Abstract
Background
After recovering from coronavirus disease 2019 (COVID-19), a considerable number of patients report long-term sequelae. The epidemiologic data vary widely in the studies published to date, depending on the study design and the patient cohorts analyzed. Using a population-based approach, we report symptoms and clinical characteristics following COVID-19 (long COVID), focusing on symptoms ≥ 12 weeks (post-COVID-19).
Methods
In three German administrative districts, all adult patients with a diagnosis of COVID-19 confirmed by polymerase chain reaction (PCR) between March and September 2020 (n = 4632) were invited to complete a questionnaire. Predictors for post-COVID-19 were identified by multiple ordinal regression analysis. Study registration: DRKS00023069.
Results
A total of 1459 patients were included in the study, 175 (12%) of whom had been hospitalized for treatment of the acute phase of COVID-19. The prevalence of post-COVID-19 was 72.6% (n = 127) and 46.2% (n = 588) for hospitalized and non-hospitalized patients, respectively. The most frequently occurring long-term symptoms were fatigue (41.5% of all symptoms ≥ 12 weeks, n = 297), physical exhaustion (40.8%, n = 292), difficulty in concentrating (30.6%, n = 219), and loss of the senses of taste (25.9%, n = 185) and smell (25.5%, n = 182). Quality of life was significantly impaired in patients with post-COVID-19. The strongest risk factors for post-COVID-19 were female sex, overall severity of comorbidities, and severity of acute COVID-19.
Conclusion
Patients who are not hospitalized also frequently experience continued symptoms following COVID-19. The heterogeneity of symptoms calls for a multidisciplinary stepped-care approach, for which identification of patients at risk is crucial. A limitation of the study is the lack of a control group.
A considerable proportion of patients infected with the novel coronavirus-2 (SARS-CoV-2) continue to experience symptoms after recovery from the acute phase of coronavirus disease 2019 (COVID-19). These long-term conditions manifest in a wide range of symptoms, such as fatigue, dyspnea, and cognitive dysfunction (1– 4). There is ongoing discussion on the definition and terminology of sequelae after COVID-19 (long COVID). In the present study, the term post-COVID-19 refers to all symptoms persisting at least 12 weeks after COVID-19, irrespective of the etiology. The 12-week duration was defined by the National Institute for Health and Care Excellence (NICE) and a consensus conference of the World Health Organization (WHO), and has been adopted in the the German clinical practice guideline (5– 7).
The reported prevalences differ by study design, the definition of post-COVID-19 applied, and the sample analyzed (8). The literature and systematic reviews report a prevalence of 33–87% in cohorts hospitalized for treatment of acute COVID-19, whereas studies with non-hospitalized patients report lower prevalences of between 2% and 53% (1, 9, 10). However, most studies have investigated small (11, 12) or non-representative samples (13, 14) or included participants without positive SARS-CoV-2 test results (15). Due to the heterogeneity in the existing evidence, the Long-COVID Forum and other groups recently defined as a research priority the identification of clinical characteristics, comorbidities, and potential risk factors of post-COVID-19, particularly in non-hospitalized or population-based cohorts (3, 16, 17).
The aims of this study were:
To report the prevalence of symptoms following COVID-19
To describe the clinical characteristics
To identify potential predictive factors in a population-based retrospective cohort in Germany
Methods
The study was conducted as a total population survey in three German administrative districts (Reutlingen, Tübingen, Enzkreis) with a total of 715 268 inhabitants. In Germany, every person who tests positive for infection with SARS-CoV-2 is registered by the local health office. These offices sent out a questionnaire to all adult patients with a positive polymerase chain reaction (PCR) test between 1 March and 30 September 2020 (n = 4632).
We asked participants retrospectively to recall the symptoms they experienced due to COVID-19 and to specify symptom duration. Based on the NICE guideline, patients were divided into the following groups:
Acute COVID-19 (symptoms for 0–4 weeks)
Ongoing COVID-19 (4–12 weeks)
Post-COVID-19 (≥ 12 weeks)
For reasons of clarity, patients without symptoms and symptoms < 12 weeks are presented in the same column of the relevant Tables. For the multiple ordinal logistic regression, we combined participants with acute and ongoing COVID-19 into one category, resulting in an outcome variable with three categories (no symptoms, symptom duration < 12 weeks, symptom duration ≥ 12 weeks). Goodness of fit was assessed by means of a likelihood ratio test comparing the fitted model to a model with varying location parameters. We conducted multiple imputation to replace missing values. Backward selection was used to select variables, taking account of p-values between 0.05 and 0.10. Age and sex were included in all models. More detail can be found in the eMethods.
Results
Study population
By 31 January 2021, a total of 1907 questionnaires (41%) had been returned. Of those, 448 were excluded (eMethods), so the final study population comprised 1459 participants.
The median age of the participants was 53 years (interquartile range [IQR] 37–62); n = 824 (56.5%) of the participants were women. This is in line with the total 4632 confirmed COVID-19 cases in the districts (median age 48 years, 52.7% women; eTable 1). The mean duration of follow-up was 219 days (standard deviation [SD] 32.6). A total of 175 (12%) participants received hospital treatment for COVID-19. Further details are shown in Table 1.
eTable 1. Confirmed cases of SARS-CoV-2 in the participating districts from 1 March to 30 September 2020, age 18 years or older*1.
| Administrative district (inhabitants) | Total number of cases | Returned questionnaires | Gender | Age (years), n | Reported deaths among positive cases | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90–99 | 100+ | |||||
|
Tübingen (228 678) |
Female | 10 | 155 | 105 | 122 | 163 | 91 | 51 | 75 | 34 | 2 | |||
| Male | 13 | 129 | 105 | 74 | 148 | 83 | 52 | 54 | 9 | 0 | ||||
| Other | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 1476*2 | 756 (51.2%) | 23 | 285 | 210 | 196 | 311 | 174 | 103 | 129 | 43 | 2 | 23 | ||
|
Enzkreis (199 556) |
Female | 8 | 126 | 101 | 121 | 125 | 56 | 34 | 48 | 29 | 1 | |||
| Male | 17 | 162 | 124 | 109 | 124 | 52 | 27 | 22 | 4 | 0 | ||||
| Other | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 1292*3 | 423 (32.7%) | 25 | 289 | 225 | 231 | 249 | 108 | 61 | 70 | 33 | 1 | 60 | ||
|
Reutlingen*5 (287 034) |
1781*4 | 728 (40.9%) | 334 | 227 | 240 | 385 | 218 | 135 | 170 | 72 | 82 | |||
*1 All 4632 persons were contacted by mail and invited to participate in the study. Reported deaths during this period by district (e8). Numbers of inhabitants as of 31 December 2019 (www.wikipedia.org).
*2 Including 73 letters that were returned undelivered
*3 Excluding 83 letters that were returned undelivered
*4 Including 84 letters that were returned undelivered
*5 No information on gender available
Table 1. Sociodemographic data, lifestyle factors and health-related quality of life of the study population (n = 1459).
|
Total study
population |
p-value*1 |
Post-COVID-19 (symptom duration ≥ 12 weeks) |
p-value*1 | |||
| No symptoms/ symptoms < 12 weeksn = 739 |
Symptoms
≥ 12 weeks (post-COVID-19) n = 720 |
Not hospitalized n = 588 | Hospitalized n = 127 | |||
| Age (years) | ||||||
| Median (IQR) | 50 (33–60) | 54 (42–64) | < 0.001*2 | 52 (37–61) | 69 (57–80) | < 0.001*2 |
| Sex | ||||||
| Male | 361 (48.8) | 226 (36.1) | < 0.001 | 196 (33.3) | 62 (48.8) | 0.004*3 |
| Female | 369 (49.9) | 455 (63.2) | 388 (66.0) | 65 (51.2) | ||
| Missing value | 9 (1.2) | 5 (0.7) | 4 (0.7) | 0 (0) | ||
| Education level | ||||||
| No qualifications | 16 (2.2) | 8 (1.1) | < 0.001 | 3 (0.5) | 5 (3.9) | < 0.001*3 |
| School certificate | 211 (28.6) | 179 (24.9) | 142 (24.1) | 36 (28.3) | ||
| Vocational training diploma | 243 (32.9) | 290 (40.3) | 228 (38.8) | 61 (48.0) | ||
| University degree | 261 (35.3) | 233 (32.4) | 207 (35.2) | 24 (18.9) | ||
| Missing value | 8 (1.1) | 10 (1.4) | 8 (1.4) | 1 (0.8) | ||
| Administrative district | ||||||
| Enzkreis | 166 (22.5) | 147 (20.4) | 0.608 | 108 (18.4) | 38 (29.9) | 0.012 |
| Reutlingen | 267 (36.1) | 272 (37.8) | 231 (39.3) | 40 (31.5) | ||
| Tübingen | 306 (41.4) | 301 (41.8) | 249 (42.3) | 49 (38.6) | ||
| Smoking status | ||||||
| Current smoker or ex- smoker | 470 (63.6) | 424 (57.5) | 0.044 | 220 (37.4) | 60 (47.2) | 0.004 |
| Never smoker | 245 (33.2) | 284 (39.4) | 354 (60.2) | 59 (46.5) | ||
| Missing value | 24 (3.2) | 22 (3.1) | 14 (2.4) | 8 (6.3) | ||
| diet | ||||||
| Conventional | 661 (89.4) | 634 (88.1) | 0.652 | 511 (86.9) | 119 (93.7) | 0.074*3 |
| Vegetarian/vegan | 67 (9.1) | 72 (10.0) | 65 (11.1) | 6 (4.7) | ||
| Missing value | 11 (1.5) | 14 (1.9) | 12 (2.0) | 2 (1.6) | ||
| Nursing home resident | ||||||
| Yes | 34 (4.6) | 13 (1.8) | 0.010 | 9 (1.5) | 4 (3.1) | 0.414*3 |
| No | 697 (94.3) | 700 (97.2) | 574 (97.6) | 122 (96.1) | ||
| Missing value | 14 (1.1) | 7 (1.0) | 5 (0.9) | 1 (0.8) | ||
| Cumulative duration of sick leave (days) | ||||||
| Median (IQR) | 14 (14–20) | 20 (14–28) | < 0.001*2 | 18 (14–26) | 45 (28–77) | < 0.001*2 |
| Health-related quality of life | ||||||
| EQ5D-5L, median (IQR) | 1.00 (0.94–1.00) | 0.93 (0.84–1.00) | < 0.001*2 | 0.94 (0.87–1.0) | 0.85 (0.62–0.92) | < 0.001*2 |
| EQ5D-VAS, median (IQR) | 90 (80–95) | 80 (65–85) | < 0.001*2 | 80 (70–90) | 70 (50–75) | < 0.001*2 |
Absolute and relative frequencies are presented as n (%) unless otherwise indicated.
Information on hospitalization was missing for five participants in the post-COVID-19 group.
EQ5D-5L, EuroQoL 5-dimension 5-level; EQ5D-VAS, EuroQoL 5-dimension visual analog scale; IQR, interquartile range
*1 Chi-square tests were used to calculate p-values unless otherwise indicated.
*2 Mann–Whitney U test.; *3 Fisher’s exact test.
Prevalence of symptoms following COVID-19
No symptoms during or after COVID-19 were reported by 8.3% (n = 121), while 33.0% (n = 481) had at least one COVID-19-related symptom for < 4 weeks (acute COVID-19), 9.4% (n = 137) had at least one symptom that lasted for 4 to < 12 weeks (ongoing COVID-19), and 49.3% (n = 720) had at least one symptom ≥ 12 weeks after the infection (post-COVID-19). Among hospitalized and non-hospitalized participants, the prevalence of post-COVID-19 was 72.6% (n = 127) and 46.2% (n = 588), respectively.
The most frequently occurring symptoms among patients with post-COVID-19 were:
Fatigue or lethargy (41.5%, n = 297)
Physical exhaustion (40.8%, n = 292)
Difficulty concentrating (30.6%, n = 219)
Loss of sense of taste (25.9%, n = 185)
Loss of sense of smell (25.5%, n = 182)
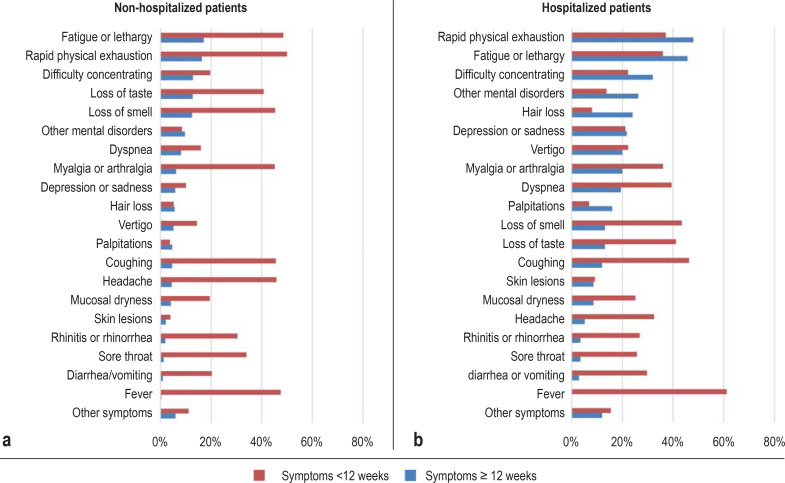
Figure 1 displays the percentage occurrence of all symptoms reported for a duration of < 12 weeks or ≥ 12 weeks.
Figure.
Prevalence of symptoms following COVID-19 in a population-based cohort. Proportions of participants who had the respective symptom for less than 12 weeks (acute and ongoing COVID-19) or for at least 12 weeks (post-COVID-19) after the infection. a) symptoms of non-hospitalized patients (n = 1273); b) symptoms of hospitalized patients (n = 175)
Table 2 shows the post-COVID-19 symptoms with immediate onset (within the first 2 weeks of the infection) versus delayed onset (at least 2 weeks after infection). Most symptoms were present from the beginning of the infection. The only symptoms that often had delayed onset were skin lesions and hair loss.
Table 2. Time of onset of symptoms*.
| Post-COVID-19 (symptoms ≥12 weeks after infection) | ||||
| Not hospitalized n = 588 | Hospitalized n = 127 | |||
| Immediate onset n (%) | Delayed onset n (%) | Immediate onset n (%) | Delayed onset n (%) | |
| Fever | 4 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea/vomiting | 8 (1.3) | 3 (0.5) | 4 (3.1) | 1 (0.8) |
| Sore throat | 15 (2.6) | 0 (0) | 5 (3.9) | 1 (0.8) |
| Rhinitis or rhinorrhea | 23 (3.9) | 1 (0.2) | 6 (4.7) | 0 (0) |
| Skin lesions | 12 (2.0) | 14 (2.3) | 6 (4.7) | 9 (7.0) |
| Headache | 45 (7,7) | 11 (1.9) | 7 (5.5) | 2 (1.6) |
| Mucosal dryness | 36 (6.1) | 13 (2.2) | 10 (7.8) | 5 (3.9) |
| Coughing | 51 (8.7) | 6 (1.0) | 19 (15.0) | 2 (1.6) |
| Palpitations | 32 (5.4) | 27 (4.6) | 21 (16.5) | 7 (5.5) |
| Vertigo | 42 (7.1) | 22 (3.7) | 24 (18.9) | 11 (8.7) |
| Depression or sadness | 38 (6.4) | 36 (6.1) | 24 (18.9) | 14 (11.0) |
| Other mental disorders | 72 (12.2) | 50 (8.5) | 30 (23.6) | 15 (11.8) |
| Myalgia or arthralgia | 65 (11.0) | 13 (2.2) | 25 (19.7) | 10 (7.8) |
| Hair loss | 17 (2.9) | 54 (9.2) | 14 (11.0) | 28 (22.0) |
| Dyspnea | 60 (10.2) | 42 (7.1) | 29 (22.8) | 5 (3.9) |
| Loss of taste | 133 (22.6) | 29 (4.9) | 16 (12.5) | 7 (5.5) |
| Loss of smell | 124 (21.1) | 35 (5.9) | 20 (15.7) | 3 (2.3) |
| Difficulty concentrating | 95 (16.1) | 68 (11.6) | 44 (34.6) | 12 (9.4) |
| Fatigue or lethargy | 174 (29.6) | 41 (7.0) | 68 (53.5) | 11 (8.6) |
| Rapid physical exhaustion | 170 (28.9) | 35 (5.9) | 72 (56.7) | 11 (8.6) |
*Symptoms with immediate onset (within 2 weeks of infection) or late onset (at least 2 weeks after infection) in non-hospitalized and hospitalized participants with symptoms persisting for at least 12 weeks (post-COVID-19).
Characteristics of patients with post-COVID-19
Patients with post-COVID-19 were older, more often female, had a significantly lower health-related quality of life (HrQoL), and had more days of sick leave (table 1). The overall burden of comorbidity correlated with a higher risk of post-COVID-19, as did most of the specific chronic conditions such as hypertension, heart and lung disease, and depression (Table 3, eTable 2). Polypharmacy and intake of most of the long-term medications also correlated with a higher risk of post-COVID-19 (etable 3).
Table 3. Comorbidities.
| Total study population | p-value*1 | Post-COVID-19 (symptom duration ≥ 12 weeks) | p-value*1 | |||
| No symptoms/ symptoms < 12 weeks n = 739 | Symptoms ≥ 12 weeks(post-COVID-19) n = 720 | Not hospitalized n = 588 | Hospitalized n=127 | |||
| Body mass index (kg/m2), median (IQR) | 25 (22–28) | 25 (22–29) | 0.646*2 | 25 (22–28) | 27 (24–31) | < 0.001*2 |
| SCQ-D score, median (IQR) | 2 (0–3.25) | 3 (1–6) | < 0.001*2 | 2.37 (0–5) | 6 (4–9) | < 0.001*2 |
| Heart problems | 69 (9.3) | 116 (16.1) | < 0.001 | 65 (11.1) | 51 (40.2) | < 0.001 |
| Hypertension | 174 (23.5) | 222 (30.8) | 0.002 | 148 (25.2) | 74 (58.3) | < 0.001 |
| Lung problems | 58 (7.8) | 114 (15.8) | < 0.001 | 72 (12.2) | 41 (32.3) | < 0.001 |
| Diabetes/blood sugar | 37 (5.0) | 63 (8.8) | 0.007 | 34 (5.8) | 29 (22.8) | < 0.001 |
| Gastrointestinal problems | 62 (8.4) | 108 (15.0) | < 0.001 | 83 (14.1) | 25 (19.7) | 0.126 |
| Kidney problems | 17 (2.3) | 27 (3.8) | 0.126 | 9 (1.5) | 18 (14.2) | < 0.001 |
| Liver problems | 3 (0.4) | 14 (1.9) | 0.007 | 7 (1.2) | 7 (5.5) | < 0.003 |
| Anemia/other blood problems | 26 (3.5) | 37 (5.1) | 0.156 | 25 (4.3) | 12 (9.4) | 0.026 |
| Cancer | 36 (4.9) | 38 (5.3) | 0.812 | 25 (4.3) | 13 (10.2) | 0.020 |
| Depression | 48 (6.5) | 85 (11.8) | < 0.001 | 63 (10.7) | 22 (17.3) | 0.027 |
| Osteoarthritis | 91 (12.3) | 167 (23.2) | < 0.001 | 117 (19.9) | 49 (38.6) | < 0.001 |
| Back pain | 191 (25.8) | 283 (39.3) | < 0.001 | 219 (37.2) | 63 (49.6) | < 0.001 |
| Rheumatism | 19 (2.6) | 39 (5.4) | 0.007 | 26 (4.4) | 13 (10.2) | 0.017 |
| Polypharmacy*3 | 30 (4.1) | 87 (12.1) | < 0.001 | 48 (8.2) | 39 (30.7) | < 0.001 |
Absolute and relative frequencies are presented as n (%) unless otherwise indicated. Information on hospitalization was missing for five patients in the post-COVID-19 group.
IQR, Interquartile range; SCQ-D, Self-Administered Comorbidity Questionnaire, German version
*1 Chi-square tests were used to calculate p-values unless otherwise indicated.
*2 Mann–Whitney U test
*3 Polypharmacy was defined as five or more medications taken on a regular basis.
eTable 2. Additional chronic conditions that were stated in the optional text boxes.
| Total study population | p-value*1 | Post-COVID-19 (symptom duration ≥ 12 weeks) | p-value*1 | |||
| No symptoms/ symptoms < 12 weeks n = 739 | Symptoms ≥ 12 weeks (post-COVID-19) n = 720 | Not hospitalized n = 588 | Hospitalized n = 127 | |||
| Allergies | 16 (2.2) | 16 (2.2) | 1.0 | 16 (2.7) | 0 (0) | 0.090*2 |
| Thyroid disorder | 20 (2.7) | 42 (5.8) | 0.004 | 36 (6.1) | 6 (4.7) | 0.679 |
| Skin lesions | 5 (0.7) | 17 (2.4) | 0.009 | 15 (2.6) | 1 (0.8) | 0.329*2 |
| Orthopedic symptoms | 11 (1.5) | 18 (2.5) | 0.191 | 12 (2.0) | 6 (4.7) | 0.110*2 |
| Neurological symptoms | 7 (0.9) | 9 (1.3) | 0.623 | 5 (0.9) | 4 (3.1) | 0.058*2 |
| Headache | 8 (1.1) | 11 (1.5) | 0.453 | 11 (1.9) | 0 (0) | 0.228*2 |
| Other chronic conditions | 28 (3.8) | 30 (4.2) | 0.789 | 21 (3.6) | 9 (7.1) | 0.086 |
Absolute and relative frequencies are presented as n (%) unless otherwise indicated. Information on hospitalization was missing for five patients in the post-COVID-19 group.
*1 Chi-square tests were used to calculate p-values unless otherwise indicated. *2 Fisher’s exact test.
eTable 3. Medications taken on a regular basis (for at least 12 months).
| Total study population | p-value*1 | Post-COVID-19 (Symptom duration ≥ 12 weeks) | p-value*1 | |||
| No symptoms/ symptoms < 12 weeks n = 739 | Symptoms ≥ 12 weeks(post-COVID-19) n = 720 | Not hospitalized n = 588 | Hospitalized n = 127 | |||
| ACE-I and ARB | 108 (14.6) | 172 (23.9) | < 0.001 | 108 (18.4) | 63 (49.6) | < 0.001 |
| Other antihypertensives | 118 (16.0) | 159 (22.1) | 0.011 | 98 (16.7) | 61 (48.0) | < 0.001 |
| Metformin | 20 (2.7) | 40 (5.6) | 0.008 | 21 (3.6) | 19 (15.0) | < 0.001 |
| Other antidiabetics | 18 (2.4) | 30 (4.2) | 0.077 | 16 (2.7) | 14 (11.0) | < 0.001 |
| Diuretics | 51 (6.9) | 79 (11.0) | 0.023 | 39 (6.5) | 41 (32.3) | < 0.001 |
| Lung inhalers | 33 (4.5) | 71 (9.9) | < 0.001 | 47 (8.0) | 22 (17.3) | < 0.001 |
| Cholesterol-reducing drugs | 53 (7.2) | 90 (12.5) | 0.002 | 53 (9.0) | 37 (29.1) | < 0.001 |
| ASA 100 | 51 (6.9) | 83 (11.5) | 0.006 | 49 (8.3) | 33 (26.0) | < 0.001 |
| Anticoagulants | 36 (4.9) | 44 (6.1) | 0.584 | 16 (2.7) | 28 (22.0) | < 0.001 |
| Opiates and morphines | 11 (1.5) | 16 (2.2) | 0.498 | 9 (1.5) | 7 (5.5) | < 0.001 |
| Other analgesics | 59 (8.0) | 83 (11.5) | 0.041 | 58 (9.9) | 25 (19.7) | < 0.001 |
| Vitamin D | 104 (14.1) | 157 (21.8) | < 0.001 | 118 (20.1) | 38 (29.9) | 0.018 |
| Antidepressants*3 | 12 (1.6) | 38 (5.3) | < 0.001 | 28 (4.8) | 10 (7.9) | 0.156 |
| Thyroid medications*3 | 68 (9.2) | 119 (15.3) | < 0.001 | 93 (15.8) | 16 (12.6) | 0.360 |
| Homeopathic preparations*3 | 7 (0.9) | 19 (2.6) | 0.015 | 14 (2.4) | 5 (3.9) | 0.323 |
| Proton pump inhibitors*3 | 15 (2.0) | 25 (3.5) | 0.092 | 20 (3.4) | 5 (3.9) | 0.766 |
| Contraceptives*3 | 16 (2.2) | 21 (2.9) | 0.407 | 20 (3.4) | 1 (0.8) | 0.149*2 |
| Vitamins and supplements*3 | 26 (3.5) | 35 (4.9) | 0.200 | 27 (4.6) | 8 (6.3) | 0.419 |
| Other medications*3 | 57 (7.7) | 86 (11.9) | 0.007 | 59 (10.0) | 27 (21.3) | < 0.001 |
Absolute and relative frequencies are presented as n (%). Information on hospitalization was missing for five patients in the post-COVID-19 group.
ACE-I, Angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; ASA 100, acetylsalicylic acid 100 milligram
*1 Chi-square tests were used to calculate p-values unless otherwise indicated. *2 Fisher’s exact test. *3 Information from the optional text boxes
Patients with post-COVID-19 more often took medication such as antibiotics, anticoagulants, or analgesics during the acute phase of acute COVID-19 (etable 4).
eTable 4. Treatment during the acute phase of COVID-19.
| Total study population | p-value*1 | Post-COVID-19 (Symptom duration ≥ 12 Wochen) | p-value*1 | |||
| No symptoms/ symptoms < 12 weeks n = 739 | Symptoms ≥ 12 weeks(post-COVID-19) n = 720 | Not hospitalized n = 588 | Hospitalized n = 127 | |||
| Hospitalization for acute COVID-19 | ||||||
| Not hospitalized | 685 (92.7) | 588 (81.7) | < 0.001 | 588 | n. a. | n.d. |
| Hospitalized*2 | 48 (6.5) | 127 (17.6) | n.d. | 127 | ||
| Intensive care unit | 6 (0.8) | 25 (3.5) | < 0.001 | n.d. | 25 | |
| Mechanical ventilation | 5 (0.7) | 20 (2.8) | n.d. | 20 | ||
| Medication for acute Covid-19 | ||||||
| No | 576 (77.9) | 412 (57.2) | < 0.001 | 386 (65.6) | 21 (16.7) | < 0.001 |
| Yes | 156 (21.1) | 295 (41.0) | 196 (33.3) | 99 (78.0) | ||
| Antibiotics | 54 (7.3) | 100 (13.9) | < 0.001 | 43 (7.3) | 57 (44.9) | < 0.001 |
| Zinc/selenium/vitamin D | 25 (3.4) | 61 (8.5) | < 0.001 | 49 (8.3) | 12 (9.4) | 0.726 |
| Anticoagulation | 20 (2.7) | 63 (8.8) | < 0.001 | 22 (3.7) | 41 (32.3) | < 0.001 |
| Cortisone | 9 (1.2) | 39 (5.4) | < 0.001 | 26 (4.4) | 13 (10.2) | 0.012 |
| Analgesics *3 | 58 (7.8) | 85 (11.8) | 0.013 | 75 (12.8) | 10 (7.9) | 0.133 |
| Common cold remedies *3 | 10 (1.4) | 18 (2.5) | 0.128 | 16 (2.7) | 2 (1.6) | 0.554*4 |
| Homeopathic preparations *3 | 3 (0.4) | 3 (0.4) | 1.000*4 | 3 (0.5) | 0 (0) | 1.000*4 |
| Other vitamins/trace elements *3 | 5 (0.7) | 10 (1.4) | 0.202 | 10 (1.7) | 0 (0) | 0.223*4 |
| Lung inhalers *3 | 5 (0.7) | 18 (2.5) | 0.006 | 15 (2.6) | 3 (2.4) | 1.000*4 |
| Others *3 | 17 (2.3) | 41 (5.7) | < 0.001 | 24 (4.1) | 17 (13.4) | < 0.001 |
Absolute and relative frequencies are presented as n (%). Information on hospitalization was missing for five patients in the post-COVID-19 group.
*1 The chi-square test was used to calculate p-values unless otherwise indicated. *2 Including intensive care unit and mechanical ventilation.
*3 Information from the optional text boxes.
*4 Fisher’s exact test
n.a., not applicable
Predictors of post-COVID-19
Univariate ordinal logistic regression identified only a small number of variables that proved to be potential predictors for long-term sequelae (table 4). The strongest predictors for post-COVID-19 identified here were:
Table 4. Parsimonious model of the multiple ordinal logistic regression*1.
| Level | OR | [95% CI] | p-value | |
| Age | Continuous | 1.003 | [0.996; 1.010] | 0.45 |
| Sex | Female | 1.805 | [1.454; 2.241] | < 0.001 |
| Male | Reference | |||
| Education level*2 | Metric | 1.147 | [1.057; 1.245] | 0.001 |
| Nursing home resident | Yes | 0.087 | [0.046; 0.165] | < 0.001 |
| No | Reference | |||
| SCQ-D*3 | Continuous | 1.096 | [1.058; 1.137] | < 0.001 |
| Hospitalized during acute COVID-19*4 | Yes | 2.014 | [1.538; 2.637] | < 0.001 |
| No | Reference | |||
| Need for analgesics during acute COVID-19*5 | Yes | 1.704 | [1.186; 2.446] | 0.004 |
| No | Reference | |||
*1 Parsimonious model after stepwise backward selection of the potential predicting variables for post-COVID-19. The outcome variable comprised three categories (1, no symptoms; 2, symptom duration < 12 weeks; 3, symptom duration = 12 weeks) with post-COVID-19 (symptom duration ≥ 12 weeks) as response (n = 1459; imputed data set with 500 imputations).
*2 Only one parameter was estimated in a linear coding of the six different educational levels.
*3 Interpretation of the OR: with each increase of one point on the 45-point scale of the SCQ-D, the likelihood of developing post-COVID-19 rises by 9.6%. In other words, the higher the overall burden of ?comorbidities (measured on the SCQ-D), the greater the likelihood of developing post-COVID-19.
*4 Interpretation of the OR: Study participants who had to be hospitalized for treatment during the acute phase of COVID-19 have a 2.01 times greater likelihood of developing post-COVID-19 than those who were not hospitalized. In other words, their risk of developing post-COVID-19 is 101% higher.
*5 Interpretation of the OR: Study participants who had to take analgesics during the acute phase of COVID-19 had a 1.7 times greater likelihood of developing p ost-COVID-19 than those who did not have to take analgesics. In other words, their risk of developing post-COVID-19 is 70% higher. This does not mean that the consumption of analgesics causes the higher risk; rather, it is a surrogate for the severity of the acute disease.
95% CI, 95% confidence interval; OR, odds ratio;
SCQ-D, Self-Administered Comorbidity Questionnaire, German version
Female sex (odds ratio [OR] 1.81, 95% confidence interval [1.45; 2.24])
The overall burden of comorbidity with an OR of 1.10 per point in the Self-Administered Comorbidity Questionnaire (SCQ-D; maximum 45 points) [1.06; 1.14)
Hospitalization for acute COVID-19 (OR 2.01 [1.54; 2.64)
The need for analgesics during acute COVID-19 (OR 1.70 [1.19; 2.45)
The last two points are surrogates for a severe course of the acute infection.
Nursing home residents had a lower risk of post-COVID-19 (OR 0.09 [0.05; 0.17]). No positive or negative association was found for smoking, specific comorbidities, regular medication, other specific medication for COVID-19, polypharmacy, or age.
Discussion
Prevalence and symptoms
The present study is one of the largest so far to investigate the prevalence of symptoms following COVID-19 in a population-based cohort. A total of 1273 (87.3%) participants were not hospitalized for treatment of their acute COVID-19.
While previous studies mostly described mixed cohorts with varying proportions of non-hospitalized and hospitalized participants, we present data for non-hospitalized and hospitalized patients separately. At least one post-COVID-19 symptom was reported by 46.2% (n = 588) of the non-hospitalized and 72.6% (n = 127) of the hospitalized patients. Compared with previous smaller studies that were also based on the NICE and WHO definition of post-COVID-19, there is good agreement (e1– e4).
The most frequently mentioned symptoms in the post-COVID-19 group were fatigue or lethargy (n = 297, 41.5%) and rapid physical exhaustion (n = 292, 40.8%), followed by difficulty concentrating (n = 219, 30.6%). Although the reported frequency of these symptoms varies very widely between studies, recent reviews confirm that fatigue is a predominant symptom (2, 3). Considering the heterogeneity of symptoms, however, it should be discussed whether these conditions represent phenotypes of one and the same entity or whether they result from different pathophysiology. In contrast, loss of the sense of taste and/or smell is thought to be a rather specific symptoms of COVID-19 and was reported to persist ≥ 12 weeks by n = 185 (25.9%) and n = 182 (25.5%), respectively. In the general population, the self-reported frequency of problems with taste and smell is 0.4% and 1.6%, respectively (e5).
Considerations on pathophysiology
Most participants in our study reported that their long-term symptoms had been present since the beginning of the infection. However, skin lesions and hair loss were mostly reported with a delay in symptom onset. Whether these differences in time of onset are due to different pathophysiological causes is currently a matter for discussion. Persistence of symptoms from the onset could support the hypothesis of residual viral particles to which the organism reacts as in the acute phase (18, 19), while delayed symptom onset could indicate immunological dysregulation (20). Moreover, direct viral toxicity the severity of the acute illness might also influence the underlying pathophysiology, the latter potentially causing hair loss and skin lesions (21).
On the other hand, some symptoms following COVID-19 may not be specific for SARS-CoV-2 but could be caused by another etiology and/or triggered by social effects of the pandemic; this is particularly likely for fatigue and mental health problems (22, 23). Some conditions reported by hospitalized patients could also be caused by post-intensive care syndrome (PICS) or post-traumatic stress disorder (PTSD) following an ICU stay (e6, e7). The lack of a control group in this study means it is not possible to differentiate between post-COVID-19 and symptoms with other causes.
Implications for health care
The quality of life was lower in the post-COVID-19 group, particularly in hospitalized participants. This is consistent with previous reports (e1, e2). Periods of sick leave were longer in patients with post-COVID-19, presumably reflecting the disabling character of the symptoms. Studies from the USA and other countries report that 40% of patients had not returned to regular work by 60 days after discharge and that 22.3% were still not back to work after a median of 7 months (15, 24). Considering the still growing number of infections, this could have a major economic impact.
The heterogeneity of symptoms calls for multidisciplinary management, coordinated by primary care specialists (5, 25). Referrals to specialists should be limited to patients with specific needs (stepped-care approach). Local cross-specialty networks with multidisciplinary boards, supplemented by specialty units from the inpatient sector, could improve the care of these patients (26– 28). In addition, the identification of patients at risk for developing post-COVID-19 will help to set the course of treatment and develop structured management strategies.
Predictors of post-COVID-19
The regression analysis revealed that hospitalization for COVID-19 and the need for analgesics during the acute phase of COVID-19 were risk factors for post-COVID-19. This suggests an association of acute infection severity with the risk of developing post-COVID-19, as previously reported by other study groups (29, 30). It is therefore plausible that convalescence is prolonged in patients who were immobilized during the acute infection or had severe pulmonary pathology resulting in post-critical illness, persisting dyspnea, PTSD, or PICS (31– 33).
Another strong predictor for post-COVID-19 was the overall burden of comorbidity. Patients with post-COVID-19 were more likely to have chronic concomitant diseases, were more often on regular medication, and had a higher rate of polypharmacy. This is in line with other studies that also show an association between preexisting comorbidity and the risk of developing post-COVID-19 (13, 34).
Consistent with previous studies, we found that female sex was associated with an increased risk of developing post-COVID-19 (4, 14, 35, 36). Other studies also reported more advanced age as an independent risk factor for post-COVID-19 (35), which was not confirmed by our multiple regression analysis.
Interestingly, we found that higher educational qualifications were also a predictor for post-COVID-19. This could not attributed to age differences and may therefore be in line with a study showing that persons with higher education levels develop depressive symptoms and a greater decline in life satisfaction during the pandemic more often than others (37). However, this could indicate that these symptoms are not specific for COVID-19.
Living in a nursing home seemed to protect against developing post-COVID-19. This may be due to a healthy patient effect, since those nursing home residents who survive COVID-19 presumably possess greater resilience (38).
Strengths and limitations
A major limitation of this study is the lack of a control group. This means that it is not possible to distinguish between symptoms specific for COVID-19 and symptoms arising from other causes.
Reports of prevalence vary substantially among previous studies owing to differences in study design. In the present study, we therefore chose a population-based approach and found an overall post-COVID-19 prevalence of 49.3% in hospitalized and non-hospitalized patients. After standardization for age, gender, and district with the data known for all of the 4632 patients contacted (etable 1), this sensitivity analysis yielded only a slight reduction in prevalence to 47.4%. Nonetheless, sampling bias is very likely and could have led to an overestimation of the prevalence of post-COVID-19, since patients with long-term sequelae would be more likely to participate than those without. The most conservative estimation would count all of the non-responders as not having post-COVID-19, resulting in a post-COVID-19 prevalence of 14.6% for non-hospitalized and 22.9% for hospitalized patients, given that 31.5% of the questionnaires distributed were included for statistical analysis.
Growing media attention to post-COVID-19 may influence both patients and medical staff and could also have led to overestimation of symptoms (39). All data derived from patients’ self-reports and may have been biased by patients having to recall their symptoms and duration of illness retrospectively. Another possible bias is the number of persons with higher educational status.
Conclusion
The data from a large population-based cohort indicate that long-term health consequences are a frequent burden even after a moderate acute course of COVID-19. Health care professionals can take advantage of these findings to improve their understanding of these patients’ symptoms and care needs and to identify patients at risk. Within the spectrum of post-COVID-19 symptoms, further studies are needed with a focus on the most disabling sequelae and should include a control group in order to distinguish between post-COVID-19 and symptoms from other causes.
Major medical and socio-economic challenges are posed when some patients are unable to attain their previous state of health or return to work owing to functional impairment and reduced quality of life (40). Interdisciplinary local networks based on a stepped-care approach will be key to the treatment strategy for this complex disease.
Supplementary Material
eMethods
Study design and study population
This retrospective cohort study was conducted as a total population survey in three administrative districts (Reutlingen, Tübingen, Enzkreis) located in southwest Germany with a total of 715 268 inhabitants. In Germany, every person who tests positive for infection with SARS-CoV-2 is registered by the local health authorities. We contacted all patients who met the inclusion criteria (a positive polymerase chain reaction [PCR] test for SARS-CoV-2 between 1 March and 30 September 2020 and age at least 18 years. A total of 4632 persons with a confirmed SARS-CoV-2 infection met these inclusion criteria. They were invited via mail by the respective local health authorities to anonymously complete a questionnaire and return it to the Institute of General Practice and Interprofessional Care using a prepaid envelope. The questionnaires were sent out on 26 October (Reutlingen, n = 1781), 12 November (Tübingen, n = 1476), and 2 December (Enzkreis, n = 1375) 2020, respectively. Two hundred forty questionnaires could not be delivered due to incorrect mailing addresses.
Of all returned questionnaires, 448 were excluded from analysis because the study participants were < 18 years of age (n = 1), had a positive test result outside of the study period (n = 17) or in the 12 weeks prior to the date the questionnaires were sent out (n = 158), did not provide information on the date of the positive test (n = 115) or symptom duration (n = 152), or returned blank questionnaires (n = 5). The final study population comprised 1459 participants.
The study was conducted in accordance with the Declaration of Helsinki, participation was voluntary, and all responses were anonymous. The study was approved by the ethics committee of the university hospital Tuebingen (reference 698/2020BO) and registered in the German Clinical Trials Registry (reference DRKS00023069).
Data collection and questionnaire
The self-developed questionnaire assessed the following categories: self-reported symptoms of COVID-19, including onset and duration of these symptoms, need for hospitalization and intubation, comorbidities, medications taken during acute COVID-19 and medications taken on a regular basis, health-related quality of life (HrQoL), lifestyle factors, and sociodemographic data.
Participants were asked retrospectively to recall symptoms they experienced due to COVID-19 and to specify whether the onset of symptoms was “immediate” or “delayed.” The latter was defined as first occurrence of symptoms 2 weeks or longer after the positive test result for SARS-CoV-2. Furthermore, for each symptom participants were asked to either specify the duration in weeks or to confirm that the symptom persisted up to the time the questionnaire was filled out. For ongoing symptoms, the duration was calculated by subtracting the date the questionnaire was sent from the date of the positive test result for SARS-CoV-2. If the onset of a symptom was marked as “delayed,” 2 weeks were subtracted from the overall symptom duration. The main outcome of this study, post-COVID-19, was defined as at least one symptom persisting for 12 weeks or longer after the initial positive test result (5).
Hospitalization for COVID-19 was defined as at least one night in the hospital. Medications for COVID-19 could be selected from a list of frequently prescribed drugs; other medications could be entered in an optional text field.
Comorbidity was ascertained using the German version of the Self-Administered Comorbidity Questionnaire (SCQ-D), a validated inventory to assess the presence of chronic conditions such as heart disease, hypertension, and diabetes from the patient’s perspective (e9, e10). The SCQ-D consists of 12 predefined medical problems with the option of specifying up to three further problems in a text field. Each condition is then rated in three categories:
1) Presence of the medical problem (yes/no)
2) Treatment for the medical problem (yes/no)
3) Functional limitation due to the medical problem (yes/no).
The minimum score of the SCQ-D is zero (all conditions absent), and the maximum score is 45 points (all conditions present, requiring treatment, and causing functional limitation). Participants were asked to provide information solely about diseases that existed before the infection with SARS-CoV-2.
The patients were asked to provide information on the medications they had been taking regularly for at least 12 months. A list of frequently prescribed drugs was provided with optional text fields. Polypharmacy was defined as five or more pharmaceuticals.
Smoking status distinguished current smokers, ex-smokers (quit smoking at least 12 months ago), and never smokers. Dietary preferences were categorized into conventional, vegetarian, or vegan diets. The body mass index (BMI) was calculated as kg/m². To assess their education level, the patients were asked to specify whether they had a graduation certificate from a lower, intermediate, or upper secondary school, a diploma in vocational training, or a university degree. They were also asked to indicate the cumulative days of sick leave if they were not able to work because of COVID-19-related symptoms.
HrQoL was assessed using the EQ5D-5L (e11). The validated instrument consists of a visual analogue scale (VAS), ranging from zero (worst imaginable health) to 100 (best imaginable health), and a descriptive part comprising five dimensions, each with five levels of severity. We calculated the value for these dimensions based on the comparative data set for the German standard population, which ranges from –0.661 (poor health) to 1 (full health) (e12).
Data analysis
According to the indicated duration of symptoms and based on the NICE guidelines (5), patients were categorized as “no symptoms” if they had no COVID-19-related symptoms, as “acute COVID-19” if they had at least one symptom for <4 weeks, as “ongoing COVID-19” if they had at least one symptom that lasted between 4 and < 12 weeks, or as “post-COVID-19” if they reported at least one symptom ≥ 12 weeks after the infection. Differences between groups in terms of categorical variables were determined using the Pearson chi-square test and Fisher’s exact test. The Kruskal–Wallis test was used for non-normally distributed continuous variables (age, BMI, SCQ-D). The significance level was set at α < 0.05 (two-sided).
A multiple ordinal regression model was used to identify predictors of post-COVID-19. For the regression analysis, the study participants with symptom durations of < 4 weeks and 4 to < 12 weeks were amalgamated into one category, creating an outcome variable with three categories (no symptoms, symptom duration of < 12 weeks, symptom duration of ≥ 12 weeks). Potential predictors for post-COVID-19 were identified from the literature and their association with the outcome was investigated by means of univariate regression analyses. All predictors that showed an association with the outcome were entered into the full model. Stepwise backward selection was used to identify those predictors that contributed to the final parsimonious model in a statistically significant way. Age was included in the parsimonious model regardless of variable selection. The majority of predictors entered into the full regression model exhibited only few missing values; the SCQ-D was the predictor with the highest proportion of missing values (23%). We conducted multiple imputation to replace missing values (number of imputation-based data sets n = 500). To test the validity of the results determined by the ordinal logistic regression, two binary logistic regression models (no symptoms and symptom duration < 12 weeks vs. symptom duration of ≥ 12 weeks; no symptoms vs. symptom duration < 12 weeks and symptom duration of ≥ 12 weeks) were calculated as a sensitivity analysis. The assumption of proportional odds was not met (chi-square [7] between 48.2 and 83.7, mean 68.7, p<0.001 for each imputation). Therefore, we present in the sensitivity analysis the two separate binary logistic regression models which are combined in the proportional odds model.
All analyses were performed using IBM SPSS Statistics for Windows, version 27.
eTable 5. Sensitivity analysis of regression*1.
| Level | No symptoms vs. any duration | Post-COVID-19 vs. no symptoms or duration <12 weeks | |||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Age | Continuous | 0.988 | [0.975; 1.001] | 0.070 | 1.006 | [0.999; 1.014] | 0.105 |
| Sex | Female | 1.556 | [1.028; 2.355] | 0.037 | 1.921 | [1.529; 2.414] | < 0.001 |
| Male | Reference | Reference | |||||
| Educational level*2 | Metric | 1.356 | [1.182; 1.556] | < 0.001 | 1.112 | [1.019; 1.214] | < 0.017 |
| Nursing home resident | Yes | 0.145 | [0.068; 0.309] | < 0.001 | 0.156 | [0.072; 0.334] | < 0.001 |
| No | Reference | Reference | |||||
| SCQ-D | Continuous | 1.012 | [0.955; 1.073] | 0.68 | 1.110 | [1.069; 1.153] | < 0.001 |
| Hospitalized during acute COVID-19 | Yes | 3.432 | [1.587; 7.422] | 0.002 | 1.839 | [1.407; 2.404] | < 0.001 |
| No | Reference | Reference | |||||
| Need for analgesics during acute COVID-19 | Yes | n.d.*3 | n.d.*3 | n.d.*3 | 1.528 | [1.059; 2.205] | 0.024 |
| No | n.d.*3 | Reference | |||||
*1 As sensitivity analysis, binary logistic regression models were calculated by combining two out of three categories that were also used in the multiple ordinal ?logistic regression (1, no symptoms; 2, symptom duration < 12 weeks; 3, symptom duration ≥ 12 weeks) (n = 1459; imputed data set with 500 imputations)
*2 Only one parameter was estimated in a linear coding of educational status with six different levels.
*3 The predictor “need for analgesics during acute COVID-19” was excluded from the analysis of no symptoms vs. any duration, because all patients who took analgesics also had symptoms (of any duration).
CI, Confidence interval; n.d., no data; OR, odds ratio; SCQ-D, Self-Administered Comorbidity Questionnaire, German version
Acknowledgments
Funding
This work was supported by the Ministry of Science, Research, and Art of Baden-Württemberg through the special funding program for COVID-19 research, a component of the medical research package to combat the SARS-CoV-2 pandemic.
Acknowledgments
We thank the health authorities in Reutlingen, Tuebingen and Enzkreis for supporting this study. We thank Dr. Hannah Fuhr for critical reading of the manuscript and Jasmin Mangold, Raphaela Samrock, and Lena Gassner for their assistance with data acquisition.
Footnotes
Conflict of interest statement
All authors declare that no conflict of interest exists.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Scientific reports. 2021;11:1–12. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. Journal of Infection. 2021;83:1–16. doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://www.nice.org.uk/guidance/ng188 . [PubMed] [Google Scholar]
- 6.World Health Organisation. A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 . doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koczulla AR, Ankermann T, Behrends U, et al. S1-Leitlinie Post-COVID/Long-COVID. Pneumologie. 2021;75:869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100899. 100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 Syndrome: The Persistent Symptoms at the Post-viral Stage of the Disease A Systematic Review of the Current Data. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.653516. 653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021:1–18. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clinical Infectious Diseases. 2021;73:e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post--COVID--19 manifestations. International Journal of Clinical Practice. 2021;75 doi: 10.1111/ijcp.13746. e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. The Lancet Regional Health-Europe. 2021;6 doi: 10.1016/j.lanepe.2021.100122. 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton A, Olliaro P, Sigfrid L, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis. 2021;21:601–602. doi: 10.1016/S1473-3099(21)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson G. Research priorities for Long Covid: refined through an international multi-stakeholder forum. BMC medicine. 2021;19:1–4. doi: 10.1186/s12916-021-01947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs JJ. Persistent SARS-2 infections contribute to long COVID-19. Medical Hypotheses. 2021;149 doi: 10.1016/j.mehy.2021.110538. 110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravioli S, Ochsner H, Lindner G. Reactivation of COVID-19 pneumonia: a report of two cases. Journal of Infection. 2020;81:e72–e73. doi: 10.1016/j.jinf.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nature Reviews Rheumatology. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battle C, Lynch C, Thorpe C, et al. Incidence and risk factors for alopecia in survivors of critical illness: A multi-centre observational study. Journal of critical care. 2019;50:31–35. doi: 10.1016/j.jcrc.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 22.van’t Leven M, Zielhuis GA, van der Meer JW, Verbeek AL, Bleijenberg G. Fatigue and chronic fatigue syndrome-like complaints in the general population. European journal of public health. 2010;20:251–257. doi: 10.1093/eurpub/ckp113. [DOI] [PubMed] [Google Scholar]
- 23.Pieh C, Budimir S, Delgadillo J, Barkham M, Fontaine JR, Probst T. Mental health during COVID-19 lockdown in the United Kingdom. Psychosomatic medicine. 2021;83:328–337. doi: 10.1097/PSY.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 24.Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Annals of Internal Medicine. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenhalgh T, Knight M, Buxton M, Husain L. Management of post-acute covid-19 in primary care. bmj. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 26.Heightman M, Prashar J, Hillman TE, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ open respiratory research. 2021;8 doi: 10.1136/bmjresp-2021-001041. e001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brigham E, O‘Toole J, Kim SY, et al. The Johns Hopkins Post-Acute COVID-19 Team (PACT): a multidisciplinary, collaborative, ambulatory framework supporting COVID-19 survivors. The American Journal of Medicine. 2021;134:462–467. doi: 10.1016/j.amjmed.2020.12.009. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkin A, Davison J, Tarrant R, et al. A Multidisciplinary NHS COVID-19 Service to Manage Post-COVID-19 Syndrome in the Community. Journal of Primary Care & Community Health. 2021;12 doi: 10.1177/21501327211010994. 21501327211010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. Journal of rehabilitation medicine. 2020;52:1–11. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 31.Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. American journal of physical medicine & rehabilitation. 2020;99 doi: 10.1097/PHM.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76:402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross--sectional evaluation. Journal of medical virology. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 36.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Prevalence and determinants of fatigue after covid-19 in non-hospitalized subjects: A population-based study. International Journal of Environmental Research and Public Health. 2021;18 doi: 10.3390/ijerph18042030. 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wanberg CR, Csillag B, Douglass RP, Zhou L, Pollard MS. Socioeconomic status and well-being during COVID-19: A resource-based examination. Journal of Applied Psychology. 2020;105 doi: 10.1037/apl0000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. New England Journal of Medicine. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young ME, Norman GR, Humphreys KR. Medicine in the popular press: the influence of the media on perceptions of disease. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003552. e3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the US workforce: prevalence and implications for lost productive work time. Journal of occupational and environmental medicine. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- E1.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA network open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. e210830-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, et al. Post-acute COVID-19 Syndrome Incidence and risk factors: a Mediterranean cohort study. Journal of Infection. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Romero-Duarte Á, Rivera-Izquierdo M, de Alba IG-F, et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC medicine. 2021;19:1–13. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. Journal of Infection. 2020;81 doi: 10.1016/j.jinf.2020.08.029. e4-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Hoffman HJ, Cruickshanks KJ, Davis B. Perspectives on population-based epidemiological studies of olfactory and taste impairment. Annals of the New York Academy of Sciences. 2009;1170 doi: 10.1111/j.1749-6632.2009.04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Kaseda ET, Levine AJ. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020;34:1498–1514. doi: 10.1080/13854046.2020.1811894. [DOI] [PubMed] [Google Scholar]
- E7.Stam HJ, Stucki G, Bickenbach J, Deltombe T. Covid-19 and Post Intensive Care Syndrome: A Call for Action. Journal of rehabilitation medicine. 2020;52 doi: 10.2340/16501977-2677. jrm00044. [DOI] [PubMed] [Google Scholar]
- E8.Health Authority of Baden-Wuerttemberg. Tagesbericht COVID-19. https://sozialministerium.baden-wuerttemberg.de/fileadmin/redaktion/m-sm/intern/downloads/Downloads_Gesundheitsschutz/200930_COVID_Tagesbericht_LGA.pdf [Google Scholar]
- E9.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- E10.Streibelt M, Schmidt C, Brunger M, Spyra K. [Comorbidity from the patient perspective – does it work? Validity of a questionnaire on self-estimation of comorbidity (SCQ-D)] Orthopade. 2012;41:303–310. doi: 10.1007/s00132-012-1901-3. [DOI] [PubMed] [Google Scholar]
- E11.EuroQol Group. EuroQol Instruments Webpage. https://euroqol.org/ [Google Scholar]
- E12.Ludwig K, von der Schulenburg J-MG, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36:663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Study design and study population
This retrospective cohort study was conducted as a total population survey in three administrative districts (Reutlingen, Tübingen, Enzkreis) located in southwest Germany with a total of 715 268 inhabitants. In Germany, every person who tests positive for infection with SARS-CoV-2 is registered by the local health authorities. We contacted all patients who met the inclusion criteria (a positive polymerase chain reaction [PCR] test for SARS-CoV-2 between 1 March and 30 September 2020 and age at least 18 years. A total of 4632 persons with a confirmed SARS-CoV-2 infection met these inclusion criteria. They were invited via mail by the respective local health authorities to anonymously complete a questionnaire and return it to the Institute of General Practice and Interprofessional Care using a prepaid envelope. The questionnaires were sent out on 26 October (Reutlingen, n = 1781), 12 November (Tübingen, n = 1476), and 2 December (Enzkreis, n = 1375) 2020, respectively. Two hundred forty questionnaires could not be delivered due to incorrect mailing addresses.
Of all returned questionnaires, 448 were excluded from analysis because the study participants were < 18 years of age (n = 1), had a positive test result outside of the study period (n = 17) or in the 12 weeks prior to the date the questionnaires were sent out (n = 158), did not provide information on the date of the positive test (n = 115) or symptom duration (n = 152), or returned blank questionnaires (n = 5). The final study population comprised 1459 participants.
The study was conducted in accordance with the Declaration of Helsinki, participation was voluntary, and all responses were anonymous. The study was approved by the ethics committee of the university hospital Tuebingen (reference 698/2020BO) and registered in the German Clinical Trials Registry (reference DRKS00023069).
Data collection and questionnaire
The self-developed questionnaire assessed the following categories: self-reported symptoms of COVID-19, including onset and duration of these symptoms, need for hospitalization and intubation, comorbidities, medications taken during acute COVID-19 and medications taken on a regular basis, health-related quality of life (HrQoL), lifestyle factors, and sociodemographic data.
Participants were asked retrospectively to recall symptoms they experienced due to COVID-19 and to specify whether the onset of symptoms was “immediate” or “delayed.” The latter was defined as first occurrence of symptoms 2 weeks or longer after the positive test result for SARS-CoV-2. Furthermore, for each symptom participants were asked to either specify the duration in weeks or to confirm that the symptom persisted up to the time the questionnaire was filled out. For ongoing symptoms, the duration was calculated by subtracting the date the questionnaire was sent from the date of the positive test result for SARS-CoV-2. If the onset of a symptom was marked as “delayed,” 2 weeks were subtracted from the overall symptom duration. The main outcome of this study, post-COVID-19, was defined as at least one symptom persisting for 12 weeks or longer after the initial positive test result (5).
Hospitalization for COVID-19 was defined as at least one night in the hospital. Medications for COVID-19 could be selected from a list of frequently prescribed drugs; other medications could be entered in an optional text field.
Comorbidity was ascertained using the German version of the Self-Administered Comorbidity Questionnaire (SCQ-D), a validated inventory to assess the presence of chronic conditions such as heart disease, hypertension, and diabetes from the patient’s perspective (e9, e10). The SCQ-D consists of 12 predefined medical problems with the option of specifying up to three further problems in a text field. Each condition is then rated in three categories:
1) Presence of the medical problem (yes/no)
2) Treatment for the medical problem (yes/no)
3) Functional limitation due to the medical problem (yes/no).
The minimum score of the SCQ-D is zero (all conditions absent), and the maximum score is 45 points (all conditions present, requiring treatment, and causing functional limitation). Participants were asked to provide information solely about diseases that existed before the infection with SARS-CoV-2.
The patients were asked to provide information on the medications they had been taking regularly for at least 12 months. A list of frequently prescribed drugs was provided with optional text fields. Polypharmacy was defined as five or more pharmaceuticals.
Smoking status distinguished current smokers, ex-smokers (quit smoking at least 12 months ago), and never smokers. Dietary preferences were categorized into conventional, vegetarian, or vegan diets. The body mass index (BMI) was calculated as kg/m². To assess their education level, the patients were asked to specify whether they had a graduation certificate from a lower, intermediate, or upper secondary school, a diploma in vocational training, or a university degree. They were also asked to indicate the cumulative days of sick leave if they were not able to work because of COVID-19-related symptoms.
HrQoL was assessed using the EQ5D-5L (e11). The validated instrument consists of a visual analogue scale (VAS), ranging from zero (worst imaginable health) to 100 (best imaginable health), and a descriptive part comprising five dimensions, each with five levels of severity. We calculated the value for these dimensions based on the comparative data set for the German standard population, which ranges from –0.661 (poor health) to 1 (full health) (e12).
Data analysis
According to the indicated duration of symptoms and based on the NICE guidelines (5), patients were categorized as “no symptoms” if they had no COVID-19-related symptoms, as “acute COVID-19” if they had at least one symptom for <4 weeks, as “ongoing COVID-19” if they had at least one symptom that lasted between 4 and < 12 weeks, or as “post-COVID-19” if they reported at least one symptom ≥ 12 weeks after the infection. Differences between groups in terms of categorical variables were determined using the Pearson chi-square test and Fisher’s exact test. The Kruskal–Wallis test was used for non-normally distributed continuous variables (age, BMI, SCQ-D). The significance level was set at α < 0.05 (two-sided).
A multiple ordinal regression model was used to identify predictors of post-COVID-19. For the regression analysis, the study participants with symptom durations of < 4 weeks and 4 to < 12 weeks were amalgamated into one category, creating an outcome variable with three categories (no symptoms, symptom duration of < 12 weeks, symptom duration of ≥ 12 weeks). Potential predictors for post-COVID-19 were identified from the literature and their association with the outcome was investigated by means of univariate regression analyses. All predictors that showed an association with the outcome were entered into the full model. Stepwise backward selection was used to identify those predictors that contributed to the final parsimonious model in a statistically significant way. Age was included in the parsimonious model regardless of variable selection. The majority of predictors entered into the full regression model exhibited only few missing values; the SCQ-D was the predictor with the highest proportion of missing values (23%). We conducted multiple imputation to replace missing values (number of imputation-based data sets n = 500). To test the validity of the results determined by the ordinal logistic regression, two binary logistic regression models (no symptoms and symptom duration < 12 weeks vs. symptom duration of ≥ 12 weeks; no symptoms vs. symptom duration < 12 weeks and symptom duration of ≥ 12 weeks) were calculated as a sensitivity analysis. The assumption of proportional odds was not met (chi-square [7] between 48.2 and 83.7, mean 68.7, p<0.001 for each imputation). Therefore, we present in the sensitivity analysis the two separate binary logistic regression models which are combined in the proportional odds model.
All analyses were performed using IBM SPSS Statistics for Windows, version 27.