Abstract
Background:
The objective of this study was to design and construct a CO2 incubator with nonmetallic walls and to investigate the viability of the cells and microwave irradiance inside this incubator.
Methods:
Because the walls of conventional incubators are made of metal, this causes scattering, reflection, and absorption of electromagnetic waves. We decided to build a nonmetallic wall incubator to examine cells under microwave radiation. Incubator walls were made using polyvinyl chloride and Plexiglas and then temperature, CO2 pressure, and humidity sensors were placed in it. Atmel® ATmega1284, a low-power CMOS 8-bit microcontroller, collects and analyzes the sensor information, and if the values are less or more than the specified limits, the command to cut off or connect the electric current to the heater or CO2 solenoid valve is sent. Using a fan inside the incubator chamber, temperature and CO2 are uniforms. The temperature of the points where the cell culture plates are placed was measured, and the temperature difference was compared. Ovarian cancer cells (A2780) were cultured in the hand-made and commercial incubators at different times, and cell viability was compared by the MTT method. Microwave radiation in the incubator was also investigated using a spectrum analyzer. The survival of cells after microwave irradiation in the incubator was measured and compared with control cells.
Results:
The data showed that there was no significant difference in temperature of different points in hand-made incubator and also there was no significant difference between the viability of cells cultured in the hand-made and commercial incubators. The survival of irradiated cells in the incubator was reduced compared to control cells, but this reduction was not significant.
Conclusion:
This incubator has the ability to maintain cells and study the effects of electromagnetic radiations on the desired cells, which becomes possible by using this device.
Keywords: Cell viability, CO2 incubator, microwave radiation, nonmetallic walls
Introduction
Radiofrequency fields are widely used in various fields such as medicine, industry, home microwave ovens, and wireless communications. The number of people who use wireless devices is increasing, and the increasing use of wireless technologies has raised concerns about the effects of electromagnetic fields.[1,2] Extensive research has been done on the effects of these waves on biological systems with conflicting results. Some researches have shown that these waves can have adverse effects on mammalian cells.[3,4,5] While others have not shown any significant effects.[6,7,8] Some studies have claimed that radiofrequency waves can interact with the body's bioelectrical signals. As a result, exposure to thousands of radiofrequency signals in daily life, which is uncontrolled and involuntary, will have health consequences.[9,10] Radiofrequency waves and electromagnetic fields can have detrimental effects on homeostasis and automatic regulation (synchronization) and biological rhythm, and alter the content of biological information exchanged between cells. Changing the content of information destroys natural environmental guidelines and interferes with the information required for natural biological regulation.[10] Some studies have also claimed that these fields can reduce cell growth and survival and induce apoptosis in cells.[11,12,13,14,15,16] In contrast, there are other studies that do not confirm the above and their results have shown that electromagnetic waves and fields have no effect on cell growth and survival.[6,7,8]
Accordingly, more researches are needed to determine the effects of these waves. The process of cell growth in the environment outside the body requires special conditions. These conditions are met by CO2 incubators. CO2 incubators are able to provide optimal conditions for cell growth. These incubators must provide the factors that affect cell growth and keep them constant. These factors include temperature of 37°C ± 0.5°C, 5% carbon dioxide, and 95% humidity.[17,18,19] An incubator must provide a uniform and constant temperature with the help of an automatic system. The air in the incubator must be circulated to distribute the temperature and carbon dioxide evenly. In order to provide the right pH in the culture medium, it is necessary to have a suitable buffering system. Most culture media contain sodium bicarbonate, which with carbon dioxide gas provides a suitable buffering system for cell culture. Therefore, in carbon dioxide incubators, it is necessary to maintain the amount of carbon dioxide inside the incubator to a certain extent. This amount of carbon dioxide is 5% for the growth of mammalian cells, which needs to be injected with an automatic system. The humidity of the incubator is usually provided by a special container in which deionized sterile water is poured. The walls of commercially available incubators are made of metal, which reduce the possibility of delivering irradiation to incubated cells. This issue is due to wave scattering and reflection from the metal wall and consequently, it dose not permit the desired radiation to reach the cells.[20,21] The necessity of evaluating the influence of electromagnetic fields on different cell lines led us to design and fabricate a new cell incubation system capable of transmitting electromagnetic fields to irradiate cells.
Materials and Methods
Incubator design
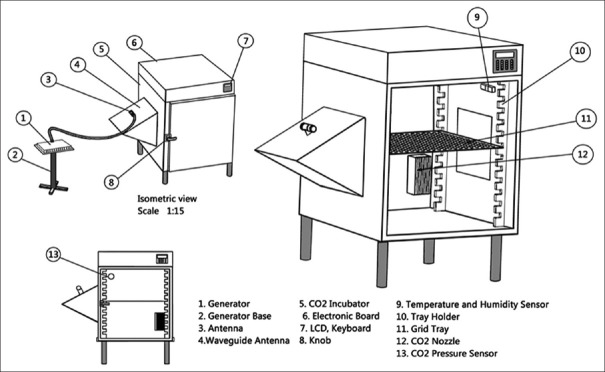
In the design of this incubator, polyvinyl chloride walls were used to eliminate scattering, absorption, and reflection of electromagnetic waves. The incubator consists of two main parts: one for cell storage and the other for electronic components and circuits. The cell storage compartment has dimensions of 50 cm × 50 cm × 50 cm, and humidity, temperature, and carbon dioxide sensors are placed inside the device chamber. A heater and a fan are also considered creating heat and air circulation inside the device. The DHT21-AM2301 digital temperature sensor module (made in ASAIR Company, China) with high-temperature measurement accuracy of 0.5°C in 0.1°C steps with a relative humidity accuracy of +/‒3% was applied.
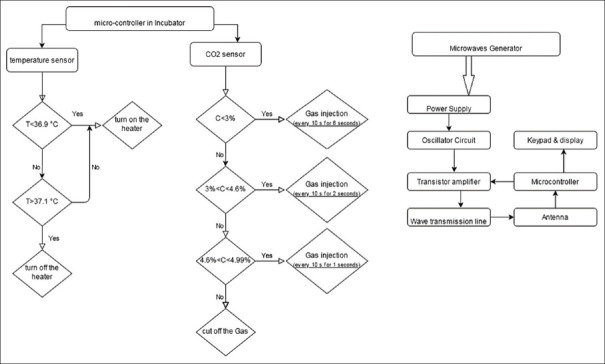
The temperature sensor constantly measures the temperature of the indoor and sends information to the Atmel® ATmega1284 as a low-power CMOS 8-bit microcontroller. The microcontroller has the ability to cut off and connect the electric current, and as soon as the temperature falls below the set limit (36.9°C), the heater turns on. If it exceeds the set limit (37.1°C), the electric current of the heater will cut off. To provide sufficient humidity in the cell culture medium, a water tank and a fan were applied. The fan was made by Ada Company with dimensions of 8 cm × 8 cm2 with a voltage of 12 volts DC and a current of 0.25A which has the speed of 100 RPM. The heat produced by the heater evenly spreads throughout the incubator space and causes water to evaporate.
To measure CO2 level, the ExplorIR®-W (formerly known as CozIR Wide Range) made in China was selected as a low-power, high-performance CO2 sensor that can measure up to 100% CO2 levels which is based on infrared LED technology and innovative optical designs. This is suitable for measuring high concentrations of CO2 in closed-loop sampling applications or battery operation in portable sampling instruments.
The microcontroller regulates the opening and closing of the solenoid valve for entering carbon dioxide. It is programmed to inject carbon dioxide at specified intervals as long as the carbon dioxide concentration falls below 5%. For concentrations <3% (which often occurs when the incubator door is opened), the CO2 is injected into the incubator every 10 s for 6 s. At concentration in the range of 3%–4.6% and 4.6%–4.99%, the injection will be occurred every 10 s for the duration of 2 and 1 s, respectively. In the concentration of 5% or more, the gas injection will be stopped.
A liquid-crystal display is placed on the incubator to show the temperature, humidity, and carbon dioxide content. On both side walls, a Plexiglass window is installed to transmit the waves and also to see the cells [Figure 1].
Figure 1.
Incubation and irradiation system of cells and its various parts
Irradiation of cells inside the incubator
The electromagnetic radiation system consists of an antenna with 14 dBi gain and a generator with the ability to adjust the continuous signal with a frequency of 100–4400MHz and a maximum output power of 33 dBm. The microwave is generated using oscillating circuits and then this frequency generated by a transistor circuit is amplified and then transmitted to the antenna section by the transmission line circuits. And then, the antenna radiates the desired frequency on the target [Figure 2].
Figure 2.
The block diagram of the microwave generator and the performance of the microcontroller in the incubator
In this study, the generator output was set to 2450MHz. The distance between the antenna and the location of the cells in the incubator was 30 cm to irradiate in the far field. The region where the electric and magnetic fields are perpendicular to each other is called far field. Practical power density measurements are possible in this area. The approximate distance of the radiation source to the far field is obtained from Eq. 1, where D is the largest antenna dimension and λ is the wavelength.[22]

Measurement of electromagnetic field power density at the location of cell culture plates
Power density was calculated using the Friis equation (Eq. 1), in which Pt is the output power of the wave generator and Gt is the antenna gain and R is the antenna distance to the location of the cells.[23,24]
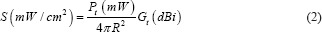
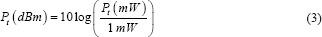
Aaronia Spectran HF-60105 spectrum analyzer (made in Germany) was used to evaluate the accuracy of the antenna output.
Temperature changes in different places inside the hand-made incubator
After starting and equilibrating the incubator temperature, temperature changes were investigated at five points at the location of the cell culture plates. For this purpose, a mercury thermometer was used which is placed inside a Falcon-containing culture medium. Temperature changes in these areas were recorded once every half hour. Temperatures were measured five times for each point and their statistical differences were investigated.
Cell viability assessment
Cell culture
The human ovarian cancer, A2780 cell line, was obtained from Pasteur Institute of Iran. These cells were cultured in RPMI-1640 medium containing 10% FBS and 1% antibiotic (penicillin-streptomycin) in a commercial incubator (Memmert type: INC 108, made in Germany) at 37°C containing 5% CO2 and 95% of the air humidity. The culture medium was changed every 48 h. After 3–4 days of initial culture, cell density was examined under a reverse microscope, and when cell density reached 70%–80%, the cells were transferred to new flasks.
Comparison of cell viability in hand-made and commercial incubators
After temperature assessment, the viability of ovarian cancer cells in hand-made and commercial incubators was measured by MTT assay. Cells were cultured at different concentrations in 96-well plates, and cell survival was measured after 12, 24, and 48 h. After the desired period, the cell culture medium was gently removed, and 2 μl of MTT solution and 90 μl of complete culture medium were added to each well. It was then incubated for 3–4 h at 37°C and away from light. The culture medium was then gently removed and 100 μl of dimethyl sulfoxide solvent was added to the formazan precipitate. After pipetting each well several times, the light absorption was read by ELISA reader (BioTek EL × 808) at 570 nm and cell survival was achieved in two incubators. Then, using statistical analysis, the growth rate in hand-made and commercial incubators was compared.
Irradiation of cells
After ensuring the correct operation of the incubator and irradiation system, the effect of 2450 MHz irradiation on ovarian cancer cells in the control and irradiation groups for 12, 24, and 48 h was compared by MTT method.
Statistical analysis of data
The results were determined based on the average of three separate tests and standard deviation. Comparisons between groups were analyzed by one-way analysis of variance and t-test. GraphPad Prism 8 software was used to evaluate the data and draw graphs. The probability of obtaining the significant difference between the groups statistically is less than 5% (P-value<0.05).
Results and Discussion
Temperature changes at different points inside the hand-made incubator
Temperature was measured at five points in the incubator, where the plates and flasks were placed for cell culture. Figure 3 shows the average temperature at these points. Statistical analysis of these points showed that temperature changes in these points are not significant (P = 0.0697). The range of temperature changes at these points was measured between 37.3 and 36.7°C, which is in the range of 37 ± 0.5°C.
Figure 3.
Comparing the temperature of the points where the cells are placed in the incubator, the temperature difference in these points is not significant
Comparison of performance of hand-made and commercial incubators
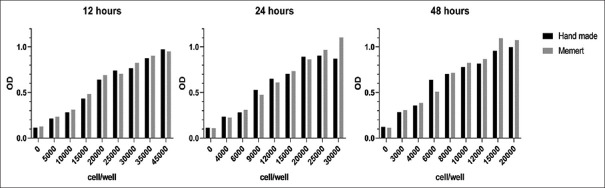
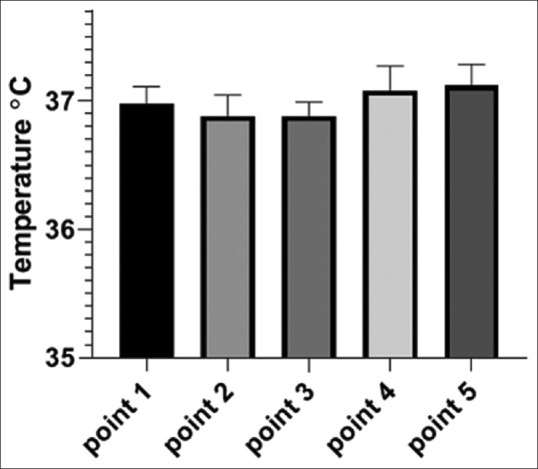
To compare the performance of hand-made incubator and commercial incubator, A2780 ovarian cancer cells were cultured and incubated for 12, 24, and 48 h in both incubators in 96-well plates with different cell concentrations. After the mentioned period, cell growth rate was compared by MTT method. Comparison was performed using t-test. With time constant, the incubator type was tested and there was no significant difference between hand-made and commercial incubators in 12, 24, and 48 h [Figure 4].
Figure 4.
Comparison of the viability of ovarian carcinoma cells in hand-made and commercial incubators that were incubated for 12, 24, and 48 h at different cell concentrations and optical density by MTT method
Power density of microwave
Power density at a distance of (R) equal to 30 cm and given output power (Pt) 33 dBm and the antenna gain (Gt) equal to 14 dBi was calculated using the Friis equation. Based on calculations, the power density was obtained 2.47 mW/cm2 and the practical measurement results with the spectrum analyzer showed the power density of 2.45 ± 0.1 mW/cm2.
Effect of 2450 MHz radiofrequency field on cell growth
A2780 cells were cultured on 96-well plates with cell concentrations of 20,000, 12,000, and 8,000 for 12, 24, and 48 h, respectively. After one night of incubation, the microwave group received 2450MHz microwave treatment for 12, 24, and 48 h and the control group was incubated without radiation for the same time. Cell growth rate in the two groups was compared by MTT method. As shown in Figure 5, after irradiation with nonionizing 2450 MHz electromagnetic waves, the viability of the cells decreased, but this decrease was not significant. The P value for 12, 24, and 48 h was 0.7, 0.657, and 0.3797, respectively.
Figure 5.
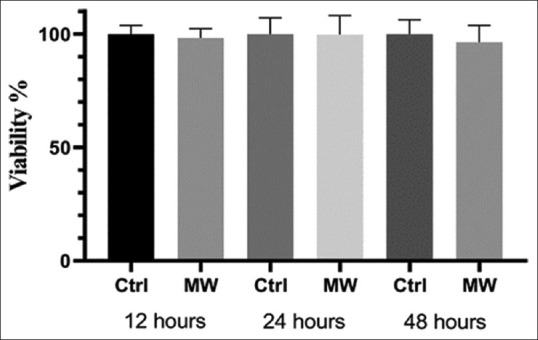
Effect of 2450 MHz microwaves on A2780 class ovarian carcinoma after 12, 24, and 48 h
Conclusion
Since the walls of the conventional incubator systems are made of metal, it is not possible to irradiate the cells from outside the incubator. If we want to apply radiation from inside the incubator, problems such as scattering and reflection of waves from the metal surfaces, the impossibility of irradiation in the area around the far field, and the possibility of contamination of the incubator are increased. Therefore, devices need to be designed and built to study these effects on biological systems more closely. The cell incubator and irradiation system developed in this study can effectively help to investigate the effects of the electromagnetic radiation. On the other hand, irradiation of cells in this incubator can be done for a long time and also the effects of low-dose ionizing electromagnetic radiation can be studied in a long time.
According to our results, this incubator has the ability to hold cell cultures and to irradiate at different times and can be used to study the effects of nonionizing electromagnetic waves.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study is taken from the PhD thesis therefore, the authors thank the office of Vice-Chancellor for Research of Jundishapur University of Medical Science, Ahvaz, Iran, for financial support with grant number (Grant Number U-95141).
References
- 1.Baohong W, Jiliang H, Lifen J, Deqiang L, Wei Z, Jianlin L, et al. Studying the synergistic damage effects induced by 1.8 GHz Radiofrequency Field Radiation (RFR) with four chemical mutagens on human lymphocyte DNA using comet assay in vitro. Mutat Res. 2005;578:149–57. doi: 10.1016/j.mrfmmm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Shekoohi-Shooli F, Mortazavi SM, Shojaei-Fard MB, Nematollahi S, Tayebi M. Evaluation of the protective role of vitamin C on the metabolic and enzymatic activities of the liver in the male rats after exposure to 2.45 GHz Of Wi-Fi routers. J Biomed Phys Eng. 2016;6:157–64. [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi G, Singh P. Cytogenetic damage in mobile phone users: Preliminary data. Int J Hum Genet. 2005;5:25. [Google Scholar]
- 4.Joubert V, Bourthoumieu S, Leveque P, Yardin C. Apoptosis is induced by radiofrequency fields through the caspase-independent mitochondrial pathway in cortical neurons. Radiat Res. 2008;169:38–45. doi: 10.1667/RR1077.1. [DOI] [PubMed] [Google Scholar]
- 5.Ledoigt G, Belpomme D. Cancer induction molecular pathways and HF-EMF irradiation. Adv Biol Chem. 2013;3:177–86. [Google Scholar]
- 6.de Gannes FP, Taxile M, Duleu S, Hurtier A, Haro E, Geffard M, et al. A confirmation study of Russian and Ukrainian data on effects of 2450 MHz microwave exposure on immunological processes and teratology in rats. Radiat Res. 2009;172:617–24. doi: 10.1667/RR1541.1. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai T, Kiyokawa T, Narita E, Suzuki Y, Taki M, Miyakoshi J. Analysis of gene expression in a human-derived glial cell line exposed to 2.45 GHz continuous radiofrequency electromagnetic fields. J Radiat Res. 2011;52:185–92. doi: 10.1269/jrr.10116. [DOI] [PubMed] [Google Scholar]
- 8.Maes A, Collier M, Verschaeve L. Cytogenetic effects of 900 MHz (GSM) microwaves on human lymphocytes. Bioelectromagnetics. 2001;22:91–6. [PubMed] [Google Scholar]
- 9.Belpomme D, Campagnac C, Irigaray P. Reliable disease biomarkers characterizing and identifying electrohypersensitivity and multiple chemical sensitivity as two etiopathogenic aspects of a unique pathological disorder. Rev Environ Health. 2015;30:251–71. doi: 10.1515/reveh-2015-0027. [DOI] [PubMed] [Google Scholar]
- 10.Sage C. The implications of non-linear biological oscillations on human Electrophysiology for Electrohypersensitivity (EHS) and Multiple Chemical Sensitivity (MCS) Rev Environ Health. 2015;30:293–303. doi: 10.1515/reveh-2015-0007. [DOI] [PubMed] [Google Scholar]
- 11.Beneduci A, Chidichimo G, De Rose R, Filippelli L, Straface SV, Venuta S. Frequency and irradiation time-dependant antiproliferative effect of low-power millimeter waves on RPMI 7932 human melanoma cell line. Anticancer Res. 2005;25:1023–8. [PubMed] [Google Scholar]
- 12.Zhu W, Zhang W, Wang H, Xu J, Li Y, Lv S. Apoptosis induced by microwave radiation in pancreatic cancer JF305 cells. Can J Physiol Pharmacol. 2014;92:324–9. doi: 10.1139/cjpp-2013-0220. [DOI] [PubMed] [Google Scholar]
- 13.Zhang KD, Tong LR, Wang SM, Peng RY, Huang HD, Dong YC, et al. Apoptosis of lewis lung carcinoma cells induced by microwave via p53 and proapoptotic proteins in vivo. Chin Med J (Engl) 2017;130:15–22. doi: 10.4103/0366-6999.196587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo H, Lin T, Wang D, Peng R, Wang S, Gao Y, et al. RKIP regulates neural cell apoptosis induced by exposure to microwave radiation partly through the MEK/ERK/CREB Pathway. Mol Neurobiol. 2015;51:1520–9. doi: 10.1007/s12035-014-8831-5. [DOI] [PubMed] [Google Scholar]
- 15.Eltiti S, Wallace D, Ridgewell A, Zougkou K, Russo R, Sepulveda F, et al. Does short-term exposure to mobile phone base station signals increase symptoms in individuals who report sensitivity to electromagnetic fields.A double-blind randomized provocation study? Environ Health Perspect. 2007;115:1603–8. doi: 10.1289/ehp.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song XL, Wang CH, Hu HY, Yu C, Bai C. Microwave induces apoptosis in A549 human lung carcinoma cell line. Chin Med J (Engl) 2011;124:1193–8. [PubMed] [Google Scholar]
- 17.Aschner M, Suñol C, Bal-Price A, editors. Canada: Humana Press; 2011. Cell Culture Techniques. [Google Scholar]
- 18.Freshney RI. 5th ed. New York: Wiley-Liss; 2005. Culture of Animal Cells: A Manual of Basic Technique. [Google Scholar]
- 19.Helgason CD, Miller CL. Totowa, NJ: Humana Press; 2005. Basic cell culture protocols. [Google Scholar]
- 20.Shishkin A, Koppel T, Mironov V, Hussainova I, Locs J, Haldre H. Microwave reflectance and transmittance properties of conductive composite materials. Energy Procedia. 2017;113:354–61. [Google Scholar]
- 21.Ulloa RZ, Santiago MG, Rueda VL. Electromagnetic Fields and Waves. London, UK: IntechOpen; 2019. The Interaction of microwaves with materials of different properties. [Google Scholar]
- 22.Cember H, Johnson TE, Alaei P. Introduction to health physics. Med Phys. 2008;35:165–78. [Google Scholar]
- 23.Shaw JA. Radiometry and the Friis transmission equation. Am J Phys. 2013;81:33–7. [Google Scholar]
- 24.Levis CA. Encyclopedia of RF and Microwave Engineering. Wiley online library; 2005. Friis free-space transmission formula. Available at onlinelibrary.wiley.com/doi/ abs/10.1002/0471654507.eme141. [Google Scholar]