A potential role of Staphylococcus aureus in bullous pemphigoid was explored by examining the colonization rate in patients with new-onset disease compared with that in age- and sex-matched controls. S. aureus colonization was observed in 85% of bullous pemphigoid lesions, 3–6-fold higher than the nares or unaffected skin from the same patients (P ≤ 0.003) and 6-fold higher than the nares or skin of controls (P ≤ 0.0015). Furthermore, 96% of the lesional isolates produced the toxic shock syndrome toxin-1 superantigen, and most of these additionally exhibited homogeneous expression of the enterotoxin gene cluster toxins. Toxic shock syndrome toxin-1–neutralizing antibodies were not protective against colonization. However, S. aureus colonization was not observed in patients who had recently received antibiotics, and the addition of antibiotics with staphylococcal coverage eliminated S. aureus and resulted in clinical improvement. This study shows that toxic shock syndrome toxin-1–positive S. aureus is prevalent in bullous pemphigoid lesions and suggests that early implementation of antibiotics may be of benefit. Furthermore, our results suggest that S. aureus colonization could provide a source of infection in patients with bullous pemphigoid, particularly in the setting of high-dose immunosuppression.
INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by autoantibodies targeting the epidermal attachment proteins, known as BP180 (collagen XVII) (Diaz et al., 1990) and BP230 (Stanley et al., 1988). BP predominantly affects the elderly (Langan et al., 2008) and manifests as localized inflammation, blisters, and erosions of the skin (Ren et al., 2017). Because the basic mechanisms driving autoantibody development are not understood, treatment consists of broad-based immunosuppression with steroids alone or in combination with additional immunomodulatory agents. This approach often results in a high degree of morbidity in the elderly population, and much of the mortality observed in BP results from infectious complications linked to the deleterious effects of treatment (Ren et al., 2017).
Staphylococcus aureus is a commensal bacterium that colonizes the respiratory tract of ~30% of the general population (Wertheim et al., 2005) and is a frequent cause of localized and systemic infections and an array of disorders induced by its secreted superantigen (SAg) toxins (Spaulding et al., 2013). These SAgs trigger massive, antigen-independent proliferation of T cells and antigen-presenting cells and have been implicated in a variety of inflammatory and autoimmune diseases (Paller et al., 2019; Spaulding et al., 2013). Within the skin, staphylococcal SAgs exert direct cytotoxic and inflammatory effects on keratinocytes, further contributing to immune dysregulation and inflammation (Schlievert et al., 2020). The goal of this study was to explore the potential role of S. aureus in BP by examining the colonization rate in patients with new-onset disease.
RESULTS
Patient population
This study included 28 patients with new-onset BP (17 male/11 female, mean age [±SD] = 76.0 ± 8.3 years, range = 61.5–90.8 years) and 28 sex- and age-matched controls (mean age [±SD] 75.9 ± 8.1 years, range = 60.2–90.3 years) who had no history of autoimmunity. Two weeks before referral to our institution, 15 of the patients with BP had not been treated, 6 had been prescribed either antibiotics or immunomodulatory agents, and 1 patient had received both treatments. Controls had not been treated with immunomodulatory agents or antibiotics in the 2-weeks before enrollment. Race was self-reported; the BP group was composed of 27 Caucasians and 1 Asian, whereas all the 28 controls were Caucasian. Bullous Pemphigoid Disease Area Index (BPDAI) scores ranged from 1 to 148 (median score = 48.5), with almost half (13 patients) presenting with severe BP (BPDAI > 56) (Lévy-Sitbon et al., 2014). As expected, BPDAI scores were strongly correlated with circulating levels of BP180 IgG (Spearman’s r = 0.6644, P < 0.0001) and, to a lesser extent, BP230 IgG (Spearman’s r = 0.4298, P = 0.0225). Individual demographic, clinical, and laboratory data are shown in Supplementary Table S1
Staphylococcal colonization
Lesional sampling was performed by gentle unroofing of an intact lesion and swabbing the interior base of the blister. Staphylococcal growth was categorized as S. aureus, coagulase-negative staphylococci (typically Staphylococcus epidermidis), or no growth. As shown in Figure 1a and Supplementary Table S2, patients with BP exhibited a high rate of colonization with S. aureus isolated from 85.7% of the lesions, 58.8% of nares, and 27.3% of unaffected skin swabs. In contrast, samples from matched controls grew primarily coagulase-negative staphylococci or had no staphylococcal growth, whereas only 10.7% of the samples obtained from the nares or skin surface (anatomically matched to their respective areas in patients with BP) grew S. aureus. Fisher’s exact test to determine relative risk and 95% confidence interval (CI) revealed that the BP lesions were over six times more likely to be colonized by S. aureus than either the skin or nares of the controls (95% CI = 2.790–15.78, P < 0.0001) Comparison of similar sites between patients with BP and controls revealed that patients with BP are 2–3-fold more likely to grow S. aureus from their nares (95% CI = 1.346–4.712, P = 0.0015) or skin (95% CI = 1.544–9.217, P = 0.0003). Finally, BP lesions were also more (3.7 or 6.5 times) likely to be colonized with S. aureus than samples from nares (95% CI = 1.199–11.88, P = 0.0300) or unaffected skin (95% CI 2.300–19.69, P = 0.0004) from the same patients. No significant association between the type of colonizing bacteria and disease severity or BP180/BP230 antibody levels was detected using a Wilcoxon rank-sum test.
Figure 1. Patients with BP exhibit an increased rate of Staphylococcus aureus colonization, and most of these isolates produce TSST-1.
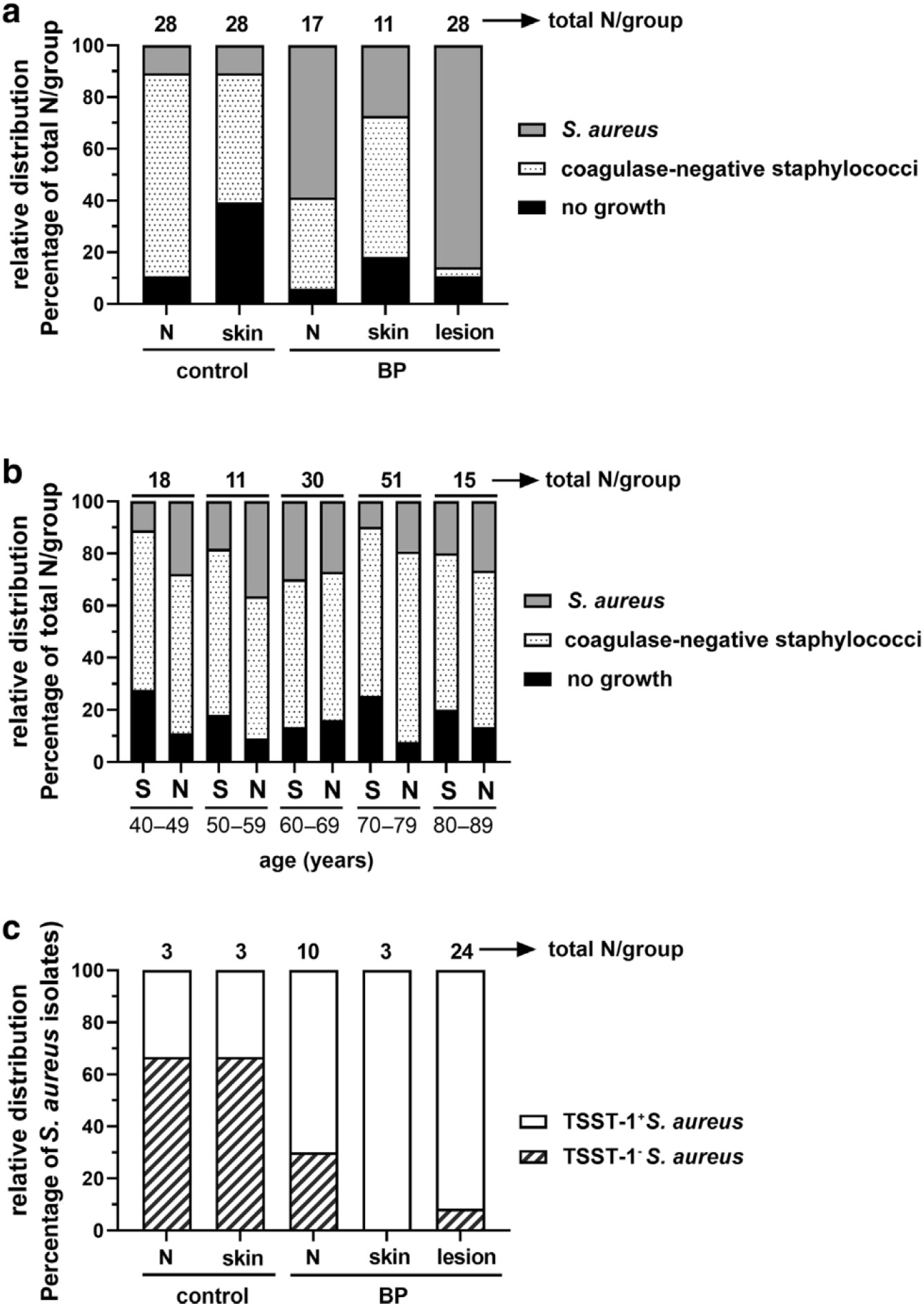
(a) Staphylococcal colonization was categorized as S. aureus (coagulase positive) or coagulase-negative staphylococci or no growth, and their relative distribution is shown as a percentage of the total number (above bars). BP lesions were six-fold more likely to be colonized by S. aureus than control Ns or S (Fisher’s exact test, 95% CI = 2.790–15.78, P < 0.0001). (b) Staphylococcal colonization of the S or Ns of 128 healthy individuals aged 40–99 years shows that bacterial distribution is not influenced by age (logistic regression models, AUC ~0.5). (c) Most S. aureus isolates from patients with BP produce TSST-1, whereas most of the control isolates do not. Using Fisher’s exact test, BP lesions were >4-fold more likely to be colonized by TSST-1+ S. aureus than control Ns (95% CI = 2.422–9.468, P < 0.0001) or S (95% CI = 2.215–9.144, P < 0.0001). AUC, area under the curve; BP, bullous pemphigoid; CI, confidence interval; N, nare; S, skin; TSST-1, toxic shock syndrome toxin-1.
Because staphylococcal colonization rates are not well-established in the elderly, we determined whether the type of colonizing bacteria changed with age in an additional cohort of 125 healthy individuals aged 40–89 years (Figure 1b). Although no significant differences in colonization were detected by age (logistic regression, area under the curve = ~0.5 for all comparisons), S. aureus colonization was more common in the nares than in the skin, as expected. These findings indicate that the average nasal carriage rate of our (Midwestern United States, Caucasian) patient population does not change dramatically with age; however, the overall S. aureus nasal carriage rate for our cohort is lower than the expected rate of 30% (Wertheim et al., 2005).
Staphylococcal production of toxic shock syndrome toxin-1
Most of the pathogenesis associated with S. aureus is linked to its production of toxic shock syndrome (TSS) toxin-1 (TSST-1) (Merriman et al., 2016). Remarkably, evaluation of TSST-1 by immunoblot revealed that most of the isolates from BP lesions (92%), nares (70%), and unaffected skin (100%) produced TSST-1 (Figure 1c). To confirm TSST-1 production in situ, TSST-1 was evaluated with fluid aspirated from intact blisters using quantitative immunoblot analysis. TSST-1 was detected in all nine samples of adequate volume (values ranged from 0.0075 to 19.4 μg/ml) at levels well above the minimum threshold needed (0.02 ng/ml) (Spaulding et al., 2013) to exert SAg effects (Supplementary Table S1). By comparison, TSST-1 production was less predominant (33%) in the S. aureus isolates obtained from controls. This translates to a >4-fold increased risk of TSST-1+ S. aureus colonization of BP lesions compared with that from the nares (95% CI = 2.422–9.468, P < 0.0001) or skin (95% CI = 2.315–9.144, P < 0.0001) of controls. Disease severity and serum BP180/BP230 antibody levels are depicted on the basis of lesional isolate in Supplementary Figure S1.
High prevalence of the enterotoxin gene cluster in TSST-1+ S. aureus isolates in BP
In addition to TSST-1, S. aureus secretes a variety of toxins that aid in colonization, tissue destruction, and subversion of the immune system (Vandenesch et al., 2012). A subset of these, known as the enterotoxin gene Cluster (EGC) (encoding SAgs G, I, M, N, O, and U), are often coexpressed in recently emergent Clonal types of S. aureus (Fischer et al., 2019; van Belkum et al., 2006). Shown in Table 1, PCR analysis of the EGC in the TSST-1+ S. aureus isolates from BP lesions revealed that 80% exhibited full expression of the EGC, 10% expressed all except M, and the remaining 10% expressed all except U. The high level of identity in EGC expression suggests that patients with BP may be colonized with a Clonal strain of S. aureus (Fischer et al., 2019). Furthermore, these isolates also consistently produced β-hemolysin but very low amounts of α-hemolysin, as is characteristic of the USA200 strains (Salgado-Pabon et al., 2014).
Table 1.
Superantigen Gene Profiling of Staphylococcus aureus Isolates from BP Lesions
| Staphylococcal SAgs1 |
|||||||
|---|---|---|---|---|---|---|---|
| G | I | M | N | O | U | TSST-1 | |
|
| |||||||
| No. (%) | 212 (100) | 21 (100) | 23 (9.5) | 21 (100) | 21 (100) | 193 (90.4) | 21 (100) |
Abbreviations: BP, bullous pemphigoid; EGC, enterotoxin gene cluster; No., number; SAg, superantigen; TSST-1, toxic shock syndrome toxin-1.
Evaluated on isolated colonies by PCR using primers specific for EGC toxins and TSST-1.
Of 28 patients with BP, TSST-1+ S. aureus was isolated from 22; 1 sample had insufficient RNA extracted, leaving 21 samples.
Two separate individuals either lack U or express M, leaving 17 patients with BP with 100% expression of the EGC plus TSST-1.
TSST-1–neutralizing antibodies
Protective levels of TSST-1–neutralizing antibodies are naturally developed in ~80% of people by the age of 12 years (Spaulding et al., 2013). Titers ≥40 are reported in healthy individuals, inCluding asymptomatic carriers, whereas levels <40 are observed in patients with menstrual TSS (Stolz et al., 1985). Overall, 16 of 28 (57%) of patients with BP exhibited protective titers of TSST-1–specific IgG, and similarly, protective titers were observed in 12 of 24 (50%) of those colonized by TSST-1+ S. aureus. Healthy controls (described in Supplementary Materials and Methods) exhibited similar levels of TSST-1 IgG, with 14 of 28 (50%) exhibiting titers ≥40 (Figure 2 and Supplementary Table S1) Spearman’s r did not reveal any association between TSST-1 IgG and TSST-1+ S. aureus colonization (r2 = 0.005, P = 0.9796) or BPDAI (r2 = 0.150,P = 0.4465).
Figure 2. TSST-1 IgG does not prevent TSST-1+ Staphylococcus aureus colonization of BP lesions.
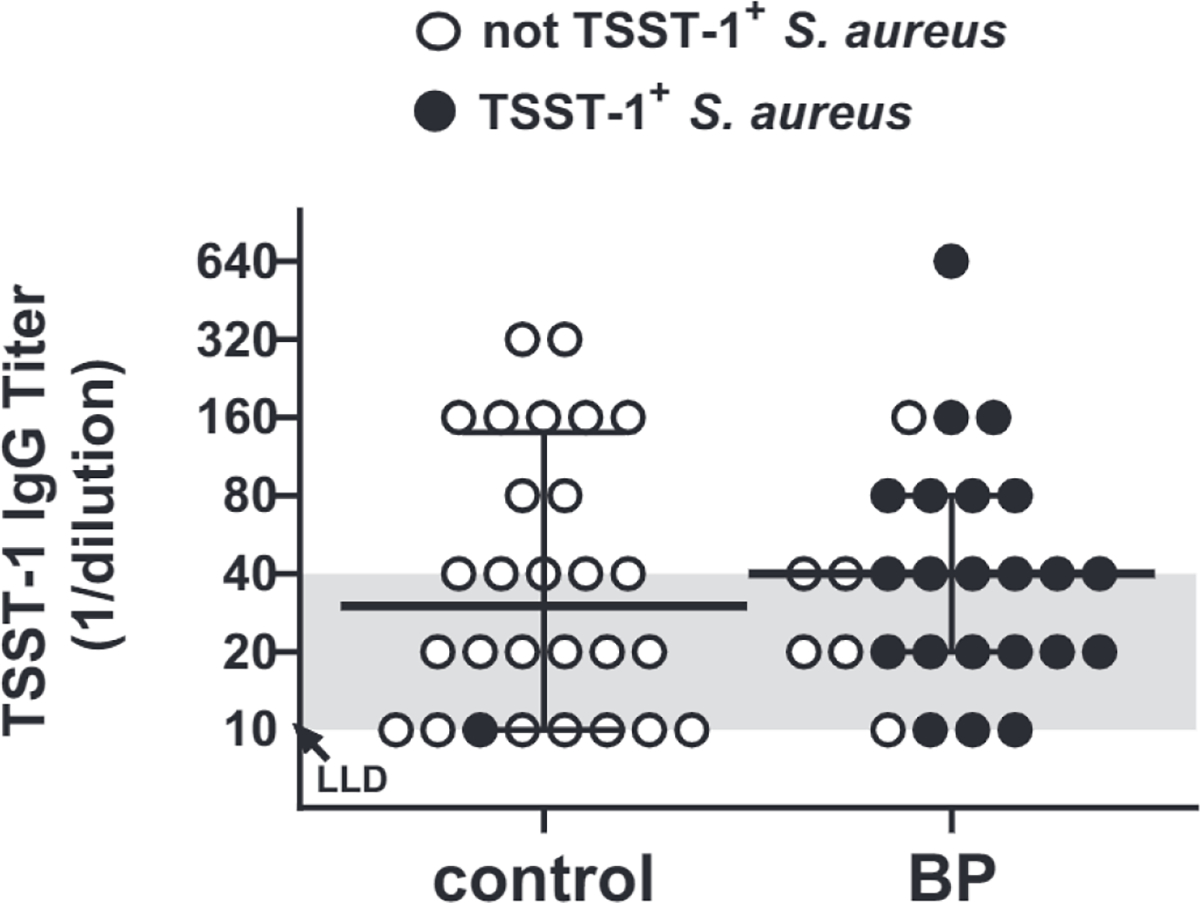
Serum titers of TSST-1–specific IgG were measured by ELISA. Titers ≤40 (shaded area) are not considered protective. TSST-1 IgG titers were similar in the sera from 28 patients with BP and 28 matched controls (Mann–Whitney test, P = 0.787), with 50% and 57% of each respective group showing protective titers. TSST-1+ S. aurous colonization (filled dot) was not correlated with the level of TSST-1 IgG (Spearman’s r2 = 0.005, P = 0.9796). Each point represents the average of triplicate wells for an individual patient, with the median indicated by the solid line. BP, bullous pemphigoid; LLD, lower limit of detection; TSST-1, toxic shock syndrome toxin-1.
Because TSST-1 IgG levels were lower than expected in our control population, we evaluated titers as a function of age in an additional 168 controls aged 40–89 years (Supplementary Figure S2). When stratified by decades of age (40–49 years, 50–59 years, and others), the frequency of individuals with protective antibody levels declined gradually as age increased, so that 68% (13/40) of subjects aged 40–49 years had titers ≥40, whereas only 47% (16/34) of subjects aged 80–89 years had protective titers. These findings indicate that although TSST-1 IgG titers wane over time, the levels observed in patients with BP are in line with those of our population of elderly individuals and thus do not explain their increased rate of TSST-1+ S. aureus colonization.
Clinical observation and application
The relevance of these findings to patient care is illustrated by the Clinical course of a man aged 67 years with a 6-month history of BP who was referred to Dermatology at the University of Iowa Hospitals & Clinics (Iowa City, IA). Six weeks before, he was hospitalized owing to the extent of his disease and started on high-dose immunosuppressive therapy (prednisone, 60 mg; mycophenolate, 3 gm/day). At the time of referral, he had a widespread cutaneous disease (BPDAI = 173) and was still forming new blisters (Figure 3 and Table 2, patient #758). He had a serum BP180 ELISA of 179 IU (normal < 9 IU) and was afebrile with circulating white blood cells of 13.2 K/mm3 and lymphocytes of 229 per mm3 (normal = 875–3,300). Lesional swabs were obtained, antibiotic therapy (Clindamycin, 450 mg, three times daily) was initiated, and his mycophenolate was decreased (1 gm/day). Lesional TSST-1+ S. aureus was confirmed, and 3 days after his first visit, the patient reported an improved condition and decreased blistering (via phone). Twenty-three days after his first visit to our Clinic, he was no longer forming new blisters, and his disease severity score had decreased to 97 (a 44% decrease). In repeat cultures, no S. aureus could be isolated from either the skin or nares.
Figure 3. Rapid reduction in disease activity with antibiotic treatment in a patient with BP colonized with TSST-1+ Staphylococcus aureus.
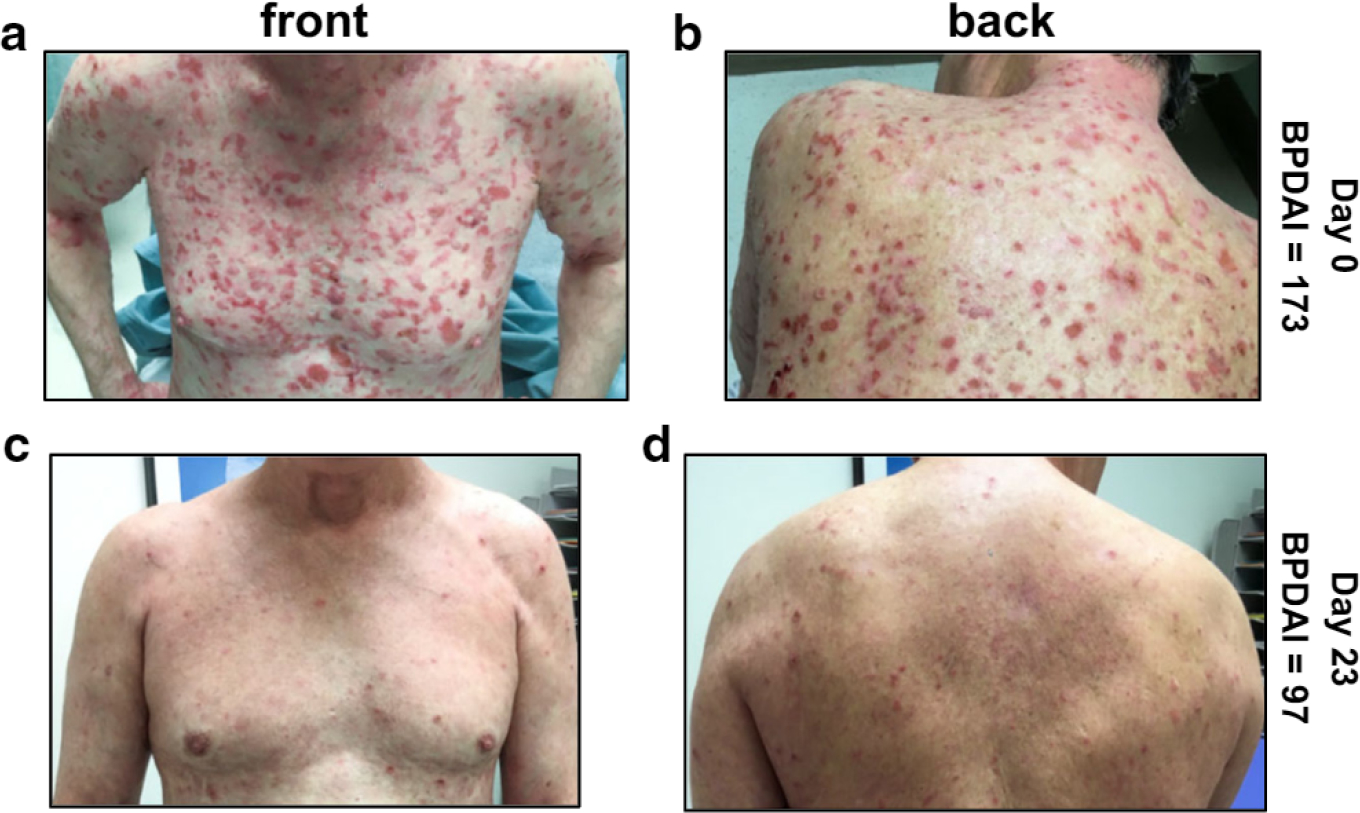
Disease activity in a male aged 67 years who had been treated with prednisone, 60 mg/day, and mycophenolate mofetil, 3 g/day, for 6 weeks (front is shown in a; back is shown in b). Bacterial swabs were obtained, and his therapeutic regimen was altered to reduce immune suppression (mycophenolate, 1 g) and inClude an antibiotic with staphylococcal coverage (Clindamycin, 450 mg, three times per day). TSST-1+ S. aurous was isolated from lesions and nares but not from the unaffected skin. (c, d) The patient reported a cessation of new blisters by day 10; by day 23, a 44% decrease in BPDAI was observed, and repeat cultures yielded no growth of S. aurous from the skin surface or the nares. The patient granted permission to publish Clinical images. BP, bullous pemphigoid; BPDAI, Bullous Pemphigoid Disease Area Index; TSST-1, toxic shock syndrome toxin-1.
Table 2.
Antibiotic Treatment of Patients with BP Eliminates Lesional Staphylococcus aureus Colonization and Is Associated with a Reduction in Disease Severity
| Patient ID | Sex/Age (y) | Before Referral1 (Drug, Daily Dose, Duration) | After Enrollment (Drug, Daily Dose) | BPDAI2 Enrollment/Follow-Up (Days Elapsed) | % Decline BPDAI3 |
|---|---|---|---|---|---|
| 758 | M/67.2 | Prednisone, 60 mg, 39 d; Mycophenolate, 3 g, 39 d |
Prednisone, 40 mg; Clindamycin, 1.35 g; Mycophenolate, 1g |
173/97 (23) | 44 |
| 781 | F/61.5 | Triamcinalone, 0.1% ointment | Clindamycin, 1.35 g | 92/44 (25) | 52 |
| 703 | F/87.1 | Betamethasone, 0.05% ointment | Doxycycline, 200 mg | 59/50 (21) | 15 |
| 807 | M/74.3 | Triamcinalone, 0.1% ointment | Doxycycline, 200 mg | 43/37 (27) | 14 |
Abbreviations: BP, bullous pemphigoid; BPDAI, Bullous Pemphigoid Disease Area Index; F, female; ID, identification; M, male.
S. aureus was isolated from lesional swabs obtained from patients with BP on the date of enrollment.
Medications prescribed by the referring physician before their initial visit to University of Iowa Hospitals & Clinics.
Disease severity was scored using the BPDAI on the date of enrollment and at the next routine visit; the number of days elapsed is indicated in the parentheses.
The percentage decline in BPDAI = (BPDAI enrollment / [BPDAI at enrollment − BPDAI at follow-up]) × 100.
Similar observations were made in an additional three patients who presented with severe BP but had not yet received any systemic immunosuppression (Table 2). Lesional TSST-1+ S. aureus was isolated from all the three patients, and only antibiotics with staphylococcal coverage (doxycycline or clindamycin) were added. In the absence of systemic immune suppression, a 14–52% decrease in disease activity was observed in all the three patients in less than a month, and TSST-1+ S. aureus was no longer detected. These findings suggest that antibiotic therapy can facilitate a reduction in immunosuppression and improve outcomes through the elimination of S. aureus colonization.
DISCUSSION
This study shows that the lesions of most patients with BP are colonized by TSST-1+ S. aureus. In addition, there is an increased prevalence of TSST-1+ S. aureus in the nares and on the surface of unaffected skin of patients with BP, compared with that in age- and sex-matched controls. These findings are significant because patients with BP are known to suffer from increased hospitalizations and mortality owing to infectious complications, including sepsis and pneumonia (Ren et al., 2017). Furthermore, recent case reports identify complications arising from methicillin-resistant S. aureus as a potential emerging problem in BP (Chen et al., 2020; Lugović-Mihić et al., 2019; Souaid et al., 2019). Our results suggest that S. aureus colonization could provide a source of infection in patients with BP, particularly in the setting of high-dose immunosuppression.
Although none of the patients with BP exhibited signs of a clinically significant infection, it remains unclear whether these patients were colonized with S. aureus before the onset of their disease. TSST-1+ S. aureus was the primary isolate found at all the three locations sampled in the BP group, whereas the controls were primarily colonized with coagulase-negative staphylococci (normal flora). Susceptibility to S. aureus colonization is complex and is dependent on a variety of factors involving its initial attachment to the skin, breach of the epidermal barrier, and immune cell responsiveness (Shukla et al., 2015), one or more of which may play a role in the lack of infectious complications among colonized patients with BP.
It is surprising that TSST-1 IgG titers did not correlate with TSST-1+ S. aureus colonization or BP severity. It is not known how circulating antibody titers translate to effective toxin neutralization in the skin; however, their complete protection of women from menstrual TSS suggests that neutralization is effective within the vaginal mucosa (Stolz et al., 1985). In BP, it is conceivable that the direct effects of TSST-1+ on keratinocytes outweigh its impact on TCR/major histocompatibility complex–mediated immune stimulation. In support of this hypothesis, a recent RNA-sequencing analysis revealed that TSST-1 treatment of primary human keratinocytes led to the dysregulation of 5,773 genes (Schlievert et al., 2020). Production of several mediators known to be upregulated in the serum and skin of patients with BP, such as IL-8 (Ameglio et al., 1998; Inaoki and Takehara, 1998), were increased in response to TSST-1 treatment (Schlievert et al., 2020). Thus, S. aureus colonization may contribute to the altered immunologic milieu observed in BP skin (D’Auria et al., 1999; Giomi et al., 2002; Kotzin et al., 1993).
To explore whether detection of S. aureus colonization and/or TSST-1 is facilitated by blister formation, suction blisters were created on four controls, including one individual who had TSST-1+ S. aureus isolated from the skin surface. No growth was observed from lesional swabs, nor was TSST-1 protein detected in the fluid aspirated from these blisters. Unfortunately, suction blisters begin to heal immediately after the vacuum is released and therefore cannot be resampled. Thus, it remains possible that TSST-1+ S. aureus colonization is a consequence of compromised barrier function and not specific to BP. This limitation could be addressed through the evaluation of skin colonization in elderly individuals with chronic skin conditions that affect barrier function, such as diabetic foot ulcers. Indeed, S. aureus is the most common pathogen isolated from diabetic foot ulcers in patients who have not been treated with topical or systemic antibiotics; however, only 8% of these express TSST-1 (Vu et al., 2014). In addition, a survey of SAg expression by S. aureus in atopic dermatitis wounds indicates that the prevalence of TSST-1 expression is decreasing in the United States (Merriman et al., 2016). These findings are in line with our sampling of affected skin from 13 individuals diagnosed with non-BP lesions (dermatitis, pustular eruptions, bullous bite reaction), which revealed that half (7, 54%) were colonized with S. aureus, but very few expressed TSST-1 (3, 23%). Taken together, these observations indicate that the prevalence of TSST-1+ S. aureus is unusually high in BP.
The TSST-1+ S. aureus isolates from BP lesions exhibited highly homogeneous expression of the EGC that is most likely of the USA200 group (Merriman et al., 2016; van Belkum et al., 2006). USA200 is a clonal group of S. aureus that is widespread in both community and healthcare settings and is the primary cause of many life-threatening infections, including TSS (Fitzgerald et al., 2001; King et al., 2016; Merriman et al., 2016; Spaulding et al., 2013). Although the molecular basis for this clonal selection remains unclear, animal studies show that full expression of the ECG plus TSST-1 gives the USA200 clone a selective advantage for colonization, even in the presence of systemic protective antibodies (Schlievert et al., 2019; Spaulding et al., 2013; Stach et al., 2016). Expanded genetic analysis is necessary to determine whether patients with BP are colonized with a clonal group of S. aureus, such as women with TSS (Fitzgerald et al., 2001), or whether it is a local strain, as intimated by the identical expression of the ECG in the isolates obtained from three controls. Going forward, whole-genome sequencing of the TSST-1+ isolates from patients with BP and controls, alongside a group of epidemiologically unrelated USA200 isolates, will determine whether a local strain of TSST-1+ S. aureus is observed in this patient cohort or whether BP is associated with a single clonal group.
A limitation is that this study was conducted at a single site; inclusion of a more geographically and racially diverse population is necessary because these factors are known to influence S. aureus SAg expression (Merriman et al., 2016; Parsonnet et al., 2005). In particular, African Americans are not typically colonized with TSST-1+ S. aureus and are not susceptible to TSST-1–associated menstrual toxic shock (Merriman et al., 2016). Despite this, the prevalence of BP is similar across all racial and geographic groups in the United States (Wertenteil et al., 2019). However, differences in the clinical course are observed in nonwhite patients with BP, such as a higher frequency of serious secondary infections (Wertenteil et al., 2019), and in the type autoimmune comorbidities observed (Narla and Silverberg, 2020). Whether African American patients with BP are colonized with TSST-1+ S. aureus will be a key factor in understanding the relevance of the current findings to BP onset and pathogenesis.
Patients with BP are treated with systemic glucocorticoids in combination with other steroid-sparing agents, typically immunosuppressants or immunomodulators (Feliciani et al., 2015). Tetracycline antibiotics (doxycycline, minocycline) are sometimes used short-term on the basis of their anti-inflammatory effects rather than of their antimicrobial functions (Feliciani et al., 2015; Schaller, 2017). A limited case series (Thomas et al., 1993) and a recent placebo-controlled study (Williams et al., 2017) provide evidence that tetracycline antibiotics are effective at reducing disease activity in BP. Unfortunately, bacterial colonization was not assessed in these studies. We propose that the antimicrobial effects of the tetracycline antibiotics play an important therapeutic role in BP through the clearance of S. aureus. This hypothesis is supported by the presence of TSST-1 protein in the blister fluid of antibiotic-treated patients (Supplementary Table S1) from whom no S. aureus was isolated. Most likely, these patients were colonized with TSST-1+ S. aureus before antibiotic treatment, and residual toxin was detected in the blister fluid.
In conclusion, this study shows that TSST-1+ S. aureus is prevalent in BP lesions and suggests that early implementation of antibiotics may be of benefit. Furthermore, our findings suggest that TSST-1+ S. aureus may play a role in the development or severity of bacterial complications seen in patients with BP.
MATERIALS AND METHODS
Patient population, enrollment criteria, and information collected
This study was approved by the University of Iowa Institutional Review Board (Institutional Review Board number 200701758) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from study participants before inclusion, and the patients granted permission for publication of the clinical images used in this manuscript. All patients with new onset of classic BP seen in the Department of Dermatology at the University of Iowa Hospitals & Clinics from March 2017 to June 2019 were offered enrollment. A total of 28 patients met our inclusion criteria of active blistering and compatible clinical, histologic, and immunologic features of BP, including deposition of IgG and/or complement C3 at the basement membrane zone of biopsied skin and circulating BP180-specific IgG. Peripheral blood was collected, and demographic information, laboratory results, and all medications taken in the 2 weeks preceding enrollment were recorded. Disease severity was scored using the standardized BPDAI (Murrell et al., 2012). A total of 28 age- and sex-matched controls with no history of autoimmune disease and no antibiotics or immunosuppressive medications in the previous 2 weeks were recruited from patients on routine visits to the clinic, primarily for skin checks and evaluation of benign lesions not known to affect the skin flora. An additional 168 controls aged 40–88 years were enrolled for examination of anti–TSST-1 IgG and staphylococcal colonization. At the time of enrollment, patients were coded numerically, and investigators conducting laboratory assays had no knowledge of the patient group and no access to their clinical information.
Sampling and staphylococcal isolation
Blister fluid was removed from intact lesions using a sterile 21-gauge needle, and colonization was assessed after the sterile opening of the blister top and gentle rotation of a swab three times on the interior base of the lesion. To control for anatomic variation in cutaneous microbiome, the site sampled for controls reflected lesional sampling site for each patient with BP, typically volar forearm, or other dry sites (Grice et al., 2009). For unaffected/control skin, a 2-cm × 2-cm area was swabbed with 10 nonoverlapping strokes. Anterior nares were sampled by 2-cm insertion and 3-second rotation of a sterile swab in first the right and then the left nostril. The swabs were streaked onto blood agar plates and were incubated for 24 hours (37 °C, 5% carbon dioxide), and staphylococci were distinguished from streptococci and lactobacilli as catalase positive. Next, S. aureus (gram positive, catalase positive, coagulase positive) was distinguished from coagulase-negative staphylococci using the slide coagulase (clumping factor) test. Multiple colonies were tested.
Bacterial toxin production
TSST-1 production by S. aureus isolates was evaluated by immunoblot, using highly purified TSST-1 as a control and monospecific (TSST-1) hyperimmune antisera (Blomster-Hautamaa and Schlievert, 1988). TSST-1 was similarly quantified in blister fluids alongside a standard curve consisting of 10-fold dilutions (10–0.001 ug/ml) (Vu et al., 2015). In this assay, no TSST-1 was detected in the controls for nonspecific interaction, including diluent or human serum. Bacterial production of cytotoxins was determined by differential lysis of sheep (beta and gamma toxins) and rabbit (alpha toxin) erythrocytes in blood agar plates.
Staphylococcal SAg gene expression
The S. aureus isolates were tested for expression of SAg genes, including the EGC and TSST-1, using PCR. Briefly, individual S. aureus colonies were selected from blood agar plates and cultured in Todd Hewitt broth (Thermo Fisher Scientific, Waltham, MA) until stationary phase, DNA was then extracted, and PCR was performed using commercial reagents (Qiagen, Germantown, MD) with primers specific for each SAg (G, I, M, N, O, X, TSST-1) and appropriate positive and negative controls, as previously described (Vu et al., 2015).
Serum TSST-1–specific IgG titers
TSST-1 titers were determined by indirect ELISA using a standard intravenous Ig preparation (Immune Globulin Intravenous [Human]; Immuno AG, Vienna, Austria) as a reference (Hudson and Hay, 1980; Park et al., 2015). Titers were recorded as the reciprocal of the last 10-fold serial dilution of serum or intravenous Ig to give a positive color reaction. In this assay, the standard intravenous Ig had a titer of 320.
Statistical analysis
Statistical analysis was performed in consultation with the University of Iowa Institute for Clinical and Translational Science. Differences in colonization or TSST-1 production were determined using a Fisher’s exact test (two-sided, P < 0.05) to determine relative risk, 95% CI, and P-value. TSST-1 titers were compared using Mann–Whitney and Kruskal–Wallis tests followed by Dunn’s multiple comparisons test, with P-value correction for comparison of age-related TSST-1 titers with those of the age group of 40–49 years. For correlation analysis, pairs of variables were evaluated using Fisher’s exact test (two categorical values), Spearman’s correlation (two continuous values), and Wilcoxon rank-sum test (one categorical, one continuous). Evaluation of the main effect or interaction between age and site as predictors of colonization (no growth, coagulase negative, or S. aureus) was performed using logistic regression models. P < 0.05 was determined to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie McKillip and Samuel Connell for their assistance with this study. This work was supported by the National Institute on Aging of the National Institutes of Health (R21AG065980; JAF, KNM, PMS); the University of Iowa, Iowa City, IA (PMS); and the National Center for Advancing Translational Sciences of the National Institutes of Health, Bethesda, MD (UL1TR002537).
Abbreviations:
- BP
bullous pemphigoid
- BPDAI
Bullous Pemphigoid Disease Area Index
- CI
confidence interval
- EGC
enterotoxin gene cluster
- SAg
superantigen
- TSS
toxic shock syndrome
- TSST-1
toxic shock syndrome toxin-1
Footnotes
CONFLICT OF INTEREST
All authors state no conflict of interest.
Disclaimer
The funding agencies had no influence in the design, interpretation, writing, or submission of this study for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2021.08.438.
Data availability statement
No large datasets were generated or analyzed during this study.
REFERENCES
- Ameglio F, D’Auria L, Bonifati C, Ferraro C, Mastroianni A, Giacalone B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol 1998;138:611–4. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol 1988;165:37–43. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang M, Chen H, Fang J. Subtherapeutic linezolid concentration in a patient with bullous pemphigoid complicated by methicillin-resistant Staphylococcus aureus infection: a case study. Ther Drug Monit 2020;42:515–7. [DOI] [PubMed] [Google Scholar]
- D’Auria L, Cordiali Fei P, Ameglio F. Cytokines and bullous pemphigoid. Eur Cytokine Netw 1999;10:123–34. [PubMed] [Google Scholar]
- Diaz LA, Ratrie H, Saunders W, Futamura S, Squiquera F, Anhalt J, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera Immunolocalization of this protein to the hemidesmosome. J Clin Investig 1990;86:1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol 2015;172:867–77. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Kilgore SH, Singh SB, Allen PD, Hansen AR, Limoli DH, et al. High prevalence of Staphylococcus aureus enterotoxin gene cluster superantigens in cystic fibrosis clinical isolates. Genes (Basel) 2019;10:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JR, Sturdevant DE, Mackie SM, Gill SR, Musser JM. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc Natl Acad Sci USA 2001;98:8821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giomi B, Caproni M, Calzolari A, Bianchi B, Fabbri P. Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci 2002;30:116–28. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324:1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Hay FC. Practical immunology. 2nd ed. Oxford, England: Black-well Scientific Publications; 1980. [Google Scholar]
- Inaoki M, Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J Dermatol Sci 1998;16:152–7. [DOI] [PubMed] [Google Scholar]
- King JM, Kulhankova K, Stach CS, Vu BG, Salgado-Pabón W. Phenotypes and virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 clonal lineages. mSphere 2016;1:e00071–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin BL, Leung DY, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol 1993;54:99–166. [DOI] [PubMed] [Google Scholar]
- Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ 2008;337:a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Sitbon C, Barbe C, Plee J, Goeldel AL, Antonicelli F, Reguiaï Z, et al. Assessment of bullous pemphigoid disease area index during treatment: a prospective study of 30 patients. Dermatology 2014;229:116–22. [DOI] [PubMed] [Google Scholar]
- Lugović-Mihić L, Duvančić T, Pavić I, Gverić-Grginić A, Šitum M, Dediol I. Baclofen-induced dyshidrosiform bullous pemphigoid in a paraplegic patient complicated by methicillin-resistant Staphylococcus aureus (MRSA) and urinary infection. Acta Dermatovenerol Croat 2019;27:184–7. [PubMed] [Google Scholar]
- Merriman JA, Mueller EA, Cahill MP, Beck LA, Paller AS, Hanifin JM, et al. Temporal and racial differences associated with atopic dermatitis Staphylococcus aureus and encoded virulence factors. mSphere 2016;1:e00295–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol 2012;66:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla S, Silverberg Jl. Associations of pemphigus or pemphigoid with autoimmune disorders in US adult inpatients. J Am Acad Dermatol 2020;82:586–95. [DOI] [PubMed] [Google Scholar]
- Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, et al. The microbiome in patients with atopic dermatitis [published correction appears in J Allergy Clin Immunol 2019;143:1660]. J Allergy Clin Immunol 2019;143:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kim JS, Woo H. Prevalence of antibody to toxic shock syndrome toxin-1 in burn patients. Ann Lab Med 2015;35:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol 2005;43:4628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Hsu DY, Brieva J, Silverberg NB, Langan SM, Silverberg Jl. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol 2017;176:87–99. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Case-Cook L, Schlievert P. Molecular analysis of staphylococcal superantigens. Methods Mol Bio 2014;1085:169–85. [DOI] [PubMed] [Google Scholar]
- Schaller M Anti-inflammatory effects of tetracyclines. J Eur Acad Dermatol Venereol 2017;31:1774. [DOI] [PubMed] [Google Scholar]
- Schlievert PM, Cahill MP, Hostager BS, Brosnahan AJ, Klingelhutz AJ, Gourronc FA, et al. Staphylococcal superantigens stimulate epithelial cells through CD40 to produce chemokines. mBio 2019;14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert PM, Gourronc FA, Leung DYM, Klingelhutz AJ. Human keratinocyte response to superantigens. mSphere 2020;5:e00803–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SK, Rose W, Schrodi SJ. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends Microbiol 2015;23:529–36. [DOI] [PubMed] [Google Scholar]
- Souaid R, Wang J, Landow SM, Noska A. Bullous pemphigoid complicated by MRSA cellulitis and bacteremia. R I Med J (2013) 2019;102:46–8. [PubMed] [Google Scholar]
- Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 2013;26:422–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach CS, Vu BG, Merriman JA, Herrera A, Cahill MP, Schlievert PM, et al. Novel tissue level effects of the Staphylococcus aureus enterotoxin gene cluster are essential for infective endocarditis. PLOS One 2016;11:e0154762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest 1988;82:1864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz SJ, Davis JP, Vergeront JM, Crass BA, Chesney PJ, Wand PJ, et al. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis 1985;151:883–9. [DOI] [PubMed] [Google Scholar]
- Thomas I, Khorenian S, Arbesfeld DM. Treatment of generalized bullous pemphigoid with oral tetracycline. J Am Acad Dermatol 1993;28:74–7. [DOI] [PubMed] [Google Scholar]
- van Belkum A, Melles DC, Snijders SV, van Leeuwen WB, Wertheim HF, Nouwen JL, et al. Clonal distribution and differential occurrence of the enterotoxin gene Cluster, egc, in carriage- versus bacteremia-associated isolates of Staphylococcus aureus. J Clin Microbiol 2006;44:1555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bicomponent leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2012;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Stach CS, Kulhankova K, Salgado-Pabón W, Klingelhutz AJ, Schlievert PM. Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes. mBio 2015;6:e02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Stach CS, Salgado-Pabón W, Diekema DJ, Gardner SE, Schlievert PM. Superantigens of Staphylococcus aureus from patients with diabetic foot ulcers. J Infect Dis 2014;210:1920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertenteil S, Garg A, Strunk A, Alloo A. Prevalence estimates for pemphigoid in the United States: a sex-adjusted and age-adjusted population analysis. J Am Acad Dermatol 2019;80:655–9. [DOI] [PubMed] [Google Scholar]
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005;5:751–62. [DOI] [PubMed] [Google Scholar]
- Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial [published correction appears in Lancet 2017;390:1948]. Lancet 2017;389:1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets were generated or analyzed during this study.


