Abstract
Widespread availability of antiretroviral therapy among pregnant women living with HIV has greatly reduced the rate of mother-to-child transmission (MTCT) of HIV across the globe. However, while UNAIDS has set targets to reduce the annual number of new pediatric HIV infections to fewer than 40,000 in 2018 and fewer than 20,000 in 2020, progress towards these targets has plateaued at an unacceptably high global estimate of greater than 160,000 children newly infected with HIV in 2018. Moreover, it has become clear that expansion of maternal ART alone will not be sufficient to close the remaining gap and eliminate MTCT of HIV. Additional strategies such as maternal or infant passive and/or active immunization that synergize with maternal ART will be required to end the pediatric HIV epidemic. In this review, we outline the landscape of existing maternal interventions and emerging maternal immune-based approaches to prevent MTCT of HIV.
Keywords: Maternal immunization, Human immunodeficiency virus, Vertical transmission, Mother-to-child transmission, Antiretroviral therapy
Introduction
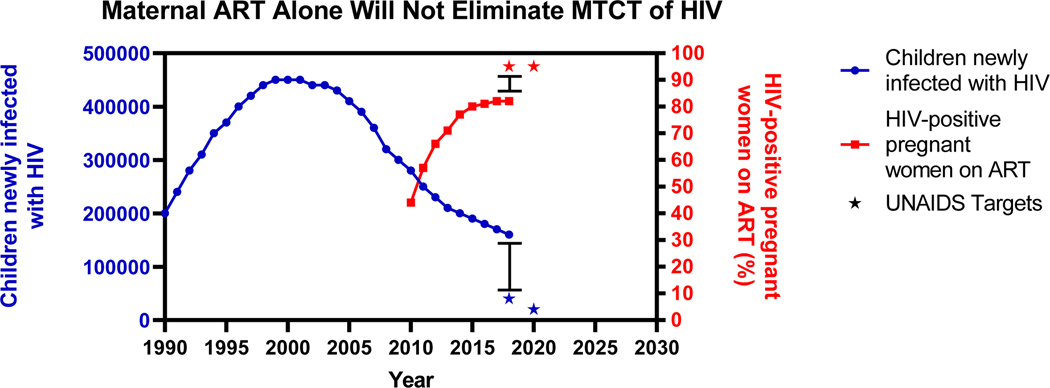
With the implementation of HIV screening during pregnancy (1) and increased access to antiretroviral therapy (ART), the risk of perinatal (in-utero and during delivery) and postnatal (during breastfeeding) transmission of HIV can reach <5% in real world settings (2). Despite this significant progress, the number of children becoming newly infected with HIV remains unacceptably high, with approximately 160,000 pediatric infections in 2018, equating to greater than 400 infections per day (3) (Figure 1). Currently available ART-based measures to prevent mother-to-child transmission (MTCT) of HIV are limited by implementation challenges, including late presentation for prenatal care (4), lack of adherence in the postpartum period (5), and fetal toxicities that can result in higher rates of infant prematurity and/or death. In addition, ART-based prevention strategies do not address the scenario of acute maternal HIV infections during pregnancy or breastfeeding. This high-risk transmission setting for infant HIV infection accounts for >50% of new pediatric infections (6). Therefore, novel immune-based strategies such as active immunization with HIV vaccines and passive immunization with broadly neutralizing antibodies (bNAbs) that could be used in conjunction with ART will be necessary to eliminate MTCT of HIV and achieve an HIV free generation. While immune-based strategies can be administered either to the mother or the infant, this review focuses on maternal interventions. Specifically, we outline the landscape of existing maternal interventions and discuss emerging maternal immune-based approaches to prevent MTCT of HIV.
Figure 1.
Maternal antiretroviral therapy (ART) alone will not eliminate mother-to-child transmission (MTCT) of HIV. Estimated global number of children newly infected with HIV (left y-axis, blue) and HIV-positive pregnant women receiving effective ART regimen (right y-axis, red) by year. Data obtained from 2019 UNAIDS Global Estimates.
Benefits, risks, and challenges associated with maternal ART regimens.
One of the biggest advances in the prevention of HIV was the discovery that maternal ART can prevent vertical transmission. The Pediatric AIDS Clinical Trials Group 076 (PACTG 076) was one of the first clinical trials which indicated that administering zidovudine to pregnant women and their infants reduces the risk of HIV MTCT by 70% (7). Since then, a few clinical trials have been conducted in pregnant and breastfeeding women from resource-limited and resource-rich settings to identify optimal ART regimens and timing of therapy initiation to efficiently reduce MTCT risk. One of these studies was the International Maternal and Pediatric Adolescent AIDS Clinical Trials (IMPAACT) “Promoting Maternal and Infant Survival Everywhere” (PROMISE) trial, which demonstrated that both zidovudine-based and tenofovir-based triple-drug ART regimens have superior efficacy, yet higher rates of adverse events as compared to zidovudine plus single-dose nevirapine (8). Therefore, the current ART recommendation during pregnancy is a triple-drug ARV regimen, comprising two nucleoside reverse transcriptase inhibitors (NRTIs) that cross the placenta and produce adequate systemic drug levels in the fetus (9, 10), in combination with either a ritonavir-boosted protease inhibitor (PI) or an integrase strand transfer inhibitor (INSTI).
Importantly, the recommended ART doses in HIV-infected pregnant women largely rely on pharmacokinetic (PK) data available from non-pregnant women. Yet, pregnancy leads to physiological alterations resulting in differential drug absorption, thereby likely impacting the PK of drugs (11). In addition, while a few clinical trials have evaluated the impact of ART combinations on MTCT during the period of pregnancy and breastfeeding; there is insufficient information on the safety and efficacy of most commonly used ART regimens in pregnant women living with HIV (12). Therefore, there is a critical need to assess the safety and efficacy of ART drug formulations and evaluating the impact of those drugs on pregnancy outcomes to ensure safer pregnancies for HIV-infected women and healthier outcomes for their infants.
The time of initiation of ART surrounding pregnancy is also a crucial factor that predicts vertical transmission risk. Administration of ART over the course of antepartum, intrapartum and postpartum periods is a more effective strategy for preventing perinatal HIV transmission than treatment during any of these periods alone. The Perinatal HIV Prevention Trial (PHPT)-5 validated that women receiving <8 weeks of ART during pregnancy had higher risk of MTCT compared to those receiving longer ART prophylaxis (13). Furthermore, the European National Study of HIV in Pregnancy and Childhood suggested that each additional week of antenatal ART can reduce the risk of HIV transmission by as much as 10% (14). Therefore, current World Health Organization (WHO) guidelines for the prevention of mother-to-child transmission (PMTCT) recommend initiation of life-long ART for all HIV-infected women, irrespective of their plasma viral load and CD4+ T cell counts, an approach known as Option B+ (15).
While combination ART has clear benefits in suppressing HIV replication in pregnant women and preventing MTCT, the adverse impacts of ART on maternal and fetal health cannot be disregarded. HIV-infected pregnant women on ART are often at increased risk for adverse pregnancy outcomes such as delivery before 37 weeks of gestation, also known as preterm birth or delivery (PTD), low birth weight (LBW) infants, small-for-gestational-age (SGA) infants and stillbirth (16). Certain ART regimens appear to be more associated with adverse outcomes than others. Notably, observations from the Pediatric HIV/AIDS Cohort Study (PHACS) Surveillance Monitoring for ART Toxicities (SMARTT) cohort (17), IMPAACT PROMISE study (8), and the IMPAACT P1025 study (18) altogether indicate that protease inhibitor-based ART formulations are associated with higher risks of PTD or LBW. In addition, non-nucleoside-based ART regimens have been associated with adverse pregnancy outcomes in South African women (19). Moreover, a nationally representative birth-outcomes surveillance study conducted in Botswana reported a near ten-fold increased prevalence of neural tube defects in newborns associated with maternal exposure to dolutegravir-based ART as compared to non-dolutegravir-based ART, from the time of conception (20). A July 2019 follow-up analysis has abated, yet not refuted this safety signal, as the prevalence of neural tube defects associated with dolutegravir-based ART is now reported to be three times as high as that of non-dolutegravir based ART (21). The identification of an ART regimen that can successfully suppress viral replication at pre-conception or during pregnancy, while having minimum adverse impact on maternal and fetal health therefore remains a critical priority in the field. Achieving this goal will require the expansion of pharmacovigilance systems in high HIV prevalence settings and the inclusion of pregnant women in clinical trials (22).
Additionally, there are significant implementation challenges associated with maternal ART regimens. Early initiation and adherence to ART leads to maternal plasma viral suppression at undetectable levels and is highly effective in reducing perinatal HIV transmission risk (23). However, if presentation to antenatal care is delayed, maternal ART efficacy may be limited during labor or delivery leading to transmission of the virus to their newborns. Additionally, lack of adherence to therapy can lead to development of drug-resistant viral strains and therapeutic failure (24), thereby resulting in MTCT of HIV. Furthermore, >50% of the annual pediatric HIV infections occur postnatally during breastfeeding from women infected with HIV (6). Despite being a high-risk HIV transmission route, breastfeeding is highly recommended in resource-limited settings to reduce infant mortality due to respiratory and diarrheal illness (25). Although triple-drug ART of HIV-infected mothers throughout breastfeeding can significantly reduce postnatal HIV transmission (26), a significant proportion of MTCT in infants occur from mothers who are newly infected with HIV during the lactation period (27). Acquiring HIV in the peripartum period or during breastfeeding commits infants to a life-long sentence of daily ART, as interruption of treatment is universally associated with the rebound of replicating virus and repopulation of viral reservoirs. Moreover, prolonged drug exposure in children who initiate ART at a young age can have long-term metabolic consequences (28), and children are at a higher risk than adults of developing triple class virologic failure (29). Thus, novel interventions to universally prevent pediatric HIV infections that do not rely on daily adherence to medications will be needed to end the pediatric HIV epidemic.
Maternal immune correlates of MTCT risk for development of immune-based interventions.
While ART can reduce MTCT risk to less than 5%, in the absence of maternal ART, less than half of infants become infected (2), suggesting that maternal immune factors may provide partial protection against the vertical transmission of HIV. Identifying these maternal immune correlates of MTCT will be critical to understanding the types of immune responses that need to be enhanced by maternal immunization strategies to prevent MTCT of HIV. As infants are passively immunized with maternal antibodies (Abs) via placental transfer prior to birth, transmission studies involving mother-infant pairs can provide a unique setting in which to examine the role of maternal Abs in infant HIV acquisition and transmission risk.
Maternal antibody specificity against HIV envelope and MTCT risk
Several studies have suggested that maternal Abs against conserved, vulnerable regions of the HIV envelope (Env) may serve as immune correlates of protection against MTCT of HIV (30, 31) (Table 1). A maternal humoral immune correlate study of subtype B HIV-infected, ART-naïve mothers in the US-based Women and Infants Transmission Study (WITS) cohort reported that the magnitude of maternal IgG antibodies specific for Env third variable loop (V3), neutralization of tier 1 viruses, and the magnitude of CD4-binding site blocking antibodies were associated with reduced risk of MTCT (32). A follow up study in the WITS cohort then mapped the specificity of maternal V3-specific IgG responses that were associated with reduced MTCT risk to the C-terminal region of the V3 loop (33). Another study linked maternal Env-specific Abs targeting the membrane-proximal external region (MPER) of gp41 with reduced risk of vertical HIV transmission among HIV subtype C infected mothers (34), and more recently gp41 ectodomain targeting Abs have been associated with increased MTCT risk (35).
Table 1.
Recently reported maternal immune correlates of mother-to-child transmission of HIV.
| Study | Cohort | Region | Population | Dominant Virus Clade | Treatment Status | Breastfeeding Status | Sample Size | Transmission Mode | Immune Correlate | MTCT Risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Permar et al. (32) | Women and Infants Transmission Study (WITS) | United States | HIV-infected mother-infant pairs | B | Naïve | No | T (n = 83); NT(n=165) | Perinatal | V3-specific IgG binding | Decreased |
| Soluble CD4- bs blocking | ||||||||||
| Tier 1A virus neutralization | ||||||||||
| Martinez et al. (33) | Women and Infants Transmission Study (WITS) | United States | HIV-infected mother-infant pairs | B | Naïve | No | T (n = 83); NT(n=165) | Perinatal | C-terminal V3- specific IgG binding and neutralization | Decreased |
| Diomede et al. (34) | Coronation Women and Children Hospital | South Africa | HIV-infected mother-infant pairs | C | sdNVP | No | I (n=40) U (n=34) | Perinatal | MPER gp41- specific IgG binding | Decreased |
| Naiman et al. (35) | Nairobi Breastfeeding Clinical Trial (NBT) | Kenya | HIV-infected mother-infant pairs | C | Naïve | Yes | T (n=21); NT (n=51) | Postnatal | gp41 ectodomain-specific IgG binding | Increased |
| Mutucumarana et al. (39) | Breastfeeding, Anti retrovirals, and Nutrition (BAN) | Malawi | HIV-infected mother-infant pairs | C | sdNVP followed by 1 week of zidovudine and lamivudine | Yes | T (n=45); NT (n=43) | Perinatal (in utero) | CD4-bs IgG binding | Increased |
| V1V2-specific IgG binding | ||||||||||
| Milligan et al. (44) | Nairobi Breastfeeding Trial (NBT) | Kenya | HIV-infected mother-infant pairs | A | Na ïve | Yes | T (n=10); NT (n = 10) | Postnatal | Maternal autologous virus neutralization | Not associated |
| Ghulam-Smith et al. (45) | Breastfeeding, Antiretrovirals, and Nutrition (BAN) | Malawi | HIV-infected mother-infant pairs | C | sdNVP followed by 1 week of zidovudine | Yes | T (n=21); NT (n=42) | Postnatal | Maternal heterologous virus neutralization | Increased |
| Mabuka et al. (46) | Nairobi Breastfeeding Trial (NBT) | Kenya | HIV-infected mother-infant pairs | A | Na ïve | Yes | T (n=9); NT (n=10) | Postnatal | Breastmilk supernatant ADCC activity | Decreased |
| Pollara et al. (42) | Breastfeeding, Antiretrovirals, and Nutrition (BAN) | Malawi | HIV-infected mother-infant pairs | C | sdNVP followed by 1 week of zidovudine and lamivudine | Yes | T (n=9); NT (n=10) | Postnatal | Breastmilk mucosal Env-specific IgA | Decreased |
| Breasmilk or plasma Env-specific IgG binding, neutralization, ADCC | Not associated | |||||||||
| Milligan et al. (43) | Nairobi Breastfeeding Trial (NBT) | Kenya | HIV-exposed infants | A | Na ïve | Yes | (n=72) | Postnatal | Passively acquired infant ADCC activity | Decreased |
Mother-to-child transmission (MTCT) Transmitting (T) Nontransmitting (NT) Infected (I) Uninfected (U) Single dose nevirapine (sdNVP) variable loop 3 (V3) CD4 binding site (CD4bs) membrane-proximal external region (MPER) variable loop 1 and variable loop 2 (V1V2) antibody dependent cellular cytotoxicity (ADCC)
Yet, not all studies have confirmed the same associations of HIV Env-specific responses and MTCT risk (36–38). Interestingly, neither maternal V3-specific IgG binding nor tier 1 virus-neutralizing responses were predictive of reduced MTCT risk in HIV, clade C virus-infected women from the Breastfeeding and Nutrition (BAN) cohort (n=88) (39). Instead, IgG against the variable loops 1 and 2 (V1V2) and anti-CD4 binding-site Abs were associated with increased risk of MTCT of HIV. These studies suggest that multiple regions of the HIV Env may be targets of potentially protective maternal humoral responses, and these targets may differ depending on virus clade, transmission mode, and maternal ART status (Table 1).
Maternal autologous virus neutralizing antibodies and MTCT risk
Similar to HIV infection in adults, most infant infections are established by a single transmitted-founder (T/F) variant, suggesting that a selective virus genetic bottleneck is involved in MTCT (40, 41). However, factors that drive this genetic bottleneck are not clear. One hypothesis is that maternal autologous virus neutralizing Abs select for neutralization escape variants that initiate infection in the infant (42). In fact, a recent study demonstrated that infant T/F viruses were more neutralization resistant against paired maternal plasma than non-transmitted maternal virus variants in the setting of peripartum transmission (43). Furthermore, linear V3-specific antibodies isolated from a non-transmitting, HIV-infected mother from the WITS cohort study neutralized her own circulating virus variants (32), suggesting that antibodies with autologous virus neutralization capacity are potentially protective in the setting of MTCT.
While neutralization resistance to paired maternal plasma neutralizing antibodies may be a defining feature of infant T/F viruses in peripartum transmission, Milligan et al. reported that maternal autologous neutralization was not associated with transmission risk during the breastfeeding period in the Nairobi Breastfeeding Trial (NBT) cohort (44). Moreover, data from the BAN cohort suggest that maternal neutralizing antibody breadth and potency are potentially associated with increased risk of postpartum transmission and adverse infant outcomes (45). Interestingly, a recent study indicated that maternal plasma broadly neutralizing antibody (bNAb) responses may select for neutralization-resistant infant transmitted-founder viruses in the setting of MTCT (Permar unpublished data, (46)). While these studies demonstrated that maternal neutralizing antibodies contribute to the selection of the infant T/F virus, whether maternal autologous virus neutralization contribute to limit vertical transmission is still unclear. Defining the precise role of neutralizing antibodies on transmission risk will be critical to inform maternal immunization strategies. Notably, the role of broadly neutralizing antibodies during MTCT should be further evaluated as many vaccine strategies under development rely on induction of bnAbs, and ongoing trials are testing the efficacy of passively administered bNAbs in the setting of MTCT.
Maternal non-neutralizing antibodies and MTCT risk
In addition to maternal neutralizing antibodies, the role of maternal non-neutralizing antibodies in MTCT risk also remains poorly defined. Mabuka et al. reported that breast milk Env-specific IgG responses with antibody-dependent cellular cytotoxicity (ADCC) activity were associated with reduced MTCT risk in a small cohort (n=19) of HIV clade A virus-infected Kenyan women (47). In contrast, a study comparing Env-specific IgG responses of clade C HIV-infected postnatally transmitting and non-transmitting mothers from the BAN cohort, reported no association between breast milk or plasma ADCC responses and postnatal transmission risk (48). Despite the contradicting outcomes, both studies reported a high frequency of breast milk samples with detectable gp120-specific ADCC activity, suggesting an important role for ADCC responses in postnatal transmission. Furthermore, Milligan et al. reported higher levels of passively acquired ADCC activity in uninfected than infected infants, and demonstrated that increased ADCC antibody activity in infected infants was associated with reduced mortality risk (49). These studies highlight the potential role for passively transferred ADCC antibodies in transmission risk and infant HIV disease progression.
Maternal innate immunity and naturally protective antiviral factors in breastmilk
Despite daily mucosal HIV exposure for a period of up to 2 years, only an estimated 10% of breastfeeding infants acquire HIV from their untreated, HIV-infected mothers, suggesting the presence of naturally protective host factors in breastmilk (50). In fact, several innate mucosal antiviral factors have been identified in breastmilk, including mucins (51), defensins (52), secretory leukocyte protease inhibitor (53), lactoferrin (54), long chain fatty acids (55), and interleukin 15 (IL-15) (56). Moreover, recent studies have identified an extracellular matrix protein naturally present in breastmilk, tenascin C, as a broad-spectrum, HIV-neutralizing host protein (57). Interestingly, the tenascin C neutralizing activity was mapped to the V3 loop of the HIV-1 Env, and thus capable of blocking chemokine coreceptor binding required for infection of target cells, similar to the activity of V3 loop-specific bNab PGT128 (58). Further study of host antiviral factors that inhibit mucosal HIV transmission will build the potential for safe and effective non-vaccine, immune-based strategies that harness the full spectrum of both the innate and adaptive arms of immunity.
Maternal immunization strategies for prevention of MTCT of HIV
Passive immunization strategies for prevention of MTCT of HIV
Maternal immunization approaches that enhance antiviral immune responses in the mother could prevent MTCT of HIV by providing passive immunization to the infant through the transfer of Abs via the placenta and/or postnatally through breastfeeding. The rhesus macaque Simian Immunodeficiency Virus (SIV) and Simian-Human Immunodeficiency Virus (SHIV) model has been integral in evaluating the efficacy of passively transferred antibodies to prevent MTCT of HIV, and a number of studies have demonstrated that passively administered HIV‐1 Env‐specific mAbs can prevent SIV/SHIV transmission to newborn nonhuman primates (59–61). To date, there have only been two completed phase III studies of passive immunization to prevent MTCT conducted in humans. Among these studies, administration of polyclonal hyperimmune globulin preparations to HIV infected pregnant women did not reduce the rate of vertical transmission (62, 63). Clinical trials are ongoing to study the safety and efficacy of VRC01-based bNAb passive immunization among several key demographics at high risk of HIV infection, including men and transgender persons who have sex with men (NCT02716675), women (NCT02568215), and pharmacokinetics and safety in HIV-exposed uninfected infants (NCT02256631). Potential rationales for maternal bNAb administration in conjunction with ART include reduction of the maternal viral reservoir, prevention of in utero and peripartum transmission, and placental transfer to the infant for protection against postnatal exposure. However, it also important to recognize potential adverse effects, such as maternal bNAb administration driving viral escape mutants that can then be transmitted to the infant.
Active immunization strategies for prevention of MTCT of HIV
A maternal vaccine capable of temporarily boosting neutralizing antibody responses against circulating viruses in HIV-infected pregnant women could effectively decrease MTCT of HIV. The safety and immunogenicity of a maternal HIV Env active vaccination regimen to reduce MTCT has previously been evaluated in a phase I clinical trial conducted by the AIDS Vaccine Evaluation Group (AVEG). The trial (AVEG 104) enrolled 26 HIV-infected pregnant U.S. women in a placebo-controlled study to evaluate the safety and immunogenicity of an MN rgp120 subunit vaccine adjuvanted with aluminum hydroxide (alum) (64). The vaccine was well tolerated with no local or systemic reactions in the mothers and no adverse outcomes in the infants attributable to the vaccine. While the small study size limited their ability to identify factors that might influence transmission, this study demonstrated that an HIV Env vaccine can be safely administered to HIV-infected pregnant women.
Yet, the authors had not assessed the ability of the vaccination regimen to induce maternal autologous virus neutralizing antibodies. In a recent follow-up study, we have found that while the vaccine increased MN gp120-specific binding responses, it failed to enhance maternal autologous virus neutralization responses (Permar unpublished data). Therefore, it is clear that more potent B cell stimulation will be required to achieve enhancement of autologous virus neutralization responses in HIV-infected pregnant women. However, elicitation of robust HIV Env-specific responses in HIV-infected pregnant women by vaccination comes with the potential risk of developing escape variants in maternal plasma that will be more fit for transmission to the infant. Thus, it will be important for vaccination studies to evaluate the impact of immunization on viral fitness and evolution. Additionally, timing of vaccine administration during pregnancy must be optimized in order to achieve maximal vaccine-elicited IgG levels prior to delivery that may be placentally transferred to the infant, thereby extending the window of protection into the breastfeeding period (65).
Conclusion
To eliminate MTCT and achieve a HIV-free generation, a combination of ART with active or passive immune-based strategies that enhance maternal immunity and provide protection to the HIV-exposed infant during the intrapartum and early breast‐feeding period will likely be necessary. To date, only limited studies have been conducted to evaluate the potential of immune-based interventions of pregnant women to prevent MTCT, and not enough effort has been employed to interrogate the impact of these interventions in resource poor settings, where MTCT is most common. Therefore, there is an urgent need to conduct both active and passive HIV immunization trials among pregnant and lactating women, particularly in the context of the widespread availability of ART. More importantly, further research to identify immune correlates of protection against HIV MTCT and address the influence of maternal neutralizing and non-neutralizing antibodies on transmission risk will guide the next generation of maternal HIV vaccine design to synergize with ART and eliminate the pediatric HIV epidemic.
Acknowledgments
S.R.P. is supported by grant R01AI122909 from the National Institutes of Health.
S.R.P. is a consultant for Merck, Sanofi, Moderna, and Pfizer CMV vaccine programs and has sponsored programs with Merck and Moderna for CMV vaccine development. All other authors have no conflicts of interest to disclose.
References
- 1.Pregnant Women, Infants and Children, CDC 2017. Available at: https://www.cdc.gov/hiv/group/gender/pregnantwomen/opt-out.html.
- 2.UNAIDS. CHILDREN AND HIV-Fact sheet 2016. Available at: https://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf.
- 3.UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet 2019. Available at: https://www.unaids.org/en/resources/fact-sheet.
- 4.Momplaisir FM, Brady KA, Fekete T, Thompson DR, Diez Roux A, Yehia BR. Time of HIV Diagnosis and Engagement in Prenatal Care Impact Virologic Outcomes of Pregnant Women with HIV. PLoS One. 2015;10:e0132262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngarina M, Popenoe R, Kilewo C, Biberfeld G, Ekstrom AM. Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Public Health. 2013;13:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourtis AP, Butera S, Ibegbu C, Belec L, Duerr A. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis. 2003;3:786–793. [DOI] [PubMed] [Google Scholar]
- 7.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. [DOI] [PubMed] [Google Scholar]
- 8.Fowler MG, Qin M, Fiscus SA, et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med. 2016;375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirt D, Urien S, Rey E, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirt D, Urien S, Ekouevi DK, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin Pharmacol Ther. 2009;85:182–189. [DOI] [PubMed] [Google Scholar]
- 11.Jeong H Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol. 2010;6:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemieniuk RAC, Lytvyn L, Mah Ming J, et al. Antiretroviral therapy in pregnant women living with HIV: a clinical practice guideline. BMJ. 2017;358:j3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lallemant M, Le Coeur S, Sirirungsi W, et al. Randomized noninferiority trial of two maternal single-dose nevirapine-sparing regimens to prevent perinatal HIV in Thailand. AIDS. 2015;29:2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–981. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Programmatic update: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 16.Kreitchmann R, Li SX, Melo VH, et al. Predictors of adverse pregnancy outcomes in women infected with HIV in Latin America and the Caribbean: a cohort study. BJOG. 2014;121:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dyke RB, Chadwick EG, Hazra R, Williams PL, Seage GR 3rd. The PHACS SMARTT Study: Assessment of the Safety of In Utero Exposure to Antiretroviral Drugs. Frontiers in immunology. 2016;7:199–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rough K, Seage GR 3rd, Williams PL, et al. Birth Outcomes for Pregnant Women with HIV Using Tenofovir-Emtricitabine. N Engl J Med. 2018;378:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramokolo V, Goga AE, Lombard C, Doherty T, Jackson DJ, Engebretsen IM. In Utero ART Exposure and Birth and Early Growth Outcomes Among HIV-Exposed Uninfected Infants Attending Immunization Services: Results From National PMTCT Surveillance, South Africa. Open Forum Infect Dis. 2017;4:ofx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zash R, Makhema J, Shapiro RL. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N Engl J Med. 2018;379:979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zash R, Holmes L, Diseko M, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N Engl J Med. 2019;381:827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zash RM. What will it take to refute the possible safety signal for dolutegravir and neural tube defects? Bjog. 2019;126:1346. [DOI] [PubMed] [Google Scholar]
- 23.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS. 2014;28:1049–1057. [DOI] [PubMed] [Google Scholar]
- 24.Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol. 2010;37:843–862, x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. J Acquir Immune Defic Syndr. 2018;77:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey JH, Marinda E, Mutasa K, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16:18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pursuing Later Treatment Options IIptftCoOHIVERE, Castro H, Judd A, et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet. 2011;377:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi P, Moschese V, Broliden PA, et al. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with the uninfected status of children born to seropositive mothers. Proceedings of the National Academy of Sciences. 1989;86:8055–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broliden PA, Moschese V, Ljunggren K, et al. Diagnostic implication of specific immunoglobulin G patterns of children born to HIV-infected mothers. AIDS (London, England). 1989;3:577–582. [DOI] [PubMed] [Google Scholar]
- 32.Permar SR, Fong Y, Vandergrift N, et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest. 2015;125:2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez DR, Vandergrift N, Douglas AO, et al. Maternal Binding and Neutralizing IgG Responses Targeting the C-Terminal Region of the V3 Loop Are Predictive of Reduced Peripartum HIV-1 Transmission Risk. J Virol. 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diomede L, Nyoka S, Pastori C, et al. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J Virol. 2012;86:4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naiman NE, Slyker J, Nduati R, Overbaugh JM. Maternal envelope gp41 ectodomain-specific antibodies are associated with increased mother-to-child transmission of HIV-1. J Infect Dis. 2019. [DOI] [PMC free article] [PubMed]
- 36.Guevara H, Casseb J, Zijenah LS, et al. Maternal HIV-1 antibody and vertical transmission in subtype C virus infection. Journal of acquired immune deficiency syndromes (1999). 2002;29:435–440. [DOI] [PubMed] [Google Scholar]
- 37.Markham RB, Gomez J, Halsey NA, et al. Maternal IgG1 and IgA antibody to V3 loop consensus sequence and maternal-infant HIV-1 transmission. The Lancet. 1994;343:390–391. [DOI] [PubMed] [Google Scholar]
- 38.Pancino G, Leste-Lasserre T, Burgard M, et al. Apparent enhancement of perinatal transmission of human immunodeficiency virus type 1 by high maternal anti-gp160 antibody titer. The Journal of infectious diseases. 1998;177:1737–1741. [DOI] [PubMed] [Google Scholar]
- 39.Mutucumarana CP, Eudailey J, McGuire EP, et al. Maternal Humoral Immune Correlates of Peripartum Transmission of Clade C HIV-1 in the Setting of Peripartum Antiretrovirals. Clin Vaccine Immunol. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad N, Baroudy BM, Baker RC, Chappey C. Genetic analysis of human immunodeficiency virus type 1 envelope V3 region isolates from mothers and infants after perinatal transmission. J Virol. 1995;69:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Parast AB, Richardson BA, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80:6525–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Smith CEP, Giorgi EE, et al. Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma. PLoS Pathog. 2018;14:e1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milligan C, Omenda MM, Chohan V, et al. Maternal Neutralization-Resistant Virus Variants Do Not Predict Infant HIV Infection Risk. MBio. 2016;7:e02221–02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghulam-Smith M, Olson A, White LF, et al. Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity. MBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez DR, Kumar A, Tu JJ, Mangold JF, Mangan RJ, Goswami R, Giorgi EE, Chen J, Mengual M, Douglas AO, Heimsath H, Saunders K Nicely NI, Eudailey J, Hernandez G, Morgan-Asiedu PK Wiehe K LaBranche C. Montefiori DC, Gao F, Permar SR Maternal Broadly Neutralizing Antibodies Select for Neutralization-Resistant Infant Transmitted/Founder HIV Variants. CELL-REPORTS-D-19–01671. 2019. [DOI] [PMC free article] [PubMed]
- 47.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8:e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollara J, McGuire E, Fouda GG, et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J Virol. 2015;89:9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fouda GG, Permar SR. Immune-based interventions to prevent postnatal HIV-1 transmission. Trends in microbiology. 2014;22:425–427. [DOI] [PubMed] [Google Scholar]
- 51.Habte HH, de Beer C, Lotz ZE, Tyler MG, Kahn D, Mall AS. Inhibition of human immunodeficiency virus type 1 activity by purified human breast milk mucin (MUC1) in an inhibition assay. Neonatology. 2008;93:162–170. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn L, Trabattoni D, Kankasa C, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. Journal of acquired immune deficiency syndromes (1999). 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 53.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. The Journal of clinical investigation. 1995;96:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmsen MC, Swart PJ, de Béthune MP, et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. The Journal of infectious diseases. 1995;172:380–388. [DOI] [PubMed] [Google Scholar]
- 55.Villamor E, Koulinska IN, Furtado J, et al. Long-chain n-6 polyunsaturated fatty acids in breast milk decrease the risk of HIV transmission through breastfeeding. The American journal of clinical nutrition. 2007;86:682–689. [DOI] [PubMed] [Google Scholar]
- 56.Walter J, Ghosh MK, Kuhn L, et al. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. The Journal of infectious diseases. 2009;200:1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fouda GG, Jaeger FH, Amos JD, et al. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18220–18225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mangan RJ, Stamper L, Ohashi T, et al. Determinants of Tenascin-C and HIV-1 envelope binding and neutralization. Mucosal immunology. 2019;12:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Rompay KK, Berardi CJ, Dillard-Telm S, et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–1259. [DOI] [PubMed] [Google Scholar]
- 60.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. [DOI] [PubMed] [Google Scholar]
- 61.Hessell AJ, Jaworski JP, Epson E, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med. 2016;22:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiehm ER, Lambert JS, Mofenson LM, et al. Efficacy of zidovudine and human immunodeficiency virus (HIV) hyperimmune immunoglobulin for reducing perinatal HIV transmission from HIV-infected women with advanced disease: results of Pediatric AIDS Clinical Trials Group protocol 185. J Infect Dis. 1999;179:567–575. [DOI] [PubMed] [Google Scholar]
- 63.Onyango-Makumbi C, Omer SB, Mubiru M, et al. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY). J Acquir Immune Defic Syndr. 2011;58:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright PF, Lambert JS, Gorse GJ, et al. Immunization with envelope MN rgp120 vaccine in human immunodeficiency virus-infected pregnant women. J Infect Dis. 1999;180:1080–1088. [DOI] [PubMed] [Google Scholar]
- 65.Eudailey JA, Dennis ML, Parker ME, et al. Maternal HIV-1 Env Vaccination for Systemic and Breast Milk Immunity To Prevent Oral SHIV Acquisition in Infant Macaques. mSphere. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]