Abstract
Background
In some areas of Germany, there is a shortage of specialist physicians for patients with inflammatory rheumatic diseases. Delegating certain medical care services to qualified, specialized rheumatological assistants (SRAs) might be an effective way to supplement the available capacity for specialized medical care.
Methods
Patients under stable treatment for rheumatoid arthritis (RA) or psoriatic arthritis (PsA) were included in this trial, which was designed to demonstrate, in a first step, the non-inferiority of a form of care involving delegation of physicians’ tasks to SRAs (team-based care), in comparison to standard care, with respect to changes in disease activity at one year. “Non-inferiority,” in this context, means either superiority or else an irrelevant extent of inferiority. In a second step, in case non-inferiority could be shown, the superiority of team-based care with respect to changes in patients’ health-related quality of life would be tested as well. Disease activity was measured with the Disease Activity Score 28, and health-related quality of life with the EQ-5D-5L. This was a randomized, multicenter, rater-blinded trial with two treatment arms (team-based care and standard care). The statistical analysis was performed with mixed linear models (DRKS00015526).
Results
From September 2018 to June 2019, 601 patients from 14 rheumatological practices and 3 outpatient rheumatological clinics in the German states of North Rhine–Westphalia and Lower Saxony were randomized to either team-based or standard care. Team-based care was found to be non-inferior to standard care with respect to changes in disease activity (adjusted difference = -0.19; 95% confidence interval [-0.36; -0.02]; p <0.001 for non-inferiority). Superiority with respect to health-related quality of life was not demonstrated (adjusted difference = 0.02 [-0.02; 0.05], p = 0.285).
Conclusion
Team-based care, with greater integration of SRAs, is just as good as standard care in important respects. Trained SRAs can effectively support rheumatologists in the care of stable patients with RA or PsA.
In some areas of Germany, there are gaps in the care of patients with inflammatory rheumatic diseases (1). The comparatively small number of specialists working in rheumatology contributes to this, as shown in the memorandum of the German Society for Rheumatology (2). Yet there is also a misallocation of rheumatology manpower, considering that routine work, documentation, etc., could most likely be carried out by specialized rheumatological assistants (SRAs), without increasing disease activity or reducing patient health-related quality of life.
The European Alliance of Associations for Rheumatology (EULAR) recommended as early as 2012 a stronger involvement of nurses to improve the care of patients with chronic inflammatory arthritis (3) (updated 2018 [4]). While in other countries (for example in the Netherlands and England) team building to include the delegation of medical tasks to staff from non-physician healthcare professions is already quite advanced in some cases (4), in Germany it has so far been slow to develop, despite the fact that special courses for medical assistants have been available since 2006. Through cooperation between the BDRh (Professional Association of German Rheumatologists), the DGRh (German Society for Rheumatology) and the Rheumatism Academy (RhAk), a curriculum was created that qualifies for the title “Specialized Rheumatological Assistant DGRh”. In April 2021, the German Medical Association decided to extend this model curriculum of continuing education to include 120 teaching units.
The project “Structured Delegation of Physician Services as Part of a Concept Based Cooperation for the Management of Inflammatory Rheumatic Diseases” (acronym: “StärkeR”) was based on the hypothesis that adequately qualified SRAs can effectively take over certain aspects of patient care. A randomized, multicenter, rater-blinded trial assessed a new team-based form of care involving the increased delegation to SRAs and compared it with standard care in patients with rheumatoid arthritis (RA) or polyarticular psoriatic arthritis (PsA). The StärkeR study explored the following hypotheses:
The team-based form of care is not inferior to standard care as regards changes in disease activity.
Changes in patient health-related quality of life are better under team-based care than under standard care.
Method
SRA training and tasks
In the first year of the project, healthcare professionals were trained to become SRAs (if they were not already). This was followed by project-related training to prepare SRAs for the requirements associated with the delegation of tasks (see eMethods).
Under the team-based form of care with increased delegation to the SRAs, the SRA became the primary contact person for patients during a one-year period. SRA tasks included:
Management of the three-monthly follow-up reviews, including history of disease progression, comorbidities and infections, vaccination status, medication use, adverse drug reactions, and capacity to work; calculation of a disease activity score (Clinical Disease Activity Index [CDAI] [5]) (eMethods).
Optimization of the treat-to-target principle (6) by discussing the calculated CDAI score during the patient’s visit to the rheumatologist: review the need for treatment adjustment in the event of deviation of disease activity from the target range (CDAI >10) or, in cases of skin involvement, >10% of skin affected by psoriasis in patients with PsA (while still adhering to the delegation concept).
Improvement of medication safety by discussing the current medication plan with the patient.
Inclusion and exclusion criteria
The study included adults
with a diagnosis of RA according to an expert (rheumatologist) opinion, based on the EULAR criteria (7), and a disease course free of complications for three months with low disease activity in the last quarter as defined by a disease activity score (DAS) 28 <3.2 (28 joints assessed, as well as erythrocyte sedimentation rate [range 0–9.4]) (8), or
with a diagnosis of polyarticular PsA according to an expert opinion, based on the CASPAR criteria (9) (CASPAR, Classification Criteria for Psoriatic Arthritis), and with a complication-free course for three months and low disease activity in the quarter before baseline (defined as for RA), and extent of skin affected by psoriasis ≤10%.
Exclusion criteria were limited legal capacity, insufficient knowledge of German, and a complicated comorbidity.
Primary endpoints
The primary endpoints were
1. change in disease activity (as measured with the DAS28) and
2. change in health-related quality of life (as measured with the EuroQol in five dimensions and five response levels [EQ-5D-5L]) (10), each after one year.
These endpoints were arranged hierarchically, meaning that examination of the second endpoint requires statistical significance of the first endpoint. We refer to the eMethods for details on determining sample size and the instruments used for assessment.
Randomization and measurement of primary and secondary endpoints
After informing the patients about the trial and obtaining their written consent, a telephone interview was conducted, followed by patient-by-patient randomization (1:1). Stratified randomization by center was used to ensure equal distribution of treatment groups within each center. The result was faxed from the data center to the practice/clinic.
At baseline and after one year, DAS28 and skin involvement due to psoriasis were assessed by a qualified assessor who was not a member of the practice team. The assessors were medical staff members of the Ruhr District Rheumatology Center and blinded to the randomization result. The EQ-5D-5L was obtained in a telephone interview by interviewers who were also blinded. The evaluating statistician was unblinded to group allocation only after database closure and finalization of the statistical evaluation plan.
Secondary analysis was conducted to determine the impact of form of care on functional capacity (11), physical activity (12), depressive tendency (13), pain intensity, fatigue, sleep disturbance, C-reactive protein (CRP), and patient satisfaction. In addition, resource use (14) was recorded in terms of health economics. The assessments took place every six months. Details regarding the assessment instruments for the secondary endpoints can be found in the eMethods.
Statistical analysis
The hierarchical evaluation of the two-part primary endpoint allows the tolerated error probability for a type-1 error to be reapplied at the second step if the previous step achieves statistical significance. For analysis of the first part of the primary endpoint, a linear mixed model was used for the data of the per-protocol population to assess non-inferiority (non-inferiority margin set at 0.4 [15], probability of error 2.5%) – in line with the recommendations of the European Medicines Agency (EMA) (16). The second part of the primary endpoint was analyzed in a linear mixed model (5% probability of error) for the intention-to-treat population. Sensitivity analyses were performed for the two primary endpoints in the other population. A more detailed description of the statistical evaluations can be found in the eMethods.
Ethical and administrative aspects
The project complied with good clinical practice. In addition to obtaining informed consent, this involved consultation with the ethics committees (in charge: Ethics Committee of the Medical Association of Westphalia-Lippe and the Westphalian Wilhelms University of Münster; file number: 2018–144-f-S, positive opinion on May 23, 2018) and monitoring of the centers. The project was funded by the Innovation Fund (grant number: 01NVF17004) and retrospectively registered with the German Clinical Trials Registry (DRKS00015526).
Results
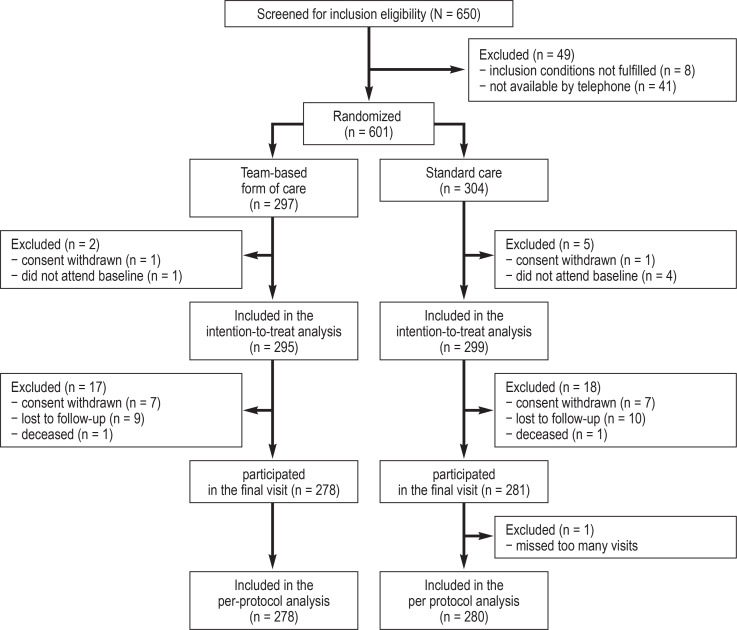
Between September 2018 and June 2019, 650 patients from 14 specialist rheumatology practices and three rheumatology outpatient clinics in North Rhine-Westphalia and Lower Saxony were screened, and 601 were randomized, with 297 patients assigned to the team-based form of care and 304 to standard care. Of these, two patients in the team-based form of care group and five in the standard care group had to be excluded from the intention-to-treat analysis because they did not attend the baseline visit or had previously withdrawn their consent. Thus, 295 patients were included for the team-based form of care and 299 for standard care. Two hundred and seventy-eight patients from the team-based form of care and 280 from standard care were available for per-protocol analysis. One death occurred in each group (cause of death: traumatic brain injury under standard care and myocardial infarction under team-based care) (figure 1).
Figure 1.
Flowchart of the project “Structured Delegation of Physician Services as Part of a Concept Based Cooperation for the Management of Inflammatory Rheumatic Diseases” (StärkeR)
Patient characteristics at baseline
There were no significant differences between the two types of care at the baseline examination. Average age was 62 years; 78% of the study participants were female, and 83% had RA. The average DAS28 score of 3.1 was in the low disease activity range (table 1).
Table 1. Patient characteristics at baseline.
|
Team-based form
of care (n = 295) |
Standard
care (n = 299) |
Total
(n = 594) |
|
| Age (years) | 62.9 (12.1) |
61.9 (12.3) |
62.4 (12.2) |
| Females (n [%]) | 224 (76) |
242 (81) |
466 (78) |
| Body Mass Index (kg/m2) | 27.5 (5.3) |
28.0 (6.0) |
27.7 (5.7) |
| CRP (mg/L) (median [interquartile range Q25-Q75]) | 3.5 (2.0–7.0) |
3.2 (1.8–7.0) |
3.4 (2.0–7.0) |
| ESR (mm/h) (median [interquartile range Q25-Q75]) | 10 (5–18) |
10 (5–18) |
10 (5–18) |
| CDAI (0–76) | 8.9 (7.2) |
8.3 (6.8) |
8.6 (7.0) |
| DAS28 (0–9.4) | 3.1 (1.2) |
3.1 (1.2) |
3.1 (1.2) |
| Type of rheumatic disease | |||
| Psoriatic arthritis (n [%]) | 50 (17) |
52 (17) |
102 (17) |
| Rheumatoid arthritis (n [%]) | 245 (83) |
247 (83) |
492 (83) |
| Patient-reported parameters | |||
| Health-related quality of life (EQ-5D-5L, ≤1) | 0.7 (0.2) |
0.7 (0.3) |
0.7 (0.3) |
| Disease activity (NRS, 0–10) | 3.6 (2.2) |
3.7 (2.3) |
3.7 (2.3) |
| Pain intensity (NRS, 0–10) | 3.7 (2.5) |
3.8 (2.4) |
3.8 (2.5) |
| Fatigue (NRS, 0–10) | 4.0 (3.0) |
3.9 (2.9) |
3.9 (2.9) |
| Sleep disturbances (NRS, 0–10) | 4.0 (3.1) |
4.2 (3.2) |
4.1 (3.2) |
| Duration of morning stiffness (minutes) | 16 (26) |
18 (31) |
17 (28) |
| Depression (PHQ-2, 0–6) | 1.5 (1.5) |
1.5 (1.5) |
1.5 (1.5) |
| Functional capacity (FFbH, 0–100) | 76.0 (21.7) |
75.7 (21.8) |
75.9 (21.7) |
| Physical activity (PRISCUS-PAQ, MET hours/week, ≥168) | 185 (10.8) |
184 (9.2) |
184 (10.0) |
Values given as mean and standard deviation, unless otherwise stated
ESR, erythrocyte sedimentation rate; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein;
DAS28, Disease Activity Score with 28 joints; EQ-5D-5L, EuroQol group questionnaire for measuring health-related quality of life in 5 dimensions, each with 5 response levels;
FFbH, Hanover Functional Status Questionnaire; MET, metabolic equivalent; n, number;
NRS, numeric rating scale; PAQ, Physical Activity Questionnaire;
PHQ-2, Patient Health Questionnaire 2 items
Primary endpoints
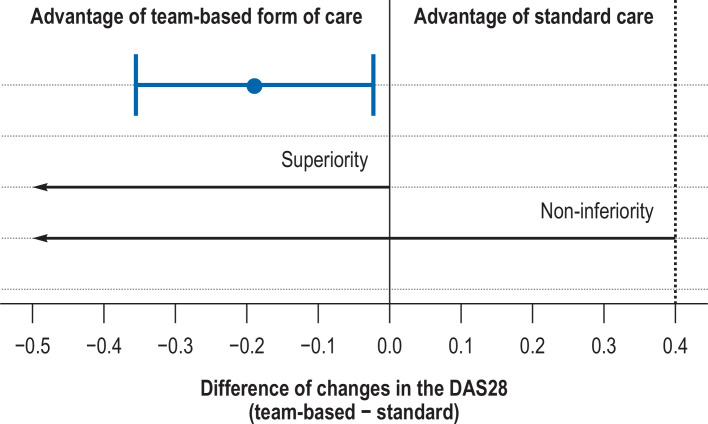
The standard care group showed an average increase of 0.04 in the DAS28 score over the course of one year (standard deviation: 1.15), whereas the team-based form of care showed a decrease of 0.14 (1.07), resulting in a difference of -0.18 in favor of the team-based care group. After adjustment in the linear mixed model, this difference changed to -0.19 (95% confidence interval: [-0.36; -0.02]), and the p value for non-inferiority was <0.001. The additional test for superiority yielded a p value of 0.025, thus demonstrating superiority in a statistical sense (Table 2, Figure 2).
Table 2. Analysis of the primary endpoints.
| Change after one year*1 per group | Group comparison | ||||
| Endpoint | Team-basedform of care | Standard care | Team-based − Standard | ||
| Observedmean value (SD) | Observedmean value (SD) | Estimated difference[95% confidence interval] | p value | D | |
| DAS28 | −0.14(1.07) | 0.04(1.15) | −0.19[−0.36; −0.02]*2 | < 0.001*4 | ↑ |
| EQ-5D-5L | 0.02(0.21) | 0.01 (0.23) | 0.02[−0.02; 0.05]*3 | 0.285 | ↑ |
*1 final value – initial value
*2 estimated using a mixed linear model in der per protocol population with adjustment for patient sex,
baseline DAS28 score, type of rheumatoid disease, and center (random effect)
*3 estimated using a linear mixed model in intention-to-treat population with adjustment for patient age and sex,
EQ-5D-5L score at baseline, type of rheumatic disease, and center (random effect)
*4 p value from test for non-inferiority, p = 0.025 in test for superiority
DAS28, Disease Activity Score with 28 joints; EQ-5D-5L, EuroQol in 5 dimensions and 5 response levels; D, direction of the estimated difference;
SD, standard deviation; ↑ indicates advantage of team-based form of care.
Figure 2.
Estimated difference and 95% confidence interval (team-based model of care - standard care) of changes in the DAS28 after one year.
“0” indicates no difference between the groups.
A negative difference signifies an advantage for the team-based form of care.
The dashed line marks the non-inferiority margin.
The arrows indicate the ranges in which the 95% confidence interval must lie in order to assume statistically significant non-inferiority or superiority of the team-based form of care.
DAS28, Disease Activity Score in 28 joints
Analysis of changes in health-related quality of life over the course of one year showed an improvement in EQ-5D-5L of 0.01 (0.23) for standard care and 0.02 (0.21) for team-based care, resulting in an overall difference of 0.01 in favor of team-based care. The linear mixed model demonstrated an estimated difference of 0.02 [-0.02; 0.05] (p = 0.285) (table 2). See eResults for full model results of the primary endpoints and sensitivity analyses.
Secondary endpoints
Statistical analysis for the secondary endpoints showed no relevant differences between the forms of care (Table 3; see eResults for sensitivity analysis in the per-protocol population).
Table 3. Analysis of the secondary endpoints.
|
Change after one year*1
per group |
Group comparison | |||
| Endpoint |
Team-based
form of care |
Standard
care |
Team based − Standard | |
|
Observed
mean value (SD) |
Observed
mean value (SD) |
Estimated difference
[95% confidence interval]*2 |
Direction | |
| CRP (mg/L)*3 | 0.0(−1.1–1.2) | 0.0(−1.5–1.4) | 0.0[−0.1; 0.2] | ↓ |
| Disease activity (NRS) | −0.2 (2.2) | −0.1 (2.5) | −0.1 [−0.5; 0.2] | ↑ |
| Pain intensity (NRS) | −0.1 (2.4) | 0.1 (2.2) | −0.2 [−0.6; 0.1] | ↑ |
| Fatigue(NRS) | −0.2 (2.3) | 0.0 (2.5) | −0.2 [−0.5; 0.2] | ↑ |
| Sleep disturbances (NRS) | −0.2 (2.6) | −0.0 (2.8) | −0.3 [−0.7; 0.1] | ↑ |
| Duration of morning stiffness (minutes) | 2.6 (30.3) | 1.4 (29.1) | 0.5 [−4.1; 5.2] | ↓ |
| Depression(PHQ-2) | −0.1 (1.5) | 0.0 (1.5) | −0.1 [−0.3; 0.1] | ↑ |
| Functional capacity (FFbH) | −2.0 (15.9) | −1.7 (13.0) | −0.1 [−2.9; 2.8] | ↓ |
| Physical activity (PRISCUS-PAQ) | −0.2(11.4) | −1.2 (10.2) | 1.6 [−0.2; 3.4] | ↑ |
*1 final value- initial value
*2 estimated using a linear mixed models in the intention-to-treat population for each endpoint with adjustment for patient age and sex, respective baseline value, and respective center (random effect)
*3 given the skewness of the distribution, median and interquartile range (Q25–Q75) of the changes are presented, and the model is calculated with log CRP.
CRP, C-reactive protein; FFbH, Hanover Functional Status Questionnaire (0–100); NRS, numeric rating scale (0–10);
PAQ, Physical Activity Questionnaire (≥ 168 MET hour/week [MET, metabolic equivalent]); PHQ-2, Patient Health Questionnaire 2 Items (0–6);
↑ indicates advantage of the team-based model of care, ↓ indicates advantage of standard care
Over the course of the study, SRAs took progressively less time to perform delegated tasks; they ultimately required a mean of 16 minutes per patient instead of the initial 26 minutes. The median physician time spent per patient after one year in the team-based care setting was six minutes, compared with 14 minutes in the standard care setting.
In the final telephone interview, 64% of the 282 accessible patients in the team-based form of care and 66% of the 286 patients in standard care reported being “very satisfied” with the opportunity to ask questions, and 32% and 29%, respectively, were “satisfied” with this. Sixty-five percent and 67% of patients were “very satisfied” with the information they received, respectively, and 28% in both groups were “satisfied”. In the final questionnaire to all participating physicians and SRAs, 70% of physicians and 93% of SRAs reported that patients would have liked the SRAs to continue involvement in their care.
The questionnaire-based patient data on the use of healthcare services did not show relevant differences between the treatment groups, either for individual elements of the service or in terms of estimating total annual costs (Table 4, details in eTable 1).
Table 4. Estimated costs per year and patient based on resource use data.
|
Costs (euros) for the same period of the
previous year at the start of the study (baseline) |
Change in costs (euros)
after one year*1 per group |
Difference in
change (euros) |
|||
| Type of service |
Team-based
care |
Standard care |
Team-based
care |
Standard care |
Standard –
team-based |
|
Observed
mean value (SD) |
Observed
mean value (SD) |
Mean value*2
[95% CI] |
Mean value*2
[95% CI] |
Estimated difference*2
[95% CI] |
|
| Total costs | 3997.08 (5307.51) | 3626.41 (4928.62) | −252.21 [−618.95; 135.71] | −203.94 [−530.42; 110.74] | 48.27 [−435.14; 531.67] |
| Medication indicated for rheumatism | 2430.94 (5191.71) | 2057.40 (4683.04) | −230.41 [−560.69; 111.54] | −229.11 [−527.45; 52.95] | 1.30 [−453.34; 455.94] |
| Remedies*3 | 430.82 (692.04) | 432.20 (726.40) | 35.64 [−46.46; 111.40] | 16.99 [−51.27; 84.00] | −18.65 [−122.99; 85.68] |
*1 final value - initial value
*2 with lower and upper confidence limits based on the t-distribution in the intention-to-treat population
*3 ergotherapy and physiotherapy
SD, standard deviation; 95% CI, 95% confidence interval
eTable 1. Estimated costs per year and patient based on resource use data.
|
Costs (euros) for the same period of
the previous year at the start of the study (baseline) |
Change in costs (euros) after one year*1 per group |
Difference in changes
(euros) |
|||
| Type of service |
Team-based
care |
Standard
care |
Team-based
care |
Standard
care |
Standard −
team-based |
|
Observed mean
value (SD) |
Observed mean
value (SD) |
Mean value*2
[95% CI] |
Mean value*2
[95% CI] |
Estimated difference*2
[95% CI] |
|
| Drugs indicated forrheumatism | 2430.94 (5 191.71) | 2057.40 (4 683.04) | −230.41 [−578.14; 104.12] | −229.11 [−507.05; 44.42] | 1.30 [−453.34; 455.94] |
| Other medications | 410.55 (421.00) | 394.90 (423.75) | −26.58 [−60.75; 11.58] | −29.67 [−66.13; 5.10] | −3.10 [−53.61; 47.41] |
| Occupational or work therapy | 82.87 (403.62) | 125.86 (494.11) | 59.15 [6.80; 110.40] | 23.94 [−15.87; 65.37] | −35.22 [−103.13; 32.70] |
| Physiotherapy, etc. | 347.95 (538.52) | 306.33 (500.09) | −23.51 [−79.36; 38.19] | −6.95 [−62.40; 47.80] | 16.56 [−60.87; 93.99] |
| Ophthalmologists | 51.56 (100.20) | 41.89 (94.79) | −3.19 [−17.25; 9.47] | 3.45 [−7.78; 14.34] | 6.64 [−10.71; 23.98] |
| Surgeons | 27.12 (162.65) | 14.93 (79.25) | −6.31 [−27.76; 12.93] | 12.50 [−3.05; 29.42] | 18.81 [−7.27; 44.89] |
| Gynecologists | 28.03 (93.53) | 23.44 (57.35) | 2.92 [−10.52; 15.03] | 2.57 [−3.51; 8.86] | −0.35 [−13.97; 13.27] |
| Primary care physicians | 241.62 (222.04) | 236.74 (202.24) | −26.17 [−49.54; −3.30] | −8.01 [−29.12; 15.61] | 18.16 [−14.51; 50.84] |
| Dermatologists | 12.63 (38.03) | 15.44 (43.54) | −1.42 [−6.07; 3.24] | 3.66 [−5.96; 15.92] | 5.08 [−7.53; 17.69] |
| Naturopaths/osteopaths | 11.20 (92.12) | 10.65 (65.02) | −2.08 [−13.87; 6.51] | 4.13 [−4.54; 13.39] | 6.20 [−7.10; 19.51] |
| ENT | 11.13 (33.76) | 12.49 (35.53) | 7.31 [1.90; 12.76] | 5.98 [0.11; 12.09] | −1.34 [−9.57; 6.90] |
| Chiropody | 67.97 (122.29) | 64.70 (124.13) | 9.96 [−3.91; 22.32] | 18.07 [7.01; 29.94] | 8.11 [−9.35; 25.58] |
| Neurologists | 47.45 (195.91) | 21.80 (72.10) | −24.88 [−49.29; −6.37] | 1.22 [−10.01; 13.55] | 26.10 [1.19; 51.01] |
| Orthopedic surgeons | 68.35 (188.45) | 63.06 (237.32) | −18.96 [−41.74; 3.74] | −6.96 [−36.06; 17.97] | 12.01 [−23.11; 47.12] |
| Psychotherapists | 14.75 (141.37) | 58.22 (415.46) | 6.24 [−11.36; 27.80] | −2.80 [−34.15; 27.99] | −9.04 [−44.84; 26.76] |
| Radiologists | 7.05 (56.72) | 5.79 (59.93) | −3.52 [−10.59; 2.94] | −2.90 [−10.44; 3.48] | 0.63 [−9.56; 10.81] |
| Speech therapy | 0.00 (0.00) | 0.00 (0.00) | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | 0.00 [NA; NA] |
| Urologists | 10.05 (38.74) | 8.50 (34.74) | −0.19 [−5.91; 6.24] | −0.54 [−5.49; 4.82] | −0.35 [−8.60; 7.89] |
| Dentists | 125.87 (203.22) | 164.25 (301.01) | 29.41 [−4.56; 61.18] | 7.48 [−33.08; 47.82] | −21.93 [−74.32; 30.46] |
*1 final value – initial value
*2 with lower and upper confidence limits based on the t-distribution in the intention-to-treat population
95% CI, 95% confidence interval; SD, standard deviation
Discussion
The aim of the StärkeR project to demonstrate the non-inferiority of team-based versus standard care in terms of disease activity in patients with stable RA or PsA in a randomized, multicenter, rater-blinded design was achieved by the present study. Several international studies (17– 19) and a meta-analysis (20), as well as a German study also supported by the Innovation Fund, came to similar conclusions as the StärkeR project (21). However, the German study had a different design without blinding of the endpoint measurement, with quarterly alternating care by the SRA and the physician in the SRA group, and the possibility of including patients with high disease activity. Overall, it can be assumed that a team-based form of care with delegation of certain physician tasks to qualified SRAs is possible in the German healthcare system and is largely equivalent to standard care.
This offers several advantages, as the freed-up physician resources can result in an improved allocation of rheumatologic expertise to the benefit of patients with urgent treatment needs. Although the total treatment time of 22 minutes for the team-based model of care was slightly longer than that for standard care, there was a physician time gain of eight minutes per patient (14 minutes of physician treatment time in standard care, six minutes in team-based care). In the German study mentioned above, the mean physician treatment time was almost as long in the delegation group, at seven minutes (21). After the familiarization period, SRAs became more efficient in everyday care, although no refresher course was offered. As expected, SRAs initially required a relatively large amount of time (mean 26 minutes) per consultation; over time, however, this eventually decreased to 16 minutes per patient.
No evidence for superiority of the team-based form of care in terms of health-related quality of life was demonstrated with respect to the second study objective. Both groups showed only a slight, clinically insignificant, improvement in EQ-5D-5L over the course of one year.
There was no difference between the treatment groups with regard to the secondary endpoints. Patient satisfaction was also comparably high. Differences in patient satisfaction as in England (22) were not evident – this was possibly a reflection of the different healthcare systems.
While cost and efficiency studies of team-based care are already available from other European countries (17, 23), such studies have so far been lacking in Germany. Resource use as assessed by health economic analysis showed no relevant differences between the treatment strategies in the StärkeR project. In particular, the type of care provided had no impact on the costs associated with prescriptions for remedies or medications.
Limitations
Limitations arise on the one hand from restricting the study to patients with the diagnoses RA and PsA. However, similar results have already been shown for patients with spondyloarthritis (24, 25) and gout (26). On the other hand, only patients with a stable disease course, i.e. with low disease activity in the previous months and without complicating comorbidities, were included in the StärkeR project. The available reports on the delegation of SRAs for patients with higher disease activity at every other consultation (21) and in patients with stable co-morbidities (17, 19) showed similar good results for delegation in terms of disease activity to our study.
The exclusion of seven patients from the intention-to-treat population for the analysis of the second primary endpoint does not constitute a limitation in our view, because only those patients were excluded who did not present at the baseline visit or who withdrew their consent immediately after randomization and before baseline. This approach is consistent with the recommendations of the International Council for Harmonisation (27). The intention-to-treat evaluation principle was not violated because the randomization result was not announced before the baseline visit and was thus unknown to the patients.
Because this was not a cluster-randomized trial that assigns entire practice teams to a particular form of care in each case, it could not be ruled out in principle in the StärkeR project that standard care patients also encountered a trained SRA in the office or outpatient clinic, which could result in a convergence of the effects of the two forms of care. Despite this possible limitation, however, our study even showed statistical superiority of SRA treatment with respect to disease activity. This underlines the reliability of the results.
Conclusion
The principles and long-term advantages of delegation to non-physician health professionals were presented by an ad hoc committee of the DGRh in 2020 (28). The results of the StärkeR project constitute a further step towards more delegation of physician tasks to qualified SRAs with the aim of increasing the capacity of rheumatological care while maintaining quality.
Supplementary Material
eMethods
Project-related specialization
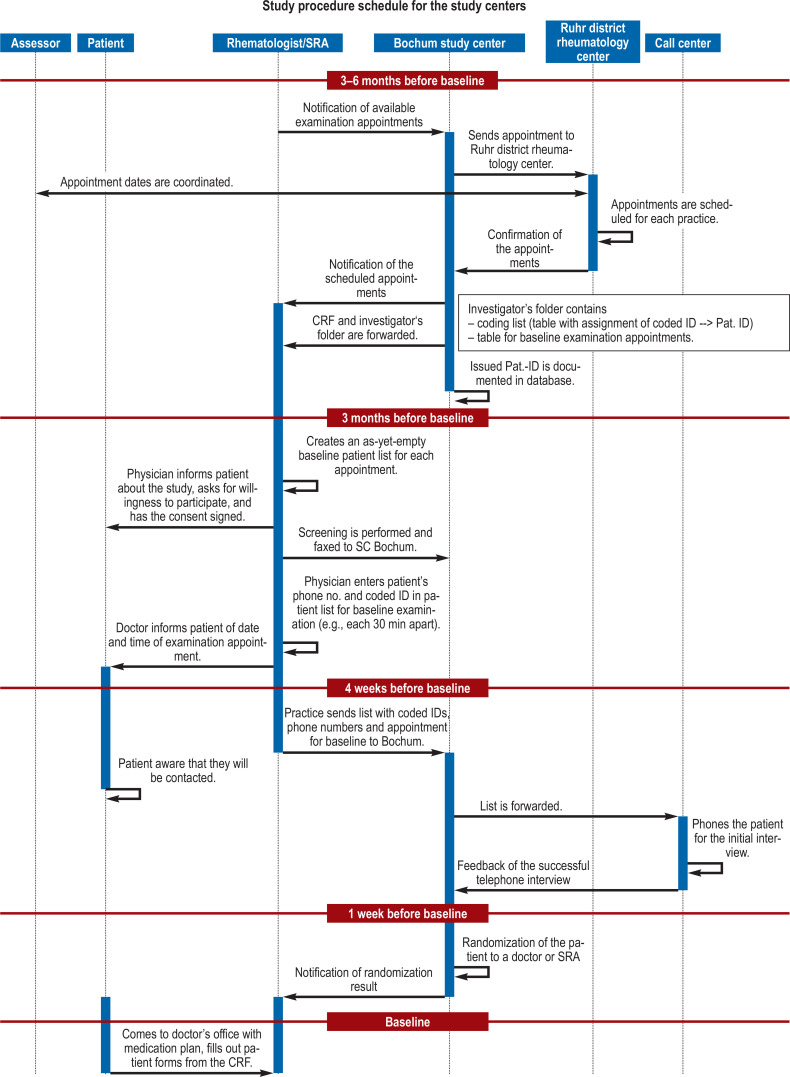
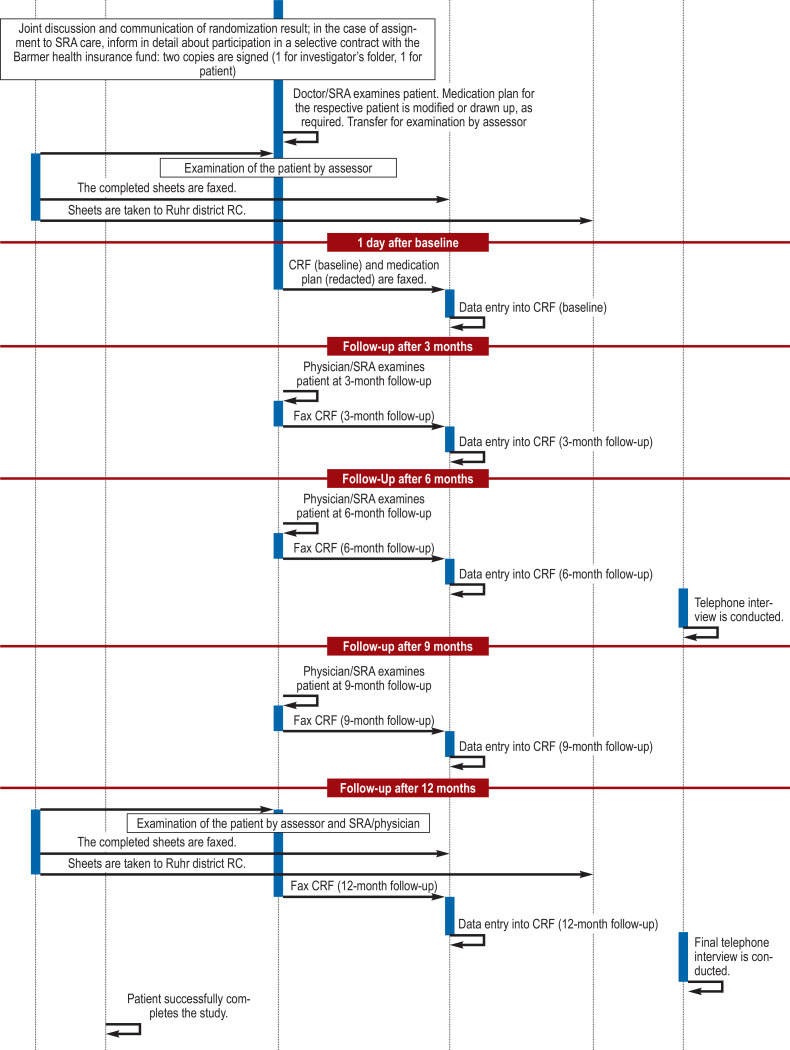
After refreshing the SRA (specialized rheumatological assistant) basic course material, which focused on the two clinical entities rheumatoid arthritis (RA) and psoriatic arthritis (PsA), the training content of the project-related specialization course for SRAs then involved presentation of the study-specific objectives and procedures. Emphasis was placed on the presentation and discussion of a flowchart demonstrating the study procedure schedule (efigure) and a checklist for interviewing patients (etable 2). This was followed by a joints examination course for the 28 joints to be evaluated.
Sample size
The sample size was calculated based on the assumption that mean changes in the DAS28 scores would not differ between the two groups after one year of intervention, with a standard deviation of 1.7 (17) and a non-inferiority margin of 0.4 (see study protocol [15]). Assuming that 5% of the final values would be missing, a total sample size of 400 patients per group was required to achieve a power of 90%. Based on a publication from Denmark (e1) (mean [standard deviation] for team-based care: 0.796 [0.158] and for standard care 0.748 [0.210]), this sample size is also able to demonstrate the superiority of health-related quality of life (EQ-5D-5L) in the team-based care group over the standard care group, with a two-tailed test at a significance level of a = 0.05 and a power of 94%. Problems with patient recruitment arose in the course of the study, however, so that only a sample size of 300 patients per group was achieved. This resulted in a power of 80% for the first test of non-inferiority in changes in disease activity over the course of one year and a power of 86% for the second test of superiority of the new model of care with regard to health-related quality of life (EQ-5D-5L).
Endpoint measurement/instruments for assessment
Disease Activity Score in 28 joints (DAS28)
Disease Activity Score 28 (DAS28) is calculated from the number of tender joints (0–28; comprising the shoulder, elbow, and wrist joints, the metacarpophalangeal and interphalangeal joints of the fingers [including the thumb interphalangeal joints], and the knee joints), the number of swollen joints (0–28), the erythrocyte sedimentation rate (mm/h), and the patient’s assessment of disease activity (numeric rating scale 0–10). The result on a scale of 0 to 9.4 can be broken down and interpreted as follows: remission: DAS28 <2.6; low disease activity: 2.6 to <3.2; moderate disease activity: 3.2 to <5.1; and high disease activity: ≥5.1.
With repeated testing at one-week intervals (test-retest reliability), DAS28 correlations ranged from 0.79–0.87, with an intraclass correlation (ICC) of 0.85. The results were similar when the tests were repeated after 24 months in patients on stable therapy.
The DAS28 recognizes between the different categories of response according to ACR (American College of Rheumatology) or EULAR and distinguishes exacerbation of RA from inactive RA. The instrument correlates with other measurements of RA disease activity and often serves as the gold standard when evaluating the quality of such measurements. The DAS28 is a predictor of radiographic outcomes and correlates with measurements of physical impairment (r = 0.70) and quality of life (36-item Short Form Health Survey [SF-36], r = 0.40–0.79). Achieving DAS28 remission is associated with improvements in physical function and health-related quality of life.
The DAS28 is highly responsive to changes in RA disease activity. The minimally important difference (MID) for the DAS28 is 1.2; EULAR considers this a good response and changes of 0.6–1.2 a moderate response (e2).
EuroQol in 5 dimensions and 5 response levels (EQ-5D-5L)
The three-level version of the EQ-5D (EQ-5D-3L) is one of the most widely used generic instruments for assessing health-related quality of life in Germany and other countries. A few years ago, the EuroQol Group introduced an improved version of this instrument to enhance its sensitivity by increasing the number of response levels per dimension from three to five. This is known as the EQ-5D-5L, the five-level version of the EQ-5D. This instrument comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), with each dimension having five levels of severity (no problems [level 1], slight [level 2], moderate [level 3], severe [level 4], and extreme problems/unable to [level 5]), thus describing a total of 3125 health states. Available evidence on the comparative responsiveness of the EQ-5D-3L and EQ-5D-5L suggests that the EQ-5D-5L
Secondary endpoints
The functional capacity of the patients was assessed using the Hanover Functional Status Questionnaire, which was developed specifically for rheumatic diseases (11). When asked about 16 activities of daily living, for example “getting dressed” or “picking up an object from the floor”, the respondents rate on a three-point scale whether they can perform these activities without difficulty, with difficulty, or not at all/only with help.
Physical activity was assessed using the PRISCUS-Physical Activity Questionnaire (PAQ). The questionnaire gathers information on activity in the categories of resting/sitting, housework, sports, gardening, and walking, and calculates total energy expenditure (in MET-hours [MET, metabolic equivalent]) per week from these activities (PRISCUS-PAQ) (12).
Depression was assessed using the PHQ-2 (13). On a four-point scale (ranging from “not at all” to “nearly every day”), patients rated how often they felt affected by the following symptoms in the previous two weeks: 1. Little interest and pleasure in activities. 2. Feeling down, depressed, hopeless. The score with values from 0 to 6 is calculated from the answers to these two questions.
Disease activity, pain intensity, exhaustion/fatigue, and sleep disturbance were assessed on a numerical rating scale ranging from 0 (not at all) to 10 (very much). The duration of morning stiffness of the joints was assessed with a simple question.
Patient satisfaction was assessed in an unstandardized manner by asking six questions: patient waiting time in the practice (15, 30, 60, 90, 120 minutes), accessibility of the physician/SRA (five-point scale from “very good” to “very poor”), satisfaction with the opportunity to ask questions, with the information received, with the relationship with the rheumatologist or SRA, and with the cooperation between the different health professionals (each on a five-point scale from “very satisfied” to “dissatisfied”).
Clinical Disease Activity Index (CDAI) – Treatment management element
The CDAI assesses disease activity of rheumatoid arthritis (RA), much like the DAS28. The instrument similarly includes the assessment of swollen (0–28) and tender joints (0–28) as well as the patient’s self-assessment of disease activity (Numeric Rating Scale [NRS], 0–10). Instead of the laboratory results ESR (erythrocyte sedimentation rate) and CRP (C-reactive protein), the score includes the physician’s assessment of disease activity (NRS 0–10). The CDAI (range 0–76) is calculated from the sum of the results.
The levels of disease activity are classified as follows: remission: ≤2.8; low disease activity: >2.8–10; moderate disease activity: >10–22; and high disease activity: >22. The score is suitable for treatment management as it can be calculated directly without the need for laboratory tests. In the StärkeR trial, it was decided that a change in therapy should be considered if CDAI >10 (e2).
Statistical analysis
The primary endpoint was evaluated hierarchically. This means that the tolerated error probability of 2.5% for one-sided and 5% for two-sided tests for a type-1 error can be reapplied at each new step, as long as the previous step achieves statistical significance. For analysis of the first part of the primary endpoint, a linear mixed model was used to assess non-inferiority, and the non-inferiority margin was set at 0.4. In addition to type of care (standard care or team-based form of care), this model included baseline DAS28 scores, type of disease (RA or psoriatic arthritis [PsA]), and patient sex as fixed effects and treatment center as a random effect. Evaluation of the per-protocol population was performed according to the recommendations of the EMA (16). The definition of the per-protocol population specified that patients were required to have participated in at least one visit in addition to the baseline and final visits. For the test of superiority of the team-based form of care over standard care in terms of health-related quality of life (changes in EQ-5D-5L [10] after one year), a similar linear mixed model was used with the baseline value of each endpoint and age as further covariates. This evaluation was based on the intention-to-treat approach. Multiple imputations were used for missing values, under the assumption they were missing at random. The evaluations were also implemented in the other population for sensitivity analysis of the two primary endpoints (eResults).
Continuous secondary endpoints were also evaluated with linear mixed models, taking into account baseline, type of care, respective center, age, and sex. For this purpose, the intention-to-treat population was used; a sensitivity analysis in the per-protocol population is presented in the eResults. The categorical secondary endpoint “patient satisfaction” was evaluated descriptively.
Health economic evaluation
In addition to testing clinical effectiveness of this new form of care involving delegation of physicians’ tasks, an economic evaluation was conducted to deal with questions of health economics. This evaluation was confined to using voluntary information submitted about the use of various resources and to the cost estimate derived from this for a selection of direct services reimbursed by the health insurance fund. Indirect costs, such as those that arise as a result of sickness-related loss of work, were excluded from the present study. Data acquisition was based on interviews with patients regarding their respective resource use utilizing the “Questionnaire on the Use of Medical and Non-medical Care Services in Old Age”, FIMA for short (14), at baseline and after 26 and 52 weeks.
Multiple imputation of missing data
Missing or non-specific answers (for example: “n.a.”) to the cost items included in the total cost estimate were quantified according to treatment groups with regard to their share of the total sample. For the evaluation of those patients with missing values in the sense of the intention-to-treat principle, multiple imputation was performed using a two-stage procedure in which the following factors were considered as model predictors:
1. In a first step, involved patients were first classified according to the probability with which they had indicated, according to the model prediction, that they had not used the resource in the relevant period. Scoring using logistic regression with the variables mentioned was used for this classification.
2. In a second step, those patients who had not been assigned zero cost in the first step were assigned values using predictive mean matching, also with regard to the variables mentioned.
The number of imputations was 20 in both cases.
Group comparison of evaluated total costs (with and without drug prescriptions)
For all the randomized patients (intention-to-treat population), the calculation of total costs was based on the following types of resources:
Drug prescriptions for fixed-price drugs were evaluated using the database of the German Institute of Medical Documentation and Information (DIMDI, 2021). Where relevant, the estimated costs for medical aids are based on the published reference prices of the German Association of Statutory Health Insurance Funds (2021) (for example, for hearing or vision aids).
In addition to these items, it was originally intended that prescribed aids, outpatient surgery/day care unit, and inpatient stays in hospitals and psychiatric institutions would also be included in the total cost calculation. However, it became clear here that the requested data are usually too non-specific for a valid cost calculation if, for example, in the case of surgical procedures not further specified or multiple aids, a very heterogeneous spectrum of possible cost effects is involved, within which actual expenditure cannot be established without knowledge of the indication. Since the validity of the results for these analyses would be severely impaired due to too much non-specific data, these items were consequently excluded from the study entirely.
Furthermore, for drugs for which no reference prices were available (e.g., for those under patent protection) and for those where BARMER Health Insurance was unable to provide cost data, we refrained from making our own estimate because the validity of the results from such constructed data would also appear doubtful.
After applying multiple imputation as stated in the statistical analysis plan, 95% confidence intervals were calculated based on the t-distribution for the mean change in costs relative to the same period of the previous year and for the difference in this change between the two types of treatment.
Total cost estimate and reference cost rates
For the estimation of total annual costs, the (inflation-adjusted) standardized valuation rates from Bock et al. (e3) and publicly available, archived reference price lists for drugs (provided by the BfArM, formerly the database of the German Institute for Medical Documentation and Information, at www.dimdi.de) were used for 2019. eTable 3 (modified from Ossendorf 2019 [e4]) provides an overview of the specific procedure for calculating costs for the items included here, as well as sources for the reference cost amounts used in each case.
is a useful improvement in measurement properties in terms of reducing ceiling effects and
provides better discrimination with greater ability to detect differences between groups as compared with the EQ-5D-3L (10).
type of ressource
questionnaire phase (whether after 26 or 52 weeks)
the attending physician (19 factor levels)
patient’s year of birth
patient’s sex
treatment arm
specialist outpatient consultations
drug prescriptions
healthcare services, including remedies
eResults
Analysis of primary endpoints—full models
eTables 4 and 5 show the full models for the primary endpoint evaluation of the DAS28 in the per-protocol population and the EQ-5D-5L in the intention-to-treat population.
Sensitivity analyses
Sensitivity analyses of the two primary endpoints each population are presented below, as well as a per-protocol analysis of the secondary endpoints.
Evaluation of the DAS28 in the intention-to-treat population showed a difference of -0.18 (95% confidence interval: [-0.35; -0.02]) in the linear mixed model after adjustment; the p value for non-inferiority was <0.001. The supplementary test for superiority yielded a p value of 0.032, so that, statistically, superiority of the team-based form of care was also shown for the sensitivity analysis in the intention-to-treat population (etable 6).
In the EQ-5D-5L analysis, the linear mixed model in the per-protocol population yielded an estimated difference of 0.02 [-0.01; 0.05] and a p value of 0.206 (etable 7). The results are comparable to the evaluation in the intention-to-treat population.
eTable 8 presents the results of the sensitivity analysis of the secondary endpoints in the per-protocol population. With the exception of physical activity, the results are comparable with those in the intention-to-treat population.
eFigure.
eFigure.
CRF, case report form; SRA, specialized rheumatological assistant; RC, rheumatology center
eTable 2. Check list for specialized rheumatological assistants (SRAs).
| Patient name: | ID – No: |
| How have you been since your last review? | |
| Have there been any changes with regard to your rheumatism? ○ What is better? ○ What is worse? |
|
| Joint complaints: ○ at rest ○ during exercise ○ in the morning ○ in the evening |
|
| Do you have problems at work? | |
| Have you been absent from work? | |
| Have you been in hospital as an inpatient? If so: when, why, for how long? | |
| Have you been to the eye doctor? | |
| Have there been any new diagnoses or symptoms? ○ fatigue ○ fever ○ infections ○ cough ○ diarrhea |
|
| Has your family doctor performed any tests on you in the meantime? ○ blood pressure ○ cholesterol ○ blood sugar ○ other laboratory tests |
|
| Do you smoke? ○ if yes, how many cigarettes/day? |
|
| Current medication plan available? | |
| If not: Which medications are you currently taking? ○ dose ○ when and how often ○ medication breaks? |
|
| Are you pregnant? Is pregnancy planned? |
|
| Current vaccination status? Please bring your vaccination card with you (1x per year) |
|
| Collect CRF questionnaires (case report form) Abnormal entries? |
|
| Examinations related to documentation in the CRF | |
| Assessment of disease activity by SRA (to calculate Clinical Disease Activity Index [CDAI]) | |
| Skin changes | |
| Blood pressure | |
| Heart rate | |
| Height Weight | |
| Preparation for examination by physician | |
| Prepare laboratory requests | |
| Prepare report for primary care physician | |
| CDAI >10? Does a change in therapy seem appropriate? |
Yes: No: |
| Prescriptions ready? | |
| Examination by physician | |
| Documentation of treatment ○ no change in treatment ○ change in treatment |
|
| Sign prescriptions | |
| Sign report | |
| Additional laboratory requests? | |
| Follow-up discussion SRA / patient | |
| Discuss medication plan, in particular, explain once again any change in therapy, mode of intake, etc. |
|
| Schedule next appointments | |
| Where applicable, hand out report and prescription | |
eTable 3. Calculation procedures of the cost types included in the total cost estimate and source of the reference cost amounts used.
| Resource | Base unit | Cost evaluation |
| Specialist outpatient consultations | Number of visits to the doctor, broken down by specialist groups | Multiplied by the mean value of contact costs for each specialist group (cf. Bock et al. 2015 [e3]) |
| Drug prescriptions | Type and number of drugs | Multiplied by the current inflation-adjusted prices for fixed-price drugs (DIMDI, 2021); otherwise, by reference costs provided by BARMER Health Insurance Fund |
| Outpatient surgical procedures/day care unit, inpatient stays in hospital, including psychiatric facilities | Number and duration of hospital stays (i.e. overnight stays) and/oroutpatient surgical procedures, stays in day care units and/or psychiatric facilities | Excluded from cost estimation due to too non-specific data |
| Healthcare services, including remedies | Type and number of services | Multiplied either by statutory health insurance reimbursement rates (if available) or by researched cost rates and estimates |
eTable 4. Analysis of the first primary endpoint DAS28—full model in the per-protocol population.
| Group comparison team-based − standard | ||||
| Model coefficient*1 | 95% confidence interval | p value | p value non-inferiority | |
| (Intercept) | 1.58 | [1.23; 1.93] | < 0.001 | |
| Baseline DAS28 | −0.41 | [−0.48; −0.34] | < 0.001 | |
| Male sex | −0.33 | [−0.53; −0.12] | 0.002 | |
| New form of care | −0.19 | [−0.36; −0.02] | 0.025*2 | < 0.001 |
| Psoriatic arthritis | −0.02 | [−0.24; 0.20] | 0.862 | |
*1 estimated using a linear mixed model with the variables listed and with a random center effect
*2 p value for superiority
DAS28, Disease Activity Score with 28 joints
eTable 5. Analysis of the second primary endpoint EQ-5D-5L—full model in the intention-to-treat population.
| Group comparison team-based − standard | |||
| Model coefficient* | 95% confidence interval | p value | |
| (Intercept) | 0.43 | [0.31; 0.56] | < 0.001 |
| Baseline EQ-5D-5L | −0.41 | [−0.48; −0.34] | < 0.001 |
| Male sex | 0.05 | [0.01; 0.09] | 0.017 |
| Age (per 10 years) | −0.03 | [−0.04; −0.01] | 0.001 |
| New form of care | 0.02 | [−0.02; 0.05] | 0.285 |
| Psoriatic arthritis | 0.00 | [−0.04; 0.05] | 0.832 |
* estimated using a linear mixed model with the variables listed and with a random center effect
EQ-5D-5L, EuroQol in 5 dimensions and 5 response levels
eTable 6. Sensitivity analysis of the first primary endpoint DAS28—full model in the intention-to-treat population.
| Group comparison team-based − standard | ||||
| Model coefficient*1 | 95% confidence interval | p value | p value non-inferiority | |
| (Intercept) | 1.56 | [1.21; 1.91] | < 0.001 | |
| Baseline DAS28 | −0.41 | [−0.48; −0.34] | < 0.001 | |
| Male sex | −0.33 | [−0.54; −0.13] | 0.002 | |
| New form of care | −0.18 | [−0.35; −0.02] | 0.032 *2 | < 0.001 |
| Psoriatic arthritis | −0.00 | [−0.23; 0.22] | 0.972 | |
*1 estimated using a linear mixed model with the variables listed and with a random center effect
*2 p value for superiority DAS28, Disease Activity Score with 28 joints
eTable 7. Sensitivity analysis of the second primary endpoint EQ-5D-5L—full model in the per-protocol population.
| Group comparison team-based − standard | |||
| Model coefficient* | 95% confidence interval | p value | |
| (Intercept) | 0.43 | [0.31; 0.55] | < 0.001 |
| Baseline EQ-5D-5L | −0.41 | [−0.48; −0.34] | < 0.001 |
| Male sex | 0.05 | [0.01; 0.09] | 0.018 |
| Age (per 10 years) | −0.03 | [−0.04; −0.01] | 0.001 |
| Neu form of care | 0.02 | [−0.01; 0.05] | 0.206 |
| Psoriatic arthritis | 0.00 | [−0.48; 0.48] | 0.937 |
* estimated using a linear mixed model with the variables listed and with a random center effect EQ-5D-5L, EuroQol in 5 dimensions and 5 response levels.
eTable 8. Sensitivity analysis of the secondary endpoints in the per-protocol population.
| Group comparison Delegation − Standard | |||
| Estimated difference*1 | 95% confidence interval | Direction | |
| CRP (mg/L)*2 | 0.1 | [−0.1; 0.2] | ↓ |
| Disease activity (NRS) | −0.1 | [−0.5; 0.2] | ↑ |
| Pain intensity (NRS) | −0.2 | [−0.6; 0.1] | ↑ |
| Fatigue (NRS) | −0.2 | [−0.5; 0.2] | ↑ |
| Sleep disturbances (NRS) | −0.3 | [−0.7; 0.1] | ↑ |
| Duration of morning stiffness (minutes) | 0.7 | [−4.1; 5.5] | ↓ |
| Depression (PHQ-2) | −0.1 | [−0.3; 0.2] | ↑ |
| Functional capacity (FFbH) | −0.2 | [−2.8; 2.5] | ↑ |
| Physical activity (PRISCUS-PAQ) | 1.6 | [−0.1; 3.3] | ↓ |
*1 estimated using a linear mixed model for each endpoint with adjustment for patient age and sex,respective baseline value, and center (random effect)
*2 given the skewness of the distribution, median and interquartile range (Q25-Q75) of changes are presented, and the model is calculated with log CRP.
CRP, C-reactive protein; FFbH: Hanover Functional Status Questionnaire (0–100);
NRS, Numeric Rating Scale (0–10);
PAQ: Physical Activity Questionnaire (≥168 MET hours/week [MET, metabolic equivalent]);
PHQ-2, Patient Health Questionnaire 2 Items (0–6);
↑ indicates the advantage of the team-based form of care, ↓ advantage of standard care
Acknowledgments
Translated from the original German by Dr. Grahame Larkin, MD.
Funding
The StärkeR project was funded by the Innovation Fund (funding code: 01NVF17004). The grant was applied for by Prof. Braun and Prof. Trampisch. Directly or indirectly, all authors were supported by Innovation Fund grants.
Acknowledgments
The authors would like to thank the patients, specialized rheumatological assistants, and assessors who participated in the StärkeR project. They would also like to thank the Rheumatism Academy (especially Prof. Erika Gromnica-Ihle MD), Dr. Gisela Reichmuth MD (for conducting the joints examination course), Dr. Ioana Andreica MD and Barbara Guminski (for organizing the assessor assignments), the AMIB employees Michelle Stein, Julien Stein, Silvia Stein, Achim Gursch, the BARMER employees Till Beckmann and Ulrich Walter as well as the employees of the call center, and finally the participating rheumatologists Dr. Ulrich Schoo MD, Dr. Ernst Gernot Scheibl MD and Dr. Albert Schmid MD.
Data sharing
Individual patient data (after anonymization) that support the findings reported in this article (text, tables, figures, and eSupplement) may be made available to academic researchers upon request in the period from three months to five years after publication of this article. Proposals should be directed to Dr. Anna Mai (mai@amib.rub.de). The application process includes scrutiny of the intended research question and research method. After approval, the data will be made available on an external website. Applicants must sign a data access agreement for access to data. The data may only be used for the purposes formulated in the evaluation plan.
Footnotes
Conflict of interest statement
Judith Günzel received reimbursement of participation fees for an event by Abbvie.
Dr. Elmar Schmitz received reimbursement of congress participation fees from Sandoz and Hexal.
The other authors confirm that there are no conflicts of interest.
References
- 1.Albrecht K, Callhoff J, Edelmann E, Schett G, Schneider M, Zink A. Klinische Remission bei rheumatoider Arthritis Daten aus der Früharthritiskohortenstudie CAPEA. Z Rheumatol. 2016;75:90–96. doi: 10.1007/s00393-015-0019-5. [DOI] [PubMed] [Google Scholar]
- 2.Zink A, Braun J, Gromnica-Ihle E, et al. Memorandum der Deutschen Gesellschaft für Rheumatologie zur Versorgungsqualität in der Rheumatologie - Update 2016. Z Rheumatol. 2017;76:195–207. doi: 10.1007/s00393-017-0297-1. [DOI] [PubMed] [Google Scholar]
- 3.van Eijk-Hustings Y, van Tubergen A, Boström C, et al. EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Dis. 2012;71:13–19. doi: 10.1136/annrheumdis-2011-200185. [DOI] [PubMed] [Google Scholar]
- 4.Bech B, Primdahl J, van Tubergen A, et al. 2018 update of the EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Dis. 2020;79:61–68. doi: 10.1136/annrheumdis-2019-215458. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Aletaha D. Scores for all seasons: SDAI and CDAI. Clin Exp Rheumatol. 2014;32:S75–S79. [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 8.Fransen J, van Riel PLCM. The disease activity score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35:745–757. doi: 10.1016/j.rdc.2009.10.001. vii-viii. [DOI] [PubMed] [Google Scholar]
- 9.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36:663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zochling J, Stucki G, Grill E, Braun J. A comparative study of patient-reported functional outcomes in acute rheumatoid arthritis. J Rheumatol. 2007;34:64–69. [PubMed] [Google Scholar]
- 12.Trampisch US, Platen P, Moschny A, Wilm S, Thiem U, Hinrichs T. Messung körperlicher Aktivität bei älteren Erwachsenen Übereinstimmung zwischen PRISCUS-PAQ und Akzelerometrie. Z Gerontol Geriatr. 2012;45:212–217. doi: 10.1007/s00391-011-0264-4. [DOI] [PubMed] [Google Scholar]
- 13.Löwe B, Kroenke K, Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J Psychosom Res. 2005;58:163–171. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Seidl H, Bowles D, Bock JO, et al. FIMA-Fragebogen zur Erhebung von Gesundheitsleistungen im Alter: Entwicklung und Pilotstudie. Gesundheitswesen. 2015;77:46–52. doi: 10.1055/s-0034-1372618. [DOI] [PubMed] [Google Scholar]
- 15.Mai A, Braun J, Reese JP, et al. Nurse-led care versus physician-led care in the management of rheumatoid arthritis and psoriatic arthritis (StaerkeR): study protocol for a multi-center randomized controlled trial. Trials. 2019;20 doi: 10.1186/s13063-019-3808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. CONSORT Group Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 17.Ndosi M, Lewis M, Hale C, et al. The outcome and cost-effectiveness of nurse-led care in people with rheumatoid arthritis: a multicentre randomised controlled trial. Ann Rheum Dis. 2014;73:1975–1982. doi: 10.1136/annrheumdis-2013-203403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Primdahl J, Sørensen J, Horn HC, Petersen R, Hørslev-Petersen K. Shared care or nursing consultations as an alternative to rheumatologist follow-up for rheumatoid arthritis outpatients with low disease activity—patient outcomes from a 2-year, randomised controlled trial. Ann Rheum Dis. 2014;73:357–364. doi: 10.1136/annrheumdis-2012-202695. [DOI] [PubMed] [Google Scholar]
- 19.Bergsten U, Almehed K, Baigi A, Jacobsson LTH. A randomized study comparing regular care with a nurse-led clinic based on tight disease activity control and person-centred care in patients with rheumatoid arthritis with moderate/high disease activity: a 6-month evaluation. Musculoskeletal Care. 2019;17:215–225. doi: 10.1002/msc.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurah de A, Esbensen BA, Roelsgaard IK, Frandsen TF, Primdahl J. Efficacy of embedded nurse-led versus conventional physician-led follow-up in rheumatoid arthritis: a systematic review and meta-analysis. RMD Open. 2017;3 doi: 10.1136/rmdopen-2017-000481. e000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeper JR, Zeidler J, Meyer SE, et al. Effect of nurse-led care on outcomes in patients with ACPA/RF-positive rheumatoid arthritis with active disease undergoing treat-to-target: a multicentre randomised controlled trial. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001627. e001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J, Thorpe R, Bird H. Outcomes for patients with RA: a rheumatology nurse practitioner clinic compared to standard outpatient care. Musculoskeletal Care. 2003;1:5–20. doi: 10.1002/msc.35. [DOI] [PubMed] [Google Scholar]
- 23.van den Hout WB, Tijhuis GJ, Hazes JMW, Breedveld FC, Vliet Vlieland TPM. Cost effectiveness and cost utility analysis of multidisciplinary care in patients with rheumatoid arthritis: a randomised comparison of clinical nurse specialist care, inpatient team care, and day patient team care. Ann Rheum Dis. 2003;62:308–315. doi: 10.1136/ard.62.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiltz U, Spiller I, Sieper J, Braun J. Ist eine Delegation ärztlicher Leistungen auf rheumatologische Fachassistenten bei der Evaluierung von Patienten mit Verdacht auf ankylosierende Spondylitis möglich? Ergebnisse der PredAS-Studie. Z Rheumatol. 2020;79:729–736. doi: 10.1007/s00393-020-00838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molto A, Gossec L, Poiraudeau S, et al. Evaluation of the impact of a nurse-led program of systematic screening of comorbidities in patients with axial spondyloarthritis: The results of the COMEDSPA prospective, controlled, one year randomized trial. Semin Arthritis Rheum. 2020;50:701–708. doi: 10.1016/j.semarthrit.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet. 2018;392:1403–1412. doi: 10.1016/S0140-6736(18)32158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ICH harmonized tripartite guideline. Statistical principles for clinical trials: E9. www.database.ich.org/sites/default/files/E9_Guideline.pdf. . 1998 [Google Scholar]
- 28.Krause A, Krüger K, Braun J, et al. Delegation ärztlicher Leistungen in der Rheumatologie. Z Rheumatol. 2020;79:47–48. doi: 10.1007/s00393-020-00862-8. [DOI] [PubMed] [Google Scholar]
- E1.Sørensen J, Primdahl J, Horn HC, Hørslev-Petersen K. Shared care or nurse consultations as an alternative to rheumatologist follow-up for rheumatoid arthritis (RA) outpatients with stable low disease-activity RA: cost-effectiveness based on a 2-year randomized trial. Scand J Rheumatol. 2015;44:13–21. doi: 10.3109/03009742.2014.928945. [DOI] [PubMed] [Google Scholar]
- E2.Johnson TM, Michaud K, England BR. Measures of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2020;72(Suppl. 10):4–26. doi: 10.1002/acr.24336. [DOI] [PubMed] [Google Scholar]
- E3.Bock JO, Brettschneider C, Seidl H, et al. Ermittlung standardisierter Bewertungssätze aus gesellschaftlicher Perspektive für die gesundheitsökonomische Evaluation. Gesundheitswesen. 2015;77:53–61. doi: 10.1055/s-0034-1374621. [DOI] [PubMed] [Google Scholar]
- E4.Ossendorf A. Krankheitskostenanalyse bei Patienten mit chronischen neuropathischen Schmerzen. Gesundheitsökonomie & Qualitätsmanagement. 2019;24:42–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Project-related specialization
After refreshing the SRA (specialized rheumatological assistant) basic course material, which focused on the two clinical entities rheumatoid arthritis (RA) and psoriatic arthritis (PsA), the training content of the project-related specialization course for SRAs then involved presentation of the study-specific objectives and procedures. Emphasis was placed on the presentation and discussion of a flowchart demonstrating the study procedure schedule (efigure) and a checklist for interviewing patients (etable 2). This was followed by a joints examination course for the 28 joints to be evaluated.
Sample size
The sample size was calculated based on the assumption that mean changes in the DAS28 scores would not differ between the two groups after one year of intervention, with a standard deviation of 1.7 (17) and a non-inferiority margin of 0.4 (see study protocol [15]). Assuming that 5% of the final values would be missing, a total sample size of 400 patients per group was required to achieve a power of 90%. Based on a publication from Denmark (e1) (mean [standard deviation] for team-based care: 0.796 [0.158] and for standard care 0.748 [0.210]), this sample size is also able to demonstrate the superiority of health-related quality of life (EQ-5D-5L) in the team-based care group over the standard care group, with a two-tailed test at a significance level of a = 0.05 and a power of 94%. Problems with patient recruitment arose in the course of the study, however, so that only a sample size of 300 patients per group was achieved. This resulted in a power of 80% for the first test of non-inferiority in changes in disease activity over the course of one year and a power of 86% for the second test of superiority of the new model of care with regard to health-related quality of life (EQ-5D-5L).
Endpoint measurement/instruments for assessment
Disease Activity Score in 28 joints (DAS28)
Disease Activity Score 28 (DAS28) is calculated from the number of tender joints (0–28; comprising the shoulder, elbow, and wrist joints, the metacarpophalangeal and interphalangeal joints of the fingers [including the thumb interphalangeal joints], and the knee joints), the number of swollen joints (0–28), the erythrocyte sedimentation rate (mm/h), and the patient’s assessment of disease activity (numeric rating scale 0–10). The result on a scale of 0 to 9.4 can be broken down and interpreted as follows: remission: DAS28 <2.6; low disease activity: 2.6 to <3.2; moderate disease activity: 3.2 to <5.1; and high disease activity: ≥5.1.
With repeated testing at one-week intervals (test-retest reliability), DAS28 correlations ranged from 0.79–0.87, with an intraclass correlation (ICC) of 0.85. The results were similar when the tests were repeated after 24 months in patients on stable therapy.
The DAS28 recognizes between the different categories of response according to ACR (American College of Rheumatology) or EULAR and distinguishes exacerbation of RA from inactive RA. The instrument correlates with other measurements of RA disease activity and often serves as the gold standard when evaluating the quality of such measurements. The DAS28 is a predictor of radiographic outcomes and correlates with measurements of physical impairment (r = 0.70) and quality of life (36-item Short Form Health Survey [SF-36], r = 0.40–0.79). Achieving DAS28 remission is associated with improvements in physical function and health-related quality of life.
The DAS28 is highly responsive to changes in RA disease activity. The minimally important difference (MID) for the DAS28 is 1.2; EULAR considers this a good response and changes of 0.6–1.2 a moderate response (e2).
EuroQol in 5 dimensions and 5 response levels (EQ-5D-5L)
The three-level version of the EQ-5D (EQ-5D-3L) is one of the most widely used generic instruments for assessing health-related quality of life in Germany and other countries. A few years ago, the EuroQol Group introduced an improved version of this instrument to enhance its sensitivity by increasing the number of response levels per dimension from three to five. This is known as the EQ-5D-5L, the five-level version of the EQ-5D. This instrument comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), with each dimension having five levels of severity (no problems [level 1], slight [level 2], moderate [level 3], severe [level 4], and extreme problems/unable to [level 5]), thus describing a total of 3125 health states. Available evidence on the comparative responsiveness of the EQ-5D-3L and EQ-5D-5L suggests that the EQ-5D-5L
Secondary endpoints
The functional capacity of the patients was assessed using the Hanover Functional Status Questionnaire, which was developed specifically for rheumatic diseases (11). When asked about 16 activities of daily living, for example “getting dressed” or “picking up an object from the floor”, the respondents rate on a three-point scale whether they can perform these activities without difficulty, with difficulty, or not at all/only with help.
Physical activity was assessed using the PRISCUS-Physical Activity Questionnaire (PAQ). The questionnaire gathers information on activity in the categories of resting/sitting, housework, sports, gardening, and walking, and calculates total energy expenditure (in MET-hours [MET, metabolic equivalent]) per week from these activities (PRISCUS-PAQ) (12).
Depression was assessed using the PHQ-2 (13). On a four-point scale (ranging from “not at all” to “nearly every day”), patients rated how often they felt affected by the following symptoms in the previous two weeks: 1. Little interest and pleasure in activities. 2. Feeling down, depressed, hopeless. The score with values from 0 to 6 is calculated from the answers to these two questions.
Disease activity, pain intensity, exhaustion/fatigue, and sleep disturbance were assessed on a numerical rating scale ranging from 0 (not at all) to 10 (very much). The duration of morning stiffness of the joints was assessed with a simple question.
Patient satisfaction was assessed in an unstandardized manner by asking six questions: patient waiting time in the practice (15, 30, 60, 90, 120 minutes), accessibility of the physician/SRA (five-point scale from “very good” to “very poor”), satisfaction with the opportunity to ask questions, with the information received, with the relationship with the rheumatologist or SRA, and with the cooperation between the different health professionals (each on a five-point scale from “very satisfied” to “dissatisfied”).
Clinical Disease Activity Index (CDAI) – Treatment management element
The CDAI assesses disease activity of rheumatoid arthritis (RA), much like the DAS28. The instrument similarly includes the assessment of swollen (0–28) and tender joints (0–28) as well as the patient’s self-assessment of disease activity (Numeric Rating Scale [NRS], 0–10). Instead of the laboratory results ESR (erythrocyte sedimentation rate) and CRP (C-reactive protein), the score includes the physician’s assessment of disease activity (NRS 0–10). The CDAI (range 0–76) is calculated from the sum of the results.
The levels of disease activity are classified as follows: remission: ≤2.8; low disease activity: >2.8–10; moderate disease activity: >10–22; and high disease activity: >22. The score is suitable for treatment management as it can be calculated directly without the need for laboratory tests. In the StärkeR trial, it was decided that a change in therapy should be considered if CDAI >10 (e2).
Statistical analysis
The primary endpoint was evaluated hierarchically. This means that the tolerated error probability of 2.5% for one-sided and 5% for two-sided tests for a type-1 error can be reapplied at each new step, as long as the previous step achieves statistical significance. For analysis of the first part of the primary endpoint, a linear mixed model was used to assess non-inferiority, and the non-inferiority margin was set at 0.4. In addition to type of care (standard care or team-based form of care), this model included baseline DAS28 scores, type of disease (RA or psoriatic arthritis [PsA]), and patient sex as fixed effects and treatment center as a random effect. Evaluation of the per-protocol population was performed according to the recommendations of the EMA (16). The definition of the per-protocol population specified that patients were required to have participated in at least one visit in addition to the baseline and final visits. For the test of superiority of the team-based form of care over standard care in terms of health-related quality of life (changes in EQ-5D-5L [10] after one year), a similar linear mixed model was used with the baseline value of each endpoint and age as further covariates. This evaluation was based on the intention-to-treat approach. Multiple imputations were used for missing values, under the assumption they were missing at random. The evaluations were also implemented in the other population for sensitivity analysis of the two primary endpoints (eResults).
Continuous secondary endpoints were also evaluated with linear mixed models, taking into account baseline, type of care, respective center, age, and sex. For this purpose, the intention-to-treat population was used; a sensitivity analysis in the per-protocol population is presented in the eResults. The categorical secondary endpoint “patient satisfaction” was evaluated descriptively.
Health economic evaluation
In addition to testing clinical effectiveness of this new form of care involving delegation of physicians’ tasks, an economic evaluation was conducted to deal with questions of health economics. This evaluation was confined to using voluntary information submitted about the use of various resources and to the cost estimate derived from this for a selection of direct services reimbursed by the health insurance fund. Indirect costs, such as those that arise as a result of sickness-related loss of work, were excluded from the present study. Data acquisition was based on interviews with patients regarding their respective resource use utilizing the “Questionnaire on the Use of Medical and Non-medical Care Services in Old Age”, FIMA for short (14), at baseline and after 26 and 52 weeks.
Multiple imputation of missing data
Missing or non-specific answers (for example: “n.a.”) to the cost items included in the total cost estimate were quantified according to treatment groups with regard to their share of the total sample. For the evaluation of those patients with missing values in the sense of the intention-to-treat principle, multiple imputation was performed using a two-stage procedure in which the following factors were considered as model predictors:
1. In a first step, involved patients were first classified according to the probability with which they had indicated, according to the model prediction, that they had not used the resource in the relevant period. Scoring using logistic regression with the variables mentioned was used for this classification.
2. In a second step, those patients who had not been assigned zero cost in the first step were assigned values using predictive mean matching, also with regard to the variables mentioned.
The number of imputations was 20 in both cases.
Group comparison of evaluated total costs (with and without drug prescriptions)
For all the randomized patients (intention-to-treat population), the calculation of total costs was based on the following types of resources:
Drug prescriptions for fixed-price drugs were evaluated using the database of the German Institute of Medical Documentation and Information (DIMDI, 2021). Where relevant, the estimated costs for medical aids are based on the published reference prices of the German Association of Statutory Health Insurance Funds (2021) (for example, for hearing or vision aids).
In addition to these items, it was originally intended that prescribed aids, outpatient surgery/day care unit, and inpatient stays in hospitals and psychiatric institutions would also be included in the total cost calculation. However, it became clear here that the requested data are usually too non-specific for a valid cost calculation if, for example, in the case of surgical procedures not further specified or multiple aids, a very heterogeneous spectrum of possible cost effects is involved, within which actual expenditure cannot be established without knowledge of the indication. Since the validity of the results for these analyses would be severely impaired due to too much non-specific data, these items were consequently excluded from the study entirely.
Furthermore, for drugs for which no reference prices were available (e.g., for those under patent protection) and for those where BARMER Health Insurance was unable to provide cost data, we refrained from making our own estimate because the validity of the results from such constructed data would also appear doubtful.
After applying multiple imputation as stated in the statistical analysis plan, 95% confidence intervals were calculated based on the t-distribution for the mean change in costs relative to the same period of the previous year and for the difference in this change between the two types of treatment.
Total cost estimate and reference cost rates
For the estimation of total annual costs, the (inflation-adjusted) standardized valuation rates from Bock et al. (e3) and publicly available, archived reference price lists for drugs (provided by the BfArM, formerly the database of the German Institute for Medical Documentation and Information, at www.dimdi.de) were used for 2019. eTable 3 (modified from Ossendorf 2019 [e4]) provides an overview of the specific procedure for calculating costs for the items included here, as well as sources for the reference cost amounts used in each case.
is a useful improvement in measurement properties in terms of reducing ceiling effects and
provides better discrimination with greater ability to detect differences between groups as compared with the EQ-5D-3L (10).
type of ressource
questionnaire phase (whether after 26 or 52 weeks)
the attending physician (19 factor levels)
patient’s year of birth
patient’s sex
treatment arm
specialist outpatient consultations
drug prescriptions
healthcare services, including remedies
eResults
Analysis of primary endpoints—full models
eTables 4 and 5 show the full models for the primary endpoint evaluation of the DAS28 in the per-protocol population and the EQ-5D-5L in the intention-to-treat population.
Sensitivity analyses
Sensitivity analyses of the two primary endpoints each population are presented below, as well as a per-protocol analysis of the secondary endpoints.
Evaluation of the DAS28 in the intention-to-treat population showed a difference of -0.18 (95% confidence interval: [-0.35; -0.02]) in the linear mixed model after adjustment; the p value for non-inferiority was <0.001. The supplementary test for superiority yielded a p value of 0.032, so that, statistically, superiority of the team-based form of care was also shown for the sensitivity analysis in the intention-to-treat population (etable 6).
In the EQ-5D-5L analysis, the linear mixed model in the per-protocol population yielded an estimated difference of 0.02 [-0.01; 0.05] and a p value of 0.206 (etable 7). The results are comparable to the evaluation in the intention-to-treat population.
eTable 8 presents the results of the sensitivity analysis of the secondary endpoints in the per-protocol population. With the exception of physical activity, the results are comparable with those in the intention-to-treat population.