Abstract
Purpose
To estimate the risk of hospital-acquired COVID-19 transmission in a population of orthopaedic trauma patients during the first wave of the pandemic.
Patients and Methods
This is a retrospective cohort study of 109 patients who underwent an emergent orthopedic procedure by a single orthopedic traumatologist between March 1, 2020 and May 15, 2020 during the first peak of the pandemic. After applying inclusion and exclusion criteria, a total of 82 patients (67 inpatients and 15 ambulatory) were identified for final analysis. The primary outcome measured was postoperative Coronavirus (COVID-19) status. Secondary outcome measures included length of stay and discharge disposition.
Results
The mean age and length of stay in the hospital group was 59.5 years (± 21.7) and 4.3 days (± 4.6), respectively, versus 47.9 years (± 9.8) in the ambulatory group. 7.3% (6/82) of the inpatients subsequently tested or screened positive for COVID-19 at 2 weeks post-operatively, compared to 0/15 ambulatory patients (P=0.58). Of the 6 inpatients who tested positive, 4 (66.7%) were discharged to a rehabilitation center. Diabetes (P=0.05), hypertension (P=0.02), and congestive heart failure (P=0.005) were associated with transmission.
Conclusion
In this analysis, there was a nosocomial transmission rate of 7% compared to zero in the ambulatory surgery center, however this was not found to be statistically significant. This data supports the use of precautions such as frequent screening, hand washing, and masks to reduce transmission when COVID-19 rates are high. There is a lower risk of nosocomial COVID-19 transmission for patients treated as an outpatient and elective surgical procedures may be safer in this setting.
Keywords: coronavirus, pandemic, infection, hospital transmission
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for the Coronavirus Disease 2019 (COVID-19) pandemic. First identified in China in late 2019, the virus quickly spread across the globe. As of April 8, 2022, there have been 494 million confirmed cases of COVID-19 and 6.1 million deaths globally. In the US, there have been 79 million cases with 976 thousand deaths.1 SARS-CoV-2 may cause a constellation of respiratory symptoms including cough and shortness of breath as well as fever and loss of taste.2,3 Ultimately the virus may cause acute respiratory distress syndrome, sepsis, or pneumonia and even death in approximately 2.2% of patients.1 The virus is transmitted via respiratory droplets, aerosolized particles, and via contact transfer.2 Social distancing along with quarantining, mask use, frequent hand washing, and avoidance of high-risk activities are key methods to prevent the spread of SARS-CoV-2.
By May 2020, there were 1.3 million confirmed cases of SARS-CoV-2 in the United States, with most of these cases concentrated in the northeast/New York metropolitan area.4,5,6 As community spread exponentially increased, the case count and rate of hospitalization was estimated to exceed and overwhelm hospital capacities. Coupled with a severely limited supply of personal protective equipment (PPE), areas such as New York City appeared to be headed towards an inevitable collapse of the healthcare system. In response to this threat, local and state guidelines were implemented to mitigate community spread. This effort became known as “flattening the curve” which included measures such as closure of non-essential businesses, local curfews, and cancellation of all elective surgical procedures.7
Being an inpatient or employee in a medical facility was considered a significant risk factor for exposure to the virus. A study analyzing 11 hospitals in London estimated 6.8% of SARS-CoV-2 cases were hospital-acquired among inpatients between March and May 2020.8 Another study from a tertiary medical center in London estimated a nosocomial transmission of 15% between March-April 2020.9 The risk of hospital-based transmission remains unknown. The purpose of this study was to estimate the rate of hospital-based transmission of the SARS-CoV-2 among inpatient orthopedic trauma patients in hospitals with a high percentage of COVID-19 patients during the height of the pandemic.
Materials and Methods
Following Rutgers University institutional review board (IRB) approval Study No. 2020001174), 109 consecutive patients who underwent surgery by the senior author, a board certified orthopedic trauma surgeon who performs nearly all emergent orthopedic operations at our hospitals, between March 1, 2020 and May 15, 2020, were retrospectively reviewed. This was a time period when the incidence of COVID-19 was at its peak in the region.5 The study group patients were admitted to one of three hospitals in central New Jersey, or were patients undergoing urgent surgery at one local ambulatory surgery center. Patients who were under the age of 18 or diagnosed with or presumed to have COVID-19 prior to their procedure were excluded from analysis. Demographic data was extracted from the electronic medical record including type of surgery, age, sex, comorbid conditions, body mass index (BMI), admission date, hospital length of stay, and discharge location. Test results for SARS-CoV-2 were also recorded at all available time points. Patients with known pre-operative positive COVID-19 tests (by polymerase chain reaction/PCR or rapid antigen) or a presumed positive clinical diagnosis were excluded from analysis (n=6). A positive clinical diagnosis made pre- or post-operatively was made when the patient met the criteria for a positive case as defined by the Centers for Disease Control and Prevention (CDC). This included exhibiting symptoms characteristic of COVID-19 such as cough, fever, shortness of breath, or chest imaging consistent with COVID-19.10 Patients who either tested positive or met the CDC criteria for a positive case were considered positive in our analyses.
In the beginning of the pandemic, testing for COVID-19 was not mandatory nor readily available in our study hospitals given the nationwide shortage of sufficient testing materials. Therefore, 25/82 of the patients in this study were not formally PCR tested and were presumed negative prior to their procedure based on lack of symptoms, chest imaging, and daily temperature screening. By April, COVID testing became mandatory for all admitted patients, and a PCR test result was required within 48–72 hours prior to any procedure.
After discharge, a telemedicine or outpatient office follow-up visit occurred at 2 weeks for each study patient, which was used to assess patient recovery as well as screen for COVID-19 status. Informed consent was acquired by all participants or their next of kin if unable to consent for themself. This was either written informed consent when the patient was met in person or verbal consent obtained over telemedicine conference, as was approved by our institution’s ethics committee. COVID status was determined either by a positive clinical diagnosis by a physician, from a positive result on a screening questionnaire, or by a positive PCR test result. Patients who presented to the office were temperature screened and responded to a questionnaire on symptoms, known sick contacts, recent travel, and any COVID test results. Patients who had telemedicine appointments were asked the same screening questions but were not temperature screened.
A second cohort of patients treated at a free-standing surgery center was also examined in a separate statistical analysis to estimate the rate of transmission in an ambulatory facility. All ambulatory patients were required to have a negative PCR test within 48 hours of their procedure.
The risk of viral transmission was then calculated for the study group as well as transmission based on in-hospital and discharge risk-factors. Fisher’s Exact test was used to determine the association between surgical setting and medical comorbidities and COVID-19 transmission. A Wilcoxon Rank Sum test was performed to determine significant differences between age, length of admission, and BMI for patients who converted COVID-19 status. Statistical significance was set at p<0.05. All statistical analyses were completed with the use of Statistical Analysis Software version 9.4 (SAS Institute, Cary, North Carolina) by a statistician.
The percentage of inpatient beds occupied by COVID-19 positive patients in each of the hospitals was tracked over the study period. Hospital 1 and 3 reported daily statistics, and these numbers were averaged over a week. Hospital 2 reported the number of COVID+ inpatients once a week. The percentage of inpatient beds occupied was then determined by dividing each weekly average by the number of inpatient beds in each of the hospitals. The designated study period is centered around the time that correlates with peak prevalence of COVID amongst our inpatient hospitals and geographic area.5
Results
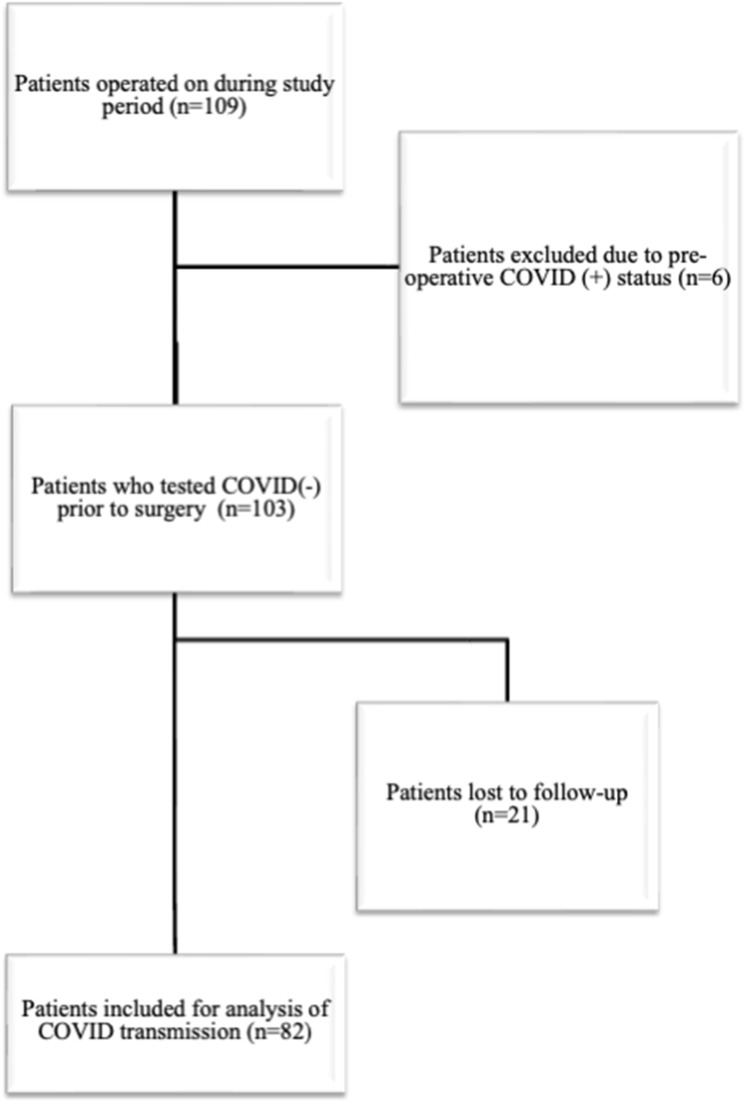
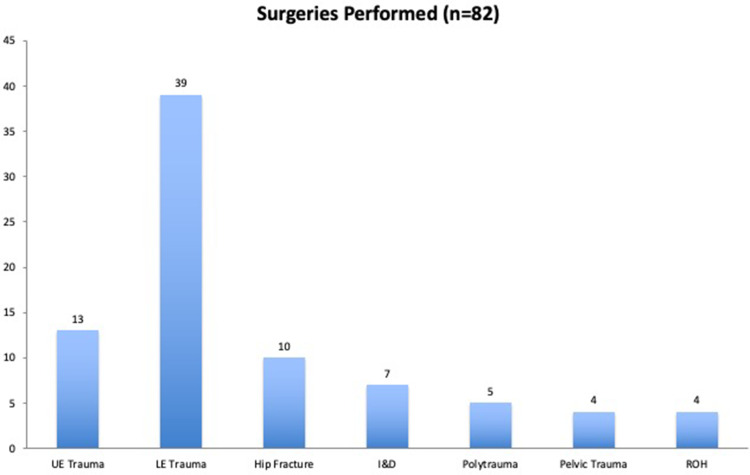
A total of 109 patients underwent an orthopedic surgical procedure during our study period. Twenty-one patients were lost to follow up or refused to participate in our screening questionnaire. Six patients were found to be COVID-19 positive on admission testing preoperatively and were excluded from further analysis of disease transmission. This left a total of 82 patients for final analysis (Figure 1). The types of cases performed are illustrated in Figure 2. Patients with more than one extremity undergoing surgery were categorized as polytrauma patients.
Figure 1.
Patients included in this study.
Figure 2.
Types of surgical cases performed during the study period.
Abbreviations: UE, upper extremity; LE, lower extremity; I&D, irrigation and debridement; ROH, removal of hardware.
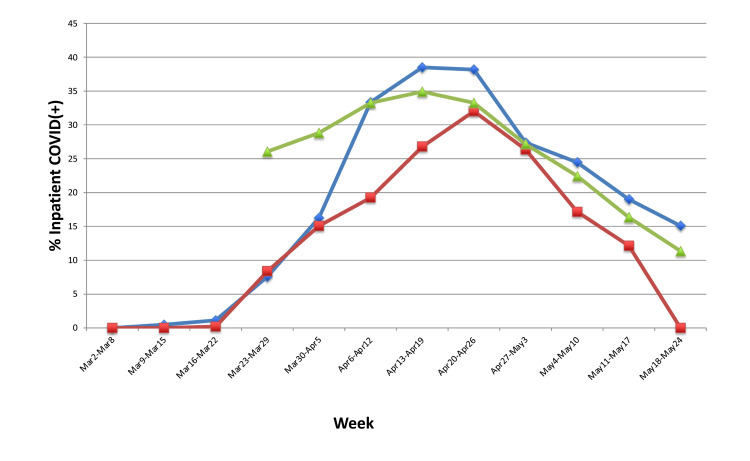
The percentage of inpatient hospital beds occupied by COVID-19 positive patients during the study period at each of the hospitals is depicted in Figure 3. Hospital 1 had the greatest percentage of COVID-19 positive patients at its peak. The maximum percentage at each of the hospitals was 38.5%, 32%, and 34.9%, respectively.
Figure 3.
The percentage of inpatient hospital beds occupied by COVID-19 positive patients in each of the hospitals over the study period.
Notes: Blue diamond, hospital 1; Red square, hospital 3; Green triangle, hospital 3. Data from hospitals 1 and 3 represent weekly averages based on daily reported values, whereas hospital 2 is based on weekly reported values.
The average age of all patients (n=103) who initially tested negative for SARS-CoV-2 was 57 years (SD 21.0, range 18–98) (Table 1). Just over half (55%) of the study population was male. The average length of admission was 4.3 days (SD 4.6, range 1–31). The majority (71.9%) of patients were discharged home, but 28.1% (29/103) were discharged to a subacute rehabilitation facility.
Table 1.
Summary of Patient Demographics
| Characteristic | All Initial COVID(-) (n = 103) |
COVID(+) Converts (n = 6) |
|---|---|---|
| Hospital, n (%) | ||
| Inpatient 1 | 63 (61.2) | 4 (66.7) |
| Inpatient 2 | 14 (13.6) | 2 (33.3) |
| Inpatient 3 | 11 (10.7) | 0 (0.0) |
| Ambulatory center | 15 (14.6) | 0 (0.0) |
| Age (years) | ||
| Mean (SD) | 57.0 (21.0) | 60.2 (17.5) |
| Range | 18–98 | 25–71 |
| Median [Q1, Q3] | 58 [43, 71] | 66.5 [63, 69] |
| Gender, n (%) | ||
| Male | 55 (53.4) | 4 (66.7) |
| Female | 48 (46.6) | 2 (33.3) |
| Body mass index (BMI) | ||
| Mean (SD) | 28.3 (7.2) | 26.3 (6.7) |
| Range | 15.2–63.7 | 21.3–38.3 |
| Median [Q1, Q3] | 27 [23.6, 32.5] | 23.2 [21.9, 30.1] |
| Note: 2 missing values | ||
| Length of admission (days) | ||
| Mean (SD) | 4.3 (4.6) | 9.8 (7.4) |
| Range | 1–31 | 2–21 |
| Median [Q1, Q3] | 3 [2, 5] | 8.5 [3, 16] |
| Note: 2 missing values | ||
| Discharge location, n (%) | ||
| Home | 74 (71.9) | 2 (33.3) |
| Rehab | 29 (28.1) | 4 (66.7) |
Abbreviations: LOA, length of admission; BMI, body mass index.
A total of 6 patients (7.3%) were tested or presumed to be COVID-19 negative pre-operatively and subsequently tested positive post-operatively, all of whom reported symptoms (Table 1). Three patients died during the study period, one of which tested positive for COVID-19 post-operatively. The mean age of these patients was 60.2 years. The average length of stay was 9.8 days and 4 of these 6 patients were discharged to a rehabilitation facility. There was no significant difference in post-operative COVID-19 status when comparing between all inpatient hospitals and the ambulatory surgery center (P=0.58).
There were significant associations between having congestive heart failure (CHF) (P=0.005) as well as diabetes (P=0.05) and converting COVID status (Table 2). There was no significant association between postoperative COVID-19 status and having a history of cerebrovascular accident (CVA), pulmonary disease, or having a psychological disorder. Although 4 of the 6 of the patients who acquired COVID-19 were discharged to a rehab facility, this proportion approached statistical significance (P=0.06). When comparing all inpatients to those who underwent a procedure at the ambulatory surgery center, the proportion of patients who converted was also not found to be significantly different (P=0.58).
Table 2.
Risk Factors Associated with Post-Operative COVID Positive
| Parameter | Converted COVID(+) (n=6) | Did Not Convert COVID(+) (n=76) | p-value |
|---|---|---|---|
| Inpatient hospitalization | 6 | 62 | 0.58 |
| Discharge to rehab | 4 | 20 | 0.06 |
| Female Sex | 2 | 33 | 1 |
| Comorbidities: | |||
| HTN | 5 | 24 | 0.02** |
| CHF | 2 | 0 | 0.005** |
| CVA | 0 | 2 | 1 |
| Pulmonary | 1 | 8 | 0.51 |
| DM | 3 | 10 | 0.05** |
| Psychologic | 0 | 5 | 1 |
Note: **Indicates statistically significant values (p<0.05).
Abbreviations: HTN, hypertension; CHF, congestive heart failure; CVA, cerebrovascular accident; DM, diabetes mellitus.
Table 3 summarizes differences in patient age, length of admission (LOA) and body mass index (BMI) between patients who converted COVID-19 status compared to those who did not. No significant differences were found between patients who did and did not convert when considering age and BMI. However, average length of admission was found to be significantly longer in patients who converted COVID-19 status (P=0.002).
Table 3.
Comparison of Age, Length of Admission, and BMI Between Patients Who Did and Did Not Convert to COVID(+)
| Converted COVID (+) | Did Not Convert COVID(+) | p -value | |
|---|---|---|---|
| Mean age | 60.2 | 54.6 | 0.28 |
| LOA | 9.8 | 3.9 | **0.02 |
| BMI | 26.3 | 29.2 | 0.25 |
Note: **Indicates statistically significant values (p<0.05).
Abbreviations: LOA, length of admission; BMI, body mass index.
Discussion
Our study identified a 7.3% rate of hospital acquired disease, despite a large percentage of positive inpatients. There is no consensus on how prevalent hospital transmission of COVID-19 is, with varying sources reporting vastly differing estimates. A study out of a large academic hospital in Massachusetts in the United States found an even lower rate of nosocomial transmission, with only 2 of 697 positive cases acquired from a hospital exposure. Russel et al hypothesized that nosocomial infections likely represent a small proportion of infections given the high rate in the community, which contrasts with a rate of up to 44% in a study out of China.11,12 Ingels et al reported a transmission rate of 12% in a population of 472 patients at a British hospital who underwent a surgical procedure within a 4-week period at the height of the pandemic, which contrasts with the National Health Service (NHS) in England’s estimate of 20% of cases being hospital acquired.13,14
The nosocomial transmission rate of the seasonal influenza is estimated to be around 1–5%, which is similar to our estimated rate of nosocomial COVID-19 transmission.15,16 However, our transmission rate COVID-19 was studied in a time when strict transmission precautions were in place. Without such precautions, the rate of COVID-19 transmission would likely be much higher compared to the flu.
During the pandemic, strict hospital regulations were enacted to reduce transmission. Daily temperature screening was required at each hospital entrance prior to entry for all patients, staff, and visitors. Public areas were closed and a 6-foot “social distancing” between persons was enforced. At each hospital, all COVID-19 positive patients as well as patients under investigation (PUI) were admitted to closed units with dedicated staff to limit hospital-based transmission of the virus. Additionally, no visitors were permitted during the study period and a mandatory mask policy was implemented in mid- to late March for all staff and patients. Each hospital had a dedicated operating room for positive or presumptive positive patients. All elective surgeries were prohibited during the study period to conserve personal protective equipment including masks, gowns, and gloves, which had become scarce.
Predictors of contracting COVID-19 in a hospital setting were pre-existing congestive heart failure, hypertension, and diabetes in this study. Previous studies have not shown that patients with these comorbidities are at a higher risk of contracting COVID-19, but the mortality rate is much higher in this population.17 While our data suggests that the rate of COVID-19 transmission is only 7% in surgical patients, it may be appropriate for surgeons to identify these risk factors in order to facilitate a joint decision regarding the risks and benefits of a procedure. Additionally, it may also be appropriate to ensure medical optimization, as well-controlled comorbidities are associated with improved prognoses in patients with COVID-19.18,19
Three patients died during the study period, two of which tested negative for COVID-19 prior to surgery and underwent intramedullary nailing (age 91, female) and hip hemiarthroplasty (age 85, female) for hip fractures. These two patients were excluded from final analysis. The third patient was considered a COVID-19 convert. This patient (age 77, male) was admitted for 9 days after undergoing intramedullary nailing, was discharged to a rehab facility, and tested positive for COVID-19 four days after discharge to rehab after exhibiting concerning symptoms. He expired on post-operative day 14. It is possible that this patient acquired COVID-19 in the hospital given the incubation period of the virus, however it is also conceivable that transmission occurred at the rehab facility, which may inflate the true hospital-based transmission rate.
In this study, we have demonstrated a low risk of acquiring symptomatic COVID-19 in orthopedic surgical patients in both in- and outpatient settings when precautions are utilized and resources are available. The difference between hospital and ambulatory surgical center transmission was not found to be significant, though this could be a result of our study being underpowered to truly detect a difference. The low rate of transmission may be a byproduct of the lessons learned as the pandemic unfolded abroad months earlier. Early adherence to infection control measures, which has been associated with minimizing risk of nosocomial COVID-19, helped to flatten the curve at our institutions.20 The early implementation of infection control measures at our study sites likely led to a relatively low rate of nosocomial infection in our study patients. It is estimated that 12% of adults in the United States have delayed urgent or emergent medical care due to concerns about COVID-19.21 The data from this study indicates that in the case of sufficient resources including PPE and hospital beds, urgent and emergent surgical procedures can be done with low risk of COVID-19 transmission. The low transmission rate in ambulatory surgery centers may support the resumption of certain elective surgical cases in this setting. These results may be used to help counsel patients undergoing orthopedic surgeries and provide reassurance.
The major strength of this study is its inclusion of patients in a region where and time when disease prevalence and incidence was high. Quarantining, social distancing, and mask-wearing were not widespread practices at the start of the pandemic, likely accelerating community spread. The geographic region included in this study captures the New York City metropolitan area, which was the epicenter of early transmission of the virus. Due to the novelty of the COVID-19 pandemic, there are currently few reports on its transmission within the hospital setting, particularly within surgical patients.
There are several limitations to our study. Namely, identification of true nosocomial transmission is difficult. Per the CDC and WHO, the initial strain of COVID-19 has an incubation period of up to 14 days, with a median time of 4–5 days from exposure to onset of symptoms.1,2 With the available testing protocols at the time of admission as well as our short-term follow up, the authors believe that the identification of COVID-19 transmission was as accurate as possible. In the early onset of the pandemic when our study period began, hospital screening was not universal and readily available. In early April, our study hospitals began universally screening all admitted patients and pre-operative testing was required prior to any procedure in the operating room. The 25 patients who underwent procedures in the beginning of our study period, were not PCR tested at that time and were presumed to be negative based on negative hospital screening, lack of symptoms, and negative admission chest radiographs, which is consistent with the CDC’s case definition. Although this introduces the possibility for false negative pre-operative values, we believed it was important to include these patients in our analysis since this would only cause us to overestimate rather than underestimate the true hospital transmission rate. It is also impossible to control and account for disease transmission outside the hospital, and it is certainly possible that the six patients who converted acquired COVID from outside the hospital. This would also inflate our number of converts and thus overestimate our true nosocomial transmission.
Our study also relies on patients reporting their symptoms accurately, which introduces bias. Testing is imperfect, and no test is 100% accurate. Using rapid COVID-19 antigen testing procedures, a Cochrane review indicates that they are accurate in correctly identifying infection in 72% of people with symptoms and 58% without symptoms. Specificity of rapid COVID-19 antigen testing is 99.5% in patients with concerning symptoms, and 98.9% in patients without concerning symptoms.22,23
There were 27 patients lost to follow-up and those who refused to participate in the screening questionnaire. The majority of patients had pre-operative PCR testing prior to their procedure as this became protocol at our hospitals. However, many patients were discharged prior to subsequent inpatient testing and their post-operative COVID status was determined by their 2 week follow-up visit, which some patients did not schedule and were not reachable by phone. If we presume that the 27 patients without follow up tested positive, the rate of nosocomial transmission in this study would increase to 30% (33/109), though unlikely. Furthermore, there were a relatively small number of patients undergoing urgent ambulatory surgery for our comparison. This number was limited by the government-mandated shutdown of elective cases that were not urgent in nature, such as fracture care. The relatively few patients in this group may have limited our statistical analysis to falsely conclude that the difference between in-hospital transmission and ambulatory surgery center transmission was not statistically significant. Additionally, we did not want to expand the duration of the study period as local COVID-19 case rates significantly dropped towards the end of the study period, representing the end of the first wave of the pandemic. For this reason, a post-hoc power analysis was performed. We would have needed 170 patients in order to achieve 80% power. Our study has a power of 61.1%. Finally, our results reflect our regional COVID-19 transmissions and although the New Jersey/New York area had one of the highest rates of the disease in the country, our results may not be generalizable to other regions.
Conclusion
The emergence of new variants of the rapidly mutating SARS-CoV-2 virus poses new challenges in treatment resistance and vaccination susceptibility. The CDC and World Health Organization (WHO) have identified several variants of concern (VOC) from the United Kingdom (UK), South Africa, Brazil, and India, all of which have since been detected in the United States by the end of January 2021.24,25 Global attention has more recently been drawn to the Delta, or B.1.617.2, and Omicron (B.1.1.529) variants. The Delta variant may be more transmissible than other variants and is comprising a growing proportion of new cases in the United States.26 Although the Omicron variant (appears to be less severe than other variants and causing fewer hospital admissions, it may be more infectious.27 The emergence and rapid global dissemination of new variants of the virus presents new concerns for the control and suppression of SARS-CoV-2.
Overall our results indicate that there is a low, but not negligible risk of transmission in the hospital setting while undergoing an orthopedic surgery when precautions are in place. These results may be used to counsel and reassure patients undergoing orthopedic surgery in the future.
Disclosure
Dr Julianne Forlizzi reports one time educational expenses from DJO, outside the submitted work; The authors reports no conflicts of interest in this work.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard [WHO web site]; 2021. Available from: https://covid19.who.int./. Accessed May 5, 2021.
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, Place S, et al.; COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–344. doi: 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bialek S, Bowen V, Chow N; CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465–471. doi: 10.15585/mmwr.mm6915e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson CN, Baumgartner J, Pichardo C, et al. COVID-19 outbreak — New York City, February 29–June 1, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1725–1729. doi: 10.15585/mmwr.mm6946a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kariya T. Rapid spread of COVID-19 in New York and the response of the community. Glob Health Med. 2020;2(2):123–126. doi: 10.35772/ghm.2020.01032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wake RM, Morgan M, Choi J, et al. Reducing nosocomial transmission of COVID-19: implementation of a COVID-19 triage system. Clin Med. 2020;20:e141. doi: 10.7861/clinmed.2020-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickman H, Rampling T, Shaw K, et al. Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London Teaching Hospital. Clin Infect Dis. 2020;72(4):690–693. doi: 10.1093/cid/ciaa816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coronavirus disease 2019 (COVID-19) 2020 interim case definition, approved April 5, 2020; 2020. Available from: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020/. Accessed October 12, 2021.
- 11.Russell T, Wu J, Clifford S, et al. Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. Lancet Public Health. 2020;6:e12–e20. doi: 10.1016/S2468-2667(20)30263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Gao Y, Wang X, et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingels A, Bibas S, Da Costa JB, et al. Surgery and COVID-19: balancing the nosocomial risk a French academic center experience during the epidemic peak. Br J Surg. 2020;107:e395–e397. doi: 10.1002/bjs.11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding L, Campbell D. Up to 20% of hospital patients with Covid-19 caught it at hospital. Guardian; 2020. Available from: https://www.theguardian.com/world/2020/may/17/hospital-patients-england-coronavirus-covid-19. Accessed June 9, 2022.
- 15.Godoy P, Torner N, Soldevila N, et al. Hospital-acquired influenza infections detected by a surveillance system over six seasons, from 2010/2011 to 2015/2016. BMC Infect Dis. 2020;20:80. doi: 10.1186/s12879-020-4792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings CN, Garg S, Nenninger EK, et al. Hospital-acquired influenza among hospitalized patients, 2011–2015. Open Forum Infect Dis. 2016;3. doi: 10.1093/ofid/ofw194.122 [DOI] [Google Scholar]
- 17.Alvarez-Garcia J, Lee S, Gupta A, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark CE, McDonagh STJ, McManus RJ, et al. COVID-19 and hypertension: risks and management. A scientific statement on behalf of the British and Irish Hypertension Society. J Hum Hypertens. 2021;35:304–307. doi: 10.1038/s41371-020-00451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e1063. doi: 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee C, Baker M, Vaidya V, et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US Academic Medical Center. JAMA Netw Open. 2020;3:e2020498–e2020498. doi: 10.1001/jamanetworkopen.2020.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeisler MÉ, Marynak K, Clarke KE, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–1257. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinnes J, Deeks JJ, Berhane S, et al.; Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;CD013705. doi: 10.1002/14651858.CD013705.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Science brief: emerging SARS-CoV-2 variants. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-emerging-variants.html#ref1. Accessed May 12, 2021. [PubMed]
- 25.World Health Organization. Tracking SARS-CoV-2 variants; 2021. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed October 9, 2021.
- 26.Lazarevic I, Pravica V, Miljanovic D, Cupic M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses. 2021;13(7):1192. doi: 10.3390/v13071192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahase E. Covid-19: hospital admission 50–70% less likely with omicron than delta, but transmission a major concern. BMJ. 2021;375:n3151. doi: 10.1136/bmj.n3151 [DOI] [PubMed] [Google Scholar]