ABSTRACT
Understanding the molecular mechanisms of herpes simplex virus 1 (HSV-1) latent infection and reactivation in neurons requires the use of in vitro model systems. Establishing a quiescent infection in cultured neurons is problematic, as any infectious virus released can superinfect the cultures. Previous studies have used the viral DNA replication inhibitor acyclovir to prevent superinfection and promote latency establishment. Data from these previous models have shown that reactivation is biphasic, with an initial phase I expression of all classes of lytic genes, which occurs independently of histone demethylase activity and viral DNA replication but is dependent on the cell stress protein DLK. Here, we describe a new model system using HSV-1 Stayput-GFP, a reporter virus that is defective for cell-to-cell spread and establishes latent infections without the need for acyclovir. The establishment of a latent state requires a longer time frame than previous models using DNA replication inhibitors. This results in a decreased ability of the virus to reactivate using established inducers, and as such, a combination of reactivation triggers is required. Using this system, we demonstrate that biphasic reactivation occurs even when latency is established in the absence of acyclovir. Importantly, phase I lytic gene expression still occurs in a histone demethylase and viral DNA replication-independent manner and requires DLK activity. These data demonstrate that the two waves of viral gene expression following HSV-1 reactivation are independent of secondary infection and not unique to systems that require acyclovir to promote latency establishment.
IMPORTANCE Herpes simplex virus-1 (HSV-1) enters a latent infection in neurons and periodically reactivates. Reactivation manifests as a variety of clinical symptoms. Studying latency and reactivation in vitro is invaluable, allowing the molecular mechanisms behind both processes to be targeted by therapeutics that reduce the clinical consequences. Here, we describe a novel in vitro model system using a cell-to-cell spread-defective HSV-1, known as Stayput-GFP, which allows for the study of latency and reactivation at the single neuron level. We anticipate this new model system will be an incredibly valuable tool for studying the establishment and reactivation of HSV-1 latent infection in vitro. Using this model, we find that initial reactivation events are dependent on cellular stress kinase DLK but independent of histone demethylase activity and viral DNA replication. Our data therefore further validate the essential role of DLK in mediating a wave of lytic gene expression unique to reactivation.
KEYWORDS: dual leucine zipper kinase, herpes simplex virus, human herpesviruses, in vitro model systems, latent infection, reactivation
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is a globally prevalent pathogen with the capacity to infect both sensory and autonomic neurons (1–4). Following neuronal infection, HSV-1 can enter a lytic replication cycle, establish a lifelong latent infection, or potentially undergo a limited amount of lytic gene expression even prior to latency establishment (5–13). While latency is largely asymptomatic, periodic reactivation of the virus can result in cutaneous lesions, keratitis, and encephalitis. Epidemiological studies have also linked HSV infection with an increased risk of developing late-onset Alzheimer’s disease (14–22).
The regulated expression of viral lytic transcripts has been well characterized following lytic infection (23–25). The HSV-1 genome enters the host cell epigenetically naked (26–28) but becomes chromatinized by histones bearing transcriptionally permissive histone modifications (29–38). Viral gene expression is initiated from the genome in response to viral transactivator and tegument protein VP16, which forms a complex with cellular factors involved in transcriptional activation, including general transcription factors, ATP-dependent chromatin remodelers, and histone-modifying enzymes to promote expression of immediate early (IE) genes (32, 39–43). Synthesis of the IE proteins is required for early (E) mRNA transcription. Products of the early viral genes enable viral DNA replication. Viral genome synthesis is a prerequisite for true-late (TL) mRNA transcription, likely due to a shift in genome accessibility and increased binding of host transcriptional machinery (44). In contrast to productive infection, during HSV-1 latency, viral lytic mRNAs are largely transcriptionally repressed, and promoters assemble into silent heterochromatin marked by the tri-methylation of histone H3 lysine 27 (H3K27me3) and di/tri-methylation of histone H3 on lysine 9 (H3K9me2/3) (45–50). The initiation of viral gene expression during reactivation is induced from a heterochromatin-associated viral genome and occurs in the absence of viral activators such as VP16. Latent HSV-1 therefore relies on host factors to act on the epigenetically silent viral genome and induce lytic gene expression.
The mechanisms that regulate entry into lytic gene expression to permit reactivation remain elusive. Using primary neuronal models of HSV-1 latent infection, reactivation has been found to progress in a two-step or bi-phasic manner. Phase I is characterized as a synchronous wave of lytic viral transcripts, occurring approximately 20 h poststimulus (25, 51–54). There is evidence that this initial induction of lytic gene expression is not dependent on the lytic transactivator VP16 or viral protein synthesis (25, 55). Instead, cellular factors, including the stress kinases dual leucine zipper kinase (DLK) and c-Jun N-terminal kinase (JNK), are required for phase I entry (52, 54). Importantly, viral gene expression occurs despite the persistence of heterochromatin on viral promoters. Instead, JNK-dependent histone phosphorylation of histone H3S10 results in a methyl/phospho switch, which can permit gene expression even while the repressive H3K9me3 histone modification is maintained (52). The second wave of viral lytic gene expression, phase II, occurs approximately 48 h poststimulus. Phase II reactivation is characterized by the full transcriptional viral cascade, including viral DNA replication, and ultimately, infectious virus production (25). In contrast to phase I, viral protein synthesis, heterochromatin removal, VP16-mediated transactivation, and viral DNA replication are required for phase II (25, 52, 54).
In vitro systems of HSV-1 latency are required to study the mechanisms of latency establishment and reactivation that cannot be easily studied in vivo (56–59). In vitro models more readily enable functional studies, and immune system components can be included to understand how the host immune response impacts latency and reactivation (60, 61). However, there are complications involved in establishing latency in vitro. As also observed in animal models, only a subpopulation of infected neurons enter a latent state, whereas other neurons become lytically infected (11, 62–64). This leads to a superinfection of the cultures and an inability to establish a latent infection. Therefore, many existing in vitro models have used viral DNA replication inhibitors, predominantly acyclovir (ACV), to establish latency in vitro (52, 65–70). ACV is proposed to inhibit viral DNA replication by incorporating into actively replicating viral genomes in lytic cells, although there is also evidence from ACV-resistant strains that ACV can also inhibit the viral DNA polymerase (71–73). There are some caveats associated with ACV use as the process of DNA replication and subsequent late gene expression cannot be studied in lytic neurons during latency establishment. Moreover, the fate of any genomes that incorporate ACV is unknown. Therefore, new model systems are required in which latency establishment can be tracked without the need for viral DNA replication inhibitors. Such a system can also be used to determine whether the use of ACV in the cultures alters mechanisms of lytic gene expression during reactivation. Here, we describe the use of a novel HSV-1 reporter virus, Stayput-GFP, which provides a powerful new methodology to investigate the establishment and reactivation from latent infection at the single neuron level without the need for DNA replication inhibitors.
RESULTS
Construction of a gH-null US11-GFP HSV-1.
To construct a recombinant HSV-1 that is deficient in cell-to-cell spread and can also be used to visualize cells containing detectable viral late protein, Us11 tagged with green fluorescent protein (GFP) (74) was inserted into an existing glycoprotein H (gH)-null virus, SCgHZ (75) (Fig. 1A). gH is essential for HSV-1 cell entry, as it mediates fusion between the virus envelope and host cell membrane (76, 77). The GFP-tagged true-late protein is a useful indicator of both lytic infection and reactivation, and Us11-GFP wild-type virus has been used as such in several HSV-1 latency systems (51, 54, 58, 65, 78, 79). The tagged virus has previously been reported to express the full complement of HSV-1 genes, as wild-type Patton strain (74).
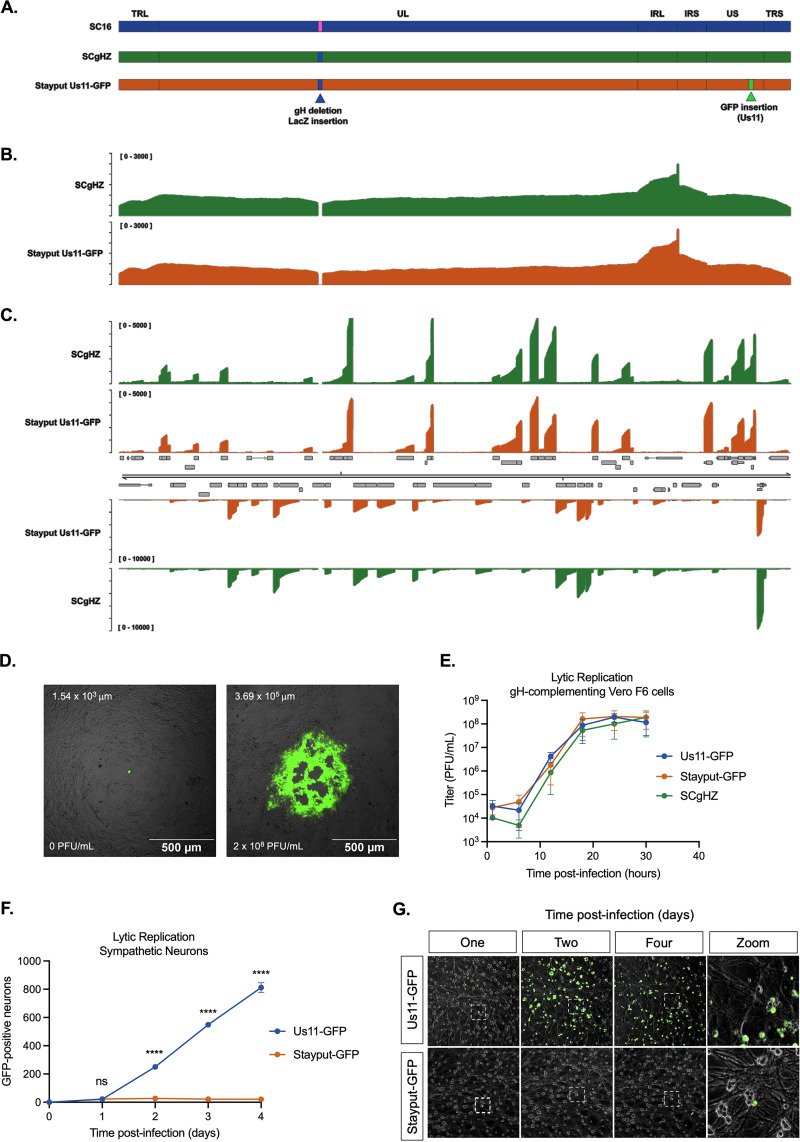
FIG 1.
Stayput-GFP replicates as wild type but is unable to spread. (A) Schematic overview of HSV-1 strain SC16, the gH-deletion mutant SCgHZ, and Stayput-GFP. The gH deletion/LacZ insertion and Us11-GFP insertion sites are shown by blue and green triangles, respectively. (B and C) Coverage plots derived from (B) nanopore gDNA sequencing and (C) nanopore direct RNA sequencing of SCgHZ and Stayput-GFP. Sequence read data were aligned against the SC16 reference genome and demonstrate a drop in coverage at the gH locus. (D) Plaque-forming assay of Stayput-GFP on Vero (left) or gH-complementing Vero-F6 (right) cells 48 h postinfection at a 10−7 dilution. The GFP-positive area in the example image is reported on the top left of each image. Titer of viral stock reported bottom left of each image. (E) Vero-F6 cells were infected with Stayput-GFP, SCgHZ, or Us11-GFP at an MOI of 5. Infectious virus was collected over time and titrated on Vero-F6 cells (n = 3 biological replicates). (F and G) Neonatal sympathetic neurons were infected at an MOI of 0.5 PFU/cell with Stayput-GFP or Us11-GFP in the absence of DNA replication inhibitors. Us11-GFP-positive neurons were counted over time (n = 3 biological replicates). Shapiro-Wilk normality test. Unpaired Student’s t test between Us11-GFP and Stayput-GFP. ****, P < 0.0001. The means and standard errors of the means (SEMs) are shown.
We first verified that the resulting virus (named Stayput-GFP) was deficient in cell-to-cell spread in nonneuronal and neuronal cells but otherwise undergoes gene expression and replication as a wild-type virus. The genome sequence and transcriptome of Stayput-GFP was validated by nanopore genomic DNA (gDNA) and direct RNA sequencing, respectively (Fig. 1B and C). As determined through plaque assay, the ability to produce infectious virus was perturbed in the gH-deletion strain (Fig. 1D) but was rescued using a previously constructed gH-complementing cell line, Vero-F6 (Fig. 1E). Importantly, replication in this cell line was indistinguishable from that of the parent SCgHZ and Us11-GFP Patton strain, which we chose for comparison as this virus has previously been used for latent infection studies in primary neurons.
To demonstrate cell-to-cell spread deficiency in neurons, we quantified GFP-positive neurons over time. Infection of murine sympathetic neurons at a multiplicity of infection (MOI) of 0.5 PFU/cell resulted in detectable GFP-positive neurons, which remained constant after 24 h postinfection (Fig. 1G), while the surrounding GFP-negative neurons remained GFP-negative (Fig. 1G). This contrasts with the wild-type US11-GFP condition, where the number of lytic-infected neurons increased substantially over time. Therefore, Stayput-GFP infection was equivalent to Us11-GFP upon initial infection but failed to spread within the neuronal culture.
Reactivation of Stayput-GFP in a primary neuronal model.
To confirm that Stayput-GFP undergoes reactivation in a manner comparable to that of the backbone SCgHZ, we infected mouse primary sympathetic neurons in the presence of viral DNA replication inhibitor ACV (52, 65–68). ACV prevents the production of infectious virus and, thus, superinfection of the cultures. Following infection, ACV was removed, and reactivation was triggered by adding a PI3-kinase inhibitor, LY294002 (Fig. 2), which mimics loss of a branch of the nerve growth factor (NGF)-signaling pathway in neurons (80) and has previously been found to induce reactivation (52, 61, 65, 79). Following the addition of the reactivation stimulus, viral gene expression increased uniformly between Stayput-GFP, SCgHZ, and wild-type Us11-GFP at 20 h poststimulus (Fig. 2C). Viral gene expression continued to increase from 20 to 48 h for all three viruses, and GFP-positive neurons were visible for both GFP-tagged viruses by 48 h (Fig. 2B and D). This indicates that even in the absence of cell-to-cell spread, reactivation progressed over a 48-h period, initiating with viral mRNA production and later detection of viral late protein.
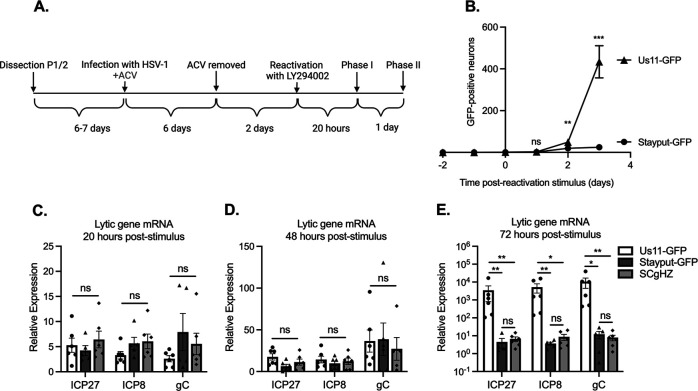
FIG 2.
Stayput-GFP in a latency and reactivation model using ACV to promote latency establishment. (A) The latency and reactivation model scheme. Neonatal sympathetic neurons were infected with Stayput-GFP, parent virus SCgHZ, or wild-type Us11-GFP at an MOI of 7.5 PFU/cell in the presence of ACV (50 μM). Then, 6 days later, ACV was removed, and 2 days later, cultures were reactivated with LY294002 (20 μM). (B) The numbers of GFP-positive neurons in a single well (containing approximately 5,000 neurons) for Stayput-GFP and wild-type Us11-GFP were counted over time. (C to E) Viral gene expression also was quantified by RT-qPCR for immediate early (ICP27), early (ICP8), and late (gC) genes at 20 h (C), 48 h (D), and 72 h (E) poststimulus. Relative expression to unreactivated samples and cellular control (mGAPDH). n = 6 biological replicates from 3 litters. Normality was determined by Kolmogorov-Smirnov test in panels B to E. Mann-Whitney (B) or Kruskal-Wallis with comparison of means (C to E); *, P < 0.05; **, P < 0.01; ***, P < 0.001. The means and SEMs are represented. Individual biological replicates are indicated in panels C to E.
By 72 h-post stimulus, GFP and viral gene transcription were significantly upregulated in wild-type Us11-GFP in comparison to Stayput-GFP or SCgHZ, suggesting that at this time point, the readout of reactivation for wild-type Us11-GFP is confounded by cell-to-cell spread (Fig. 2B and E). We are therefore unable to differentiate between genuine reactivation and downstream cell-to-cell spread using a wild-type virus. Previous attempts to reduce cell-to-cell spread include using pooled human gamma globulin or the viral DNA packaging inhibitor WAY-150138 (65, 81–83). However, using Stayput-GFP offers a built-in mechanism to prevent cell-to-cell spread during reactivation and permit quantification of the progression to reactivation at the single-neuron level.
Stayput-GFP can be used to create a quiescence model of neuronal infection in the absence of viral DNA replication inhibitors.
When wild-type virus is used for neuronal infection, superinfection of the cultures occurs (Fig. 1F), and a latent infection cannot be established. By infecting with Stayput-GFP, we posited that we could create a model of latency establishment without the use of DNA replication inhibitors. Following the infection of neonatal sympathetic neurons at an MOI of 7.5 PFU/cell, the number of GFP-positive neurons emerged by 1 day postinfection, increased until 3 days postinfection, and then decreased until reaching zero by 30 days postinfection (Fig. 3A). The peak number of GFP-positive neurons was approximately 1,000. Given that 5,000 neurons were plated per well, this equates to approximately 20% of the population of neurons that progress to become Us11-GFP-positive. The length of time required for Us11-GFP to be lost from the cultures was surprising and may result from the previously characterized restricted cell death in neurons (84) or a gradual shutoff in protein synthesis in a subpopulation of neurons that survive even late gene expression. Single-cell tracking demonstrates that at least a proportion of neurons that become GFP-positive also end up staining positive for cell death marker SYTOX Orange (Fig. 3B and C). These data suggest that most neurons that undergo a lytic infection end up undergoing cell death. However, accurately tracking all neurons over time to determine whether all GFP-positive neurons do die is problematic because individual neurons shift over time. Therefore, we cannot definitively conclude that all GFP-positive neurons undergo cell death.
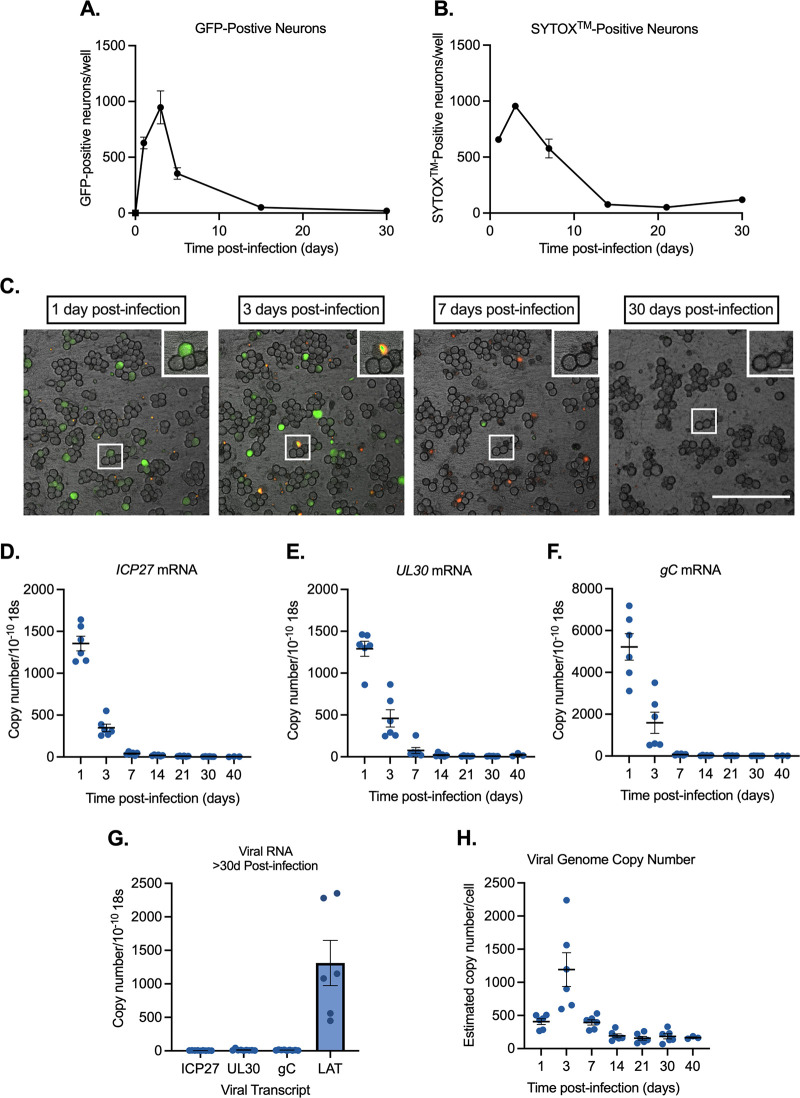
FIG 3.
Stayput-GFP can be used to create a quiescence model in the absence of viral DNA replication inhibitors in neonatal sympathetic neurons. Neonatal sympathetic neurons were infected with Stayput-GFP at an MOI of 7.5 PFU/cell, and the numbers of Us11-GFP-positive neurons were quantified. (A) n = 9 biological replicates from 3 litters. (B) SYTOX Orange-positive neurons were also quantified over time (n = 3). (C) Following infection, the same field of view was imaged to track GFP and SYTOX Orange (250 μm scale bar for field of view [FOV], 25 μm scale bar for zoom) over time. (D to G) Lytic (D to F) and latent (G) viral transcripts (n = 6) were quantified up to 40 days postinfection. (H) Viral DNA load (n = 6) was also quantified up to 40 days postinfection. Individual biological replicates along with the means and SEMs are shown.
All classes of lytic viral gene expression emerged by 1-day postinfection and then decreased over the span of 30-days postinfection (Fig. 3D to F). In contrast to the lytic transcripts, latency-associated transcript (LAT) expression was maintained over the infection scheme of 30 to 40 days and was approximately 400-fold higher than lytic transcripts from 30 days onward (Fig. 3G). This indicated that LAT-positive neurons persisted over this period, likely reflecting entry into quiescence. In agreement with this hypothesis, at 40 days postinfection there were approximately 200 viral DNA copies per cell, demonstrating that viral genomes persist (Fig. 3H). Together, these data show that infection of sympathetic neurons with a cell-to-cell defective virus results in a remaining population of neurons containing viral genomes and the LAT transcript. Notably, this mimics events following in vivo infection of mice.
The ability of HSV-1 to undergo reactivation decreases with length of time infected.
The presence of viral genomes and LAT transcripts suggested that HSV-1 had established a quiescent infection 30 days postinfection. Therefore, we hypothesized that some genomes enter latency, which is defined by an ability to reactivate in response to a stimulus. We thus attempted to reactivate cultures with LY294002 (20 μM). However, we were unable to detect an increase in GFP-positive neurons after the addition of the trigger (Fig. 4A). We were also unable to detect a change in immediate early, early, or late transcripts (data not shown). This was unexpected, as LY294002 has repeatedly been shown to elicit robust reactivation in vitro and was able to induce Stayput-GFP in a model using ACV to promote latency establishment (Fig. 2). Therefore, we sought to determine whether the inability to induce reactivation was due to the lack of ACV or the more prolonged time between initial infection and the addition of the reactivation stimulus. We infected neonatal cultures in the presence of ACV and reactivated over increasing lengths of time. ACV was removed from all cultures 6 days postinfection. We found that the number of GFP-positive neurons following addition of LY294002 decreased as the length of time infected increased (Fig. 4B). This was likely not due to a loss of viral genomes, as viral genome copy number and LAT expression remained constant over this period (Fig. 3G and H) and therefore instead reflected a more repressed viral genome unable to undergo reactivation upon PI3-kinase inhibition.
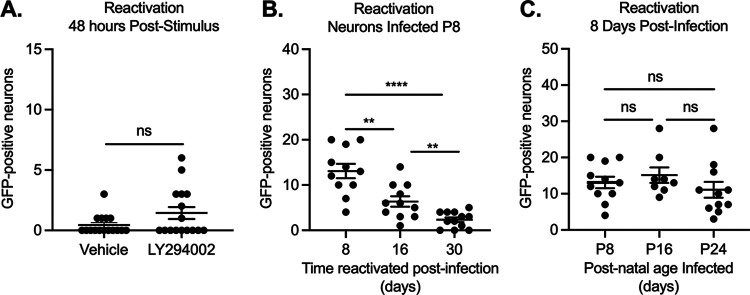
FIG 4.
Reactivation decreases with length of time infected. Sympathetic neurons were infected with Stayput-GFP at an MOI of 7.5 PFU/cell and were treated with LY294002 when GFP-positive neurons were no longer detected (approximately 30 days postinfection). GFP-positive neurons were quantified over time; (A) peak GFP (48 h poststimulus) is represented. Neonatal SCGs were infected at age postnatal day 8 (P8) with Stayput-GFP in the presence of ACV. (B) ACV was removed 6 days postinfection, and reactivation was triggered at the indicated times postinfection. (C) Neonatal SCGs were infected as described above after different lengths of time in vitro, representing indicated postnatal ages, and reactivated 8 days postinfection with LY294002. n = 12 biological replicates from 3 litters. Normality was determined by the Kolmogorov-Smirnov test. Unpaired Student’s t test (B) or the Mann-Whitney test (A and C) was used based on normality of data. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Individual biological replicates along with the means and SEMs are shown.
Neurons are known to undergo intrinsic maturation, even in culture (85, 86). Therefore, the increased age of the neuron could have also impacted the ability of HSV-1 to undergo reactivation. Hence, we investigated how reactivation changed with increased neuronal maturation (Fig. 4C). We infected cultured neurons with Stayput-GFP at an MOI of 7.5 at the postnatal (P) ages of P8, P16, and P24 and then reactivated 8 days later. These postnatal ages of infection were chosen to reactivate at the same ages of reactivation in Fig. 4B. Importantly, we did not detect a decrease in reactivation output as the age of the neuron increased. Together, these data indicate that the decreased ability of Stayput-GFP to reactivate in a model that did not require ACV to establish quiescence was due to the longer time frame of infection and was not associated with a lack of ACV in the cultures or increased age of the neuron.
Viral gene expression can be induced following long-term quiescent infection when multiple triggers are combined.
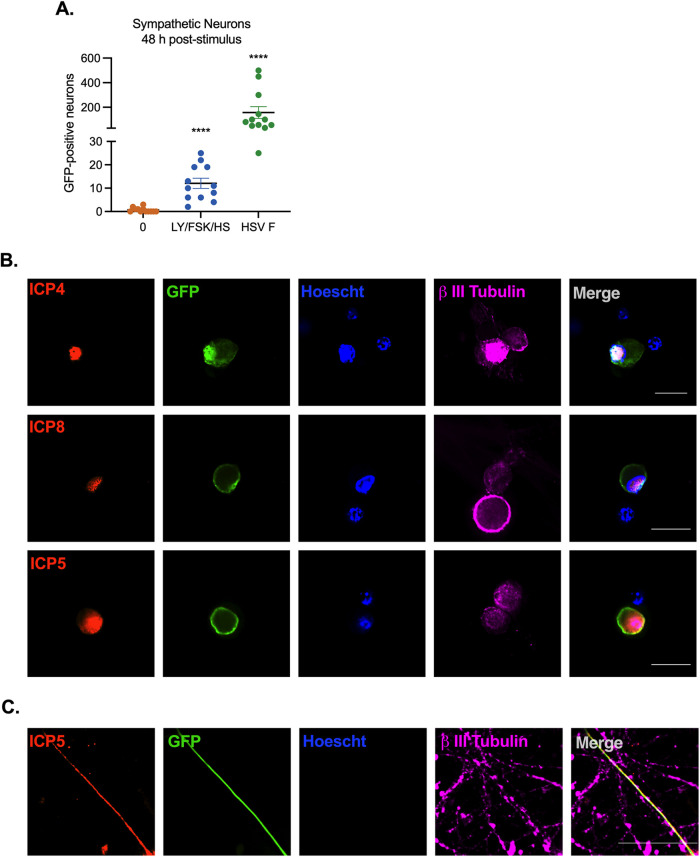
We next sought to determine whether other known stimuli of HSV-1 reactivation could induce Us11-GFP expression, indicative of entry into reactivation. We attempted a number of triggers, including forskolin (87–90) and heat shock/hyperthermia (91–98), which are both known inducers of HSV-1 reactivation. Alone, these stimuli did not induce Us11-GFP expression or viral lytic mRNA induction (data not shown). However, when heat shock (43°C for 3 h) and forskolin (60 μM) and LY294002 (20 μM) (both pulsed for 20 h) were combined, Us11-GFP positive neurons were detected at 48 h poststimulus, indicating progression to reactivation (Fig. 5A). Superinfection was also used, as this can induce rapid and robust reactivation, likely resulting in delivery of viral tegument proteins. In comparison to superinfection, the combined heat shock/forskolin/LY294002 trigger resulted in reduced entry into reactivation/Us11-GFP expression, indicating that only a subpopulation of neurons undergo reactivation with this combined trigger, which is consistent with previous studies investigating the mechanism of HSV-1 reactivation both in vivo and in vitro (25, 66, 99, 100). Importantly, GFP-positive neurons at 48 h poststimulus were also positive for viral immediate early protein ICP4, early protein ICP8, as well as late capsid protein ICP5 based on immunofluorescence staining (Fig. 5B). ICP8 staining appeared to form replication compartments, suggesting viral DNA replication occurs at this time. ICP5 capsid staining was also detectable in GFP-positive axons (Fig. 5C).
FIG 5.
Viral protein synthesis can be induced from neurons quiescently infected with Stayput-GFP using a combination of stimuli. Neonatal sympathetic neurons or adult sensory neurons were infected at an MOI of 7.5 PFU/cell with Stayput-GFP in the absence of viral DNA replication inhibitors. Following the loss of GFP, signaling quiescence of the culture, wells were reactivated with a variety of triggers, including combinations of LY294002 (20 μM), forskolin (60 μM), and heat shock (43°C for 3 h), as well as a superinfection with untagged F strain at an MOI of 10 PFU/cell. (A) GFP was quantified over time, and the peak GFP, at 48 h poststimulus, is depicted. n = 12 biological replicates. (B to C) Neurons were fixed at 48 h poststimulus and stained with viral immediate early (ICP4), early (ICP8) (B), or late (ICP5) (C) protein (red), GFP (green), Hoescht (blue), and β II tubulin (magenta). 25-μm scale bar.
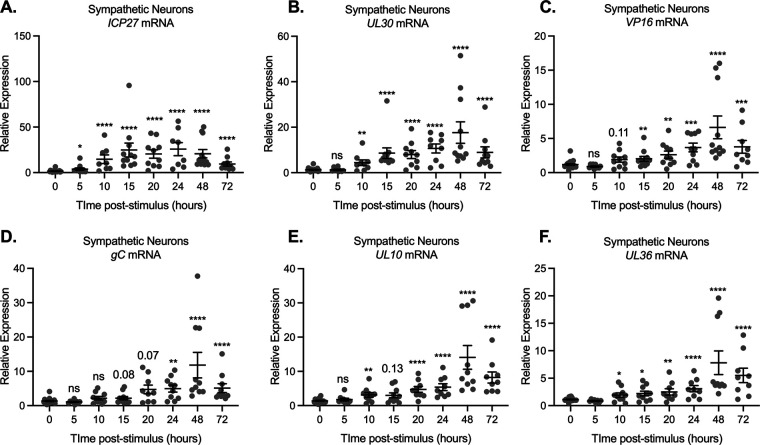
We went on to investigate whether viral mRNA expression was induced prior to the detection of Us11-GFP-positive neurons. Using the triple stimulus, we detected an increase in viral lytic transcripts as early as 10 h poststimulus, which continued to increase, peaking at 48 h poststimulus (Fig. 6). Detection of immediate early (Fig. 6A) and early transcripts (Fig. 6B) was slightly more robust than that of late transcripts (Fig. 6C to F). Because of the less robust increase in late gene expression, multiple late genes were analyzed, including the leaky late transcript VP16 and true-late transcripts encoding gC, UL10, and UL36. All late transcripts analyzed were not significantly increased beyond the latent samples until 20 h poststimulus (Fig. 6C to F).
FIG 6.
Viral gene expression can be restarted following latency establishment. Neonatal sympathetic neurons or adult sensory neurons were infected at an MOI of 7.5 PFU/cell with Stayput-GFP in the absence of viral DNA replication inhibitors. Following the loss of GFP, signaling quiescence of the culture, wells were reactivated with a variety of triggers, including combinations of LY294002 (20 μM), forskolin (60 μM), and heat shock (43°C for 3 h). (A to F) Immediate early (A), early (B), and late (C to F) viral transcripts were investigated over time following the stimulus. Reactivated samples were compared to latent samples described as 0 h poststimulus. n = 9 biological replicates from 3 litters. Mann-Whitney against 0 h (A to D). Normality was determined by the Kolmogorov-Smirnov test; *, P < 0.05; **,P < 0.01; ***, P < 0.001; ****, P < 0.0001. Individual biological replicates along with the means and SEMs are shown.
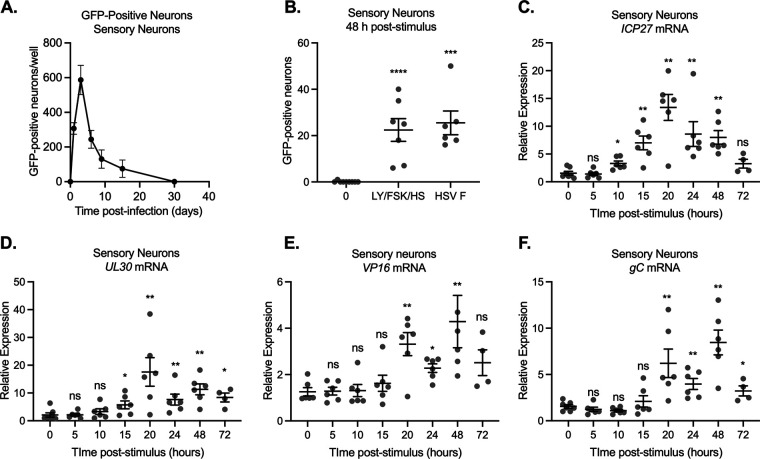
We were also interested to determine whether a similar quiescent infection could be established in adult sensory neurons and whether the kinetics of viral lytic gene expression were similar between sensory and sympathetic cultures. Following infection with Stayput-GFP in primary trigeminal ganglia (TG) neurons, GFP also increased by 1 day postinfection and then was lost over time (Fig. 7A). Assuming 5,000 neurons per well, Us11-GFP-positive neurons account for approximately 12% of the culture. GFP was repeatedly lost within 15 days, a shorter period than that which is observed in neonatal sympathetic neurons. We also confirmed that the triple combinatorial stimulus elicits robust GFP reemergence in adult TGs (Fig. 7B). Similar to what we observed in the sympathetic neurons, Us11-GFP-positive neurons were detected by 48 h poststimulus. Intriguingly, superinfection only induced GFP expression to equivalent levels as the triple stimuli, which may be reflective of the repressive nature of the subpopulation of mature sensory neurons to lytic replication and reactivation (62, 63). The kinetics of viral lytic gene expression in sensory neurons following the reactivation stimulus mirrored those in sympathetic neurons, with an IE and E gene expression robustly induced by 15 h poststimuli (Fig. 7C and D) and late gene expression by 20 h (Fig. 7E and F). Together, these data indicate that in vitro models of latency and reactivation can be established in sympathetic and sensory neurons in the absence of ACV, and reactivation can be induced using a combination of triggers. In both models, a wave of lytic mRNA expression was detected at 15 to 20 h poststimulus, which was approximately 24 h prior to the detection of Us11-GFP-positive neurons.
FIG 7.
Reactivation from quiescently infected adult sensory trigeminal ganglia neurons. Neurons isolated from the trigeminal ganglia (TG) of female mice were infected with Stayput-GFP at an MOI of 7.5 PFU/cell. (A) The resolution of lytic infection was monitored over time by imaging and counting GFP-positive neurons. n = 12 biological replicates from 3 dissections. The mean and SEM are shown. Following the loss of GFP, signaling quiescence of the culture, wells were reactivated with a combination of LY294002 (20 μM), forskolin (60 μM), and heat shock (43°C for 3 h), as well as a superinfection with untagged F strain at an MOI of 10 PFU/cell. (B) GFP was quantified over time, and the peak GFP, at 48 h poststimulus, is depicted. n = 9 biological replicates. (C to F) Immediate early (C), early (D), and late (E and F) viral transcripts were investigated over time following the stimulus. n = 6 replicates. Mann-Whitney test against 0 h; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Individual biological replicates along with the means and SEMs are shown.
Neurons infected with Stayput-GFP undergo a DLK-dependent phase I reactivation.
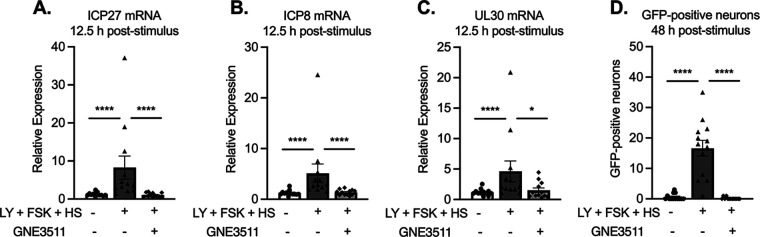
Phase I reactivation has largely been investigated using in vitro models in which ACV has been used to promote latency establishment. In addition, phase I has been found to occur with the single triggers of forskolin or LY294002 (25, 52, 54). The requirement of multiple triggers for reactivation suggested that multiple cell-signaling pathways converged to have a synergistic effect and induce reactivation in this more repressive model. Therefore, we were interested to determine whether the characteristics of phase I reactivation occurred in the model of quiescent infection established in the absence of ACV and using the more robust trigger to induce reactivation. Potential phase I viral transcription was investigated at 12.5 h poststimulus by reverse transcription-quantitative PCR (RT-qPCR), and phase II was investigated when Us11-GFP-positive neurons could be detected (48 h poststimulus). A characteristic of phase I expression is the requirement of the stress kinase dual leucine zipper kinase (DLK) (52, 54). Therefore, using the DLK-specific inhibitor GNE3511, 4 μM (101), we investigated the effect of DLK on reactivation in our system. We found that upregulation of immediate early/early transcripts 12.5 h poststimulus was eliminated with the addition of the DLK inhibitor (Fig. 8A to C). Further, full reactivation, demonstrated by peak GFP expression at 48 h, was also reduced to baseline levels upon the addition of the DLK inhibitor (Fig. 8D). Therefore, using our new model of HSV-1 latency, our data demonstrate that the initiation of viral lytic gene expression is dependent on DLK activity.
FIG 8.
Reactivation is dependent on DLK. Cultures were infected with Stayput-GFP at an MOI of 7.5 PFU/cell in the absence of ACV. Following loss of GFP, cultures were reactivated with a combination of LY294002, forskolin, and heat shock in the presence of DLK inhibitor GNE-3511 (4 μM). (A to D) Immediate early (ICP27) and early (ICP8/UL30) viral genes (A to C) were investigated at 12.5 h poststimulus, and GFP was counted over time (D). Peak GFP, consistently around 48 h poststimulus, is presented. n = 9 biological replicates from 3 litters. Normality was determined by the Kolmogorov-Smirnov test. Mann-Whitney test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The mean and SEM are shown.
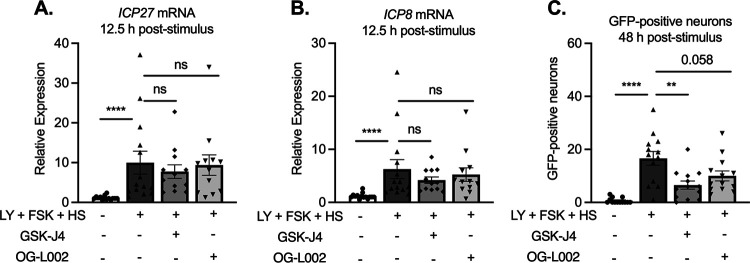
In addition to the dependence on DLK activity, a further characteristic of phase I gene expression is the induction of viral mRNA transcripts independent of the activity of histone demethylase enzymes required for full reactivation. Therefore, we also triggered reactivation in the presence of demethylase inhibitors. GSK-J4 is known to specifically inhibit the H3K27 histone demethylases UTX and JMJD3 (102) and has previously been found to inhibit HSV-1 reactivation but not phase I gene expression (52, 54). OG-L002 is an LSD1-specific inhibitor. LSD1 has previously been shown to be involved in removal of H3K9me2 from HSV-1 genomes, and its activity is required for full reactivation but not phase I gene expression (41, 52, 54). The initial expression of lytic transcripts at 12.5 h poststimulation was not inhibited by either OG-L002 or GSK-J4 (Fig. 9A and B). Full reactivation was reduced in the presence of OG-L002 or GSK-J4 as demonstrated by GFP-positive neurons at 48 h (Fig. 9C). For OG-L002, the reduction in the numbers of GFP-positive neurons was not significant, indicating that removal of H3K9me2 may not be as important as removal of H3K27me3 in this model system. Importantly, the initiation of gene expression in a manner that is independent of histone demethylase activity occurs when reactivation is induced by a triple stimulus and in a primary neuronal model in which latency was established without ACV.
FIG 9.
The early phase of lytic gene expression following a reactivation stimulus is independent of demethylase activity. Cultures were infected with Stayput-GFP at an MOI of 7.5 PFU/cell. Following loss of GFP, cultures were reactivated with a combination of LY294002, forskolin, and heat shock in the presence of H3K27 demethylase inhibitor GSK-J4 (2 μM) or H3K9 demethylase inhibitor OG-L002 (20 μM). Immediate early (ICP27) and early (ICP8/UL30) viral genes (A to C) were investigated at 12.5 h poststimulus, and GFP was counted over time. Peak GFP is presented. n = 3 biological replicates from 3 litters. Normality was determined by the Kolmogorov-Smirnov test. Mann-Whitney; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The mean and SEM are shown.
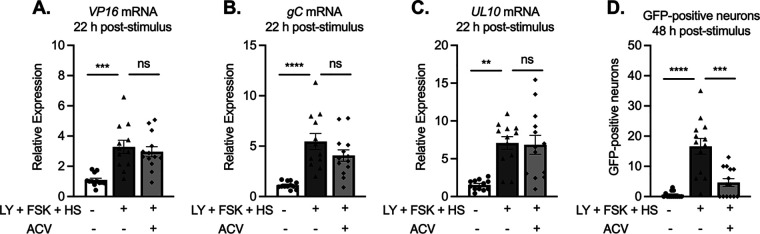
During phase I, there is no detectable replication of viral genomes even though true late gene expression occurs (52, 54). In addition, using ACV models to establish latency, late gene expression has previously been found to occur at equivalent levels when viral DNA replication is inhibited during reactivation. Although we did observe late gene expression during the initial period of lytic gene induction (12.5 to 20 h), the increase was less robust than that for IE and E genes and appeared slightly delayed, not reaching significance for all analyzed late transcripts until 24 h poststimulus (Fig. 6C to F). Therefore, we investigated whether this late gene induction was dependent on viral DNA replication by reactivating in the presence of ACV. The addition of ACV did not inhibit the induction of lytic transcripts at 22 h poststimulus. Importantly, we included multiple true late genes in this analysis, and all were induced to equivalent levels in the presence and absence of ACV (Fig. 10A to C). Therefore, the initial expression of late genes following a reactivation stimulus is independent of viral DNA replication. However, the addition of ACV did inhibit entry in full reactivation, demonstrated by Us11-GFP positive neurons at 48 h poststimulus, indicating that ACV was capable of blocking robust late gene expression at this late time point (Fig. 10D). In summary, using a model system in which a quiescent infection is established without the need for ACV, all the previous characteristics of phase I gene expression (dependence on DLK and independence of histone demethylase activity and viral DNA replication) were still observed.
FIG 10.
Differential dependence on viral DNA replication between phase I and II reactivation. Cultures were infected with Stayput-GFP at an MOI of 7.5 PFU/cell in the absence of ACV. Following loss of GFP, cultures were reactivated with a combination of LY294002, forskolin, and heat shock in the presence of ACV (50 μM). (A to D) Late (VP16, gC, UL10) genes (A to C) were investigated at 22 h poststimuli. GFP was counted over time, and peak GFP is presented (D). n = 12 biological replicates. Normality was determined by the Kolmogorov-Smirnov test. Mann-Whitney; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The mean and SEM are shown.
DISCUSSION
We envision multiple uses for the Stayput-GFP virus model developed here for investigating HSV-1 neuronal infection in vitro. The Stayput-GFP virus is advantageous in models that otherwise use DNA replication inhibitors to promote latency establishment because it allows for the separation of initial viral gene expression/protein synthesis events and readouts from events that result from cell-to-cell spread. In addition, even in systems where ACV is used, there can be low levels of lytic replication or spontaneous reactivation after removal of ACV from cultures. The use of Stayput-GFP helps limit the confounding effects of spontaneous reactivation events by inhibiting subsequent cell-to-cell spread, while at the same time identifying neurons that escape quiescence. Further, the GFP tag serves as an imaging indicator in real time of when de novo lytic infection is resolved and latency is considered established. We are also able to track viral DNA replication and downstream late viral transcription and protein synthesis during the latency establishment process in vitro. The fate of lytic neurons, whether they undergo cell death or turn off gene expression programs and enter the latency pool, can also be investigated by tracking GFP and cell death at a single-cell level.
There are also some limitations to our system. Although there is some discrepancy in what defines reactivation (103), it is ultimately defined by the production of infectious virus. Due to the nature of the gH-deletion virus, de novo virus is by design noninfectious, and we are unable to demonstrate reactivation in its strictest definition. That said, we can readily demonstrate the reemergence of all classes of viral gene transcripts, synthesis of viral capsid protein, and replication compartment formation.
An intriguing finding in our study is that reactivation output decreases as length of infection increases. A potential explanation is that the viral genome becomes increasingly chromatinized over time, leading to a more repressive phenotype. In support of this hypothesis, the association of the facultative heterochromatin mark H3K27me3 with the HSV-1 genome increases dramatically between 10 and 15 days postinfection in vivo (46). The kinetics of H3K27me3 deposition remain to be investigated in vitro, but if they mirror in vivo observations, this could suggest that active chromatinization and reinforcement of silencing continues even after initial shut-down of viral gene expression. In the cellular context, H3K27me3 is linked with the recruitment of canonical polycomb repressor complex 1 (cPRC1), which may reinforce silencing through long-range chromosomal interactions or 3D compaction (50, 104, 105). It is therefore possible that even following H3K27me3 formation on the genome, there are additional layers of protein recruitment that build up over time. In addition, it is also possible that the accumulation of viral noncoding RNAs expressed in latency could impact cellular pathways, resulting in decreased signaling to the viral genome for reactivation. The use of the Stayput-GFP model system will permit these different avenues to be explored.
Our model system recapitulates the hallmarks of reactivation phase I, which has previously been explored in in vitro systems using a DNA replication inhibitor. These data are interesting considering the discrepancies in conclusions drawn about reactivation between in vitro and in vivo modeling. There is evidence ex vivo for a phase I, as all classes of viral gene are expressed in a disordered, noncascade fashion when a combination of explant and nerve-growth factor deprivation is used (106). However, in other models of reactivation ex vivo, there is evidence that phase I-like gene expression may not occur, especially from studies investigating the requirement for histone demethylase inhibitors. These latter experiments used explant (axotomy) to induce reactivation and found that the earliest induction of lytic gene expression is dependent on H3K9 demethylase activity (40, 41). In a recent study from our lab, we have found that phase I reactivation can occur ex vivo when axotomy is combined with PI3-kinase inhibition, although with more rapid kinetics than those observed here (107). These discrepancies may result from the different trigger used to induce reactivation or currently unknown effects of latency established in vivo. Importantly, here, we have demonstrated that any potential differences between in vivo and in vitro observations on the mechanisms of reactivation do not result from the use of ACV to establish a quiescent infection. It is worth noting that despite decreases in GFP-positive neurons following reactivation in the presence of either H3K9 or H3K27 demethylase inhibitor, some neurons still become GFP-positive, especially in the OG-L002 (H3K9 demethylase inhibitor)-treated neurons. Although viral genomes have previously been shown to be enriched for both H3K9me2/3 and H3K27me3 in latency (45, 47, 49, 52), the relationship between the different modifications and whether they exist on the same or different genomes is not known. In addition, whether reactivation occurs from genomes differentially enriched for H3K9me2/3 or H3K27me3 when different triggers of reactivation are used has not been investigated. One possible explanation is that the triple stimuli used here may be a more effective reactivation trigger for H3K27me3-enriched genomes. Further work using the Stayput-GFP model system will help determine whether there is heterogeneity in the heterochromatin on viral genomes and the role of removal of specific types of heterochromatin for reactivation in response to different stimuli.
Phase I, in addition to occurring synchronously and independently of histone demethylases, also occurs in the absence of viral protein synthesis. In an in vitro model employing ACV during latency establishment and stimulus LY294002 during reactivation, it is demonstrated that initial viral transcription occurs before the appearance of viral late protein synthesis and, specifically, independently of viral transactivator VP16 (25, 51). Therefore, cellular host factors must be responsible for instigating the initial reactivation process. Evidence from an in vitro model system has demonstrated that these events are in fact navigated by cellular proteins JNK and DLK (52). Interestingly, host cell proteins may also be implicated in restricting the full reactivation process, including Gadd45b, which appears to antagonize the HSV-1 late expression program to prevent full reactivation (51). Interestingly, Gadd45b mRNA is increased in response to LY294002 only in infected neurons, suggesting perhaps that a viral factor may be mediating the gatekeeping from phase I to phase II reactivation.
In our system, phase I gene expression was dependent on the neuronal regulator of JNK activity, DLK, highlighting the central role of DLK in HSV-1 reactivation. DLK is a cell protein implicated in neuronal stress signaling upstream of cellular protein JNK (108). It has previously been found to be essential for HSV-1 reactivation following PI3-kinase inhibition (52), as well as neuronal hyperexcitability through forskolin (54). However, it has not, until now, been shown to be central to reactivation mediated by heat shock. Although heat shock has been used as a trigger for HSV-1 reactivation (91–98), the downstream molecular events following this stimulus are not well elucidated. Multiple studies have demonstrated that heat shock during reactivation leads to the upregulation of heat shock proteins, although none of them are knowing to relate to DLK. Following hyperthermia-induced reactivation in vivo, heat shock proteins HSP60 and HSP40 have been demonstrated to be upregulated (98). Components of the heat shock response pathway have also been demonstrated to be upregulated by LY294002 treatment in an in vitro system (51), including HSP70. In fact, in this same system, treatment with cultures of heat shock factor 1 (HSF-1) activator compound causes robust reactivation. Outside of the virological context, heat shock protein chaperone HSP90 has been shown to bind and maintain DLK stability in vivo, and it is specifically required for DLK function following axon injury signaling (109). It is a possibility that heat shock in our system is enhancing the function of DLK. Therefore, multiple signals may converge on DLK, which is then able to activate JNK and histone phosphorylation and to promote lytic gene expression from the heterochromatin-associated viral genome for reactivation to occur. Indeed, synergy has been demonstrated to enhance DLK activity in neurons (110). This central role for DLK is especially important, as it is largely a neuron-specific protein that regulates the response to multiple forms of stress (111) and is therefore a potential target for novel therapeutics that would prevent HSV-1 gene expression and, ultimately, reactivation.
MATERIALS AND METHODS
Reagents.
The compounds used in the study are as follows: acyclovir (acycloguanosine), LY 294002, forskolin, GNE-3511, GSK-J4, and OG-L002. Compound concentrations were used based on previously published 50% infective concentrations (IC50s) and assessed for neuronal toxicity using the cell body and axon health and degeneration index as previously published (54).
Preparation of HSV-1 virus stocks.
Stayput-GFP, as well as SCgHZ, is propagated and titrated on previously constructed gH-complementing F6 cells, which contain copies of the gH gene under the control of an HSV-1 gD promoter, as described in reference 75. Vero F6 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FetalPlex (Gemini Bio-Products) and 250 μg/mL of G418/Geneticin (Gibco).
HSV-1 stocks of eGFP-Us11 Patton were grown and titrated on Vero cells obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FetalPlex (Gemini Bio-Products) and 2 mM l-glutamine. eGFP-Us11 Patton (HSV-1 Patton strain with enhanced GFP [eGFP] reporter protein fused to true late protein Us11) (74) was kindly provided by Ian Mohr at New York University.
Viral genomic DNA sequencing.
Genomic DNA was isolated from stocks of SCgHZ and Stayput-GFP using the Qiagen DNeasy blood and tissue kit. Then, 250 ng of gDNA was used as input for the SQK-LSK109 genomic DNA using the ligation protocol (Oxford Nanopore Technologies Ltd.), and resulting libraries were loaded onto individual R9.4.1 Flongles for 20 h of sequencing. Resulting raw fast5 data sets were base called using Guppy v4.2.2 (-f FLO-MIN106 -k SQK-RNA002), with only reads passing filter used for subsequent analyses. De novo assembly was performed as follows. First, HSV-1 reads were extracted from the total pool by aligning them against the HSV-1 strain SC16 reference genome (MN159383.1) using MiniMap2. HSV-1 reads shorter than 1,000 nucleotides (nt) were subsequently filtered out prior to performing de novo assembly with Canu (112). Canu yielded a single circular contig for both SCgHZ and Stayput-GFP, which was manually linearized at the canonical HSV-1 start site. Four rounds of genome polishing were performed using Racon (113) before a final round of SNP calling was achieved using medaka (https://github.com/nanoporetech/medaka).
Construction of Stayput-GFP.
Stayput-GFP was created by inserting an eUs11-GFP tag into the previously created gH-deficient HSV-1 SCgHZ virus (strain SC16) through cotransfection of SCgHZ viral DNA and pSXZY-eGFP-Us11 plasmid (74) in Vero F6 cells. Transfections were carried out in 6-well plates using 2 μg of viral DNA and 2 μg of pSXZY-eGFP-Us11 and 8 μL of jetPRIME (Polyplus). GFP-positive plaques were subjected to 4 rounds of plaque purification.
Direct RNA sequencing.
For both SCgHZ and Stayput-GFP, normal human dermal fibroblasts (passage 13) were infected at an MOI of 10. At 6 h postinfection, the supernatant was removed and cells were washed once with phosphate-buffered saline (PBS) prior to lysing with 8 mL TRIzol. Total RNA was extracted according to TRIzol manufacturer’s instructions and eluted in nuclease-free water. Nanopore direct RNA-Seq libraries were prepared for each sample using ~500 ng of poly(A) RNA that was previously isolated from 30 μg of total RNA using the Dynabeads mRNA purification kit (Invitrogen, 61006). The poly(A) RNA was spiked with 0.5 μL of a synthetic enolase 2 (ENO2) calibration RNA (Oxford Nanopore Technologies Ltd.), and direct RNA-Seq libraries were prepared according to the standard SQK-RNA002 protocol (Oxford Nanopore Technologies Ltd.). Raw fast5 data sets were then base called using Guppy v4.2.2 (-f FLO-MIN106 -k SQK-RNA002), with only reads passing filter used for subsequent analyses. Sequence reads were aligned against the Stayput-GFP genome using MiniMap2 (114) (-ax splice -k14 -uf –secondary=no), with subsequent parsing through SAMtools and BEDtools (115, 116). Here, sequence reads were filtered to retain only primary alignments (alignment flag 0 [top strand] or 16 [bottom strand]). Figures associated with this study were generated using the R packages Gviz (117) and GenomicRanges (118).
Single-step growth curve.
A total of 1 × 105 Vero F6 cells were seeded per well in a 24-well plate. Then, 24 h later, when the cells had reached approximately 90% confluence, cells were infected with Stayput-GFP, SCgHZ, or wild-type Patton Us11-GFP at an MOI of 5 PFU/cell for 1 h at 37°C with gentle rocking (60 rpm). Inoculum was then removed and replaced with 400 μL of medium. At the specified time points, 400 μL of 9% sterile milk was added to the well, and the plates were frozen at −80C. Following three freeze-thaw cycles, titrations were carried out on Vero F6 cells.
Primary neuronal cultures.
Sympathetic neurons from the superior cervical ganglia (SCG) of postnatal day-0 to -2 (P0 to P2) CD1 mice (Charles River Laboratories) were dissected as previously described (52). Sensory neurons from the trigeminal ganglia (TG) of adult (P21 to -24) CD1 mice (Charles River Laboratories) were dissected as previously described (119). Rodent handling and husbandry were carried out under animal protocols approved by the Animal Care and Use Committee of the University of Virginia (UVA). Ganglia were briefly kept in Leibovitz’s L-15 medium with 2.05 mM l-glutamine before dissociation in collagenase type IV (1 mg/mL) followed by trypsin (2.5 mg/mL) for 20 min each at 37°C. Dissociated ganglia were triturated, and approximately 5,000 neurons per well were plated onto rat tail collagen in a 24-well plate. Sympathetic neurons were maintained in CM1 (neurobasal medium supplemented with PRIME-XV IS21 neuronal supplement [Irvine Scientific], 50 ng/mL mouse NGF 2.5S, 2 mM l-glutamine, and Primocin). Aphidicolin (3.3 mg/mL) was added to the CM1 for the first 5 days postdissection to select against proliferating cells. Sensory neurons were maintained in the same medium supplemented with GDNF (50 ng/mL; PeproTech 450-44), and more aphidicolin (6.6 mg/mL) was used for the first 5 days, as more nonneuronal cells tend to be dissected with this neuron type.
Establishment and reactivation of latent HSV-1 infection in primary neurons.
Neonatal SCGs were infected at postnatal days 6 to 8 with either Us11-GFP, SCgHZ, or Stayput-GFP at an MOI of 7.5 PFU/cell assuming 5,000 cells/well in Dulbecco’s Phosphate Buffered Saline (DPBS) + CaCl2 + MgCl2 supplemented with 1% fetal bovine serum, 4.5 g/L glucose, and either with or without 10 mM acyclovir (ACV) for 3 h at 37°C. Postinfection, inoculum was replaced with CM1 with or without 50 mM ACV. For infections with ACV, the ACV was washed out 5 to 6 days postinfection. Reactivation was carried out with LY294002 (20 μM), forskolin (60 μM; pulsed for 20 h), and heat shock (3 h at 43°C) in BrainPhys (Stem Cell Technologies) supplemented with 2 mM l-glutamine, 10% fetal bovine serum, mouse NGF 2.5S (50 ng/mL), and Primocin. Reactivation was quantified by counting the number of GFP-positive neurons or performing reverse transcription-quantitative PCR (RT-qPCR) for HSV-1 lytic transcripts.
Immunofluorescence.
Neurons were fixed for 15 min in 4% formaldehyde and blocked in 5% bovine serum albumin and 0.3% Triton X-100 and incubated overnight in primary antibody. Following primary antibody treatment, neurons were incubated for 1 h in Alexa Fluor 488-, 555-, and 647-conjugated secondary antibodies for multicolor imaging (Invitrogen). Nuclei were stained with Hoechst 33258 (Life Technologies). Images were acquired using an sCMOS charge-coupled device camera (pco.edge) mounted on a Nikon Eclipse Ti inverted epifluorescent microscope using NIS-Elements software (Nikon). Images were analyzed using ImageJ.
Analysis of mRNA expression and viral genome copy number.
To assess expression of HSV-1 lytic mRNA, total RNA was extracted from approximately 5,000 neurons using the Quick-RNA miniprep kit (Zymo Research) with an on-column DNase I digestion. mRNA was converted to cDNA using the Maxima cDNA synthesis kit (Thermo Fisher) using random hexamers for first-strand synthesis and equal amounts of RNA (20 to 30 ng/reaction). To assess viral DNA load, total DNA was extracted from approximately 5,000 neurons using the Quick-DNA Miniprep Plus kit (Zymo Research). qPCR was carried out using PowerUp SYBR green master mix (Thermo Scientific). Relative mRNA/DNA copy numbers were determined using the comparative threshold cycle (ΔΔCT) method normalized to mRNA levels in latently infected samples. Viral RNAs were normalized to mouse reference gene 18s rRNA. All samples were run in duplicate on an Applied Biosystems QuantStudio 6 Flex real-time PCR system, and the mean fold change was compared to the calculated reference gene. Exact copy numbers were determined by comparison to standard curves of known DNA copy number of viral genomes or plasmids containing 18S rRNA (p18S.1 tag was a gift from Vincent Mauro; Addgene plasmid no. 51729; http://n2t.net/addgene:51729; RRID:Addgene_51729) (120).
Statistical analysis.
All statistical analyses were performed using Prism v8.4. The normality test and statistical test used for each figure are denoted in the figure legends. Individual biological replicates are plotted, each corresponding to a single well of cells/neurons. All neuronal experiments were repeated from pooled neurons from at least 3 litters.
Data availability.
All nanopore sequencing data sets associated with this study are available via the European Nucleotide Archive under the accession no. PRJEB51869.
ACKNOWLEDGMENTS
We thank Ian Mohr at New York University for the gift of the Us11-GFP virus and the pSXZY-GFP-Us11 plasmid. We thank Gary Cohen at the University of Pennsylvania for SCgHZ and Vero F6 cells.
This work was supported by National Institutes of Health grants NS105630 (A.R.C.); T32GM008136 (S.D.); AI130618, AI147163, and AI151358 (A.C.W.); the Owens Family Foundation (A.R.C.); and a German Centre for Infection Research (DZIF) associate professorship (D.P.D.).
Contributor Information
Anna R. Cliffe, Email: cliffe@virginia.edu.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic
REFERENCES
- 1.Richter ER, Dias JK, Gilbert JE, II, Atherton SS. 2009. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 200:1901–1906. 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baringer JR, Pisani P. 1994. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol 36:823–829. 10.1002/ana.410360605. [DOI] [PubMed] [Google Scholar]
- 3.Baringer JR, Swoveland P. 1973. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med 288:648–650. 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 4.Warren KG, Brown SM, Wroblewska Z, Gilden D, Koprowski H, Subak-Sharpe J. 1978. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med 298:1068–1069. 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Tscharke DC. 2020. Herpes simplex virus latency is noisier the closer we look. J Virol 94:e01701-19. 10.1128/JVI.01701-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom DC. 2016. Alphaherpesvirus latency: a dynamic state of transcription and reactivation. Adv Virus Res 94:53–80. 10.1016/bs.aivir.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Speck PG, Simmons A. 1991. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol 65:4001–4005. 10.1128/JVI.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speck PG, Simmons A. 1992. Synchronous appearance of antigen-positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J Gen Virol 73:1281–1285. 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 9.Proenca JT, Nelson D, Nicoll MP, Connor V, Efstathiou S. 2016. Analyses of herpes simplex virus type 1 latency and reactivation at the single cell level using fluorescent reporter mice. J Gen Virol 97:767–777. 10.1099/jgv.0.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillet S, Naas T, Crepin S, Roque-Afonso AM, Lafay F, Efstathiou S, Labetoulle M. 2006. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. J Virol 80:9310–9321. 10.1128/JVI.02615-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proenca JT, Coleman HM, Connor V, Winton DJ, Efstathiou S. 2008. A historical analysis of herpes simplex virus promoter activation in vivo reveals distinct populations of latently infected neurones. J Gen Virol 89:2965–2974. 10.1099/vir.0.2008/005066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proenca JT, Coleman HM, Nicoll MP, Connor V, Preston CM, Arthur J, Efstathiou S. 2011. An investigation of herpes simplex virus promoter activity compatible with latency establishment reveals VP16-independent activation of immediate-early promoters in sensory neurones. J Gen Virol 92:2575–2585. 10.1099/vir.0.034728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SH, Kramer MF, Schaffer PA, Coen DM. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J Virol 71:5878–5884. 10.1128/JVI.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, Lu RB, Chang HA, Kao YC, Yeh HW, Chiang WS, Chou YC, Tsao CH, Wu YF, Chien WC. 2018. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections: a nationwide, population-based cohort study in Taiwan. Neurotherapeutics 15:417–429. 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letenneur L, Peres K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, Orgogozo JM, Gauthier S, Dartigues JF. 2008. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PLoS One 3:e3637. 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Chiara G, Piacentini R, Fabiani M, Mastrodonato A, Marcocci ME, Limongi D, Napoletani G, Protto V, Coluccio P, Celestino I, Li Puma DD, Grassi C, Palamara AT. 2019. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog 15:e1007617. 10.1371/journal.ppat.1007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzhaki RF. 2014. Herpes simplex virus type 1 and Alzheimer's disease: increasing evidence for a major role of the virus. Front Aging Neurosci 6:202. 10.3389/fnagi.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 349:241–244. 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- 19.Koelle DM, Magaret A, Warren T, Schellenberg GD, Wald A. 2010. APOE genotype is associated with oral herpetic lesions but not genital or oral herpes simplex virus shedding. Sex Transm Infect 86:202–206. 10.1136/sti.2009.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel AJ, Eslick GD. 2015. Herpes viruses increase the risk of Alzheimer’s disease: a meta-analysis. J Alzheimers Dis 47:351–364. 10.3233/JAD-140822. [DOI] [PubMed] [Google Scholar]
- 21.Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, Reiman EM, Schadt EE, Ehrlich ME, Gandy S, Dudley JT. 2018. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99:64–82.e7. 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopatko Lindman K, Hemmingsson ES, Weidung B, Brännström J, Josefsson M, Olsson J, Elgh F, Nordström P, Lövheim H. 2021. Herpesvirus infections, antiviral treatment, and the risk of dementia-a registry-based cohort study in Sweden. Alzheimers Dement (N Y) 7:e12119. 10.1002/trc2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honess RW, Roizman B. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol 14:8–19. 10.1128/JVI.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honess RW, Roizman B. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA 72:1276–1280. 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. 2012. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog 8:e1002540. 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine AF, Bachenheimer SL. 1990. Intracellular organization of herpes simplex virus type 1 DNA assayed by staphylococcal nuclease sensitivity. Virus Res 16:275–292. 10.1016/0168-1702(90)90053-e. [DOI] [PubMed] [Google Scholar]
- 27.Leinbach SS, Summers WC. 1980. The structure of herpes simplex virus type 1 DNA as probed by micrococcal nuclease digestion. J Gen Virol 51:45–59. 10.1099/0022-1317-51-1-45. [DOI] [PubMed] [Google Scholar]
- 28.Pignatti PF, Cassai E. 1980. Analysis of herpes simplex virus nucleoprotein complexes extracted from infected cells. J Virol 36:816–828. 10.1128/JVI.36.3.816-828.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conn KL, Hendzel MJ, Schang LM. 2008. Linker histones are mobilized during infection with herpes simplex virus type 1. J Virol 82:8629–8646. 10.1128/JVI.00616-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conn KL, Hendzel MJ, Schang LM. 2011. Core histones H2B and H4 are mobilized during infection with herpes simplex virus 1. J Virol 85:13234–13252. 10.1128/JVI.06038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monier K, Armas JC, Etteldorf S, Ghazal P, Sullivan KF. 2000. Annexation of the interchromosomal space during viral infection. Nat Cell Biol 2:661–665. 10.1038/35023615. [DOI] [PubMed] [Google Scholar]
- 32.Herrera FJ, Triezenberg SJ. 2004. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J Virol 78:9689–9696. 10.1128/JVI.78.18.9689-9696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kent JR, Zeng PY, Atanasiu D, Gardner J, Fraser NW, Berger SL. 2004. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J Virol 78:10178–10186. 10.1128/JVI.78.18.10178-10186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacasse JJ, Schang LM. 2010. During lytic infections, herpes simplex virus type 1 DNA is in complexes with the properties of unstable nucleosomes. J Virol 84:1920–1933. 10.1128/JVI.01934-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutluay SB, Doroghazi J, Roemer ME, Triezenberg SJ. 2008. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 373:239–247. 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutluay SB, Triezenberg SJ. 2009. Regulation of histone deposition on the herpes simplex virus type 1 genome during lytic infection. J Virol 83:5835–5845. 10.1128/JVI.00219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J, Fraser NW. 2008. Temporal association of the herpes simplex virus genome with histone proteins during a lytic infection. J Virol 82:3530–3537. 10.1128/JVI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cliffe AR, Knipe DM. 2008. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol 82:12030–12038. 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan D, Wang M, Cheng A, Jia R, Yang Q, Wu Y, Zhu D, Zhao X, Chen S, Liu M, Zhang S, Ou X, Mao S, Gao Q, Sun D, Wen X, Liu Y, Yu Y, Zhang L, Tian B, Pan L, Chen X. 2020. The role of VP16 in the life cycle of alphaherpesviruses. Front Microbiol 11:1910. 10.3389/fmicb.2020.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Y, Vogel JL, Arbuckle JH, Rai G, Jadhav A, Simeonov A, Maloney DJ, Kristie TM. 2013. Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency. Sci Transl Med 5:167ra5. 10.1126/scitranslmed.3005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Vogel JL, Narayanan A, Peng H, Kristie TM. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat Med 15:1312–1317. 10.1038/nm.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayanan A, Ruyechan WT, Kristie TM. 2007. The coactivator host cell factor-1 mediates Set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection. Proc Natl Acad Sci USA 104:10835–10840. 10.1073/pnas.0704351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. 2003. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev 17:896–911. 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dremel SE, DeLuca NA. 2019. Herpes simplex viral nucleoprotein creates a competitive transcriptional environment facilitating robust viral transcription and host shut off. Elife 8:e51109. 10.7554/eLife.51109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang QY, Zhou C, Johnson KE, Colgrove RC, Coen DM, Knipe DM. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc Natl Acad Sci USA 102:16055–16059. 10.1073/pnas.0505850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cliffe AR, Coen DM, Knipe DM. 2013. Kinetics of facultative heterochromatin and polycomb group protein association with the herpes simplex viral genome during establishment of latent infection. mBio 4:e00590-12. 10.1128/mBio.00590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cliffe AR, Garber DA, Knipe DM. 2009. Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. J Virol 83:8182–8190. 10.1128/JVI.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knipe DM, Cliffe A. 2008. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6:211–221. 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 49.Kwiatkowski DL, Thompson HW, Bloom DC. 2009. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. J Virol 83:8173–8181. 10.1128/JVI.00686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dochnal SA, Francois AK, Cliffe AR. 2021. De novo polycomb recruitment: lessons from latent herpesviruses. Viruses 13:1470. 10.3390/v13081470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu HL, Srinivas KP, Wang S, Chao MV, Lionnet T, Mohr I, Wilson AC, Depledge DP, Huang TT. 2022. Single-cell transcriptomics identifies Gadd45b as a regulator of herpesvirus-reactivating neurons. EMBO Rep 23:e53543. 10.15252/embr.202153543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cliffe AR, Arbuckle JH, Vogel JL, Geden MJ, Rothbart SB, Cusack CL, Strahl BD, Kristie TM, Deshmukh M. 2015. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 18:649–658. 10.1016/j.chom.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cliffe AR, Wilson AC. 2017. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J Virol 91:e01419-16. 10.1128/JVI.01419-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuddy SR, Schinlever AR, Dochnal S, Seegren PV, Suzich J, Kundu P, Downs TK, Farah M, Desai BN, Boutell C, Cliffe AR. 2020. Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1. Elife 9:e58037. 10.7554/eLife.58037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steiner I, Spivack JG, Deshmane SL, Ace CI, Preston CM, Fraser NW. 1990. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol 64:1630–1638. 10.1128/JVI.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson AC, Mohr I. 2012. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 20:604–611. 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lachmann R. 2003. Herpes simplex virus latency. Expert Rev Mol Med 5:1–14. 10.1017/S1462399403006975. [DOI] [PubMed] [Google Scholar]
- 58.Pourchet A, Modrek AS, Placantonakis DG, Mohr I, Wilson AC. 2017. Modeling HSV-1 latency in human embryonic stem cell-derived neurons. Pathogens 6:24. 10.3390/pathogens6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzich JB, Cliffe AR. 2018. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology 522:81–91. 10.1016/j.virol.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzich JB, Cuddy SR, Baidas H, Dochnal S, Ke E, Schinlever AR, Babnis A, Boutell C, Cliffe AR. 2021. PML-NB-dependent type I interferon memory results in a restricted form of HSV latency. EMBO Rep 22:e52547. 10.15252/embr.202152547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linderman JA, Kobayashi M, Rayannavar V, Fak JJ, Darnell RB, Chao MV, Wilson AC, Mohr I. 2017. Immune escape via a transient gene expression program enables productive replication of a latent pathogen. Cell Rep 18:1312–1323. 10.1016/j.celrep.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J Virol 85:6669–6677. 10.1128/JVI.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cabrera JR, Charron AJ, Leib DA. 2018. Neuronal subtype determines herpes simplex virus 1 latency-associated-transcript promoter activity during latency. J Virol 92:e00430-18. 10.1128/JVI.00430-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arthur JL, Scarpini CG, Connor V, Lachmann RH, Tolkovsky AM, Efstathiou S. 2001. Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro. J Virol 75:3885–3895. 10.1128/JVI.75.8.3885-3895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, Chao MV. 2010. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe 8:320–330. 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanez AA, Harrell T, Sriranganathan HJ, Ives AM, Bertke AS. 2017. Neurotrophic factors NGF, GDNF and NTN selectively modulate HSV1 and HSV2 lytic infection and reactivation in primary adult sensory and autonomic neurons. Pathogens 6:5. 10.3390/pathogens6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilcox CL, Johnson EM, Jr.. 1988. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol 62:393–399. 10.1128/JVI.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilcox CL, Smith RL, Freed CR, Johnson EM, Jr.. 1990. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci 10:1268–1275. 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thellman NM, Botting C, Madaj Z, Triezenberg SJ. 2017. An immortalized human dorsal root ganglion cell line provides a novel context to study herpes simplex virus 1 latency and reactivation. J Virol 91:e00080-17. 10.1128/JVI.00080-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards TG, Bloom DC. 2019. Lund human mesencephalic (LUHMES) neuronal cell line supports herpes simplex virus 1 latency in vitro. J Virol 93:e02210-18. 10.1128/JVI.02210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Clercq E. 2008. The discovery of antiviral agents: ten different compounds, ten different stories. Med Res Rev 28:929–953. 10.1002/med.20128. [DOI] [PubMed] [Google Scholar]
- 72.De Clercq E, Holy A. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discov 4:928–940. 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 73.Morfin F, Thouvenot D. 2003. Herpes simplex virus resistance to antiviral drugs. J Clin Virol 26:29–37. 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 74.Benboudjema L, Mulvey M, Gao Y, Pimplikar SW, Mohr I. 2003. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J Virol 77:9192–9203. 10.1128/jvi.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol 66:341–348. 10.1128/JVI.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hilterbrand AT, Heldwein EE. 2019. Go go gadget glycoprotein!: HSV-1 draws on its sizeable glycoprotein tool kit to customize its diverse entry routes. PLoS Pathog 15:e1007660. 10.1371/journal.ppat.1007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner A, Bruun B, Minson T, Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol 72:873–875. 10.1128/JVI.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu HL, Shiflett LA, Kobayashi M, Chao MV, Wilson AC, Mohr I, Huang TT. 2019. TOP2beta-dependent nuclear DNA damage shapes extracellular growth factor responses via dynamic AKT phosphorylation to control virus latency. Mol Cell 74:466–480.e4. 10.1016/j.molcel.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi M, Kim JY, Camarena V, Roehm PC, Chao MV, Wilson AC, Mohr I. 2012. A primary neuron culture system for the study of herpes simplex virus latency and reactivation. J Vis Exp. 10.3791/3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao MV. 2003. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309. 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 81.van Zeijl M, Fairhurst J, Jones TR, Vernon SK, Morin J, LaRocque J, Feld B, O’Hara B, Bloom JD, Johann SV. 2000. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J Virol 74:9054–9061. 10.1128/jvi.74.19.9054-9061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newcomb WW, Brown JC. 2002. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. J Virol 76:10084–10088. 10.1128/jvi.76.19.10084-10088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pesola JM, Zhu J, Knipe DM, Coen DM. 2005. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J Virol 79:14516–14525. 10.1128/JVI.79.23.14516-14525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hollville E, Romero SE, Deshmukh M. 2019. Apoptotic cell death regulation in neurons. FEBS J 286:3276–3298. 10.1111/febs.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Annis RP, Swahari V, Nakamura A, Xie AX, Hammond SM, Deshmukh M. 2016. Mature neurons dynamically restrict apoptosis via redundant premitochondrial brakes. FEBS J 283:4569–4582. 10.1111/febs.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kole AJ, Swahari V, Hammond SM, Deshmukh M. 2011. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 25:125–130. 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith RL, Pizer LI, Johnson EM, Jr, Wilcox CL. 1992. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology 188:311–318. 10.1016/0042-6822(92)90760-M. [DOI] [PubMed] [Google Scholar]
- 88.Colgin MA, Smith RL, Wilcox CL. 2001. Inducible cyclic AMP early repressor produces reactivation of latent herpes simplex virus type 1 in neurons in vitro. J Virol 75:2912–2920. 10.1128/JVI.75.6.2912-2920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Regge N, Van Opdenbosch N, Nauwynck HJ, Efstathiou S, Favoreel HW. 2010. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro. PLoS One 5:e13076. 10.1371/journal.pone.0013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danaher RJ, Jacob RJ, Miller CS. 2003. Herpesvirus quiescence in neuronal cells. V: forskolin-responsiveness of the herpes simplex virus type 1 alpha0 promoter and contribution of the putative cAMP response element. J Neurovirol 9:489–497. 10.1080/13550280390218797. [DOI] [PubMed] [Google Scholar]
- 91.Moriya A, Yoshiki A, Kita M, Fushiki S, Imanishi J. 1994. Heat shock-induced reactivation of herpes simplex virus type 1 in latently infected mouse trigeminal ganglion cells in dissociated culture. Arch Virol 135:419–425. 10.1007/BF01310025. [DOI] [PubMed] [Google Scholar]
- 92.Halford WP, Gebhardt BM, Carr DJ. 1996. Mechanisms of herpes simplex virus type 1 reactivation. J Virol 70:5051–5060. 10.1128/JVI.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Halford WP, Schaffer PA. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol 75:3240–3249. 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kushnir AS, Davido DJ, Schaffer PA. 2010. Role of nuclear factor Y in stress-induced activation of the herpes simplex virus type 1 ICP0 promoter. J Virol 84:188–200. 10.1128/JVI.01377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sawtell NM, Thompson RL. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol 66:2150–2156. 10.1128/JVI.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perng GC, Osorio N, Jiang X, Geertsema R, Hsiang C, Brown D, BenMohamed L, Wechsler SL. 2015. Large amounts of reactivated virus in tears precedes recurrent herpes stromal keratitis in stressed rabbits latently infected with herpes simplex virus. Curr Eye Res 41:284–291. 10.3109/02713683.2015.1020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimomura Y, Higaki S, Watanabe K. 2010. Suppression of herpes simplex virus 1 reactivation in a mouse eye model by cyclooxygenase inhibitor, heat shock protein inhibitor, and adenosine monophosphate. Jpn J Ophthalmol 54:187–190. 10.1007/s10384-010-0803-3. [DOI] [PubMed] [Google Scholar]
- 98.Hill JM, Lukiw WJ, Gebhardt BM, Higaki S, Loutsch JM, Myles ME, Thompson HW, Kwon BS, Bazan NG, Kaufman HE. 2001. Gene expression analyzed by microarrays in HSV-1 latent mouse trigeminal ganglion following heat stress. Virus Genes 23:273–280. 10.1023/a:1012517221937. [DOI] [PubMed] [Google Scholar]
- 99.Sawtell NM, Thompson RL. 2004. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J Virol 78:7784–7794. 10.1128/JVI.78.14.7784-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thompson RL, Preston CM, Sawtell NM. 2009. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog 5:e1000352. 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel S, Cohen F, Dean BJ, De La Torre K, Deshmukh G, Estrada AA, Ghosh AS, Gibbons P, Gustafson A, Huestis MP, Le Pichon CE, Lin H, Liu W, Liu X, Liu Y, Ly CQ, Lyssikatos JP, Ma C, Scearce-Levie K, Shin YG, Solanoy H, Stark KL, Wang J, Wang B, Zhao X, Lewcock JW, Siu M. 2015. Discovery of dual leucine zipper kinase (DLK, MAP3K12) inhibitors with activity in neurodegeneration models. J Med Chem 58:401–418. 10.1021/jm5013984. [DOI] [PubMed] [Google Scholar]
- 102.Heinemann B, Nielsen JM, Hudlebusch HR, Lees MJ, Larsen DV, Boesen T, Labelle M, Gerlach LO, Birk P, Helin K. 2014. Inhibition of demethylases by GSK-J1/J4. Nature 514:E1–E2. 10.1038/nature13688. [DOI] [PubMed] [Google Scholar]
- 103.Sawtell NM, Thompson RL. 2016. Herpes simplex virus and the lexicon of latency and reactivation: a call for defining terms and building an integrated collective framework. F1000Res 5:2038. 10.12688/f1000research.8886.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS. 2004. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell 14:637–646. 10.1016/j.molcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Chadwick BP, Willard HF. 2003. Barring gene expression after XIST: maintaining facultative heterochromatin on the inactive X. Semin Cell Dev Biol 14:359–367. 10.1016/j.semcdb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 106.Du T, Zhou G, Roizman B. 2011. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci USA 108:18820–18824. 10.1073/pnas.1117203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitford AL, Clinton CA, Lane KE, Dochnal SA, Suzich JB, Cliffe AR. 2022. Herpes simplex virus reactivation induced ex vivo involves a DLK-dependent but histone demethylase-independent wave of lytic gene expression. bioRxiv. 10.1101/2022.02.25.481951. [DOI] [PMC free article] [PubMed]
- 108.Tedeschi A, Bradke F. 2013. The DLK signalling pathway: a double-edged sword in neural development and regeneration. EMBO Rep 14:605–614. 10.1038/embor.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karney-Grobe S, Russo A, Frey E, Milbrandt J, DiAntonio A. 2018. HSP90 is a chaperone for DLK and is required for axon injury signaling. Proc Natl Acad Sci USA 115:E9899–E9908. 10.1073/pnas.1805351115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Summers DW, Frey E, Walker LJ, Milbrandt J, DiAntonio A. 2020. DLK activation synergizes with mitochondrial dysfunction to downregulate axon survival factors and promote SARM1-dependent axon degeneration. Mol Neurobiol 57:1146–1158. 10.1007/s12035-019-01796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirai S, Kawaguchi A, Suenaga J, Ono M, Cui DF, Ohno S. 2005. Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr Patterns 5:517–523. 10.1016/j.modgep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27:722–736. 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vaser R, Sovic I, Nagarajan N, Sikic M. 2017. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res 27:737–746. 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heng L. 2016. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics 32:2103–2110. 10.1093/bioinformatics/btw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hahne F, Ivanek R. 2016. Visualizing genomic data using Gviz and Bioconductor. Methods Mol Biol 1418:335–351. 10.1007/978-1-4939-3578-9_16. [DOI] [PubMed] [Google Scholar]
- 118.Lawrence M, Huber W, Pages H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, Carey VJ. 2013. Software for computing and annotating genomic ranges. PLoS Comput Biol 9:e1003118. 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malin SA, Davis BM, Molliver DC. 2007. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2:152–160. 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 120.Burman LG, Mauro VP. 2012. Analysis of rRNA processing and translation in mammalian cells using a synthetic 18S rRNA expression system. Nucleic Acids Res 40:8085–8098. 10.1093/nar/gks530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All nanopore sequencing data sets associated with this study are available via the European Nucleotide Archive under the accession no. PRJEB51869.