Abstract
Root-adhering soil (RAS) forms the immediate environment where plants take up water and nutrients for their growth. We report the effect of an exopolysaccharide (EPS)-producing rhizobacterium (strain YAS34) on the physical properties of sunflower (Helianthus annuus L.) RAS, associated with plant growth promotion, under both water stress and normal water supply conditions. Strain YAS34 was isolated as a major EPS-producing bacterium from the rhizoplane of sunflowers grown in a French dystric cambisol. Strain YAS34 was assigned to the Rhizobium genus by 16S ribosomal DNA gene sequencing. Inoculation of sunflower seeds and soil with strain YAS34 caused a significant increase in RAS per root dry mass (dm) (up to 100%) and a significant increase in soil macropore volume (12 to 60 μm in diameter). The effect of inoculation on sunflower shoot dm (up to +50%) and root dm (up to +70%) was significant under both normal and water stress conditions. Inoculation with strain YAS34 modified soil structure around the root system, counteracting the negative effect of water deficit on growth. Using [15N]nitrate, we showed that inoculation made the use of fertilizer more effective by increasing nitrogen uptake by sunflower plantlets.
Soil structure has a strong impact on a range of processes influencing crop yield. The basic units of soil structure, named aggregates, comprise solid material and pores. These aggregates determine the mechanical and physical properties of soil such as retention and movement of water, aeration, and temperature (16). Aggregate formation is an important factor controlling germination and root growth (17).
Several studies have shown that formation of stable aggregates strongly depends on both the nature and the content of organic matter (10, 12, 14, 18, 29). Unstable aggregates generally have a lower content of organic matter than do stable ones (24). Plant roots contribute to soil organic material, and thereby to soil aggregate stability, directly through the root material itself (36) and indirectly through stimulation of microbial activity in the rhizosphere (4). It is generally believed that microbial action on soil aggregation is due to the production of exopolysaccharides (EPS) (25). This is supported by experimental observations demonstrating that the amendment of soil with microbial EPS results in an increased soil aggregation (14, 26).
The influence of microbes on aggregate stability has largely been studied in bulk soil (15, 25, 34). Relatively little attention has been paid to the influence of microorganisms, particularly EPS-producing rhizobacteria, on the aggregation of root-adhering soil (RAS) (3, 36). Understanding the effects of microorganisms on RAS aggregation is important because RAS forms the immediate environment where plants take up water and nutrients for their growth. Factors liable to change the physical properties of RAS can be expected to modify absorption of water and minerals by plants. In previous work, we found that inoculation of wheat with Paenibacillus polymyxa (selected for its nitrogen fixation activity in wheat rhizosphere) in a silty topsoil increased the RAS mass-to-root tissue (RT) mass ratio (RAS/RT ratio) by 57% (20). We recently demonstrated that the EPS (levan) produced by P. polymyxa is implicated in the aggregation of RAS on wheat (7). The same effect on wheat was observed after inoculation with Pantoea agglomerans, with additional evidence of the importance of bacterial activity in the regulation of water content of the rhizosphere by improved soil aggregation (3).
The purpose of this study was to determine the influence of inoculation with a rhizobacterium selected for its EPS production on soil aggregation of sunflower RAS, and its consequences for water and nitrogen uptake of sunflower plants, under both water stress and normal water supply conditions.
MATERIALS AND METHODS
Soil.
A silty sand clay soil (24.7% sand, 53.1% silt, and 18.3% clay) (dystric cambisol), with a field capacity of 18.2%, was collected from the upper layer (0 to 30 cm) at the CETIOM (Centre Technique Interprofessionnel des Oléagineux Métropolitains) experimental station (Saint-Florent-sur-Cher, France). The station had been under sunflower-wheat crop rotation for 10 years. Soil was sieved (<2-mm pore size) and stored at 4°C until used. Total carbon and nitrogen concentrations were 1.0 and 0.11%, respectively, total cation exchange capacity (0.5 M ammonium chloride) was 14.9 meq per 100 g of dry soil, and pH was 6.8 (1:1, soil/water ratio).
Bacterial strain.
Bacterial strain YAS34 was selected from the indigenous sunflower taproot microflora for its ability to produce large amounts of gel-forming EPS (2) on RCV-glucose medium (3). The sunflower plantlets were grown in the soil of Saint-Florent-sur-Cher for 4 weeks. Strain YAS34 was phenotypically identified using the Biolog GN MicroPlate system (Biolog Inc., Hayward, Calif.) based on bacterial ability to metabolize 95 carbon sources. The Biolog GN microplates were inoculated with strain YAS34 according to the recommendations of the manufacturer. The microplates were incubated for 4 and 24 h at 30°C and then read with a 590-nm filter (microplate reader, Dynatech MR 5000). The results were analyzed with Biolog GN database version 3.0 to assign strain YAS34.
Genotypic characterization of strain YAS34 was also carried out by sequencing the entire small-subunit (16S) ribosomal DNA (rDNA) gene as previously described (1). Strain YAS34 was grown in modified Luria-Bertani broth (1 g of tryptone, 0.5 g of yeast extract, 0.5 g of NaCl, and 4 g of glucose, all per liter). The 16S rDNA gene was amplified by PCR, with the primer pairs fD1 and rD1 (37), and sequenced (R. D. Anderson, D. T. Minnick, M. Veigl, and W. D. Sedwick, 1992, United States Biochemical Corp. publication).
Bacterial inoculation and growth conditions.
Strain YAS34 was grown overnight in 300 ml of modified Luria-Bertani broth at 30°C. The culture was harvested in late log phase (107 to 108 CFU ml−1), washed twice, and resuspended in 300 ml of sterile distilled water (SDW). Sunflower seeds (Helianthus annuus L., cv. Albena, Rustica Prograin Génétique, Mondonville, France) (75 to 85 mg per seed) were decorticated and surface sterilized after incubation (1 h) under vacuum in 2% (vol/vol) calcium hypochlorite solution. Seeds were rinsed three times with SDW, immersed in 3% (vol/vol) hydrogen peroxide solution for 30 min, and washed again three times with SDW. The sterilized seeds were inoculated with strain YAS34 by incubating them with 10 ml of bacterial suspension (107 CFU per seed) in a sterile plastic tube (15 ml). Noninoculated control seeds were incubated with 10 ml of SDW. The tubes were gently shaken on an orbital shaker for 2 h before the seeds were planted (one seed per pot) at a depth of 2 cm. In addition, 5 kg of nonsterile air-dried soil was inoculated by spraying it with 290 ml of the bacterial suspension (107 to 108 CFU ml−1) and then placed in plastic pots (6 cm in diameter by 19 cm in height). Each pot was filled with 250 g of soil from Saint-Florent-sur-Cher. A noninoculated control soil (5 kg) was sprayed with 290 ml of SDW. Twenty pots containing inoculated soil and inoculated seeds and 20 pots with uninoculated soil and uninoculated seeds were placed in a controlled-environment chamber under a 26°C/18°C day/night cycle and with a 16-h/8-h light/dark cycle (350-μmol m−2 s−1 light intensity).
Soil moisture was adjusted to 70% of water-holding capacity (WHC) (soil moisture, 13.3% of dry weight; matric potential, −0.20 MPa) and maintained constant during the experiment by daily sprinkling with SDW. Ten days after sowing, 10 of the 20 replicates were submitted to water stress by discontinuing watering. Fourteen days after planting (4 days of water stress), plantlets were harvested (experiment 1). To evaluate the consequences of inoculation for nitrogen uptake by the plant, an independent experiment (experiment 2) was performed using exactly the same protocol, except that each pot received 6.7 mg of N at sowing. Nitrogen was applied to soil as Ca (15NO3)2 (26.9 atom% 15N excess). After 4 days of water stress, soil moisture in the pots was about 9.5% of dry weight (50% of WHC; matric potential, −0.60 MPa) in experiment 1 and 7.5% of dry weight (40% of WHC; matric potential, −1.0 MPa) in experiment 2.
Harvesting and determination of RAS.
Ten plantlets per treatment were sampled. Plant watering was stopped 24 to 36 h before harvesting to facilitate the separation of RAS from bulk soil. Roots with adhering soil were carefully separated from bulk soil by gentle mechanical agitation (Agitest; Stuart Scientific) for 1 min. RAS was removed from RT by washing them in SDW. RAS dry mass (dm) and root dm were measured after 24 h at 105°C, and the RAS/RT ratio was calculated.
Estimation of strain YAS34 population in RAS and on roots.
Three replicates per treatment were randomly selected for the detection and enumeration of strain YAS34 in RAS and RT fractions. Serial dilutions (in 0.85% KCl) of root macerates and soil suspensions were performed, and aliquots of each dilution were plated on RCV-glucose medium. Bacteria were incubated at 30°C for 72 h, and mucoid colonies (similar to strain YAS34) were counted as CFU. To make sure that mucoid colonies originated from the strain YAS34 inoculum, about 10% of the isolates counted as similar to YAS34 at the last dilution were randomly selected and genotyped by a DNA fingerprinting method based on rep-PCR with enterobacterial repetitive intergenic consensus (ERIC) sequences as primers (35). The procedure of in vitro amplification of the DNA by PCR and conditions for electrophoresis are described in detail elsewhere (19). The gels containing ethidium bromide were photographed under UV illumination with Polaroid 665 film (instant camera system, Polaroid MP4+).
Pore size distribution.
Mercury intrusion porosimetry was performed to measure pore size distributions in RAS aggregates (5). RASs of three root systems per treatment were pooled and placed in an intrusion porosimeter (Carlo Erba, series 200), associated with the Macropore Unit Series 120 connected to an IBM PC computer, according to the previously described mercury intrusion porosimetry method (3). The mercury intrusion porosimetry method is based on the principle that mercury requires an overpressure (from 1.25 × 10−3 to 200 MPa) to enter a pore of air-dried millimetric soil aggregates, previously outgassed at room temperature for 2 h. The pore radii intruded range from 4 to 106 nm. A value for the surface tension of mercury of 0.48 N m−1 and a contact angle of 141° were used with the Washburn equation (5), assuming cylindrical pores in the calculation.
Nitrogen analyses.
Four pots per treatment were randomly selected after 14 days of plant growth. From each pot, four compartments were sampled: nonadhering soil, RAS, RT, and shoots. The samples were finely ground, and nitrogen analyses were carried out using the Kjeldahl-Olsen method. Isotopic analyses were performed by mass spectrometry (Micromass VG 622), using the Ross and Martin method as described by Guiraud (21).
Statistical analysis.
Analyses of variance were performed with Statgraphics software (version 5.0; STSC Software Products). The least significant difference (LSD) and Newman-Keuls tests were used for multiple-range analyses.
Nucleotide sequence accession number.
The strain YAS34 was deposited at the Collection Nationale de Culture Microbienne, Institut Pasteur, Paris, France, under no. I-1809. The accession number of the complete 16S rDNA sequence of strain YAS34 in the GenBank database is AF239242.
RESULTS
Isolation and identification of strain YAS34.
The EPS-producing strain YAS34 was isolated from the rhizoplane of sunflowers (about 5 × 106 CFU g of root dm−1) growing in a dystric cambisol (Saint-Florent-sur-Cher). This strain is gram negative, catalase positive, and oxidase negative. Using the Biolog GN MicroPlate system, strain YAS34 was identified as Pantoea agglomerans (gamma-Proteobacteria subdivision). However, molecular identification based on sequencing of the total 16S rDNA gene of strain YAS34 (accession no. AF239242) revealed that strain YAS34 belongs to the genus Rhizobium (alpha-Proteobacteria subdivision). Comparison of the complete 16S rDNA sequence with data bank sequences (GenBank and EMBL) showed that strain YAS34 had identity levels of 99.5% with Rhizobium sp. strain USDA 1920 (GenBank accession no. U89823), 98.9% with Rhizobium sp. strain OK50 (GenBank accession no. D14515), 98.0% with Rhizobium gallicum R602T (GenBank accession no. U86343), and 97.8% with Rhizobium mongolense USDA 1844T (GenBank accession no. U89817). On the basis of 16S rDNA analysis, it was concluded that strain YAS34 is unlike any of the previously described species of Rhizobium and so will be named in this work Rhizobium sp. strain YAS34 rejecting unambiguously the phenotypic identification provided by the Biolog system.
Rhizosphere and root colonization with the Rhizobium sp. strain YAS34.
Neither water limitation (data not shown) nor inoculation with strain YAS34 modified the total number of culturable bacteria (enumerated on nutrient agar or RCV-glucose medium) on RT and RAS fractions (Table 1). The number of EPS-producing bacteria (per gram of root dm) on roots was 10 times higher than that in RAS. Colonies of Rhizobium sp. strain YAS34 were identified using ERIC-PCR genomic fingerprinting of randomly selected mucoid colonies (phenotypically similar to Rhizobium sp. strain YAS34). The results showed that the ERIC-PCR patterns of 95% of mucoid YAS34-like colonies were identical to that of strain YAS34. Consequently, we conclude that 95% of mucoid colonies in both experiments (experiments 1 and 2) originated from the inoculated strain YAS34. Although the inoculation did not modify the total number of culturable bacteria, the percentage of Rhizobium sp. population (consisting of isolates with the same ERIC genotype as strain YAS34) in the inoculated treatments was four to eight times higher than that in uninoculated controls, making up to 54% of root-associated bacteria enumerated on RCV-glucose medium (Table 1).
TABLE 1.
Colonization of RAS and RT of sunflower by Rhizobium sp. strain YAS34 in two independent experiments
| Part colonized | Expt no.c | Total culturable bacteriaa(n = 6)
|
Rhizobium sp. (%)b(n = 3)
|
||
|---|---|---|---|---|---|
| Control | Inoculated | Control | Inoculated | ||
| RAS | 1 | 8.1 ± 0.3 | 7.9 ± 0.5 | 3 ± 1 | 23 ± 5 |
| 2 | 7.8 ± 0.3 | 7.6 ± 0.3 | 5 ± 1 | 38 ± 3 | |
| Roots | 1 | 9.7 ± 0.3 | 9.5 ± 0.5 | 8 ± 4 | 31 ± 7 |
| 2 | 8.3 ± 0.6 | 8.3 ± 0.5 | 8 ± 1 | 54 ± 5 | |
Counted on nutrient agar medium (in log CFU per gram dm); 6 out of 20 plantlets were analyzed (n = 6).
Isolates with the same ERIC-PCR profile as Rhizobium sp. strain YAS34 (in percentage of total culturable bacteria counted on RCV-glucose medium); 3 out of the 20 plantlets were analyzed (n = 3).
1 = 16-day-old plantlets, without nitrogen supply; 2 = 15-day-old plantlets, with nitrogen supply.
Effect of inoculation and water limitation on RAS mass.
The quantitative effect of these two factors on RAS was estimated by the RAS/RT ratio for each sunflower plantlet, where RAS was the dm of RAS and RT was the root dm. In both experiments, with (experiment 2) and without (experiment 1) nitrogen supply, the average RAS/RT ratio decreased significantly in water stress conditions, up to 50% in uninoculated samples and up to 40% in inoculated samples (Table 2). Under both water supply conditions, the mass of RAS of inoculated plantlets was significantly higher than that of the uninoculated control treatment (Table 2). In the experiment without additional nitrogen supply (experiment 1), inoculation of sunflowers with strain YAS34 increased the RAS/RT ratio by 70 and 104% under normal and water stress conditions, respectively (Table 2). In the experiment with nitrogen supply (experiment 2), the increase in the RAS/RT ratio due to inoculation was lower but still significant: +22% under normal water conditions and +52% under water limitation (Table 2).
TABLE 2.
Effects of soil water content and inoculation of sunflowers with Rhizobium sp. strain YAS34 on plant growth parameters and the production of RAS in two independent experimentsa
| Compartment | Expt no.b | Value for treatment groupc:
|
LSD | n | Significance of effectd:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| NI/NWS | Inoc/NWS | NI/WS | Inoc/WS | Inoculation | Water stress | Interaction | ||||
| Shoots | 1 | 41.6 a | 48.2 b | 40.2 a | 43.7 a | 4.0 | 9 | *** | * | NS |
| 2 | 75.1 a | 114.5 c | 81.1 a | 91.8 b | 8.2 | 12 | *** | ** | *** | |
| RT | 1 | 14.8 ab | 17.9 b | 11.5 a | 14.8 ab | 3.8 | 9 | * | * | NS |
| 2 | 51.0 a | 89.2 c | 59.2 ab | 65.2 b | 8.3 | 12 | *** | ** | *** | |
| RAS | 1 | 287 b | 565 c | 109 a | 281 b | 92 | 9 | *** | *** | NS |
| 2 | 714 b | 1,555 c | 488 a | 878 b | 189 | 7 | *** | *** | ** | |
| RAS/RT ratio | 1 | 19.4 b | 32.8 c | 9.5 a | 19.4 b | 5.0 | 9 | *** | *** | NS |
| 2 | 14.0 b | 17.1 c | 9.1 a | 13.8 b | 2.2 | 7 | *** | *** | NS | |
Abbreviations: NI, noninoculated; NWS, non-water stressed; Inoc, inoculated; WS, water stressed; NS, nonsignificant.
Experiment 1, 16-day-old plantlets without nitrogen supply; experiment 2, 15-day-old plantlets with nitrogen supply.
For shoots, RT, and RAS, values are shown as milligrams per plant; for RAS/RT ratio, values are milligrams to milligrams. Values followed by the same letter(s) indicate homogeneous groups (P < 0.05 [LSD]) after line-by-line variance analysis.
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
RAS porosity.
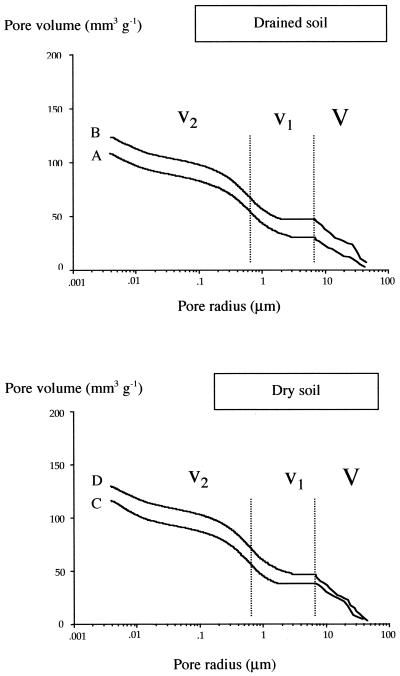
The curves fitted in Fig. 1 describe the influence of water stress and inoculation with strain YAS34 on the distribution of pore volume of sunflower RAS in experiment 1. The four cumulative curves were trimodal. Phase I corresponded to the mercury entrance in main pore volumes with a neck throat radius ranging from 6 to 40 μm (V, macropores). The second (v1) and third (v2) phases represented mercury entrance in pores with a radius ranging from 0.6 to 6 μm (v1, mesopores) and a radius of less than 0.6 μm (v2, micropores) (Fig. 1). The effects of water stress and of bacterial inoculation were mainly observed on volumes of macropores ranging from 6 to 30 μm in pore radius (V). For example, in the absence of inoculation, the water stress increased the macropore volume from about 30 mm3 g−1 in the control to 40 mm3 g−1 in water stress conditions (curve A versus curve C, Fig. 1). This increase in macropore volumes was responsible for the increase (110 versus 120 mm3 g−1) of the cumulative pore volume (V + v1 + v2). The main effect of inoculation with strain YAS34 on RAS porosity was observed in normal water conditions. This effect was an increase in cumulative pore volume (from 110 to 130 mm3 g−1), resulting from an increase in macropore volume (from 30 to 50 mm3 g−1) (Fig. 1). The mercury porosimetry curves of the inoculated treatment were identical in normal and water stress conditions (curves B and D).
FIG. 1.
Effect of inoculation with Rhizobium sp. strain YAS34 and soil hydric treatment on the cumulative mercury pore volume of RAS aggregates. (A) Noninoculated control treatment in drained soil; (B) inoculated treatment in drained soil; (C) noninoculated control treatment in dry soil; (D) inoculated treatment in dry soil.
Plant growth.
In experiment 1 (no nitrogen supply), there was an overall negative effect of water stress (for 4 days) on sunflower growth. However, inoculated plantlets, whatever the water conditions, showed a significantly greater shoot dm (+20%, P < 0.001) than did the uninoculated controls (Table 2). There was also a significant effect of inoculation on root dm (P < 0.05). In experiment 2 (in the presence of nitrogen supply), water stress caused a significant decrease in shoot and root dm's only in the inoculated treatments (Table 2). Inoculated-stressed plantlets had 20 and 27% lower root and shoot dm's, respectively, compared with inoculated nonstressed plantlets. However, as in the previous experiment (experiment 1), after 14 days of growth, inoculated sunflower plantlets showed a significant increase in shoot dm compared with uninoculated controls: +10 and +50% increase under water stress and normal water supply conditions, respectively (P < 0.001). This significant stimulatory effect was also detected for root dm (+70%) under normal water supply conditions (Table 2). The greater dry matter of inoculated plantlets might explain their sensitivity to water stress, compared with uninoculated plantlets.
Nitrogen uptake.
In noninoculated control plantlets, water stress had no significant effect on the total nitrogen content (QN) or the percentage of fertilizer N recovery (FNR) (Table 3). Four days of water stress significantly decreased the QN of inoculated sunflower plantlets (−14% in shoots). In spite of this reduction, the QN of shoots from inoculated plantlets subjected to water stress conditions was significantly higher than that of uninoculated controls grown under normal and water-stressed conditions (+12%) (Table 3). No significant difference was detected for the nitrogen content of roots in dry conditions between inoculated and uninoculated treatments (Table 3). However, in normal water supply conditions, the roots of inoculated plantlets showed a significant increase in nitrogen content (approximately +57%, P < 0.05). The same trend was also reflected in 15N uptake: 6.5% of the 15N-labeled nitrogen was recovered in sunflower plantlets of uninoculated controls compared with 9.0 and 15.4% in inoculated stressed plantlets and inoculated nonstressed plantlets, respectively (Table 3). This enhancement of 15N uptake by sunflower plantlets due to inoculation with Rhizobium sp. strain YAS34 was concomitant with a significant increase in 15N content in RAS from the inoculated nonstressed treatment (three times higher), compared with noninoculated controls (Table 3). No significant effect of inoculation on 15N content in RAS was detected under water stress conditions. In bulk soil, neither inoculation nor water stress had any effect on nitrogen content (data not shown).
TABLE 3.
Effects of soil water content and inoculation of sunflowers with Rhizobium sp. strain YAS34 on nitrogen uptake by the plant in experiment 2a
| Compartment | Value type | Value for treatment groupd:
|
LSD | n | Significance of effecte:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| NI/NWS | Inoc/NWS | NI/WS | Inoc/WS | Inoculation | Water stress | Interaction | ||||
| Shoots | QNb | 3.30 a | 4.43 c | 3.35 a | 3.80 b | 0.27 | 4 | *** | ** | ** |
| % FNRc | 6.68 a | 15.36 c | 6.46 a | 9.04 b | 2.15 | 4 | *** | *** | *** | |
| Roots | QN | 0.88 a | 1.38 b | 1.03 a | 1.10 a | 0.27 | 4 | ** | NS | * |
| % FNR | 2.09 a | 5.04 b | 2.38 a | 2.88 a | 1.17 | 4 | *** | * | ** | |
| RAS | QN | 1.05 ab | 1.98 c | 0.73 a | 1.20 b | 0.34 | 4 | *** | *** | NS |
| % FNR | 0.31 a | 0.94 b | 0.15 a | 0.33 a | 0.20 | 4 | *** | *** | ** | |
Abbreviations: NI, noninoculated; NWS, non-water stressed; Inoc, inoculated; WS, water stressed; NS, nonsignificant.
QN is in milligrams of N per plantlet.
FNR = (QNsample × Esample)/(QNfertilizer × Efertilizer) where QN is the total nitrogen in a sample or applied fertilizer and E is the isotopic excess of 15N (percent).
Values followed by the same letter(s) indicate homogeneous groups (P < 0.05 [LSD]) after line-by-line variance analysis.
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Colonization of sunflower rhizosphere by Rhizobium sp. strain YAS34.
Rhizobium sp. strain YAS34 was found naturally associated with sunflower roots at high frequency (5 × 106 CFU g of root dm−1), and we showed that inoculation could improve this association. Rhizobium sp. strain YAS34 was able to colonize the rhizosphere of sunflowers and to persist in it for at least 2 weeks at a high level (up to 30% of total culturable bacteria counted on RCV-glucose) (Table 1). Recent experiments have shown that rhizobia are also good colonizers of nonlegume roots such as rice (Y. G. Yanni, R. Y. Rizk, V. Corich, A. Squartini, and F. B. Dazzo, Proc. 15th Symbiotic Nitrogen Fixation Conf., p. 17, 1995 [abstract]) and canola, maize, and lettuce (9, 27). Interestingly, the Rhizobium sp. strain YAS34 population was still high after 4 days of water stress, in both rhizoplane and RAS fractions. This survival ability under water-limited conditions may be due, at least in part, to EPS production. The EPS layer may maintain a hydrated microenvironment around microorganisms during desiccation (13). Hartel and Alexandre (22) and later Roberson and Firestone (30) demonstrated that desiccation survival of Rhizobium sp. and Pseudomonas sp., respectively, required an increased EPS production.
Effect of inoculation on aggregation and porosity of RAS.
As expected, the increase in the EPS-producing strain YAS34 population in the sunflower rhizosphere after inoculation significantly increased the RAS/RT ratio, whatever the water conditions. Similar results were obtained for wheat plantlets inoculated with either P. polymyxa (20) or Pantoea agglomerans (3). This significant increase in RAS mass around the roots of sunflower plantlets inoculated with Rhizobium sp. strain YAS34 could be the result of either an increase in soil adhesion to roots or a higher soil aggregate stability around roots, or both. This aggregation effect of strain YAS34 may be due to EPS production. Purified xanthan and alginate (produced by Xanthomonas sp. and Azotobacter vinelandii, respectively) can improve aggregate formation (11). The polysaccharides are apparently adsorbed on soil particle surfaces and cement particles together (6, 13). On the other hand, it was shown previously that microbial biomass and polysaccharide production are increased in association with the stimulation of microbial populations in the rhizosphere of various plants (23).
The factors that favor root cap polysaccharide production may be expected to improve soil adhesion to roots and/or RAS aggregation. Hence, the effect of Rhizobium sp. strain YAS34 in sunflower rhizosphere on soil aggregation may also be partly indirect, through a stimulation of root exudation.
On plantlets subjected to water stress, whatever the inoculation treatment, the RAS/RT ratio values were lower than those of plantlets growing under normal water supply conditions. Of particular interest in this study is the finding that this RAS/RT ratio of stressed and inoculated plantlets is as great as that of noninoculated nonstressed plantlets (Table 2). This suggests that inoculation of sunflowers with strain YAS34 may limit the negative effect of dry conditions on RAS aggregation. This effect may also be related to the production of EPS by strain YAS34. Microbial EPS may both increase WHC of soil (31) and reduce water loss during desiccation (30).
Concomitantly with this bacterial effect on the RAS/RT ratio, a significant increase in soil macropore volume (corresponding to 12 to 60 μm in pore diameter) associated with an increase in total pore volume was observed at harvesting (day 14), whatever the water supply conditions (Fig. 1). Exactly the same increase in soil macropore volume due to the inoculation was detected at an intermediate stage (day 12 [data not shown]). According to Wu et al. (38), pores between aggregates of 120 to 600 μm (diameter) should be in the range of 12 to 60 μm (diameter). According to the model of Oades and Waters (28), roots and fungal hyphae contribute to the formation of macroaggregates (diameter >250 μm), whereas formation of meso- and microaggregates (diameter <250 μm) involves plant and microbial debris and bacteria. Our results suggest that bacteria, probably via their EPS production, also contribute to macroaggregate formation. Theoretically, new aggregates can be obtained from either breakdown of larger aggregates or accretion of mesoaggregates (33). Concerning the increase in the RAS/RT ratio, the second process seems to be a better explanation for the increased RAS macroporosity observed after inoculation with strain YAS34. By colonizing mesopores, the inoculated bacteria may bind these aggregates into larger ones through their EPS production, generating a new macroporosity (Fig. 1).
Plant growth and N assimilation.
Rhizobia are well known for their beneficial effect on plant growth resulting from symbiotic N2 fixation with legumes. There is also some evidence in the literature that some rhizobia are able to colonize nonlegume roots and promote their growth (32; Yanni et al., Proc. 15th Symbiotic Nitrogen Fixation Conf.). Chabot et al. (8) attributed promotion of maize and lettuce growth observed in the field after inoculation with Rhizobium leguminosarum bv. phaseoli to phosphate solubilization, whereas Noel et al. (27) suspected indoleacetic acid production for promotion of lettuce growth by R. leguminosarum, in gnotobiotic conditions. The stimulation of sunflower N uptake after inoculation with Rhizobium sp. strain YAS34 may be explained by the increase in RAS macroporosity (12 to 60 μm) (Fig. 1) and increased plant growth due to the production of plant growth hormones. According to Oades and Waters, in these pores the water transfer depends on plant root suction (28). A better water supply may also enhance plant growth, especially in water-limiting conditions, and so enhance the nitrogen uptake. Another hypothesis to be tested is that strain YAS34 can enhance the diffusion rate of nitrate through its EPS toward plant roots, as has been demonstrated previously for glucose in EPS-amended clay, compared with pure clay (13). Among the main results is the finding that stressed sunflower plantlets inoculated with strain YAS34 had as much shoot dry matter as the uninoculated nonstressed control. Strain YAS34 seems to modify soil structure around the root system in such a way that the water supply, plant growth, and N uptake of sunflowers are increased, counteracting the effect of water deficit on growth. Further work under “real” conditions is needed to check for the rhizosphere and root colonization of strain YAS34 and its effects on plant growth.
Conclusions.
We demonstrate that Rhizobium sp. strain YAS34, specifically selected for EPS production, acts as a plant growth-promoting rhizobacterium with a nonlegume, even in dry conditions. The main effects of sunflower inoculation with Rhizobium sp. strain YAS34 were the increase of RAS mass, macropore volume of RAS, and N uptake by the plant and finally plant growth. Strain YAS34 was also able to relieve the effect of water stress on sunflower growth, which is particularly important in Mediterranean areas, where crops are often subjected to lengthy dry periods. Most of these inoculation effects may be related to EPS production. To confirm the involvement of EPS in this process, further experiments will be performed with a knockout mutant of strain YAS34 deficient in EPS production.
ACKNOWLEDGMENTS
This work was partly supported by CETIOM (Y.A.), for which we thank A. Merrien.
We thank the CETIOM team of Saint-Florent-sur-Cher for soil sampling. We also thank G. Guiraud for supervision of 15N experiments and M. Ryder (CSIRO, Adelaide, Australia) for critically reading the manuscript.
REFERENCES
- 1.Achouak W, Christen R, Barakat M, Martel M-H, Heulin T. Burkholderia caribensis sp. nov., an exopolysaccharide-producing bacterium isolated from vertisol microaggregates in Martinique. Int J Syst Bacteriol. 1999;49:787–794. doi: 10.1099/00207713-49-2-787. [DOI] [PubMed] [Google Scholar]
- 2.Alami Y, Heulin T, Milas M, de Baynast R, Heyraud A, Villain A. Polysaccharide, microorganism and method for obtaining same, composition containing it and application. European patent 97-1624 970212. August 1998. [Google Scholar]
- 3.Amellal N, Burtin G, Bartoli F, Heulin T. Colonization of wheat roots by EPS-producing Pantoea agglomerans and its effect on rhizosphere soil aggregation. Appl Environ Microbiol. 1998;64:3740–3747. doi: 10.1128/aem.64.10.3740-3747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angers D A, Mehuys G R. Effects of cropping on carbohydrate content and water stable aggregation of a clay soil. Can J Soil Sci. 1989;69:373–380. [Google Scholar]
- 5.Bartoli F, Philippy R, Burtin G. Aggregation in soil with small amounts of swelling clay. I. Aggregate stability. J Soil Sci. 1991;39:593–616. [Google Scholar]
- 6.Ben-Hur M, Letey J. Effect of exopolysaccharides, clay dispersion, and impact energy on water infiltration. Soil Sci Soc Am J. 1989;53:233–238. [Google Scholar]
- 7.Bezzate S, Aymerich S, Chambert R, Czarnes S, Berge O, Heulin T. Disruption of the Paenibacillus polymyxa levansucrase gene impairs ability to aggregate soil in the wheat rhizosphere. Environ Microbiol. 2000;2(3):333–342. doi: 10.1046/j.1462-2920.2000.00114.x. [DOI] [PubMed] [Google Scholar]
- 8.Chabot R, Antoun H, Cescas M P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar phaseoli. Plant Soil. 1996;184:311–321. [Google Scholar]
- 9.Chabot R, Antoun H, Kloepper J W, Beauchamp C J. Root colonization of maize and lettuce by bioluminescent Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1996;62:2767–2772. doi: 10.1128/aem.62.8.2767-2772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaney K, Swift R S. The influence of organic matter on aggregate stability of some British soils. J Soil Sci. 1984;35:223–230. [Google Scholar]
- 11.Chaney K, Swift R S. Studies on aggregate stability. I. Reformation of soil aggregates. J Soil Sci. 1986;37:329–335. [Google Scholar]
- 12.Chenu C. Clay or sand polysaccharide associations as models for the interface between micro-organisms and soil: water-related properties and microstructure. Geoderma. 1993;56:143–156. [Google Scholar]
- 13.Chenu C, Roberson E B. Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol Biochem. 1996;28:877–884. [Google Scholar]
- 14.Cheshire K V. Nature and origin of carbohydrates in soils. New York, N.Y: Academic Press, Inc.; 1979. [Google Scholar]
- 15.Degens B P, Sparling G P, Abbott L K. The contribution from hyphae, roots and organic carbon constituent to aggregation of a sandy loam under long-term clover-based and grass pastures. Eur J Soil Sci. 1994;45:459–468. [Google Scholar]
- 16.Dickson E L, Rasiah V, Groenevelt P H. Comparison of four prewetting techniques in wet aggregate stability determination. Can J Soil Sci. 1990;71:67–72. [Google Scholar]
- 17.Dinel H, Levesque P E M, Jambu P, Righi D. Microbial activity and long-chain aliphatics in the formation of stable soil aggregates. Soil Sci Soc Am J. 1992;56:1455–1463. [Google Scholar]
- 18.Elustondo J, Angers D A, Laverdière M R, N'Dayegamiye A. Etude comparative de l'agrégation et de la matière organique associée aux fractions granulométriques de sept sols sous culture de maïs en prairie. Can J Soil Sci. 1990;70:395–402. [Google Scholar]
- 19.Frey P, Frey-Klett P, Garbaye J, Berge O, Heulin T. Metabolic and genotypic fingerprinting of fluorescent Pseudomonas associated with the Douglas fir-Laccaria bicolor mycorhizosphere. Appl Environ Microbiol. 1997;63:1852–1860. doi: 10.1128/aem.63.5.1852-1860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouzou L, Burtin G, Philippy R, Bartoli F, Heulin T. Effect of inoculation with Bacillus polymyxa on soil aggregation in the wheat rhizosphere: preliminary examination. Geoderma. 1993;56:479–490. [Google Scholar]
- 21.Guiraud G. Contribution du marquage isotopique à l'évaluation des transfert d'azote entre les compartiments organiques et minéraux dans les systèmes sol-plante. Ph.D. thesis. Paris, France: Université Pierre et Marie Curie; 1984. [Google Scholar]
- 22.Hartel P G, Alexandre M. Role of extracellular polysaccharide production and clays in the desiccation tolerance of cowpea Bradyrhizobia. Soil Sci Soc Am J. 1986;50:1193–1198. [Google Scholar]
- 23.Haynes R J, Francis G S. Changes in microbial biomass C, soil carbohydrate composition and aggregates stability induced by growth of selected crop and forage species under field conditions. J Soil Sci. 1993;44:665–675. [Google Scholar]
- 24.Haynes R J, Swift R S. Stability of soil aggregates in relation to organic constituents and soil water content. J Soil Sci. 1990;41:73–83. [Google Scholar]
- 25.Lynch J M, Bragg E. Microorganisms and soil aggregate stability. Adv Soil Sci. 1985;2:133–171. [Google Scholar]
- 26.Martens D A, Frankenberger W R T., Jr Soil saccharide extraction and detection. Plant Soil. 1993;149:145–147. [Google Scholar]
- 27.Noel T C, Sheng C, Yost C K, Pharis R P, Hynes M F. Rhizobium leguminosarum as plant growth-promoting rhizobacterium: direct growth promotion of canola and lettuce. Can J Microbiol. 1996;42:279–283. doi: 10.1139/m96-040. [DOI] [PubMed] [Google Scholar]
- 28.Oades J M, Waters A G. Aggregate hierarchy in soils. Aust J Soil Res. 1991;29:815–828. [Google Scholar]
- 29.Reid J B, Goss M J. Effect of living roots of different plant species on the aggregate stability of two arable soils. J Soil Sci. 1981;32:521–541. [Google Scholar]
- 30.Roberson E B, Firestone M. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992;58:1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanmunagathan R T, Oades J M. Effect of dispersible clay on the physical properties of the B horizon of red-brown earth. Aust J Soil Res. 1982;20:315–324. [Google Scholar]
- 32.Terouchi N, Syono K. Rhizobium attachment and curling in asparagus, rice and oat plants. Plant Cell Physiol. 1990;31:119–127. [Google Scholar]
- 33.Tespra R. Formation of new aggregates and weed seed behaviour in a coarse and in a fine-textured loam soil: a laboratory experiment. Soil Tillage Res. 1990;15:285–296. [Google Scholar]
- 34.Tisdall J M, Oades J M. The effects of crop rotation on aggregation in a red-brown earth. Aust J Soil Res. 1980;18:423–434. [Google Scholar]
- 35.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt M, McCully M E, Jeffree C E. Plant and bacterial mucilages of the maize rhizosphere: comparison of their soil binding properties and histochemistry in a model system. Plant Soil. 1993;151:151–165. [Google Scholar]
- 37.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Vomocil J A, Childs S W. Pore size, particle size, aggregate size, and water retention. Soil Sci Soc Am J. 1990;54:952–956. [Google Scholar]