Abstract
The Hippo pathway has emerged as a crucial integrator of signals in biological events from development to adulthood and in diseases. Although extensively studied in Drosophila and in cell cultures, major gaps of knowledge still remain on how this pathway functions in mammalian systems. The pathway consists of a growing number of components, including core kinases and adaptor proteins, which control the subcellular localization of the transcriptional co-activators Yap and Taz through phosphorylation of serines at key sites. When localized to the nucleus, Yap/Taz interact with TEAD transcription factors to induce transcriptional programs of proliferation, stemness, and growth. In the cytoplasm, Yap/Taz interact with multiple pathways to regulate a variety of cellular functions or are targeted for degradation. The Hippo pathway receives cues from diverse intracellular and extracellular inputs, including growth factor and integrin signaling, polarity complexes, and cell–cell junctions. This review highlights the mechanisms of regulation of Yap/Taz nucleocytoplasmic shuttling and their implications for epithelial cell behavior using the lung as an intriguing example of this paradigm.
This article is categorized under:
Gene Expression and Transcriptional Hierarchies > Regulatory Mechanisms
Signaling Pathways > Cell Fate Signaling
Establishment of Spatial and Temporal Patterns > Cytoplasmic Localization
Keywords: epithelial differentiation, Hippo signaling, lung development, nucleocytoplasmic shuttling, organogenesis, stemness, Taz, Yap
1 |. INTRODUCTION
Epithelial cells play a major role as an interface between the body and the external environment in functions as diverse as water and nutrient absorption, metabolism, gas exchange and by acting as a protective barrier. The establishment and maintenance of barrier function are of pivotal importance for organ integrity. For this, a multitude of signals must be continuously monitored and integrated so that the epithelium can respond appropriately to the dynamic changes or local perturbations in the cellular environment. The Hippo pathway has been increasingly recognized as a key integrator of these signals in biological processes. The Hippo cascade of kinases integrates information from environmental and cell-type specific signals, local extracellular matrix (ECM) components as well as mechanical forces to modulate the subcellular localization of Yap/Taz effector molecules and alter cell behavior. In this review, we will discuss how these mechanisms ultimately influence expansion, specification, and differentiation of epithelial cells in various contexts. We review information and gaps of knowledge about the function and regulation of Hippo-Yap/Taz gathered from a variety of biological systems and we discuss how these mechanisms ultimately influence epithelial cell behavior in various contexts, in particular the lung.
2 |. OVERVIEW OF THE HIPPO-YAP SIGNALING PATHWAY
The main core components of the Hippo pathway comprise two serine/threonine kinases, Mst1/2 and Lats1/2 (Drosophila Hippo [Hpo] and Warts [Wts], respectively), their adaptor proteins Mob1A/B (Drosophila Mob) and Sav1 (Drosophila Sal-vador [Sav]), and the transcriptional co-activators Yap (Drosophila Yorkie [Yki]) and Taz (Figure 1). When the kinases are inactive, Yap/Taz localize to the nucleus, where they canonically cooperate with TEA domain transcription factor family members (TEAD1–4, Drosophila Scalloped [Sd]) to activate transcriptional programs. Serine phosphorylation by Lats1/2 leads to Yap/Taz sequestration to the cytoplasm where Yap/Taz are degraded or retained to interact with cytoplasmic components of other pathways. The outcome depends on the particular residues phosphorylated. For example, LATS-mediated phosphorylation of Yap on S127 or TAZ on S89 promotes sequestration allowing cytoplasmic interactions. Indeed, mutations in these residues render Yap/Taz unable to undergo phosphorylation, resulting in nuclear accumulation and constitutively activated signaling. By contrast phosphorylation of Yap S381 or Taz S311 promotes Casein Kinase 1 (CK1) phosphorylation and recruitment of the SCFb-TRCP E3 ligase of the ubiquitination pathway, leading to Yap/Taz degradation (B. Zhao, Li, Tumaneng, Wang, & Guan, 2010). Importantly, while the majority of Yap/Taz post-translational modifications involve serine phosphorylation, there are notable exceptions (Varelas, 2014). For example, there is evidence that Yap/Taz can undergo tyrosine phosphorylation resulting in repression of their transcriptional activity or undergo lysine methylation by the lysine methyltransferase SETD7 to promote Yap cytoplasmic retention (Jang et al., 2012; Levy, Adamovich, Reuven, & Shaul, 2008; Oudhoff et al., 2013; Zaidi et al., 2004).
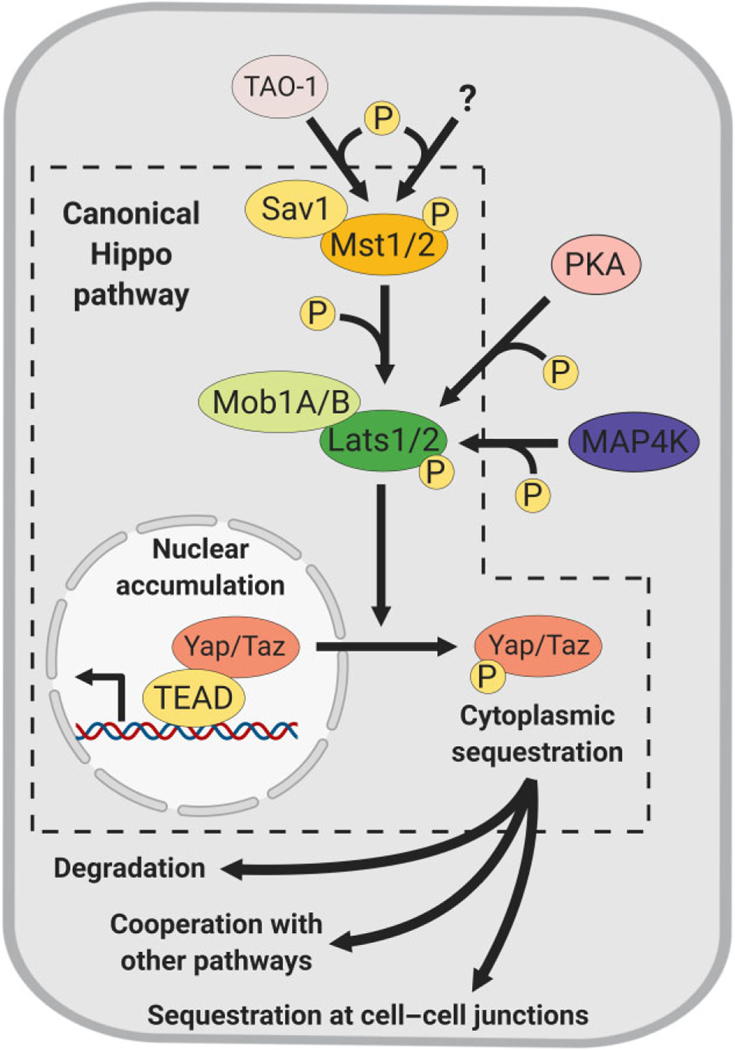
FIGURE 1.
Overview of the Hippo-Yap/Taz signaling pathway. Core components of the mammalian Hippo pathway and their regulation through phosphorylation events. (Left, canonical Hippo pathway): Hippo signaling inactive, nuclear Yap/Taz accumulation and activation of TEAD-mediated transcriptional programs. Yap/Taz phosphorylation by the Hippo kinases (ultimately by Lats1/2) leading to cytoplasmic sequestration for interactions with other pathways, degradation and other functions (bottom). Mst1/2 and Lats1/2 are also activated by non-canonical kinases Tao-1, PKA and MAP4K (top and right)
The activity of Hippo enzymes is also exquisitely modulated by a number of phosphorylation events. Lats1/2 are canonically phosphorylated by Mst1/2, and less frequently by other kinases, such as MAP4Ks and PKA (M. Kim et al., 2013; Meng et al., 2015; Pan et al., 2015). Notably, Mst1/2-Hpo activity is regulated by a remarkably conserved mechanism. For example, in Drosophila the polarity protein Par1 phosphorylates Hpo at S30, hampering its interaction with Sav1 to repress Hpo (Mst1/2) activity. Analysis of the Par1 human homologs MARK1 and MARK4 in HEK293T cells shows similar inhibitory function on Mst1/2 activity (H. L. Huang et al., 2013). Mst1/2 and Lats1/2 activities are further enhanced by their adaptor proteins, Sav1 and Mob1A/B, respectively. Sav1 inhibits the PP2A phosphatase complex STRIPAK (striatininteracting phosphatase and kinase) from dephosphorylating Mst1/2 (Bae et al., 2017), while Mob1A/B acts as a scaffold to potentiate the phosphorylation of Lats1/2 by Mst1/2 (Ni, Zheng, Hara, Pan, & Luo, 2015). Inactivation of any of these components results in Hippo loss of function, Yap/Taz nuclear accumulation and activation of their transcriptional targets.
2.1 |. Dynamics of Yap/Taz subcellular shuttling
Despite the accumulated evidence that nucleocytoplasmic shuttling is a critical determinant of Yap/Taz function, its operating mechanisms are still little understood. While Yap/Taz subcellular localization is commonly perceived as being static and dependent on cytosolic retention factors, such as the 14–3-3 protein (Kanai et al., 2000; Ren, Zhang, & Jiang, 2010; B. Zhao, Xiong, et al., 2008), recently a more dynamic model is gaining traction. Here Yap/Taz molecules continuously translocate between nucleus and cytoplasm, and sequestration to a particular subcellular compartment is dependent on changes in nuclear import or export rates (Figure 2) (Ege et al., 2018; Manning et al., 2018). Whether one or the other is the determinant event, is still contentious.
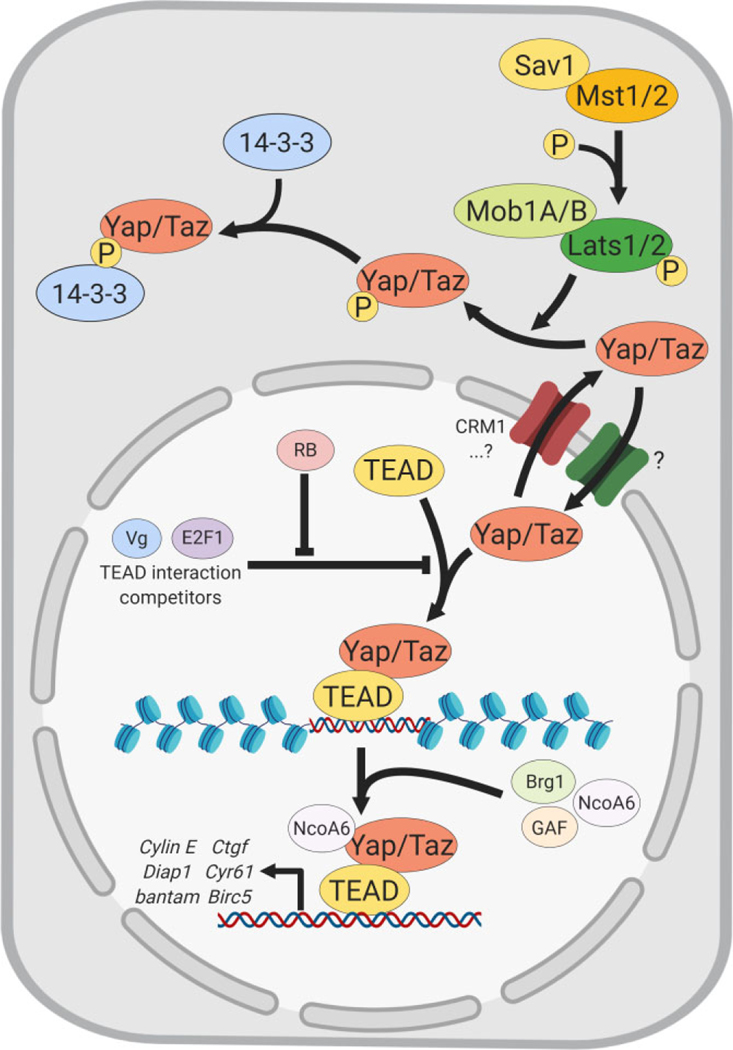
FIGURE 2.
Nuclear Yap/Taz, target gene induction and nuclear import/export balance. Model proposed for continuous Yap/Taz nucleocytoplasmic shuttling, nuclear accumulation or cytoplasmic sequestration regulated by nuclear import/export rates. TEAD can regulate Yap/Taz nuclear export rate by mechanisms such as modulation of the nuclear pore channel CRM-1 or by physically occluding a nuclear export signal (NES) at the TEAD-Yap/Taz interface (not represented). Yap/Taz-TEAD interactions can be blocked by competitors (Vg, E2F1), which bind to TEAD instead of Yap/Taz. RB (retinoblastoma) relieves such competition. TEAD-Yap/Taz recruit chromatin remodelers (Brg, GAF, NCo6A) and induce downstream gene targets
A study in Drosophila suggested that the nuclear import rate of Yki (Yap), regulated through Warts (Lats)-mediated phosphorylation, is of pivotal importance for shuttling (Manning et al., 2018). However, another study in the mammalian fibroblast cell lines NF1 and CAF1 showed no significant difference in Yap nuclear import rate. Rather, shuttling appeared to depend ultimately on the Yap nuclear export rate, as determined by fluorescence recovery after photobleaching (FRAP) analysis of nucleocytoplasmic diffusion rates (Ege et al., 2018). NF1 and CAF1 exhibited preferential cytoplasmic or nuclear Yap, respectively. Interestingly, the study showed that the differential Yap subcellular localization stemmed from differential stability of Yap-TEAD interactions in these cells. In CAF1 cells TEAD-binding to Yap increased its nuclear retention and reduced the fraction of free nuclear Yap available for export, thus ultimately influencing Yap nuclear export but not import.
There is also evidence that Yap/Taz are actively exported to the cytoplasm through the nuclear channel CRM-1 (XPO-1; Figure 2). CRM-1 knockdown or Leptomycin B-induced blockade causes Yap/Taz nuclear accumulation (Ege et al., 2018; Kofler et al., 2018; Ren et al., 2010). Intriguingly, Taz nuclear import was found to depend on an atypical C-terminus nuclear localization signal (NLS), and to be regulated through RhoA activation in a phosphorylation-independent fashion (Kofler et al., 2018). Deletion of this C-terminal NLS inhibits Taz nuclear accumulation. Once in the nucleus, Taz-TEAD interactions suppress nuclear efflux through physical occlusion of a CRM-1-dependent Taz nuclear export signal (NES) by TEAD (Kofler et al., 2018). This NES resides in the Taz-TEAD interaction domain. In Drosophila Yki nuclear export has been shown to also depend on other factors. For example, Yki can interact directly with the Wnt-related protein Dishevelled (Dvl), which has a NES (Lee et al., 2018).
Together, these studies provide valuable insights into the dynamics of Yap/Taz subcellular shuttling, demonstrating that shuttling is continuous but tightly regulated.
3 |. TRANSCRIPTIONAL ROLES OF YAP/TAZ
The Hippo pathway was first described from a genetic screen in Drosophila to identify regulators of cell growth. Mutations in Hippo, Warts, Mob, or Sav resulted in nuclear accumulation of Yki and concomitant organ overgrowth. Thus, nuclear Yki was suspected to label cells undergoing active proliferation. Since then, a substantial body of work has confirmed these findings in vivo and in vitro, elucidating binding partners and transcriptional targets. However, broader functions of nuclear Yap have been increasingly recognized, as will be discussed in this section.
3.1 |. Mechanisms of Yap-mediated gene transcription
Since Yap does not have a DNA-binding site, it has to interact with partner transcription factors to induce gene expression (Yagi, Chen, Shigesada, Murakami, & Ito, 1999). Several context-specific partner transcription factors have been identified, concomitant with unique gene expression profiles (Oh et al., 2013; C. Zhu, Li, & Zhao, 2014). Thus, Yap-induced gene expression is directed by the factor it binds to and the cellular context in which this binding occurs. For example, in the mammalian lung epithelium Yap interacts with the transcription factor p63 to induce a basal cell transcription program, while in breast cancer Yap interacts with Smads to induce and maintain tumorigenic phenotypes (Hiemer, Szymaniak, & Varelas, 2014; R. Zhao et al., 2014). However, TEADs are widely recognized as the most common Yap interaction partners in the contexts of development, cancer, and epithelial–mesenchymal transition (Hiemer et al., 2014; Ivanek et al., 2014; J. Y. Kim et al., 2016; Mahoney, Mori, Szymaniak, Varelas, & Cardoso, 2014; Miesfeld et al., 2015; Stein et al., 2015; Y. Yang, Wu, et al., 2015).
TEADs are potent Yap binding partners interacting through an α-helix and a Ω-loop in Yap. Point mutations in these regions abrogate Yap-TEAD interactions and inhibit Yap-induced gene expression (Goulev et al., 2008; Mesrouze et al., 2017; Oh et al., 2013; Vassilev, Kaneko, Shu, Zhao, & Depamphilis, 2001; Wu, Liu, Zheng, Dong, & Pan, 2008; L. Zhang et al., 2008; B. Zhao, Ye, et al., 2008). ChIP-seq studies on anti-TEAD siRNA-treated samples show that TEAD knockdown obliterates Yap ChIP-seq peaks (Stein et al., 2015). Notably, Yap-TEAD (or Yki-Sd) binding and ultimately function can be modulated by other transcriptional co-activators (Figure 2). For example, in Drosophila, Yki-Sd interactions and target gene expression can be repressed by binding of Sd to Vestigial (Vg) or E2F1 (Koontz et al., 2013; P. Zhang, Pei, et al., 2017). To relieve this repression, the retinoblastoma family protein RB interacts with E2F1 to allow Yki-Sd interaction (Figure 2). The Yki-Sd complex then binds to DNA to induce gene expression directly. Such mechanisms appear to be conserved, since similar interactions have also been identified in the human MCF7 cell line (Y. Zhang, Jiang, et al., 2017).
Commonly assayed Yap/Taz target genes including Cyclin-E, Diap1, and bantam in Drosophila (J. Huang, Wu, Barrera, Matthews, & Pan, 2005; Thompson & Cohen, 2006), or Birc5, Cyr61, and Ctgf in mice (Rosenbluh et al., 2012; H. Zhang, Pasolli, & Fuchs, 2011; B. Zhao, Ye, et al., 2008) induce cell proliferation and survival (Figure 2). A number of studies identify nuclear Yap as a driver of cell proliferation, survival and organ growth. However, Yap target genes are not restricted to these categories, being also found to be quite diverse and context-dependent, as demonstrated by the Yap regulation of Ajuba, Ankrd1, Areg, and SGK1 (Dupont et al., 2011; Lange et al., 2015; Yoo, Kim, Chung, Hwang, & Lim, 2017; J. Zhang et al., 2009). For example, while Sox9 is upregulated by Yap in hepatocytes, there is no evidence that in the developing lung epithelium Yap gain or loss of function has any obvious impact in Sox9 expression (Lin et al., 2017; Mahoney et al., 2014; van Soldt et al., 2019; Yimlamai et al., 2014). Thus, a central question revolves around the ability of Yap to induce gene expression in a context-dependent manner. There is evidence that this context-dependency is due to differentially available binding partners for Yap. For example, Yap associates with TEAD in intestinal stem cells to drive proliferation, but cooperates with Klf4 in differentiating intestinal epithelium to drive a goblet cell differentiation program (Imajo, Ebisuya, & Nishida, 2015). In addition, the chromatin landscape may drive differential Yap target gene expression (Figure 2).
Significant differences in the Yap-DNA binding landscape can be noted between transformed and nontransformed mammalian cells (Stein et al., 2015). There is also evidence of species-specific preferential Yap-DNA binding, as studies in mammalian cells revealed that Yap binds at active distal enhancers, enriched in H3K27 acetylation marks, while in Drosophila Yap was found at active promoters (H3K4me3+; Eissenberg & Shilatifard, 2010) (Creyghton et al., 2010; Stein et al., 2015). Intriguingly, regardless of the species, Yki/Yap associates with the chromatin remodeling factors GAGA factor (GAF) and Brahma (BRM, mammalian Brg1). In Drosophila, Yki was also found to recruit NcoA6, a subunit of the Trithorax methyltransferase (Oh et al., 2013, 2014; Y. Zhu et al., 2015). Thus, a potential model posits that Yki/Yap is recruited to DNA through sequence-specific binding partners, and then recruits chromatin remodeling factors to induce transcription. Additional studies are required to elucidate the precise mechanisms by which Yap-induced gene expression is regulated.
3.2 |. Roles in the maintenance of stemness and progenitor status
A significant body of work has been devoted to the understanding of the role of Yap in progenitor, stem, and induced pluripotent stem (iPS) cells. Initially described to play a role in cell fate decisions of mesenchymal stem cells, Yap and Taz were later reported in intestinal stem cells, human embryonic stem (ES) cells, iPS reprogramming, trophoblast/ inner cell mass formation, and in basal cells of the lung and skin, among other contexts (Camargo et al., 2007; J. H. Hong et al., 2005; Karpowicz, Perez, & Perrimon, 2010; Kohlmaier et al., 2010; Lian et al., 2010; Nishioka et al., 2009; Staley & Irvine, 2010; Varelas et al., 2008; Volckaert et al., 2011; H. Zhang et al., 2011; R. Zhao et al., 2014). Here, Yap is often associated with its role in induction of proliferation and survival genes, or the activation of pathways, such as Wnt, which ultimately leads to these responses through feedback loop interactions. Indeed, the role of Yap in maintenance of stemness is not necessarily a cell-autonomous process, and may entail the maintenance of a niche (Barry et al., 2013; Camargo et al., 2007; Hou et al., 2019; Konsavage & Yochum, 2013; Veltri, Lang, & Lien, 2018; Volckaert et al., 2017). Below we illustrate this paradigm in basal cells.
Basal cells are multipotent progenitors of a variety of adult tissues, including the skin, esophagus, lung, and prostate. They are characterized largely by expression of p63 and cytokeratins Krt5 and Krt14, as well as a basal position in the epithelium (Aaron, Franco, & Hayward, 2016; S. Liu, Zhang, & Duan, 2013; Rock, Randell, & Hogan, 2010; Y. Zhang, Jiang, et al., 2017). Yap was found to expand basal cells when overexpressed or to decrease their number upon Yap inhibition. In the skin this effect is at least in part mediated through direct Yap-TEAD induction of Cyr61 and Ctgf. In airways the Yap-mediated expansion of basal cells and pseudostratification results from Yap-p63 interactions (Elbediwy, Vincent-Mistiaen, Spencer-Dene, et al., 2016; Koster, Kim, Mills, DeMayo, & Roop, 2004; Lange et al., 2015; Romano, Ortt, Birkaya, Smalley, & Sinha, 2009; Schlegelmilch et al., 2011; H. Zhang et al., 2011; R. Zhao et al., 2014). Interestingly, there is evidence that Yap acts in a non-cell-autonomous fashion to maintain the integrity of the basal cell layer during homeostasis and injury repair. This is achieved through a Yap-Wnt-Fgf10 feedback loop established between the airway basal cells and the adjacent mesenchyme, which constitutes a stromal niche. In this model Hippo signaling is downregulated in basal cells following injury, leading to nuclear Yap accumulation and induction of Wnt7b expression. This likely involves Yap-Nkx2.1 interactions, as Nkx2.1 is known to regulate Wnt7b in the lung epithelium and at least one of the Hippo effectors (Taz) is reported to directly bind to Nkx2.1. Wnt7b diffuses to the mesenchyme where it induces Fgf10 expression. Secreted Fgf10 then activates epithelial Fgfr2 signaling, expanding basal cells. Fgfr2 signaling is crucial here since targeted deletion of Fgfr2 ablates basal cells (Balasooriya, Goschorska, Piddini, & Rawlins, 2017; Hou et al., 2019; K. S. Park et al., 2004; Volckaert et al., 2011, 2017; Volckaert & De Langhe, 2015; Weidenfeld, Shu, Zhang, Millar, & Morrisey, 2002).
Whether a similar Hippo-Wnt-Fgf feedback loop plays a role in the maintenance of basal cells in other tissues is unclear. For example, in the interfollicular epidermis (IFE) activation of Wnt signaling is known to promote basal cell expansion. In addition, Fgf7 (also known as KGF) secreted by the dermal mesenchyme activates epithelial Fgfr2, eliciting IFE basal cell proliferation (Guo, Yu, & Fuchs, 1993; Koh et al., 2013; Choi et al., 2013). However, there is no evidence of Yap-Wnt or Wnt-Fgf7 crosstalk in this system in spite of the availability of these components.
3.3 |. Exceptions to the rule: nuclear Yap in cell specification and differentiation
While nuclear Yap has been typically associated with maintenance of stemness, there is also evidence of transcriptional roles for Yap in differentiation. This is exemplified by the role of Yap in the developing retinal pigment epithelium (RPE), a cell sheet associated with the neural retina (NR) (J. Y. Kim et al., 2016; Miesfeld et al., 2015). An involvement of Hippo-Yap in ocular development came from reports of an association between Sveinsson’s chorioretinal atrophy (SCRA) in humans and a TEAD1 missense mutation that abolishes TEAD-Yap/Taz interactions (Bokhovchuk et al., 2019; Kitagawa, 2007). Indeed, studies in zebrafish showed that Yap interacts with TEAD1 in RPE progenitors to specify RPE cell fate (Miesfeld et al., 2015; Miesfeld & Link, 2014). Loss of Yap, or its ability to interact with TEAD1, was shown to result in RPE loss due to disruption of cell fate rather than defective cell proliferation or survival. By contrast, a report in Yap-deficient mice showed defects in proliferation and survival as well as evidence of progenitor cell trans-differentiation into NR. As shown by the appearance of NR markers, such as Chx10 and b-tubulin III, (J. Y. Kim et al., 2016). It is unclear whether this disparity represents species-specific differences in ocular development RPE.
An additional example of a requirement of nuclear Yap in differentiation has been reported in the developing lung. Early during branching morphogenesis, when the lung epithelium is patterned into distal Sox9+ and proximal Sox2+ (airway) compartments, Yap is preferentially localized to the nucleus of distal bud tips. Lineage studies show that tip cells function as progenitors for the cell types of both the distal and proximal epithelial compartments (Rawlins, Clark, Xue, & Hogan, 2009; Y. Yang et al., 2018). Disruption of Yap in the lung epithelium of ShhCre; Yapf/f mice show that, although not required for specification of the distal progenitors, Yap is crucial to form the Sox2+ epithelial compartment. Indeed, analysis of Yap-deficient lungs in vivo reveals a markedly truncated Sox2+ domain (airways) associated with large cyst-like dilated Sox9+ distal buds (Lin et al., 2017; Mahoney et al., 2014; van Soldt et al., 2019). Interestingly, there is evidence suggesting that the Yap effects in Sox2 specification and airway morphogenesis are not fully interdependent. Embryonic lung explants from ShhCre; Yapf/f mice in culture show the characteristic shortened Sox2+ domain but not the aberrant cysts and severe distal morphogenetic abnormalities seen in vivo (Mahoney et al., 2014).
At later developmental stages, when distal alveolar saccules start to form in the lung, Yap becomes localized to the nucleus of distal epithelial progenitors. Notably, nuclear Yap remains in the population that differentiates selectively into alveolar type 1 (AT1) cells, the flat and large cells that cover the vast surface of the gas-exchange region of the lung postnatally. By contrast, Yap becomes largely cytoplasmic in the distal progenitors differentiating into the surfactant-producing alveolar type 2 (AT2) cells. Intriguingly, preventing Yap nucleocytoplasmic shuttling by epithelial deletion of Mst1/2 or Lats1/2, or by expression of a mutant Yap unable to properly undergo phosphorylation (e.g., YapS127A or Yap5SA), induces an ectopic AT1 program of distal differentiation in airways and disrupts the balance of AT1 versus AT2 cells in the distal saccules (Lange et al., 2015; Lin, Yao, & Chuang, 2015; Nantie et al., 2018; van Soldt et al., 2019). Altogether, these studies reveal that Yap is crucial for proper induction of context-specific transcriptional programs that control not only proliferation and stemness, but also differentiation.
4 |. HIPPO IN CELL FATE COMMITMENT AND MATURATION:CYTOPLASMIC SEQUESTRATION OF YAP/TAZ
Studies in a variety of biological systems show that stem and progenitor cells typically maintain nuclear Yap, but as cells commit to a fate and differentiate, Yap is sequestered to the cytoplasm through activation of Hippo kinases. A significant contribution of Hippo-activating signals arises from cell–cell and cell–matrix contacts. Below we summarize these modes of Hippo activation and propose an integrated model to explain how they cooperate. In doing so we also discuss how Hippo activation and cytoplasmic Yap sequestration can lead to cell fate commitment and differentiation in vivo.
4.1 |. Cues from cell–cell contacts: contact inhibition and establishment of apical-basal polarity
As cells proliferate, their crowding will result in the establishment of cell–cell contacts, which have long been recognized to inhibit cell proliferation (Abercrombie, 1979). Termed contact inhibition, this event is intimately linked with activation of Hippo kinases and Yap cytoplasmic sequestration, thus inhibiting Yap transcriptional activity (B. Zhao et al., 2007).
In the epithelium, cell–cell contact is largely established through adherens junctions (AJs) and tight junctions (TJs): multiprotein complexes located in the subapical domain of the lateral cell membrane (Campbell, Maiers, & DeMali, 2017) (Figure 3). The primary components of epithelial AJs are E-cadherin, β-catenin, and α-catenin (Aberle et al., 1994). TJs form after establishment of AJs. They are composed of claudin, occludin, and ZO-1, allowing intercellular exchange of water, ions, and macromolecules, as well as serving to restrict intermixing of apical and basolateral lipids (Campbell et al., 2017). Cell-cell contacts are critical for the establishment of apical-basal polarity, cell fate and differentiation. In addition, the atypical cadheri Fat and Dachsous localized just apically of the AJ, form heterophilic bonds and establish planar cell polarity (Thomas & Strutt, 2012).
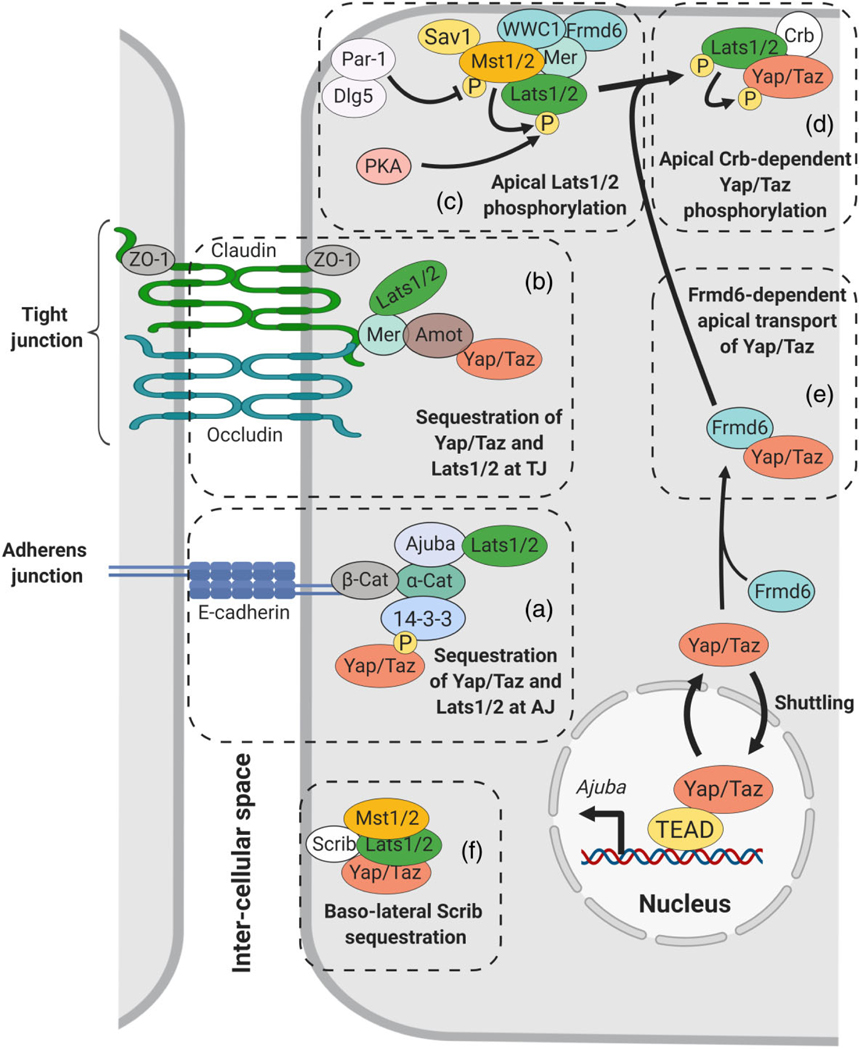
FIGURE 3.
Yap/Taz cytoplasmic sequestration by cell–cell junctions and polarity determinants. (a,b) Cell–cell-contacts establish adherens junction (AJ) and tight junctions (TJ). At the AJ α-catenin (α-Cat) bound to β-catenin (β-Cat)-E-cadherin functions as a scaffold for interactions of Ajuba with Lats1/2 and 14–3–3 with phospho-Yap/Taz, leading to local cytoplasmic sequestration. Merlin (Mer) sequestered to TJ (Claudin/Occludin/ZO1) functions as a scaffold for Lats1/2, Amot-Yap/Taz and Par3 (not represented). (c–e) At the apical domain Merlin-WWC1-Frmd6 form an apical scaffold for Merlin recruitment of Mst1/2 and Lats1/2 and their subsequent phosphorylation. Par-1 inhibits Mst1/2 activity by both destabilizing Sav1 and phosphorylating Mst1/2 at Serine 30 residues. Dlg5 reinforces these Par-1 effects. Lats1/2 may also be phosphorylated by PKA. Also at the apical domain, phosphorylated Lats1/2 recruited by Crumbs3 (Crb3) promotes efficient phosphorylation of Yap/Taz transported apically in part by direct interaction with Frmd6. (f) At the basal-lateral domain, Scribble sequesters Mst1/2, Lats1/2, and Yap/Taz preventing their phosphorylation or nuclear shuttling
Junctional and polarity complexes have all been linked with cell density-dependent regulation of Hippo-Yap signaling (Benham-Pyle, Pruitt, & Nelson, 2015; Delanoë-Ayari, AL Kurdi, Vallade, Gulino-debrac, & Riveline, 2004; Gottardi, Wong, & Gumbiner, 2001; N.-G. Kim, Koh, Chen, & Gumbiner, 2011; Perrais, Chen, Perez-Moreno, & Gumbiner, 2007; Sarpal, Yan, Kazakova, Sheppard, & Tepass, 2019; Schlegelmilch et al., 2011; Silvis et al., 2011; Szymaniak, Mahoney, Cardoso, & Varelas, 2015; Varelas et al., 2010; C.-C. Yang, Graves, et al., 2015; Zhou et al., 2018). E-cadherin and β-catenin are crucial for contact inhibition such that their depletion stimulates cell proliferation. Conversely, E-cadherin can also mediate mechanical tension-induced Yap-dependent cell cycle entry, which is likely to be reinforced by the clustering of adhesion proteins upon mechanical tension. Claudin-18 knockout in the alveolar epithelium of the lung increases Yap nuclear accumulation and proliferation. However, their effects are mediated through interacting proteins, rather than the junctional proteins themselves. Thus, the effects of AJ components can be mediated through α-catenin (Figure 3a,b), which interacts directly with 14–3-3-bound phospho-Yap, restricting it to the cytoplasm. α-Catenin also interacts with Ajuba LIM protein (Drosophila Jub), which, through Ajuba-Lats1/2 interaction, inhibits Lats1/2 activity and causes Yap nuclear localization in an actin cytoskeletal tension-dependent fashion. Notably, Ajuba is a target of Yap in bronchiolar epithelial cells, thus establishing a positive feedback loop that reinforces Yap nuclear localization (Das Thakur et al., 2010; Hirata, Samsonov, & Sokabe, 2017; Lange et al., 2015; Rauskolb, Sun, Sun, Pan, & Irvine, 2014; Sarpal et al., 2019; Schlegelmilch et al., 2011; Silvis et al., 2011; B. Yang, Sheetz, et al., 2015).
Another interacting partner of α-catenin and a critical Hippo-Yap regulator is Merlin (Mer, also known as Nf2) (Figure 3c), a tumor suppressor related to the ezrin, radixin, moesin (ERM) family of proteins encoded by the neurofi-bromatosis type 2 gene NF2 with prominent roles in stabilization of junctional complexes and mediation of contact inhibition that appear conserved between Drosophila and mammals (Cooper & Giancotti, 2014; Gladden, Hebert, Schneeberger, & McClatchey, 2010; Lallemand, Curto, Saotome, Giovannini, & McClatchey, 2003; Morrison et al., 2001; Okada, Lopez-Lago, & Giancotti, 2005; Slocum et al., 2007; Yin et al., 2013; N. Zhang et al., 2010). Merlin deficiency results in destabilization of AJs and tissue overgrowth (Lallemand et al., 2003). Merlin forms an apical scaffold in cooperation with FERM-domain protein 6 (Frmd6, Drosophila Expanded, Ex) and WWC1 (Drosophila Kibra) to bind and recruit Lats1/2 to the apical domain where Merlin promotes Lats1/2 phosphorylation by Mst1/2-Sav1 (Genevet, Wehr, Brain, Thompson, & Tapon, 2010; M. Kim et al., 2013; Yin et al., 2013; J. Yu et al., 2010). At TJs Merlin interacts with Amot, a member of the angiomotion family of proteins (Amot, Amotl1, Amotl2, and Amotp130) and a major regulator of Hippo-Yap signaling (Figure 3b). In complex with Merlin, Amot induces a conformational change that promotes Merlin-Lats1/2 binding. However, Amot can also physically interact with Yap to sequester it to TJs. Crucially, Merlin and Amot interact with polarity proteins. For example, interactions of Amot with Pals1 and Patj, proteins of the apical Crumbs polarity complex, stabilize the TJ, promoting cell adhesion (Duquesne et al., 2015; Gladden et al., 2010; Y. Li et al., 2015; Varelas et al., 2010; Wang, Huang, & Chen, 2011; Wells et al., 2006; Yi et al., 2011; B. Zhao et al., 2011).
Polarity proteins themselves are major regulators of Hippo-Yap signaling. Three major polarity protein groups/complexes exist: (a) apically localized Crumbs polarity complex (Crumbs [Crb], Pals1, and Patj), (b) the apically localized Par protein complex, which comprises the scaffold proteins Par3 and Par6, and the atypical kinase C (aPKC), and (c) laterally localized Scribble (Scrib), Discs-Large (Dlg), and Lethal Giant Larvae (Lgl) (Assémat, Bazellières, Pallesi-Pocachard, Le Bivic, & Massey-Harroche, 2008; Martin-Belmonte & Mostov, 2008; Rodriguez-Boulan & Macara, 2014). Hippo regulation by these proteins is remarkably conserved and relevant in multiple biological processes. In Drosophila imaginal disc epithelial cells, Yki is recruited to Crb by Expanded, which binds Yki directly and translocates it to the cytoplasm, sequestering it at Crb, among other subcellular domains (Badouel et al., 2009; Ling et al., 2010). Studies in mice show that Crb3 interacts directly with Lats1/2 and acts as a scaffold for the efficient phosphorylation of Yap (Figure 3d). Indeed, Crb3 deletion in lung progenitors results in nuclear Yap localization and causes massive epithelial expansion in the airway epithelium, disrupting the pseudostratified epithelial architecture (Szymaniak et al., 2015; Varelas et al., 2010).
Scribble participates in the inhibition of Yap transcriptional activity in the zebrafish pronephros and was revealed to promote the formation of an inhibitory Taz-Lats1/2-Mst1/2 complex in breast cancer cells (Figure 3f). Consequently, loss of Scribble destabilizes this complex, allowing Taz nuclear accumulation (Cordenonsi et al., 2011; Skouloudaki et al., 2009). In contrast to Crb and Scribble, Par1 (mammalian MARK), a kinase from the Par protein family, activates Yap through destabilization of Sav1 and inactivation of Mst1/2 by phosphorylation at S30, inducing Yap target gene expression (H. L. Huang et al., 2013; Kwan et al., 2016; Rodriguez-Boulan & Macara, 2014). Unexpectedly, the Par1-Sav1 interaction may be promoted by both Scribble and Scribble family member Dlg5, since Par1 knockdown in the engineered intestinal epithelial cell line W4 leads to Scribble mislocalization, suggesting Par1-Scribble interactions, while Dlg5 acts as a scaffold for Par1-Sav1 (Kwan et al., 2016; Mohseni et al., 2014).
Lastly, the atypical cadherins Fat4 (Drosophila Fat) and Dsch1 (Drosophila Dachsous) interact with AJ and TJ and are regulators of planar cell polarity (PCP), reviewed in Thomas and Strutt (2012), and Hippo signaling (Cappello et al., 2013; Ragni et al., 2017; Cho et al., 2006; Cho & Irvine, 2004; Das et al., 2013; Ma et al., 2016; Mao et al., 2011; Silva, Tsatskis, Gardano, Tapon, & McNeill, 2006; Tyler & Baker, 2007; Willecke et al., 2006). Fat4 and Dsch1 localize to opposite sides of the lateral cell membranes, enabling cell-to-cell heterotypic interactions to control transcriptional programs of growth. Studies in Drosophila found that Fat/Dachsous and Warts regulate common gene targets (e.g., diap1 and CyclinE), suggesting an epistatic link between these pathways. In mammals, Fat4 or Dsch1 mutation caused Yap-dependent overgrowth phenotypes, which may be mediated by Amotl1. While Fat4/Dsch1 and their Hippo-activating effect are common to Drosophila and mammals, downstream signaling mediators are not, as Drosophila lacks Amot, while mammals lack Dachs (Chen et al., 2013). In Drosophila, Dachs mediates Fat/Dachsous signaling, since Dachs deletion suppresses tissue overgrowth phenotypes in Fat loss-of-function mutants (Blair & McNeill, 2018; Mao et al., 2006). Dachs regulates Warts activity both directly and indirectly. Direct interaction with Dachs renders Warts inactive through the reversion of a Mats-induced conformational change. Dachs inhibits Warts activity indirectly by sequestering Warts to the subapical domain in complex with Expanded. Deletion of Fat abolishes this apical sequestration (Bennett & Harvey, 2006; Cho et al., 2006; Oh, Reddy, & Irvine, 2009; Vrabioiu & Struhl, 2015; Willecke et al., 2006).
4.2 |. Cues from cell–matrix contacts: integrins, focal adhesion, the actin cytoskeleton and mechanical tension
The basement membrane provides an anchoring point for the epithelium as well as an interface for crosstalk between the epithelium and the stroma. Focal adhesion and integrin proteins are key components of this interface. Integrin signaling is a major regulator of Hippo activity, controlling cell behavior in a context and cell type-specific fashion. Generally viewed as conduits for mechanotransduction, integrins are heterodimeric transmembrane proteins that consist of α and β subunits (Sun, Guo, & Fässler, 2016) and mediate adherence to the basement membrane, linking the ECM to the actin cytoskeleton (Figure 4). The actin cytoskeleton itself is a major determinant of Yap subcellular localization, though the precise effect appears to be dependent on cell density.
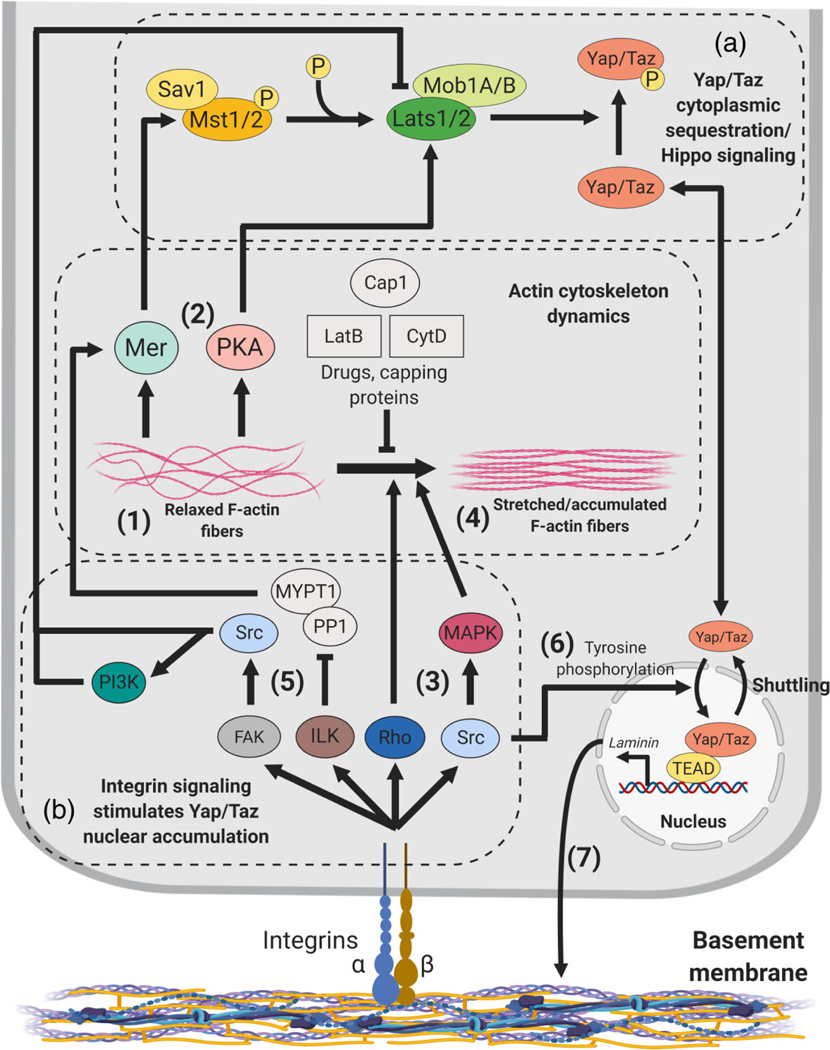
FIGURE 4.
Regulation Yap/Taz subcellular localization by Cell-ECM signaling and the actin cytoskeleton. (a) Yap/Taz phosphorylation/cytoplasmic sequestration is favored in the context of a relaxed Actin fiber cytoskeleton (1), which stimulates Mer or PKA activity, promoting Mst1/2 and Lats1/2 activity, respectively (2). (b) Integrin-mediated Yap/Taz nuclear accumulation. Actin-dependent regulation of Yap/Taz subcellular localization: mechanotransduction through Rho or Src-MAPK activity (3), leading to stretching or accumulation of actin fibers (4). Actin-independent mechanisms: Integrin-ILK or integrin-FAK-induced Src blocking Mer activity (5). Src tyrosine phosphorylation of Yap also induces Yap transcriptional activity (6). Yap/Taz induction of laminin expression and basement membrane deposition (7)
Given the physical linkage between integrins and the actin cytoskeleton, it is unsurprising that integrin signaling modulates Yap subcellular localization predominantly by altering cytoskeletal tension (Figure 4). Elegant experiments have demonstrated that matrix stiffness determines Yap localization such that cells plated on stiff substrate accumulate Yap in the nucleus, whereas cells on soft substrate sequester Yap in the cytoplasm (Aragona et al., 2013; Das, Fischer, Pan, & Waterman, 2016; Dupont et al., 2011; Janody et al., 2011; Sansores-Garcia et al., 2011; Shih, Tseng, Lai, Lin, & Lee, 2011; Wada, Itoga, Okano, Yonemura, & Sasaki, 2011). Increased ECM stiffness can induce malignant phenotypes in MCF10A mammary epithelial cells (Chaudhuri et al., 2014). The effect of matrix stiffness on Yap subcellular localization was shown to be dependent on cell spreading and suggested to be modulated by cytoskeletal tension (B. Yang, Sheetz, et al., 2015).
There is evidence suggesting that Rho GTPase and myosin motors regulate Yap subcellular localization (Das et al., 2016). Cytoskeletal tension is controlled by Rho GTPases through bundling and accumulation of actin filaments. The activity of myosin motors produces tension in the contractile actomyosin (composed of F-actin and non-muscle myosin II) by pulling on the actin fibers that connect cellular structures. Actomyosin tension is critical in the establishment of AJs, so that myosin motors indirectly contribute to Yap cytoplasmic sequestration through AJ-mediated contact inhibition of growth (Miyake et al., 2006). Multiple studies implicate F-actin-Rho GTPases in the mechanism by which mechanical tension controls Yap subcellular localization. Dissolution of F-actin polymers by cytochalasin D or Latrunculin A or B treatment, or inhibition of Rho GTPase by C3 treatment, lead to sequestration of Yap in the cytoplasm and inhibition of malignant phenotypes (Chaudhuri et al., 2014; Das et al., 2016; Dupont et al., 2011; Ege et al., 2018; Elbediwy, Vincent-Mistiaen, Spencer-Dene, et al., 2016; Sansores-Garcia et al., 2011). In addition, actin-capping enzymes, which regulate F-actin polymerization in vivo, were found to control Yap/Taz/Yki nuclear accumulation in both Drosophila and mammalian cells. Loss of Capulet (mammalian Cap1) or Capping Protein αβ heterodimer caused F-actin accumulation, Hippo kinase deactivation and Yki target gene expression (Aragona et al., 2013; Janody et al., 2011; Sansores-Garcia et al., 2011). Alternatively, disruption of the actin cytoskeleton releases F-actin-bound Merlin promoting Lats1/2 activity (James, Manchanda, Gonzalez-Agosti, Hartwig, & Ramesh, 2001; Yin et al., 2013). Downstream of actin reorganization, cyclic AMP-dependent protein kinase (PKA) has been shown to transduce the mechanical signal to the Hippo pathway by phosphorylating Lats1/2 (M. Kim et al., 2013). Interestingly, F-actin-dependent mechanisms can control Yap subcellular localization independent of Hippo kinases. For example, studies in mesenchymal stem cells and human microvascular endothelial cells show that disruption of Rho and the actin cytoskeleton inhibits YAP/TAZ transcriptional activity. However, LATS1/2 inactivation has only a marginal effect on the YAP/TAZ inactivation mediated by changes in mechanical cues (Dupont et al., 2011).
A Hippo-independent mechanism that has recently gained traction involves “nuclear flattening.” Studies in mammalian cells (epithelial and mesenchymal) growing in nonconfluent conditions or on soft substrates have identified actin cytoskeletal fibers running from basally located focal adhesions and integrins to the nucleus, and connected with the nuclear envelope. Contractile forces generated by actomyosin activity can press the nucleus down and flatten it, opening up nuclear pores that allow Yap import to the nucleus (Driscoll, Cosgrove, Heo, Shurden, & Mauck, 2015; Elosegui-Artola et al., 2017; Shiu, Aires, Lin, & Vogel, 2018). However, it is unclear why Yap nuclear export is not similarly affected. Another example of tension-based Hippo-independent control of Yap shuttling was described in mammalian cell lines, such as HaCaT and MDCK (Furukawa, Yamashita, Sakurai, & Ohno, 2017; Hirata et al., 2017). Here, upon confluence and establishment of sufficient tension on the circumferential bundle of actomyosin fibers that surrounds the cell and connects AJs, Merlin is released from the AJs. Free Merlin shuttles between the nucleus and cytoplasm due to its nuclear localization and export sequences (NLS, NES). In the nucleus Merlin interacts with Yap, subsequently shuttling Yap into the cytoplasm. Indeed, mutation of all three of Merlin NES’s abrogates Merlin shuttling and allows Yap nuclear accumulation. The mechanism that mediates Merlin release from AJs is unknown and potentially involves α-catenin conformational changes (Dobrokhotov, Samsonov, Sokabe, & Hirata, 2018). It is well-established that α-catenin is subjected to forces generated from actomyosin fibers so that increasing actomyosin tension could alter α-catenin conformation (Yao et al., 2014; Yonemura, Wada, Watanabe, Nagafuchi, & Shibata, 2010). Since Merlin directly interacts with α-catenin, it is possible that α-catenin conformational changes allow Merlin release.
While mechanical tension regulates Yap subcellular localization, there is evidence that Yap itself regulates mechanical tension. One study described a Yap-regulated mechanism that controls cell alignment required for 3D body shape acquisition. Here, Yap induces Arhgap18, a Rho activator, leading to actin accumulation, increased actomyosin tension and Fibronectin accumulation (Porazinski et al., 2015). Another example comes from lung development, where Yap induces MLCK-mediated cytoskeletal tension in epithelial cells of the distal bud tips to ensure normal branching morphogenesis through the induction of Ahrgef17, a Rho inhibitor (Lin et al., 2017). Thus Yap induces both Rho activators and inhibitors, a striking paradox that might depend on context. How context might determine the induction of either Rho activator or inhibitor is as yet unresolved.
Integrins also regulate Hippo activity through mechanisms unrelated to mechanical tension (Figure 4b). In the colon cancer cell line HCT116 integrin linked kinase (ILK) inhibits Hippo signaling by inactivating Merlin through inhibition of the myosin light-chain phosphatase MYPT1-PP1 (Serrano, McDonald, Lock, Muller, & Dedhar, 2013). Integrin-Hippo interactions may also involve signaling through Src (Drosophila dcsk) (Ege et al., 2018; Elbediwy, Vincent-Mistiaen, Spencer-Dene, et al., 2016; Enomoto & Igaki, 2012; N.-G. Kim & Gumbiner, 2015; P. Li et al., 2016; McLachlan, Kraemer, Helwani, Kovacs, & Yap, 2007; Si et al., 2017). In MCF10A cells, focal adhesion kinase (FAK)-activated Src promotes Yap transcriptional activity through PI3K-PDK1 signaling, but also through tyrosine phosphorylation and inactivation of Lats1/2. Src tyrosine phosphorylation was also found to activate Yap in mouse epidermal epithelial cells. In the developing Drosophila imaginal disc epithelium, Src inhibits the Hippo pathway through JNK signaling, preventing tumorigenic overgrowth. In addition to activating Src, FAK itself may also promote MAPK-mediated F-actin accumulation through PKA (Ege et al., 2018; Howe & Juliano, 2000; Miyazu, Sokabe, Naruse, Matsushita, & Wang, 2002). Lastly, there is evidence of an Integrin-Hippo positive feedback loop in transformed MCF10A cells. In this line integrin interactions with Laminin-511 causes Taz nuclear accumulation and induction of Laminin-511 gene expression. This results in increased deposition of Laminin-511 available to interact with integrin, perpetuating the cycle (Chang et al., 2015; Pouliot & Kusuma, 2013).
Hippo regulation by cell shape and mechanical tension has been demonstrated in vivo in the alveolar epithelium of the lung. The flat and thin alveolar type 1 (AT1) cell occupies a remarkably large surface area of the lung (Herriges & Morrisey, 2014). Yap accumulates in the nucleus of these cells, in stark contrast to the cytoplasmic Yap localization in the cuboidal alveolar type 2 (AT2) cells (Z. Liu et al., 2016;Nantie et al., 2018; van Soldt et al., 2019). Mechanical tension plays a significant role in perinatal differentiation of these cell types (J. Li et al., 2018). In mouse embryos, it has been proposed that late in gestation amniotic fluid enters the lung, triggered by intrauterine fetal breathing movements, expanding the distal saccules and stretching the epithelial cells. Prospective AT2 cells escape stretching by extruding from the epithelium. Although Hippo-Yap has not been causally implicated in these morphogenetic changes, recent evidence demonstrates nuclear accumulation occurring selectively in epithelial cells undergoing flattening (van Soldt et al., 2019). Furthermore, Yap knockout prevents AT1 cells from forming, while expression of a constitutively nuclear Yap increases their number (Nantie et al., 2018; van Soldt et al., 2019).
How components of the integrin-Src signaling influence Yap activity is less clear in Drosophila. In flies, Src functions in an integrin-independent manner to inactivate Yap by directly phosphorylating Wts (Lats) possibly downstream of Dachs, a component of Fat/Dachsous signaling (Kwon et al., 2015; Si et al., 2017; Stewart, Li, Huang, & Xu, 2003). This mechanism has not been described in mammals. In addition, recent studies in the developing lung delineate an ILK-mediated mechanism that induces cytoplasmic sequestration of Yap (Volckaert et al., 2017, 2019). Here, ILK promotes epithelial-mesenchymal crosstalks to alter the signaling environment, leading to the cytoplasmic sequestration of Yap and induction of fate in airway epithelial cells.
4.3 |. Integration of junctional, polarity and basal cues
While the effects of mechanical contacts and associated signaling pathways appear at first disparate, an integrative mechanism emerges from these observations. Stimuli from the basal lamina have been typically associated with promotion of nuclear Yap localization, while establishment of cell–cell contacts, apical–basal or planar polarity override these signals, inducing cytoplasmic sequestration (Elbediwy, Vincent-Mistiaen, Spencer-Dene, et al., 2016; Elbediwy, Vincent-Mistiaen, & Thompson, 2016). The F-actin cytoskeleton, coupled to both basal and intracellular junctions, functions as an intermediary sensor of cell crowding through the interpretation of cytoskeletal tension as cell stretch. For example, a sudden reduction in cell number increases cytoskeletal tension, inducing nuclear Yap accumulation and proliferation, to restore the number of cells in the tissue. The model is consistent with observations about how Yap is expressed in specific cell types: (a) basal cells retain nuclear Yap due to integrin-mediated contact with the basal lamina; (b) parabasal and luminal cell types in the stratified epithelia of skin or esophagus acquire cytoplasmic Yap localization due to loss of contact with the basement membrane, acquiring concomitantly extensive cell–cell contacts. This releases Hippo kinases inhibition, while promoting their activation through AJ and TJ-mediated contact inhibition; and (c) luminal (columnar) cells in simple or pseudostratified epithelia sequester Yap to the cytoplasm due to establishment of polarity and Crb-mediated Hippo activation.
5 |. COOPERATION BETWEEN HIPPO-YAP/TAZ SIGNALING AND INTRACELLULAR AND EXTRACELLULAR SIGNALING PATHWAYS IN EPITHELIAL DIFFERENTIATION
Significant Hippo-Yap/Taz regulatory input comes from interactions with various ligand-receptor signaling cascades. Nearly all major pathways have been found to interact with Hippo-Yap/Taz/Yki signaling to regulate biological functions. A selected number of these pathways will be subsequently discussed, highlighting their impact on Yap sub-cellular localization and function.
5.1 |. Wnt signaling
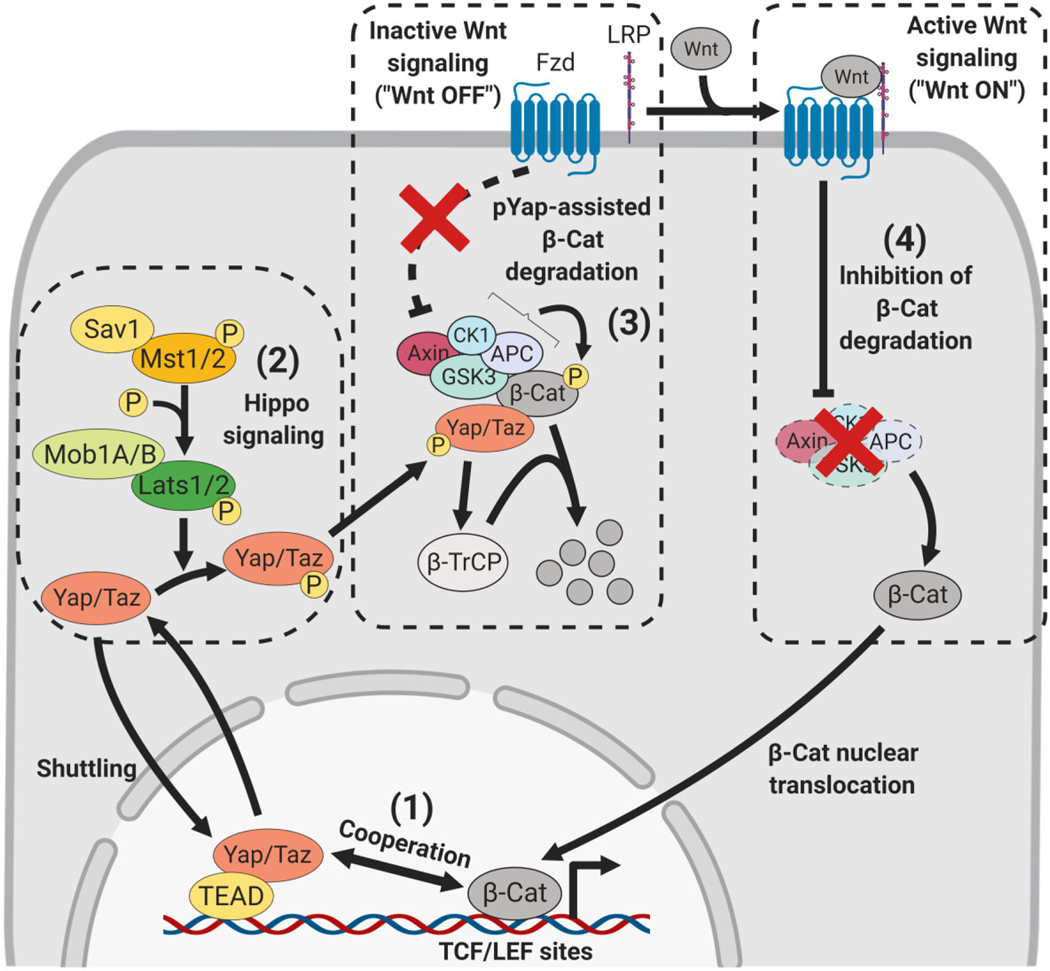
The Wnt signaling pathway, named after Drosophila Wingless and mammalian homolog Int-1, is omnipresent in embryonic development and plays major roles in adult homeostasis and disease. When unstimulated (“Wnt OFF”), the canonical Wnt transcriptional effector β-catenin is degraded by the proteasome after phosphorylation by the β-catenin destruction complex and ubiquitination by β-TrCP (Axin2, APC, CK1, and GSK3; Figure 5). When stimulated through Wnt binding to the Frizzled/LRP co-receptor complex (“Wnt ON”), the β-catenin destruction complex is inhibited, stabilizing β-catenin, which then localizes to the nucleus to induce target gene expression (Clevers, 2006).
FIGURE 5.
Hippo-Yap crosstalk with Wnt signaling. Hippo-Wnt crosstalk occurs at multiple levels. Yap/Taz and β-catenin (β-Cat) cooperate in the nucleus to induce Wnt target genes (1). Hippo kinase activity phosphorylates Yap/Taz (2). When Wnt signaling is inactive (“Wnt OFF” (3)), phospho-Yap/Taz induce β-catenin degradation by interacting with β-catenin directly and recruiting β-TrCP (3), leading to β-catenin ubiquitination and degradation. When Wnt signaling is active (“Wnt ON” (4)), the β-catenin degradation complex is inhibited, allowing β-catenin nuclear translocation and induction of canonical Wnt targets
Wnt and Hippo-Yap/Taz interact at multiple levels (Figure 5). Nonphosphorylated Yap can potentiate canonical Wnt targets through cooperation with β-catenin in the nucleus of 293T cells (Heallen et al., 2011). Studies in 293T cells and in the developing Xenopus embryo in vivo show that phosphorylated Yap inhibits β-catenin activity directly by sequestering β-catenin to the cytoplasm (Imajo, Miyatake, Iimura, Miyamoto, & Nishida, 2012). Phospho-Yap was also shown to augment β-catenin degradation through interaction with the β-catenin destruction complex in cultured 293 cells, as well as in intestinal epithelial cells in vivo (Azzolin et al., 2012, 2014). Here, β-catenin directly interacts with Yap/Taz, retaining them in the destruction complex. Then, Yap/Taz recruit β-TrCP to the destruction complex and augments β-catenin degradation. Intriguingly, in 293 cells the association of Yap with the destruction complex and β-catenin appears to depend on Yap methylation at K494 by SETD7, since mutation of this residue abolishes Yapβ-catenin interaction (Oudhoff et al., 2016). Upon Wnt signaling or Axin1/2 or APC knockdown Yap/Taz are dislodged from the β-catenin destruction complex, and potentiate Wnt signaling by blocking β-TrCP recruitment, promoting β-catenin nuclear translocation (Azzolin et al., 2012, 2014). Viewed from a different perspective, Wnt signaling promotes Yap/Taz target gene induction by inducing Yap/Taz release from the β-catenin destruction complex. This notion is further supported by evidence describing Wnt-regulated Lats1/2 activity through a Rho GTPase-dependent mechanism in in vitro adipogenesis of 3T3-L1 cell lines (H. W. Park et al., 2015), while during osteogenic differentiation canonical Wnt signaling was shown to dephosphorylate Taz through PP1A activation (Byun et al., 2014). In colorectal cancer, β-catenin was shown to directly induce Yap expression, suggesting a positive feedback loop that reinforces Yap and β-catenin target gene induction (Konsavage, Kyler, Rennoll, Jin, & Yochum, 2012). Finally, the adaptor protein Mob1A/B was found to regulate Wnt target gene expression in a Yap-dependent manner in the intestinal epithelium, such that Mob1A/B depletion resulted in the ablation of the intestinal stem cell pool, although a molecular mechanism was not described (Bae et al., 2018). Together these studies demonstrate extensive Hippo-Wnt crosstalk during both active and inactive Wnt signaling. Active Wnt signaling promotes proliferation through Yap and β-catenin-mediated gene induction, while Wnt signaling inactivation promotes quiescence and differentiation by sequestering Yap and β-catenin to the cytoplasm.
5.2 |. Tgfβ signaling
The transforming growth factor beta (Tgfβ) superfamily of signaling pathways encompasses Tgfβ, Bmp and activin signaling pathways. The core components of the Tgfβ subfamily consist of the ligands Tgfβ1–3, and the serine–threonine kinase receptors Tgfβr1 (also known as ALK5) and Tgfβr2 (Figure 6a). Activation of this pathway has been intricately linked with the development of a number of organs, as well as the pathogenesis of fibrotic diseases and cancer. Ligandreceptor binding causes Tgfβ receptor heterodimerization and induction of Tgfβr1 phosphorylation by Tgfβr2. Tgfβr1 subsequently recruits and phosphorylates Smad2/3, allowing for formation of a heterocomplex of Smad2/3 with co-Smad Smad4. The complex then translocates to the nucleus to induce Tgfβ-mediated transcriptional programs alone or in cooperation with other factors (F. Huang & Chen, 2012).
FIGURE 6.
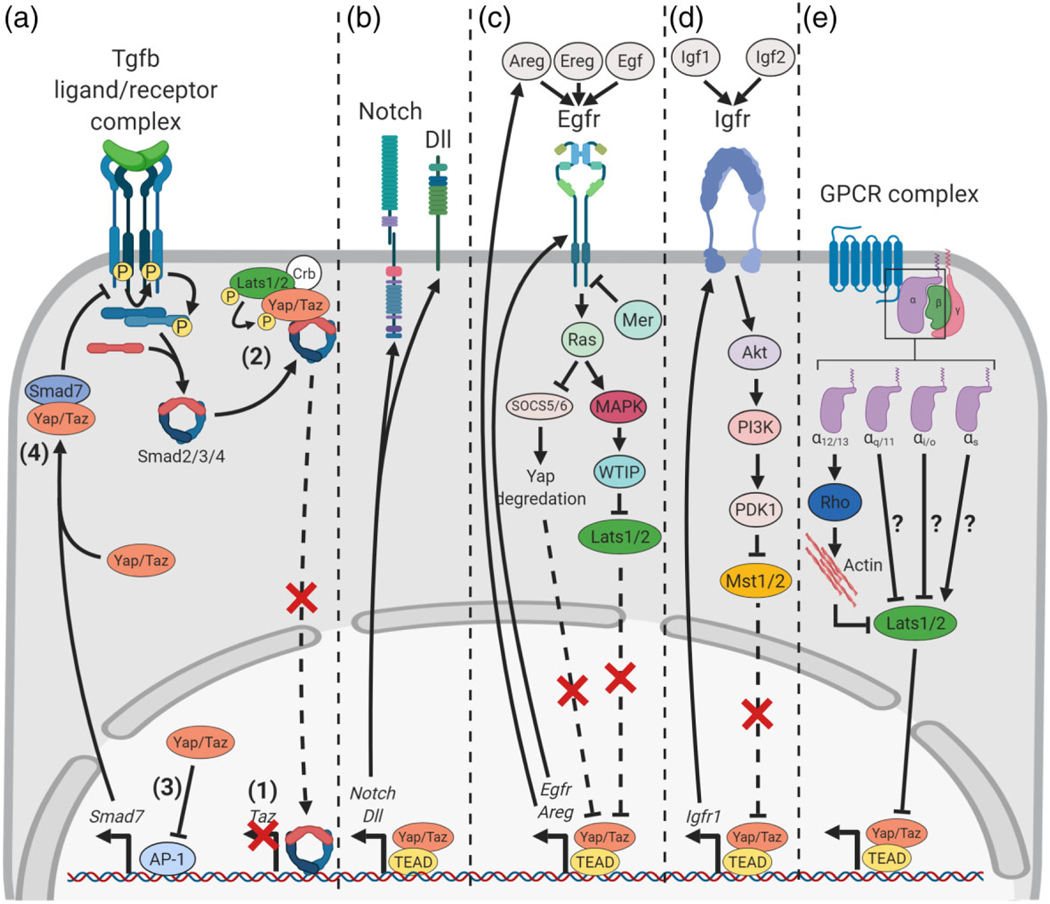
Hippo-Yap/Taz crosstalks with Tgfβ, Notch, Egf, Igf, GPRCs. (a) Tgfβ signaling: (1) Tgfβ-Smad2/3/4 signaling induces Taz. (2) Crumbs-dependent Yap/Taz phosphorylation, subsequent binding to Smad2/3/4 and sequestration to cytoplasm prevents Tgfβ target gene expression. (3) Nuclear Yap inhibits AP-1-induced Smad7 induction. (4) Yap-Smad7 interactions inhibits Tgfβ signaling. (b) Notch signaling: induction of Notch receptor and ligand expression by nuclear Yap/Taz. (c) Egf signaling: Egfr binding to Areg, Ereg or Egf induces a signaling cascade that inhibits both the degradation of Yap/Taz and the activity of Lats1/2, which promotes nuclear Yap/Taz accumulation and transcriptional activity. Yap/Taz targets include Egfr and Areg, establishing a positive feedback loop. (d) Igf signaling: Igf-Igfr activation inhibits Mst1/2 activity through an Akt-PI3K-PDK1 signaling cascade resulting in Yap/Taz nuclear accumulation and induction of Igfr1 expression. This further inhibits Yap signaling through a positive feedback loop. (e) GPCR signaling employs an intracellular trimeric Gprotein complex for downstream signaling. Four different α subunits participate in this complex, of which three promote nuclear Yap accumulation and one (Gαs) inhibits Yap nuclear accumulation through mechanisms still unclear. Gα12/13 inhibits Lats1/2 activity through Rho-mediated F-Actin accumulation
Hippo-Yap/Taz and Tgfβ signaling pathways converge at several levels (Figure 6a). At a transcriptional level, Tgfβ signaling induces Taz (Miranda et al., 2017; Varelas et al., 2008). In human ES cells this establishes a feedback loop, as Taz directly controls Smad subcellular localization in a Tgfβ-dependent manner (Varelas et al., 2008). Subsequent studies showed that in the epithelial Eph4 cell line Smad subcellular localization was Hippo-dependent (Varelas et al., 2010). Disruption of junctional complexes through calcium depletion or α-catenin ablation abrogated Hippo-dependent contact inhibition, allowing nuclear accumulation of both Yap/Taz and Smads, promoting Tgfβ signaling and epithelial-to-mesenchymal transition. The Crumbs polarity complex has been implicated in this mechanism, such that acquisition of apical-basal polarity and establishment of the Crumbs complex activates Hippo kinases, sequestering Yap/Taz and Smad concurrently to the cytoplasm. However, another study asserted a Crumbs-independent mechanism using a variety of cell lines, including Eph4 cells. Here, apical-basal polarity leads to sequestration of Tgfβ receptors to the basolateral aspect of Eph4 cells, which disrupts Tgfβ ligand-receptor interactions, since Tgfβ ligand is only available apically (Nallet-Staub et al., 2015). From these observations an overarching model emerges in which Crumbs-medicated apical-basal polarity gradually sequesters Yap/Taz and Smads to the cytoplasm. As the cell fully polarizes, Tgfβ receptors become sequestered to the basolateral aspect of epithelial cells and Tgfβ signaling is fully attenuated (Narimatsu, Samavarchi-Tehrani, Varelas, & Wrana, 2015). It is also worth noting that Yap/Taz inhibit Tgfβ signaling through Smad7, an inhibitor of Tgfβ signaling (Aragón et al., 2012; Ferrigno et al., 2002; Qin, Xia, Fisher, Voorhees, & Quan, 2018). Yap interacts directly with Smad7 in HaCaT keratinocytes, potentiating the inhibitory effect of Smad7 on Tgfβ signaling. Furthermore, at low Yap/Taz protein levels inhibition of AP-1-induced Smad7 expression is alleviated, allowing efficient Tgfβ signaling inhibition by Smad7 in human skin primary fibroblasts. Together, these studies highlight the cooperative action of Hippo and Tgfβ signaling in the promotion of quiescence and differentiation.
5.3 |. Other signaling pathways: Notch, Egf and Igf
A variety of other pathways, such as Notch, Egf and Igf, has been shown to converge with Hippo signaling to regulate epithelial cell behavior (Figure 6). Notch signaling is initiated when cell surface ligands (Delta; mammalian Delta-like, or Jagged) and Notch receptors bind on neighboring cells. Two cleavage events allow the Notch intracellular domain to translocate to the nucleus and induce target genes such as Hey and Hes, by interacting with transcription factor Rbpj (recombining binding protein suppressor of hairless) and nuclear effector Maml (mastermind-like) (Guruharsha, Kankel, & Artavanis-Tsakonas, 2012; Siebel & Lendahl, 2017). Hippo-Yap/Taz and Notch signaling converge at both the transcriptional level, where Yap/Taz induce Notch ligands or receptors, and at the protein level, cooperating in the transcriptional regulation of common targets (Totaro, Castellan, Di Biagio, & Piccolo, 2018) (Figure 6b). While these modes of Hippo-Notch crosstalk have been reported in a variety of cell types, few reports in epithelia exist (de Lima, Bonnin, Birchmeier, & Duprez, 2016; Rayon et al., 2014; Slemmons et al., 2017; Tschaharganeh et al., 2013; Watanabe et al., 2017; Yimlamai et al., 2014; J. Yu, Poulton, Huang, & Deng, 2008). In epidermal basal cells, Hippo-mediated mechanotransduction accumulates Yap in the nucleus and induces Notch ligand Delta-like 1 (Dll1). Yap-induced Dll1 prevents these cells from expressing Notch, but activates Notch signaling in neighbor cells to induce their differentiation (Totaro et al., 2017). This mechanism was also demonstrated in intestinal organoids, wherein cells have different levels of nuclear Yap. Since Yap induces Dll1, this results in different Dll1 expression levels among cells. Cells with low Dll1 expression levels then upregulate Notch and activate Notch signaling in these cells, promoting their differentiation into Paneth cells (Serra et al., 2019). Furthermore, villin-gp130Act transgenic mice, expressing a constitutively active form of the IL-6 co-receptor gp130 in intestinal epithelial cells, show a decrease in secretory cell type diversity (Taniguchi et al., 2015). Because villin-gp130Act intestinal epithelial cells have increased levels of nuclear Yap, Notch receptors and ligands, it was suggested that Yap-mediated intestinal regeneration relies on Notch signaling to reconstitute the normal intestinal epithelium. Yap has an additional role in the transient emergence and expansion of the so-called “revival stem cells” in vitro, which have been suggested to reconstitute the crypt-base columnar stem cells and repopulate the injured intestinal epithelium (Ayyaz et al., 2019). However, Hippo-Notch crosstalk is also likely to have a role during differentiation of the embryonic lung. For example, Yap deletion in epithelial progenitors of the developing airways results in an imbalance in secretory versus multiciliated differentiation and a resulting excess in multiciliated cells (van Soldt et al., 2019), a phenotype typically seen in Notch-deficient mutant mice (Mori et al., 2015; Tsao et al., 2009). How Notch and Yap interact in this context is, however, unclear. In summary, Hippo-Notch crosstalk is context-dependent and frequently comprises direct induction of Notch genes by Yap.
The Hippo pathway converges in a more defined fashion with epidermal growth factor (Egf) signaling (Figure 6c). By binding to Egfr, Egf, Epiregulin (Ereg), and Amphiregulin (Areg) activate Ras signaling, promoting proliferation and survival. Early studies noted that Merlin is involved in contact-dependent Egfr inhibition through Egfr compart-mentalization and internalization in a number of cell lines, including liver-derived epithelial cells (Cole, Curto, Chan, & McClatchey, 2007; Curto, Cole, Lallemand, Liu, & McClatchey, 2007). A subsequent study reported that, in both Drosophila and mammalian epithelial cell lines, Egfr-Ras-MAPK signaling activates Yap by inhibiting Lats1/2 through its interaction with the Ajuba family protein Wilms tumor interacting protein (Wtip) (Reddy & Irvine, 2013), but also though the depletion of ubiquitin ligase complex substrate recognition factors SOCS5/6. Without SOCS5/6, Yap can no longer be adequately degraded, resulting in its nuclear accumulation and induction of Areg expression, perpetuating a tumorigenic positive feedback loop (X. Hong et al., 2014). Unexpectedly, Yap-induced Areg expression can increase proliferation non-cell-autonomously, as shown in MCF10A breast epithelial cells (J. Zhang et al., 2009). Furthermore, in the developing mouse submandibular gland, Yap induces Ereg to specify ductal epithelial progenitor cell identity (Szymaniak et al., 2017). A link between Ereg and Yap was also established in intestinal regeneration, although the mechanism remains unclear (Wrana, Inanlou, Khomchuk, Gregorieff, & Liu, 2015). Another study showed that Egfr is a Yap target in mouse mammary epithelial (NMuMG) cells (Y. Liu et al., 2017). Together, these studies provide evidence for the convergence of Egf and Hippo signaling at multiple levels to stimulate proliferation and growth.
Relatively fewer reports have described Hippo-Igf crosstalk (Figure 6d). Igf signaling is activated by binding of Igf-1 or Igf-2 ligands with Igf-1 receptor (Igfr1), promoting cell survival through PI3K and Akt signaling (Brouwer-Visser & Huang, 2015; Vincent & Feldman, 2002). Studies in cardiac regeneration highlighted Hippo-Igf convergence at the transcriptional level, such that Yap induces Igfr1 in cardiomyocytes to induce proliferation and, consequently, heart growth (Xin et al., 2011, 2013). A study in Drosophila reported that Igfr1 activates Yki by inhibiting Hippo through Akt and PDK1 in a PI3K-mediated mechanism (Straßburger, Tiebe, Pinna, Breuhahn, & Teleman, 2012). Thus, Igf signaling typically induces nuclear Yap, although no data in epithelial cells is available.
Recent studies have begun to characterize Hippo-Hgf and Hippo-Fgf crosstalks. Hgf induces nuclear Yap localization in MDCK cells through several pathways that inhibit Hippo kinase activity, including Src activation (Farrell et al., 2014). In agreement with these data, Hgf signaling in the pancreatic cancer cell line Panc-1 leads to Yap nuclear accumulation and HIF1α stabilization, ultimately promoting glucose metabolism through increased Hexokinase 2 (HK2) activity, which was speculated to facilitate cancer stem cell properties, such as increased self-renewal ability (Yan et al., 2018). Regarding Hippo-Fgf crosstalk, evidence suggests that activation of Fgf receptors induces Yap gene expression and nuclear accumulation in a cholangiocarcinoma model, resulting in increased cell proliferation. In turn, Fgfr expression is induced by Yap in cooperation with the transcription factor T-box 5 (Tbx5) (Rizvi et al., 2016). In the developing lens epithelium Fgf signaling-induced proliferation is mediated through nuclear accumulation and transcriptional activity of Yap (Dawes, Shelley, McAvoy, & Lovicu, 2018). Together these studies suggest that Hgf and Fgf signaling stimulate Yap nuclear accumulation and transcriptional activity.
5.4 |. Intracellular metabolic pathways
Hippo interactions with metabolic pathways in the regulation of epithelial cell behavior has received substantial interest in the recent literature. A pioneering study demonstrated that Taz subcellular localization is mTOR-dependent in the hepatocellular carcinoma cell line HepG2 (Chiang & Martinez-Agosto, 2012). Mechanistic links were subsequently described in MCF10A cells, wherein Yap downregulated the mTOR inhibitor PTEN (phosphatase and tensin homolog) in a miR-29-dependent manner (Tumaneng et al., 2012) (Figure 7a). This mechanism implied that Yap subcellular localization is a determinant of mTOR activity. Indeed, both Hippo-dependent and independent mechanisms have been reported. For example, Sav1, the Hippo/Mst1/2 cofactor, represses Akt–mTOR activation in DLD-1 epithelial colorectal cancer cells in a Yap-dependent manner. Importantly, Sav1 downregulation is a potential driver of colorectal tumorigenesis (S. Zhang, Gao, et al., 2017). A Lats-independent mechanism was demonstrated in dental transit amplifying epithelia, where an Integrin α3-FAK-Cdc42-PP1a signaling cascade (Hu et al., 2017) dephosphorylates Yap, promoting nuclear Yap and mTOR activity.
FIGURE 7.
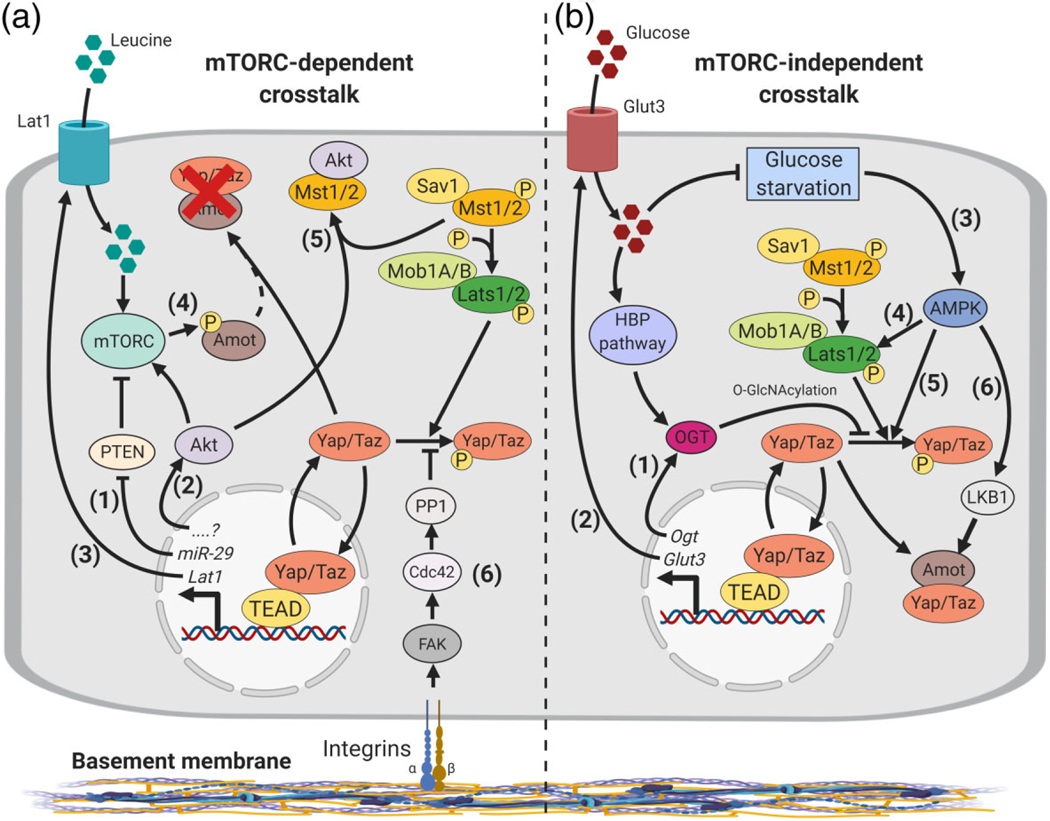
Hippo-Yap/Taz crosstalks with metabolic pathways. (a) mTORC-related metabolic pathways and Yap/Taz interact at multiple levels. Nuclear Yap/Taz: (1) induces mTORC activity through miR-29 induction, which inhibits PTEN, a potent mTORC inhibitor; (2) enhances Akt activity by a currently unclear mechanism leading to increased mTORC activity; (3) induces expression of Lat1, a Leucine transporter, increasing amino acid uptake, which further stimulates mTORC activity. mTORC sustains nuclear Yap/Taz by inhibiting Amot through phosphorylation, which disables Amot-Mst1/2 inhibitory interaction (4). This effect is enhanced by Akt inhibition of Mst1/2 activity (5). Integrin-FAK-Cdc42-PP1 signaling inhibits Yap/Taz phosphorylation leading to nuclear accumulation enhancing mTORC activity (6). (b) mTORC-independent pathways and Hippo. Nuclear Yap/Taz: (1) upregulates Ogt leading to O-GlcNAcylation of Yap/Taz and inhibition of Lats1/2-mediated phosphorylation; (2) upregulates Glut3, increasing glucose uptake, which further stimulates Yap/Taz O-GlcNAcylation and prevents glucose starvation. Glucose starvation inhibits Yap/Taz nuclear accumulation by activating AMPK (3). AMPK promotes Yap/Taz cytoplasmic sequestration by activating Lats1/2 (3), directly phosphorylating Yap/Taz (4) and promoting Amot-Yap/Taz inhibitory interaction through LKB1 (5)
PTEN-independent mechanisms have also been described (Figure 7a). In 293a cells, Yap/Taz control mTORC1 activity though upregulation of the L-type amino acid transporter 1 (Lat1, also known as Slc7a5), a leucine transporter that increases amino acid uptake and activates mTORC1 (Hansen, Ng, Lam, Plouffe, & Guan, 2015). Other mechanisms include inhibitory interactions of Mst1 with Akt in a glioma model (Gao et al., 2014), and mTOR-dependent Yap autophagy in a tuberous sclerosis complex (TSC) model (Liang et al., 2014), which blunts Yap transcriptional activity in a Hippo-independent manner. Intriguingly, angiomotin family proteins may also be coopted by mTORC to regulate Yap transcriptional output: in glioblastoma, Amotl2 is phosphorylated by mTORC2, blocking its ability to bind and repress Yap, thus promoting Yap transcriptional activity (Artinian et al., 2015).
Metabolic pathways other than mTOR also cooperate with Hippo-Yap/Taz (Figure 7b). Glucose starvation, an important cellular stressor, was found to regulate AMPK to induce Yap cytoplasmic sequestration (DeRan et al., 2014; Mo et al., 2015; Nguyen, Babcock, Wells, & Quilliam, 2013; Wang et al., 2015). AMPK directly phosphorylates Yap at S94, interrupting Yap-TEAD interactions, but also directly activates Lats1/2. AMPK phosphorylates and stabilizes Amotl1, a mechanism mediated by LKB1 that results in Yap cytoplasmic sequestration in a range of nonepithelial cell lines. In addition, the glucose sensing hexosamine biosynthetic pathway promotes Yap transcriptional activity by disrupting Yap-Lats1/2 interaction through O-GlcNAcylation of Yap. Moreover, a Yap mutation that abrogates its O-GlcNAcylation inhibits tumorigenic growth of L3.6 pancreatic cancer cells (Peng et al., 2017). Intriguingly, there is evidence of positive feedback loops between Hippo and these pathways, such that Yap nuclear accumulation reinforces both its own nuclear localization as well as cellular metabolic activity. For example, there is evidence that mTORC upregulates Yap to induce glucose transporter 3 (Glut3), promoting glucose metabolism and OGT, ultimately responsible for O-GlcNAcylation of Yap (Liang et al., 2014; Peng et al., 2017; Wang et al., 2015).
Together, these studies demonstrate that pathways that control cellular metabolism and energy stress are intricately linked with Hippo signaling. Nutrient or energy deficit sequesters Yap to the cytoplasm, while energy abundance allows Yap nuclear accumulation. Subsequent Yap transcriptional activity amplifies metabolic activity. Thus, these pathways cooperate to synchronize cellular energy demand and production.
5.5 |. G-protein-coupled receptor signaling
The family of G-protein-coupled receptors (GPCRs) constitutes a class of multifunctional, diverse extracellular signaling receptors. Activated by an extensive family of ligands, such as thrombin, lysophosphatidic acid (LPA), gastrin-releasing peptide, and endothelin, these receptors are characterized by seven membrane-spanning domains (Figure 6e). When activated, the heterotrimeric Gαβγ complex, held together by GDP, interacts with the GPCR, promoting the exchange of GDP with GTP. This induces the release of Gα, of which there are four subtypes (α12/13, αq/11, αi/o, and αs), from Gβγ, and consequent signal transduction (Dorsam & Gutkind, 2007; Hilger, Masureel, & Kobilka, 2018).
An extensive study surveyed representative members of GPCR subgroups and showed consistent interactions with Hippo-Yap signaling in a G-protein α-subunit-dependent fashion (F. X. Yu et al., 2012). Initially this study reported transient Yap/Taz dephosphorylation after adding serum to a diverse array of cell lines, including MCF10A. Further analyses demonstrated that the serum-components LPA and sphingosine-1-phosphate (S1P) signal through the GPCRs LPA1 and S1P2 to dephosphorylate Yap and augment Yap transcriptional activity. These effects were mediated by the α12/13 G-protein subunit to RhoA, modulating actin dynamics to inhibit Lats1/2 activity. Overexpressing a breadth of GPCRs, as well as constitutively active Gα subunits, showed that only signaling through Gαs stimulated Lats1/2. This indicated that the majority of GPCR signaling likely promotes Yap nuclear accumulation and transcriptional activity. Subsequent studies corroborated the Yap-activating role of S1P (Miller et al., 2012), and identified activating mutations in G-protein subunits in cancer (Cai & Xu, 2013; Feng et al., 2014, 2019; Jang et al., 2012; Van Raamsdonk et al., 2009; F. X. Yu et al., 2014). Thus, GPCR signaling is a significant regulator of Hippo signaling, typically associated with Yap nuclear accumulation and transcriptional activity.
6 |. AFTER SPECIFICATION: HIPPO-YAP/TAZ IN EPITHELIAL CELL MATURATION
There is evidence that cytoplasmic sequestration of Yap/Taz regulates unique aspects of differentiation of the mature epithelium. This is well illustrated by their reported role in the formation of primary cilia or multicilia in epithelial progenitors during organogenesis (Grampa et al., 2016; Habbig et al., 2011; Hossain et al., 2007; J. Kim et al., 2015; M. Kim, Kim, Lee, Kim, & Lim, 2014). Analysis of mouse mutants carrying a germline deletion of Taz revealed cystic kidneys and epithelial tubules with fewer and shorter primary cilia, a phenotype associated with downregulation of ciliary genes. The requirement of Taz for proper integrity and function of monocilia suggested its potential involvement in the pathogenesis of kidney glomerulocystic disease. The association of Hippo-Yap/Taz activity with ciliary function was strengthened further by evidence that in HEK293T and MCF-7 cells the ciliary protein Nephronophthisis 4 (NPHP4) can inhibit the Yap association with Lats1 to promote Yap transcriptional activity. Additional studies identified biochemical interactions with other ciliary proteins, such as NIMA related kinase 8 (Nek8, also known as NPHP9), resulting in downregulation of Hippo signaling and ciliogenesis in other cell types. Two molecular mechanisms directly involving Mst1/2-Sav1 in promoting primary ciliogenesis have also been described. One interaction involves Mst1/2-Sav1-mediated inhibition of an Aurora Kinase A (AURKA)-HDAC6 complex, inducing dissociation of the cilia-disassembly complex. Mst1/2-Sav1 also associates with the NPHP transition-zone complex, which promotes ciliary localization of multiple cilia-related cargoes.
Formation of mature multicilia in epithelial cells undergoing multiciliated cell differentiation is also dependent on endogenous Yap. Yap knockdown prevents multiciliogenesis in airway epithelial progenitors differentiating in air-liquid interface culture (Mahoney et al., 2014). Consistent with this, prenatal disruption of endogenous Yap in airway epithelial progenitors also disrupts multiciliogenesis (van Soldt et al., 2019). Interestingly, in the developing airway epithelial progenitors Yap is essentially cytoplasmic and this subcellular localization is crucial for differentiation. If endogenous Yap is substituted by a Yap transgene that is unable to undergo nucleocytoplasmic shuttling and remains constitutively active in the nucleus, multiciliogenesis is abrogated. In these mutants, airway progenitors lose their identity and turn on a program of distal AT1 cell fate ectopically at proximal sites. These data suggest that, during normal lung development, cytoplasmic sequestration of Yap in the airway epithelium is critical to allow proper region-specific cell differentiation.
7 |. CONCLUSION
Our understanding of Hippo-Yap signaling has expanded greatly in recent years, illuminating an intriguing context-dependent diversity of the effects in cellular behavior. Whether cells will be instructed to migrate and proliferate, or be stationary, quiescent and differentiate, appears to depend on a unique local network of Yap/Taz interactions with other pathways, cell adhesion and structural proteins among other molecules discussed here. The very dynamic nature of processes, such as those during organogenesis, makes the Hippo pathway ideally suited as a switch that rapidly alters Yap/Taz subcellular localization, allowing interactions with different partners for proper cellular responses. In spite of this significant context-dependence, a set of regulatory stimuli can be discerned that broadly govern the subcellular localization of Yap in epithelial cells. Overall Yap nuclear accumulation is induced by cues from the basal lamina, cytoskeletal tension, nutrient availability and growth factor or GPCR signaling, while cytoplasmic Yap sequestration is generally the result of the establishment of cell–cell junctions, attainment of apical-basal polarity, or nutrient depletion.
While the integrative function of Hippo-Yap signaling appears at first overly elaborate and complex, it is also exquisitely intuitive. Yap signaling is coupled to intracellular sensory pathways, forming feedback loops that enable controlled proliferation or another cell behavior to properly respond to a given environmental cue. For example, growth factor signaling may promote nuclear Yap for expansion of a particular cell population. However, this will also require activation of metabolic pathways to fuel proliferation as well as nutrient sensing to monitor and control energy availability and consumption, thus synchronizing cellular energy demand and production. Nutrient depletion sequesters Yap to the cytoplasm to reduce energy consumption. Cell–cell contact, which signals cellular crowding, sequesters Yap to the cytoplasm and attenuates proliferation. This may trigger quiescence or differentiation, potentially determined by the extent to which these cells contact the basal lamina. In tissues, such as the airway epithelium, this sets apart basal cells from luminal cells and highlights the potential responses of the epithelium in response to injury.
However, knowledge of Hippo crosstalks with other key pathways, such as Igf, Hgf, and Fgf, is currently rudimentary. This is particularly surprising in regard to Hippo-Igf, given the widespread function of Igf signaling in development and homeostasis and its anti-apoptotic and pro-proliferative effects, which align well with the Yap canonical function. This is also true for Hippo-Fgf crosstalk, given the widespread function of Fgf signaling in development. In addition, a large part of the studies discussed here were performed in vitro using various cell lines, some of them transformed or cancer-derived cells. In the present review we aimed to emphasize the role of Hippo in epithelial behavior but much of the information available is on non-epithelial cell lines. Given the prominent context-dependent responses of Hippo-Yap/Taz, care should be exercised in interpreting these results.
Current studies typically refer to phospho-Yap as “inactive”, suggesting that it does not have a function per se. In fact, accumulated evidence suggest that phospho-Yap has important roles as a bona fide regulator of epithelial differentiation and maturation. Further studies clarifying the roles of phospho-Yap are therefore necessary and are likely to uncover another layer of previously unrecognized Yap/Taz interacting proteins and roles in development, homeostasis and disease states.
Another current gap of knowledge is on the precise mechanism regulating the dynamics of nucleocytoplasmic shuttling of Yap/Taz and their transient preferential localization in subpopulations of cells. A better understanding of the mechanisms that underlie the context-dependence of Yap target induction is particularly important with respect to tumorigenesis. Hippo control over Yap subcellular localization is frequently abolished due to mutations or hypermethylation of Hippo components (Bao, Hata, Ikeda, & Withanage, 2011). Consistent with this nuclear Yap is often correlated with poor prognosis (Poma, Torregrossa, Bruno, Basolo, & Fontanini, 2018). While aberrant Yap transcriptional activity is typically reported in cancer, it is unclear whether a common Yap tumorigenic transcriptional profile exists.
Perhaps as a consequence of these complexities and uncertainties, there are currently limited options for pharmacological manipulation of key Hippo signaling components in vivo. Future studies addressing these issues will open new perspectives for effectively targeting this pathway in biological processes, including human conditions, such as cancer.
ACKNOWLEDGMENT
We thank the Cardoso’s lab for helpful discussion. Figures were created with BioRender.com.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Number: NIH-NHLBI R35-HL135834–01
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIRES ARTICLES
The regulation and function of the Hippo pathway in heart regeneration
Mechanotransduction: A major regulator of homeostasis and development
Kidney: Polycystic kidney disease
Normal morphogenesis of epithelial tissues and progression of epithelial tumors
REFERENCES
- Aaron LT, Franco OE, & Hayward SW (2016). Review of prostate anatomy and embryology and the etiology of benign prostatic hyper-plasia. The Urologic Clinics of North America, 43, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie M. (1979). Contact inhibition and malignancy. Nature, 281, 259–262. [DOI] [PubMed] [Google Scholar]
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, & Hoschuetzky H. (1994). Assembly of the cadherin-catenin complex in vitro with recombinant proteins. Journal of Cell Science, 107(Pt 1), 3655–3663. [DOI] [PubMed] [Google Scholar]
- Aragón E, Goerner N, Xi Q, Gomes T, Gao S, Massagué J, & MacIas MJ (2012). Structural basis for the versatile interactions of Smad7 with regulator WW domains in TGF-β pathways. Structure, 20, 1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, … Piccolo S. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell, 154, 1047–1059. [DOI] [PubMed] [Google Scholar]
- Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, & Gera J. (2015). Phosphorylation of the Hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. The Journal of Biological Chemistry, 290, 19387–19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, & Massey-Harroche D. (2008). Polarity complex proteins. Biochimica et Biophysica Acta – Biomembranes, 1778, 614–630. [DOI] [PubMed] [Google Scholar]
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, … Gregorieff A. (2019). Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature, 569, 121–125. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, … Piccolo S. (2014). YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell, 158, 157–170. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, … Piccolo S. (2012). Role of TAZ as mediator of wnt signaling. Cell, 151, 1443–1456. [DOI] [PubMed] [Google Scholar]
- Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, & McNeill H. (2009). The FERM-domain protein expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Developmental Cell, 16, 411–420. [DOI] [PubMed] [Google Scholar]
- Bae JS, Jeon Y, Kim SM, Jang JY, Park MK, Kim IH, … Lee H. (2018). Depletion of MOB1A/B causes intestinal epithelial degeneration by suppressing Wnt activity and activating BMP/TGF-β signaling. Cell Death & Disease, 9, 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SJ, Ni L, Osinski A, Tomchick DR, Brautigam CA, & Luo X. (2017). SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. Elife, 6, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasooriya G, Goschorska M, Piddini E, & Rawlins EL (2017). FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development, 144, 1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Hata Y, Ikeda M, & Withanage K. (2011). Mammalian Hippo pathway: From development to cancer and beyond. Journal of Biochemistry, 149, 361–379. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, … Camargo FD (2013). Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature, 493, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, & Nelson WJ (2015). Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science (80-), 348, 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, & Harvey KF (2006). Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Current Biology, 16, 2101–2110. [DOI] [PubMed] [Google Scholar]
- Blair S, & McNeill H. (2018). Big roles for fat cadherins. Current Opinion in Cell Biology, 51, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhovchuk F, Mesrouze Y, Izaac A, Meyerhofer M, Zimmermann C, Fontana P, … Chène P. (2019). Molecular and structural characterization of a TEAD mutation at the origin of Sveinsson’s chorioretinal atrophy. The FEBS Journal, 286, 2381–2398. [DOI] [PubMed] [Google Scholar]
- Brouwer-Visser J, & Huang GS (2015). IGF2 signaling and regulation in cancer. Cytokine & Growth Factor Reviews, 26, 371–377. [DOI] [PubMed] [Google Scholar]
- Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, & Hong JH (2014). Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death and Differentiation, 21, 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, & Xu Y. (2013). The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Communication and Signaling, 11, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, & Brummelkamp TR (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology, 17, 2054–2060. [DOI] [PubMed] [Google Scholar]
- Campbell HK, Maiers JL, & DeMali KA (2017). Interplay between tight junctions & adherens junctions. Experimental Cell Research, 358, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S, Gray MJ, Badouel C, Lange S, Einsiedler M, Srour M, … Robertson SP (2013). Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nature Genetics, 45, 1300–1308. [DOI] [PubMed] [Google Scholar]
- Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, … Mercurio AM (2015). A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes & Development, 29, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Koshy ST, Branco da Cunha C, Shin J-W, Verbeke CS, Allison KH, & Mooney DJ (2014). Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Materials, 13, 1–35. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Chen Q, Bossuyt W, Kopp A, Halder G, McNeill H, … Pan D. (2013). An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene, 33, 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J, & Martinez-Agosto JA (2012). Effects of mTOR inhibitors on components of the Salvador-Warts-Hippo pathway. Cell, 1, 886–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, & Irvine KD (2006). Delineation of a fat tumor suppressor pathway. Nature Genetics, 38, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Cho E, & Irvine KD (2004). Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development, 131, 4489–4500. [DOI] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, et al. (2013). Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell, 13, 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/β-catenin signaling in development and disease. Cell, 127, 469–480. [DOI] [PubMed] [Google Scholar]
- Cole BK, Curto M, Chan AW, & McClatchey AI (2007). Localization to the cortical cytoskeleton is necessary for Nf2/Merlin-dependent epidermal growth factor receptor silencing. Molecular and Cellular Biology, 28, 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, & Giancotti FG (2014). Molecular insights into NF2/Merlin tumor suppressor function. FEBS Letters, 588, 2743–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, … Piccolo S. (2011). The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell, 147, 759–772. [DOI] [PubMed] [Google Scholar]
- Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America, 107, 21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, & McClatchey AI (2007). Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. The Journal of Cell Biology, 177, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Fischer RS, Pan D, & Waterman CM (2016). YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II and phospho-YAP-independent pathway during extracellular matrix mechanosensing. The Journal of Biological Chemistry, 291, 6096–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, … Carroll TJ (2013). Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nature Cell Biology, 15, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, & Longmore GD (2010). Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Current Biology, 20, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Shelley EJ, McAvoy JW, & Lovicu FJ (2018). A role for Hippo/YAP-signaling in FGF-induced lens epithelial cell proliferation and fibre differentiation. Experimental Eye Research, 169, 122–133. [DOI] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, et al. (2014). Energy stress regulates Hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Reports, 9, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima JE, Bonnin MA, Birchmeier C, & Duprez D. (2016). Muscle contraction is required to maintain the pool of muscle progenitors via yap and notch during fetal myogenesis. eLife, 5, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoë-Ayari H, Al Kurdi R, Vallade M, Gulino-debrac D, & Riveline D. (2004). Membrane and acto-myosin tension promote clustering of adhesion proteins. Proceedings of the National Academy of Sciences of the United States of America, 101, 2229–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrokhotov O, Samsonov M, Sokabe M, & Hirata H. (2018). Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clinical and Translational Medicine, 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, & Gutkind JS (2007). G-protein-coupled receptors and cancer. Nature Reviews. Cancer, 7, 79–94. [DOI] [PubMed] [Google Scholar]
- Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, & Mauck RL (2015). Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophysical Journal, 108, 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, … Piccolo S. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474, 179–183. [DOI] [PubMed] [Google Scholar]
- Duquesne S, Liu K, Agarwala S, König S, Link S, Eimer S, … Lecaudey V. (2015). Amotl2a interacts with the Hippo effector Yap1 and the Wnt/β-catenin effector Lef1 to control tissue size in zebrafish. eLife, 4, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege N, Dowbaj AM, Jiang M, Howell M, Hooper S, Foster C, … Sahai E. (2018). Quantitative analysis reveals that actin and Srcfamily kinases regulate nuclear YAP1 and its export. Cell Systems, 6, 692–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, & Shilatifard A. (2010). Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Developmental Biology, 339, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, … Thompson BJ (2016). Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development, 143, 1674–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A, Vincent-Mistiaen ZI, & Thompson BJ (2016). YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. BioEssays, 38, 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui-Artola A, Andreu I, Beedle AE, Lezamiz A, Uroz M, Kosmalska AJ, … Roca-Cusachs P. (2017). Force triggers YAP nuclear entry by mechanically regulating transport across nuclear pores. Cell, 171, 1397–1410.e14. [DOI] [PubMed] [Google Scholar]
- Enomoto M, & Igaki T. (2012). Src controls tumorigenesis via JNK-dependent regulation of the Hippo pathway in Drosophila. EMBO Reports, 14, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J, Kelly C, Rauch J, Kida K, García-Muñoz A, Monsefi N, … von Kriegsheim A. (2014). HGF induces epithelial-tomesenchymal transition by modulating the mammalian Hippo/MST2 and ISG15 pathways. Journal of Proteome Research, 13, 2874–2886. [DOI] [PubMed] [Google Scholar]
- Feng X, Arang N, Rigiracciolo DC, Lee JS, Yeerna H, Wang Z, … Gutkind JS (2019). A platform of synthetic lethal gene interaction networks reveals that the GNAQ uveal melanoma oncogene controls the Hippo pathway through FAK. Cancer Cell, 35, 457–472.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, … Gutkind JS (2014). Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell, 25, 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, & Mauviel A. (2002). Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-β/Smad signaling. Oncogene, 21, 4879–4884. [DOI] [PubMed] [Google Scholar]
- Furukawa KT, Yamashita K, Sakurai N, & Ohno S. (2017). The epithelial circumferential actin belt regulates YAP/TAZ through nucleocytoplasmic shuttling of Merlin. Cell Reports, 20, 1435–1447. [DOI] [PubMed] [Google Scholar]
- Gao J, Liu X, Zhou X, Shi Y, Yu R, Wang Y, … Chao Y. (2014). Mst1 regulates glioma cell proliferation via the AKT/mTOR signaling pathway. Journal of Neuro-Oncology, 121, 279–288. [DOI] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, & Tapon N. (2010). Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Developmental Cell, 18, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, & McClatchey AI (2010). The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Developmental Cell, 19, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, & Gumbiner BM (2001). E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. The Journal of Cell Biology, 153, 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, & Zider A. (2008). SCALLOPED interacts with YORKIE, the nuclear effector of the Hippo tumor-suppressor pathway in Drosophila. Current Biology, 18, 435–441. [DOI] [PubMed] [Google Scholar]
- Grampa V, Delous M, Zaidan M, Odye G, Thomas S, Elkhartoufi N, … Saunier S. (2016). Novel NEK8 mutations cause severe syndromic renal cystic dysplasia through YAP dysregulation. PLoS Genetics, 12, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Yu QC, & Fuchs E. (1993). Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. The EMBO Journal, 12, 973–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, & Artavanis-Tsakonas S. (2012). The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nature Reviews. Genetics, 13, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbig S, Bartram MP, Müller RU, Schwarz R, Andriopoulos N, Chen S, … Schermer B. (2011). NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. The Journal of Cell Biology, 193, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Ng YLD, Lam WLM, Plouffe SW, & Guan KL (2015). The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Research, 25, 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, & Martin JF (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science (80-), 332, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, & Morrisey EE (2014). Lung development: Orchestrating the generation and regeneration of a complex organ. Development, 141, 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemer SE, Szymaniak AD, & Varelas X. (2014). The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells. The Journal of Biological Chemistry, 289, 13461–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger D, Masureel M, & Kobilka BK (2018). Structure and dynamics of GPCR signaling complexes. Nature Structural & Molecular Biology, 25, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Samsonov M, & Sokabe M. (2017). Actomyosin contractility provokes contact inhibition in E-cadherin-ligated keratinocytes. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, … Yaffe MB (2005). TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science (80-), 309, 1074–1078. [DOI] [PubMed] [Google Scholar]
- Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, Voorhoeve PM, & Cohen SM (2014). Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. The EMBO Journal, 33, 2447–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, … Hunziker W. (2007). Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proceedings of the National Academy of Sciences of the United States of America, 104, 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Wu Q, Sun X, Chen H, Li Y, Zhang Y, … Jiang M. (2019). Wnt/Fgf crosstalk is required for the specification of basal cells in the mouse trachea. Development, 146, dev171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK, & Juliano RL (2000). Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nature Cell Biology, 2, 593–600. [DOI] [PubMed] [Google Scholar]
- Hu JKH, Du W, Shelton SJ, Oldham MC, DiPersio CM, & Klein OD (2017). An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell, 21, 91–106.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, & Chen Y-G (2012). Regulation of TGF-β receptor activity. Cell & Bioscience, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HL, Wang S, Yin MX, Dong L, Wang C, Wu W, … Zhang L. (2013). Par-1 regulates tissue growth by influencing Hippo phosphorylation status and Hippo-Salvador Association. PLoS Biology, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, & Pan D. (2005). The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell, 122, 421–434. [DOI] [PubMed] [Google Scholar]
- Imajo M, Ebisuya M, & Nishida E. (2015). Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nature Cell Biology, 17, 7–19. [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, & Nishida E. (2012). A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. The EMBO Journal, 31, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanek R, Diepenbruck M, Waldmeier L, Berninger P, Arnold P, Christofori G, & van Nimwegen E. (2014). Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. Journal of Cell Science, 127, 1523–1536. [DOI] [PubMed] [Google Scholar]
- James MF, Manchanda N, Gonzalez-Agosti C, Hartwig JH, & Ramesh V. (2001). The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. The Biochemical Journal, 356, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EJ, Jeong H, Han KH, Kwon HM, Hong J-H, & Hwang ES (2012). TAZ suppresses NFAT5 activity through tyrosine phosphorylation. Molecular and Cellular Biology, 32, 4925–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janody F, Gaspar P, Fernandez BG, Jezowska B, Rebelo SR, & Bras-Pereira C. (2011). Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Journal of Cell Science, 124, e1. [DOI] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, … Yaffe MB (2000). TAZ: A novel transcriptional coactivator regulated by interactions with 14–3-3 and PDZ domain proteins. The EMBO Journal, 19, 6778–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, & Perrimon N. (2010). The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Journal of Cell Science, 123, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee J-K, … Kim J. (2015). Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nature Communications, 6, 6781. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park R, Lee JHJ, Shin J, Nickas J, Kim S, & Cho SH (2016). Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Developmental Biology, 419, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim M, Lee MS, Kim CH, & Lim DS (2014). The MST1/2-SAV1 complex of the Hippo pathway promotes ciliogenesis. Nature Communications, 5, 1–14. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, … Lim D-S (2013). cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. The EMBO Journal, 32, 1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N-G, & Gumbiner BM (2015). Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. The Journal of Cell Biology, 210, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N-G, Koh E, Chen X, & Gumbiner BM (2011). E-cadherin mediates contact inhibition of proliferation through Hippo signalingpathway components. Proceedings of the National Academy of Sciences of the United States of America, 108, 11930–11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M. (2007). A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the cofactors YAP and TAZ. Biochemical and Biophysical Research Communications, 361, 1022–1026. [DOI] [PubMed] [Google Scholar]
- Kofler M, Speight P, Little D, Di Ciano-Oliveira C, Szászi K, & Kapus A. (2018). Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nature Communications, 9, 4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh WLC, van Amerongen R, Tan SH, Lim X, Kuo CJ, Nusse R, … Klein AM (2013). Interfollicular epidermal stem cells selfrenew via autocrine Wnt signaling. Science (80-), 342, 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmaier A, Shaw RL, Veelken C, Polesello C, Tapon N, & Edgar BA (2010). The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Journal of Cell Science, 123, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsavage WM, Kyler SL, Rennoll SA, Jin G, & Yochum GS (2012). Wnt/β-catenin signaling regulates yes-associated protein (YAP) gene expression in colorectal carcinoma cells. The Journal of Biological Chemistry, 287, 11730–11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsavage WM, & Yochum GS (2013). Intersection of Hippo/YAP and Wnt/beta-catenin signaling pathways. Acta Biochimica et Biophysica Sinica, 45, 71–79. [DOI] [PubMed] [Google Scholar]
- Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, … Pan D. (2013). The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Developmental Cell, 25, 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, & Roop DR (2004). P63 is the molecular switch for initiation of an epithelial stratification program. Genes & Development, 18, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan J, Sczaniecka A, Arash EH, Nguyen L, Chen C-C, Ratkovic S, … Vasioukhin V. (2016). DLG5 connects cell polarity and Hippo signaling protein networks by linking PAR-1 with MST1/2. Genes & Development, 30, 2696–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Waghmare I, Verghese S, Singh A, Singh A, & Kango-Singh M. (2015). Drosophila C-terminal Src kinase regulates growth via the Hippo signaling pathway. Developmental Biology, 397, 67–76. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, & McClatchey AI (2003). NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes & Development, 17, 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Sridharan A, Xu Y, Stripp BR, Perl A-K, & Whitsett JA (2015). Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. Journal of Molecular Cell Biology, 7, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim NH, Cho ES, Yang JH, Cha YH, Kang HE, … Yook JI (2018). Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner. Nature Communications, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, & Shaul Y. (2008). Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Molecular Cell, 29, 350–361. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Z, Chu Q, Jiang K, Li J, & Tang N. (2018). The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Developmental Cell, 44, 297–312.e5. [DOI] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, & Vasioukhin V. (2016). αE-catenin inhibits a Src–YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes & Development, 30, 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou H, Li F, Chan SW, Lin Z, Wei Z, … Zhang M. (2015). Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Research, 25, 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, … Guan KL (2010). The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes & Development, 24, 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, … Pende M. (2014). Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. The Journal of Experimental Medicine, 211, 2249–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yao E, & Chuang P-T (2015). A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Developmental Biology, 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yao E, Zhang K, Jiang X, Croll S, Thompson-Peer K, & Chuang P-T (2017). YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife, 6, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, … Pan D. (2010). The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proceedings of the National Academy of Sciences of the United States of America, 107, 10532–10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang H, & Duan E. (2013). Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. International Journal of Molecular Sciences, 14, 10869–10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He K, Hu Y, Guo X, Wang D, Shi W, … Song J. (2017). YAP modulates TGF-β1-induced simultaneous apoptosis and EMT through upregulation of the EGF receptor. Scientific Reports, 7, 45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, … Tang N. (2016). MAPK-mediated YAP activation controls mechanical-tensioninduced pulmonary alveolar regeneration. Cell Reports, 16, 1810–1819. [DOI] [PubMed] [Google Scholar]
- Ma L, Cui J, Xi H, Bian S, Wei B, & Chen L. (2016). Fat4 suppression induces Yap translocation accounting for the promoted proliferation and migration of gastric cancer cells. Cancer Biology & Therapy, 17, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X, & Cardoso WV (2014). The Hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Developmental Cell, 30, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning SA, Dent LG, Kondo S, Zhao ZW, Plachta N, & Harvey KF (2018). Dynamic fluctuations in subcellular localization of the Hippo pathway effector Yorkie in vivo. Current Biology, 28, 1651–1660.e4. [DOI] [PubMed] [Google Scholar]
- Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, … Irvine KD (2011). Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development, 138, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Rauskolb C, Cho E, Hu W-L, Hayter H, Minihan G, … Irvine KD (2006). Dachs: An unconventional myosin that functions downstream of fat to regulate growth, affinity and gene expression in Drosophila. Development, 133, 2539–2551. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, & Mostov K. (2008). Regulation of cell polarity during epithelial morphogenesis. Current Opinion in Cell Biology, 20, 227–234. [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, & Yap AS (2007). E-cadherin adhesion activates c-Src signaling at cell–cell contacts. Molecular Biology of the Cell, 18, 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, … Guan KL (2015). MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nature Communications, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesrouze Y, Bokhovchuk F, Meyerhofer M, Fontana P, Zimmermann C, Martin T, … Chène P. (2017). Dissection of the interaction between the intrinsically disordered YAP protein and the transcription factor TEAD. eLife, 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld JB, Gestri G, Clark BS, Flinn MA, Poole RJ, Bader JR, … Link BA (2015). Yap and Taz regulate retinal pigment epithelial cell fate. Journal of Cell Science, 128, e1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld JB, & Link BA (2014). Establishment of transgenic lines to monitor and manipulate Yap/Taz-Tead activity in zebrafish reveals both evolutionarily conserved and divergent functions of the Hippo pathway. Mechanisms of Development, 133, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, Deran M, Wu C, Su AI, Bonamy GMC, … Wu X. (2012). Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chemistry & Biology, 19, 955–962. [DOI] [PubMed] [Google Scholar]
- Miranda MZ, Bialik JF, Speight P, Dan Q, Yeung T, Szászi K, … Kapus A. (2017). TGF-β1 regulates the expression and transcriptional activity of TAZ via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. The Journal of Biological Chemistry, 292, 14902–14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, & Yonemura S. (2006). Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Experimental Cell Research, 312, 1637–1650. [DOI] [PubMed] [Google Scholar]
- Miyazu M, Sokabe M, Naruse K, Matsushita E, & Wang JG (2002). Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochemical and Biophysical Research Communications, 288, 356–361. [DOI] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, … Guan KL (2015). Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nature Cell Biology, 17, 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, … Camargo FD (2014). A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nature Cell Biology, 16, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X, … Cardoso WV (2015). Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development, 142, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, … Herrlich P. (2001). The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes & Development, 15, 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallet-Staub F, Yin X, Gilbert C, Marsaud V, BenMimoun S, Javelaud D, … Mauviel A. (2015). Cell density sensing alters TGF-β signaling in a cell-type-specific manner, independent from Hippo pathway activation. Developmental Cell, 32, 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantie LB, Young RE, Paltzer WG, Zhang Y, Johnson RL, Verheyden JM, & Sun X. (2018). Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing. Development, 145. 10.1242/dev.163105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M, Samavarchi-Tehrani P, Varelas X, & Wrana JL (2015). Distinct polarity cues direct Taz/Yap and TGFβ receptor localization to differentially control TGFβ-induced Smad signaling. Developmental Cell, 32, 652–656. [DOI] [PubMed] [Google Scholar]
- Nguyen HB, Babcock JT, Wells CD, & Quilliam LA (2013). LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene, 32, 4100–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Zheng Y, Hara M, Pan D, & Luo X. (2015). Structural basis for Mob1-dependent activation of the core Mst–Lats kinase cascade in Hippo signaling. Genes & Development, 29, 1416–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue KI, Adachi K, Kiyonari H, Ota M, Ralston A, … Sasaki H. (2009). The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental Cell, 16, 398–410. [DOI] [PubMed] [Google Scholar]
- Oh H, Reddy BVVG, & Irvine KD (2009). Phosphorylation-independent repression of Yorkie in Fat-Hippo signaling. Developmental Biology, 335, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Slattery M, Ma L, Crofts A, White KP, Mann RS, & Irvine KD (2013). Genome-wide Association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Reports, 3, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Slattery M, Ma L, White KP, Mann RS, & Irvine KD (2014). Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Reports, 8, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, & Giancotti FG (2005). Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. The Journal of Cell Biology, 171, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudhoff MJ, Braam MJS, Freeman SA, Wong D, Rattray DG, Wang J, … Zaph C. (2016). SETD7 controls intestinal regeneration and tumorigenesis by regulating Wnt/β-catenin and Hippo/YAP signaling. Developmental Cell, 37, 47–57. [DOI] [PubMed] [Google Scholar]
- Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH, … Zaph C. (2013). Control of the Hippo pathway by Set7-dependent methylation of Yap. Developmental Cell, 26, 188–194. [DOI] [PubMed] [Google Scholar]
- Pan D, Liu B, Wang W, Zheng Y, Uster E, & Deng H. (2015). Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Developmental Cell, 34, 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo J-S, Plouffe SW, … Guan KL (2015). Alternative Wnt signaling activates YAP/TAZ. Cell, 162, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Whitsett JA, Di Palma T, Hong JH, Yaffe MB, & Zannini M. (2004). TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. The Journal of Biological Chemistry, 279, 17384–17390. [DOI] [PubMed] [Google Scholar]
- Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X, … Pei H. (2017). Regulation of the Hippo-YAP pathway by glucose sensor OGlcNAcylation. Molecular Cell, 68, 591–604.e5. [DOI] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M, & Gumbiner BM (2007). E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Molecular Biology of the Cell, 18, 2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poma AM, Torregrossa L, Bruno R, Basolo F, & Fontanini G. (2018). Hippo pathway affects survival of cancer patients: Extensive analysis of TCGA data and review of literature. Scientific Reports, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, … Furutani-Seiki M. (2015). YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature, 521, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot N, & Kusuma N. (2013). Laminin-511: A multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis. Cell Adhesion & Migration, 7, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Xia W, Fisher GJ, Voorhees JJ, & Quan T. (2018). YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Communication and Signaling, 16, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni CV, Diguet N, Le Garrec JF, Novotova M, Resende TP, Pop S, et al. (2017). Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth. Nature Communications, 8, 14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Sun S, Sun G, Pan Y, & Irvine KD (2014). Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell, 158, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, & Hogan BLM (2009). The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development, 136, 3741–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, … Manzanares M. (2014). Notch and Hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental Cell, 30, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BVVG, & Irvine KD (2013). Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Developmental Cell, 24, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Zhang L, & Jiang J. (2010). Hippo signaling regulates Yorkie nuclear localization and activity through 14–3-3 dependent and independent mechanisms. Developmental Biology, 337, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Yamada D, Hirsova P, Bronk SF, Werneburg NW, Krishnan A, … Gores GJ (2016). A Hippo and fibroblast growth factor receptor autocrine pathway in cholangiocarcinoma. The Journal of Biological Chemistry, 291, 8031–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH, & Hogan BLM (2010). Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Disease Models & Mechanisms, 3, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, & Macara IG (2014). Organization and execution of the epithelial polarity programme. Nature Reviews. Molecular Cell Biology, 15, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano RA, Ortt K, Birkaya B, Smalley K, & Sinha S. (2009). An active role of the ΔN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One, 4, e5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. (2012). β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell, 151, 1457–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, Sasaki H, & Halder G. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway. The EMBO Journal, 30, 2325–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal R, Yan V, Kazakova L, Sheppard L, & Tepass U. (2019). Role of α-catenin and its mechanosensing properties in the regulation of Hippo/YAP-dependent tissue growth. bioRxiv, 567750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, … Camargo FD (2011). Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell, 144, 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra D, Mayr U, Boni A, Lukonin I, Rempfler M, Challet Meylan L, … Liberali P. (2019). Self-organization and symmetry breaking in intestinal organoid development. Nature, 569, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano I, McDonald PC, Lock F, Muller WJ, & Dedhar S. (2013). Inactivation of the Hippo tumour suppressor pathway by integrinlinked kinase. Nature Communications, 4, 2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu JY, Aires L, Lin Z, & Vogel V. (2018). Nanopillar force measurements reveal Actin-cap-mediated YAP mechanotransduction. Nature Cell Biology, 20, 262–271. [DOI] [PubMed] [Google Scholar]
- Si Y, Ji X, Cao X, Dai X, Xu L, Zhao H, … Zhao B. (2017). Src inhibits the Hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Research, 77, 4868–4880. [DOI] [PubMed] [Google Scholar]
- Siebel C, & Lendahl U. (2017). Notch signaling in development, tissue homeostasis, and disease. Physiological Reviews, 97, 1235–1294. [DOI] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, & McNeill H. (2006). The tumor-suppressor gene fat controls tissue growth upstream of expanded in the Hippo signaling pathway. Current Biology, 16, 2081–2089. [DOI] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien W-H, Klezovitch O, Rudakova GM, Camargo FD, … Vasioukhin V. (2011). α-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Science Signaling, 4, ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouloudaki K, Puetz M, Simons M, Courbard J-R, Boehlke C, Hartleben B, … Walz G. (2009). Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proceedings of the National Academy of Sciences of the United States of America, 106, 8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmons KK, Crose LES, Riedel S, Sushnitha M, Belyea B, & Linardic CM (2017). A novel notch–YAP circuit drives stemness and tumorigenesis in embryonal rhabdomyosarcoma. Molecular Cancer Research, 15, 1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum KL, Huang J, Magendantz M, Michaud NA, Crowley D, Kruger GM, … Jacks T. (2007). The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proceedings of the National Academy of Sciences of the United States of America, 104, 3261–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, & Irvine KD (2010). Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Current Biology, 20, 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, … Bauer A. (2015). YAP1 exerts its transcriptional control via TEADmediated activation of enhancers. PLoS Genetics, 11, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RA, Li DM, Huang H, & Xu T. (2003). A genetic screen for modifiers of the Lats tumor suppressor gene identifies C-terminal Src kinase as a regulator of cell proliferation in Drosophila. Oncogene, 22, 6436–6444. [DOI] [PubMed] [Google Scholar]
- Straßburger K, Tiebe M, Pinna F, Breuhahn K, & Teleman AA (2012). Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Developmental Biology, 367, 187–196. [DOI] [PubMed] [Google Scholar]
- Sun Z, Guo SS, & Fässler R. (2016). Integrin-mediated mechanotransduction. The Journal of Cell Biology, 215, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymaniak AD, Mahoney JE, Cardoso WV, & Varelas X. (2015). Crumbs3-mediated polarity directs airway epithelial cell fate through the Hippo pathway effector YAP. Developmental Cell, 34, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymaniak AD, Mi R, McCarthy SE, Gower AC, Reynolds TL, Mingueneau M, … Varelas X. (2017). The Hippo pathway effector YAP is an essential regulator of ductal progenitor patterning in the mouse submandibular gland. eLife, 6, e23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, De Jong PR, Lian I, Yu FX, … Karin M. (2015). A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature, 519, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, & Strutt D. (2012). The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Developmental Dynamics, 241, 27–39. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, & Cohen SM (2006). The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell, 126, 767–774. [DOI] [PubMed] [Google Scholar]
- Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, … Piccolo S. (2017). YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature Communications, 8, 15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro A, Castellan M, Di Biagio D, & Piccolo S. (2018). Crosstalk between YAP/TAZ and Notch signaling. Trends in Cell Biology, 28, 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P-N, Vasconcelos M, Izvolsky KI, Qian J, Lu J, & Cardoso WV (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development, 136, 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, … Breuhahn K. (2013). Yes-associated protein up-regulates jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology, 144, 1530–1542.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YR, Tseng KF, Lai HY, Lin CH, & Lee OK (2011). Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of Bone and Mineral Research, 26, 730–738. [DOI] [PubMed] [Google Scholar]
- Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, … Guan K-L (2012). YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nature Cell Biology, 14, 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DM, & Baker NE (2007). Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Developmental Biology, 305, 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, … Bastian BC (2009). Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature, 457, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soldt BJ, Qian J, Li J, Tang N, Lu J, & Cardoso WV (2019). Yap and its subcellular localization have distinct compartmentspecific roles in the developing lung. Development, 146. 10.1242/dev.175810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X. (2014). The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development, 141, 1614–1626. [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, … Wrana JL (2008). TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biology, 10, 837–848. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, … Wrana JL (2010). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Developmental Cell, 19, 831–844. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, & Depamphilis ML (2001). TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes & Development, 15, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri A, Lang C, & Lien WH (2018). Concise review: Wnt signaling pathways in skin development and epidermal stem cells. Stem Cells, 36, 22–35. [DOI] [PubMed] [Google Scholar]
- Vincent AM, & Feldman EL (2002). Control of cell survival by IGF signaling pathways. Growth Hormone & IGF Research, 12, 193–197. [DOI] [PubMed] [Google Scholar]
- Volckaert T, & De Langhe S. (2015). Wnt and FGF mediated epithelial–mesenchymal crosstalk during lung development. Developmental Dynamics, 244, 342–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, & De Langhe S. (2011). Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. The Journal of Clinical Investigation, 121, 4409–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Yuan T, Chao C-M, Bell H, Sitaula A, Szimmtenings L, … De Langhe SP (2017). Fgf10-Hippo epithelial–mesenchymal crosstalk maintains and recruits lung basal stem cells. Developmental Cell, 43, 48–59.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Yuan T, Yuan J, Boateng E, Hopkins S, Zhang J-S, … De Langhe S. (2019). Hippo signaling promotes lung epithelial lineage commitment by curbing Fgf10 and β-catenin signaling. Development, 146. 10.1242/dev.166454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, & Struhl G. (2015). Fat/Dachsous signaling promotes Drosophila wing growth by regulating the conformational state of the NDR kinase Warts. Developmental Cell, 35, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K-I, Itoga K, Okano T, Yonemura S, & Sasaki H. (2011). Hippo pathway regulation by cell morphology and stress fibers. Development, 138, 3907–3914. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, & Chen J. (2011). Angiomotin-like proteins associate with and negatively regulate YAP1. The Journal of Biological Chemistry, 286, 4364–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, & Chen J. (2015). AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nature Cell Biology, 17, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Miyasaka KY, Kubo A, Kida YS, Nakagawa O, Hirate Y, … Ogura T. (2017). Notch and Hippo signaling converge on Strawberry Notch 1 (Sbno1) to synergistically activate Cdx2 during specification of the trophectoderm. Scientific Reports, 7, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenfeld J, Shu W, Zhang L, Millar SE, & Morrisey EE (2002). The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. The Journal of Biological Chemistry, 277, 21061–21070. [DOI] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, … Pawson T. (2006). A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell, 125, 535–548. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen C, Tao C, … Halder G. (2006). The fat cadherin acts through the Hippo tumor-suppressor pathway to regulate tissue size. Current Biology, 16, 2090–2100. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Inanlou MR, Khomchuk Y, Gregorieff A, & Liu Y. (2015). Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature, 526, 715–718. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, & Pan D. (2008). The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental Cell, 14, 388–398. [DOI] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, … Olson EN (2013). Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America, 110, 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, … Olson EN (2011). Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Science Signaling, 4, ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Chen LF, Shigesada K, Murakami Y, & Ito Y. (1999). A WW domain-containing Yes-associated protein (YAP) is a novel transcriptional co-activator. The EMBO Journal, 18, 2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Jiang Z, Cheng L, Chen K, Zhou C, Sun L, … Lei J. (2018). Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1α. Experimental Cell Research, 371, 63–71. [DOI] [PubMed] [Google Scholar]
- Yang B, Sheetz M, Park S, Cui Y, Lee K, Hameed FM, & Pan CQ (2015). Cyclic stretching of soft substrates induces spreading and growth. Nature Communications, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-C, Graves HK, Moya IM, Tao C, Hamaratoglu F, Gladden AB, & Halder G. (2015). Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proceedings of the National Academy of Sciences of the United States of America, 112, 1785–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Riccio P, Schotsaert M, Mori M, Lu J, Lee D-K, … Cardoso WV (2018). Spatial–temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Developmental Cell, 44, 752–761.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu T, Wang G, Liang Z, Cui L, Liu C-Y, … Mei Z. (2015). Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial–mesenchymal transition and metastasis in a YAP-independent manner. Oncogene, 35, 2789–2800. [DOI] [PubMed] [Google Scholar]
- Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, … Yan J. (2014). Force-dependent conformational switch of α -catenin controls vinculin binding. Nature Communications, 5. [DOI] [PubMed] [Google Scholar]
- Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, … Kissil JL (2011). A tight junction-associated Merlinangiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell, 19, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, … Camargo FD (2014). Hippo pathway activity influences liver cell fate. Cell, 157, 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, & Pan D. (2013). Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell, 154, 1342–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, & Shibata M. (2010). α-Catenin as a tension transducer that induces adherens junction development. Nature Cell Biology, 12, 533–542. [DOI] [PubMed] [Google Scholar]
- Yoo G, Kim T, Chung C, Hwang D-S, & Lim D-S (2017). The novel YAP target gene, SGK1, upregulates TAZ activity by blocking GSK3β-mediated TAZ destabilization. Biochemical and Biophysical Research Communications, 3–9, 650–656. [DOI] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, … Guan KL (2014). Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell, 25, 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, … Guan KL (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell, 150, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Poulton J, Huang YC, & Deng WM (2008). The Hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS One, 3, e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, & Pan D. (2010). Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Developmental Cell, 18, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, Van Wijnen AJ, Stein JL, … Stein GS (2004). Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. The EMBO Journal, 23, 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, & Fuchs E. (2011). Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proceedings of the National Academy of Sciences of the United States of America, 108, 2270–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ji J-Y, Yu M, Overholtzer M, Smolen GA, Wang R, … Haber DA (2009). YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nature Cell Biology, 11, 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, & Jiang J. (2008). The TEAD/TEF family of transcription factor scalloped mediates Hippo signaling in organ size control. Developmental Cell, 14, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, … Pan D. (2010). The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental Cell, 19, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Pei C, Wang X, Xiang J, Sun BF, Cheng Y, … Yuan Z. (2017). A balance of Yki/Sd activator and E2F1/Sd repressor complexes controls cell survival and affects organ size. Developmental Cell, 43, 603–617.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Gao Y, Chang W, Fu Y, Jiang J, Zhang X, & Zhao C. (2017). SAV1 represses the development of human colorectal cancer by regulating the Akt-mTOR pathway in a YAP-dependent manner. Cell Proliferation, 50, e12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang M, Kim E, Lin S, Liu K, Lan X, & Que J. (2017). Development and stem cells of the esophagus. Seminars in Cell & Developmental Biology, 66, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, & Guan KL (2011). Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & Development, 25, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang C-Y, & Guan K-L (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFb-TRCP. Genes & Development, 24, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, … Guan KL (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & Development, 21, 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Xiong Y, Zhao S, Guan K-L, Lei Q-Y, Zhang H, … Bai F. (2008). TAZ promotes cell proliferation and epithelial– mesenchymal transition and is inhibited by the Hippo pathway. Molecular and Cellular Biology, 28, 2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, … Guan KL (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes & Development, 22, 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, … Rajagopal J. (2014). Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Developmental Cell, 30, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, Yu F, … Borok Z. (2018). Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. The Journal of Clinical Investigation, 128, 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Li L, & Zhao B. (2014). The regulation and function of YAP transcription co-activator. Acta Biochimica et Biophysica Sinica, 47, 16–28. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li D, Wang Y, Pei C, Liu S, Zhang L, … Zhang P. (2015). Brahma regulates the Hippo pathway activity through forming complex with Yki-Sd and regulating the transcription of Hrumbs. Cellular Signalling, 27, 606–613. [DOI] [PubMed] [Google Scholar]