA (Honda and Honda, 1999). In our in-gel SOD activity assay we observe a slightly more intense SOD band in the daf-2 mutants compared to that observed in the wild-type control supporting the reported increase in sod mRNA (Figure 1C). Taken together our observations raise a strong possibility that only one SOD enzyme is naturally active starting from the L4 stage and throughout adulthood in C. elegans.
The obvious question then is which one of the five SOD proteins remains active in adult C. elegans? We previously mentioned that the single active C. elegans SOD enzyme lines up nicely with the mitochondrial SOD2 band of Drosophila and E. coli (Figure 1A). This suggests that either one of the two mitochondrial SOD proteins (SOD-2 or SOD-3) or may be both are represented in this single SOD active enzyme band. It has been reported that these two SOD proteins arise from a gene duplication event (Hunter et al., 1997) and the predicted molecular weights of C. elegans SOD-2 and SOD-3 are 24.5 and 24.6kD respectively (Table 1) which matches very closely with the Drosophila and E. coli SOD2 proteins that are 24.7 and 25.2kD respectively (Table 1). The only other C. elegans SOD of similar molecular weight is SOD-4b (a.k.a Sod4–2) isoform that weighs 23.3 kD (Table 1). However, SOD-4b with its large trans-membrane domain should remain associated with the cell membrane, (Fujii et al., 1998) and therefore it is very likely eliminated with the membrane fraction during protein extraction. On the other hand, the three cytoplasmic SOD proteins in C. elegans, SOD-1, SOD-4a and SOD-5 are significantly smaller in molecular weight compared to the mitochondrial SODs weighing 18.7, 18.2 and 18.5kD respectively (Table 1) and thus not likely to contribute to high molecular weight SOD band.
Table 1.
Molecular weights of SOD enzymes isomers in C. elegans, D. melanogaster and E. coli
| Organism C. elegans |
Protein Length (aa) | Subunit Mol.wt (Estimated)/ | Isoelectric Point (pI) |
|---|---|---|---|
| SOD-3/MnSOD | 218 | 24.6 kD | 8.43 |
| (Inducible; Hunter et al., 2015) | Tetrameric | ||
| SOD-2/MnSOD (constitutive) | 221 | 25.5 kD Tetrameric |
7.77 |
| SOD-1, Isoform a/Cu-Zn SOD | 180 | 18.7 kD Dimeric |
6.14 |
| SOD-1, Isoform b/Cu-Zn SOD | 158 | 16.2 kD Dimeric |
6.14 |
| SOD4, Isoform a | 176 | 18.2 kD Dimeric (?) |
6.43 |
| SOD4, Isoform b | 221 | 23.3 kD Dimeric (?) |
6.22 |
| SOD-5 | 178 | 18.5 kD | 5.42 |
| D. melanogaster | |||
| SOD-2/MnSOD | 217 | 24.7 kD | 8.10 |
| SOD-1, PA | 153 | Tetrameric 15.7 kD |
6.06 |
| Cu-Zn SOD | Dimeric | ||
| E. coli | |||
| SOD-2/MnSOD | 206 | 25.2 kD Dimeric and Tetrameric |
6.88 |
| FeSOD | 192 | 21.9 kD | 5.91 |
| SOD-1 (Cu-Zn SOD) | 173 | 15.6 kD Dimeric |
6.53 |
Most subunit information obtained from Sheng et al (2014)
Interestingly, the single SOD band in C. elegans was previously designated as the active SOD1 enzyme (Jenese and Cullota, 2005). To resolve this issue we used various single and multiple sod mutants in our in-gel SOD activity assay. The quintuple sod knockout mutant worms [sod-2(ok1030) I; sod-5(tm1146) sod-1(tm783) II; sod-4(gk101) III; sod-3(tm760) X] (Van Raamsdonk and Hekimi, 2012) displays no active SOD enzyme (Supplemental Figure 1). Protein extracts from three single sod null alleles provided a plausible answer. sod-1(tm776) and sod-3(gk235) mutant homozygotes clearly display the single SOD band as in wild type, whereas the sod-2(ok1030) mutant is completely lacking an active SOD band (Figure 2B).
FIGURE 2:
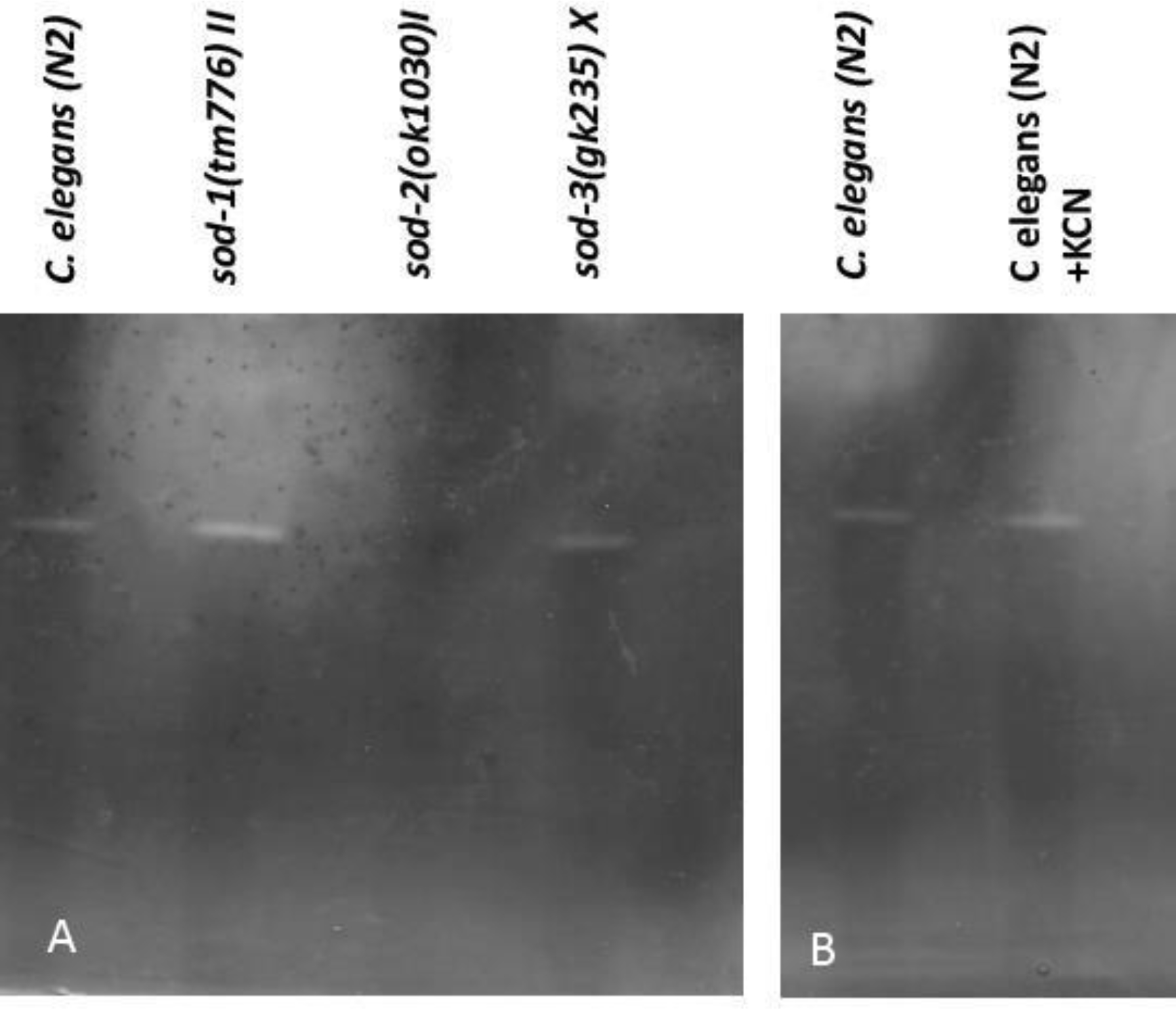
SOD activity profiles in different C. elegans sod mutants. (A) No active SOD appears in the sod2(ok1030)I null mutant whereas both the sod-1(tm776)II and sod-3(gk235)X null mutants still possess the single active form of SOD observed in wild type animals. (B) Treatment of wild-type C. elegans with KCN does not inhibit this SOD activity.
The above observation clearly suggests that the active SOD enzyme in C. elegans is the mitochondrial SOD-2 protein, however as SOD-2 and SOD-4b are quite close in terms of molecular weight there remains the possibility that the single active band may be arising from an active sod-4b allele. We did not think this was likely due to the fact that SOD-4b is a trans-membrane protein and all membrane bound proteins should be eliminated during the extraction procedure. However, to clearly test whether the single active SOD band in C. elegans is due to SOD-4b we treated wild-type protein extract with KCN. SOD-4b isoform is a Cu-ZnSOD (Fujii et al., 1998) and Cu-ZnSOD activities are inhibited by KCN. Incubation of C. elegans extract in the presence of 10 mM KCN solution failed to inhibit the SOD active band proving thereby that the active C. elegans SOD protein it is not a Cu-ZnSOD enzyme and thus not SOD-4b (Figure 2B).
Our attempt to profile the active SOD enzymes in C. elegans led to the finding that the mitochondrial SOD-2 enzyme is the solely active SOD enzyme that is present atleast throughout the adult life span of this organism. Since the mitochondria is the main source of superoxide radicals in the cell due to the increased oxygen metabolism in this organelle, keeping the mitochondrial enzyme in an active state makes more sense for any aerobic organism. Why the C. elegans SOD-2 enzyme appears relatively less active compared to other organisms remains unclear (Figure 1A). The nonessential nature of this lone active SOD2 enzyme is demonstrated as the sod-2 deletion mutant positively influences the life span by surviving longer than the wild type and also exhibits other mutant phenotypes such as slow development, a low brood size, and slow defecation (Raamsdonk and Hekimi, 2009). Unlike any other aerobes, total abrogation of all SOD enzymes is tolerated by C. elegans, as well as it is completely resistant to hypoxic environment (Mehta et al., 2009). In fact, hypoxia imposes a positive influence on C. elegans life span. In sum, these observations challenge the necessity of the SOD system as the primary defense mechanism towards ROS detoxification in C. elegans. An important question that remains is what has taken the place of the SOD system requirement in C. elegans?
Supplementary Material
SUPPLEMENTAL FIGURE: No active SOD enzyme is present in the quintuple sod deletion line [sod-2(ok1030) I; sod-5(tm1146) sod-1(tm783) II; sod-4(gk101) III; sod-3(tm760) X], whereas sod-3(gk235) and sod1(tm776)II null alleles individually carry the single active form of SOD.
Acknowledgement:
We thankfully acknowledge the National Science Foundation grant 1832026 and NIH grant R25AG047843 awarded to AD. LMF and AKA are supported by a Department of Defense grant W911NF1810465 awarded to AKA. Authors are thankful to Mr. Aon Ali for finishing the KCN sensitivity experiment. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (PO OD10440).
REFERENCES:
- Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998; 53: B240–B244. [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M (2015) A transparent window into biology: a primer on Caenorhabditis elegans. Genetics 200;387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Yang M, and O’Halloran TV (2006) Activation of superoxide dismutases: Putting the metal to the pedal. Biochim Biophys Acta. 1763: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC (2000) Superoxide dismutase, oxidative stress, and cell metabolism. Curr Top Cell Regul. 36:117–32. [DOI] [PubMed] [Google Scholar]
- Doonan R, McElwee JJ, Matthijssens F, Walker GA, Hou- thoofd K, Back P, Matscheski A, Vanfleteren JR, and Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22: 3236–3241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics 2003; 165:2295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I Superoxide radical and superoxide dismutases. Annu. Rev. Biochem 64:97–112; 1995. [DOI] [PubMed] [Google Scholar]
- Fujii M, Ishi N, Joguchi A, Yasuda K, and Ayusawa D (1998) A novel superoxide dismutase gene encoding membrane bound and extracellular isoforms by alternate splicing in Caenorhabditis elegans. DNA Research 5, 25–30. [DOI] [PubMed] [Google Scholar]
- Godenschwege T, Forde R, Davis CP, Paul A, Beckwith K, Duttaroy A (2009) Mitochondrial superoxide radicals differentially affect muscle activity and neural function. Genetics 183: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Bannister WH and Hunter GJ (1997) Cloning, expression, and characterization of two manganese superoxide dismutases from Caenorhabditis elegans. J.Biol.Chem 272: 28652–28659. [DOI] [PubMed] [Google Scholar]
- Hunter G J, Trinh Chi H., Rosalin Bonetta, Stewart Emma E., Cabelli Diane E., and Therese Hunter(2015) The structure of the Caenorhabditis elegans manganese superoxide dismutase MnSOD-3-azide complex. Protein Science 24:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y and Honda S 1999. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 13: 1385–1393. [PubMed] [Google Scholar]
- Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interfer- ence-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endog- enous oxidative stress. Proc Natl Acad Sci USA 2002; 99:16162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN and Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 126: 365–79, 2005. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr, Dionne L, Lu N, et al. Neurodegeneration, myocardial injury and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA 1996; 93:9782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, 26. Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature Gene 1995; 11:376–81. [DOI] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, et al. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009; 324: 1196–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missirlis F; Hu J; Kirby K; Hilliker AJ; Rouault TA; Phillips JP Compartment- speci!c protection of iron–sulfur proteins by superoxide dismutase. J. Biol. Chem 278:47365–47369; 2003. [DOI] [PubMed] [Google Scholar]
- Muller FL, Liu Y, and Van Remmen H 2004. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem 279: 49064–49073. [DOI] [PubMed] [Google Scholar]
- Paul et al. (2007) Mech Aging and Development 128:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JP; Campbell SD; Michaud D; Charbonneau M; Hilliker AJ A null mutation of cSOD in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. U. S. A 86:2761–2765; 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raamsdonk JM, and Hekimi S (2012) Superoxide dismutase is dispensable for normal animal lifespan. PNAS 109, 5785–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, and Imai H. (2017) Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in Caenorhabditis elegans. JBC 292(36) 14804–14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, and Valentine JS (2014) Superoxide dismutases and superoxide reductases. Chem. Rev 114: 3854–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John G, Steinman HM. Periplasmic copper–zinc superoxide dismutase of Legionella pneumophila: role in stationary-phase survival. J Bacteriol 1996;178:1578–1584. [PubMed: 8626284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S (2009) Deletion of the Mitochondrial Superoxide Dismutase sod-2 Extends Lifespan in Caenorhabditis elegans. PLoS Genet 5(2): e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Potter SZ. Copper–zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem 2005;74:563–593. [DOI] [PubMed] [Google Scholar]
- Weydert CJ, & Cullen JJ (2010). Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature protocols, 5(1), 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger RA; Fridovich I Superoxide dismutase: organelle specificity. J. Biol. Chem 248:4793–4696; 1973. [PubMed] [Google Scholar]
- Yanase S, Yasuda K, and Ishii N 2002. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech. Ageing Dev 123: 1579–1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE: No active SOD enzyme is present in the quintuple sod deletion line [sod-2(ok1030) I; sod-5(tm1146) sod-1(tm783) II; sod-4(gk101) III; sod-3(tm760) X], whereas sod-3(gk235) and sod1(tm776)II null alleles individually carry the single active form of SOD.


