Abstract
Trametes hirsuta and a purified laccase from this organism were able to degrade triarylmethane, indigoid, azo, and anthraquinonic dyes. Initial decolorization velocities depended on the substituents on the phenolic rings of the dyes. Immobilization of the T. hirsuta laccase on alumina enhanced the thermal stabilities of the enzyme and its tolerance against some enzyme inhibitors, such as halides, copper chelators, and dyeing additives. The laccase lost 50% of its activity at 50 mM NaCl while the 50% inhibitory concentration (IC50) of the immobilized enzyme was 85 mM. Treatment of dyes with the immobilized laccase reduced their toxicities (based on the oxygen consumption rate of Pseudomonas putida) by up to 80% (anthraquinonic dyes). Textile effluents decolorized with T. hirsuta or the laccase were used for dyeing. Metabolites and/or enzyme protein strongly interacted with the dyeing process indicated by lower staining levels (K/S) values than obtained with a blank using water. However, when the effluents were decolorized with immobilized laccase, they could be used for dyeing and acceptable color differences (ΔE*) below 1.1 were measured for most dyes.
It is known that 90% of reactive textile dyes entering activated sludge sewage treatment plants will pass through unchanged and will be discharged to rivers (34). Not all dyes currently used could be degraded and/or removed with physical and chemical processes, and sometimes the degradation products are more toxic (40). The traditional textile finishing industry consumes about 100 liters of water to process about 1 kg of textile materials. New closed-loop technologies such as the reuse of microbially or enzymatically treated dyeing effluents could help to reduce this enormous water consumption.
Several combined anaerobic and aerobic microbial treatments have been suggested to enhance the degradation of textile dyes (5, 23, 32). However, under anaerobic conditions, azo-reductases usually cleave azo dyes into the corresponding amines, many of which are mutagenic and/or carcinogenic (10, 11, 32). Furthermore, azo reductases have been shown to be very specific enzymes, thus cleaving only azo bonds of selected dyes (50, 51). By contrast, laccases act oxidatively and less specifically on aromatic rings, thus having potential to degrade a wider range of compounds (43).
Laccases are involved in the biodegradation of lignins, which constitute the main noncarbohydrate component in wood and are among the most abundant groups of biopolymers in the biosphere. A great number of white-rot fungi have been reported to produce the lignin-degrading enzymes laccase, lignin peroxidases, and manganese peroxidases, or at least one of these enzymes (15, 16, 44).
Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) have very broad substrate specificity with respect to the electron donor. They catalyze the removal of a hydrogen atom from the hydroxyl group of ortho- and para-substituted mono- and polyphenolic substrates and from aromatic amines by one-electron abstraction to form free radicals capable of undergoing further depolymerization, repolymerization, demethylation, or quinone formation (43, 49). Oxidation of methoxyhydroquinones during lignin degradation followed by autooxidation of the resulting methoxysemiquinones results in the formation of superoxide anion radicals, which can undergo further reactions (21). The rather broad substrate specificity of laccases may be additionally expanded by addition of redox mediators, such as ABTS [2,2′-azino bis[3-ethylbenzthiazolinesulfonic acid], 1-hydroxybenzotriazole, or compounds secreted by lignolytic fungi (6, 16, 27, 35, 47).
Laccases can be used for the treatment of effluents from pulp mills or from other industries containing chlorolignins or phenolic compounds (4, 8). The enzymes render phenolic compounds less toxic via degradation or polymerization reactions and/or cross-coupling of pollutant phenols with naturally occurring phenols (25, 28, 45). Several processes using laccases as well as immobilized laccases have been developed for the treatment of phenolic effluents and polycylic aromatic hydrocarbons (3, 9, 12, 13).
In this study, we have assessed the potential of Trametes hirsuta and a laccase from this organism to continuously degrade textile dyes. We examined for the first time the reuse of enzymatically decolorized dyeing liquors for dyeing and the toxicity of the degradation products.
MATERIALS AND METHODS
Production of enzymes.
The medium for cultivation of T. hirsuta (BT 2566) contained 4.5% (wt/vol) wheat bran flakes, 1.5% yeast extract, 1% glucose, 0.25% NH4Cl, 0.05% thiamine dichloride, 0.2% KH2PO4, 0.05% MgSO4 · 7H2O, 0.01% CaCl2, and 0.05% KCl. Tap water was used for preparation of the medium, and the pH was adjusted to 5.0 by using NaOH or HCl. Incubation was carried out at 30°C on a rotary shaker (150 rpm) in cotton-plugged 250-ml Erlenmeyer flasks containing 100 ml of media. Flasks were inoculated with 1-cm2 agar pieces from an actively growing fungus on PDA agar. Cultures were harvested after 10 days, filtered, and clarified by centrifugation at 7,800 × g for 20 min to remove the mycelia, and the clear supernatant was used for the enzyme activity assay and for further purification. The predominant laccase (molecular mass, 45 kDa; isoelectric point, 3.5) from T. hirsuta was concentrated using acetone precipitation and ultrafiltration (30 kDa), and it was purified as described previously (20).
Enzyme immobilization.
Alumina pellets were silanized at 45°C for 24 h in a 2.5% (vol/vol) solution of γ-aminopropyltriethoxy silane in acetone. The silanized pellets were washed with distilled water and immersed in 2% (vol/vol) aqueous glutaraldehyde for 2 h at 20°C. Thereafter, the pellets were incubated with 60 mg of the crude enzyme preparation (obtained after acetone precipitation and ultrafiltration of the culture filtrate) per liter for 5 h at 20°C. The immobilized enzyme pellets were washed with potassium phosphate buffer (100 mM, pH 7.0) and kept refrigerated until further use. Immobilized protein was determined by protein analysis according to the method of Bradford by using bovine serum albumin for the calibration (7).
Enzyme assay.
Laccase activity was determined using 2,6-dimethoxyphenol (DMP) as a substrate as described before (14). The reaction mixture contained 50 mM sodium malonate (pH 4.5) and 1 mM DMP. The formation of 2,2′,6,6′-demethoxydiphenoquinone (orange/brownish) at 30°C was followed spectrophotometrically at 468 nm, and laccase activity was calculated from the molar extinction coefficient (ɛ) of 49.6 mM−1 cm−1 (46). Inhibition of the laccase was determined using the enzyme assay described above after 5 min of preincubation of the enzyme with the inhibitors.
Microbial treatment of textile dyes and dyeing effluents.
T. hirsuta was cultivated as described above, and the mycelium was collected by filtration under aseptic conditions and washed twice with 300 ml of sterilized distilled water. A sample of 1.5 g (wet weight) of mycelium was incubated for 8 days with different dyes (final concentration, 0.25 mM) as described for cultivation except that only glucose was used as a carbon source. Sterile controls without inoculum were also maintained under the same conditions. Growth of the fungus was inhibited with antibiotics to determine whether decolorization was due to metabolic activity of the organism or due to other phenomena. A mixture of 100 mg of benylate liter−1, 1,000 mg of cycloheximide liter−1, and 300 mg of streptomycin antibiotic solution liter−1 was prepared. A volume of 10% (vol/vol) of this solution was added to the incubation mixtures. After 10 days, all incubation mixtures were filtered using 0.22-μm-pore-size filter paper, the decolorization efficiency was determined spectrophotometrically at the absorption maximum of each dye, and concentrations were calculated from calibration curves. Adsorption of dyes to the mycelium was determined by solubilization of the dyes with water or organic solvents. Adsorbed dye was washed off the mycelium twice with 10 ml of water. The amount of dye adsorbed was calculated from the absorptions of the supernatants. In case of the dye Basic red 9 base, the mycelium was washed with water, 50% (vol/vol) methanol, ethanol, and acetone. The mycelium was then suspended in 4 N NaOH and disintegrated by ultrasonification. After centrifugation, the absorption of the solubilized dye in the supernatant was measured (17).
Enzymatic treatment of textile dyes.
Typically, 4-ml test tubes or 300-ml Erlenmeyer flasks containing 0.25 mM dye and 10 nkat of laccase ml−1 in 50 mM sodium acetate buffer (pH 5.0) were incubated on a rotary shaker at 30°C for 10 h. Heat-inactivated enzymes were used as control, and decolorization was followed spectrophotometrically.
An enzyme reactor was constructed using a cross-flow membrane ultrafiltration system from Filtron (30-kDa exclusion; Northborough, Mass.). The dye solution (0.25 mM dye in 50 mM sodium acetate buffer [pH 5.0]) was pumped (dual-piston pump) into a column (15 by 300 mm, 50-ml active volume) at a flow rate of 0.1 ml min−1, and mixing was performed with a peristaltic pump at a flow rate of 1 ml min−1. Initially, the column was filled with 7 nkat of laccase ml−1 in 50 mM sodium acetate buffer (pH 5.0). Alternatively, the dye solutions described above were continuously pumped (0.1 ml min−1, dual-piston pump) through a column (15 by 300 mm) filled with 50 ml of immobilized laccase corresponding to about 70 nkat of laccase activity. Both column reactors and the flow cell were kept at 30°C. Decolorization was monitored on a spectrophotometer equipped with a flow cell. The effects of commercial dyeing additives (Ciba), salts, and known laccase inhibitors on the immobilized laccase were determined analogously using DMP as a substrate.
Dyeing experiments.
Bleached cotton fabrics were dyed in liquors containing enzymatically decolorized (exactly 80% decolorization) dyes by using an Ahiba Spectradye dyeing apparatus (Datacolor International, Lucerne, Switzerland) at a liquor-to-good ratio of 20:1 (40 rpm; step 1, temperature was raised from 20 to 60°C in 20 min; step 2, 60°C, 60 min). All chemicals used are listed in Table 1. Dyed fabrics were washed at the same liquor ratio with the nonionic detergent Hostapal CV (1 g liter−1 for 30 min at 90°C to remove the unfixed dye. Diode array spectrums (TIDAS instrument; J&M, Aalen, Germany) of dyes both in standard dye baths and in dye baths containing enzymatically decolorized dyes were recorded. Color differences of the dyed fabrics were determined using a reflectance-measuring apparatus (Spectraflash 600; Datacolor) according to the CIELAB color difference concept at standard illuminant D65 (large area of observation of the sample, specularity excluded, d/8, D65/10°) with a color tolerance interval of 1 CIELAB unit. Color deviation was also quantified by calculating the staining level K/S values from reflectance measurements on the dyed fabrics.
TABLE 1.
Experimental conditions for dyeing with dyes from different classes
| Experimental condition | Dye class (dyes)a
|
||||
|---|---|---|---|---|---|
| Monochlorotriazine (R. Blue 198, R. Green 19) | Vinylsulfone/bifunctional (R. Orange 16, R. Black 5, EB Red 3BS) | Direct (D. Blue 199, D. Yellow 86) | Sulfur (Hydrozol, Schwarz B) | R. Yellow 160 | |
| Concn (% owf)b | 3 | 3 | 2 | 2 | 4 |
| Na2SO4 (g liter−1) | 60 | 50 | 20 | 30 | |
| Na2CO3 (g liter−1) | 20 | 20 | 5 | 20 | |
| Na2S (g liter−1) | 4 | ||||
| NaCl (g liter−1) | 10 | ||||
| Temp (°C) | 80 | 60 | 90 | 90 | 60 |
| Time (min) | 60 | 60 | 60 | 40 | 60 |
R, Reactive; D, Direct; EB Red 3BS, Everzol Brilliant Red 3BS.
1% owf corresponds to 10 mg of dye over 1 g of fiber.
Detoxification experiments.
Solutions of dyes were treated with the T. hirsuta laccase as described above to yield exactly 90% decolorization. The aquatic toxicities of samples were evaluated based on their inhibitory effects on the oxygen consumption rate of Pseudomonas putida. The experiments were conducted according to the German standard methods for the examination of water, wastewater, and sludge (bioassays, group L 27 DIN 38 412) using the OXI 3000 from WTW (Weinheim, Germany) for automated measurements of the oxygen consumption rate. Detoxification was expressed as the decrease (expressed as a percentage) of the inhibitory effect on the oxygen consumption rate between a control and the treated sample.
Results are given as the average of two separate experiments.
RESULTS
Triarylmethane, indigoid, azo, and anthraquinonic dyes were degraded by T. hirsuta and a purified laccase from this organism (Fig. 1; Table 2). Except for Reactive Black 5 and Basic Red 9, a similar pattern was observed for microbial and enzymatic degradation, suggesting that the extracellular laccase seems to be mainly responsible for degradation of the dyes. Anthraquinonic dyes and indigo carmine (Acid Blue 74) were degraded more than twofold faster than the azo dyes by both T. hirsuta and the purified laccase. Adsorption of dyes to the mycelium did not contribute to microbial decolorization since a maximum of 1.5% of the dye added was bound to the mycelium except for Basic Red 9, for which 7% of the dye had bound to the mycelium after incubation of 6 days. When growth of T. hirsuta was inhibited by antibiotics, no decolorization was measured after the same incubation period (data not shown).
FIG. 1.
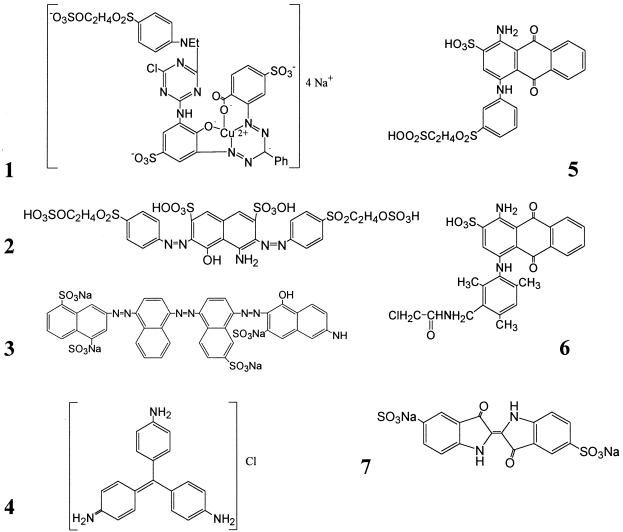
Textile dyes used in the this study. Color index names are given in Tables 2 and 4.
TABLE 2.
Initial decolorization rates of textile dyes with T. hirsuta and a laccase from T. hirsuta
| Dye | Structure no.a | Initial decolorization rates (mean ± SD)
|
|
|---|---|---|---|
| Laccase (nmol s−1 ml−1) | T. hirsuta (pmol s−1 ml−1) | ||
| Reactive Blue 221 | 1 | 6.7 ± 0.3 | 19.4 ± 1.0 |
| Reactive Black 5 | 2 | 1.3 ± 0.2 | 15.9 ± 1.7 |
| Direct Blue 71 | 3 | 3.7 ± 0.6 | 16.2 ± 2.3 |
| Basic Red 9 Base | 4 | 3.3 ± 0.4 | 44.5 ± 2.9 |
| Reactive Blue 19 | 5 | 8.8 ± 0.5 | 29.1 ± 1.7 |
| Acid Blue 225 | 6 | 10.6 ± 0.7 | 30.9 ± 2.6 |
| Acid Blue 74 | 7 | 9.3 ± 0.4 | 41.7 ± 2.8 |
Structure numbers correspond to Fig. 1.
Continuous decolorization was achieved with a membrane reactor. All enzyme was retained in the reactor; however, the membrane had to be cleaned or replaced periodically because of plugging with enzyme protein. Furthermore, an up-scaling of this system would be limited by the cost of membrane and throughput. To overcome these limitations and to increase enzyme stabilities, the laccase was immobilized on alumina support; 89% of the enzyme preparation bound to the carrier corresponding to 0.14 mg g alumina−1, and 68% of the laccase activity was retained in the alumina pellets, which was measured in a column reactor. Using this column reactor, the stabilities of the immobilized enzyme and the pure laccase were compared. In general, the overall lifetime of the immobilized laccase column seems to be determined by the stability of the enzyme under industrial conditions (pH, temperature, additives) rather than by physical phenomena, such as clogging of the column.
The immobilized enzyme was slightly more stable at 60°C and pH 4.5 (half-life, 13.0 h) than the free enzyme (half-life, 11.5 h). Various known laccase inhibitors, salts and textile dyeing auxiliaries, which are usually applied in combination with textile dyes were tested for potential inhibitory effects on the immobilized and free enzyme. Sodium azide was the strongest inhibitor, with a 50% inhibitory concentration (IC50) of 0.002 mM for both the immobilized and free laccases. The immobilized laccase was less vulnerable (e.g., IC50 of up to 3.5-fold higher than that for DCC) to all other laccase inhibitors than the free enzyme. The effect of dyeing auxiliaries on the laccase was determined at a fixed concentration of 2 g liter−1, which is commonly applied in the industry. Sequestering agents and anionic surfactants caused less than 20% inhibition, while wetting and soaping agents and cationic surfactants did not have any significant inhibitory effect on the free and immobilized laccases from T. hirsuta (Table 3).
TABLE 3.
Inhibition of laccase and immobilized laccase from T. hirsuta by various chemical substances
| Laccase inhibitors | IC50 or % inhibition (mean ± SD)
|
|
|---|---|---|
| Laccase | Immobilized laccase | |
| TGAa | 500 μM ± 15 μM | 600 μM ± 14 μM |
| NaN3 | 2.1 μM ± 0.1 μM | 2.2 μM ± 0.1 μM |
| NaF | 80 μM ± 2 μM | 200 μM ± 8 μM |
| NaCl | 50 mM ± 1 mM | 85 mM ± 2 mM |
| NaBr | 190 mM ± 8 mM | 340 mM ± 14 mM |
| DDCa | 1.2 mM ± 0.1 mM | 4.2 mM ± 0.2 mM |
| EDTA | >200 mM | >200 mM |
| Dyeing auxiliaries (2 g liter−1) | ||
| Cibacel DBC (sequestering agent) | 16.8% ± 0.7% | 13.2% ± 0.5% |
| Univadine PA (anionic surfactant) | 17.9% ± 0.9% | 18.1% ± 1.2% |
| Tinegal MR (cationic surfactant) | 5.2% ± 0.2% | 6.4% ± 0.3% |
| Albegal FFA (wetting agent) | 2.3% ± 0.2% | 0.0% |
| Cibapon R (soaping agent) | 2.1% ± 0.1% | 0.0% |
DCC, diethyldithiocarbamate; TGA, thioglycolic acid.
Various textile dyes were decolorized with the immobilized laccase, and the toxicity of the degradation products was determined (Table 4). The extent of decolorization was standardized to 80% for all dyes. Under these conditions, the anthraquinonic dyes were detoxified to about 80%, while the toxicity of the azo dyes Reactive Black 5 and Direct Blue 71 decreased only by 13 and 40%, respectively. The copper containing dye Reactive Blue 221 was detoxified only to a very low extent by the enzyme treatment (4.1%).
TABLE 4.
Detoxification of textile dyes with an immobilized laccase from T. hirsutaa
| Dye | Structure no.b | % Detoxification (mean ± SD) |
|---|---|---|
| Reactive Blue 221 | 1 | 4.1 ± 0.4 |
| Reactive Black 5 | 2 | 11.4 ± 1.4 |
| Direct Blue 71 | 3 | 39.0 ± 1.0 |
| Basic Red 9 Base | 4 | 62.2 ± 3.2 |
| Reactive Blue 19 | 5 | 84.4 ± 4.4 |
| Acid Blue 225 | 6 | 78.0 ± 3.0 |
| Acid Blue 74 | 7 | 53.7 ± 3.7 |
Determination of the toxicity was based on the inhibition of the oxygen consumption rate of P. putida.
Structure numbers correspond to Fig. 1.
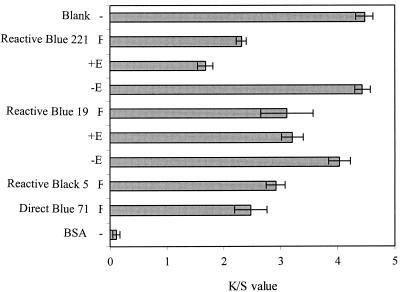
The fungus T. hirsuta, a laccase, and an immobilized laccase from this organism were compared for their ability to decolorize textile dye solutions to an extent which would allow reuse of the solutions in the dyeing process. T. hirsuta was incubated with various textile dyes, and the decolorized solutions were used for dyeing. Reactive Yellow 160 was chosen for dyeing with the decolorized solutions to ensure detection of even small interferences in the dyeing process, which could otherwise not be seen when using dark colors. Obviously, the fungus produced metabolites and/or degradation products which strongly interfered with the subsequent dyeing process, and only K/S values between 55 and 69% of those of the blank (fabric dyed in a standard dye bath prepared with water) were reached (Fig. 2).
FIG. 2.
K/S values of fabrics dyed with Reactive Yellow 160 in dye baths prepared with water (blank) or in solutions containing various dyes decolorized with T. hirsuta (F) or with a laccase from T. hirsuta. The laccase treatment was performed in an enzyme reactor in which the enzyme was retained (−E) or in Erlenmeyer flasks (+E).
Two recalcitrant textile dyes (Reactive Blue 19 and 221) that are difficult to remove from effluents were chosen to assess the decolorization potential of the T. hirsuta laccase for reuse of the solution for dyeing. Degradation products or the enzyme present in the decolorized solutions obviously interfered with dyeing, since the K/S values of fabrics dyed in a dyebath prepared with these solutions were significantly lower than those obtained using water (Fig. 2). An even more pronounced interference was measured when the same amount of protein (bovine serum albumin) instead of enzyme was present in the solution used for dyeing. However, when the decolorization was carried out in a reactor retaining the enzyme, the resulting solution yielded K/S values in dyeing which were only 1% (decolorized Reactive Blue 19) and 10% (Reactive Blue 221) lower than that of the blank. Based on these promising results, further experiments were carried out with an immobilized laccase.
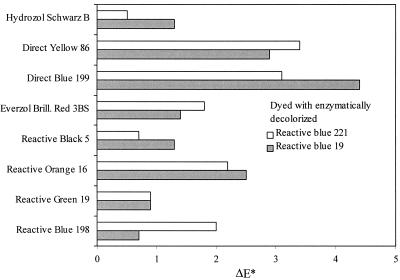
In a second step, the effect of using dye solutions decolorized with immobilized laccase for dyeing was studied in more detail in terms of acceptability of the resulting color difference. The shift of the coordinates of the color in the cylindrical color spaces L*, a*, and b*, based on the theory that color is perceived by black-white (L), red-green (a), and yellow-blue (b) sensations (22), was summarized by the ΔE* value. The value of ΔE* represents the overall color difference between the sample and the standard (Fig. 3). The ΔE* values for more than half of the combinations of decolorized dyes and dyeing processes were in the acceptable range of about 1.1. Dyeing in bright colors with decolorized dye solutions yielded worse results than dyeing in dark colors. Diode Array spectra of Reactive Green 19, Reactive Black 5, and Everzol Brilliant Red 3BS in solutions of enzymatically decolorized dyes did not show any shift but an increase in the absorption maximum, and thus, no change of the color in the dyeing solution was detected (data not shown). For Direct Blue 199 and Direct Yellow 86, a decrease and shift of the absorption maximum was observed. Correlation between absorbency measurement in solution and reflectance measurements on dyed fabrics was evident (18).
FIG. 3.
ΔE* values of fabrics dyed with various dyes in dye baths prepared with water or solutions of Reactive Blue 221 and Reactive Blue 19 decolorized with an immobilized laccase from T. hirsuta.
DISCUSSION
Decolorization of dyes by T. hirsuta was mainly ascribed to extracellular laccase activity, which is in agreement with results reported previously for T. hispida (36). 1-Amino-subtituted antraquinoid dyes were good substrates for the T. hirsuta laccase, and they were degraded to a similar extent. Out of the two azo dyes, Direct Blue 71 was the preferred substrate for the T. hirsuta laccase, which might be due to limited accessibility of the −OH and −NH2 groups in Reactive Black 5. However, for smaller substrates, the electronic contribution of substituents on the aromatic ring seemed to be more important than steric effects (48). Electron-donating methyl and methoxy substituents seemed to enhance laccase activity, while electron-withdrawing chloro, fluoro, and nitro substituents inhibited oxidation of azophenols and other substituted phenols and phenol analogs by fungal laccases (10, 48).
The T. hirsuta laccase retained 50% of its activity at 50 mM NaCl, while the immobilized enzyme tolerated a higher NaCl concentration (IC50 = 85 mM NaCl). Other authors have reported a wide IC50 range, between 0.4 and 600 mM for Cl−, for fungal laccases (48). It has been suggested that the magnitude of inhibition of laccases by halides depends on the accessibility of the copper atoms and can thus vary between different laccases and inhibitors. In plant laccases, the channels to the T2/T3 sites seem to be wider than those in fungal laccases, which are inhibited to a lower extent. Among various halides, F− was the strongest inhibitor for the T. hirsuta laccase, followed by Cl− and Br−. The same trend has been previously observed for a number of fungal laccases (48).
Additives used during textile processing can significantly affect enzyme activities (29). Anionic surfactants, which inhibited the T. hirsuta laccase by about 20%, seem to interact with the positively charged side chain of an amino acid after penetration of the 3-dimensional protein structure by the hydrophobic tail. In contrast, cationic surfactants did not show any significant denaturation effect. The T. hirsuta laccase was inhibited by 17% when incubated with a sequestering agent which is used for the inactivation of copper and iron during textile processing. Many laccases are inhibited by metal chelators, such as EDTA, and more specifically by copper chelators, such as diethyldithiocarbamate (DDC) (4, 19). Type one copper is exposed to solvents and can be easily removed by complexons (49). EDTA at a concentration of up to 200 mM showed no effect on the T. hirsuta laccase. However, DDC (IC50 = 1.2 mM) strongly inhibited the enzyme like many other fungal laccases, such as those from Pycnoporus cinnabarinus (17), from Botrytis cinerea (39), from Pleurotus ostreatus, and from Trametes versicolor (4), for which the IC50s were below 1 mM.
Interestingly, the immobilized T. hirsuta laccase was less sensitive to some inhibitors, such as F− and DCC, than the free enzyme. Similar findings have been reported for an immobilized laccase from Phlebia radiata, which was much less vulnerable to inhibitors than the free enzyme (38). The introduction of covalent bonds during immobilization usually enhances stabilities of enzymes due to the limitation of conformational changes.
Immobilization of fungal laccases on various carrier materials, such as activated carbon (13), agarose (35), Eupergit C (12), Sepharose (30), and porosity glass (37, 38), has been shown to increase stabilities of the enzyme at high pH and tolerance to elevated temperatures and to make the enzyme less vulnerable to inhibitors, such as Cu chelators. The effectivities of all these immobilization techniques varied between 70 to 98% of the protein immobilized and 67 to 96% of laccase activity recovery (13, 33, 38). Similarly, in our experiments, 89% of the protein was immobilized on alumina and 68% of the laccase activity was recovered.
Previously, it was found that a considerable number of textile wastewaters reacted toxically and mutagenically (26, 31). Toxicity assays using bacteria or daphnia have been found to be more sensitive than testing methods with fish (26). Various examples for application of the luminescent bacteria test in textiles have been discussed (42). Alternatively, the oxygen consumption rate of P. putida has been used as a parameter to monitor detoxification (24). Using a respiration-inhibition test, it has been found that anarobic degradation of azo dyes rendered the effluents more toxic by generating amines, while a second aerobic treatment eliminated this toxicity (32). Although microbial treatment of textile effluents has been found to reduce ecotoxicity (2, 32), there is no information available on detoxification of textile effluents with enzymes. Only recently have laccases been used to reduce the toxicity of wood hydrolysates for yeast fermentation (28). Using the P. putida test, we found that the toxicity of several dyes, including azo compounds, was reduced by the laccase treatment. A reaction mechanism has been recently suggested for the degradation of azo dyes by laccases involving the conversion of azo-nitrogen into molecular nitrogen (10). In our experiments, there was no strict correlation between decolorization and detoxification, indicating that dye degradation products were still toxic in some cases. Reactive Blue 221, a copper-containing dye, retained most of its toxicity after the enzyme treatment.
Reusing the laccase-treated effluent in the dyeing process, we found that enzyme protein obviously strongly interfered with the dyeing process while promising results were obtained with the immobilized enzyme. This was indicated by ΔE* values of the dyed fabrics below 1.1, which is acceptable to the industry (1, 22, 41). Shifts in the dye absorption maximum of the solution could be caused by binding of auxochromes to the dye molecules. Alternatively, degradation products and/or protein could cause aggregation of dye molecules, preventing the dye uptake to the fabric, which would cause larger color failure. In both cases, adjustments of the standard dyeing protocols would certainly improve redyeing and allow partial recycling of dyeing additives.
In this paper, we have shown that both water consumption and effluent toxicity in textile dyeing could be reduced by “enzyme remediation” with laccases. Further studies focusing on the nature of degradation products and their role in the dyeing process should be carried out.
REFERENCES
- 1.Baumann W, Brossmann R, Groebel B T, Kleinemeier N, Krayer M, Leaver A T, Oesch H P. Determination of relative color strength and residual color difference by means of reflectance measurements. Part II. Determination of residual color difference. Text Chem Color. 1987;19:21–22. [Google Scholar]
- 2.Boe R W, Boardman G D, Dietrich A M, Michelsen D L, Padaki M. Pilot scale study on anaerobic treatment of a textile wastewater. Hazard Ind Wastes. 1993;25:218–227. [Google Scholar]
- 3.Böhmer S, Messner K, Srebotnik E. Oxidation of phenanthrene by a fungal laccase in the presence of 1-hydroxybenzotriazole and unsaturated lipids. Biochem Biophys Res Commun. 1998;244:233–238. doi: 10.1006/bbrc.1998.8228. [DOI] [PubMed] [Google Scholar]
- 4.Bollag J M, Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984;48:849–854. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortone G. Effects of an anaerobic zone in a textile wastewater treatment plant. Water Sci Technol. 1995;32:133–140. [Google Scholar]
- 6.Bourbonnais R, Paice M G, Reid I D, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-Azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Brenna O, Bianchi E. Immobilized laccase for phenolic removal in must and wine. Biotechnol Lett. 1994;16:35–40. [Google Scholar]
- 9.Call H P, Mucke I. Process development and mechanisms in the mediated bleaching of pulps by laccase. Abstr. Pap Am Chem Soc. 1996;211:147. CELL. [Google Scholar]
- 10.Chivukula M, Renganathan V. Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl Environ Microbiol. 1995;61:4374–4377. doi: 10.1128/aem.61.12.4374-4377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung K T, Stevens S E. Degradation of azo dyes by environmental microorganism and helminths. Environ Toxicol Chem. 1993;12:2121–2132. [Google Scholar]
- 12.D'Annibale A, Rita Stazi S, Vinciguerra V, Giovannozzisermanni G. Oxirane-immobilized Lentinula edodes laccase: stability and phenolics removal efficiency in olive mill wastewater. J Biotechnol. 2000;77:265–273. doi: 10.1016/s0168-1656(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Burns R G. Covalent immobilization of laccase on activated carbon for phenolic effluent treatment. Appl Microbiol Biotechnol. 1992;37:474–479. [Google Scholar]
- 14.de Jong E, de Vries F P, Field J A, Van der Zwan R P, de Bont J A M. Isolation and screening of basidiomycetes with high peroxidative activity. Mycol Res. 1992;96:1098–1104. [Google Scholar]
- 15.Dey S, Maiti T K, Bhattacharyya B C. Production of some extracellular enzymes by a lignin peroxidase producing brown rot fungus Polyporus ostreiformis and its comparative abilities for lignin degradation and dye decolorization. Appl Environ Microbiol. 1994;60:4216–4218. doi: 10.1128/aem.60.11.4216-4218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggert C, Temp U, Eriksson K E. Laccase-producing white-rot fungus lacking lignin peroxidase and manganese peroxidase. ACS Symp Ser. 1996;655:130–150. [Google Scholar]
- 17.Eggert C, Temp U, Eriksson K E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ericson A C, Posner S. Relative absorbance and reflectance measurements of dye solutions and dyed fabrics. Text Chem Color. 1996;28:23–27. [Google Scholar]
- 19.Givaudan A, Effosse A, Faure D, Potier P, Bouillant M L, Bally R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in nonmotile strains of Azospirillum lipoferum. FEMS Microbiol Lett. 1993;108:205–210. [Google Scholar]
- 20.Goncalves M L, Steiner W. Purification and characterization of laccase from a newly isolated wood-decaying fungus. ACS Symp Ser. 1996;655:258–266. [Google Scholar]
- 21.Guillén F, Muñoz C, Gómez-Toribio V, Martínez A T, Martínez M J. Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl Environ Microbiol. 2000;66:170–175. doi: 10.1128/aem.66.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harold R W. Textiles: appearance analysis and shade sorting. Text Chem Color. 1987;19:23–31. [Google Scholar]
- 23.Haug W, Schmidt A, Nortemann B, Hempel D C, Stolz A, Knackmuss H J. Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-aminonaphthalene-2-sulfonate degrading bacterial consortium. Appl Environ Microbiol. 1991;57:3144–3149. doi: 10.1128/aem.57.11.3144-3149.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hund K, Traunspurger W. Ecotox-evaluation strategy for soil bioremediation exemplified for a PAH-contaminated site. Chemosphere. 1994;29:371–390. doi: 10.1016/0045-6535(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 25.Huttermann A, Haars A, Herche C. Polymerization of water insoluble lignins by Fomes annosus. Holzforschung. 1980;34:64–66. [Google Scholar]
- 26.Jaeger I, Gartiser S, Willmund R. Testing effluents of the textile refining industry with biological methods. Acta Hydrochim Hydrobiol. 1996;24:22–30. [Google Scholar]
- 27.Johannes C, Majcherczyk A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol. 2000;66:524–528. doi: 10.1128/aem.66.2.524-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jönsson L J, Palmqvist E, Nilvebrant N O, Hahnhagerdal B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl Microbiol Biotechnol. 1998;49:691–697. [Google Scholar]
- 29.Li Y, Hardin I R. Enzymatic scouring of cotton—surfactants, agitation, and selection of enzymes. Text Chem Color. 1998;30:23–29. [Google Scholar]
- 30.Milstein O, Huttermann A, Majcherczyk A, Schulze K, Fruend R, Luedermann H D. Transformation of lignin-related compounds with laccase in organic solvents. J Biotechnol. 1993;30:37–47. [Google Scholar]
- 31.Odeigah P G. Genotoxic effects of 2 industrial effluents & EMS in Clarias lazera. Food Chem Toxic. 1995;33:501–505. doi: 10.1016/0278-6915(95)00019-x. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill C, Lopez A, Esteves S, Hawkes F, Hawkes D L, Wilcox S. Azo-dye degradation in an anaerobic-aerobic treatment system operating on simulated textile effluent. Appl Biochem Biotechnol. 2000;53:249–254. doi: 10.1007/s002530050016. [DOI] [PubMed] [Google Scholar]
- 33.Osiadacz J, Al-Adhami A, Bajraszewska D, Fischer P, Peczynska-Czoch W. On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol. 1999;72:141–149. [Google Scholar]
- 34.Pierce J. Colour in textile effluents—the origins of the problem. J Soc Dyers Colourists. 1994;110:131–134. [Google Scholar]
- 35.Reyes P, Pickard M A, Vazquez-Duhalt R. Hydroxybenzotriazole increases the range of textile dyes decolorized by immobilized laccase. Biotechnol Lett. 1999;21:875–880. [Google Scholar]
- 36.Rodriguez E, Pickard M A, Vazquez-Duhalt R. Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol. 1999;38:27–32. doi: 10.1007/pl00006767. [DOI] [PubMed] [Google Scholar]
- 37.Rogalski J, Dawidowicz A L, Jozwik E, Leonowicz A. Immobilization of laccase from Cerrena unicolor on controlled porosity glass. J Mol Catal B Enzym. 1999;6:29–39. [Google Scholar]
- 38.Rogalski J, Jozwik E, Hatakka A, Leonowicz A. Immobilization of laccase from Phlebia radiata on controlled porosity glass. J Mol Catal A Chem. 1995;95:99–108. [Google Scholar]
- 39.Slomczynski D, Nakas J P, Tanenbaum S W. Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol. 1995;61:907–912. doi: 10.1128/aem.61.3.907-912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spadaro J T, Lorne I, Renganathan V. Hydroxyl radical mediated degradation of azo dyes: evidence for benzene generation. Environ Sci Technol. 1994;28:1389–1393. doi: 10.1021/es00056a031. [DOI] [PubMed] [Google Scholar]
- 41.Steen D. Acceptabilité Colorimétrique, Comparaison des Équations CMC et CIE94. L'Industrie Textile. 1998;1300:55–58. [Google Scholar]
- 42.Teichmann R. Toxicity determination with the luminescent bacteria test—applications examples in textiles. Melliand Textilberichte. 1995;76:1106–1108. [Google Scholar]
- 43.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 44.Tuor U, Winterhalter K, Fiechter A. Enzymes of white rot fungi involved in lignin degradation and ecological determinants for wood decay. J Biotechnol. 1995;41:1–17. [Google Scholar]
- 45.Ullah M A, Bedford C T, Evans C S. Reactions of pentachlorophenol with laccase from Coriolus versicolor. Appl Microbiol Biotechnol. 2000;53:230–234. doi: 10.1007/s002530050013. [DOI] [PubMed] [Google Scholar]
- 46.Wariishi H, Valli K, Gold M H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- 47.Wong Y, Yu J. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 1999;33:3512–3520. [Google Scholar]
- 48.Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- 49.Yaropolov A I, Skorobogatko O V, Vartanov S S, Varfolomeyev S D. Laccase properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol. 1994;49:257–280. [Google Scholar]
- 50.Zimmermann T, Gasser F, Kulla H G, Leisinger T. Comparison of two bacterial azoreductases acquired during adaptation to growth on azo dyes. Arch Microbiol. 1984;138:37–43. doi: 10.1007/BF00425404. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann T, Kulla H G, Leisinger T. Properties of purified orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem. 1982;129:197–203. doi: 10.1111/j.1432-1033.1982.tb07040.x. [DOI] [PubMed] [Google Scholar]