Abstract
Background:
Bullous pemphigoid (BP) responds to a variety of immunosuppressive agents and usually controls, but does not cure, the disease. Omalizumab, Food and Drug Administration—approved for asthma, selectively suppresses the activity of IgE, an important immunoglobulin in the pathogenesis of BP.
Objective:
We wished to determine if systemic omalizumab would have a therapeutic effect in patients with BP.
Methods:
We treated 6 patients with BP using omalizumab and followed up their disease for up to 42 months.
Results:
Although variable, 5 of the 6 patients with BP received therapeutic benefit from systemic omalizumab (the sixth terminated treatment because of intercurrent illness) with less use of other immunosuppressants, inhibition of new bullae, less pruritus, and dramatic decreases in eosinophil counts. None of the patients had untoward side effects from omalizumab.
Limitations:
This was an open, uncontrolled study.
Conclusions:
Omalizumab neutralizes the activity of IgE in patients with BP and improves the control of their disease activity. (J Am Acad Dermatol 2014;71:468–74.)
Keywords: autoimmunity, bullous pemphigoid, IgE, omalizumab, pruritus
Bullous pemphigoid (BP) is an acquired autoimmune bullous disease characterized by autoantibodies against 2 skin basement membrane zone (BMZ) proteins: type XVII collagen, a 180-kd protein (bullous pemphigoid antigen 2), and the 230-kd BP antigen (bullous pemphigoid antigen 1) found within the hemidesmosomes of basal keratinocytes.1,2 Patients with BP develop tense skin blisters on inflammatory skin, and often experience pruritus and urticaria-like erythematous skin lesions. IgG is the predominant antibody against the BMZ components, but studies have shown that a majority (70%) of patients with BP also have elevated levels of serum IgE,3–7 and 25% of patients with BP also have IgE deposits at the BMZ.8 Standard therapies for BP consist of corticosteroids and immunosuppressants, which are associated with significant morbidity.9 Therefore, a general treatment goal for BP is to use the lowest possible dose of systemic steroids. Nevertheless, nonsteroidal immunosuppressive agents also carry significant potential side effects, especially with prolonged use.
Omalizumab, a treatment for asthma,10 is a humanized monoclonal antibody that blocks the binding of IgE to its receptors. Compared with systemic corticosteroids, methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil, the mechanism of action of omalizumab is more selective. Therefore, omalizumab may have a more benign side-effect profile than the conventional agents for BP. Herein, we report 6 cases that illustrate the use of omalizumab in the management of BP. Preliminary results on patients 1 and 4 were previously reported11,12 and updated information on their long-term treatment is contained in this report.
CASE REPORTS
A summary of the salient features of the cases are provided in Table I. Detailed reports of the cases follow.
Table I.
Summary of bullous pemphigoid patients treated with OMZ
| Patient no. | Patient age, sex, and disease duration | Problems and comorbidities | IgE levels at start of OMZ therapy, IU/mL | Eosinophil counts* | Regimen | Response | Final dose |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 70 y, Female, BP × 1 y | Steroid-refractory disease. Osteoporosis. Poor control on prednisone (40 mg daily), azathioprine (150 mg daily), minocycline 200 mg daily. | 222 | Elevated: 3.4 × 103/μL | 300 mg SC q2 wk | 1 wk After OMZ administration, significant decrease in itching, reduction of blister count by 44%. By 16 wk, BSA involvement decreased from 50% to 5%. Patient disease free for 15 wk after OMZ d/c and then flared. Repeated OMZ monotherapy led to clearance of disease. Remained symptom free for 5 mo. Flare was refractory to OMZ. | d/c’ed |
| 2 | 78 y, Female, BP × 1.5 y | Steroid-refractory disease. Osteoporosis. Flared on prednisone taper, intense pruritus, niacinamide (2 g), doxycycline (200 mg). | 1835 | Not elevated: 1.8%, 0.12 × 103/μL | 300 mg SC q6 wk, q8, q6, then q4 wk; maintenance dose of 300 mg every 4 wk for 20 mo | Successful taper off prednisone after 3 mo on OMZ. D/c’ed because of insurance. Relapse with new blister formation. OMZ restarted and titrated to effect. Patient has been symptom free for 20 mo on 300 mg q4 wk. | 300 mg Q4 wk |
| 3 | 72 y, Female, BP × 3.5 y | Multiple failed attempts at prednisone taper. Osteoporosis. | 1181 | Elevated: 19%, 5.4 × 103/μL | 375 mg SC q4 wk | Successfully tapered off prednisone in 2 mo. Symptom free since. | 375 mg Q4 wk |
| 4 | 76 y, Female, BP × 6 mo | Steroid-refractory disease. Steroid psychosis. Failed intensive regimen of plasmapheresis, cyclophosphamide, and azathioprine. | 287 | Elevated: 31.9%, 1.64 × 103/μL | 300 mg SC q4 wk | Cessation of pruritus and new blister formation within 24 h after OMZ administration. Symptom free on OMZ during taper and as monotherapy for a total of 42 mo. | 300 mg Q4-Q8 wk |
| 5 | 86 y, Female, long-standing BP | Steroid-refractory disease. | 2135 | Elevated: 1.81 × 103/μL | 375 mg SC q2 wk | 1 wk After OMZ administration, 22% reduction in blister count, d/c’ed because of intercurrent exacerbation of COPD 2/2 to discontinuation of prednisone. No adverse effects caused by OMZ. | N/A |
| 6 | 55 y, Female, BP × 7 mo | Failed prednisone taper, followed by steroid-refractory disease. | 5821 | Elevated: 17.7 × 103/μL | 375 mg SC q2 wk | 1 wk After OMZ administration, cessation of new blister formation, 30% reduction in BSA involvement, all clear after 3 wk; taper begun after 3 doses of OMZ; disease free for 3 mo, OMZ d/c’ed because of insurance, relapsed. Resumed prednisone, azathioprine, and minocycline. | N/A |
The asthma dosing nomogram was used to obtain the starting dose and dosing interval for patients 1, 5, and 6. It was used for the initial dose of OMZ for patients 2, 3, and 4. The subsequent doses in these patients were titrated to effect and/or availability of the medication. In patients 2, 3, 5, and 6, the initial IgE levels exceeded the maximum asthma nomogram values (600–700 IU/mL), and the maximum recommended dose appropriate for the patients’ weight was used.
BP, Bullous pemphigoid; BSA, body surface area; COPD, chronic obstructive pulmonary disease; d/c, discontinue; N/A, not applicable; OMZ, omalizumab; Q, every; SC, subcutaneous injection.
In patients 1, 5, and 6, only the absolute eosinophil counts were available. In patients 2, 3, and 4, both the relative and absolute eosinophil counts were reported. Reference ranges: <7% and <0.18 × 103/μL, respectively.
Patient 1
This female patient’s initial treatment was previously reported as part of a trial of omalizumab as monotherapy for BP (IND 100569).11 During the trial, her body surface involvement with urticarial plaques declined from 50% to 5%, and only a small number of 4- to 6-mm erosions remained (Fig 1). The patient remained clear for 15 weeks, at which point she noticed increased pruritus and a recurrence of skin lesions. Omalizumab was reinstituted at the same dose, the pruritus subsided, and the blisters resolved within 2 weeks. After a 6-dose cycle her skin was clear and omalizumab was discontinued. The patient remained clear for 5 months until she flared with a recurrence of pruritus and blistering. Omalizumab was again reinstituted at the same dose; however, after an initial decrease in her eosinophil count and some improvement in symptoms, her disease recurred while on omalizumab, the medication was discontinued, and she was treated with prednisone and azathioprine.
Fig 1.
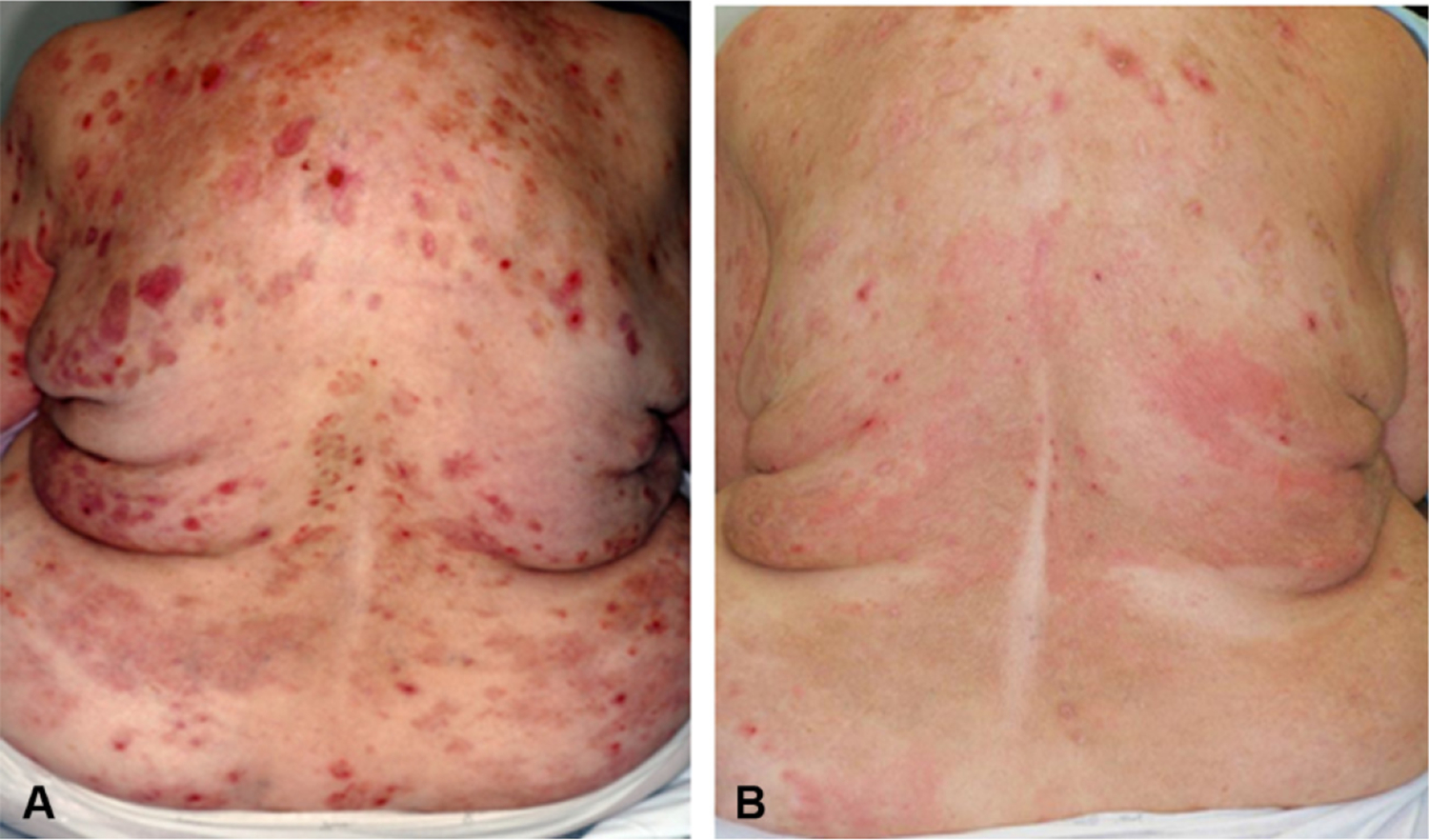
Response of a patient with bullous pemphigoid (BP) after 6 omalizumab injections as monotherapy. A, Involvement of the back of a steroid-refractory patient with BP before omalizumab treatment. B, Four months after beginning omalizumab as monotherapy, the inflammatory plaques have largely resolved, leaving postinflammatory hyperpigmentation and a small number of erosions and mild, transient erythema. The patient cleared completely after a second cycle of omalizumab treatment.
Patient 2
A 78-year-old woman was referred to University of Southern California for BP. She had pruritus for 18 months when the diagnosis of BP was made by histology, IgG at the BMZ of biopsied skin by direct immunofluorescence (IF), and elevated enzyme- linked immunosorbent assay (ELISA) to bullous pemphigoid 180 kDa antigen (BP180) (90 U, normal <9 U) and bullous pemphigoid 230 kDa antigen (BP230) (115 U, normal <9 U). On 20 mg of prednisone per day, her BP improved, but was still present. Therapy was augmented with niacinamide (2 g daily) and doxycycline (200 mg daily), but her pruritus continued. The addition of multiple antihistamines did not control her symptoms. Despite the addition of 50 mg daily of azathioprine she continued to have persistent pruritus when her prednisone was decreased below 15 mg daily. Because of her disabling pruritus, elevated IgE (615 IU/mL), and osteoporosis associated with continued prednisone use, the decision was made to initiate therapy with omalizumab, 300 mg calculated by weight and IgE level, every 6 weeks. Three months after initiation of omalizumab therapy, she was able to discontinue the use of prednisone and azathioprine. However, she was not able to obtain her next scheduled dose of omalizumab because of difficulty with approval from her insurance company, and subsequently had a relapse of her BP symptoms. Omalizumab was then reinstituted at 300 mg every 8 weeks. New blister formation ceased, but she continued to experience pruritus. Treatment cycles were shortened to every 6 weeks, and her pruritus improved, but did not resolve completely. Treatment cycles were further shortened to every 4 weeks, and she now experiences only intermittent pruritus that is controlled readily with antihistamines fexofenadine (180 mg daily) and hydroxyzine (50 mg at bedtime as needed). We have continued her on this omalizumab regimen, and she has remained free of skin lesions for the last 20 months.
Patient 3
A 72-year-old woman presented with a 7-month history of highly pruritic plaques that evolved into tense blisters on erythematous bases. Histology of a lesion showed a subepidermal bulla with an eosinophilic infiltrate and trace IgG was observed at the dermoepidermal junction by direct IF. When referred to University of Southern California, she was on 60 mg of prednisone per day and her indirect IF and ELISA results were negative. Because of the patient’s hesitancy to start another immunosuppressive agent, confirmed osteoporosis with a history of fracture of the 12th thoracic vertebrae, and persistently elevated eosinophil counts and IgE levels, the decision was made to institute a trial of omalizumab. Two months after the initiation of therapy with omalizumab, prednisone was successfully tapered off for the first time since her diagnosis 3–5 years prior. She has remained disease free on the above regimen for the past 12 months.
Patient 4
Patient 4 was previously reported12 with limited follow-up, and we update her care here. A 76-year-old Caucasian woman presented with a 6-month history of highly pruritic blisters. She was given a diagnosis at an outside hospital with BP based on histology and direct IF. She was initially treated with 60 mg of oral prednisone, but she subsequently became agitated and abusive while on steroids, and experienced an exacerbation of her essential tremor. Further attempts to control her disease with prednisone (40 mg) and azathioprine (100 mg/d) failed, so she was admitted to the University of Southern California Norris Comprehensive Cancer Center for more intensive therapy. Because of her elevated IgE levels (287 IU/mL), high eosinophil count (l640/mm3), complications related to immunosuppression, and recalcitrant disease, omalizumab (300 mg by subcutaneous injection) was initiated. Her pruritus and new blisters almost immediately resolved, and her eosinophil count decreased to 60/mm3 (0.8%) within the first day. She was discharged on 80 mg of oral prednisone daily augmented with 100 mg of oral azathioprine daily with scheduled omalizumab injections every 4 to 8 weeks.
Over the next 2 years, she received subcutaneous omalizumab injections every 4 to 8 weeks and was gradually tapered off steroids and the other immunosuppressants. Although she had no skin lesions, she continued to experience periodic pruritus. Approximately 42 months after initiating omalizumab, she developed urticarial plaques and a single 12-mm tense bulla on her left thigh. Oral prednisone (20 mg/d) and mycophenolate mofetil (1 g/d) were reinstituted, and it was recommended to her referring physician that intravenous rituximab be started.
Patient 5
An 86-year-old patient was also enrolled in a trial of omalizumab (Investigator New Drug 100569) and was treated as a monotherapy using the asthma dosing nomogram at a dose of 375 mg every 2 weeks. Before omalizumab treatment, her initial blister count was 49 (individual vesicles and bullae). One week after her first injection, her blister count had decreased to 40. After this visit, and before her next injection, the patient had an exacerbation of pre-existing chronic obstructive lung disease requiring hospitalization and omalizumab was stopped. The opinion of her pulmonologist was that these complications were not related to the omalizumab, but more likely from the previous termination of her prednisone.
Patient 6
A 55-year-old woman was referred for a blistering rash. A biopsy specimen was consistent with BP with IgG and C3 at the BMZ by direct IF and indirect IF was positive (1:10 dilution) on the roof of salt-split skin. On examination, over 80% of her body surface was involved with widespread urticarial plaques, vesicles, and bullae. Prednisone was initiated at 100 mg/d and azathioprine at 100 mg/d. Despite plasmapheresis, prednisone (100 mg/d), and azathioprine (100 mg/d), her eosinophil count continued to increase, peaking at 17,700/mm3, and new bullae formed daily. Treatment was initiated with omalizumab at 375 mg every 2 weeks. Two weeks after her first injection her eosinophil count was 40/mm3 and it remained in the normal range throughout her omalizumab treatment. Six doses of omalizumab were given, at which time the patient was clear of lesions and prednisone had been tapered to 15 mg/d and the azathioprine was tapered to 50 mg. Her insurance company then denied further treatments with omalizumab. She did well for 3 months with further tapering of her prednisone to 10 mg daily, when she relapsed and was treated with increased prednisone, minocycline, and azathioprine. The only side effect during her treatment was epigastric pain and mild elevation of the liver enzymes (aspartate aminotransferase-41, alanine aminotransferase-63), which responded to a decrease in her azathioprine dose from 100 mg to 50 mg.
DISCUSSION
All of our patients with BP had typical lesions with urticarial plaques, vesicles, and bullae; reported severe pruritus; and were found to have elevated IgE or eosinophil counts. Five of 6 of our patients responded to treatment with omalizumab and none had adverse reactions to the drug. In 3 of our patients, omalizumab was sufficient as monotherapy (patients 1, 2, and 3). In patient 1, discontinuation and reinstitution of omalizumab seemed to correlate with clinical disease exacerbations and subsequent disease remissions, suggesting that continuous therapy with omalizumab may be superior to intermittent dosing. In 2 other patients (patients 4 and 6), omalizumab was used successfully as a steroid-sparing agent in the induction and maintenance of remission. One patient (patient 6) was able to reduce her steroids while on omalizumab, but had an exacerbation of her BP when omalizumab became unavailable. These observations illustrate the use of omalizumab in the management of BP, even in those patients whose disease is refractory to steroids and other immunosuppressants.
In patients 1, 5, and 6, sequential eosinophils counts, BP180 and BP230 ELISA data, and disease severity scores (as defined13) were available. The patients experienced significant improvement in the BP before any significant changes in their circulating autoantibodies, which gradually declined over several months. The parameter that followed disease activity the most closely during omalizumab therapy was the eosinophil count. Summary data on patient 1, who received several cycles of omalizumab separated by intervening flares, are shown in Fig 2. It illustrates the relative lack of correlation between the disease activity and BP180 or BP230 ELISA but generally a parallel increase and decrease of the eosinophil counts with disease activity.
Fig 2.
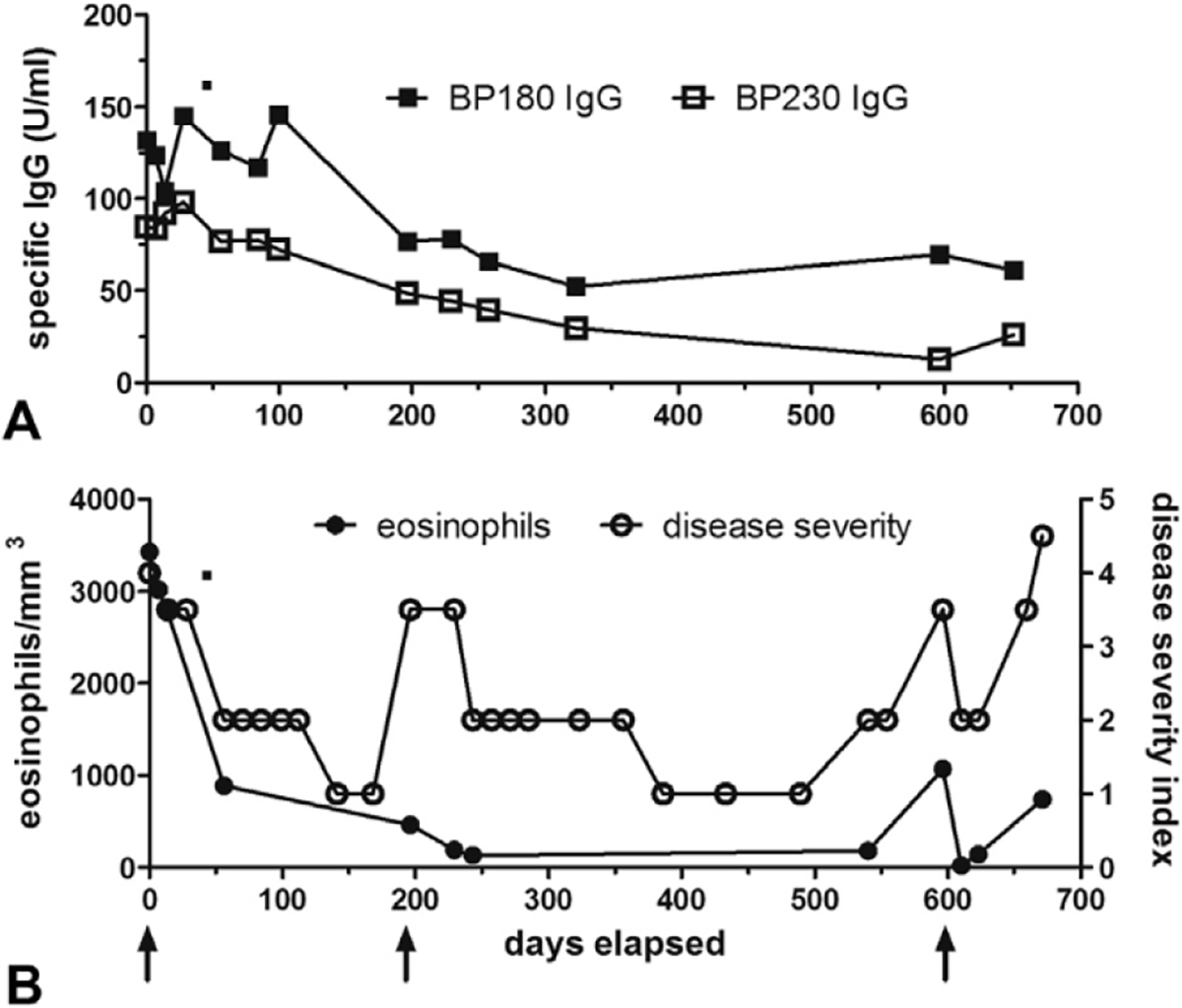
Patient 4: autoantibody levels, eosinophil counts, and disease severity throughout omalizumab therapy. Patient 4 was assessed during 3 separate courses of omalizumab; arrows indicate the first dose of omalizumab in her treatment cycles. Bullous pemphigoid (BP)180 and BP230 IgG (A) autoantibody levels were measured in serum samples by enzyme-linked immunosorbent assay (MBL International, Woburn, MA). Disease activity was assessed on a 4-point scale: 4.5/4.0 = generalized disease >10% total body square area with/without immunosuppressive medications; 3–5/3 0 = localized disease <10% total body square area with/without medications; 2 = remission with medications; 1 = remission without medications. Peripheral eosinophil counts (B) paralleled disease activity more closely than autoantibody levels.
In asthma studies the only statistically significant adverse reaction compared with placebo was hypersensitivity reactions to the medication including anaphylaxis (<0.1%).14,15 Because many of these patients have an allergic diathesis, it is not clear whether patients with BP will have the same risk of anaphylaxis. However, because of the anaphylaxis warning for omalizumab, we supply all patients with an epinephrine autoinjector. Neoplasms were also numerically higher in omalizumab-treated patients compared with control subjects, although this was not statistically significant and a larger, more recent analysis did not reveal an increased risk of malignancy.16 None of our patients had any side effects attributable to omalizumab.
Initial omalizumab dosing in our patients was based on the asthma nomogram. This nomogram uses body weight and total IgE levels to determine the dose and can vary from 150 mg to 375 mg. The dosing interval can vary from every 2 weeks or every 4 weeks depending on these parameters. Because IgE levels cannot be accurately measured after treatment with omalizumab,17 when dosage adjustments were made in these patients they were made by altering the interval between injections, as was done with several of the patients we describe herein. Because the recommended dosing of omalizumab has been based on its use in asthma, the ideal regimen in BP has yet to be established.
With the relatively small number of patients treated, it is not possible to make strong conclusions about the ideal patient for omalizumab treatment. Until a larger, controlled trial is performed, it seems most prudent to use omalizumab in patients with recalcitrant disease who have elevated IgE levels, eosinophilia, or both.
Although the mechanism of action of omalizumab in patients with BP is not completely understood, the rationale for omalizumab in the treatment of BP is based on the observations that: (1) IgE levels are elevated in most patients with untreated BP3–7; (2) patients with BP express IgE autoantibodies against BP antigens5–7; and (3) the developing knowledge that IgE autoantibodies can replicate the early phases of lesion development in BP.5,18,19 The pharmacokinetics of omalizumab indicate that free IgE decreases within hours of the first dose.15 Down-regulation of the IgE receptor and circulating eosinophils has also been demonstrated in asthmatics after omalizumab treatment.17 Given the striking eosinophilic infiltrate in BP,20 increased mast cells in BP lesions, and the close association between eosinophils and mast cell activation via IgE antibodies,21 we postulate that omalizumab likely interferes with these interactions.
CAPSULE SUMMARY.
A role lgE class autoantibodies in bullous pemphigoid has been demonstrated
Herein, 6 patients with pemphigoid were treated with omalizumab, an anti-lgE monoclonal antibody
Five of 6 patients had a good response to omalizumab, suggesting that targeting of IgE may be a strategy for the treatment of bullous pemphigoid.
Acknowledgments
This material is based on work supported in part by VA Merit Review grant 1101CX000317-01 (Dr Fairley) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development 1BX001680-01.
Abbreviations used:
- BMZ
basement membrane zone
- BP
bullous pemphigoid
- BP180
bullous pemphigoid 180 kDa antigen
- BP230
bullous pemphigoid 230 kDa antigen
- ELISA
enzyme-linked immunosorbent assay
- IF
immunofluorescence
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterizatrion of bullous pemphigoid antigen: a unique basement membrane zone protein of stratified squamous epithelia. Cell 1981;24:897–903. [DOI] [PubMed] [Google Scholar]
- 2.Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogeneity of the bulllous pemphigoid antigens as detected by immunoblotting. J Immunol 1986;136:1231–5. [PubMed] [Google Scholar]
- 3.Arbesman CE, Wypych Jl, Reisman RE, Beutner EH. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch Dermatol 1974;110:378–81. [PubMed] [Google Scholar]
- 4.Bowszyc-Dmochowska M, Silny W, Dmochowski M. Detection of lgG4 deposits using a single-step direct immunofluorescence on natrium chloride-separated skin and elevated levels of serum total IgE in active bullous pemphigoid. J Invest Dermatol 2000;114:804a. [Google Scholar]
- 5.Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, et al. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol 2003;120: 784–8. [DOI] [PubMed] [Google Scholar]
- 6.Delaporte E, Dubost-Brama A, Ghohestani R, Nicolas JF, Neyrinck JL, Bergoend H, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol 1996;157: 3642–7. [PubMed] [Google Scholar]
- 7.Christophoridis S, Budinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA, and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol 2000;143:349–55. [DOI] [PubMed] [Google Scholar]
- 8.Provost TT, Tomasi TB Jr. Immunopathology of bullous pemphigoid: basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol 1974;18: 193–200. [PMC free article] [PubMed] [Google Scholar]
- 9.Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G. British Association of Dermatologists’ guidelines for the management of bullous pemphigoid. Br J Dermatol 2012;167:1200–14. [DOI] [PubMed] [Google Scholar]
- 10.Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy 2009;64:1728–36. [DOI] [PubMed] [Google Scholar]
- 11.Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009; 123:704–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.London VA, Kim GH, Fairley JA, Woodley DT. Successful treatment of bullous pemphigoid with omalizumab. Arch Dermatol 2012;148:1241–3. [DOI] [PubMed] [Google Scholar]
- 13.Messingham KAN, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP 180-specific IgE production in bullous pemphigoid. J Immunol Methods 2009;346:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-lgE antibody. N Engl J Med 1999;341: 1966–73. [DOI] [PubMed] [Google Scholar]
- 15.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004;170:583–93. [DOI] [PubMed] [Google Scholar]
- 16.Busse W, Buhl R, Fernandez-Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol 2012; 129:983–9. [DOI] [PubMed] [Google Scholar]
- 17.Corren J, Shapiro G, Reimann J, Deniz Y, Wong D, Adelman A, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol 2008;121:506–11. [DOI] [PubMed] [Google Scholar]
- 18.Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol 2007;127:1167–74. [DOI] [PubMed] [Google Scholar]
- 19.Fairley JA, Burnett CT, Fu C-L, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol 2007;127:2605–11. [DOI] [PubMed] [Google Scholar]
- 20.Wintroub BU, Mihm MC Jr, Goetzl EJ, Soter NA, Austen KF. Morphologic and functional evidence for release of mast-cell products in bullous pemphigoid. N Engl J Med 1978;298: 417–21. [DOI] [PubMed] [Google Scholar]
- 21.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 2010;125:S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


