Rheumatology key message.
Baricitinib administration was highly effective for treating pericardial effusion in a patient with Aicardi–Goutières syndrome.
Dear Editor, Aicardi–Goutières syndrome (AGS) was initially described as an early-onset progressive encephalopathy with severe neurological symptoms, acquired microcephaly, basal ganglia calcification, leukoencephalopathy, cerebral atrophy and chronic cerebrospinal fluid (CSF) pleocytosis [1]. Subsequently, autoinflammatory systemic manifestations (e.g. recurrent sterile fevers, chilblain-like lesions, hepatitis) and elevated CSF IFN-α activity were also reported [1].
Nine genes that encode proteins involved in nucleotide metabolism and/or sensing (TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, IFIH1, LSM11 and RNU7-1) have been associated to AGS [2]. Mutations in these genes result in constitutive induction of type I IFN and upregulation of IFN-stimulated genes (ISGs) [3]. The IFN signature (IS) measures the expression of ISG in peripheral blood and is a useful marker for disease activity [3].
Janus kinase (JAK) inhibitors may be effective in blocking IFN activation in patients with AGS [4, 5]. In one patient with AGS and an IFIH1 mutation, an open-label trial of ruxolitinib, a JAK 1/2 blocker, resulted in substantial developmental gains against a background of previous regression, combined with a decrease in IS [4]. An open-label trial of baricitinib, another JAK 1/2 blocker, in 35 patients with genetically confirmed AGS (including 8 patients with IFIH1 mutations), showed overall clinical improvement (including a likely improvement in neurologic function) and a decrease in IS [5]. In this report we describe an AGS patient with IFIH1 mutation treated with baricitinib and show new potential clinical benefits.
The patient was the only child of healthy non-consanguineous parents, born after an uneventful pregnancy and delivery. The child had normal development until 12 months, when he presented with neurological regression leading to spastic-dystonic tetraparesis that required intrathecal baclofen, intellectual disability and progressive microcephaly. Neuroimaging showed bilateral calcifications in the basal ganglia, subcortical white matter and cerebellar folia. Over the course of the disease he developed recurrent episodes of fever, sometimes associated with an isolated and self-limited blister-like ear lesion. He also developed chronic hypertension and chronic pericardial effusion refractory to standard treatment with aspirin, ibuprofen and colchicine. A de novo heterozygous missense mutation in the IFIH1 gene (NM_022168, c.1009C>G, p.Arg337Gly) that has previously been reported as a pathogenic variant was identified [3].
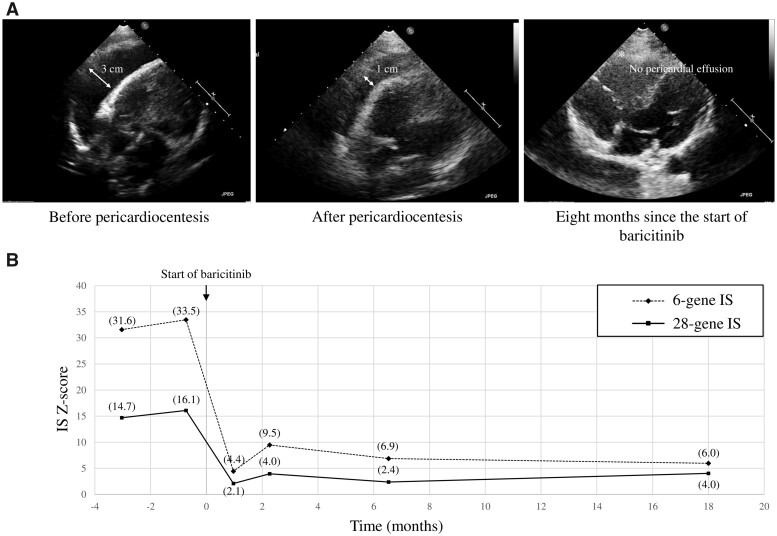
At 16 years of age, the neurological symptoms were stable, although the patient experienced daily low-grade fever and chronic hypertension requiring treatment with enalapril and carvedilol. Despite receiving steroids, the echocardiography showed an anterior echo-free space of 30 mm (Fig. 1A). Pericardiocentesis was performed that reduced the anterior echo-free space to 10 mm (Fig. 1A).
Fig. 1.
Echocardiograms and IFN signature before and after baricitinib treatment
(A) Echocardiograms showing the evolution of pericardial effusion before pericardiocentesis, after pericardiocentesis and after 8 months of treatment with baricitinib. (B) IS before starting baricitinib therapy and after 1, 2, 6 and 18 months of treatment.
With the aim of treating the comorbidities associated with the systemic inflammatory manifestations, we prescribed baricitinib under a compassionate use protocol after obtaining informed consent from the parents. Oral baricitinib was initiated at 2 mg twice a day. After the first week of therapy the patient remained afebrile and enalapril was discontinued <1 month later due to normalization of arterial tension. The pericardial effusion decreased significantly until it resolved completely (Fig. 1A). The carvedilol has also been discontinued and the improvements in systemic manifestations have been maintained 18 months after treatment initiation. No neurological changes were seen and no adverse effects have occurred to date.
Peripheral blood samples were collected using PAXgene Blood RNA Tubes (Qiagen, Venlo, The Netherlands) before starting baricitinib therapy and after 1, 2, 6 and 18 months of treatment (Fig. 1B). Total RNA was extracted using the PAXgene Blood RNA Kit (Qiagen). RNA samples were quantified using Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and RNA integrity was checked with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Expression levels of 28 ISGs (CXCL10, DDX60, EPSTI1, BGP1, HERC5, HERC6, IFI27, IFI44, IFI44L, IFI6, IFIT1, IFIT2, IFIT3, IFIT5, ISG15, LAMP3, LY6E, MX1, OAS1, OAS2, OAS3, OASL, RSAD2, RTP4, SIGLEC1, SOCS1, SPATS2L and USP18) and 4 housekeeping genes (ALAS1, HPRT1, TBP and TUBB) were measured using the nCounter Digital Analyzer (NanoString, Seattle, WA, USA) [6]. The type I IS was calculated using the median of the Z scores [7] of the six genes mainly involved in AGS previously reported by Rice et al. [3] and the median of the Z scores [7] of the 28 genes described by Kim et al. [6]; both were considered positive if >1.96 (s.d. 2) [7]. Expression of all ISs was highly elevated and declined markedly after initiation of baricitinib (Fig. 1B).
In conclusion, baricitinib administration in a patient with AGS and an IFIH1 mutation was well tolerated and had a beneficial effect on several inflammation-mediated comorbidities, including pericardial effusion. Further studies in large series of patients are needed to confirm these findings. It would be particularly interesting to investigate whether early treatment with JAK inhibitors in patients with AGS could modify neurological outcomes. The IS was a useful marker for disease activity and a decrease in IS correlated with clinical response to treatment.
Funding: This study was supported by the projects PI18/00486 and PI21/00316 (to T.A.) and PI19/01567 (to A.M.V.), integrated in the Plan Nacional de I+D+I and cofinanced by the ISCIII Subdirección General de Evaluación y Formento de la Investigación Sanitaria and the Fondo Europeo de Desarrollo Regional through the Pla estratègic de recerca i innovació en salut, Departament de Salut, Generalitat de Catalunya (SLT006/17/00362 to T.A.), Mutua Madrileña Foundation (AP162572016 to T.A.), Fundació Marató de TV3 (37/C/2021 to T.A.), 2021 Invest AEP Grant (PI047351 to T.A.) and a Torrons Vicens Foundation grant (PFNR0144 to T.A.).
Disclosure statement: The authors have declared no conflicts of interests.
Data availability statement
The data that support this article are available from the corresponding author upon reasonable request.
Contributor Information
Dídac Casas-Alba, Pediatric Neurology Department, Hospital Sant Joan de Déu.
Alejandra Darling, Pediatric Neurology Department, Hospital Sant Joan de Déu.
Eva Caballero, Neuroimmunology Program, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic, University of Barcelona.
Anna Mensa-Vilaró, Immunology Department, Hospital Clínic.
Joaquim Bartrons, Cardiology Department.
Jordi Antón, Pediatric Rheumatology Department.
Àngels García-Cazorla, Inborn Errors of Metabolism Unit, Neurology Department, Hospital Sant Joan de Déu, University of Barcelona; Institut de Recerca Sant Joan de Déu, Barcelona; Centre for Biomedical Network Research on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid, Spain.
Adeline Vanderver, Division of Neurology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Thaís Armangué, Neuroimmunology Program, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic, University of Barcelona; Centre for Biomedical Network Research on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid, Spain; Pediatric Neuroimmunology Unit, Neurology Department, Sant Joan de Déu Children’s Hospital, University of Barcelona, Barcelona, Spain.
References
- 1. Livingston JH, Crow YJ. Neurologic phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi–Goutières syndrome and beyond. Neuropediatrics 2016;47:355–60. [DOI] [PubMed] [Google Scholar]
- 2. Uggenti C, Lepelley A, Depp M et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat Genet 2020;52:1364–72. [DOI] [PubMed] [Google Scholar]
- 3. Rice GI, Del Toro Duany Y, Jenkinson EM et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 2014;46:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kothur K, Bandodkar S, Chu S et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology 2018;90:289–91. [DOI] [PubMed] [Google Scholar]
- 5. Vanderver A, Adang L, Gavazzi F et al. Janus kinase inhibition in the Aicardi–Goutières syndrome. N Engl J Med 2020;383:986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim H, De Jesus AA, Brooks SR et al. Development of a validated interferon score using nanostring technology. J Interf Cytokine Res 2018;38:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armangue T, Orsini JJ, Takanohashi A et al. Neonatal detection of Aicardi Goutières syndrome by increased C26:0 lysophosphatidylcholine and interferon signature on newborn screening blood spots. Mol Genet Metab 2017;122:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this article are available from the corresponding author upon reasonable request.