Abstract
Synthetic cannabinoid receptor agonists (SCRAs) are a large group of abused psychoactive compounds that elicit numerous toxic effects not observed with cannabis, including death. Abuse of third-generation SCRA 5F-MDMB-PINACA (also known as 5F-ADB) has been associated with over 40 fatalities. This SCRA is metabolized to several active phase I metabolites, including excessively high post-mortem serum concentrations of an ester hydrolysis metabolite, 5F-MDMB-PINACA-M7 (M7). Although high serum concentrations of M7 (and other active metabolites) have been suggested to contribute to 5F-MDMB-PINACA toxicity, the affinity of M7 for CB1 receptors is unknown and more complete pharmacodynamic characterization of 5F-MDMB-PINACA and its active metabolites is needed. Competition binding and G-protein modulation studies presented here confirm reports that 5F-MDMB-PINACA and a second N-5-hydroxypentyl metabolite (M2) exhibit nM affinity and act as high efficacy agonists at CB1 receptors. Also as previously published, M7 exhibits high efficacy at CB1 receptors; however, demonstrated here for the first time, M7 retains only low μΜ affinity. Empirically derived Kb values indicate rimonabant differentially antagonizes G-protein activation produced by 5F-MDMB-PINACA, relative to Δ9-THC (THC) or its metabolites. Chronic administration of 5F-MDMB-PINACA and metabolites results in CB1 down-regulation, but only 5F-MDMB-PINACA produces desensitization. Although low CB1 affinity/potency of M7 precluded in vivo studies, both M2 and THC produce locomotor suppression and CB1-mediated dose-dependent hypothermia and analgesia in mice. Collectively, these data confirm and extend previous studies suggesting that 5F-MDMB-PINACA is metabolized to active compounds exhibiting atypical pharmacodynamic properties at CB1 receptors, that may accumulate with parent drug to produce severe toxicity.
Keywords: synthetic cannabinoids, metabolites, toxicity, cb1 receptors, G-protein coupled receptors, novel psychoactive substances
Synthetic cannabinoid receptor agonists (SCRAs) are currently one of the largest classes of novel psychoactive substances making up over 30% of newly identified compounds worldwide in 2018 (Xu et al., 2019). These compounds are used recreationally and are typically mixtures of SCRAs that are found in products such as “K2” or “Spice,” sold in head shops or online (Le Boisselier et al., 2017). The popularity of these products has grown due to their ability to mimic or produce greater effects than those of Δ9-THC (THC; the psychoactive component of cannabis), while also being difficult to detect (Cooper, 2016). However, unlike THC, SCRA use is often accompanied by a multitude of adverse effects such as seizures, hyperemesis, and even death (Ford et al., 2017b). In attempt to regulate and schedule these illegal substances, the United States and other governments around the world are continually identifying SCRAs that are synthesized by clandestine laboratories. However, due to the numerous structural modifications which characterize these compounds, the rate of new SCRA production outpaces identification (Brents and Prather, 2014). Thus, new generations of dangerous SCRAs are being designed, synthesized, and marketed in ever changing products for abuse and to evade detection.
5F-MDMB-PINACA (also known as 5F-ADB) is a third-generation SCRA associated with numerous deaths, in which this compound is listed as a contributor or the sole cause (Boland et al., 2020). Furthermore, a multitude of other third generation SCRAs, in addition to 5F-MDMB-PINACA, has been identified and is associated with fatalities (Al-Matrouk et al., 2019). The cause of death accompanying SCRA abuse is often varied, usually associated with organ failure including respiratory, cardiac, and liver (Solimini et al., 2017; Ivanov et al., 2019). The mechanisms underlying organ failure are poorly understood and it is also unknown whether SCRAs are directly responsible for mortality (Ivanov et al., 2019). However, a role for SCRAs in mediating toxicity might be implicated because both subtypes of cannabinoid receptors (cannabinoid type-1; CB1R and cannabinoid type-2; CB2R) are highly expressed in the brain and many peripheral organs associated with failure (Solimini et al., 2017).
Definitive answers concerning mechanisms underlying SCRA toxicity are lacking due to a paucity of thoroughly designed studies. The fact that that these compounds produce differential toxic effects, including DNA damage and cytotoxicity, also complicates interpretation of available data (Koller et al., 2013). However, our laboratory and others have reported that phase I metabolism of SCRAs results in production of a number of active, rather than inactive, metabolites that may play a role in the unusually severe adverse effects and fatalities associated with abuse of these compounds (Brents et al., 2011, 2012; Cannaert et al., 2016; Rajasekaran et al., 2013; Tai and Fantegrossi, 2017). Forty-three deaths have been attributed to 5F-MDMB-PINACA abuse and abnormally high concentrations, as high as 166 ng/ml, of a phase I ester hydrolysis metabolite (M7) have been reported in post-mortem blood samples (Boland et al., 2020). In addition to M7, 5F-MDMB-PINACA is metabolized to several other phase I metabolites, including both N-4-hydroxypentyl and N-5-hydroxypentyl (M2) metabolites (Gamage et al., 2019). Interestingly, all 3 of these metabolites exhibit agonist activity at CB1 receptors (Gamage et al., 2019; Lie et al., 2021), and the N-4-hydroxypentyl metabolite and M2 also retain high nM affinity for CB1 receptors (Gamage et al., 2019). Although high serum concentrations of M7 (and other active metabolites) have been suggested to contribute to 5F-MDMB-PINACA toxicity (Lie et al., 2021), the affinity of M7 for CB1 receptors is currently unknown and more complete pharmacodynamic characterization of 5F-MDMB-PINACA and its active metabolites is needed. Thus, the purpose of this study was to confirm previous reports of active 5F-MDMB-PINACA metabolites and extend these findings by providing more complete characterization of the pharmacodynamic properties of these compounds.
These studies were conducted by first employing in vitro studies to compare the affinity and activity of THC, 5F-MDMB-PINACA, and commercially available 5F-MDMB-PINACA phase I metabolites M2 and M7. Second, experiments were performed to determine the Kb value for the CB1 antagonist rimonabant to antagonize G-protein activation produced by all compounds. Third, the effect of chronic administration of 5F-MDMB-PINACA and metabolites on CB1 receptor down-regulation and desensitization in transfected CHO cells was examined. Finally, in vivo cannabimimetic effects of THC and M2 were determined in mice. These data confirm and extend previous studies suggesting that 5F-MDMB-PINACA is metabolized to active compounds that retain CB1 receptor affinity and exhibit atypical pharmacodynamic properties, and thus accumulation with parent drug may contribute to severe toxic effects often observed with this drug of abuse.
MATERIALS AND METHODS
Materials
All drugs used for in vitro studies were stored at –20°C and diluted to 10 mM stock solutions containing 100% dimethyl sulfoxide (DMSO), except for 5F-MDMB-PINACA (N-[[1-(5-fluoropentyl)-1H-indazole-3-yl]carbonyl]-3-methyl-d-valine, methylester), which was diluted to a 1-mM stock. 5F-MDMB-PINACA (provided as the S-enantiomer) was obtained from Cerilliant (Round Rock, Texas). Two commercially available 5F-MDMB-PINACA metabolites 5F-MDMB-PINACA-M2 (methyl (S)-2-(1-(5-hydroxypentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate) and 5F-MDMB-PINACA-M7 [(S)-2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoic acid)] (also S-enantiomers) were purchased from Cayman Chemical (Ann Arbor, Michigan). THC was provided by the National Institute on Drug Abuse (NIDA, Bethesda, Maryland). Rimonabant was a generous gift, synthesized and provided by Thomas E. Prisinzano, Ph.D. (Department of Medicinal Chemistry, University of Kentucky School of Pharmacy, Lexington, Kentucky). GTPγS and GDP used in [35S]GTPγS binding assays were purchased from Thermo Fisher Scientific (Waltham, Massachusetts) and diluted in water to a 10-mM stock solution and stored at −20°C. Radioligands [3H]CP-55,940 (174.6 Ci/mmol) and [35S]GTPγS (1250 Ci/mmol) used for receptor binding and G-protein activation studies, respectively, were obtained from PerkinElmer (Waltham). In vivo experiments using rimonabant and 5F-MDMB-PINACA-M2 were diluted to a 0.3-mg/ml concentration in a vehicle containing a ratio of 1 (ethanol):1 (tween):18 (saline) and stored at 4°C.
Cell culture
CHO-hCB1 cells purchased from DiscoverRx Corporation (Fremont, California) were cultured as described previously (Ford et al., 2017a) in HAM’s F-12 K media (ATCC, Manassas, Virginia) containing 10% FetalPlex (Gemini Bioproducts, Sacramento, California), penicillin (0.05 IU/ml)/streptomycin (50 μg/ml) (Invitrogen, Carlsbad, California), and 250 μg/ml of Geneticin (G418; Sigma-Aldrich, St Louis, Missouri). Cells were maintained in a 37°C humidified incubator with 5% CO2, and only cells from passages 5–15 were used in all studies.
In experiments where CHO-hCB1 cells were chronically treated with SCRAs, cells were cultured until 80%–90% confluent and then washed with warmed serum/antibiotic-free media 3 times, followed by incubation with a high concentration of selected SCRAs for 24 h. After chronic drug exposure, to remove residual SCRA used for treatment, cells were rinsed 3 times by 5-min incubations in warmed serum/antibiotic-free media in a humidified 37°C incubator with 5% CO2. Finally, rinsed cells were harvested using 1× trypsin in PBS (10 mM)/EDTA (1 mM), and then processed to make membrane homogenates as described in the following section.
Membrane homogenates
Whole brains collected from CD-1 female mice were snap-frozen in liquid nitrogen and stored at −80°C until homogenates were prepared. CHO-hCB1 cells were harvested from T175 flasks using 1× trypsin in PBS (10 mM)/EDTA (1 mM) and kept at −80°C until use. Brains or cell pellets were thawed on ice, pooled, and suspended in ice-cold homogenization buffer (50 mM HEPES, pH 7.4, 3 mM MgCl2, and 1 mM EGTA) (Brents et al., 2011). Pooled samples were then transferred to a 40-ml Dounce glass homogenizer and homogenized using 10 strokes of a glass plunger, and then subjected to 40 000 × g for 10 min at 4°C. Supernatants were discarded and pellets resuspended in ice-cold homogenization buffer, and the process was repeated twice more. After final centrifugation, pellets were resuspended in ice-cold 50 mM HEPES, pH 7.4, to an approximate concentration of 5 mg/ml, aliquoted, and stored at –80°C. Protein concentrations were determined by the BCA Protein Assay obtained from Thermo Fisher Scientific.
Competition receptor binding
Mouse brain (50 µg) or CHO-hCB1 (50 µg) homogenates were incubated with 0.1 nM of the radiolabeled high-affinity non-selective CB1/CB2 ligand [3H]CP-55,940 and increasing concentrations (10−12–10−5 M; range differed for individual compounds examined, as indicated in figure legends) of non-radioactive test compounds, as described previously (Brents et al., 2012). To achieve equilibrium, assay mixes were incubated for a duration of 15 min at 37°C in an assay buffer containing MgCl2 (5 mM), Tris (50 mM), pH 7.4, and bovine serum albumin (0.05%) in a total volume of 1 ml. Non-specific binding was defined as radioactivity remaining after co-incubation of [3H]CP-55,940 with the non-radioactive high-affinity CB1/CB2 ligand WIN-55,212-2 (10−5 M). Specific binding was determined by subtracting non-specific from total binding. For detection of specific binding in membranes prepared from chronically treated cells, 0.2 nM [3H]CP-55,940 was incubated with WIN-55,212-2 (10−5 M). All reactions were conducted in triplicate and binding terminated by rapid filtration through Whatman GF/B glass fiber filters (Brandel, Gaithersburg, Maryland). Following 4 washes with an ice-cold buffer (50 mM Tris, pH 7.4, and 0.05% bovine serum albumin), filters were punched out into 7 ml vials into which 4 ml of RPI Bio-Safe II scintillation fluid (RPI Research Products, Mount Prospect, Illinois) was added. After overnight incubation, radioactivity was quantified using liquid scintillation spectrophotometry.
[35S]GTPγS binding assays
[35S]GTPγS binding assays were performed as previously described (Rajasekaran et al., 2013). Briefly, mouse brain (25 µg) or CHO-hCB1 (50 µg) homogenates were incubated with increasing drug concentrations (10−11–10−4 M; range differed for individual compounds examined, as indicated in figure legends) in a reaction mixture containing [35S]GTPγS (0.1 nM), GDP (10 µM), HEPES (20 mM), MgCl2 (10 mM), NaCl (100 mM), adenosine deaminase (20 units/l), and bovine serum albumin (0.05%). The final volume for all reactions was 1 ml and samples were incubated for 30 min at 30°C. Non-specific binding was defined as radioactivity remaining after co-incubation of [35S]GTPγS with non-radioactive GTPγS (10 µM). Specific binding was determined by subtracting non-specific from total binding. For experiments to determine Kb values for the selective CB1R antagonist/inverse agonist rimonabant to reverse G-protein activation by SCRAs, the concentration of SCRAs required to elicit 80% G-protein activation (ED80; as determined from concentration-effect curves) was incubated with increasing concentrations of rimonabant (10−12–10−6). Depending on specific experimental conditions examined, rimonabant was either added at the same time as agonists, or pre-incubated with membranes for 10 min prior to agonist addition (see Figure 3 legend for details). All reactions were conducted in triplicate and binding terminated by rapid filtration through Whatman GF/B glass fiber filters (Brandel, Gaithersburg, Maryland). Following 4 washes with an ice-cold buffer (50 mM Tris, pH 7.4, and 0.05% bovine serum albumin), filters were punched out into 7 ml vials into which 4 ml of RPI Bio-Safe II scintillation fluid was added. After overnight incubation, radioactivity was quantified using liquid scintillation spectrophotometry.
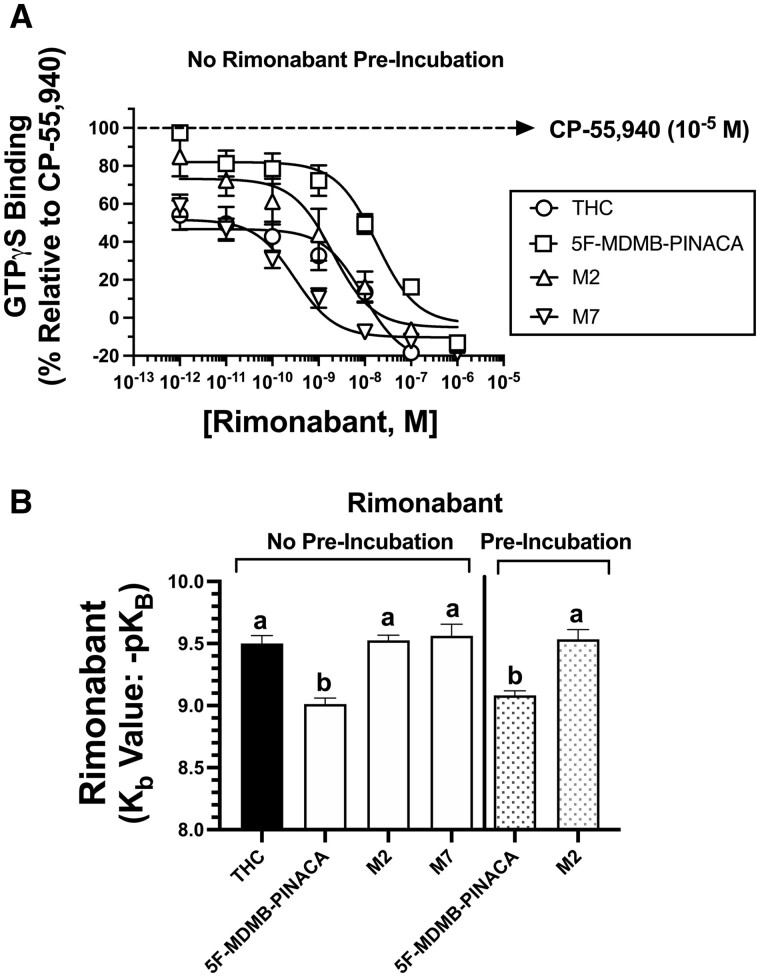
Figure 3.
K b determination of the CB1R-selective antagonist rimonabant for reversal of G-protein activation produced by SCRAs. A, Rimonabant reversal of G-protein activation by SCRAs was conducted by examining [35S]GTPγS binding produced by coincubation 5F-MDMB-PINACA (10−7 M), M2 (10−6 M), M7 (10−5 M), or THC (10−5 M) with increasing concentrations (10−12–10−6 M) of rimonabant in mouse brain homogenates. (Note: Rimonabant and agonists were added at the same time.) B, Calculation of antagonist-dissociation constants (Kb values) reveals G-protein activation by 5F-MDMB-PINACA requires greater concentrations of rimonabant than that needed for reversal of THC, M2, or M7, when rimonabant was added at the same time as agonists (left panel) or if pre-incubated for 10-muin prior to addition of agonists (right panel). Data points presented are the mean ± SEM of a minimum of 3 experiments, each conducted in triplicate. Specific IC50, Kb, and pKb values are listed and statistically compared in Table 3. a,bBars designated with different letters are significantly different (one-way ANOVA, followed by Tukey’s post hoc comparisons, p < 0.01).
Animal care and use
Male NIH Swiss mice (Charles River Laboratories Inc., Wilmington, Massachusetts), weighing approximately 30–40 g, were housed at 3 animals per plexiglass cage (15.24 × 25.40 × 12.70 cm) in a temperature-controlled room at the University of Arkansas for Medical Sciences (UAMS). Rooms were maintained at an ambient temperature of 22°C ± 2°C at 45%–50% humidity. Lights were set to a 12-h light/dark cycle. Animals were fed rodent chow (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St Louis) and water ad libitum until immediately before testing. Mice were acclimated to the laboratory environment 2 days prior to experiments and tested in groups of 6 per condition. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health (NIH). Experimental protocols were approved by the Animal Care and Use Committee at the UAMS (Animal Use Protocol No. 3155).
Biotelemetry of locomotor activity and core body temperature
Following appropriate anesthetization with ketamine (100 mg/kg, intraperitoneally [i.p.]) and xylazine (10 mg/kg, i.p.), the abdominal area of each mouse was shaved and sanitized with iodine swabs. A rostral-caudal cut approximately 1.5 cm in length was made with skin scissors, providing access to the intraperitoneal cavity, as described previously in Ford et al. (2019). A cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter, Mini Mitter, Bend, Oregon) was then inserted and the incision was closed using absorbable 5-0 chromic gut suture material. At least 7 days were imposed between surgery and experimental observation of drug effects to allow incisions to heal and mice to recover normal body weights. Following surgery, implanted mice were individually housed in plexiglass cages (15.24 × 25.40 × 12.70 cm) for the duration of all locomotor activity experiments. Implanted transmitters produced position-dependent signals that were sent to a receiver (model ER-4000 Receiver; Starr Life Sciences Corp., Oakmont, Pennsylvania) underneath cages, which were converted by an interfaced computer to locomotor counts. Mice were randomly assigned to experimental groups as cohorts were received from the supplier. Every 5 min, data updates were collected via the computer from probes for locomotor activity (in cm). For acclimation, on day 1 mice were injected (i.p.) with saline and on day 2 with vehicle. Following acclimation mice were tested on day 3 with THC or 5F-MDMB-PINACA-M2. All daily injections were given i.p. immediately followed by a biotelemetry session. Measurements during sessions were taken every 5 min for 8 h via the Vital View data acquisition system (Starr Life Sciences Corp., Oakmont). Antagonist studies employing rimonabant were conducted similarly by administering either rimonabant or vehicle (i.p.) 60 min prior to SCRA injections, followed by biotelemetry sessions. After 30 min, mice were removed from their cages and temperatures determined using a rectal thermometer (model BAT-12, PhysiTemp, Clifton, New Jersey) equipped with a Ret-3 mouse probe (model 50314, Stoelting Co., Dale, Illinois).
Catalepsy and warm water tail-withdraw
In separate groups of animals, catalepsy was measured using the horizontal bar test by non-blinded investigators, which employed a cylindrical steel bar (0.5 cm in diameter) that was supported 4.0 cm above a covered platform, as previously described in Brents et al. (2012). Thirty minutes after drug injections, mice were placed into a species-atypical position with hind limbs on the platform and forelimbs on the horizontal bar. Upon placement on the catalepsy bar, a timer was started and counted until the mouse removed both paws from the bar. The maximum time allowed on the bar was 20 s.
Following catalepsy testing, mice were subjected to the Warm Water Tail Withdraw assay. Tail withdrawal latency was conducted, as previously described in Marshell et al. (2014), in which mice were immobilized in one hand of the investigator, allowing the tail to hang freely. The distal 5 cm portion of the tail was submerged into a Stable Temp (Cole-Parmer, Vernon Hills, Illinois) heat-controlled water bath maintained at 50°C. Mice were then able to remove their tails from the water bath at any point, with the latency to tail-withdrawal measured by a stopwatch. A maximal latency cutoff time of 15 s was used to minimize tissue damage. Base-line latency measurements obtained prior to injections ranged from 3 to 5 s. Antagonist studies employing rimonabant were conducted by administering either rimonabant or vehicle 60 min prior to SCRA injections, followed by catalepsy and tail-withdrawal sessions.
Statistical analysis
Curve fitting and statistical analyses for in vitro experiments were performed using GraphPad Prism version 8.3 (GraphPad Software Inc., San Diego, California). Non-linear regression for one-site competition was used to determine IC50 values for receptor binding studies. The Cheng-Prusoff equation (Cheng and Prusoff, 1973) was used to convert the experimental IC50 values to Ki values, a quantitative measure of receptor affinity. Curve fitting of concentration-effect curves via non-linear regression was also employed to determine the EC50 (a measure of potency) and Emax (a measure of efficacy) for [35S]GTPγS experiments. A power equation (Cheng, 2001) based on the Cheng-Prusoff equation was used to determine Kb values of rimonabant, derived from the IC50 of rimonabant required to antagonize an EC80 concentration of SCRAs examined. Data are expressed as mean ± SEM. A one-way ANOVA, followed by Tukey’s Multiple Comparison post hoc test, was employed to determine statistical significance (p < .05) between 3 or more groups.
All in vivo statistical calculations were performed using GraphPad Prism version 8.3. Analysis of locomotor activity employed the trapezoidal rule to calculate the area under the curve from 0 to 8 h. Total locomotor counts were also summed from 0 to 8 h. Rectal temperature (in °C) and tail withdrawal latencies (in seconds) were presented as mean ± SEM from a minimum of 6 mice. For locomotor activity, rectal temperature, and locomotor data, statistical significance (p < .05) was determined using a one-way ANOVA, followed by Tukey’s HSD post hoc test.
RESULTS
5F-MDMB-PINACA Metabolites Retain Affinity for Mouse CB1 Receptors
We have previously demonstrated by saturation binding experiments using the radiolabeled, high affinity cannabinoid agonist [3H]CP-55,940, that mouse brain homogenates employed for these experiments contain a CB1R density of 2.44 pmol/mg protein, to which [3H]CP-55,940 binds with a Kd of 0.37 nM (N = 3) (Brents et al., 2012). To determine the affinity (Ki) of THC, 5F-MDMB-PINACA, and 5F-MDMB-PINACA metabolites M2 and M7 (Figure 1A) for CB1Rs, initial competition receptor binding studies with [3H]CP-55,940 were conducted (Figure 1B and Table 1). Specifically, the ability of increasing concentrations of each compound to displace [3H]CP-55,940 from CB1Rs present in mouse brain homogenates was examined. 5F-MDMB-PINACA bound to CB1Rs with high affinity (0.42 nM, N = 3, Table 1). Interestingly, M2 also displayed nM affinity (25 nM, N = 3) for CB1Rs, similar to that of the reference compound THC (34 nM, N = 3). The affinity of M7 for CB1Rs was significantly lower (Table 1, p < .05), but still in the μM range (1.9 µM, N = 3), predicting that if abnormally high concentrations of this metabolite are formed (Boland et al., 2020; Lie et al., 2021), physiologically relevant in vivo CB1R-mediated effects might nonetheless be observed.
Figure 1.
Affinity determination (Ki) of SCRAs for CB1Rs expressed in mouse brain used in this study. A, Synthetic and phytocannabinoids examined in this study are structurally diverse. B, Competition receptor binding studies were conducted by examining binding of [3H]CP-55,940 (0.1 nM) to CB1Rs in mouse brain homogenates in the presence of increasing concentrations of THC (10−11–10−6 M), 5F-MDMB-PINACA (10−12–10−6 M), M2 (10−11–10−6 M), and M7 (10−10–10−5 M). These results demonstrate that 5F-MDMB-PINACA binds with high sub-nM affinity and metabolites of this synthetic cannabinoid retain nM (M2) and μM affinities (M7). The reference compound THC exhibits nM affinity similar to that of M2. Data points presented are the mean ± SEM of a minimum of 3 experiments, each conducted in triplicate. The Cheng-Prusoff equation (Cheng and Prusoff, 1973) was used to convert the experimental IC50 values obtained from competition receptor binding experiments to Ki values, a quantitative measure of receptor affinity. Specific Ki values are listed and statistically compared in Table 1.
Table 1.
CB1R Affinity (Ki) in Mouse Brain Membranes
| Drug | [3H]CP-55,940 Binding |
||
|---|---|---|---|
|
mCB1
| |||
| K i (nM) | pKi (−log Ki) | N | |
| THC | 34 | 7.469 ± 0.147a | 3 |
| 5F-MDMB-PINACA | 0.42 | 9.377 ± 0.273b | 3 |
| M2 | 25 | 7.602 ± 0.156a | 3 |
| M7 | 1929 | 5.715 ± 0.407c | 3 |
pKi values designated by different letters are significantly different (one-way ANOVA, Tukey’s post hoc test, p < 0.05).
5F-MDMB-PINACA Metabolites Retain High Agonist Efficacy at Mouse CB1 Receptors
The activity of THC, 5F-MDMB-PINACA, M2, and M7 at CB1Rs expressed in mouse brain homogenates was next determined by employing the [35S]GTPγS binding assay, which measures G-protein activation. All compounds examined produced concentration-dependent increases in [35S]GTPγS binding, with a rank order of potency of 5F-MDMB-PINACA (2.3 nM) > 5F-MDMB-PINACA-M2 (305 nM) = THC (408 nM) > 5F-MDMB-PINACA-M7 (5.5 µM) (Figure 2A and Table 2). As anticipated, the potency for G-protein activation produced by these compounds occurred with the same rank order as that determined for CB1R affinity (Figure 1B and Table 1). For comparison of activity, a receptor saturating concentration (10 µM; 10 000-fold greater than its Ki value) of the well-characterized full CB1R agonist CP-55,940 produced 101% ± 2.9% (N = 4) increase in [35S]GTPγS binding in mouse brain homogenates. 5F-MDMB-PINACA and both metabolites also acted as agonists with high efficacy at CB1Rs when compared with CP-55,940, producing EMAX values of equal to or greater than 100% G-protein activation (Figure 2A and Table 1). Importantly, both 5F-MDMB-PINACA metabolites M2 and M7 produced significantly greater G-protein activation relative to that produced by the partial agonist THC (Table 2; p < .05). Confirming that all compounds activated G-proteins via specific interaction with CB1Rs, co-incubation with the CB1R-selective antagonist rimonabant (1 µM), significantly attenuated increases in [35S]GTPγS binding produced by each of these agonists (Figure 2B). Collectively, these data indicate that 5F-MDMB-PINACA metabolites retain high agonist efficacy at mouse CB1 receptors and M2 exhibits potency for G-protein activation in the nM range similar to that of THC.
Figure 2.
Determination of activity of 5F-MDMB-PINACA and metabolites by examining CB1R-induced G-protein activation in mouse brain homogenates. A, G-protein activation studies were conducted by examining [35S]GTPγS binding produced by coincubation with increasing concentrations of THC (10−10–10−5 M), 5F-MDMB-PINACA (10−11–10−6 M), M2 (10−10–10−5 M), and M7 (10−10–10−4 M) in mouse brain homogenates. These results demonstrate that 5F-MDMB-PINACA and metabolites act as high efficacy agonists compared with the lower efficacy agonist THC. Similar to the rank order of CB1 receptor affinity observed in Figure 1B (Table 1), 5F-MDMB-PINACA activates G-proteins most potently, while M2 and THC are similar in potency, and M7 is the least potent. B, G-protein activation produced by SCRAs was CB1R-dependent, as indicated by significant reversal of drug effects (ED80 concentration of each) when co-incubated with the CB1-selective antagonist rimonabant (1 µM). Data points presented are the mean ± SEM of a minimum of 3 experiments, each conducted in triplicate. Specific EMAX and ED50 values are listed and statistically compared in Table 2. **Significantly different from G-protein activation produced by respective drugs tested in the absence of rimonabant (non-paired t-test, p < 0.01).
Table 2.
Potency (EC50) and Efficacy (Emax) of G-Protein Activation in Mouse Membranes
| Drug | [35S]GTPγS |
|||
|---|---|---|---|---|
| EC50 (nM) | pEC50 (−log EC50) | E MAX (% CP-55,940) | N | |
| THC | 408 | 6.39 ± 0.287a | 63.7 ± 5.2a | 3 |
| 5F-MDMB-PINACA | 2.3 | 8.64 ± 0.342b | 117 ± 4.3b | 3 |
| M2 | 305 | 6.52 ± 0.226a | 107 ± 2.2b | 3 |
| M7 | 5544 | 5.26 ± 0.312c | 107 ± 10.9b | 3 |
pEC50 and EMAX values designated by different letters are significantly different from values in the same column (one-way ANOVA, Tukey’s post hoc test, p < 0.05).
Rimonabant Differentially Antagonizes SCRA-Induced G-Protein Activation
A measure of the potency of CB1R antagonism for rimonabant was investigated by determining the antagonist dissociation constant (eg, Kb) for reversal of G-protein activation produced by THC, 5F-MDMB-PINACA, M2, and M7 (Figure 3A and Table 3). Initially, the ability of increasing concentrations of rimonabant (10−12–10−6) to reduce [35S]GTPγS binding induced by a single EC80 concentration of each SCRA (as determined from Figure 2A) in mouse brain homogenates was conducted. Co-incubation of rimonabant (added at the same time as agonists) with all compounds produced a concentration-dependent decrease in SCRA-induced [35S]GTPγS binding. IC50 values for rimonabant, derived from these concentration-effect curves, were converted to a measure of antagonist potency (eg, Kb values) by employing a modified function of the Cheng-Prusoff equation (Figure 3B; Cheng, 2001). Employing these conditions, Kb values for rimonabant reversal of G-protein activation produced by THC (0.32 nM), M2 (0.30 nM), and M7 (0.27 nM) were similar. However, rimonabant exhibited significantly reduced potency for antagonism of [35S]GTPγS binding induced by 5F-MDMB-PINACA (Kb = 0.98 nM) relative to other SCRAs examined (Figure 3B and Table 3; p < .05).
Table 3.
Rimonabant Antagonism (Kb) of Cannabinoid G-Protein Activation in Mouse Membranes
| Drug | IC50 (nM) | K b (nM) | pKb (−log Kb) | N | Rimonabant Pre-Incubation |
|---|---|---|---|---|---|
| THC | 9.27 | 0.32 | 9.50 ± 0.06a | 5 | None |
| 5F-MDMB-PINACA | 37.9 | 0.98 | 9.01 ± 0.05b | 7 | None |
| M2 | 11.7 | 0.30 | 9.53 ± 0.04a | 4 | None |
| M7 | .913 | 0.27 | 9.56 ± 0.09a | 4 | None |
| 5F-MDMB-PINACA | 43.8 | 0.83 | 9.08 ± 0.04a | 5 | 10 min |
| M2 | 10.4 | 0.30 | 9.53 ± 0.08b | 4 | 10 min |
pKb values designated by different letters are significantly different from values in the same column (one-way ANOVA, Tukey’s post hoc test, p < 0.05).
Since [35S]GTPγS binding are not equilibrium assays, it is possible that the observed differences in Kb values for rimonabant antagonism of 5F-MDMB-PINACA, relative to the other SCRAs tested, might be due to differences in CB1 receptor on/off rates between SCRAs examined. Therefore, additional experiments were conducted in which concentrations of rimonabant were incubated for 10-min prior to addition of 5F-MDMB-PINACA and M2. Employing these conditions, it would be predicted that results would be dependent only on the agonist reaching binding equilibrium in the assay, not both agonist and antagonist. Similar to conditions when rimonabant and agonists were added at the same time, these pre-incubation studies revealed that Kb values for reversal of 5F-MDMB-PINACA and M2 G-protein activation by rimonabant were significantly different from each other. Furthermore, Kb values for antagonism of 5F-MDMB-PINACA and M2 did not differ between experimental designs, regardless if rimonabant was pre-incubated for 10-min or added at the same time as agonists (Figure 3B and Table 3).
Chronic 5F-MDMB-PINACA and Metabolites Produce Differential Effects on CB1R Down-Regulation and Desensitization
Chronic exposure to agonists of G-protein-coupled receptors (GPCRs), such as CBRs, results in 2 adaptive cellular processes, receptor down-regulation (Ferguson and Caron, 1998) and desensitization (Gainetdinov et al., 2004). In the present study, the following experiments were conducted to compare the ability of 5F-MDMB-PINACA and metabolites to produce CB1R down-regulation and desensitization in CHO-hCB1 cells.
5F-MDMB-PINACA and Metabolites Produce CB1 Down-Regulation
To determine a time course for CB1R down-regulation, CHO-hCB1 cells were incubated with a relatively high concentration of CP-55,940 (10−7 M; approximately 100-fold greater that its Ki value) for 0, 0.5, 1, 3, 12, and 24 h, followed by rigorous washing to remove residual drug and homogenized for determination of CB1R density by radioligand receptor binding employing [3H]CP-55,940 (Figure 4A). As anticipated, prolonged exposure to the full CB1R agonist CP-55,940 produced a time-dependent reduction in receptor density beginning as early as 30-min, with a maximal and near complete reduction of 98.8% ± 1.2% by 12 h. In subsequent experiments, to compare maximal CB1R down-regulation following exposure to different SCRAs, CHO-hCB1 cells were incubated for 24-h with a high concentration of CP-55,940 (10−7 M), 5F-MDMB-PINACA (10−7 M; approximately 200-fold greater than its Ki value), M2 (10−5 M; approximately 400-fold greater than its Ki value), or M7 (10−5 M; approximately 5-fold greater than its Ki value, concentration limited due to M7 solubility) (Figure 4B; right panel). Following washing to remove residual drug and harvesting of cells, chronic treatment with all drugs produced significant reductions in CB1R density. However, while CP-55,940 (81.7% ± 5.9%), 5F-MDMB-PINACA (58.4% ± 2.6%), and M7 (63.6% ± 8.3%) produced similar levels of CB1R down-regulation, chronic M2 treatment resulted in less reduction of CB1R density (36.3% ± 9.2%). The observed reductions in CB1R density are likely not due to presence of residual drug, because 1 or 10 min exposure to CP-55,950 (10−7 M; followed by the washing procedure) does not alter CB1R binding relative to vehicle-treated cells (Figure 4B; left panel).
Figure 4.
Effect of chronic SCRA treatment on CB1R density. A, To determine a time-course for CB1R down-regulation, CHO-hCB1 cells were incubated with a relatively high concentration of CP-55,940 (10−7 M; approximately 100-fold greater than its Ki value) for 0, 0.5, 1, 3, 12 and 24 h, followed by rigorous washing to remove residual drug and homogenized for determination of CB1R density by radioligand receptor binding employing [3H]CP-55,940. B, To compare the degree of CB1R down-regulation produced by SCRAs examined, CHO-hCB1 cells were incubated for 24-h (right panel) with a high concentration of CP-55,940 (10−7 M), 5F-MDMB-PINACA (10−7 M; approximately 200-fold greater than its Ki value), M2 (10−5 M; approximately 400-fold greater than its Ki value), or M7 (10−5 M; approximately 5-fold greater than its Ki value, concentration limited due to M7 solubility) and receptor density was quantified. To test for potential residual agonist effects present during membrane preparation on receptor binding, CHO-hCB1 cells were also incubated with CP-55,940 (10−7 M) for 1 and 10 min, or no agonist at all (Vehicle) prior to cell harvesting (left panel). Data points presented are the mean ± SEM of a minimum of 3 experiments, each conducted in triplicate. **Significantly different from 100% (one-sample t-test, p < 0.01). a-cBars designated with different letters are significantly different (one-way ANOVA, followed by Tukey’s post hoc comparisons, p < 0.01).
5F-MDMB-PINACA But Not Metabolites Produce CB1R Desensitization
Homogenates obtained from CHO-hCB1 cells treated with CP-55940, 5F-MDMB-PINACA, M2, or M7 were also used to quantify levels of CB1R desensitization by employing radiolabeled [35S]GTPγS binding assays (Figure 5). To first determine a time-course for CB1R desensitization, CHO-hCB1 cells were incubated with a relatively high concentration of CP-55,940 (10−7 M; approximately 100-fold greater that its Ki value) for 0, 0.5, 1, 3, 12, and 24 h, followed by rigorous washing to remove residual drug and homogenized for determination of G-protein activation produced by challenge with a concentration of CP-55,940 demonstrated to produce a maximal effect (10−7 M). Similar to that observed for receptor down-regulation, chronic treatment with CP-55,940 produced a time-dependent reduction in CB1R-mediated [35S]GTPγS binding, beginning around 30 min, with maximal desensitization of 93.3% ± 6.7% by 12 h (Figure 5A). Interestingly, a significant rebound, or increase, in G-protein activation was observed when cells were treated with CP-55,940 for 24 h. To compare CB1R desensitization produced by all SCRAs, identical conditions were used to those described for CB1R down-regulation experiments (Figure 4B), in which CHO-hCB1 cells exposed to high concentrations of SCRAs for 24 h (Figure 5B; right panel). In cells chronically exposed to either CP-55,940 (10−7 M) or 5F-MDMB-PINACA (10−7 M), maximal G-protein activation produced by a CP-55,940 (10−5 M) challenge was decreased similarly by approximately 25%–50% compared with the vehicle-treated cells. In marked contrast, prolonged treatment with M2 (10−5 M) or M7 (10−5 M) did not result in any desensitization and, in fact, in cells chronically treated with M7, G-protein activation was slightly (although insignificantly) increased by 13.7% ± 7.9%. The observed CB1R-desensitization is likely not due to presence of residual drug, because 1 min exposure to CP-55,940 (10−7 M; followed by the washing procedure) does not alter CP-55,94-induced [35S]GTPγS binding relative to vehicle-treated cells (Figure 5B; left panel). In summary, these experiments indicate that although chronic exposure of CHO-hCB1 cells to CP-55,940 and 5F-MDMB-PINACA produces both CB1R down-regulation and desensitization, prolonged treatment with 5F-MDMB-PINACA metabolites M2 and M7 unexpectedly results in CB1R down-regulation but not desensitization.
Figure 5.
Effect of chronic SCRA treatment on CB1R function. A, To determine a time-course for CB1R desensitization, CHO-hCB1 cells were incubated with a relatively high concentration of CP-55,940 (10−7 M; approximately 100-fold greater than its Ki value) for 0, 0.5, 1, 3, 12, and 24 h, followed by rigorous washing to remove residual drug. Cells were harvested, homogenized, and G-protein activation studies conducted by examining CP-55,940-mediated (10−7 M) [35S]GTPγS binding. B, To compare the degree of CB1R desensitization produced by SCRAs examined, CHO-hCB1 cells were incubated for 24-h (right panel) with a high concentration of CP-55,940 (10−7 M; approximately 100-fold greater than its Ki value), 5F-MDMB-PINACA (10−7 M; approximately 200-fold greater than its Ki value), M2 (10−5 M; approximately 400-fold greater than its Ki value), or M7 (10−5 M; approximately 5-fold greater than its Ki value, concentration limited due to M7 solubility). Following chronic treatment, cells were harvested, rigorously washed, membranes prepared, and G-protein activation studies quantified by examining [35S]GTPγS binding in response to a CP-55,940 (10−7 M) challenge. To test for potential residual agonist effects present during membrane preparation on G-protein activation studies, CHO-hCB1 cells were also incubated with CP-55,940 (10−7 M) for 1-min or no agonist at all (Vehicle) prior to cell harvesting (left panel). Data points presented are the mean ± SEM of a minimum of 3 experiments, each conducted in triplicate. **Significantly different from 100% (one-sample t-test, p < .01). a-cBars designated with different letters are significantly different (one-way ANOVA, followed by Tukey’s post hoc comparisons, p < .01).
The 5F-MDMB-PINACA Metabolite M2 Elicits Cannabimimetic Effects in Vivo
The cannabinoid tetrad is a standard in vivo test to quantify cannabimimetic properties of drugs, which includes measurement of 4 parameters: locomotor activity, core temperature, analgesia, and catalepsy (Wiley and Martin, 2003). It has been well established that cannabinoid CB1R agonists can elicit effects in all 4 tetrad parameters. Importantly, low CB1 affinity and potency of M7 precluded in vivo studies with this metabolite; however, M2 was evaluated in the full cannabinoid tetrad in mice to detect cannabimimetic properties.
Locomotor activity
Mice were injected i.p. with THC (3 mg/kg), M2 (3 mg/kg), saline or vehicle, and locomotor activity measured for 8-h by biotelemetry (Figure 6A). Both THC and M2 reduced locomotor activity, with maximal effects of M2 occurring 30 min post-injection, and a total duration of action of approximately 1 h. Decreased locomotor activity elicited by M2 was greater than that produced by THC, which likely reflects higher versus lower agonist efficacy of M2 at CB1 receptors relative to THC, respectively (Figure 2A and Table 2). To determine if M2 reduction in locomotor activity was CB1R-mediated, mice were injected with rimonabant (3 mg/kg) or saline 60 min prior to M2 (3 mg/kg) injection, followed by measurement of locomotor activity for 2 h (Figure 6B). Rimonabant pretreatment did not significantly reverse effects elicited by M2 on total locomotor activity, but did increase total locomotor counts during the 2-h observation period. In our laboratory, rimonabant never fully reverses cannabinoid-elicited locomotor suppression in mice (eg, see Ford et al., 2017a, Figure 9D; Pinson et al., 2020, Figure 11). A likely explanation for this might be that rimonabant exhibits its own effects on locomotor activity at doses similar to those which antagonize other cannabinoid agonist effects in the tetrad assay. In any case, whether activity is scored manually (as quadrant crossings) from overhead recorded videos of behavior, or quantified by our radiotelemetry system or photobeam boxes, locomotor suppression induced by THC or other SCRAs is never fully reversed by rimonabant.
Figure 6.
Effect of SCRAs on locomotor activity in mice. A, Mice were implanted with biotelemetry probes and locomotor activity monitored for 2 h following injection with saline (open circles), vehicle (filled squares), M2 (3 mg/kg; filled triangles), or THC (3 mg/kg; open triangles). Indicative of in vivo activity, both THC and the M2 metabolite reduced locomotor activity. In fact, at the doses tested, M2 produced greater reduction in locomotor activity than did THC. B, Pretreatment of mice with rimonabant (3 mg/kg) prior to M2 injection did not alter total locomotor activity (expressed as total distance traveled in cm over 2 h), indicative of a non-CB1-mediated effect (non-paired t-test, p < .05).
Rectal temperature
To examine SCRA effects on rectal temperature, mice were pretreated with vehicle or rimonabant (3 mg/kg; i.p.) and injected with M2 (3 mg/kg) 1 h later, followed by temperature measurement via rectal probe 30 min after M2 injection (Figure 7A). In vehicle pretreated mice, M2 produced a significant (p < .001) drop in temperature of approximately 4°C. However, in mice pretreated with rimonabant, no change in temperature was observed. These results suggest that hypothermia produced by M2 is mediated by CB1R activation.
Figure 7.
Effect of SCRAs on rectal temperature and warm water tail withdrawal in mice. Mice were pretreated with 5F-MDMB-PINACA-M2 (3 mg/kg) in the absence and presence of rimonabant (3 mg/kg) and activity in 2 additional measures of the cannabinoid tetrad was examined. Thirty minutes after injection, M2 decreased rectal temperature (A) and increased tail-withdrawal latency (B) in vehicle-injected, but not mice pretreated with rimonabant, indicative of a CB1-mediated effect. ***Significantly different from pre-injection values (two-way ANOVA, followed by Sidak’s post hoc multiple comparisons test, p < .001).
Analgesia
The capacity of SCRAs to induce analgesia was measured in a warm water tail withdrawal assay. Specifically, mice were pretreated with vehicle or rimonabant (3 mg/kg; i.p.) and injected with M2 (3 mg/kg) 1 h later. Thirty-minutes after M2 injection, the time (in seconds) required for mice to withdraw their tails from a 50°C water bath was measured (Figure 7B). M2 injection produced a significant (p < .0002) increase tail withdrawal latency in mice pretreated with vehicle, but not with rimonabant, indicative of a CB1R-mediated analgesic effect.
Catalepsy
To measure catalepsy, 30 min after (i.p.) drug injections, mice were placed into a species-atypical position with hind limbs on the platform and forelimbs on the horizontal bar. At the dose of M2 tested (3 mg/kg), mice immediately removed their paws from the bar, indicative that M2 produced no measurable signs of catalepsy. Although not detected at the dose examined, it is possible that M2 might produce catalepsy at higher doses.
DISCUSSION
In the present study, initial in vitro studies compared the affinity and activity of THC, 5F-MDMB-PINACA, and 2 commercially available 5F-MDMB-PINACA phase I metabolites M2 and M7. Competition binding and G-protein modulation studies detailed here confirm previous studies that 5F-MDMB-PINACA and M2 exhibit high nM affinity for CB1 receptors (Gamage et al., 2019) and also act as high efficacy CB1 agonists (Gamage et al., 2019; Lie et al., 2021). Also consistent with another recent report (Lie et al., 2021), M7 was shown to exhibit high agonist efficacy, but was shown for the first time here, to also retain only low μM affinity for CB1 receptors. Additional pharmacodynamic characterization found that rimonabant differentially antagonizes G-proteins activated by 5F-MDMB-PINACA, relative to THC and its metabolites, when quantified by empirically derived Kb values. Prolonged treatment of CHO-hCB1 cells with 5F-MDMB-PINACA and metabolites produces CB1R down-regulation, but unexpectedly M2 an M7 failed to desensitize CB1Rs. Finally, in vivo studies in mice show that M2 and THC produce locomotor suppression, as well as dose-dependent CB1-mediated hypothermia and analgesia in mice. Collectively, these data confirm and extend suggestions made by previous studies (Gamage et al., 2019; Lie et al., 2021) that 5F-MDMB-PINACA is metabolized to active compounds exhibiting atypical pharmacodynamic properties at CB1 receptors, that may accumulate with parent drug to produce severe toxicity.
THC, the major psychoactive component in cannabis, has been used for centuries, producing little to no adverse effects (Zuardi, 2006). However, recent abuse of 5F-MDMB-PINACA has been associated with severe toxicity and death (Yeter and Erol Öztürk, 2019). While the mechanisms responsible for 5F-MDMB-PINACA toxicity are unknown, based on recent autopsy reports, a potential contributing factor could be extremely high levels of active phase I metabolites, such as M7 reported in blood of abusers. For example, M7 has been quantified in blood at concentrations as high as 166 ng/ml (eg, 457 nM), in contrast to relatively low levels of the 5F-MDMB-PINACA parent compound of 2.2 ng/ml (eg, 6 nM) (Boland et al., 2020). Given that M7 has been shown to retain affinity (Table 1) and exhibit high agonist efficacy at CB1 receptors (Table 2; Lie et al., 2021), such high serum concentrations acting in conjunction with 5F-MDMB-PINACA might contribute to toxicity. Furthermore, M2 (Table 1; Gamage et al., 2019) and an additional 5F-MDMB-PINACA metabolite (N-4-hydroxypentyl) not examined here (Gamage et al., 2019) exhibit high nM affinity (19–25 nM) for hCB1Rs, similar to that of THC. M2 (Table 2; Gamage et al., 2019; Lie et al., 2021), M7 (Table 2; Lie et al., 2021), and 4OH-pentyl-5F-MDMB-PINACA (Gamage et al., 2019) all also act as high efficacy hCB1R agonists, relative to the lower efficacy agonist THC. In contrast, THC is metabolized to 2 major metabolites, only one of which is active (11-hydroxy-THC or 11-OH-THC) and a second inactive compound (11-nor-9-carboxy-THC or THC-COOH) (Schwilke et al., 2009). Therefore, accumulation of the 5F-MDMB-PINACA parent drug with its metabolites exhibiting high affinity and that act as high efficacy hCB1R agonists could contribute to enhanced adverse effects associated with 5F-MDMB-PINACA abuse relative to that of THC and other SCRAs.
The classic view of GPCR activation is described by a 2-state model in which receptors exist primarily in 2 conformations, consisting of an inactive or active state. Agonists preferentially bind and enrich the proportion of receptors in the active state, while inverse agonists stabilize the inactive state, and neutral antagonists have equal affinity for both receptor conformations (Smith et al., 2018). However, recent work indicates that GPCRs instead exist in multiple active conformations (Latorraca et al., 2017) and that some individual agonists can differentially bias receptor signaling toward β-arrestin or G-protein-dependent pathways, to produce distinct intracellular effects (Ford et al., 2019). Due to considerable structural diversity, it is possible that a subset of SCRAs may function as biased agonists (Wouters et al., 2020), selectively enhancing receptor signaling toward pathways that may result in pronounced adverse effects. Interestingly, evidence that SCRAs bind to distinct CB1R conformations is supported by results presented here, in that rimonabant exhibits significantly reduced potency for antagonism of G-protein activation produced by 5F-MDMB-PINACA, relative to other SCRAs examined. Furthermore, it is tempting to speculate that such distinct binding properties of 5F-MDMB-PINACA may also contribute to the extreme toxicity observed for this compound, relative to other abused SCRAs. Finally, these observations should be considered, and could be potentially important, given the proposed use of rimonabant for treatment of acute SCRA overdose (Pryce and Baker, 2017).
Prolonged exposure of GPCRs to agonists leads to 2 important adaptive processes known as desensitization and downregulation (Rajagopal and Shenoy, 2018; Tsao et al., 2001). Desensitization occurs in response to chronic agonist stimulation, during which GPCRs are initially phosphorylated, recruit β-arrestin, and are no longer able to activate G-proteins, resulting in reduced intracellular signaling. Upon continued agonist exposure, signaling is further reduced by internalization and degradation of GCPRs via a process known as down-regulation. Both desensitization and down-regulation contribute to development of tolerance, necessitating increases in dose to achieve desired effects, and in many cases, ultimately leading to drug dependence. In the present study, as anticipated, chronic exposure to both SCRAs CP-55,940 and 5F-MDMB-PINACA resulted in CB1R down-regulation and desensitization. In marked contrast, although prolonged exposure to both 5F-MDMB-PINACA metabolites (M2 and M7) also reduced CB1R density (eg, down-regulation), chronic treatment with either metabolite did not result in reduced CB1R signaling (eg, desensitization). It is interesting that no desensitization occurs following prolonged exposure to M2 or M7, in spite of a loss of over 60% of hCB1R density. However, this is likely explained by the presence of spare receptors in transfected cell lines (such as CHO-hCB1) expressing high receptor levels (Stephenson, 1997). However, it should also be considered that residual activity after the significant CB1 down-regulation presented here might provide information about either remaining spare receptor density, desensitization, or a mixture of both. Most importantly, dependence has been observed following chronic abuse of SCRAs in humans (Zimmermann et al., 2009), with abrupt discontinuation often resulting in seizures, cardiovascular, and respiratory risk (Cooper, 2016). Therefore, chronic use of SCRAs such as 5F-MDMB-PINACA, metabolized to active compounds that fail to desensitize CB1Rs (eg, M2 and M7), might result in increased abuse liability and/or rapid development of dependence.
Phase I metabolism results in molecular modifications that reduce lipophilicity and enhance elimination of drugs. Therefore, although active metabolites of 5F-MDMB-PINACA (and other SCRAs) are formed, due to potential unfavorable pharmacokinetic properties, the efficacy of such compounds quantified in vitro might not be directly translated to activity observed in vivo (Singh, 2006). If so, the contribution of such metabolites to adverse effects might be of questionable consequence. M7 could not be evaluated for activity in vivo due to low CB1 receptor affinity and potency. However, results presented in the present study suggest that in vitro observations demonstrating similar CB1R affinity and high agonist efficacy of the active M2 metabolite, relative to the lower efficacy agonist THC, are indeed reflected by in vivo effects in the cannabinoid tetrad. For example, the high efficacy agonist M2 (3 mg/kg) elicits greater reduction in locomotor activity than that produced by an equivalent dose of THC (3 mg/kg). Similar agreement between in vitro and in vivo measures of efficacy has also been reported for the SCRA JWH-018, in which the active phase I metabolite (JWH-018-M1) produced significantly higher levels of G-protein activation, as well as greater decreases in locomotor activity and reduction in core body temperature than THC (Brents et al., 2011). Collectively, these data suggest that the high efficacy SCRA metabolite M2 predicted from in vitro experiments is indeed able to elicit activity in vivo, and thus contribute to adverse effects often observed in abusers.
It is interesting to note that M2 did not elicit effects in all tetrad parameters. However, while catalepsy was not observed at the dose of M2 examined, it is possible (perhaps likely) that M2 might produce effects in this tetrad parameter if tested at higher doses. It would have also been very informative to evaluate the parent 5F-MDMB-PINACA compound in the tetrad for comparative purposes with M2. 5F-MDMB-PINACA unfortunately could not be obtained commercially, as it is a Schedule I compound in the United States and was unavailable from any of our usual sources. Although previously published data report that 5F-ADB (referred to as 5F-MDMB-PINACA in the paper) dose-dependently suppressed locomotor activity in mice and elicited THC-like discriminative stimulus effects in rats, but cataleptic effects were not assessed (see Gatch and Forster, 2019).
In summary, data presented here confirm and extend previous studies (Gamage et al., 2019; Lie et al., 2021) suggesting that 5F-MDMB-PINACA is metabolized to active compounds exhibiting atypical pharmacodynamic properties at CB1 receptors, that may accumulate with parent drug to produce severe toxicity. Specifically, by potentially binding to distinct receptor conformations, 5F-MDMB-PINACA may accumulate with active metabolites (which fail to desensitize CB1Rs), to enhance signaling pathways responsible for producing adverse effects. Future research directions should include study of additional structurally distinct classes of SCRAs to determine if similar findings might be observed and to provide novel mechanistic insight, aimed to better understand and ultimately treat SCRA-mediated toxicity.
FUNDING
These experiments were conducted in part, by funding provided by National Institutes of Health/National Institute on Drug Abuse grant DA039143.
DECLARATION OF CONFLICTING INTERESTS
The authors declare that there are no conflicts of interest.
Contributor Information
Christian V Cabanlong, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, Arkansas 72205, USA.
Lauren N Russell, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, Arkansas 72205, USA.
William E Fantegrossi, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, Arkansas 72205, USA.
Paul L Prather, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, Arkansas 72205, USA.
REFERENCES
- Al-Matrouk A., Alqallaf M., AlShemmeri A., BoJbarah H. (2019). Identification of synthetic cannabinoids that were seized, consumed, or associated with deaths in Kuwait in 2018 using GC-MS and LC-MS-MS analysis. Forensic Sci. Int. 303, 109960. [DOI] [PubMed] [Google Scholar]
- Boland D. M., Reidy L. J., Seither J. M., Radtke J. M., Lew E. O. (2020). Forty-three fatalities involving the synthetic cannabinoid, 5-fluoro-adb: Forensic pathology and toxicology implications. J. Forensic Sci. 65, 170–182. [DOI] [PubMed] [Google Scholar]
- Brents L. K., Gallus-Zawada A., Radominska-Pandya A., Vasiljevik T., Prisinzano T. E., Fantegrossi W. E., Moran J. H., Prather P. L. (2012). Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem. Pharmacol. 83, 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents L. K., Prather P. L. (2014). The k2/spice phenomenon: Emergence, identification, legislation and metabolic characterization of synthetic cannabinoids in herbal incense products. Drug Metab. Rev. 46, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents L. K., Reichard E. E., Zimmerman S. M., Moran J. H., Fantegrossi W. E., Prather P. L. (2011). Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS ONE 6, e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannaert A., Storme J., Franz F., Auwarter V., Stove C. P. (2016). Detection and activity profiling of synthetic cannabinoids and metabolites with a newly developed bio-assay. Anal Chem. 88, 11476–11485. [DOI] [PubMed] [Google Scholar]
- Cheng H. C. (2001). The power issue: Determination of Kb or Ki from IC50. A closer look at the Cheng-Prusoff equation, the schild plot and related power equations. J. Pharmacol. Toxicol. Methods 46, 61–71. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- Cooper Z. D. (2016). Adverse effects of synthetic cannabinoids: Management of acute toxicity and withdrawal. Curr. Psychiatr. Rep. 18, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. S. G., Caron M. G. (1998). G protein-coupled receptor adaptation mechanisms. Semin. Cell Dev. Biol. 9, 119–127. [DOI] [PubMed] [Google Scholar]
- Ford B. M., Cabanlong C. V., Tai S., Franks L. N., Penthala N. R., Crooks P. A., Prather P. L., Fantegrossi W. E. (2019). Reduced tolerance and asymmetrical crosstolerance to effects of the indole quinuclidinone analog PNR-4-20, a G protein-biased cannabinoid 1 receptor agonist in mice: Comparisons with delta(9)-tetrahydrocannabinol and JWH-018. J. Pharmacol. Exp. Ther. 369, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B. M., Franks L. N., Tai S., Fantegrossi W. E., Stahl E. L., Berquist M. D., Cabanlong C. V., Wilson C. D., Penthala N. R., Crooks P. A., et al. (2017a). Characterization of structurally novel G protein biased CB1 agonists: Implications for drug development. Pharmacol. Res. 125, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B. M., Tai S., Fantegrossi W. E., Prather P. L. (2017b). Synthetic pot: Not your grandfather’s Marijuana. Trends Pharmacol. Sci. 38, 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov R. R., Premont R. T., Bohn L. M., Lefkowitz R. J., Caron M. G. (2004). Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144. [DOI] [PubMed] [Google Scholar]
- Gamage T. F., Farquhar C. E., McKinnie R. J., Kevin R. C., McGregor I. S., Trudell M. L., Wiley J. L., Thomas B. F. (2019). Synthetic cannabinoid hydroxypentyl metabolites retain efficacy at human cannabinoid receptors. J. Pharmacol. Exp. Therap. 368, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M. B., Forster M. J. (2019). Cannabinoid-like effects of five novel carboxamide synthetic cannabinoids. Neurotoxicology 70, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I. D., Stoykova S., Ivanova E., Vlahova A., Burdzhiev N., Pantcheva I., Atanasov V. N. (2019). A case of 5F-ADB/FUB-AMB abuse: Drug-induced or drug-related death? Forensic Sci. Int. 297, 372–377. [DOI] [PubMed] [Google Scholar]
- Koller V. J., Zlabinger G. J., Auwarter V., Fuchs S., Knasmueller S. (2013). Toxicological profiles of selected synthetic cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB(1). Arch. Toxicol. 87, 1287–1297. [DOI] [PubMed] [Google Scholar]
- Latorraca N. R., Venkatakrishnan A. J., Dror R. O. (2017). GPCR dynamics: Structures in motion. Chem. Rev. 117, 139–155. [DOI] [PubMed] [Google Scholar]
- Le Boisselier R., Alexandre J., Lelong-Boulouard V., Debruyne D. (2017). Focus on cannabinoids and synthetic cannabinoids. Clin. Pharmacol. Ther. 101, 220–229. [DOI] [PubMed] [Google Scholar]
- Lie W., Cheong E. J. Y., Goh E. M. L., Moy H. Y., Cannaert A., Stove C. P., Chan E. C. Y. (2021). Diagnosing intake and rationalizing toxicities associated with 5F-MDMB-PINACA and 4F-MDMB-BINACA abuse. Arch. Toxicol. 95, 489–508. [DOI] [PubMed] [Google Scholar]
- Marshell R., Kearney-Ramos T., Brents L. K., Hyatt W. S., Tai S., Prather P. L., Fantegrossi W. E. (2014). In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: Inhalation versus intraperitoneal injection. Pharmacol. Biochem. Behav. 124, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson A., Yarbrough A. L., Bush J. M., Cabanlong C. V., Shoeib A., Jackson B. K., Fukuda S., Gogoi J., Fantegrossi W. E., McCain K., et al. (2020). Metabolism, CB1 cannabinoid receptor binding and in vivo activity of synthetic cannabinoid 5F-AKB48: Implications for toxicity. Pharmacol. Biochem. Behav. 195, 172949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce G., Baker D. (2017). Antidote to cannabinoid intoxication: the CB(1) receptor inverse agonist, AM251, reverses hypothermic effects of the CB(1) receptor agonist, CB-13, in mice. Br. J. Pharmacol. 174, 3790–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S., Shenoy S. K. (2018). GPCR desensitization: Acute and prolonged phases. Cell Signal. 41, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran M., Brents L. K., Franks L. N., Moran J. H., Prather P. L. (2013). Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol. Appl. Pharmacol. 269, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwilke E. W., Schwope D. M., Karschner E. L., Lowe R. H., Darwin W. D., Kelly D. L., Goodwin R. S., Gorelick D. A., Huestis M. A. (2009). Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 55, 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. S. (2006). Preclinical pharmacokinetics: An approach towards safer and efficacious drugs. Curr. Drug Metab. 7, 165–182. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Lefkowitz R. J., Rajagopal S. (2018). Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 17, 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimini R., Busardo F. P., Rotolo M. C., Ricci S., Mastrobattista L., Mortali C., Graziano S., Pellegrini M., di Luca N. M., Palmi I. (2017). Hepatotoxicity associated to synthetic cannabinoids use. Eur. Rev. Med. Pharmacol. Sci. 21, 1–6. [PubMed] [Google Scholar]
- Stephenson R. P. (1997). A modification of receptor theory. Br. J. Pharmacol. 120, 106–120; discussion 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S., Fantegrossi W. E. (2017). Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. Curr. Top. Behav. Neurosci. 32, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P., Cao T., von Zastrow M. (2001). Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol. Sci. 22, 91–96. [DOI] [PubMed] [Google Scholar]
- Wiley J. L., Martin B. R. (2003). Cannabinoid pharmacological properties common to other centrally acting drugs. Eur. J. Pharmacol. 471, 185–193. [DOI] [PubMed] [Google Scholar]
- Wouters E., Walraed J., Robertson M. J., Meyrath M., Szpakowska M., Chevigné A., Skiniotis G., Stove C. (2020). Assessment of biased agonism among distinct synthetic cannabinoid receptor agonist scaffolds. ACS Pharmacol. Transl. Sci. 3, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. Q., Zhang W. F., Li J., Wang J. F., Qin S. Y., Lu J. H. (2019). Analysis of AMB-FUBINACA biotransformation pathways in human liver microsome and zebrafish systems by liquid chromatography-high resolution mass spectrometry. Front. Chem. 7, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeter O., Erol Öztürk Y. (2019). Detection and quantification of 5F-ADB and its methyl ester hydrolysis metabolite in fatal intoxication cases by liquid chromatography-high resolution mass spectrometry. Forensic Sci. Int. 302, 109866. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. S., Winkelmann P. R., Pilhatsch M., Nees J. A., Spanagel R., Schulz K. (2009). Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Dtsch. Arztebl. Int. 106, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi A. W. (2006). History of cannabis as a medicine: A review. Rev. Bras Psiquiatr. 28, 153–157. [DOI] [PubMed] [Google Scholar]