Abstract
Mechanistic evaluations of processes that underlie organism-level physiology often require reductionist approaches. Dermal fibroblasts offer one such approach. These cells are easily obtained from minimally invasive skin biopsy, making them appropriate for the study of protected and/or logistically challenging species. Cell culture approaches permit extensive and fine-scale sampling regimes as well as gene manipulation techniques that are not feasible in vivo. Fibroblast isolation and culture protocols are outlined here for primary cells, and the benefits and drawbacks of immortalization are discussed. We show examples of physiological metrics that can be used to characterize primary cells (oxygen consumption, translation, proliferation) and readouts that can be informative in understanding cell-level responses to environmental stress (lactate production, heat shock protein induction). Importantly, fibroblasts may display fidelity to whole animal physiological phenotypes, facilitating their study. Fibroblasts from Antarctic Weddell seals show greater resilience to low temperatures and hypoxia exposure than fibroblasts from humans or rats. Fibroblast oxygen consumption rates are not affected by temperature stress in the heat-tolerant camel, whereas similar temperature exposures depress mitochondrial metabolism in fibroblasts from rhinoceros. Finally, dermal fibroblasts from a hibernator, the meadow jumping mouse, better resist experimental cooling than a fibroblast line from the laboratory mouse, with the hibernator demonstrating a greater maintenance of homeostatic processes such as protein translation. These results exemplify the parallels that can be drawn between fibroblast physiology and expectations in vivo, and provide evidence for the power of fibroblasts as a model system to understand comparative physiology and biomedicine.
Keywords: Dermal fibroblast, Primary cell culture, Skin biopsy, Environmental stress, Immortalization
1. Introduction
Understanding biochemical and physiological responses in complex biological systems requires increasingly mechanistic evaluations. Gold standard approaches to understand gene function require knock in and knock down experiments, which are often complicated or unfeasible in non-model organisms (reviewed in (Fanter et al., 2022)). Mammalian cell culture is a well established branch of biological science with a long history of service to basic research and medical advancement (Abbas et al., 2021). At its most basic, cell culture is the isolation of cells from the host organism and their propagation in a controlled, artificial environment – the foundations of which have not changed since the establishment of Earle’s mouse fibroblast Strain L in 1948 (Sanford et al., 1948), and Eagle’s development of a defined cell culture medium in 1955 (Eagle, 1955). While this reductionist approach separates the cell from organismal physiology, it also allows great freedom and control in experimental design and enables research approaches that are not possible in vivo. In broad strokes, primary cell culture begins with the isolation of tissue from the study organism, the disaggregation of tissue to individual cells, and the maintenance of those cells for their useful (but finite) lifetime in culture conditions (Freshney, 2015). In practice, a population of primary cells stops proliferating after a certain number of population doublings, a phenomenon commonly referred to as senescence (Cristofalo and Pignolo, 1993). In some cases, the proliferative ability and natural aging of the cells may be a phenotype of interest (e.g. (Murakami et al., 2003; Pickering et al., 2015)), but this natural limitation can be overcome through the process of immortalization which is detailed below. Immortalized cells embody tradeoffs inherent in the use of cell culture as a research tool - their rapid proliferation and immortal nature are convenient and can allow the perpetual maintenance of cells from rare or endangered species, but the genetic alterations causing immortalization may also affect the phenotype of interest to the comparative physiologist. Despite possible limitations, the use of cell culture opens doors to mechanistic studies that are not possible in the intact animal.
Dermal fibroblasts from non-model organisms could provide a bridge between in vivo approaches and functional molecular biology, supporting experiments that take first steps toward understanding genome to phenome (e.g. (Murakami et al., 2003)), as well as mechanisms of species responses and resilience to stressor or disease (Ryder and Onuma, 2018). Such an approach will build on the historical utility of human fibroblasts in medical research. Primary fibroblasts have been used to successfully investigate or diagnose human medical disorders, with examples including Werner’s syndrome of premature aging (Cohen and Sinclair, 2001), many inborn errors of metabolism such as primary carnitine deficiency (Magoulas and El-Hattab, 2012), and even psychiatric disorders (Mesdom et al., 2020). Here we report methodology for isolating and culturing dermal fibroblasts from a range of mammals, and present examples of data demonstrating the links between primary fibroblasts and organismal phenotypes. These data types are illustrative of experimental approaches that can be performed in primary cell culture, but are not exhaustive. We also discuss considerations for experimental design in cell culture systems.
Relevant cell types are a key experimental foundation for exploring species-specific physiology, molecular biology, and responses to perturbation (e.g. (Jimenez et al., 2014a)). Cell culture systems must be experimentally tractable but also physiologically informative. Fibroblasts are an interesting candidate cell type to consider, as they are found within many tissues and organs, including the largest organ in the body – the skin. Logistically, ideal cell type(s) should have high expansion potential and be easily cultured. Fibroblasts are straightforward to proliferate in culture, often forming the cause of cell type contamination in more sensitive primary cultures (e.g. kidney, heart). To apply modern molecular techniques to non-model species, including endangered animals, it is also an important consideration to choose a cell type that can be collected via non-lethal samples such as biopsies or blood draw. Dermal fibroblasts are ideal in this regard, as they can be isolated and expanded as explants from a minimally invasive skin biopsy (Mestre-Citrinovitz et al., 2016; Siengdee et al., 2018; Wang et al., 2021).
2. Materials and methods
2.1. Protocol for isolation and culture of primary dermal fibroblasts
Establishing primary dermal fibroblasts from skin biopsies is a well-established technique that has been robustly described for humans (e.g. (Jones and Wise, 1997)), other mammals (e.g. (Abade dos Santos et al., 2021; Mestre-Citrinovitz et al., 2016; Ryder and Onuma, 2018)), birds (e.g. (Harper et al., 2011; Ryder and Onuma, 2018)), and vertebrate ectotherms (e.g. (Webb et al., 2014)). Enzymatic digestion of the biopsy prior to plating is common, but not universal (e.g. (Vangipuram et al., 2013; Villegas and McPhaul, 2005)). Below, we present an example of a protocol, optimized widely in mammalian samples, for collecting and handling a skin biopsy, then processing the sample to isolate dermal fibroblasts from the enzymatically digested skin explant (Fig. 1).
Fig. 1.
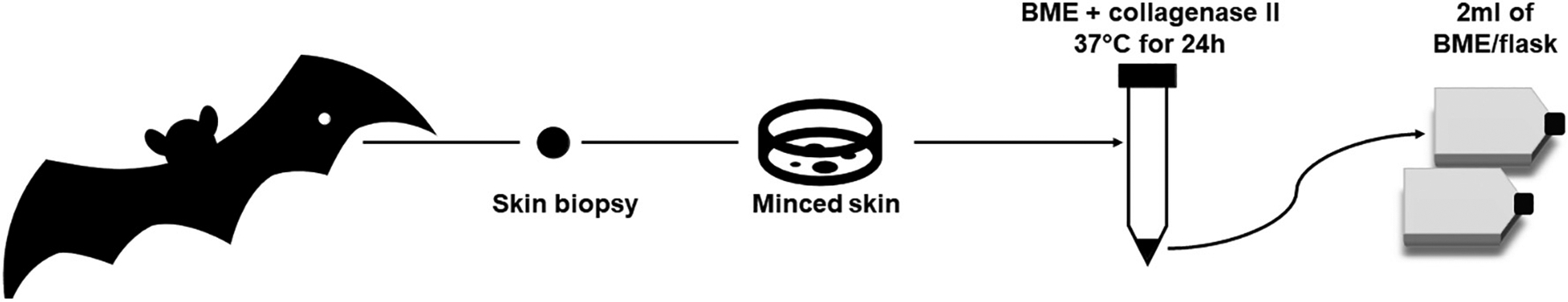
Schematic of primary fibroblast isolation protocol from a mammalian skin biopsy.
2.1.1. Skin biopsy
Prepare skin site by removing hair with depilatory cream or razor and disinfecting the skin with antimicrobial soap, isopropanol, or 70% ethanol. For live animal biopsy, provide local analgesic as appropriate and use a sterile biopsy punch (1–8 mm diameter), for freshly euthanized animals a similarly sized piece of skin can be obtained with scissors. Sterilize all instruments and collect the skin sample using aseptic technique. In field settings, bleach is also useful for decontamination. After collection, spray the biopsy with 70% ethanol on both sides, or briefly immerse, then place in 5 mL of ice cold growth media (e.g. complete BME media; Gibco #21010046 with 20% fetal bovine serum (FBS; BenchMark, Gemini Bio #100–106), 1% l-glutamine, 1% penicillin/streptomycin) until processing. Mammalian biopsies may be stored in cold media for several days (48–72 h) before processing (Jones and Wise, 1997), and avian skin samples are reported to retain viability up to 24 h after collection (Calhoon et al., 2013). This feature makes dermal fibroblasts particularly appealing for studies of remote species, where cell culture facilities are not readily available near field sites of sample collection.
2.1.2. Fibroblast isolation and preservation
To isolate primary fibroblasts from a skin sample, transfer explant to a sterile petri dish and mince with a sterile razor or scalpel blade. Transfer to a sealed tube containing 1:6 10 mg/mL collagenase type II and complete BME media and incubate overnight at 37 °C. After incubation, centrifuge at 1600 g for 10 min. Aspirate supernatant, add 5 mL of sterile DBPS, centrifuge at 1600 g for 10 min. Repeat the washing procedure twice. After final aspiration of supernatant, resuspend the digested skin sample with 4–6 mL of fresh warm complete BME and plate in 2–3 25 cm2 flasks, each with 2 mL of media plus cell suspension. A low volume is essential initially to ensure the minced explant adheres to the flask. Dermal fibroblasts grow as a monolayer in standard cell culture plasticware with no requirements for coating substrates (e.g., extracellular matrix proteins or poly-d-lysine). Place flasks into a cell culture incubator at 37 °C in a humidified, 5% CO2 environment. After 3–4 days, gently add another 2 mL of complete BME. Change media once outgrowth is observed (3–7 days). When cells reach 80–90% confluence, they can be passaged or cryopreserved at 6 × 105 cells/mL in a solution of 40% BME, 50% fetal bovine serum (FBS) and 10% dimethylsulfoxide and freeze slowly, at a rate no greater than −1 °C/min.
2.2. Experimental cell systems in mammals
In addition to describing a culture protocol and considerations for the use of dermal fibroblasts, we present experimental data to support their application in comparative physiology and biomedicine. Each example includes data from one specific experiment and an associated cell physiological endpoint, to highlight a range of possible approaches. A thorough mechanistic investigation of cell physiology responses to ex vivo stimuli in a comparative system would necessarily incorporate a suite of endpoints and experimental manipulations.
2.2.1. Mammalian fibroblast culture
Primary fibroblasts included in experiments presented here were derived from mammalian skin explants in all cases. For Weddell seals (Leptonychotes weddellii), skin samples were obtained in conjunction with collection of muscle biopsies as part of another project (National Marine Fisheries Service permits #19439, #18662, Antarctic Conservation Act #2016–005). Adult seals were anesthetized with ketamine and midazolam (Mellish et al., 2010) and local analgesia was applied to the biopsy site with lidocaine. Skin samples were obtained with a 6 mm biopsy punch (Milltex). Humpback whale (Megaptera novaeangliae) fibroblasts were collected for another project by biopsy dart and developed under National Marine Fisheries Service parts permit #22479. Dromedary camel (Camelus dromedarius) and Southern white rhinoceros (Ceratotherium simum simum) primary cells were obtained from the San Diego Zoo Wildlife Alliance. Primary fibroblasts from Sprague Dawley rat (Rattus norvegicus), 13-lined ground squirrel (Ictidomys tridecemlineatus), and meadow jumping mouse (Zapus hudsonius) were isolated from skin samples collected immediately after euthanasia. Human fibroblasts and an established, immortalized laboratory mouse (Mus musculus) fibroblast cell line (NIH-3 T3) were purchased from ATCC. All procedures involving animals were authorized by institutional animal care and use committees.
2.2.2. Growth media for primary cells
Fibroblasts were isolated and proliferated in complete BME for rat, 13-lined ground squirrel, and Weddell seal. Human fibroblasts were thawed in FibroLife serum free media (Lifeline, #L-0001) then proliferated in BME. Humpback whale cells were cultured in DMEM:F-12 (Gibco #10565–018) supplemented with 10% FBS, 1% HEPES (Gibco #15630–080), and 1× antimycotic/antibiotic (Gibco #15–240–096). Rhinoceros cells were cultured in FGM-2 complete media (Lonza #CC-3131). Camel cells were cultured in either FGM-2 media or BME (with only 10% FBS, otherwise the same as complete BME).
2.2.3. Fibroblast immortalization
Primary meadow jumping mouse fibroblast cultures were immortalized using SV40 Large T Antigen. A commercial cell line optimized for lentiviral production (Lenti-X 293 T, Clon-Tech) was transiently transfected at 80% confluency with a mixture of lentiviral DNA constructs (Lois et al., 2002), including the SV40 Large T Antigen coding sequence, using TransIT-Lenti Transfection Reagent (6603, Mirus). The Lenti-X 293 T cells used for viral packaging were incubated for 48 h and the overlying culture medium (containing virus) was harvested and 0.45 μm filtered. The filtered culture medium containing viral particles was supplemented with 10 μg/mL polybrene and added to proliferating meadow jumping mouse dermal fibroblasts. Fresh growth medium was added after 4 h. The virally-transduced dermal fibroblasts were propagated and cryogenically stored for later experimental use.
2.3. Physiological endpoints
2.3.1. Crystal violet assay for cell content and proliferation
Crystal violet stain was used to determine relative number of adherent fibroblasts, following the protocol of Feoktistova et al. (2016). Cells were fixed with methanol then incubated for 30 min with a crystal violet solution (0.5% crystal violet, 20% methanol). Crystal violet solution was aspirated, and cells were washed twice to remove excess stain. The dye remaining in cells reflects the amount of DNA present (a proxy for the number of cells present), which can be used to evaluate relative cell number and proliferation rates. This was quantified by measuring absorbance at 585 nm of the stain in triplicate, dissolved in 1 mL 0.01% acetic acid and 50% EtOH solution per well (volumes are indicated for a 12 well plate).
2.3.2. Lactate measurement
Lactate (mmol/L) was assayed in duplicate from cell culture supernatant using a Nova sport lactate meter. Lactate levels were normalized to absorbance of crystal violet assay in the same wells as a metric for number of cells present.
2.3.3. Oxygen consumption rates (OCR)
Mitochondrial oxygen consumption rates were determined using a Seahorse XFp (Agilent, Santa Clara, CA). Seahorse extracellular flux (XF) analyzer can provide an array of metabolic data by tracking oxygen consumption and extracellular acidification rates in plate-based assays (see (Divakaruni et al., 2014) for a review of methodology). In our analysis, we seeded 0.8 × 105 cells/well in duplicate per individual into 6-well Seahorse XFp culture plates and allowed the cells to adhere overnight. Cells were then subject to temperature exposure (32 °C, 37 °C, or 41 °C) in these culture plates. Immediately prior to the start of the temperature exposure, media was refreshed with growth media (80 μL). After temperature experiments, the standard medium was aspirated and replaced with Seahorse XF Assay Medium containing 8 mmol/L glucose, 1 mmol/L sodium pyruvate and 2 mmol/L glutamine, pH = 7.4. The plate was then incubated at 37 °C without CO2 for 60 min. OCR was measured immediately following this incubation at baseline, then after inhibition of mitochondrial respiration via injection of rotenone plus antimycin A (1 μM), to quantify non-mitochondrial respiration background. Following OCR measurements, protein concentration was determined in each well (BCA assay, Pierce) for normalization to protein content (μg/μL). Basal OCR, normalized to cellular protein content, was calculated as baseline OCR minus non-mitochondrial respiration (after addition of electron transport chain inhibitors rotenone and antimycin A), using Report Generator software (Agilent).
2.3.4. Translation rate determination by metabolic labeling and CLICK chemistry
Cells were seeded (1.5 × 106 cells per 10 cm dish) 24 h prior to metabolic labeling of proteins at a range of temperatures (37, 32, 27, 22, 17, 12, and 6 °C) relevant to hibernating mammals (Cranford, 1983). This method is based on detection of newly translated proteins using CLICK chemistry (Presolski et al., 2011) and their visualization via fluorescence following PAGE (Calve et al., 2016). The base labeling medium consisted of DMEM without methionine or cysteine (D0422, Sigma) with 10% dialyzed fetal bovine serum (F0392, Sigma), 4 mM glutamine, and 0.2 mM cysteine. The base medium was completed by adding 1 mM of the methionine analog L-homopropargylglycine (HPG, 1067, Click Chemistry Tools), or 1 mM L-methionine for control samples. The cells were washed twice with 10 mL PBS to remove any residual growth medium and incubated for 150 min at the designated temperature with labeling media that were pre-equilibrated to temperature in refrigerated CO2 incubators. The CO2 percentage was reduced with temperature according to Henry’s Law to maintain constant pH in the growth medium. To harvest, the cells were rapidly washed twice with ice-cold PBS on ice and harvested by scraping into ice-cold RIPA buffer containing 1× protease (cOmplete, Roche) and phosphatase inhibitors (PhosSTOP, Roche). Lysis was completed by sonication, the protein concentration of each whole cell lysate was determined by BCA assay, and 50 μg of protein from each sample were used in a CLICK chemistry reaction (Click-&-Go Protein Reaction Buffer Kit, 1262, Click Chemistry Tools) to link a fluorophore (AF 647, 1301, Click Chemistry Tools) to the HPG-labeled proteins. The processed samples were run on TGX Stain-Free Precast Protein Gels (Bio-Rad). The gels were imaged at 647 nm on a Bio-Rad Gel Documentation instrument, then exposed to activate the Stain-Free gel stain and total protein was imaged. Densitometry of each lane was performed using Bio-Rad Image Lab software for each imaging channel, and the labeled protein content was normalized to total protein per lane. The linearity of this detection method was determined by mixing known percentages of labeled and unlabeled protein lysates prior to the CLICK reaction and SDS-PAGE visualization.
2.3.5. Western blotting
For all samples, protein concentrations were determined by BCA (Pierce). For blots of ribosomal protein S6 (rpS6) of meadow jumping mouse (hibernator) and lab mouse (non-hibernator) cell lines, 20 μg protein per sample was resolved by SDS-PAGE on 10% gels, transferred to PVDF membrane, and blocked using 5% nonfat dry milk in TBS-T. The membranes were probed for S6 Ribosomal Protein phosphorylated on Ser235/236 (1:2000, #4858, Cell Signaling Technologies), incubated with anti-rabbit HRP-conjugated 2° antibody, and visualized with ECL reagent on a BioRad Gel Documentation instrument. Membranes were then stripped, re-probed with antibody against total S6 Ribosomal Protein (1:1000, #2217, Cell Signaling Technologies), incubated with anti-rabbit HRP-conjugated 2° antibody, and visualized as above.
For samples obtained from 13-lined ground squirrel primary dermal fibroblasts, 5 μg protein per sample was prepared in loading buffer (Bolt, Invitrogen), resolved by SDS-PAGE on 4–12% gradient gels (Bolt Bis-Tris plus, Invitrogen), then transferred to a PVDF membrane. Total protein transferred to the membrane was detected with Revert total protein stain (Licor) and quantified at 700 nm on an Odyssey scanner. After destaining, the membranes were blocked for 1 h in Intercept blocking buffer (Licor) then probed with HSP70 1° antibody overnight at 4 °C (1:1000, #ADI-SPA-812, Enzo Life Sciences). Membranes were then rinsed and probed with anti-rabbit 680RD-conjugated 2° antibody (Licor) and visualized as above. HSP70 expression was normalized to total protein.
2.4. Experimental design
2.4.1. Hypoxia exposure
Hypoxia tolerance of diving mammals is one feature of interest in comparative physiology and biomedicine that we investigated via cell-level responses to low oxygen. Primary dermal fibroblasts from n = 5 human, n = 5 rat, n = 4 humpback whale, and n = 3 Weddell seal (all individual biological replicates) were exposed to experimental hypoxia (0.5%O2) and species differences in cellular lactate production were evaluated as a metric of reliance on anaerobic metabolism. Fibroblasts were proliferated in species-specific growth media in a humidified incubator (37 °C, 21% O2, 5% CO2). Cells were plated in a 12-well plate at a concentration of 0.5 × 105 cells/well and allowed to adhere. 24–48 h prior to hypoxia exposure, media was exchanged to experimental media (DMEM containing 1 g/L glucose, Gibco #11965–092, 10% FBS, 1% l-glutamine, 1% penicillin/streptomycin). Hypoxic exposure was initiated by placing cells in an InVivo 400 hypoxia workstation under the following ambient conditions: 37°, 0.5% O2, 5% CO2, with 50% humidity. Once under a hypoxic atmosphere, cells were provided deoxygenated experimental media. After a 6 h incubation, lactate was measured inside the hypoxia workstation from the cell culture media. Plates were removed after lactate measurement and cell density was measured via crystal violet staining. Lactate concentration of supernatant (mmol/L) was normalized to cell density based on a crystal violet assay.
2.4.2. Temperature exposures
Many mammals experience daily or seasonal heterothermy, exposing cells to varied temperatures. The impact of temperature on cell stress responses and viability is another topic of interest in comparative physiology and biomedicine, which we considered by comparing proliferation rates and metrics of cell metabolism in species which naturally experience heterothermy, with those that do not. We present data for three comparisons.
Antarctic Weddell seals experience regional heterothermy, with skin temperatures routinely falling to near freezing temperatures (Hindle et al., 2015). Proliferation rates of dermal fibroblasts were evaluated under cold exposure in Weddell seals, humans, and rats, as a potential metric of species differences in cellular health and function. Primary fibroblasts from all three species were seeded into 12 well plates and incubated at either 37, 25, or 4 °C for 18 h (6 technical replicates of 1 biological individual per treatment, except Weddell seal with 3 technical replicates for the 37 °C condition). Cellular DNA was quantified following each exposure with crystal violet stain. For each species and temperature, cell proliferation rate over 18 h was calculated using the percent change in crystal violet assay, all normalized to human fibroblast proliferation at 37 °C.
As an indicator of cell metabolic responses to heat stress, we compared fibroblasts from a daily heterotherm (dromedary camel) to a homeotherm (Southern white rhinoceros). Fibroblasts from n = 5 individual camels and n = 5 individual rhinoceros were plated in 6-well Seahorse XFp culture plates and exposed to 32 °C, 37 °C, or 41 °C for 24 h in the same humidified incubator as during standard culture and proliferation (21% O2, 5% CO2). Basal OCR was calculated and compared in each species across the three temperature treatments.
To gain insight into the maintenance of homeostatic processes during heterothermy, we evaluated two metrics of protein synthesis at low temperatures in a non-hibernator (mouse) and a hibernator (meadow jumping mouse). Immortalized fibroblasts were used for this experiment. Translation rates in a mouse cell line (NIH-3 T3) were compared to immortalized dermal fibroblasts from the jumping mouse following exposure to a range of temperatures (37, 32, 27, 22, 17, 12, and 6 °C) relevant to hibernating mammals (n = 3 for each temperature). Cells were seeded (1.5 × 106 cells per 10 cm dish) 24 h prior to metabolic labeling of proteins, and translation was assayed via CLICK chemistry as described above. Following label incorporation during cold exposure, cells were washed twice with ice cold DPBS, then harvested for Western immunoblot analysis by scraping into ice cold RIPA buffer containing 1× protease (cOmplete, Roche) and phosphatase inhibitors (PhosSTOP, Roche). As a second metric of translational depression in the cold, mouse and jumping mouse fibroblast cell lines were probed for phosphorylated and total ribosomal protein S6 as described above. Phosphorylation of rpS6 correlates with translation of ribosomal protein subunits and has been shown to be torpor responsive in other hibernators (McMullen and Hallenbeck, 2010). Cells from the hibernator and non-hibernator were cold-exposed (at 6 °C) for 1, 2, 4, 8, and 24 h, and compared to a pre-exposure control, as well as a post-exposure control obtained after one hour of recovery at 37 °C.
2.4.3. Effect of passage number
Passage number may affect the physiology of primary cells, and is an important metric to note and validate in experiments with new cell types or species. Pre-testing of key experimental endpoints over relevant passage numbers is important to assure reproducible results. To demonstrate this validation, we first compared the inducible heat shock response in primary dermal fibroblasts of 13-lined ground squirrels (n = 4), harvested after heat exposure (24 h at 41 °C) at early passage (P 4) and mid-passage (P 10). Inducible heat shock proteins underlie an organism’s response to heat stress (Hassan et al., 2019; Riabowol et al., 1988), and the differential production of heat shock proteins in isolated fibroblasts has been previously linked to organismal tolerance to hot environments (Shandilya et al., 2020). Induction of HSP70 in response to high temperature (41 °C) was used as a metric of cell physiology that could be tracked to compare different culture conditions. Cells were seeded into 6 well plates with species-specific growth medium and incubated at 37 °C until they reached 80% confluency. Immediately prior to the start of the temperature exposure, media was refreshed with growth media. Replicates of plated cells were harvested at 37 °C as a pre-exposure control. At 24 h in 41 °C, cells were washed twice in DPBS, then harvested for Western immunoblot analysis in ice cold RIPA buffer with EDTA (BP-115D, Boston Bioproducts) containing 1× protease and phosphatase inhibitors (HALT, ThermoFisher). Samples were then probed for expression of inducible heat shock protein HSP70 as described above. As a second example, we compared the basal OCR of primary dermal fibroblasts of the rhinoceros (n = 3–5) at low (P 4–6), mid (P 9–12) and late-passage stages (P 15–19) using the same approach described above. Mitochondrial function is considered a highly sensitive marker of cellular stress, and altered metabolic capacity has been shown between proliferating and differentiated cells (reviewed in (Divakaruni et al., 2014)) and at high passage numbers in model systems (e.g., (Fiechtner et al., 2022)).
2.5. Statistical analysis
Values are presented as mean ± standard deviation (SD). Shapiro–Wilk tests were performed initially for each variable to confirm normality. For the hypoxia exposure experiment, relative lactate production was calculated over 18 h for four species (Weddell seal, humpback whale, human, rat) and compared with 1-way ANOVA and Tukey pairwise posthoc tests. Cell proliferation at cold temperatures (37, 25 and 4 °C conditions in three species, Weddell seal, human, rat) was evaluated by 2-way ANOVA (species * temperature) with Tukey posthoc tests. Differences in mitochondrial respiration rates (OCR) were evaluated independently in camel and rhinoceros across temperature exposures using one-way ANOVA with repeated measures. Species differences in translation rates (between Zapus and the mouse 3 T3 cell line) across cooling temperatures were determined for each temperature point with a two-sided t-test. Effect of passage number on induction of HSP70 after temperature treatment in 13-lined ground squirrel fibroblasts was evaluated with a paired, two-sided t-test to compare the responses of fibroblasts from the same 3 individuals at each passage. Differences between rhinoceros fibroblast OCR across three stages of passage (early, mid, late) were tested with a mixed effect model to account for partially repeated measures. Analyses were performed using GraphPad Prism and R.
3. Results & discussion
3.1. Dermal fibroblasts can be indicators of biochemical specializations
Although dermal fibroblasts may not be directly associated with physiological specializations in many biomedical models or species of interest for comparative physiology, all cells contain the genomic information that reflects an evolutionary endpoint for the organism and has undergone natural selection. This information may illuminate whole-organism responses that are shared across all cell types, such as metabolism (Jimenez et al., 2014b), mechanisms that are activated to sustain homeostasis, or to allow tolerance or resilience during stressful conditions. For example, primary dermal fibroblasts from different species demonstrate innate resistance to oxidative stress in culture conditions in a manner proportional to longer organismal lifespans (Jimenez, 2018; Kapahi et al., 1999), demonstrating that the manifestation of organismal phenotypes might be built upon cell-level response mechanisms. In biomedicine, while fibroblasts are an established model system for studying skin cancer (e.g. (Berning et al., 2015)) and are a key component of lab-developed skin substitutes (e.g. (Kisiel and Klar, 2019)) they can also be induced into other cell types for study, including neurons (Erharter et al., 2019; Shrigley et al., 2018). Skin fibroblasts may also bear biochemical signatures that reflect human disease states that manifest at the whole-body level. For example, elevation of phospholipase C activity in the vascular wall is associated with hypertension in rats (Uehara et al., 1988) and a correspondingly increased phospholipase C activity can be detected in skin fibroblasts isolated from human patients with essential hypertension (Kosugi et al., 2003). Similarly, patients with coronary spastic angina show positive correlations between phospholipase C activity in skin fibroblasts and basal coronary arterial tone as well as constrictor response (Okumura et al., 2000). Enhanced phospholipase C activity in these patients is related to a structural variant in the phospholipase C-δ1 isoform affecting intracellular calcium levels, and is a proposed mechanism explaining enhanced vasoconstrictor activity of coronary vessels in these patients (Nakano et al., 2002).
Here, we also provide several examples of ex vivo recapitulation of environmental stressors in dermal fibroblasts from comparative physiology systems, indicating that for some conditions, even dermal fibroblasts retain fundamental elements of organismal phenotype.
3.1.1. Hypoxia tolerance
Seals are underwater hunters that are specialized for long breath-holds and impressive hypoxia tolerance. One element of this hypoxia tolerance is the ability to maintain aerobic metabolism even at low levels of cellular oxygen (Kanatous et al., 2002; Trumble and Kanatous, 2012). While arterial (Qvist et al., 1986) and tissue (Guppy et al., 1986) oxygen levels decline during the first minutes of diving, lactate does not become detectable at high circulating levels until dives approach or exceed ~20 min (Kooyman et al., 1980; Williams et al., 2004). Over a 6-h hypoxia exposure, primary dermal fibroblasts from Weddell seals make significantly less lactate (F3,12 = 6.89, p = 0.006) than fibroblasts from humans (posthoc p = 0.006) or rats (posthoc p = 0.02) (Fig. 2A), indicating that, like the whole animal, Weddell seal fibroblasts may possess metabolic specializations that extend aerobic ATP generation even under conditions of low oxygen, and may be a valuable model for the study of hypoxia tolerance. Humpback whale fibroblasts generated an intermediate amount of lactate to the seal and the two terrestrial mammals (Fig. 2A). While the rates and timecourse of diving lactate production is not known for humpback whales, this cell culture finding is consistent with known life history traits of whales versus seals. Humpback whales are substantially larger animals, yet dive for shorter durations (4–8 min) and shallow depths (<200 m) compared to the much smaller Weddell seal (Ponganis, 2015), suggesting an intermediate level of hypoxia tolerance.
Fig. 2.
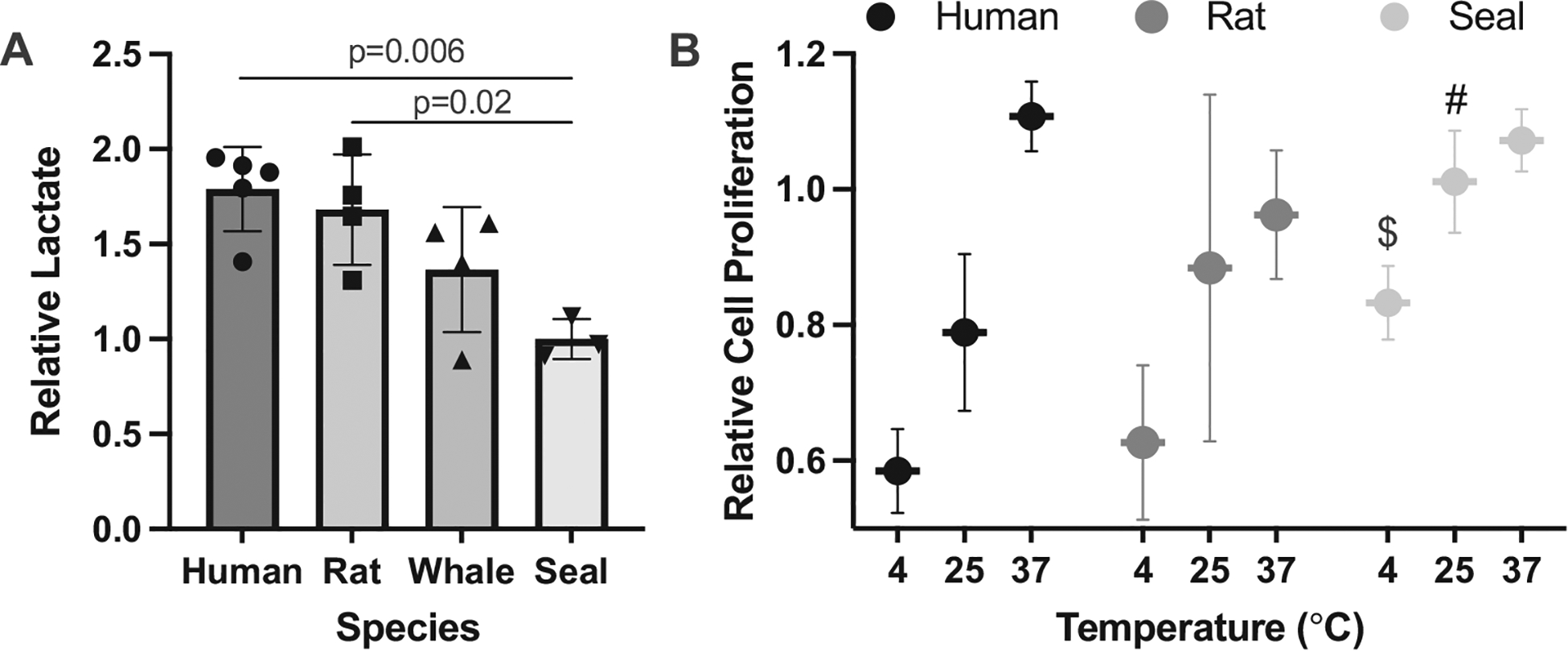
(A) Lactate production of primary fibroblasts from humans, rats, humpback whales, and Weddell seals exposed to hypoxia (0.5% O2) for 6 hours. Relative lactate production is percent increase over the experimental period, normalized to absorbance of crystal violet stain in the same wells as a metric for number of cells present. Lactate production differed between species (F3,12 = 6.89, p = 0.006) with significant pairwise posthoc differences between the Weddell seal and both the human and the rat. (B) Relative cell proliferation in human, rat and Weddell seal fibroblasts after 18-h incubation at 37 °, 25 ° or 4 °C differed between species (F2,42 = 7.6, p = 0.0015) and across temperatures (F2,42 = 38.5, p < 0.0001) with a significant interaction term between these factors (F4,42 = 2.71, p < 0.04). Relative proliferation was detected as percent change in crystal violet stain, and plotted as normalized to human fibroblast proliferation at 37°C). # denotes a significant posthoc difference between Weddell seal and humant at 25 °C (p = 0.006), $ denotes a significant posthoc differences between Weddell seal and both human (p = 0.002) and rat (p = 0.01) at 4°C.
3.1.2. Temperature tolerance
Skin cells from an Antarctic seal are routinely exposed to extremely cold temperatures in air and water, and the temperature of the skin itself can drop to near-freezing (Hindle et al., 2015). When compared to rat or human dermal fibroblasts, Weddell seal fibroblasts proliferate and better survive cold temperature exposure compared to fibroblasts from either humans or rats (species effect F2,42 = 73.6, p = 0.0015; Fig. 2B). While proliferation and survival declines with temperature in all species (temperature effect F2,42 = 38.5, p < 0.0001), fibroblasts from Weddell seals continue to proliferate at 25 °C (95% of cells compared to human proliferation at 37 °C) and retain a higher survival capacity at 4 °C compared to both rat (posthoc p = 0.01) and human (posthoc p = 0.002; Fig. 2B), consistent with specialization of the whole organism to a polar environment.
Mammals are core homeotherms, however many species are specialized to inhabit high temperature environments (Walsberg, 2000). Several species exhibit body temperature lability, allowing core temperature to climb by several degrees on a short-term basis. While camels can use daily hyperthermia (Grigg et al., 2009; Schmidt-Nielsen et al., 1956), rhinoceros keep their body temperature fairly constant by using behavioral and ventilation rate adjustments (Hiley, 1972). We therefore investigated if the difference in body temperature lability in camels versus rhinoceros is apparent in the metabolic activity of their dermal fibroblasts at different temperatures. While mitochondria respiration in camels is unaltered from baseline levels at either 32 or 41 °C (F2,12 = 1.59, P = 0.25, Fig. 3A), primary rhinoceros fibroblasts have temperature sensitive mitochondria, exhibiting metabolic rate depression outside of normothermic temperatures (F2,12 = 8.12, P < 0.006; Fig. 3B). Our results from dermal fibroblasts provide the first indication that these species show differences in temperature sensitivity at the cellular level that could reflect similarities with whole-organism responses. Non-optimal temperature can affect ATP synthesis by inactivating thermosensitive complexes in the respiratory chain which slows down electron transport and by enhancing ROS production which leads to oxidative damage in the mitochondrial membranes (Belhadj-Slimen et al., 2014). Mechanisms that could be involved in differential mitochondrial thermosensitiviness in camels versus rhinoceros include heat shock proteins associated with the mitochondrial fraction as well as antioxidant defenses (Belhadj-Slimen et al., 2014; Samali et al., 2001). Thermal sensitivity of metabolic rate may also be afforded via differential membrane composition between species (Hulbert and Else, 1999), as membrane fluidity (affected by composition of the lipid bilayer including the proportion of polyunsaturated fatty acids) is temperature-sensitive, and may act as a metabolic pacemaker.
Fig. 3.
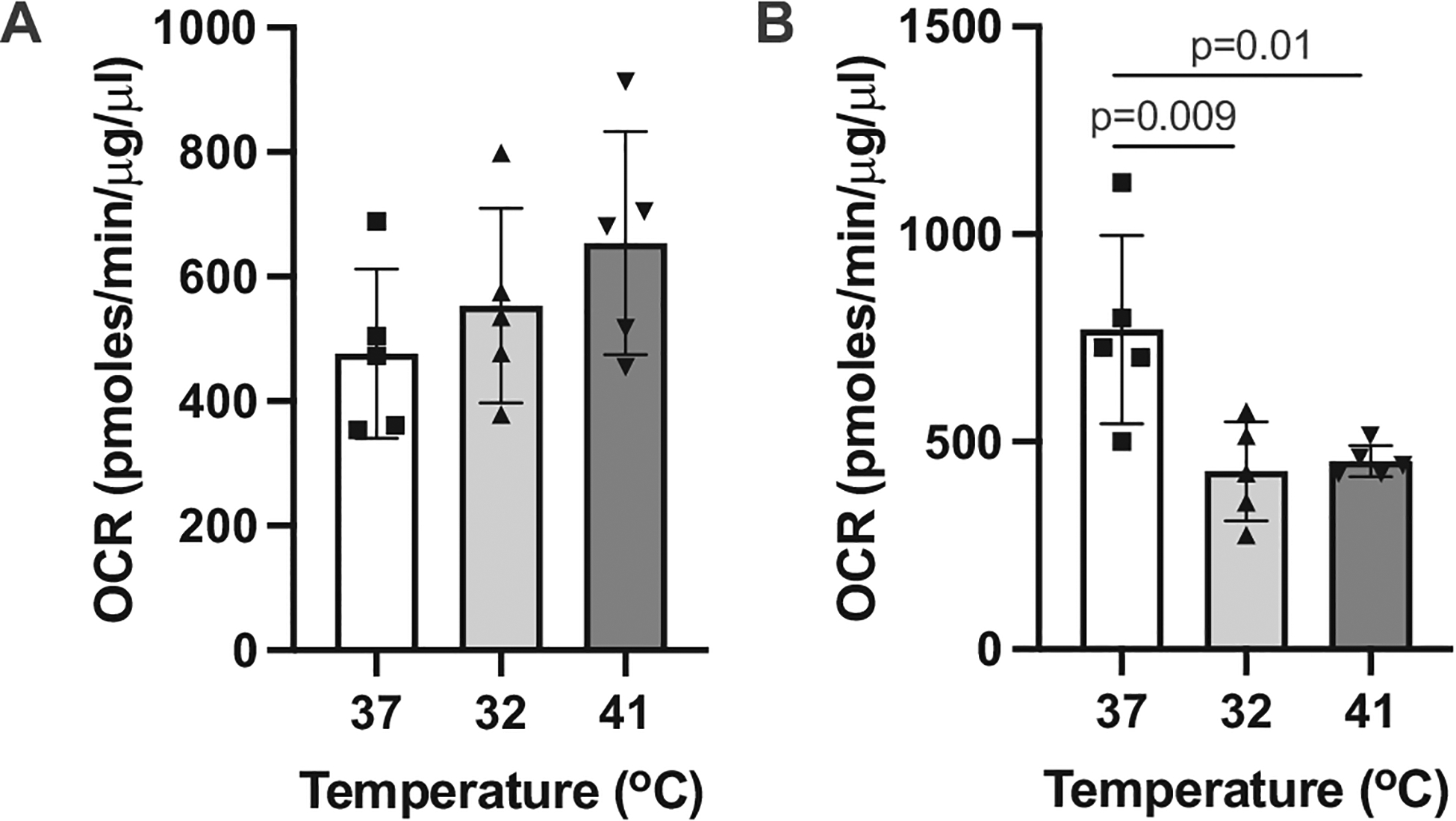
Oxygen consumption rate (OCR) of primary fibroblasts from (A) dromedary camel (n = 5) and (B) Southern white rhinoceros (n = 5) subject to a 24-hour exposure at 37°, 32 ° or 41 °C. Only rhinoceros fibroblast metabolism was affected by temperature exposure (one-way ANOVA F2, 12 = 8.12, p = 0.006).
Some mammals also exhibit body temperature lability during the hypothermic state of torpor. Deep hibernators tolerate near-freezing body temperatures that would be lethal to a comparable non-hibernator. The low body temperatures (at or below 4 °C) experienced by deep hibernators may require adaptation at the cellular and molecular level to allow for proper cell and tissue function at abnormally low temperatures. Previous research has shown that protein translation can be slowed and even stopped at low, hibernation-relevant temperatures (van Breukelen and Martin, 2001). We used immortalized dermal fibroblasts from a small rodent hibernator, the meadow jumping mouse, to investigate the temperature-dependence of protein translation, with an established laboratory mouse fibroblast cell line (NIH-3 T3) as a control. Our results from metabolic labeling experiments confirmed that global protein translation is slowed as temperature falls in both species (Fig. 4). Interestingly, fibroblasts from the hibernating species are better able to maintain protein translation at intermediate temperatures (significant species differences at 27 °C (p = 0.006), 17 °C (p = 0.004), and 12 °C (p = 0.01) were identified by t-test; Fig. 4B), suggesting that an ability to maintain protein translation during the critical transitions during torpor entry and arousal may be maintained in isolated fibroblasts.
Fig. 4.
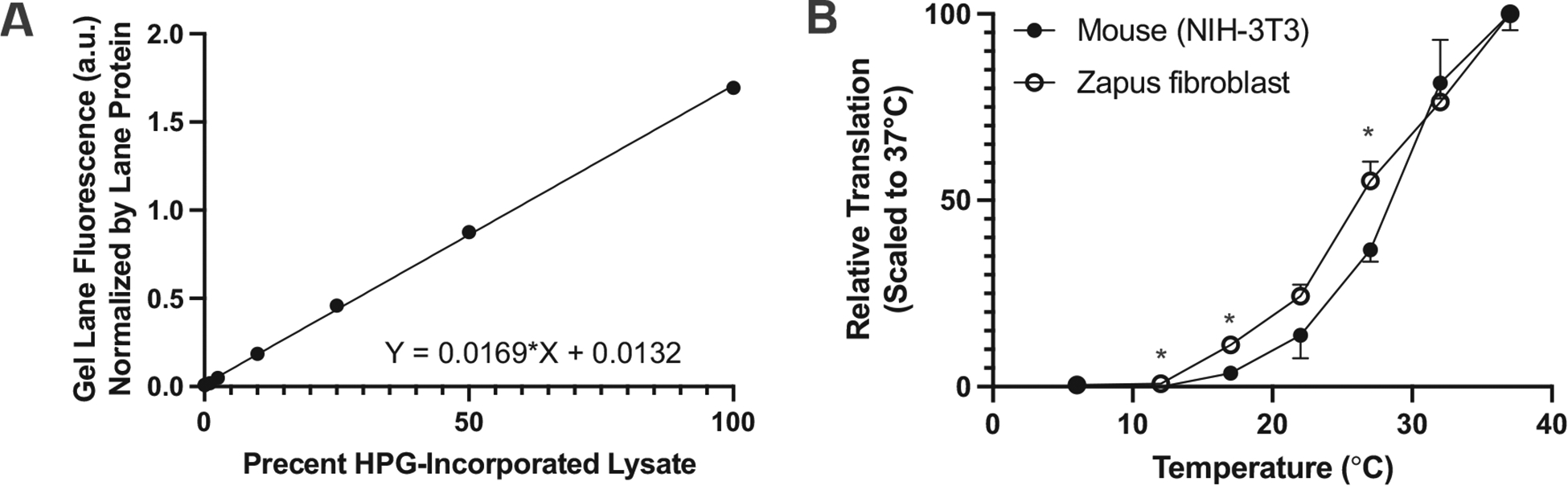
Metabolic labeling of proteins to determine temperature dependence of global protein translation in cell lines. (A) Linearity of method for quantifying incorporation of L-homopropargylglycine (HPG) into protein in live cells via CLICK chemistry and fluorescence quantification of labeled protein following PAGE. Simple linear regression r2 = 0.0996, p < 0.0001. (B) Temperature dependence of relative rates of global translation in meadow jumping mouse and lab mouse cells (n = 3 technical replicates per species cell line). Significant species differences in translation rates at each time point (two-sided t-test) are denoted *.
Ribosomal protein S6 (rpS6) is part of the 40S (small) ribosome subunit in higher eukaryotes, and this highly conserved protein is downstream of the mTOR signaling pathway and has been implicated in control of protein translation, cell size, and whole animal glucose homeostasis (Ruvinsky and Meyuhas, 2006). Phosphorylation of rpS6 correlates with the translation of ribosomal protein subunits, translation elongation factors, and proteins involved in cell cycle progression. It was noted that rpS6 becomes dephosphorylated during torpor in hibernating ground squirrels (McMullen and Hallenbeck, 2010), a state in which little to no protein translation takes place (Frerichs et al., 1998). We investigated the phosphorylation state of rpS6 in mouse and meadow jumping mouse fibroblasts held at 6 °C and found that cold exposure alone is sufficient to cause dephosphorylation of rpS6 in both species, but at different rates (Fig. 5). Meadow jumping mouse fibroblasts show a slower time course of dephosphorylation, suggesting greater cold tolerance at the cellular level in the hibernating species. This finding correlates with the greater ability of meadow jumping mouse fibroblasts to maintain protein translation at intermediate temperatures (Fig. 4). The use of fibroblasts in time course experiments highlights the convenience in working in a reductionist cell culture model, as it can be impractical or impossible to perform repeated sampling or induce significant, prolonged body temperature changes in live mammals.
Fig. 5.
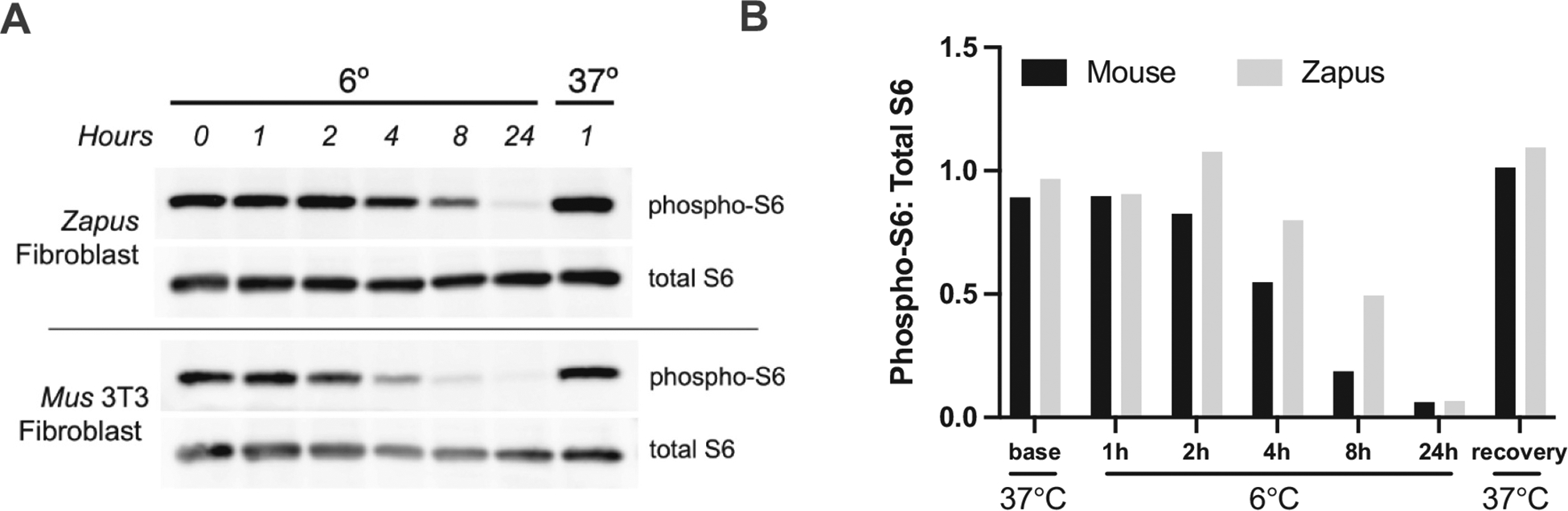
(A) Time course of cold-induced dephosphorylation of ribosomal protein S6 in meadow jumping mouse (Zapus, hibernator) and lab mouse (Mus, non-hibernator) cell lines. Recovery was for one hour at 37 °C after 24 hours cold exposure. Cells from the hibernator species exhibit slower loss of S6 phosphorylation, relative to total S6 protein pool (B), suggestive of adaptation to reduced body temperature during torpor.
3.1.3. Tissue or cell type specificity of responses
Different organs have different susceptibilities to environmental stress (e.g., (Ali et al., 1997)) and this is likely true of different cell types. Quantifying responses within a species from fibroblast response may not be appropriate for all experimental questions, however we suggest that this approach can reveal species-level differences in homeostasis mechanisms and protection against environmental extremes that are relevant for studying comparative physiology as well as human biomedicine.
3.2. Logistical and experimental considerations
3.2.1. The dermal fibroblast cell type
Fibroblasts derived from skin have a spindle shaped morphology and express cell surface markers including fibronectin, vimentin and CD90 (Harris, 1994) and these markers are distinct from fibroblasts isolated from other organs and tissues. Establishing primary fibroblast cultures from tissue samples results in a heterogenous population of cells. Fibroblasts also demonstrate a degree of spatial heterogeneity, even in skin (Driskell and Watt, 2015; Lynch and Watt, 2018; Philippeos et al., 2018), therefore the location of skin biopsied for the initial establishment of cultures can affect cellular physiology and should be noted. It is not generally a concern for skin fibroblast cultures that preparations could be contaminated by other cell types. Dermal tissue is composed mainly of fibroblasts, along with blood vessels and dendritic cells. The epidermal layer of skin is mainly composed of keratinocytes, melanocytes, and Langherans cells (Rittié and Fisher, 2005); all require specialized culture conditions with growth factors and are not likely to proliferate as contaminants alongside fibroblasts cultured in Eagle media types.
3.2.2. Limitations of single cell type monolayers to understand emergent traits
There are a number of considerations when simplifying in vivo physiology to an ex vivo experimental system. Cells grown in a monolayer represent a single proliferating population that has been removed from its in vivo signaling system. This means that the experimental system does not reflect any complexities or emergent physiological properties that may arise in tissues or organs that are composed of many cell types. Experimental approaches to address this at the cellular or tissue level are the development of 3D culture systems (e.g. (Berning et al., 2015)), or co-culture between dependent cell types (e.g. (Allah et al., 2017; Schutte et al., 2021)). Indeed, fibroblasts often form the basis for these more complex culture systems.
3.2.3. Culture conditions: oxygen
Oxygen levels in cell culture is often a consideration, as many incubators do not regulate atmospheric oxygen (Toussaint et al., 2011). Oxygen conditions also regulate and are regulated by cell physiology. Hypoxic conditions stimulate cellular proliferation rates, and cell surface oxygen tension varies according to cell density as well as physiological state, such as healing (Tokuda et al., 2000). Dermal fibroblasts are exposed to higher in vivo oxygen levels than many other cell types due to cutaneous oxygen uptake (Stücker et al., 2002). Nonetheless, culturing cells at atmospheric oxygen levels (21%) may dampen proliferation and hasten senescence (Saretzki et al., 1998; von Zglinicki et al., 1995), as well as induce hyperoxic stress (e.g. (Hafner et al., 2017) in cardiac myoblasts). Importantly, oxygen sensitivity of primary fibroblasts may be species-specific (Patrick et al., 2016).
3.2.4. Culture conditions: temperature
Temperature is a primary driver of cellular processes and whole-body metabolic rates. For every 10 °C decrease in temperature, most enzymatic reactions will decrease by 2 to 3-fold (Q10 effect). An important question in studying thermal physiology is whether biological rates of cultured cells are driven entirely by thermodynamics or whether they retain any part of the organism’s thermoregulatory or temperature-sensitive phenotype. Despite species differences in translation rates between mouse and jumping mouse fibroblasts across a temperature gradient, both species exhibit a consistent temperature-dependence for this homeostatic process across the assayed range (Fig. 4).
Endotherms defend core temperature, creating a relatively stable internal cellular environment. However, precision of body temperature regulation in individuals can range from a couple of degrees, mostly related to circadian rhythm and rest phase, to dramatic daily and/or seasonal fluctuations that can resemble vertebrate ectotherms (e.g. (Levesque et al., 2021)). Cells are typically cultured in thermally stable environments, eliminating naturally occurring temperature variation which may impact cell physiological performance. In vivo plasticity of performance curves can also buffer variation in reaction rates arising from Q10 impacts of altered body temperature (Seebacher and Little, 2017), a strategy which cannot be replicated in cell culture. Even during a strictly homeothermic period, environmental conditions such as hypoxia can lower body temperature set-point and shift the lower critical limit of the thermal neutral zone (Branco et al., 2006). The implication for cell culture experiments is that aspects of the cellular environment in vivo can co-vary, and knowledge of whole-animal physiology will be needed to guide experimental design.
3.2.5. Culture conditions: media
Optimal growth media can differ substantially by species. Glucose content of culture media is particularly important for proliferation, for example high glucose media increases toxicity from hyperoxia in some species (Buranasin et al., 2018). The researcher may choose to proliferate cells in species-specific growth media, but then to conduct experiments in different media (either experiment-specific media, or in order to conduct experiments for many species in the same media). An important consideration is to allow cells to stabilize physiologically in experimental media prior to assays. For example, changing the glucose concentration of media within 24 h of an experiment impairs mitochondrial oxygen consumption rates, a highly sensitive marker of cell physiology (Harper et al., 2011).
3.2.6. Influence of primary cell passage number on phenotype
The culture of primary dermal fibroblasts occurs in three phases (reviewed in (Rittié and Fisher, 2005)): 1) Establishment of cellular monolayer from skin biopsy explant; 2) proliferation and expansion; 3) slowing of cell division rates and replicative senescence. While dermal fibroblasts can offer proliferation potential past 20 passages, their initial culture quality, species-specific requirements for optimal growth media, and culture in too-low or too-high confluence conditions can reduce the viable life of primary cultures.
Senescent fibroblasts bear a number of molecular markers that differentiate them from expanding cells (e.g. (Cristofalo and Pignolo, 1996)). Key metrics of cellular physiology are also altered at high passage number. The heat shock response, specifically the expression of HSP70 mRNA and protein, as well as the binding activity of heat shock transcription factor 1 is reduced in senescent T lymphocytes (Effros et al., 1994) and in late stage proliferation/senescent fibroblasts (Gutsmann-Conrad et al., 1998).
However, within the presumed second phase of culture identified above (between P2 and P20 or beyond) cellular phenotypes in dermal fibroblasts are anticipated to be fairly stable and comparable. For instance, no differences in lipid composition were found in human fibroblasts of P18-P43 (Polgar et al., 1978). On the other hand, Calhoon et al. (2013) found that lipid composition can change or remain unaltered among passages (P0-P4) depending on the species. Heat shock response, measured as the induction of HSP70 protein, demonstrated a robust increase at 41 °C in fibroblasts from 13-lined ground squirrels assayed at P10 versus P4 (t = 1.12, df = 2, P = 0.38, Fig. 6A and B). Oxygen consumption rates, a highly sensitive metric of cell health and physiological status, was unchanged in rhinoceros dermal fibroblasts at early (P4-P6, n = 3), mid (P9-P12, n = 5) and late passage (P15-P19, n = 5) (F1,5 = 3.46, P = 0.12, Fig. 6C).
Fig. 6.
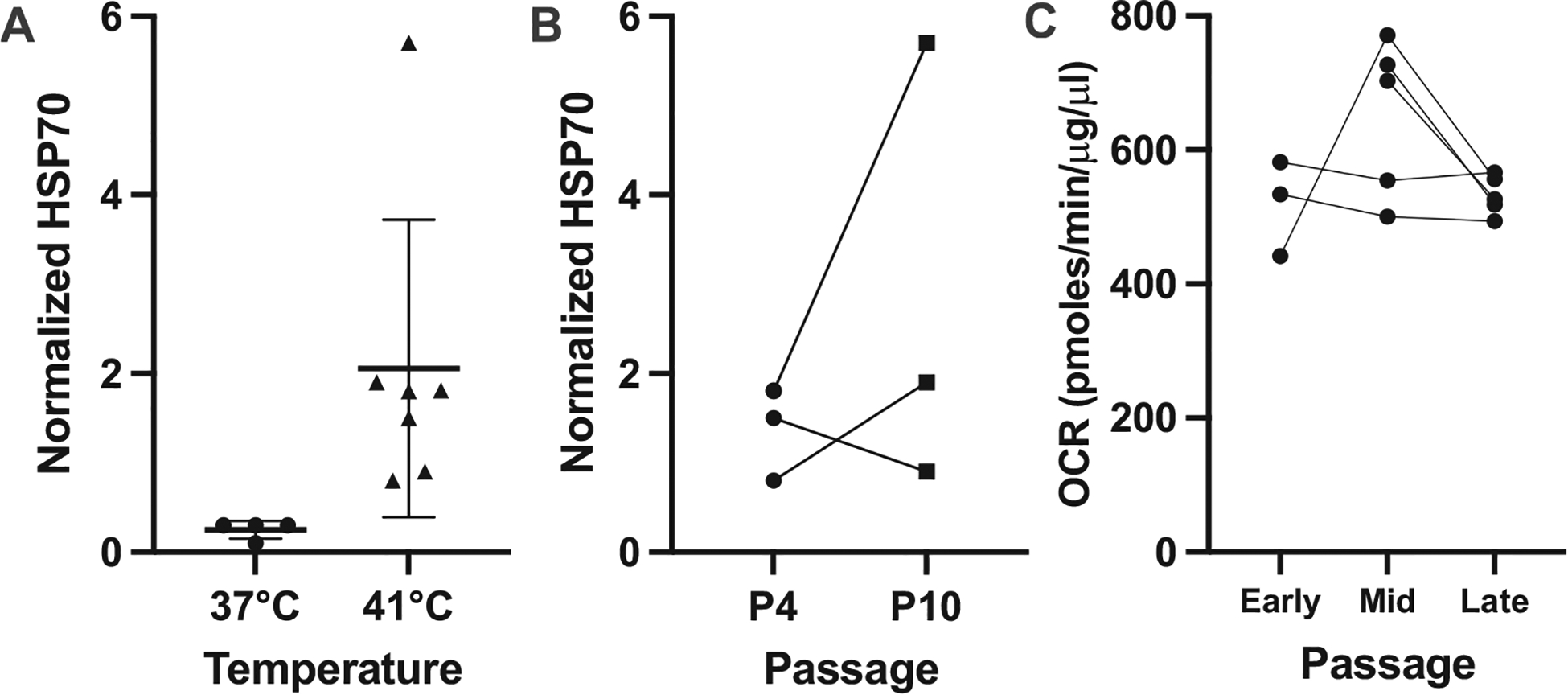
Consistent physiological responses are observed across passage number in mammalian primary fibroblasts. (A) Heat shock 70 induction in 13-lined ground squirrel primary fibroblast exposed to 24 hours at 41 °C compared to 37 °C baseline. (B) Paired comparison of HSP70 induction in early passage (P4) versus mid-passage (P10) 13-lined ground squirrel fibroblasts (n = 3 individuals) after 24 hour exposure to 41 °C (two-sided t = 1.118, df = 2, p = 0.38). (C) Oxygen consumption rate (OCR) of Southern white rhinoceros primary fibroblasts did not differ across passage number (n = 3 individuals for Early passage (P4,5,6), n = 5 paired samples in Mid (P9,10,12) and Late (P15,16,17,19) passages; Mixed effect model F1,5 = 3.46, P = 0.12).
3.2.7. Immortalization
In practice, primary cultures of dermal fibroblasts from endangered or non-model species are a limited resource due to the difficulty in obtaining starting material and the finite proliferative capacity of the cells themselves. Immortalization of precious primary cultures circumvents the eventual death of the culture and allows the researcher to expand cell number without limitation and to select clones or otherwise establish a stable, fast-growing cell culture for convenient experimental use.
Replicative senescence is the eventual loss of proliferative ability affecting fibroblast cultures (Cristofalo and Pignolo, 1993). The senescence, or the cessation of growth in culture, is controlled by tumor suppressor genes such as p53 and Rb that act to stop the cell cycle, and can result from the progressive shortening and then loss of telomeres with each cell division. The most common methods of immortalization include the use of viral vectors to genetically alter the cell to bypass the causes of replicative senescence. The Large T antigen of simian virus 40 (SV40) is a protein that inhibits the p53 and Rb family of tumor suppressors (Ahuja et al., 2005); when the SV40 Large T gene is introduced into the primary call via viral vector, its expression results in escape from replicative senescence and the immortalization of the culture of infected cells. Another common method of immortalization is the introduction of the human telomerase gene (hTERT); telomerase is not normally expressed in somatic cells, but its exogenous expression, in some cases in combination with p53 or Rb knockdown, results in immortalization of the culture (Colgin and Reddel, 1999; Yang et al., 2007). In some cases, continued culture (serial passage) of a primary cell line allows for spontaneous immortalization of the culture through the selection and expansion of those rare cells that have spontaneously acquired mutations affecting cell cycle control and thus by-passing replicative senescence (Amand et al., 2016; Xu, 2005).
The use of immortalized cells allows convenient experimentation with the caveat that the researcher has introduced changes into the cell line that may affect a phenotype of interest. The immortalization procedures discussed here directly or indirectly result in the inhibition of key tumor suppressor genes, which play an important role in cell cycle checkpoints and in various stress responses (Passos et al., 2010). In addition to playing a role in the heat shock response (Gong et al., 2019), the p53 tumor suppressor pathway is involved in the cold shock response, a stress response that occurs at the cellular level when the cells are exposed to hypothermic conditions and then re-warmed (Rieder and Cole, 2002). The cold shock response has been shown to be mediated via p21, a direct target of the p53 tumor suppressor gene (Matijasevic et al., 1998). Compromise of p53 during the immortalization process may thus affect cell biological functions of interest, and must be considered when choosing an appropriate cell model. For example, the SV40-immortalized fibroblasts used here to study protein translation rates in the cold (Fig. 4) would likely be unsuited for investigation of heat shock or cold shock responses due to the effect of immortalization on normal p53 function. Unmodified primary fibroblasts would be more appropriate for such studies, as were used in the current heat shock experiments (Fig. 6).
3.2.8. Choice of cell types and controls for multi-species comparisons
Once the scientific question has been identified, the researcher is faced with multiple considerations when choosing cell lines for use in comparative studies across multiple species. Cell lines should be as closely matched to the study objectives and each other as possible. Areas of consideration include the species of origin, the tissue of origin, whether the cultures are primary or immortalized, and if the method of immortalization could influence the phenotype of interest. In some cases, the availability of cell lines is an important factor, and the researcher may benefit in including a well characterized, established mouse or human cell line as an anchor point due to the large body of literature and established experimental protocols available for those cell types. For comparisons across many species or for the purpose of evaluating evolutionary adaptation within or across lineages, phylogenetically informed analyses should be considered (Garland Jr et al., 2005; Rezende and Diniz-Filho, 2011; Uyeda et al., 2021). In addition to careful choice of species, the number of biological replicates should be given ample consideration due to the significant heterogeneity inherent in a primary culture (Sorrell and Caplan, 2004). Such replicates should include individual organisms; if clonal populations are derived from a single individual, it may be important to investigate multiple clones to capture the spectrum of variability within the cells of a single individual.
3.3. Generating hypotheses about whole organism function using cells in environmental extremes
Despite limitations, cell culture approaches with skin fibroblasts provide new avenues for investigation in both comparative physiology models and biomedicine. Cell culture provides an ability for researchers to use tools that have not been previously possible, such as gene manipulation approaches, because they are expensive, logistically difficult, or impossible in whole animals. Working with cells also allows the direct assay of cell-level processes (e.g. transcription and translation rates) that may provide important windows into physiological and biochemical mechanism. For example, differences in the translation responses of mouse versus jumping mouse fibroblasts were assayed using CLICK chemistry, which is not a viable approach in vivo (Fig. 4). Cell culture enables high-resolution (and high-quantity) sampling strategies that are a further challenge in vivo. For example, the timecourse details of stress response markers can be assayed in cells to better understand the details of the response, or to inform the optimal time windows for subsequent in vivo sampling. Timecourse sampling over a cold-exposure and recovery in mouse and jumping mouse fibroblasts revealed differential responses in ribosome subunit phosphorylation that likely reflect species differences in translational activity (Fig. 5).
More broadly, cell culture approaches enable the study of exposure to environmental conditions beyond what is possible in whole animals. Fibroblasts from skin biopsies show several physiological responses that are relevant at the organismal level. Temperature responses in hibernators can be compared in cell culture against control non-hibernators, even though it would not be viable in vivo to cool both species to target assay temperatures. Importantly, lab-based approaches also allow researchers to escape the restrictions that accompany working with remote or logistically challenging species that may only be accessible in the field at certain times of year.
Acknowledgements
We gratefully acknowledge the collaboration of the San Diego Zoo for fibroblast stocks from rhino and camel, and Dr. Jose Pablo Vazquez Medina for dermal fibroblasts from humpback whales. We thank Annabelle J. Batten and Ethan A. Brem for technical support.
Funding
This work was supported by the National Science Foundation [grant number 2022046], the National Institutes of Health [grant number DP5OD021365], and the Sara and Frank McKnight Fund for Biomedical Research.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abade dos Santos FA, Carvalho C, Almeida I, Fagulha T, Rammos F, Barros SC, Henriques M, Luís T, Duarte MD, 2021. Simple method for establishing primary leporidae skin fibroblast cultures. Cells 10, 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas M, Moradi F, Hu W, Regudo KL, Osborne M, Pettipas J, Atallah DS, Hachem R, Ott-Peron N, Stuart JA, 2021. Vertebrate cell culture as an experimental approach–limitations and solutions. Comp. Biochem. Physiol. B: Biochem. Mol. Biol 254, 110570. [DOI] [PubMed] [Google Scholar]
- Ahuja D, Sáenz-Robles MT, Pipas JM, 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24, 7729–7745. [DOI] [PubMed] [Google Scholar]
- Ali A, Fernando P, Smith WL, Ovsenek N, Lepock JR, Heikkila JJ, 1997. Preferential activation of HSF-binding activity and hsp70 gene expression in Xenopus heart after mild hyperthermia. Cell Stress Chaperones 2, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allah NUM, Berahim Z, Ahmad A, Kannan TP, 2017. Biological interaction between human gingival fibroblasts and vascular endothelial cells for angiogenesis: a co-culture perspective. Tissue Eng. Regener. Med 14, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amand MMS, Hanover JA, Shiloach J, 2016. A comparison of strategies for immortalizing mouse embryonic fibroblasts. J. Biol. Methods 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadj-Slimen I, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M, 2014. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth 30, 513–523. [DOI] [PubMed] [Google Scholar]
- Berning M, Prätzel-Wunder S, Bickenbach JR, Boukamp P, 2015. Three-dimensional in vitro skin and skin cancer models based on human fibroblast-derived matrix. Tissue Eng. Part C: Method 21, 958–970. [DOI] [PubMed] [Google Scholar]
- Branco LG, Gargaglioni LH, Barros RC, 2006. Anapyrexia during hypoxia. J. Therm. Biol 31, 82–89. [Google Scholar]
- Buranasin P, Mizutani K, Iwasaki K, Pawaputanon Na Mahasarakham C, Kido D, Takeda K, Izumi Y, 2018. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One 13, e0201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon E, Miller M, Jimenez A, Harper J, Williams J, 2013. Changes in cultured dermal fibroblasts during early passages across five wild bird species. Can. J. Zool 91, 653–659. [Google Scholar]
- Calve S, Witten AJ, Ocken AR, Kinzer-Ursem TL, 2016. Incorporation of non-canonical amino acids into the developing murine proteome. Sci. Rep 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Sinclair DA, 2001. Recombination-mediated lengthening of terminal telomeric repeats requires the Sgs1 DNA helicase. Proc. Natl. Acad. Sci 98, 3174–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LM, Reddel RR, 1999. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev 9, 97–103. [DOI] [PubMed] [Google Scholar]
- Cranford JA, 1983. Body temperature, heart rate and oxygen consumption of normothermic and heterothermic western jumping mice (Zapus princeps). Comp. Biochem. Physiol. A Comp. Physiol 74, 595–599. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Pignolo RJ, 1993. Replicative senescence of human fibroblast-like cells in culture. Physiol. Rev 73, 617–638. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Pignolo RJ, 1996. Molecular markers of senescence in fibroblast-like cultures. Exp. Gerontol 31, 111–123. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M, 2014. Analysis and interpretation of microplate-based oxygen consumption and pH data. In: Methods in Enzymology. Elsevier, pp. 309–354. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Watt FM, 2015. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25, 92–99. [DOI] [PubMed] [Google Scholar]
- Eagle H, 1955. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–504. [DOI] [PubMed] [Google Scholar]
- Effros RB, Zhu X, Walford RL, 1994. Stress response of senescent T lymphocytes reduced hsp70 is independent of the proliferative block. J. Gerontol 49, B65–B70. [DOI] [PubMed] [Google Scholar]
- Erharter A, Rizzi S, Mertens J, Edenhofer F, 2019. Take the shortcut–direct conversion of somatic cells into induced neural stem cells and their biomedical applications. FEBS Lett 593, 3353–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanter C, Madelaire C, Genereux DP, van Breukelen F, Levesque D, Hindle A, 2022. Epigenomics as a paradigm to understand the nuances of phenotypes. J. Exp. Biol 225, jeb243411. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Leverkus M, 2016. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc 2016 pdb. prot087379. [DOI] [PubMed] [Google Scholar]
- Fiechtner MŠBPB, Sandbichler MEAM, Höckner M, 2022. Aging cell culture-genetic and metabolic effects of passage number on zebrafish Z3 cells. Cell. Physiol. Biochem 56, 50–65. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM, 1998. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc. Natl. Acad. Sci 95, 14511–14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney RI, 2015. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. John Wiley & Sons. [Google Scholar]
- Garland T Jr., Bennett AF, Rezende EL, 2005. Phylogenetic approaches in comparative physiology. J. Exp. Biol 208, 3015–3035. [DOI] [PubMed] [Google Scholar]
- Gong L, Zhang Q, Pan X, Chen S, Yang L, Liu B, Yang W, Yu L, Xiao Z-X, Feng X-H, 2019. p53 protects cells from death at the heatstroke threshold temperature. Cell Rep 29, 3693–3707 e3695. [DOI] [PubMed] [Google Scholar]
- Grigg G, Beard L, Dörges B, Heucke J, Coventry J, Coppock A, Blomberg S, 2009. Strategic (adaptive) hypothermia in bull dromedary camels during rut; could it increase reproductive success? Biol. Lett 5, 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guppy M, Hill R, Schneider R, Qvist J, Liggins G, Zapol W, Hochachka P, 1986. Microcomputer-assisted metabolic studies of voluntary diving of Weddell seals. Am. J. Phys. Regul. Integr. Comp. Phys 250, R175–R187. [DOI] [PubMed] [Google Scholar]
- Gutsmann-Conrad A, Heydari AR, You S, Richardson A, 1998. The expression of heat shock protein 70 decreases with cellular senescencein vitroand in cells derived from young and old human subjects. Exp. Cell Res 241, 404–413. [DOI] [PubMed] [Google Scholar]
- Hafner C, Wu J, Tiboldi A, Hess M, Mitulovic G, Kaun C, Krychtiuk KA, Wojta J, Ullrich R, Tretter EV, 2017. Hyperoxia induces inflammation and cytotoxicity in human adult cardiac myocytes. In: Shock: Injury, Inflammation, and Sepsis: Laboratory and Clinical Approaches, 47, pp. 436–444. [DOI] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA, 2011. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J. Exp. Biol 214, 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, 1994. Fibroblasts and their transformations: the connective-tissue cell family. Mol. Biol. Cell 1179, 1193. [Google Scholar]
- Hassan F-U, Nawaz A, Rehman MS, Ali MA, Dilshad SM, Yang C, 2019. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr 5, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley PG, 1972. A Comparison of Thermoregulation in a Number of East African Animals.
- Hindle AG, Horning M, Mellish J-AE, 2015. Estimating total body heat dissipation in air and water from skin surface heat flux telemetry in Weddell seals. Anim. Biotel 3, 50. [Google Scholar]
- Hulbert A, Else PL, 1999. Membranes as possible pacemakers of metabolism. J. Theor. Biol 199, 257–274. [DOI] [PubMed] [Google Scholar]
- Jimenez AG, 2018. “The same thing that makes you live can kill you in the end”: exploring the effects of growth rates and longevity on cellular metabolic rates and oxidative stress in mammals and birds. Integr. Comp. Biol 58, 544–558. [DOI] [PubMed] [Google Scholar]
- Jimenez AG, Cooper-Mullin C, Anthony NB, Williams JB, 2014a. Cellular metabolic rates in cultured primary dermal fibroblasts and myoblast cells from fast-growing and control Coturnix quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol 171, 23–30. [DOI] [PubMed] [Google Scholar]
- Jimenez AG, Van Brocklyn J, Wortman M, Williams JB, 2014b. Cellular metabolic rate is influenced by life-history traits in tropical and temperate birds. PLoS One 9, e87349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GE, Wise CJ, 1997. Establishment, maintenance, and cloning of human dermal fibroblasts. In: Pollard JW, Walker JM (Eds.), Basic Cell Culture Protocols. Humana Press, Totowa, NJ, pp. 13–21. [DOI] [PubMed] [Google Scholar]
- Kanatous S, Davis R, Watson R, Polasek L, Williams T, Mathieu-Costello O, 2002. Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J. Exp. Biol 205, 3601–3608. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB, 1999. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med 26, 495–500. [DOI] [PubMed] [Google Scholar]
- Kisiel MA, Klar AS, 2019. Isolation and culture of human dermal fibroblasts. In: Skin Tissue Engineering. Springer, pp. 71–78. [DOI] [PubMed] [Google Scholar]
- Kooyman G, Wahrenbrock E, Castellini M, Davis R, Sinnett E, 1980. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: evidence of preferred pathways from blood chemsitry and behavior. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol 138, 335–346. [Google Scholar]
- Kosugi T, Osanai T, Kamada T, Nakano T, Okumura K, 2003. Phospholipase C activity is enhanced in skin fibroblasts obtained from patients with essential hypertension. J. Hypertens 21, 583–590. [DOI] [PubMed] [Google Scholar]
- Levesque D, Nowack J, Boyles J, 2021. Body temperature frequency distributions: a tool for assessing thermal performance in endotherms? Front. Physiol 1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D, 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872. [DOI] [PubMed] [Google Scholar]
- Lynch MD, Watt FM, 2018. Fibroblast heterogeneity: implications for human disease. J. Clin. Invest 128, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoulas PL, El-Hattab AW, 2012. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orph. J. Rare Dis 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matijasevic Z, Snyder JE, Ludlum DB, 1998. Hypothermia causes a reversible, p53-mediated cell cycle arrest in cultured fibroblasts. Oncol. Res. Featur.Preclin. Clin. Cancer Ther 10, 605–610. [PubMed] [Google Scholar]
- McMullen DC, Hallenbeck JM, 2010. Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J. Comp. Physiol. B 180, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellish J-AE, Tuomi PA, Hindle AG, Horning M, 2010. Chemical immobilization of Weddell seals (Leptonychotes weddellii) by ketamine/midazolam combination. Vet. Anaesth. Analg 37, 123–131. [DOI] [PubMed] [Google Scholar]
- Mesdom P, Colle R, Lebigot E, Trabado S, Deflesselle E, Fève B, Becquemont L, Corruble E, Verstuyft C, 2020. Human dermal fibroblast: a promising cellular model to study biological mechanisms of major depression and antidepressant drug response. Curr. Neuropharmacol 18, 301–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre-Citrinovitz AC, Sestelo AJ, Ceballos MB, Barañao JL, Saragüeta P, 2016. Isolation of primary fibroblast culture from wildlife: the Panthera onca case to preserve a South American endangered species. Curr. Protocol Mol. Biol 116, 28.27. 21–28.27. 14. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller RA, 2003. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J 17, 1565–1576. [DOI] [PubMed] [Google Scholar]
- Nakano T, Osanai T, Tomita H, Sekimata M, Homma Y, Okumura K, 2002. Enhanced activity of variant phospholipase C-δ1 protein (R257H) detected in patients with coronary artery spasm. Circulation 105, 2024–2029. [DOI] [PubMed] [Google Scholar]
- Okumura K, Osanai T, Kosugi T, Hanada H, Ishizaka H, Fukushi T, Kamada T, Miura T, Hatayama T, Nakano T, 2000. Enhanced phospholipase C activity in the cultured skin fibroblast obtained from patients with coronary spastic angina: possible role for enhanced vasoconstrictor response. J. Am. Coll. Cardiol 36, 1847–1852. [DOI] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick A, Seluanov M, Hwang C, Tam J, Khan T, Morgenstern A, Wiener L, Vazquez JM, Zafar H, Wen R, 2016. Sensitivity of primary fibroblasts in culture to atmospheric oxygen does not correlate with species lifespan. Aging (Albany NY) 8, 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, 2018. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Investig. Dermatol 138, 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Lehr M, Kohler WJ, Han ML, Miller RA, 2015. Fibroblasts from longer-lived species of primates, rodents, bats, carnivores, and birds resist protein damage. J. Gerontol. Ser. A Biomed. Sci. Med. Sci 70, 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar P, Taylor L, Brown L, 1978. Plasma membrane associated metabolic parameters and the aging of human diploid fibroblasts. Mech. Ageing Dev 7, 151–160. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, 2015. Diving Physiology of Marine Mammals and Seabirds. Cambridge University Press. [Google Scholar]
- Presolski SI, Hong VP, Finn M, 2011. Copper-catalyzed azide–alkyne click chemistry for bioconjugation. Curr. Protocol Chem. Biol 3, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM, 1986. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J. Appl. Physiol 61, 1560–1569. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Diniz-Filho JAF, 2011. Phylogenetic analyses: comparing species to infer adaptations and physiological mechanisms. Compr. Physiol 2, 639–674. [DOI] [PubMed] [Google Scholar]
- Riabowol KT, Mizzen LA, Welch WJ, 1988. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science 242, 433–436. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, 2002. Cold-shock and the mammalian cell cycle. Cell Cycle 1, 168–174. [PubMed] [Google Scholar]
- Rittíe L, Fisher GJ, 2005. Isolation and culture of skin fibroblasts. In: Fibrosis Research. Springer, pp. 83–98. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O, 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci 31, 342–348. [DOI] [PubMed] [Google Scholar]
- Ryder OA, Onuma M, 2018. Viable cell culture banking for biodiversity characterization and conservation. Ann. Rev. Anim. Biosci 6, 83–98. [DOI] [PubMed] [Google Scholar]
- Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo A-P, Orrenius S, 2001. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford KK, Earle WR, Likely GD, 1948. The growth in vitro of single isolated tissue cells. J. Natl. Cancer Inst 9, 229–246. [PubMed] [Google Scholar]
- Saretzki G, Feng J, Zglinicki TV, Villeponteau B, 1998. Similar gene expression pattern in senescent and hyperoxic-treated fibroblasts. J. Gerontol. Ser. A Biol. Med. Sci 53, B438–B442. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR, 1956. Body temperature of the camel and its relation to water economy. Am. J. Physiol. Legacy Content 188, 103–112. [DOI] [PubMed] [Google Scholar]
- Schutte SC, Kadakia F, Davidson S, 2021. Skin-nerve co-culture systems for disease modeling and drug discovery. Tissue Eng. Part C: Method 27, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Little AG, 2017. Plasticity of performance curves can buffer reaction rates from body temperature variation in active endotherms. Front. Physiol 8, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya UK, Sharma A, Sodhi M, Mukesh M, 2020. Heat stress modulates differential response in skin fibroblast cells of native cattle (Bos indicus) and riverine buffaloes (Bubalus bubalis). Biosci. Rep 40. BSR20191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrigley S, Pircs K, Barker RA, Parmar M, Drouin-Ouellet J, 2018. Simple generation of a high yield culture of induced neurons from human adult skin fibroblasts. J. Vis. Exp 132, e56904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siengdee P, Klinhom S, Thitaram C, Nganvongpanit K, 2018. Isolation and culture of primary adult skin fibroblasts from the Asian elephant (Elephas maximus). PeerJ 6, e4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI, 2004. Fibroblast heterogeneity: more than skin deep. J. Cell Sci 117, 667–675. [DOI] [PubMed] [Google Scholar]
- Stücker M, Struk A, Altmeyer P, Herde M, Baumgärtl H, Lübbers DW, 2002. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J. Physiol 538, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda Y, Crane S, Yamaguchi Y, Zhou L, Falanga V, 2000. The levels and kinetics of oxygen tension detectable at the surface of human dermal fibroblast cultures. J. Cell. Physiol 182, 414–420. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Weemaels G, Debacq-Chainiaux F, Scharffetter-Kochanek K, Wlaschek M, 2011. Artefactual effects of oxygen on cell culture models of cellular senescence and stem cell biology. J. Cell. Physiol 226, 315–321. [DOI] [PubMed] [Google Scholar]
- Trumble SJ, Kanatous SB, 2012. Fatty acid use in diving mammals: more than merely fuel. Front. Physiol 3, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Ishii M, Ishimitsu T, Sugimoto T, 1988. Enhanced phospholipase C activity in the vascular wall of spontaneously hypertensive rats. Hypertension 11, 28–33. [DOI] [PubMed] [Google Scholar]
- Uyeda JC, Bone N, McHugh S, Rolland J, Pennell MW, 2021. How should functional relationships be evaluated using phylogenetic comparative methods? A case study using metabolic rate and body temperature. Evolution 75, 1097–1105. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL, 2001. Translational initiation is uncoupled from elongation at 18 C during mammalian hibernation. Am. J. Phys. Regul. Integr. Comp. Phys 281, R1374–R1379. [DOI] [PubMed] [Google Scholar]
- Vangipuram M, Ting D, Kim S, Diaz R, Schüle B, 2013. Skin punch biopsy explant culture for derivation of primary human fibroblasts. JoVE e3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas J, McPhaul M, 2005. Establishment and culture of human skin fibroblasts. Curr. Protocol Mol. Biol 71, 28.23. 21–28.23. 29. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Döcke W, Lotze C, 1995. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res 220, 186–193. [DOI] [PubMed] [Google Scholar]
- Walsberg GE, 2000. Small mammals in hot deserts: some generalizations revisited. Bioscience 50, 109–120. [Google Scholar]
- Wang T, Li Z, Wei J, Zheng D, Wang C, Xu C, Chen W, Wang B, 2021. Establishment and characterization of fibroblast cultures derived from a female common hippopotamus (Hippopotamus amphibius) skin biopsy. Cell Biol. Int 45 (7), 1571–1578. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Zychowski GV, Bauman SW, Higgins BM, Raudsepp T, Gollahon LS, Wooten KJ, Cole JM, Godard-Codding CI, 2014. Establishment, characterization, and toxicological application of loggerhead sea turtle (Caretta caretta) primary skin fibroblast cell cultures. Environ. Sci. Technol 48, 14728–14737. [DOI] [PubMed] [Google Scholar]
- Williams TM, Fuiman LA, Horning M, Davis RW, 2004. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol 207, 973–982. [DOI] [PubMed] [Google Scholar]
- Xu J, 2005. Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr. Protocol Mol. Biol 70, 28.21. 21–28.21. 28. [DOI] [PubMed] [Google Scholar]
- Yang G, Rosen DG, Mercado-Uribe I, Colacino JA, Mills GB, Bast RC Jr., Zhou C, Liu J, 2007. Knockdown of p53 combined with expression of the catalytic subunit of telomerase is sufficient to immortalize primary human ovarian surface epithelial cells. Carcinogenesis 28, 174–182. [DOI] [PubMed] [Google Scholar]


